Efficiency and Persistence of Movento® Treatment against Myzus persicae and the Transmission of Aphid-Borne Viruses
Abstract
1. Introduction
2. Results
2.1. Efficiency of a Movento® Treatment against Aphids
2.2. Persistence of Movento® Protection against Aphids
2.3. Impact of Movento® on Viral Infection
2.4. Behavior of M. persicae on Treated/Untreated Plants
3. Discussion
4. Materials and Methods
4.1. Plants, Aphids, and Viruses
4.2. Viruliferous Aphids to Inoculate Plants
4.3. Plant/Aphid Interactions
4.4. Serological Detection of Viruses in Plant Samples
4.5. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sacristán, S.; Díaz, M.; Fraile, A.; García-Arenal, F. Contact Transmission of Tobacco Mosaic Virus: A Quantitative Analysis of Parameters Relevant for Virus Evolution. J. Virol. 2011, 85, 4974–4981. [Google Scholar] [CrossRef]
- Maree, H.; Almeida, R.; Bester, R.; Chooi, K.M.; Cohen, D.; Dolja, V.; Fuchs, M.; Golino, D.; Jooste, A.; Martelli, G.; et al. Grapevine Leafroll-Associated Virus 3. Front. Microbiol. 2013, 4, 82. [Google Scholar] [CrossRef]
- Riedle-Bauer, M.; Suarez, B.; Reinprecht, H.J. Seed Transmission and Natural Reservoirs of Zucchini Yellow Mosaic Virus in Cucurbita Pepo Var. Styriaca J. Plant Dis. Prot. 2002, 109, 200–206. [Google Scholar]
- Schellenberger, P.; Sauter, C.; Lorber, B.; Bron, P.; Trapani, S.; Bergdoll, M.; Marmonier, A.; Schmitt-Keichinger, C.; Lemaire, O.; Demangeat, G.; et al. Structural Insights into Viral Determinants of Nematode Mediated Grapevine Fanleaf Virus Transmission. PLoS Pathog. 2011, 7, e1002034. [Google Scholar] [CrossRef] [PubMed]
- Han, C.G.; Li, D.W.; Wang, D.Y.; Yang, L.L.; Yu, J.L.; Cai, Z.N.; Liu, Y. Functional Analysis of Beet Necrotic Yellow Vein Virus (BNYVV) RNA4 in Fungal Transmission. Chin. Sci. Bull. 2002, 47, 1281–1284. [Google Scholar] [CrossRef]
- Abt, I.; Souquet, M.; Angot, G.; Mabon, R.; Dallot, S.; Thébaud, G.; Jacquot, E. Functional Transcomplementation between Wheat Dwarf Virus Strains in Wheat and Barley. Viruses 2020, 12, 34. [Google Scholar] [CrossRef]
- Pleydell, D.R.J.; Soubeyrand, S.; Dallot, S.; Labonne, G.; Chadœuf, J.; Jacquot, E.; Thébaud, G. Estimation of the Dispersal Distances of an Aphid-Borne Virus in a Patchy Landscape. PLoS Comput. Biol. 2018, 14, e1006085. [Google Scholar] [CrossRef] [PubMed]
- Demangeat, G.; Voisin, R.; Minot, J.-C.; Bosselut, N.; Fuchs, M.; Esmenjaud, D. Survival of Xiphinema Index in Vineyard Soil and Retention of Grapevine Fanleaf Virus Over Extended Time in the Absence of Host Plants. Phytopathology 2005, 95, 1151–1156. [Google Scholar] [CrossRef]
- Hauser, S.; Stevens, M.; Beuve, M.; Lemaire, O. Biological Properties and Molecular Characterization of Beet Chlorosis Virus (BChV). Arch. Virol. 2002, 147, 745–762. [Google Scholar] [CrossRef]
