Effect of In Vitro Culture of Long Shoot Tip on Variant Structure and Titer of Grapevine Viruses
Abstract
1. Introduction
2. Results
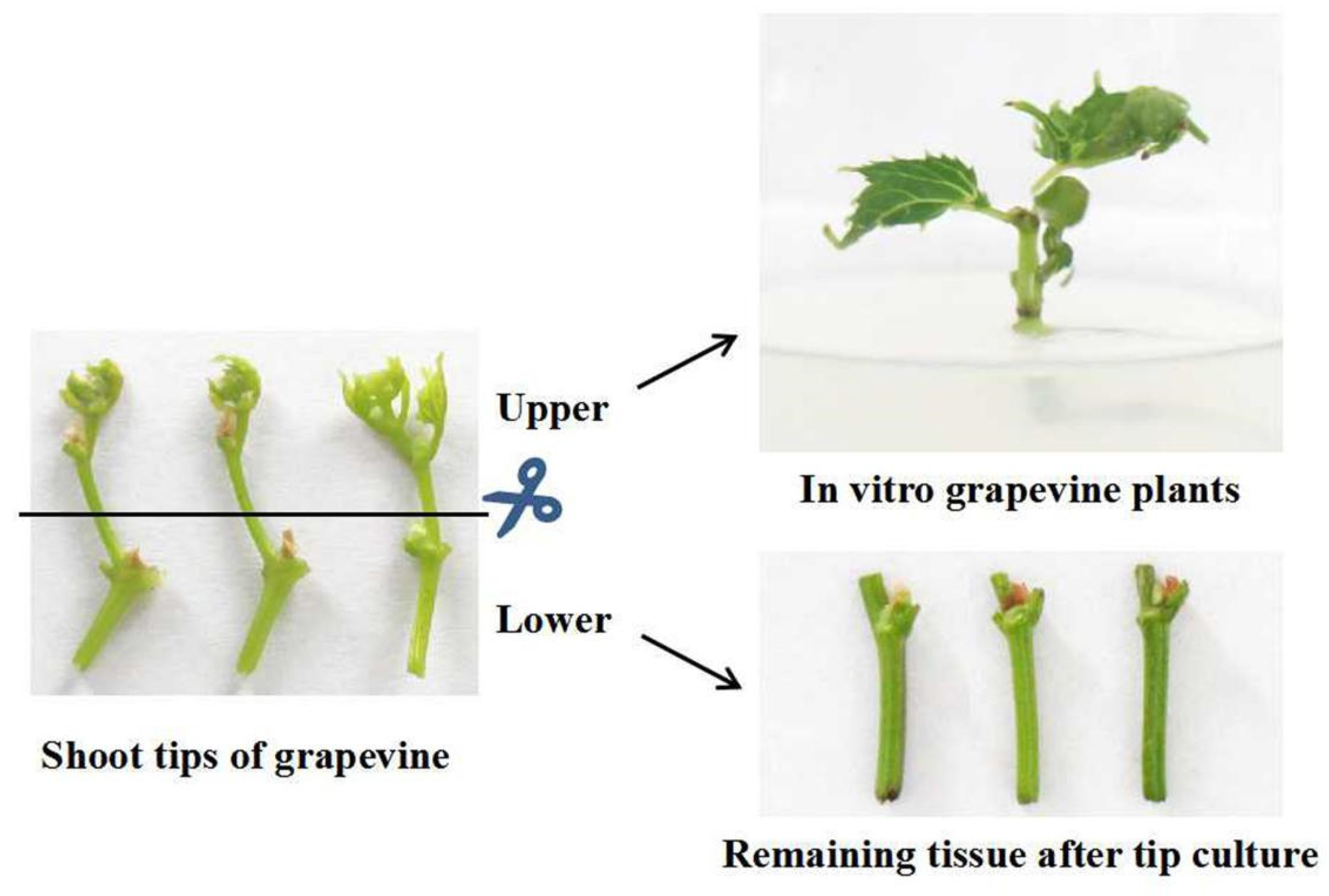
2.1. Survival of Shoot Tips
2.2. Virus Detection of In Vitro Grapevine Plantlets
2.3. Diversity of In Vitro Grapevine Plantlets
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Establishment of In Vitro Cultures
4.3. RNA Extraction, cDNA Synthesis and Regular PCR Amplification
4.4. Cloning and Sequencing
4.5. Quantitative Real-Time PCR (qPCR)
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Espinosa-Leal, C.A.; Puente-Garza, C.A.; García-Lara, S. In vitro plant tissue culture: Means for production of biological active compounds. Planta 2018, 248, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Doran, P.M. Foreign protein degradation and instability in plants and plant tissue cultures. Trends Biotechnol. 2006, 24, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Mourad, A. Elimination of grapevine bois noir phytoplasma by tissue culture coupled or not with heat therapy or hot water treatment. Adv. Crop Sci. Technol. 2013, 1, 1–4. [Google Scholar] [CrossRef]
- Wang, M.R.; Li, B.Q.; Feng, C.H.; Wang, Q.C. Culture of shoot tips from adventitious shoots can eradicate apple stem pitting virus but fails in apple stem grooving virus. Plant Cell Tiss. Org. Cult. 2016, 125, 283–291. [Google Scholar] [CrossRef]
- 5. Sim, S.T.; Khuu, N.; Shoulders, J.R.; Pudlo, W.; Hoang, N.H.; Golino, D.A. Elimination of rose viruses using microshoot tip tissue culture. Acta Hortic. 2019, 1232, 241–246. [Google Scholar] [CrossRef]
- Kang, H.C.; Shin, J.; Kim, D.H.; Park, S.J.; Han, C.L. Elimination of apple stem grooving virus from ‘mansoo’ pear (Pyrus pyrifolia L.) by an antiviral agent combined with shoot tip culture. J. Plant Biotechnol. 2016, 43, 391–396. [Google Scholar]
- Hu, G.J.; Dong, Y.F.; Zhang, Z.P.; Fan, X.D.; Ren, F.; Li, Z.N. Efficacy of virus elimination from apple by thermotherapy coupled with in vivo shoot-tip grafting and in vitro meristem culture. J. Phytopathol. 2017, 165, 701–706. [Google Scholar] [CrossRef]
