Abstract
Astragalus membranaceus (A. membranaceus), a traditional Chinese medicine, has gained increasing recognition for its potential in treating central nervous system (CNS) disorders. This review aims to systematically integrate the mechanisms of action of A. membranaceus and its bioactive compounds on CNS diseases, with a focus on exploring its therapeutic potential and introducing related health food products. We conducted a comprehensive literature search in PubMed and Web of Science from January 2015 through July 2025. Our analysis reveals that A. membranaceus and its bioactive compounds, particularly A. membranaceus IV (AS-IV) and A. membranaceus polysaccharides (APS), exert multifaceted neuroprotective effects. These effects encompass the mitigation of neuroinflammation, oxidative stress, apoptosis, and ferroptosis, as well as the regulation of autophagy and protection of the blood–brain barrier. The therapeutic potential of A. membranaceus is linked to the modulation of key signaling pathways, such as NF-κB, Nrf2, and PI3K/Akt. Furthermore, based on the concept of “homology of medicine and food,” A. membranaceus is being developed into various health food formulations, offering a promising strategy for the adjuvant treatment and preventive care of CNS diseases. In conclusion, A. membranaceus represents a promising, multi-target pharmacological agent for CNS disorders, yet further high-quality clinical studies are warranted to validate its efficacy and safety in humans.
1. Introduction
Central Nervous System (CNS) disorders continue to climb up the global burden of disease list, and it has been reported that the number of deaths in 2019 alone is as high as 10 million []. For instance, the occurrence of neurodegenerative diseases like Parkinson’s disease (PD) continues to rise annually, leading to patient distress [,]. The severity of CNS disease is not only reflected in the direct destruction of neurological function, but also in the chain reaction of multiple systems. For instance, hepatic encephalopathy, a severe issue associated with advanced liver disease, can quickly result in reduced consciousness, coma, and potentially death []. These conditions not only endanger individuals’ lives and health but also impose a significant burden on society and families due to their high rates of disability and long-term care needs []. Hence, patients with CNS disorders are in urgent need of novel therapeutic approaches. Given their origin from natural resources and low toxicity, traditional Chinese medicines (TCM) have gradually gained wide recognition in the preventive and therapeutic fields.
Astragalus membranaceus (A. membranaceus), the dried root of A. membranaceus (Fisch.) Bge. var. Mongholicus (Bge.) [] is renowned as “the holy medicine” for enhancing qi. First mentioned in Shennong Ben Cao Jing [], it has a long history of medicinal use and is one of the most commonly used TCM for CNS disorders (Figure 1) []. Recent pharmacological research indicates that A. membranaceus offers therapeutic benefits through multiple targets and pathways for preventing and treating CNS diseases, due to its diverse bioactive compounds. Until now, researchers have identified more than 200 compounds from A. membranaceus, comprising polysaccharides, saponins, flavonoids, alkaloids, among others, which form a treasure trove of structurally diverse and functionally rich natural small molecules []. Among them, A. membranaceus IV (AS-IV) and A. membranaceus polysaccharides (APS) have attracted the most attention because of their outstanding biological activities, which together constitute the “main force” of A. membranaceus in exerting therapeutic and pharmacological effects []. AS-IV, a form of AST, is vital for its anti-inflammatory and neuroprotective properties by inhibiting NF-κB and other pathways []. Cycloastragenol (CAG) is a potent derivative of AS-IV, known for its ability to reduce inflammation and improve lipid metabolism []. APS is a water-soluble, chemically complex heteropolysaccharide that comprises portions of heteropolysaccharides, glucans, and both neutral and acidic polysaccharides, and it has notable effects on modulating immune cell activity [,]. These representative active ingredients work together to carry the scientific basis for the overall efficacy of A. membranaceus.
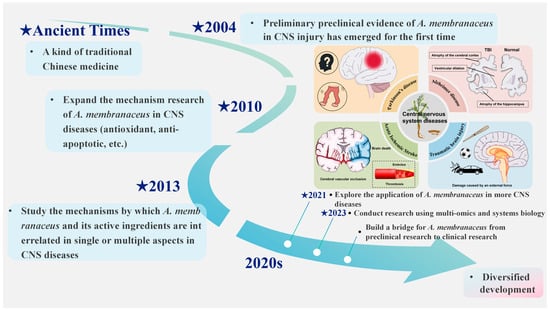
Figure 1.
A. membranaceus as a treatment option for central nervous system disorders. This figure is original and was created by the authors for this publication.