- Chesnais, Q.; Caballero Vidal, G.; Coquelle, R.; Yvon, M.; Mauck, K.; Brault, V.; Ameline, A. Post-Acquisition Effects of Viruses on Vector Behavior Are Important Components of Manipulation Strategies. Oecologia 2020, 194, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Mauck, K.E.; Chesnais, Q.; Shapiro, L.R. Chapter Seven—Evolutionary Determinants of Host and Vector Manipulation by Plant Viruses. In Advances in Virus Research; Malmstrom, C.M., Ed.; Environmental Virology and Virus Ecology; Academic Press: Cambridge, MA, USA, 2018; Volume 101, pp. 189–250. [Google Scholar]
- Tsvetkov, N.; Samson-Robert, O.; Sood, K.; Patel, H.S.; Malena, D.A.; Gajiwala, P.H.; Maciukiewicz, P.; Fournier, V.; Zayed, A. Chronic Exposure to Neonicotinoids Reduces Honey Bee Health near Corn Crops. Science 2017, 356, 1395–1397. [Google Scholar] [CrossRef]
- Wood, T.J.; Goulson, D. The Environmental Risks of Neonicotinoid Pesticides: A Review of the Evidence Post 2013. Environ. Sci. Pollut. Res. Int. 2017, 24, 17285–17325. [Google Scholar] [CrossRef]
- Bass, C.; Denholm, I.; Williamson, M.S.; Nauen, R. The Global Status of Insect Resistance to Neonicotinoid Insecticides. Pestic. Biochem. Physiol. 2015, 121, 78–87. [Google Scholar] [CrossRef]
- Bass, C.; Puinean, A.M.; Zimmer, C.T.; Denholm, I.; Field, L.M.; Foster, S.P.; Gutbrod, O.; Nauen, R.; Slater, R.; Williamson, M.S. The Evolution of Insecticide Resistance in the Peach Potato Aphid, Myzus Persicae. Insect Biochem. Mol. Biol. 2014, 51, 41–51. [Google Scholar] [CrossRef]
- Namara, L.M.; Gauthier, K.; Walsh, L.; Thébaud, G.; Gaffney, M.; Jacquot, E. Management of Yellow Dwarf Disease in Europe in a Post-Neonicotinoid Agriculture. Pest Manag. Sci. 2020, 76, 2276–2285. [Google Scholar] [CrossRef]
- Hauer, M.; Hansen, A.L.; Manderyck, B.; Olsson, Å.; Raaijmakers, E.; Hanse, B.; Stockfisch, N.; Märländer, B. Neonicotinoids in Sugar Beet Cultivation in Central and Northern Europe: Efficacy and Environmental Impact of Neonicotinoid Seed Treatments and Alternative Measures. Crop Prot. 2017, 93, 132–142. [Google Scholar] [CrossRef]
- Jactel, H.; Verheggen, F.; Thiéry, D.; Escobar-Gutiérrez, A.J.; Gachet, E.; Desneux, N. Alternatives to Neonicotinoids. Environ. Int. 2019, 129, 423–429. [Google Scholar] [CrossRef]
- Hossain, R.; Menzel, W.; Lachmann, C.; Varrelmann, M. New Insights into Virus Yellows Distribution in Europe and Effects of Beet Yellows Virus, Beet Mild Yellowing Virus, and Beet Chlorosis Virus on Sugar Beet Yield Following Field Inoculation. Plant Pathol. 2020, 70, 584–593. [Google Scholar] [CrossRef]
- Hauser, S.; Stevens, M.; Mougel, C.; Smith, H.G.; Fritsch, C.; Herrbach, E.; Lemaire, O. Biological, Serological, and Molecular Variability Suggest Three Distinct Polerovirus Species Infecting Beet or Rape. Phytopathology 2000, 90, 460–466. [Google Scholar] [CrossRef]
- Scheets, K.; Miller, W.A.; Somera, M. Abolish the Family Luteoviridae (Tolivirales) and Move Its Genera to the Familes Tombusviridae (Tolivirales) and Solemoviridae (Sobelivirales). Available online: https://ictv.global/ictv/proposals/2020.026P.R.Abolish_Luteoviridae.zip (accessed on 9 November 2021).