- Bettoni, J.C.; Fazio, G.; Carvalho Costa, L.; Hurtado-Gonzales, O.P.; Rwahnih, M.A.; Nedrow, A.; Volk, G.M. Thermotherapy followed by shoot tip cryotherapy eradicates latent viruses and apple hammerhead viroid from in vitro apple rootstocks. Plants 2022, 11, 582. [Google Scholar] [CrossRef]
- Camborde, L.; Tournier, V.; Noizet, M.; Jupin, I. A Turnip yellow mosaic virus infection system in Arabidopsis suspension cell culture. FEBS Lett. 2007, 581, 337–341. [Google Scholar] [CrossRef][Green Version]
- Shih, S.M.; Doran, P.M. In vitro propagation of plant virus using different forms of plant tissue culture and modes of culture operation. J. Biotechnol. 2009, 143, 198–206. [Google Scholar] [CrossRef]
- Scorza, R.; Callahan, A.; Dardick, C.; Ravelandro, M.; Polak, J.; Malinowski, T.; Zagrai, I.; Cambra, M.; Kamenova, I. Genetic engineering of plum pox virus resistance: ‘Honey Sweet’ plum-from concept to product. Plant Cell, Tiss. Org. Cult. 2013, 115, 1–12. [Google Scholar] [CrossRef]
- Svensson, D.; Perveen, R.; Hirschfeld, C.; Robel, J.; Mühlbach, H.P. In vitro cultures of Sorbus aucuparia sustain replication of European mountain ash ringspot-associated virus (EMARaV). Plant Cell, Tiss. Org. Cult. 2014, 119, 441–445. [Google Scholar] [CrossRef]
- Ramgareeb, S.; Snyman, S.J.; Antwerpen, T.V.; Rutherford, R.S. Elimination of virus and rapid propagation of disease-free sugarcane (Saccharum spp. cultivar NCo376) using apical meristem culture. Plant Cell, Tiss. Org. Cult. 2010, 100, 175–181. [Google Scholar] [CrossRef]
- Špak, J.; Pavingerova, D.; Přibylová, J.; Špaková, V.; Sedlak, J. Blueberry red ringspot virus eliminated from highbush blueberry by shoot tip culture. Plant Protect. Sci. 2014, 50, 174–178. [Google Scholar] [CrossRef]
- Azad, M.; Hossen, M.I.; Khatun, Z.; Eaton, E.J.; Soren, E.B. Generation of virus free potato plantlets through meristem culture and their field evaluation. Am. J. Plant Sci. 2020, 11, 1827–1846. [Google Scholar] [CrossRef]