Currently, the body of research concerning A. membranaceus remains relatively sparse, with a notable absence of systematic reviews addressing its role and potential mechanisms in CNS disorders. Consequently, this study seeks to conduct a comprehensive review of the active constituents of A. membranaceus and elucidate their molecular mechanisms in the context of CNS disease treatment. The findings aim to furnish novel insights and references for future investigations into A. membranaceus and to inform the development of innovative strategies for the prevention and management of CNS disorders.
2. Methods
2.1. Literature Search
PubMed and Web of Science were searched for reports on the effects of A. membranaceus and its components on CNS diseases using the following search keywords: “A. membranaceus”, “Astragali Radix”, “AS-IV”, “APS”, “CNS diseases”, and “pharmacology”. The search period spanned from January 2015 to July 2025, with a focus on incorporating high-impact studies from the past decade.
2.2. Inclusion Criteria
The inclusion criteria encompassed studies focusing on A. membranaceus and its active constituents in relation to CNS disorders, including AD, TBI, PD, and SCI, as well as pharmacological investigations of A. membranaceus.
2.3. Exclusion Criteria
Studies that were duplicates, incomplete, lacked ethical approval, were presented as conference abstracts, appeared as brief newsletters, or were published in languages other than English were excluded.
3. Results
Two researchers independently executed the search strategy using the specified keywords and screened titles, abstracts, and full texts against the inclusion and exclusion criteria, resulting in 218 eligible publications. The literature search and screening process is summarized in Figure 2.
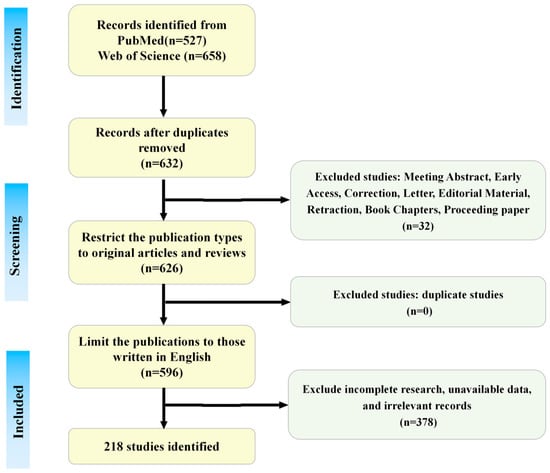
Figure 2.
The literature search and screening flowchart. This figure is original and was created by the authors for this publication.
4. Impact of A. membranaceus and Its Bioactive Components on the CNS
A. membranaceus and its bioactive constituents exert curative effects on CNS diseases via multiple pathways and targets (Table 1).

Table 1.
Summary of Neuroprotective Mechanisms of Major Bioactive Compounds from A.membranaceus.
4.1. Anti-Neuroinflammation
Neuroinflammation is a key pathogenetic mechanism in many CNS diseases, such as cerebral infarction, spinal cord injury (SCI), and Alzheimer’s disease (AD). It has long since ceased to be a “bystander” to CNS disease and is one of the central mechanisms driving disease onset, progression, and determining prognosis []. One of the primary causes of neuroinflammation is the activation of microglia []. When tissues are inflamed, macrophages of the activated M1 phenotype secrete inflammatory and chemotactic proteins to help the host resist the infection and then transform into the activated M2 phenotype to repair the damaged tissues [,]. Nuclear factor-κB (NF-κB) acts as a key transcriptional regulator in the inflammatory response []. Upon activation, it enhances the secretion of pro-inflammatory molecules, including IL-6, IL-8, TNF-α, IL-1β, COX-2, and iNOS, thereby intensifying the inflammatory cascade []. NLRP3, a coreceptor in inflammatory vesicles, is linked to inflammation in various CNS disorders and can trigger local and systemic inflammatory responses. Inhibiting NLRP3 can significantly reduce inflammation and protect the CNS (Figure 3) [,].
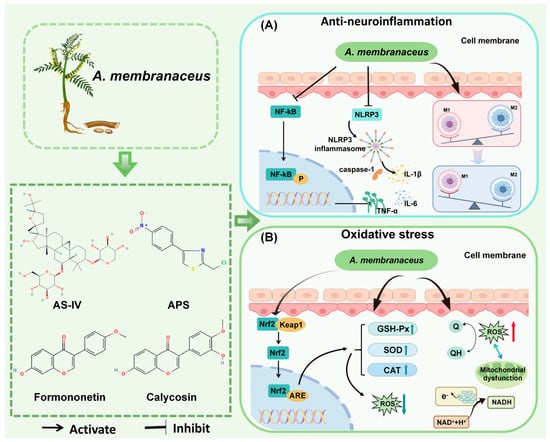
Figure 3.