- Chesnais, Q.; Couty, A.; Uzest, M.; Brault, V.; Ameline, A. Plant Infection by Two Different Viruses Induce Contrasting Changes of Vectors Fitness and Behavior. Insect Sci. 2019, 26, 86–96. [Google Scholar] [CrossRef]
- Kozłowska-Makulska, A.; Beuve, M.; Syller, J.; Szyndel, M.S.; Lemaire, O.; Bouzoubaa, S.; Herrbach, E. Aphid Transmissibility of Different European Beet Polerovirus Isolates. Eur. J. Plant Pathol. 2009, 125, 337–341. [Google Scholar] [CrossRef]
- ANSES Rapport d’appui scientifique et technique Demande n 2016-SA-0057—Volet impact agricole. Risques et bénéfices relatifs des alternatives aux produits phytopharmaceutiques comportant des néonicotinoïdes. Rapport d’appui scientifique et technique sur l’impact Agricole. 2018, p. 149. Available online: https://agritrop.cirad.fr/588091/ (accessed on 9 November 2021).
- Congdon, B.S.; Baulch, J.R.; Coutts, B.A. Novel Sources of Turnip Yellows Virus Resistance in Brassica and Impacts of Temperature on Their Durability. Plant Dis. 2021, PDIS10202312RE. [Google Scholar] [CrossRef]
- Souquet, M.; Pichon, E.; Armand, T.; Jacquot, E. Fine Characterization of a Resistance Phenotype by Analyzing TuYV-Myzus Persicae-Rapeseed Interactions. Plants 2021, 10, 317. [Google Scholar] [CrossRef]