- Guerra, P.A.; Souza, E.H.; Andrade, E.C.; Max, D.A.S.; Oliveira, R.S.; Souza, F.V.D. Comparison of shoot tip culture and cryotherapy for eradication of ampeloviruses associated with Pineapple mealybug wilt in wild varieties. Vitro Cell Dev. Biol.-Plant 2020, 56, 903–910. [Google Scholar] [CrossRef]
- Yan, K.; Du, X.; Mao, B. Production of virus-Free chrysanthemum (Chrysanthemum morifolium Ramat) by tissue culture techniques. Methods in Molecular Biology 2022, 2400, 171–186. [Google Scholar]
- Tello, J.; Roux, C.; Chouiki, H.; Laucou, V.; Sarah, G.; Weber, A.; Santoni, S.; Flutre, T.; Pons, T.; This, P.; et al. A novel high-density grapevine (Vitis vinifera L.) integrated linkage map using GBS in a half-diallel population. Theor. Appl. Genet. 2019, 132, 2237–2252. [Google Scholar] [CrossRef]
- Blaisdell, G.K.; Zhang, S.; Rowhani, A.; Klaassen, V.; Almeida, R. Trends in vector-borne transmission efficiency from coinfected hosts: Grapevine leafroll-associated virus-3 and grapevine virus A. In Eur. J. Plant Pathol.; 2020; Volume 156, pp. 1163–1167. [Google Scholar]
- Fuchs, M. Grapevine viruses: A multitude of diverse species with simple but overall poorly adopted management solutions in the vineyard. J. Phytopathol. 2020, 102, 643–653. [Google Scholar] [CrossRef]
- Messmer, N.; Bohnert, P.; Schumacher, S. Studies on the occurrence of viruses in planting material of grapevines in southwestern Germany. Viruses 2021, 13, 248. [Google Scholar] [CrossRef]
- Han, Y.; Shi, X.; Yang, G.; Wang, X.; Liu, K.; Feng, X.; Ni, J. Shoot tip tissue virus-free culture and RT-PCR detection of GLRaV-3 in red globe grape. Chin. Agric. Sci. Bull. 2011, 27, 198–202. [Google Scholar]
- Hu, G.J.; Dong, Y.F.; Zhang, Z.P.; Fan, X.D.; Ren, F. Efficiency of chemotherapy combined with thermotherapy for eliminating grapevine leafroll-associated virus 3 (GLRaV-3). Sci. Hortic. 2020, 271, 109462. [Google Scholar] [CrossRef]
- Hu, G.J.; Dong, Y.F.; Zhang, Z.P.; Fan, X.D.; Ren, F. Elimination of grapevine fleck virus and grapevine rupestris stem pitting-associated virus from vitis vinifera ‘87-1′ by ribavirin combined with thermotherapy. J. Integr. Agr. 2021, 20, 2463–2470. [Google Scholar] [CrossRef]