Mechanisms of A. membranaceus in Combating Neuroinflammation and Oxidative Stress. (A) Anti-neuroinflammation pathway. A. membranaceus components, notably Astragaloside IV (AS-IV) and A. membranaceus polysaccharides (APS), exert potent anti-inflammatory effects primarily by inhibiting the NF-κB signaling pathway. This inhibition results in a decrease in the production of key pro-inflammatory cytokines, including TNF-α, IL-1β, and IL-6. Furthermore, these bioactive compounds suppress the activation of the NLRP3 inflammasome, a critical complex that drives the maturation and secretion of IL-1β, thereby mitigating a central inflammatory cascade in CNS disorders. Concurrently, A. membranaceus promotes the polarization of microglia/macrophages from a pro-inflammatory M1 phenotype towards an anti-inflammatory and reparative M2 phenotype, contributing to the resolution of neuroinflammation. (B) Antioxidant stress pathway. A. membranaceus and its flavonoid constituents, such as Calycosin and Formononetin, activate the central antioxidant regulator Nrf2. Upon activation, Nrf2 translocates to the nucleus and binds to the Antioxidant Response Element (ARE), initiating the transcription of a battery of cytoprotective and antioxidant enzymes. This includes the upregulation of Superoxide Dismutase (SOD), Catalase (CAT), and Glutathione Peroxidase (GSH-Px). These enzymes work in concert to neutralize reactive oxygen species (ROS): SOD catalyzes the conversion of superoxide anions into hydrogen peroxide, which is then subsequently degraded to water (H2O) by CAT and GSH-Px. This enhanced antioxidant capacity effectively reduces oxidative damage to cellular components, a common feature in various CNS diseases. This figure is original and was created by the authors for this publication.
4.1.1. Regulatory Effects of A. membranaceus Components (AS-IV, APS) on Microglia/Macrophages
Suppressing microglial overactivation represents a promising therapeutic strategy for various neurological disorders. AS-IV has been identified as a possible neuroprotective and anti-inflammatory compound for CNS disorders. In a mouse model of cerebral ischemia, AS-IV demonstrated anti-inflammatory properties by converting microglia/macrophages from the M1 to the M2 phenotype through a mechanism dependent on peroxisome proliferator-activated receptor γ (PPARγ) [,]. It simultaneously decreases pro-inflammatory elements like IL-1β, IL-6, TNF-α, MyD88, NF-κB, and TLR4, while boosting anti-inflammatory components such as ARG-1, CD206, and IL-10 [,]. APS also reverses M1/M2 polarization, promotes ATP degradation to release anti-inflammatory factors, and inhibits P2X7R expression, providing neuroprotection in the cerebral cortex of MCAO rats [,].
4.1.2. Inhibitory Effect of AS-IV on NLRP3 Inflammatory Body and Related Damage
NLRP3 levels were notably increased in brain tissues affected by hypoxic–ischemic conditions, and its overexpression exacerbated the degree of brain tissue damage. Inhibition of its activity became an important way to protect the CNS []. In the rat MCAO/R model, AS-IV decreased LOC102555978 expression to lessen cerebral infarction volume and suppress inflammation and cell death []. Furthermore, in a mouse model of depression caused by repeated restraint stress (RRS) and LPS, AS-IV administration significantly increased GSK3β phosphorylation and inhibited NLRP3 inflammatory vesicles, and decreased the levels of inflammatory factors, such as TNF-α and IL-1β []. The combination of AS-IV and hydroxy safflower yellow A treatment in ischemic brain showed a significant increase in NLRP3/ASC/IL-1β/Caspase-1/1/GSDMD protein expression, which was significantly more pronounced, indicating that the combination suppressed NLRP3 inflammasome-mediated cellular pyroptosis, reduced the inflammatory response, and mitigated brain tissue damage [].
4.1.3. Regulatory Effect of A. membranaceus on Astrocytes
Astrocytes are the “igniters, amplifiers, and regulators” of the immune cascade in a variety of human CNS diseases []. Research conducted in vitro has demonstrated that activated astrocytes produce high levels of inflammatory mediators, including IL-1β and TNF-α [,]. AS-IV significantly inhibited penicillin-induced expression of inflammatory factors and p-MAPK family proteins in astrocytes, and significantly alleviated neurological damage in epileptic mice []. AS-IV also inhibited the senescence of astrocytes and prevented dopaminergic neuronal damage in PD by promoting mitochondrial autophagy, thereby avoiding the degeneration of dopaminergic neurons in PD and demonstrating significant therapeutic potential []. APS protects astrocytes from OGD/R-induced neuroinflammation to a certain extent by blocking the HMGB1/RAGE/NF-κB/NLRP3 signaling pathway [].