- Nauen, R.; Reckmann, U.; Thomzik, J.; Thielert, W. Biological Profile of Spirotetramat (Movento)—A New Two Way Systemic (Amimobile) Insecticide against Sucking Pests. Bayer Crop Sci. J. 2007, 61, 245–278. [Google Scholar]
- Brueck, E.; Elbert, A.; Fischer, R.; Krueger, S.; Kuehnhold, J.; Klueken, A.M.; Nauen, R.; Niebes, J.-F.; Reckmann, U.; Schnorbach, H.-J.; et al. Movento (R), an Innovative Ambimobile Insecticide for Sucking Insect Pest Control in Agriculture: Biological Profile and Field Performance. Crop Prot. 2009, 28, 838–844. [Google Scholar] [CrossRef]
- Shannag, H.K.; Capinera, J.L. Comparative Effects of Two Novel Betaproteobacteriabased Insecticides on Myzus Persicae (Hemiptera: Aphididae) and Phenacoccus Madeirensis (Hemiptera: Pseudococcidae). Fla. Entomol. 2018, 101, 212–218. [Google Scholar] [CrossRef]
- Arnaudov, V.; Petkova, R. Spirotetramat (Movento®): New Systemic Insecticide for Control of Green Peach Aphid, Myzus Persicae (Sulzer) (Hemiptera: Aphidae) on Peach. Bulg. J. Agric. Sci. 2020, 26, 431–434. [Google Scholar]
- Brault, V.; Uzest, M.; Monsion, B.; Jacquot, E.; Blanc, S. Aphids as Transport Devices for Plant Viruses. C. R. Biol. 2010, 333, 524–538. [Google Scholar] [CrossRef]