- Li, C.; Fan, X.D.; Zhang, Z.P.; Hu, G.J.; Ren, F.; Zhang, B.D.; Dong, Y.F. Analysis of virus infection status based on RT-PCR detection of 6 flavoring grape varieties. China Fruits 2021, 2, 66–69. [Google Scholar]
- Wu, B.; Xu, D.K.; Jiang, S.S.; Zhang, M.; Wang, S.J.; Ma, Y.L.; Xin, Z.M. Distribution and pathogen identification of grape main virus diseases in Shandong province. Shandong Agric. Sci. 2019, 51, 73–77. [Google Scholar]
- Asakura, I.; Hoshino, Y. Evaluation of plant regeneration ability of different explants and establishment of an efficient regeneration system using immature seeds in actinidia kolomikta, a cold-hardy kiwifruit relative. Sci. Hortic. 2017, 220, 275–282. [Google Scholar] [CrossRef]
- Amano, R.; Momoi, R.; Omata, E.; Nakahara, T.; Kimura, S. Molecular and biochemical differences in leaf explants and the implication for regeneration ability in Rorippa Aquatica (Brassicaceae). Plants 2020, 9, 1372. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, A.; Kishine, S.; Hirosawa, T. Efficient plant regeneration by smashing callus in rice (Oriza Sativa L.) cell culture. Tissue Cult. Lett. 2010, 13, 291–295. [Google Scholar] [CrossRef]
- Alkhazindar, M. Elimination of tomato ppotted wilt virus (genus Tospovirus) from infected tomato plants by meristem tip culture. Egypt. J. Bot. 2015, 55, 149–159. [Google Scholar]
- Klimek-Chodacka, M.; Kadluczka, D.; Lukasiewicz, A.; Malec-Pala, A.; Grzebelus, E. Effective callus induction and plant regeneration in callus and protoplast cultures of Nigella Damascena L. Plant Cell Tiss. Org. Cult. 2020, 143, 693–707. [Google Scholar] [CrossRef]
- Moya, A.; Elena, S.F.; Bracho, A.; Miralles, R.; Barrio, E. The evolution of RNA viruses: A population genetics view. Proc. Natl. Acad. Sci. USA 2000, 97, 6967–6973. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, J.M.; Moya, A.; Sanjuán, R. Following the very initial growth of biological RNA viral clones. J. Gen. Virol. 