4.1.4. Regulatory Effect of A. membranaceus on Neutrophils
Neutrophils are integral components of the innate immune system, serving as the initial responders to sites of tissue injury or pathogen invasion, where they participate in the inflammatory response []. This characteristic underlies their extensive infiltration and activation within brain tissue during the inflammatory response to cerebral ischemia/reperfusion. Consequently, inhibiting this pathway may provide adequate protection against CNS diseases []. AS-IV mitigates the accumulation of neutrophils in the brain parenchyma by reducing the concentration of MPOs in brain tissue, the percentage of CD11b/CD18-positive neutrophils, and neutrophil-associated molecules in brain tissue up to 24 h post-reperfusion, thereby enhancing neurological outcomes. This reduction in accumulation within the brain parenchyma contributes to improved neurological outcomes and a decrease in infarct volume [].
4.1.5. Regulatory Effects of A. membranaceus Through Multiple Inflammatory Signaling Pathways
Inflammatory signaling pathways play a central role in the pathogenesis and progression of neurological disorders. In the context of traumatic brain injury (TBI), AS-IV significantly contributes to minimizing neuroinflammation and brain damage by downregulating the expression of inflammatory markers, including IL-6, IL-1β, and TNF-α, through the PERK pathway []. Moreover, addressing PERK-mediated stress in the endoplasmic reticulum reduces neuroinflammation and improves depressive-like symptoms []. In the PC12 neuronal inflammation model induced by 1 mg/mL LPS, pretreatment with AS-IV significantly reduced TNF-α, IL-1β, and TLR4 levels and inhibited the IL-17 signaling pathway []. APS also suppresses TNF-α, IL-1β, and TLR4 through the NF-κB and MAPK (ERK, JNK) pathways [].
Although numerous preclinical studies have demonstrated the potent anti-neuroinflammatory effects of AS-IV and APS in regulating microglial polarization, inhibiting NLRP3 inflammasome, and regulating astrocyte activation, these findings, derived primarily from animal models and in vitro experiments, require validation in human studies. For instance, AS-IV demonstrated the ability to transform from an M1 to an M2 phenotype in the MCAO model [], but its immunomodulatory effect in human ischemic stroke remains unclear. In addition, the regulatory results of pathways such as NF-κB and NLRP3 in various studies exhibit specific heterogeneity, suggesting that multiple factors, including model type, administration timing, and dose, may influence their effects. Future studies on human cells and clinical samples are needed to clarify their precise role in the complex neuroinflammatory network.
4.2. Oxidative Stress
Oxidative stress is characterized by the disruption of cellular mitochondria and the endoplasmic reticulum due to damaging stimuli, leading to the excessive production of reactive oxygen species (ROS) and reactive nitrogen species (RNS) []. This disruption disturbs the balance between oxidative and antioxidant systems, resulting in the accumulation of oxidative products and a decrease in reductase activity. This series of events ultimately triggers the peroxidation of DNA/RNA, proteins, and lipids, as well as tissue damage, culminating in a pathological state [,,]. Numerous studies have demonstrated that ROS levels are significantly elevated in the pathophysiology of conditions such as glioma, AIS, AD, and TBI, among others. Common types of ROS include H2O2, -OH, and O2− []. Maintaining moderate levels of reactive oxygen species during neuronal development is essential for executing critical physiological functions and participating in complex signaling processes (Figure 3) [].
4.2.1. A. membranaceus Regulates the Expression Levels of Antioxidant Enzymes
In the CNS, superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) are crucial, with SOD converting superoxide anion to H2O2 and GSH-Px further converting H2O2 to H2O and O2, thereby scavenging ROS and free radicals and attenuating CNS oxidative stress damage. Increasingly accumulating evidence suggests that AS-IV can improve the operations of SOD and GSH-Px while reducing the accumulation of oxidative products, such as ROS, in CNS disease models, including ischemia–reperfusion and experimental autoimmune encephalomyelitis, thereby exerting its biological effects against lipid peroxidation [,,,,,]. In addition to the above results, AS-IV reduced the expression of NADPH oxidase 2/4 (NOX2/4) and increased the total antioxidant capacity (T-AOC) in the brain tissues of Acute Ischemic Stroke (AIS) mice []. Calycosin (CA), a flavonoid component of A. membranaceus, showed potential neuroprotective effects by decreasing the expression of MDA, NO, and LDH and inhibiting oxidative stress in a SCI model in combination with rehabilitation training [].