- EFSA. Evaluation of the Data on Clothianidin, Imidacloprid and Thiamethoxam for the Updated Risk Assessment to Bees for Seed Treatments and Granules in the EU. EFSA Support. Publ. 2018, 15, 1378E. [Google Scholar] [CrossRef]
- Yu, Y.; Shen, G.; Zhu, H.; Lu, Y. Imidacloprid-Induced Hormesis on the Fecundity and Juvenile Hormone Levels of the Green Peach Aphid Myzus Persicae (Sulzer). Pestic. Biochem. Physiol. 2010, 98, 238–242. [Google Scholar] [CrossRef]
- Panter, S.N.; Jones, D.A. Age-Related Resistance to Plant Pathogens. In Advances in Botanical Research; Academic Press: Cambridge, MA, USA, 2002; Volume 38, pp. 251–280. [Google Scholar]
- Lindblad, M.; Sigvald, R. Temporal Spread of Wheat Dwarf Virus and Mature Plant Resistance in Winter Wheat. Crop Prot. 2004, 23, 229–234. [Google Scholar] [CrossRef]
- Salvalaggio, A.E.; López Lambertini, P.M.; Cendoya, G.; Huarte, M.A. Temporal and Spatial Dynamics of Tomato Spotted Wilt Virus and Its Vector in a Potato Crop in Argentina. Ann. Appl. Biol. 2017, 171, 5–14. [Google Scholar] [CrossRef]
- Powell, G.; Tosh, C.; Hardie, J. Host Plant Selection by Aphids: Behavioral, Evolutionary, and Applied Perspectives. Annu. Rev. Entomol. 2006, 51, 309–330. [Google Scholar] [CrossRef] [PubMed]
- Labonne, G.; Yvon, M.; Quiot, J.B.; Avinent, L.; Llacer, G. Aphids as potential vectors of plum pox virus: Comparison of methods of testing and epide-miological consequences. Acta Hortic. 1995, 207–218. [Google Scholar] [CrossRef]