2005, 86, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Simmons, H.E.; Holmes, E.C.; Stephenson, A.G. Rapid turnover of intra-host genetic diversity in zucchini yellow mosaic virus. Virus Res. 2011, 155, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.J.; Wang, G.P.; Hong, N. Effect of in vitro sub-culture of sandy pear on variant structure of apple stem grooving virus. Trop. Plant Pathol. 2019, 44, 302–307. [Google Scholar] [CrossRef]
- Schneider, W.L.; Roossinck, M.J. Evolutionarily related Sindbis-like plant viruses maintain different levels of population diversity in a common host. J. Virol. 2000, 74, 3130–3134. [Google Scholar] [CrossRef][Green Version]
- Murakishi, H.H.; Hartmann, J.X.; Pelcher, L.E.; Beachy, R.N. Improved inoculation of cultured plant cells resulting in high virus titer and crystal formation. Virology 1970, 41, 365–367. [Google Scholar] [CrossRef]
- Zelcer, A.; van Adelsberg, J.; Leonard, D.A.; Zaitlin, M. Plant cell suspension cultures sustain long-term replication of potato spindle tuber viroid. Virology 1981, 109, 314–322. [Google Scholar] [CrossRef]
- Bradamante, G.; Scheid, O.M.; Incarbone, M. Under siege: Virus control in plant meristems and progeny. Plant Cell 2021, 33, 2523–2537. [Google Scholar] [CrossRef]
- Waigmann, E.; Ueki, S.; Trutnyeva, K.; Citovsky, V. The ins and outs of nondestructive cell-to-cell and systemic movement of plant viruses. Crit. Rev. Plant Sci. 2004, 23, 195–250. [Google Scholar] [CrossRef]
- Hu, G.J.; Dong, Y.F.; Zhang, Z.P.; Fan, X.D.; Ren, F.; Li, Z.N.; Zhang, S.N. Elimination of Grapevine rupestris stem pitting-associated virus from Vitis vinifera ‘Kyoho’ by an antiviral agent combined with shoot tip culture. Sci. Hortic. 2018, 229, 99–106. [Google Scholar] [CrossRef]
- Osman, F.; Leutenegger, C.; Golino, D.; Rowhani, A. Comparison of low-density arrays, RT-PCR and real-time taqman RT-PCR in detection of grapevine viruses. J. Virol. Methods 2008, 149, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Minafra, A.; Saldarelli, P.; Grieco, F.; Martelli, G.P. Nucleotide sequence of the 3′ terminal region of the RNA of two filamentous grapevine viruses. Arch. Virol. 