4.2.2. The Regulation of Mitochondrial Function by A. membranaceus
Abnormal mitochondrial function is regarded as a central causative factor of oxidative stress []. An intrinsic key factor driving a series of complex pathological alterations, which play a pivotal role in the process of neurological injury, ionic homeostasis imbalance, and impaired nerve regeneration []. A. membranaceus and its active ingredients have been shown to have significant potential to modulate various mitochondrial functions. AS-IV regulates mitochondrial permeability transition, upregulates Bc1-2 levels, inhibits Caspase-3 activation, and attenuates neurological damage caused by AD, among others []. The extract from A. membranaceus root can counteract oxidative damage in the brain tissue of epileptic mice caused by pentylenetetrazole (PTZ) by enhancing mitochondrial complex activity and membrane potential, thereby providing anticonvulsant effects [].
4.2.3. Nrf2 Plays a Core Role in the Antioxidant Stress Resistance of A. membranaceus
Nrf2 is a key transcription factor that regulates the expression of genes encoding antioxidant enzymes, which are essential for cellular defense against oxidative stress. It binds to Keap1, enters the nucleus, and activates genes like HO-1 and SOD [,]. Activating Nrf2 may offer new treatments for neurological disorders. AS-IV shields cortical neurons from OGD/R damage through the activation of EGFR-Nrf2 signaling []. AS-IV protects neurons from OGD/R damage via EGFR-Nrf2 signaling and, with Panax ginseng’s (Rg1), activates the Nrf2/HO-1 pathway to combat oxidative stress []. Studies suggest that Formononetin (FMN), an isoflavonoid in A. membranaceus, offers neuroprotection by increasing Nrf2 expression in rats suffering from TBI, thereby regulating redox homeostasis []. Interestingly, the Chinese herbal formula Huangqi Guizhi WuWu Tang (HGWD) reduced oxaliplatin-induced neurotoxicity, primarily by regulating antioxidant precursors and essential molecules in the PI3K/Akt-Nrf2 pathway, which subsequently decreased the oxidative response triggered by paclitaxel in the CNS [].
A. membranaceus and its bioactive components have demonstrated significant antioxidant potential in various CNS disease models by enhancing the activities of antioxidant enzymes such as SOD and GSH-Px, regulating mitochondrial function, and activating the Nrf2 pathway. However, it is not clear whether these effects have the same efficacy in the human body. For example, AS-IV protects cortical neurons in the OGD/R model through EGFR-Nrf2 signaling [], but whether the regulatory mechanism of this pathway in human neurons is consistent remains to be verified. Moreover, it remains unclear whether long-term or high-dose administration of A. membranaceus antioxidants might induce compensatory oxidative stress or disrupt redox homeostasis.
4.3. Anti-Apoptosis
Apoptosis is a self-initiated cell death process triggered by normal cells in both physiological and pathological states, playing a vital role in maintaining biological balance []. Genes that control apoptosis can be divided into three groups: pro-apoptotic genes (e.g., Fas, Bax, ICE, p53) [,], anti-apoptotic genes (e.g., bcl-2, EIB, IAP) [], and genes with bidirectional regulatory functions (e.g., c-myc) []. These genes determine cell fate through a sophisticated network; however, it is the caspase cascade that ultimately carries out programmed cell death, and this pathway is regarded as the common endpoint for the convergence of apoptotic signals []. Upon further study, researchers found that apoptosis, as seen in neuronal cells, is at the core of the dramatic decline in neurological function and the rapid deterioration of the disease (Figure 4).
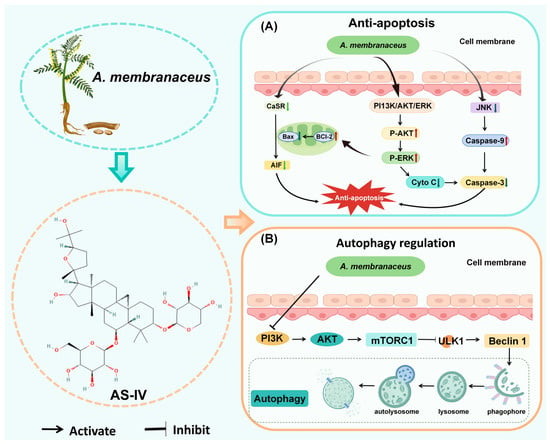
Figure 4.