- Clark, M.F.; Adams, A.N. Characteristics of the Microplate Method of Enzyme-Linked Immunosorbent Assay for the Detection of Plant Viruses. J. Gen. Virol. 1977, 34, 475–483. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
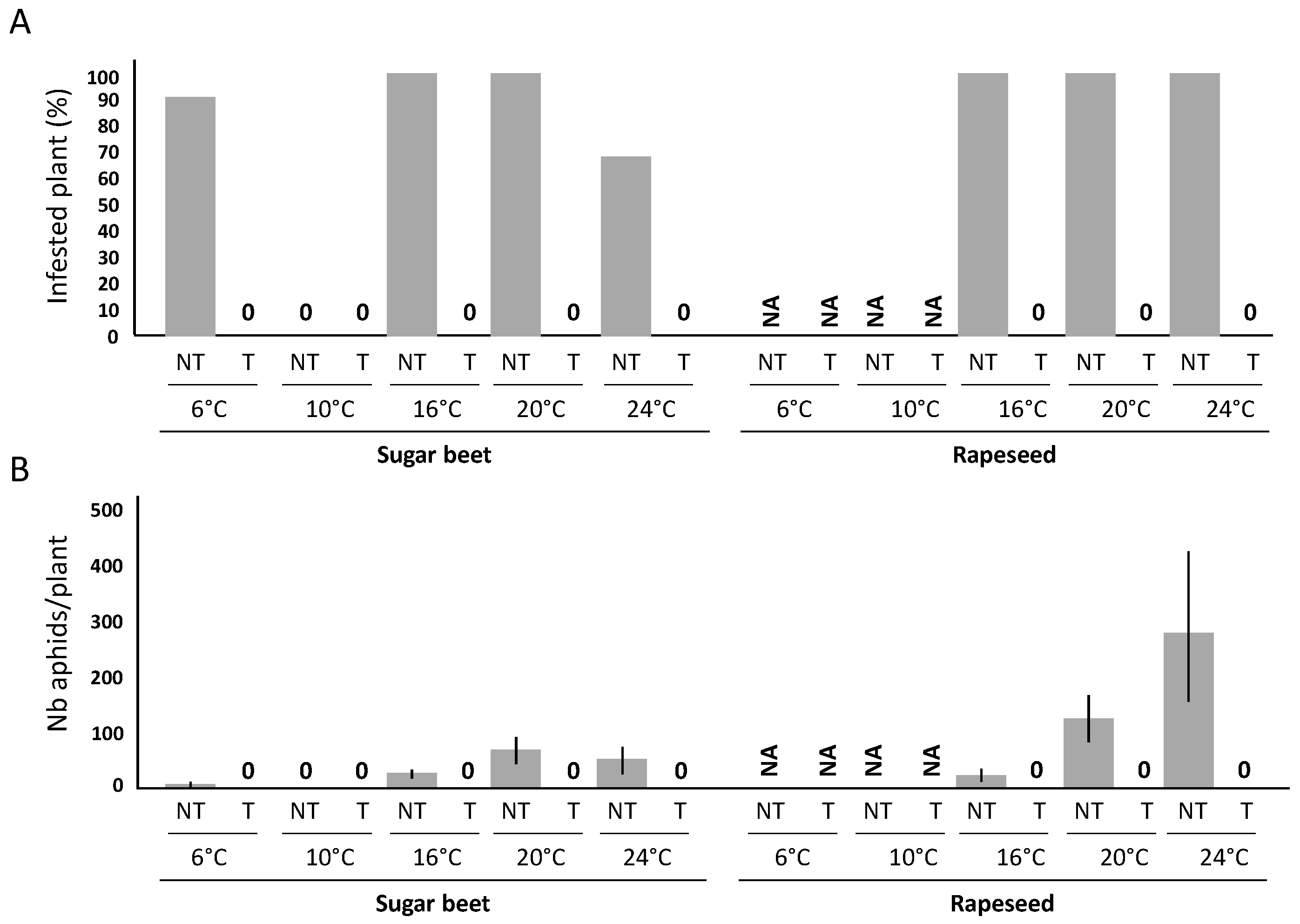
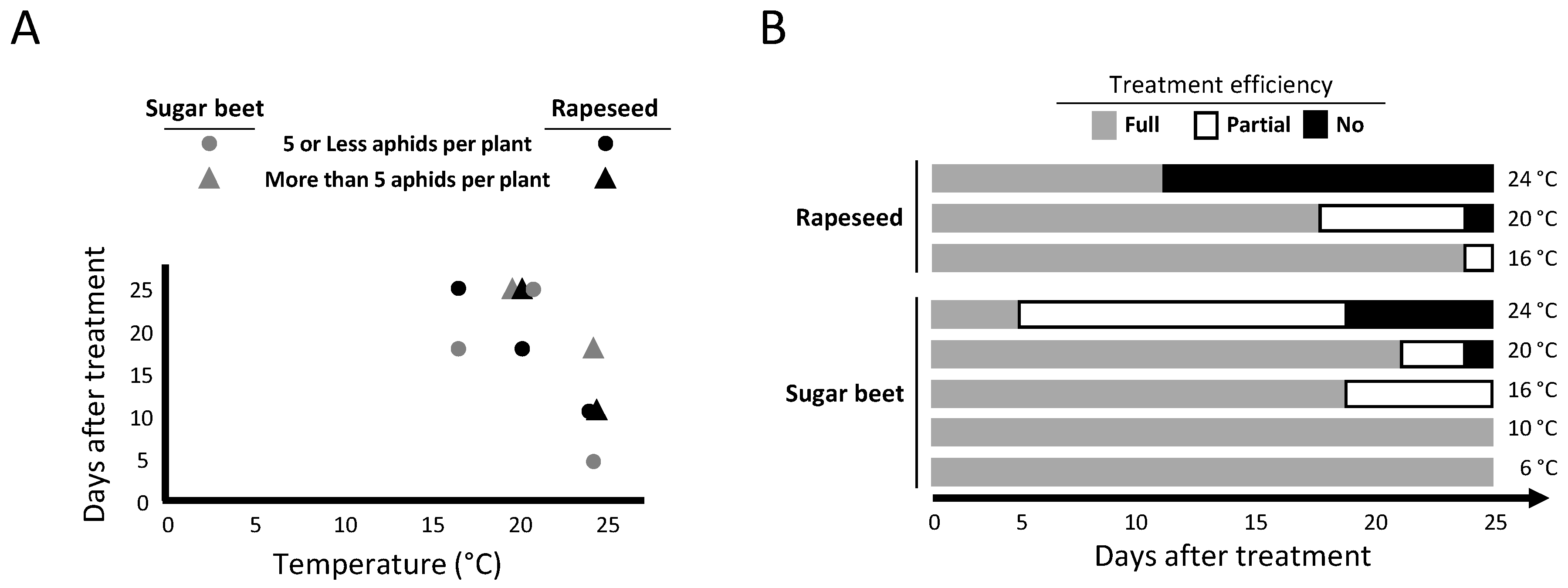
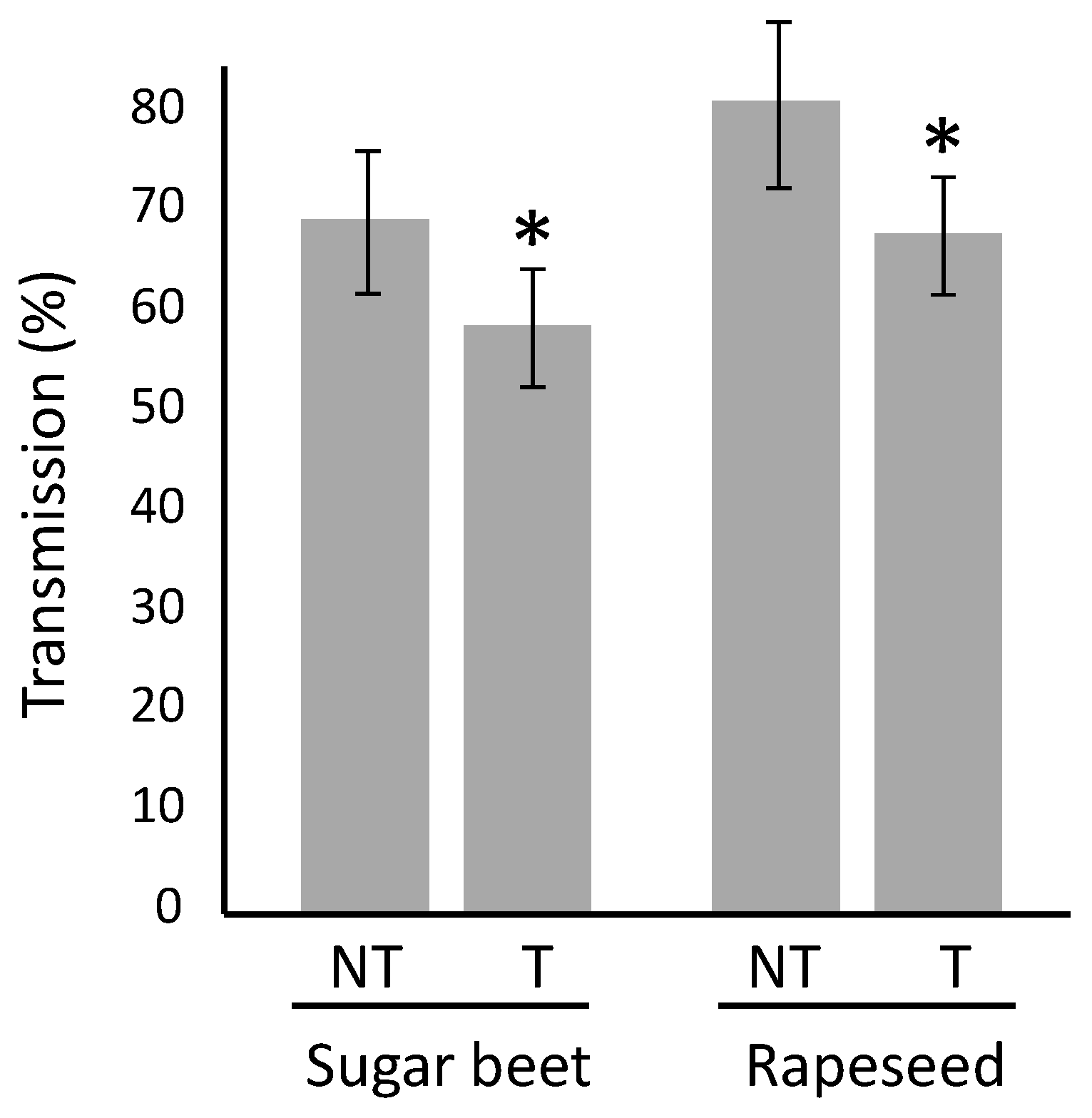
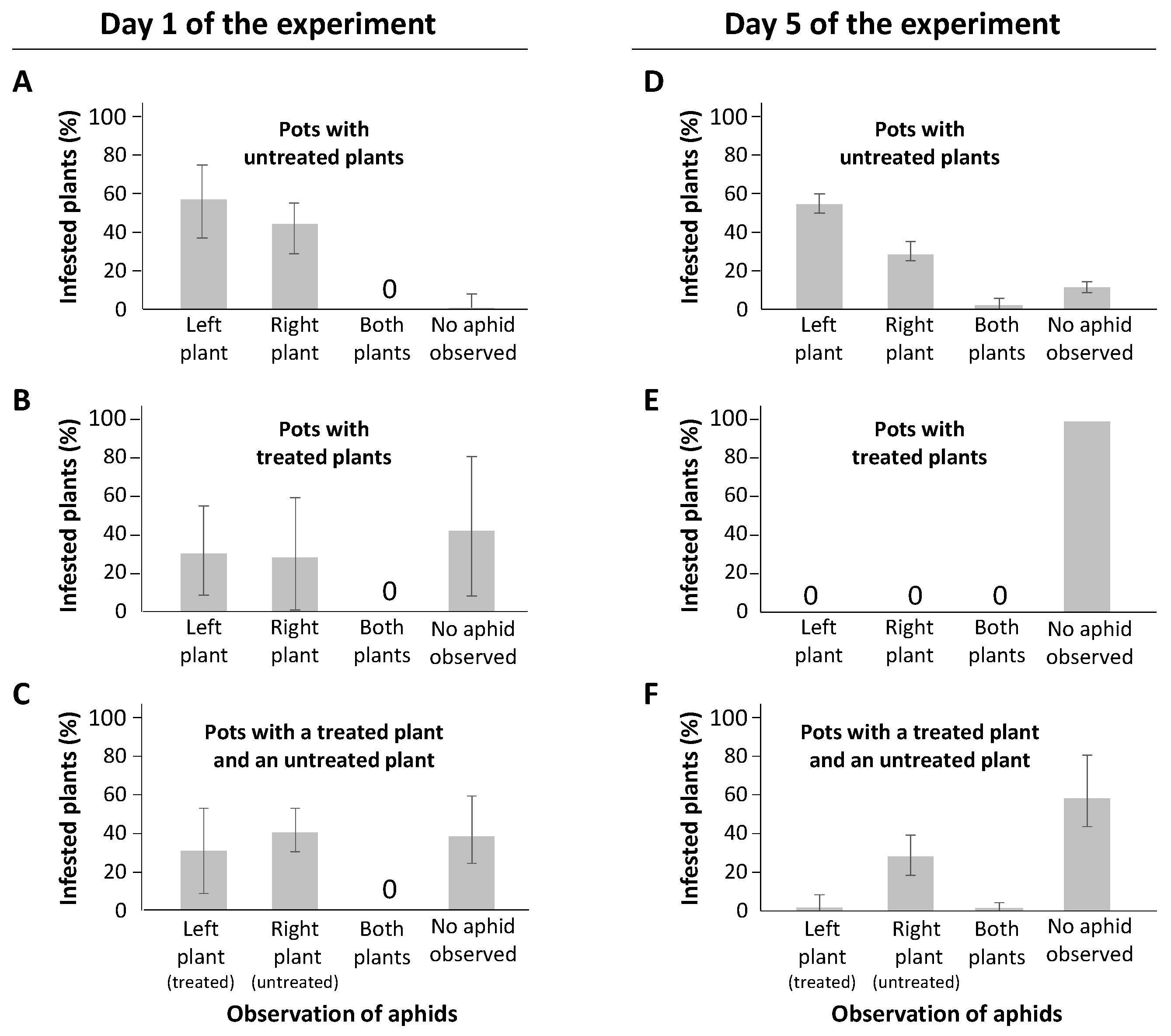

| 6 °C | 10 °C | 16 °C | 20 °C | 24 °C | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T | NT | T | NT | T | NT | T | NT | T | NT | ||
| Sugar beet | D1 | 3/10 (30%) | 2/6 (33.3%) | 1/10 (10%) | 6/6 (100%) | 10/10 (100%) | 6/6 (100%) | 10/10 (100%) | 6/6 (100%) | 7/10 (70%) | 5/6 (83.3%) |
| D4 | 4/10 (40%) | 2/6 (33.3%) | 8/10 (80%) | 2/6 (33.3%) | 9/10 (90%) | 6/6 (100%) | 9/10 (90%) | 6/6 (100%) | 10/10 (100%) | 6/6 (100%) | |
| D8 | / | / | / | / | 10/10 (100%) | 6/6 (100%) | 10/10 (100%) | 6/6 (100%) | 7/10 (70%) | 4/6 (66.6%) | |
| D11 | 5/10 (50%) | 2/6 (33.3%) | 8/10 (80%) | 6/6 (100%) | 8/10 (80%) | 6/6 (100%) | 8/10 (80%) | 5/6 (83.3%) | 6/10 (60%) | 5/6 (83.3%) | |
| D14 | 10/10 (100%) | 1/6 (16.7%) | 7/10 (70%) | 2/6 (33.3%) | 4/10 (40%) | 6/6 (100%) | 5/10 (50%) | 6/6 (100%) | 6/10 (60%) | 5/6 (83.3%) | |