1994, 137, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Nolasco, G.; Mansinho, A.; Santos, M.T.; Soares, C.; Sequeira, Z.; Sequeira, C.; Sequeira, O.A. Large scale evaluation of primers for diagnosis of rupestris stem pitting associated virus-1. Eur. J. Plant Pathol. 2000, 106, 311–318. [Google Scholar] [CrossRef]
- Gambino, G.; Gribaudo, I. Simultaneous detection of nine grapevine viruses by multiplex reverse transcription-polymerase chain reaction with coamplification of a plant RNA as internal control. Phytopathology 2006, 96, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.D.; Dong, Y.F.; Zhang, Z.P.; Ren, F.; Hu, G.J.; Zhou, J. Detection and sequence analysis of grapevine leafroll-associated virus 2 isolates from China. J. Phytopathol. 2015, 163, 978–986. [Google Scholar] [CrossRef]
- Turturo, C.; Saldarelli, P.; Dong, Y.; Digiaro, M.; Martelli, G.P. Genetic variability and population structure of grapevine leafroll-associated virus 3 isolates. J. Gen. Virol. 2005, 86, 217–224. [Google Scholar] [CrossRef]
- Poojari, S.; Alabi, O.J.; Okubara, P.A.; Naidu, R.A. SYBR® Green-based real-time quantitative reverse-transcription PCR for detection and discrimination of grapevine viruses. J. Virol. Methods 2016, 235, 112–118. [Google Scholar] [CrossRef]
- Beuve, M.; Sempé, L.; Lemaire, O. A sensitive one-step real-time RT-PCR method for detecting grapevine leafroll-associated virus 2 variants in grapevine. J. Virol. Methods 2007, 141, 117–124. [Google Scholar] [CrossRef]
- Bester, R.; Pepler, P.T.; Burger, J.T.; Maree, H.J. Relative quantitation goes viral: An RT-qPCR assay for a grapevine virus. J. Virol. Methods 2014, 210, 67–75. [Google Scholar] [CrossRef]
- Wang, J.; Xi, D.; Liu, J.; Chen, K.; Li, H.; Liu, X.; Yan, S.; Ercisli, S.; Lin, H. Genetic variability in grapevine virus A from Vitis vinifera L.× Vitis labrusca L. in Sichuan, China. Turk. J.Biol. 2012, 36, 542–551. [Google Scholar] [CrossRef]
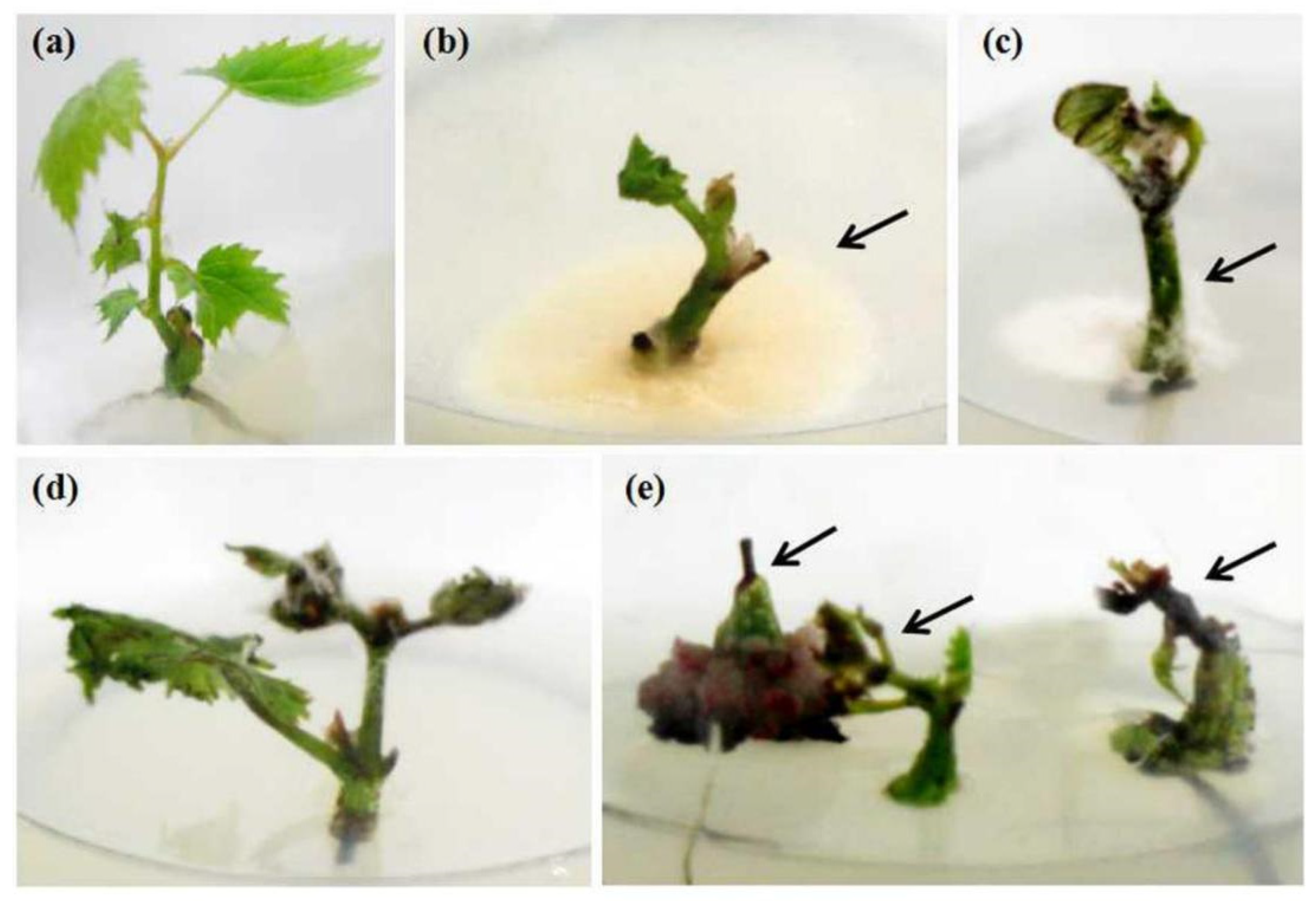
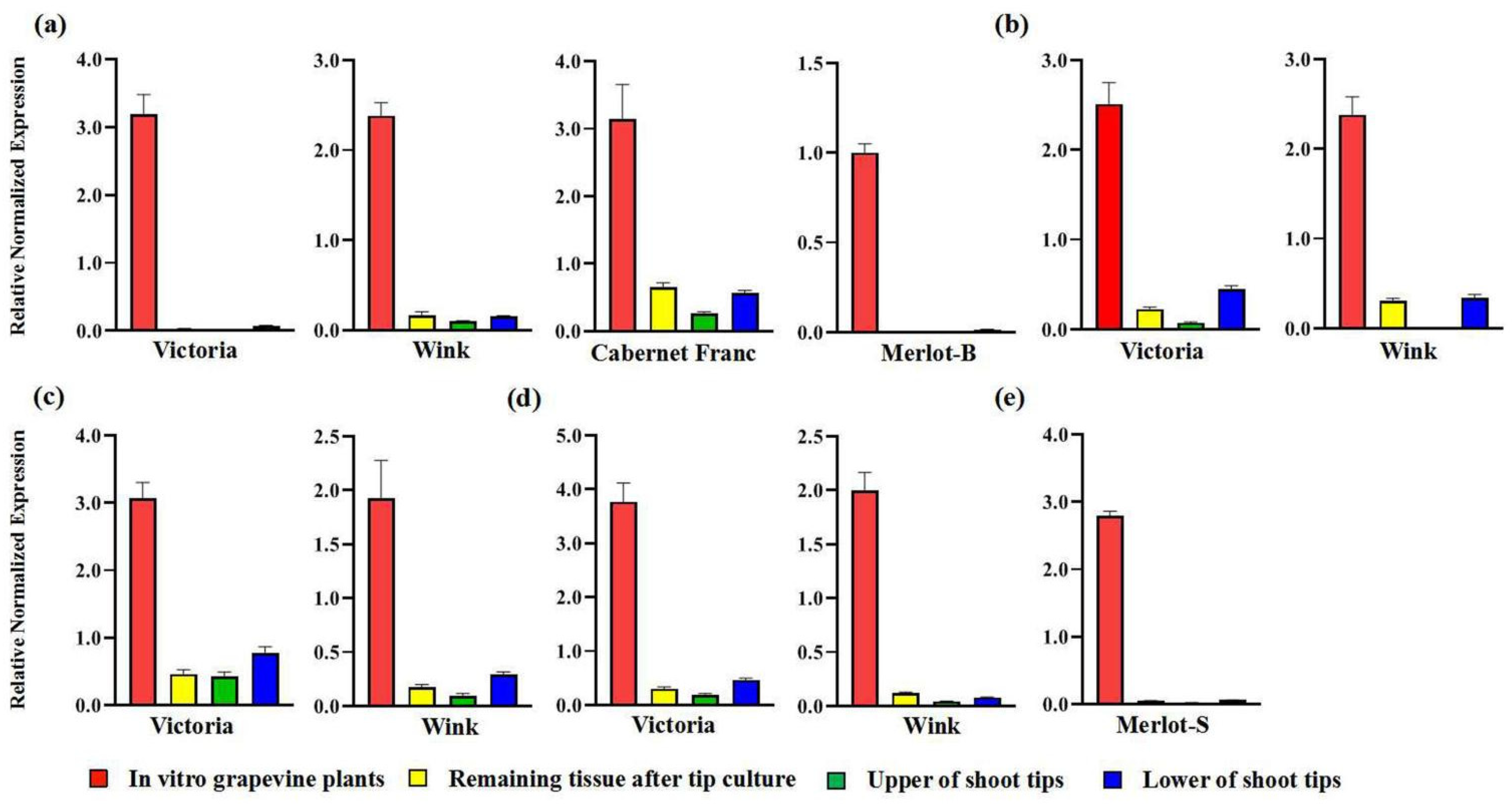
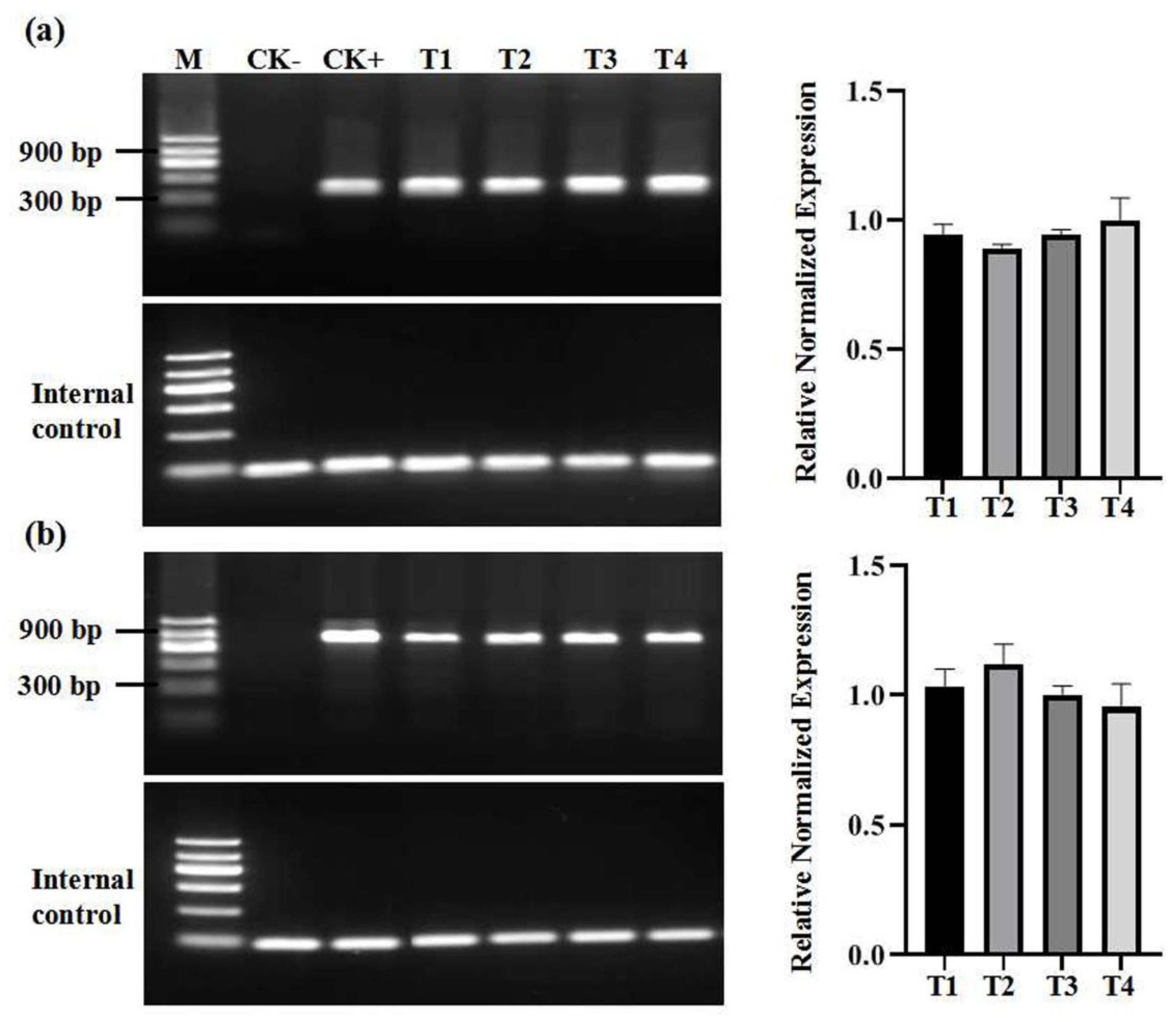