A. membranaceus exerts anti-apoptotic and autophagy regulatory effects. (A) Anti-apoptotic signaling pathways. AS-IV inhibits neuronal apoptosis through multiple pathways. AS-IV suppresses the phosphorylation of c-Jun N-terminal kinase (JNK) and downregulates the expression of the calcium-sensing receptor (CaSR), thereby preventing the activation of pro-apoptotic cascades. Concurrently, it activates the pro-survival PI3K/Akt and ERK signaling pathways. The net effect is a shift in the mitochondrial balance towards cell survival, characterized by a decrease in the Bax/Bcl-2 ratio, inhibition of cytochrome c (Cyto C) and apoptosis-inducing factor (AIF) release from mitochondria, and subsequent suppression of the key executioner caspase, caspase-3. (B) Regulation of autophagy. A. membranaceus components modulate the highly conserved autophagy pathway to exert neuroprotective effects. A key mechanism involves the inhibition of the PI3K/Akt/mTORC1 signaling axis. By reducing mTORC1 activity, A. membranaceus relieves its suppression on the ULK1 complex and Beclin-1, thereby initiating autophagosome formation. This promotes the sequestration of damaged organelles and protein aggregates into a phagophore, which matures into an autophagosome and subsequently fuses with a lysosome to form an autolysosome, where cargo is degraded and recycled. The regulation of this process helps maintain cellular homeostasis in neurons under stress. This figure is original and was created by the authors for this publication.
4.3.1. A. membranaceus Inhibits JNK Phosphorylation
Activation of c-Jun N-terminal kinase (JNK) leads to the initiation of apoptosis []. Inhibition of JNK phosphorylation may be a key pathway for protecting against neuronal apoptosis. AST attenuates neuronal apoptosis in ischemia/reperfusion rats through this mechanism, increasing the expression of phosphorylated extracellular signal-regulated kinase (p-ERK) and phosphorylated Akt []. Sun’s team experimentally verified that AS-IV, administered orally for 3 days, in addition to down-regulating p-JNK and upregulating p-ERK, p Akt, in addition to down-regulating p-JNK, upregulating p-ERK, p-ERK, and Klotho, and inhibiting oxidative stress and pro-inflammatory factor release, AS-IV also protects the developing brain from anesthesia-induced neuronal apoptosis [].
4.3.2. A. membranaceus Regulates the Expression of CaSR
In cerebral ischemia–reperfusion injury (CIRI), the calcium-sensitive receptor (CaSR) is an essential G-protein-coupled receptor that exacerbates secondary neurological injury upon activation. Increasing evidence suggests that AS-IV inhibits apoptosis and attenuates neurological deficits secondary to CIRI by down-regulating the expression of CaSR and apoptotic proteins such as Fas, FasL, Bid, and Caspase-8. It also attempts to inhibit cleaved caspase-3 and upregulate the Bax/Bcl-2 ratio, suggesting that the dynamic regulation of CaSR may be one of the targets of AS-IV’s action to exert neuroprotective effects and inhibit apoptosis [,]. AS-IV not only reduces apoptotic protein expression but also inhibits endoplasmic reticulum stress-related proteins like eIF2a, Bip, and CHOP, thereby decreasing cerebral infarction by preventing endothelial cell apoptosis [].
4.3.3. A. membranaceus Inhibits Apoptosis Through Other Pathways
Research has demonstrated that AS-IV provides anti-apoptotic benefits in the hippocampus of AD rats, thereby enhancing cognitive function by reducing Bax and caspase-3 concentrations []. Wang et al. discovered that AST suppressed key proteins in the Fas/Fasl-VDAC1 pathway in Aβ1-42-damaged C8D1A cells, thereby reducing apoptosis and enhancing cognitive function in hippocampal astrocytes, which helps alleviate cognitive deficits in AD mice []. Notably, PPARγ is a key molecular switch for the treatment of AD, and its inhibition reduces AβO-induced apoptosis of HT22 cells by AS-IV [].
A. membranaceus components exert anti-apoptotic effects through mechanisms such as inhibiting JNK phosphorylation, down-regulating CaSR expression, and regulating the Bax/Bcl-2 ratio. These findings have been repeatedly verified in models such as ischemia–reperfusion and AD. However, the apoptotic pathway may play a dual role at different stages of the disease. Excessive inhibition of apoptosis may hinder the necessary cell clearance process. For example, AS-IV alleviates apoptosis in the CIRI model by inhibiting CaSR, but the physiological role of CaSR during the neural repair period has not been fully considered []. Furthermore, most studies have focused on a single pathway, lacking a systematic exploration of the interactions between apoptosis and other forms of cell death, such as autophagy and pyroptosis. In the future, it will be necessary to combine multi-omics technologies to reveal the regulatory nodes within the complete biological network.
4.4. Autophagy Regulation
Autophagy maintains cellular balance by removing damaged organelles, faulty proteins, and pathogens through lysosomal degradation and recycling [,]. Numerous studies have demonstrated that abnormalities in autophagy and endolysosomal pathways, associated with neuronal dysfunction, are intricately linked to the pathogenesis of CNS disorders. Consequently, the regulation of autophagy may play a crucial role in the therapeutic strategies for CNS diseases (Figure 4) [,,].