| D18 | 0/10 (0%) | 0/6 (0%) | 1/10 (10%) | 6/6 (100%) | 8/10 (80%) | 6/6 (100%) | 7/10 (70%) | 4/6 (66.6%) | 3/10 (30%) | 4/6 (66.6%) | |
| D21 | 2/10 (20 %) | 4/6 (66.6%) | 4/10 (40%) | 6/6 (100%) | 8/10 (80%) | 6/6 (100%) | 2/10 (20%) | 1/6 (16.7%) | 9/10 (90%) | 3/6 (50%) | |
| D25 | 0/10 (0%) | 3/6 (50%) | 6/10 (60%) | 1/6 (16.7%) | 7/10 (70%) | 5/6 (83.3%) | 9/10 (90%) | 6/6 (100%) | 5/10 (50%) | 0/6 (0%) | |
| Rapeseed | D1 | / | / | / | / | 8/10 (80%) | 5/6 (83.3%) | 8/10 (80%) | 5/6 (83.3 %) | 7/10 (70%) | 6/6 (100%) |
| D4 | / | / | / | / | 9/10 (90%) | 6/6 (100%) | 9/10 (90%) | 5/6 (83.3%) | 4/10 (40%) | 1/6 (16.7%) | |
| D8 | / | / | / | / | 8/10 (80%) | 5/6 (83.3%) | 8/10 (80%) | 5/6 (83.3%) | 4/10 (40%) | 4/6 (66.6%) | |
| D11 | / | / | / | / | 9/10 (90%) | 5/6 (83.3%) | 7/10 (70%) | 6/6 (100%) | 4/10 (40%) | 3/6 (50%) | |
| D14 | / | / | / | / | 4/10 (40%) | 6/6 (100%) | 6/10 (60%) | 5/6 (83.3%) | 3/10 (30%) | 1/6 (16.7%) | |
| D18 | / | / | / | / | 5/10 (50%) | 4/6 (66.6%) | 8/10 (80%) | 4/6 (66.6%) | 2/10 (20%) | 1/6 (16.7%) | |
| D21 | / | / | / | / | 6/10 (60%) | 4/6 (66.6%) | 5/10 (50%) | 2/6 (33.3%) | 7/10 (70%) | 1/6 (16.7%) | |