| Cultivars | Origins | In Vivo | In Vitro | ||
|---|---|---|---|---|---|
| Infected Virus | No. of Dissected Tips | No. of Survival Tips (%) | Infected Virus | ||
| Cabernet Franc | Ningxia, China | GRSPaV, GRLaV-2 | 20 | 10 (50.0) | GRSPaV, GRLaV-2 |
| Cabernet Gernischt | Liaoning, China | GRSPaV, GRLaV-3, GFkV | 20 | 0 | / |
| Cabernet Sauvignon | Tianjin, China | GRSPaV, GRLaV-2, GFkV | 20 | 0 | / |
| Wink | Guangxi, China | GRSPaV, GRLaV-2, GRLaV-3, GFkV | 20 | 12 (60.0) | GRSPaV, GRLaV-2, GRLaV-3, GFkV |
| Victoria | Guangxi, China | GRSPaV, GRLaV-2, GRLaV-3, GFkV | 20 | 14 (70.0) | GRSPaV, GRLaV-2, GRLaV-3, GFkV |
| Merlot | Beijing, China | GRSPaV | 20 | 16 (80.0) | GRSPaV |
| Merlot | Sichuan, China | GRLaV-1, GVA | 20 | 12 (60.0) | GRLaV-1, GVA |
| Infected Virus | Cultivars | No. of Clones Sequenced | Identities (%) | Consistency (%) | Mutation Frequency/nt | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| In Vivo | In Vitro | Between Group | In Vivo | In Vitro | Between Group | In Vivo | In Vitro | Uncorrected Difference | Corrected Difference a | |||
| GRLaV-2 | Wink | 15 | 99.7–100.0 | 99.4–100.0 | 99.4–100.0 | 99.94 | 99.96 | 99.97 | 1.7 × 10−4 | 3.5 × 10−4 | 1.8 × 10−4 | 0 |
| Victoria | 15 | 99.5–100.0 | 99.2–100.0 | 99.2–100.0 | 99.89 | 99.91 | 99.90 | 8.7 × 10−4 | 8.7 × 10−4 | 0 | 0 | |
| Cabernet Franc | 13 | 98.9–100.0 | 99.5–100.0 | 98.9–100.0 | 99.79 | 99.85 | 99.73 | 8.1 × 10−4 | 8.1 × 10−4 | 0 | 0 | |
| GRLaV-3 | Wink | 15 | 99.7–100.0 | 99.5–100.0 | 99.5–100.0 | 99.93 | 99.93 | 99.93 | 7.1 × 10−4 | 7.1 × 10−4 | 0 | 0 |
| Victoria | 17 | 99.5–100.0 | 99.2–100.0 | 99.2–100.0 | 99.87 | 99.87 | 99.87 | 8.1 × 10−4 | 6.3 × 10−4 | 0 | 0 | |
| GRSPaV | Wink | 16 | 99.2–100.0 | 99.2–100.0 | 99.1–100.0 | 99.89 | 99.88 | 99.83 | 6.2 × 10−4 | 7.0 × 10−4 | 0.8 × 10−4 | 0 |
| Merlot-B | 16 | 99.8–100.0 | 99.6–100.0 | 99.7–100.0 | 99.94 | 99.93 | 99.93 | 9.7 × 10−4 | 9.7 × 10−4 | 0 | 0 | |
| Viruses | Primers | Sequences (5′-3′) | Size (bp) | Reference |
|---|---|---|---|---|
| Regular PCR | ||||
| GFkV | C1/R | TGGTCCTCGGCCCAGTGAAAGTA | 344 | [42] |