A. membranaceus Regulates Autophagy via AMPK and mTOR
Adenosine-activated protein kinase (AMPK) is a crucial energy sensor that helps maintain cellular energy balance []. AMPK activity regulates a broad spectrum of metabolic processes, encompassing the modulation of autophagy, mitophagy, and various other mechanisms []. Rapamycin, a principal regulator of cellular metabolism [], exerts its inhibitory effects on autophagy by phosphorylating and inactivating critical regulatory proteins, including ULK1, Beclin-1, UVRAG, and TFEB, as well as by suppressing the expression of autophagy-related proteins []. Consequently, AMPK and mTOR are proposed to play pivotal roles in regulating autophagy. Neuronal apoptosis is a major cause of secondary injury after SCI. Research conducted by Lin’s team demonstrated that the intraperitoneal administration of AS-IV can induce autophagy in neuronal cells through the mTORC1 signaling pathway, thereby conferring protection against apoptosis by modulating autophagy. This suggests that AS-IV holds promise as a novel therapeutic agent for SCI []. Researchers found that AST plays a key neuroprotective role by stimulating the PI3K/Akt-mTOR pathway-mediated autophagy and upregulating the levels of autophagy flux-associated proteins in APP/PS1 mice, which represents a new mechanism of AST for treating AD through autophagy regulation []. Hao et al. demonstrated that AS-IV can attenuate autophagy activity by inhibiting the AMPK/mTOR pathway, thereby reducing mitochondrial-mediated apoptosis. This action mitigates cellular injury in SH-SY5Y cells and the MCAO model, thereby exerting neuroprotective effects and positioning AS-IV as a potential therapeutic candidate for AIS []. Zhang and colleagues identified a novel mechanism for treating ischemia/reperfusion (I/R) injury, wherein AS-IV inhibits apoptosis by enhancing P62-LC3-mediated autophagy and increasing the expression of LC3II/LC3I in HT22 cells following oxygen-glucose deprivation/reperfusion (OGD/R) treatment. This suggests that the promotion of autophagy regulation by AS-IV represents a novel therapeutic mechanism for I/R injury []. Furthermore, AS-IV modulates the AMPK-MTORC1-ULK pathway to enhance autophagy, as evidenced by reduced P-mTOR levels and increased levels of LC3, P-AMPK, and P-ULK in retinal tissues, thereby exerting a therapeutic effect on traumatic optic neuropathy (TON) [].
Autophagy has a “double-edged sword” characteristic in CNS diseases, and its regulation requires a high degree of spatiotemporal specificity. For instance, in the SCI model, AS-IV promotes autophagy and reduces apoptosis by inhibiting mTORC1 [], but in the AIS model, it weakens autophagy to protect neurons by inhibiting AMPK/mTOR []. These seemingly contradictory findings suggest that the effects of A. membranaceus may be highly context-dependent, varying with the specific disease model or stage. Currently, there is no systematic study comparing the dynamic effects of A. membranaceus on autophagic flux at different disease stages, nor has its interaction with processes such as inflammation and oxidative stress been elucidated. In the future, models that more closely resemble human diseases should be constructed to assess the clinical translational potential of autophagy regulation.
4.5. Anti-Ferroptosis
Ferroptosis is a regulated form of programmed cell death that results from iron-triggered phospholipid peroxidation [,]. Since brain tissue is rich in lipids and iron and has a high rate of oxygen consumption, which makes ferroptosis particularly strong, a defense strategy against ferroptosis may be an important tool in the treatment of CNS diseases (Figure 5) [].
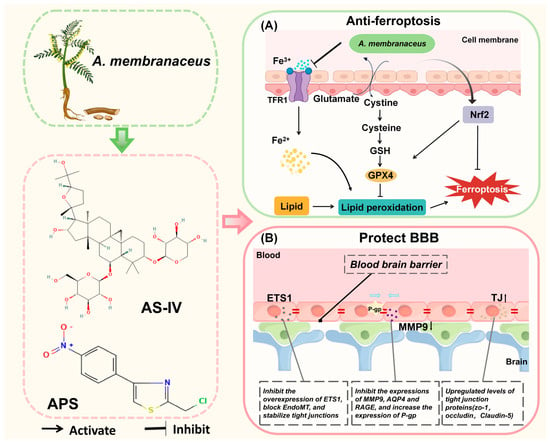
Figure 5.