| D25 | / | / | / | / | 9/10 (90%) | 6/6 (100%) | 3/10 (30%) | 3/6 (50%) | 7/10 (70%) | 3/6 (50%) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Armand, T.; Korn, L.; Pichon, E.; Souquet, M.; Barbet, M.; Martin, J.-L.; Devavry, M.; Jacquot, E. Efficiency and Persistence of Movento® Treatment against Myzus persicae and the Transmission of Aphid-Borne Viruses. Plants 2021, 10, 2747. https://doi.org/10.3390/plants10122747
Armand T, Korn L, Pichon E, Souquet M, Barbet M, Martin J-L, Devavry M, Jacquot E. Efficiency and Persistence of Movento® Treatment against Myzus persicae and the Transmission of Aphid-Borne Viruses. Plants. 2021; 10(12):2747. https://doi.org/10.3390/plants10122747
Chicago/Turabian StyleArmand, Thomas, Luâna Korn, Elodie Pichon, Marlène Souquet, Mélissandre Barbet, Jean-Laurent Martin, Magalie Devavry, and Emmanuel Jacquot. 2021. "Efficiency and Persistence of Movento® Treatment against Myzus persicae and the Transmission of Aphid-Borne Viruses" Plants 10, no. 12: 2747. https://doi.org/10.3390/plants10122747
APA StyleArmand, T., Korn, L., Pichon, E., Souquet, M., Barbet, M., Martin, J.-L., Devavry, M., & Jacquot, E. (2021). Efficiency and Persistence of Movento® Treatment against Myzus persicae and the Transmission of Aphid-Borne Viruses. Plants, 10(12), 2747. https://doi.org/10.3390/plants10122747