| V1/R | GGCCAGGTTGTAGTCGGTGTTGTC | |||
| GVA | H587 | GACAAATGGCACACTACG | 429 | [43] |
| C995 | AAGCCTGACCTAGTCATCTTGG | |||
| GRSPaV | RSP52 | TGAAGGCTTTAGGGGTTAG | 905 | [44] |
| RSP53 | CTTAACCCAGCCTTGAAAT | |||
| GRLaV-1 | L1A | TCTTTACCAACCCCGAGATGAA | 232 | [45] |
| L1B | GTGTCTGGTGACGTGCTAAACG | |||
| GRLaV-2 | L2HSPL | CARAAYAATTCGGCGTACAT | 386 | [46] |
| L2HSPR | TAATTGGCRGGYACYGAACA | |||
| GRLaV-3 | LR3PU | CGCTCATGGTGAAAGCAGACG | 653 | [47] |
| LR3PD | CTTAGAACAAAAATATGGAGCAG | |||
| Quantitative real-time PCR | ||||
| GFkV | F1 | TCAAGGACTCCGTCACCTACA | 110 | This study |
| R1 | AGGATGGAGCCGCAGAT | |||
| GRSPaV | Y-cpf1 | GCACGTCACTGCTCTGATGTTGG | 170 | [41] |
| Y-cpr1 | GTCTCCAGATGGATGTTCCACACGAT | |||
| GRLaV-1 | GLRaV-1F | GTGGAGAGTATGATTCCGTGGTCAC | 267 | [48] |
| GLRaV-1R | CACTGGCACGTTAACTTGAGGTCG | |||
| GRLaV-2 | RL2 P19 | CTAACAATTTCTTCTTTGGATCGCAT | 155 | [49] |
| RL2 P24 | AGAATGTCTTCAGCTTCATAAGGAG | |||
| GRLaV-3 | LR3-F1 | GGGRACGGARAAGTGTTACC | 143 | [50] |
| LR3-R1 | TCCAAYTGGGTCATRCACAA | |||
| Internal control | Vivi-18Sf | AAGCCCGATCCAGCAATA | 176 | [51] |
| Vivi-18Sr | GCCCTTTACGCCCAGTCA | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, G.; Dong, Y.; Zhang, Z.; Fan, X.; Ren, F. Effect of In Vitro Culture of Long Shoot Tip on Variant Structure and Titer of Grapevine Viruses. Plants 2022, 11, 1907. https://doi.org/10.3390/plants11151907
Hu G, Dong Y, Zhang Z, Fan X, Ren F. Effect of In Vitro Culture of Long Shoot Tip on Variant Structure and Titer of Grapevine Viruses. Plants. 2022; 11(15):1907. https://doi.org/10.3390/plants11151907
Chicago/Turabian StyleHu, Guojun, Yafeng Dong, Zunping Zhang, Xudong Fan, and Fang Ren. 2022. "Effect of In Vitro Culture of Long Shoot Tip on Variant Structure and Titer of Grapevine Viruses" Plants 11, no. 15: 1907. https://doi.org/10.3390/plants11151907
APA StyleHu, G., Dong, Y., Zhang, Z., Fan, X., & Ren, F. (2022). Effect of In Vitro Culture of Long Shoot Tip on Variant Structure and Titer of Grapevine Viruses. Plants, 11(15), 1907. https://doi.org/10.3390/plants11151907