A. membranaceus exerts anti-ferroptosis and anti-blood–brain barrier damage effects. (A) Inhibition of ferroptosis. A. membranaceus and its bioactive compounds, such as total flavonoids (TFA) and AS-IV, combat ferroptosis, an iron-dependent form of cell death. They upregulate the system Xc-transporter (comprising the SLC7A11 subunit), which enhances cystine uptake and its reduction to cysteine for the synthesis of the key antioxidant glutathione (GSH). Concurrently, they activate the Nrf2 signaling pathway, which promotes the expression of glutathione peroxidase 4 (GPX4). GPX4 utilizes GSH to reduce toxic lipid hydroperoxides (L-OOH) to non-toxic lipid alcohols (L-OH), thereby halting the lipid peroxidation chain reaction that drives ferroptosis. Additionally, modulation of iron metabolism (e.g., via Transferrin Receptor 1, TFR1) may contribute to reducing the intracellular pool of reactive Fe2+. (B) Protection of the Blood–Brain Barrier (BBB). A. membranaceus components preserve BBB integrity through several concerted actions. Astragalus polysaccharides (APS) inhibit the transcription factor ETS1, thereby blocking Endothelial-to-Mesenchymal Transition (EndoMT) and helping to stabilize tight junctions (TJ). Key active ingredients, such as AS-IV and Cycloastragenol (CAG), upregulate the expression of tight junction proteins, including Zonula Occludens-1 (ZO-1), occludin, and Claudin-5, which seal the paracellular space. Furthermore, they protect the BBB by inhibiting the expression and activity of matrix metalloproteinase-9 (MMP-9) and Aquaporin-4 (AQP4), which are involved in basement membrane degradation and edema formation, respectively []. They also favorably modulate transporters by upregulating P-glycoprotein (P-gp) for efflux and downregulating the Receptor for Advanced Glycation End-products (RAGE) for reduced influx. This figure is original and was created by the authors for this publication.
4.5.1. The Regulation of GPX4 by A. membranaceus
Glutathione peroxidase 4 (GPX4) serves a distinctive role as a critical negative regulator of ferroptosis, primarily through its ability to convert lipid hydroperoxides into non-toxic lipid alcohols, thus interrupting the lipid peroxidation chain reaction. The proper functioning of GPX4 is crucial for regulating ferroptosis. Inhibition of GPX4 may increase cellular susceptibility to ferroptosis []. Total flavonoids (TFA) extracted from A. membranaceus inhibited ferroptosis by upregulating the SLC7A11/GPX-4 axis, as well as by increasing intracellular glutathione levels and GSH/GSSG ratio. Additionally, researchers found that the use of the flavonoids extracted from A. membranaceus as a dietary supplement might be beneficial for PD patients []. Zhang et al. demonstrated that AS-IV enhanced the sensitivity of SLC7A11 and GPX4, both of which serve as indicators for evaluating ferroptosis. Additionally, AS-IV activated the Nrf2/HO-1 signaling pathway, thereby inhibiting ferroptosis and mitigating neuronal death in the MCAO model by modulating critical nodes within this pathway []. Interestingly, AS-IV administration in subarachnoid hemorrhage (SAH) similarly activates this pathway, inhibiting lipid peroxide accumulation and blocking the ferroptosis process in SAH []. Mai’s research team created selenium nanoparticles (TSIIA/TMP/APS@Se NPs) containing APS, Tan-shinoneIA, and tetramethylpyrazine, which effectively inhibited ferroptosis. These nanoparticles reversed the decline in neuronal numbers and GPX4 levels after SCI and significantly reduced 4-hydroxynonenal, a marker of lipid peroxidation, potentially improving functional recovery after SCI [].
4.5.2. A. membranaceus Relies on NADPH to Function
The commonality of enzymes and metabolites necessary for resistance to lipid peroxidation during ferroptosis lies in their dependence on the key cellular reducing agent, NADPH []. In HT22 cells, AS modulators restored the balance of brain iron metabolism and stabilized lipid peroxidation by reducing NADPH oxidase 4 (NOX4) and activating the NOX4/Nrf2 signaling pathway. Peroxidation (LPO) homeostasis and inhibited ferrocyte apoptosis in SAMP8 mice, a mechanism that suggests the potential of AS modulators to inhibit ferroptosis and improve AD symptoms, making them a potential therapeutic candidate [].
A. membranaceus components inhibit ferroptosis by upregulating GPX4, activating the Nrf2/HO-1 pathway, and regulating NOX4, etc., showing good prospects in models such as SAH, PD, and AD. However, ferroptosis, as a new form of programmed cell death, has not yet been fully elucidated in its molecular mechanism, and it remains unclear whether A. membranaceus components have direct targets in this process. For example, AS-IV inhibits ferroptosis in the MCAO model through the Nrf2/HO-1 pathway [], but whether this pathway is its primary mechanism of action remains controversial. Additionally, there is currently limited research on how the interaction between ferroptosis and other modes of cell death, such as apoptosis and autophagy, changes under the intervention of A. membranaceus. In the future, it is necessary to combine gene editing and lipidomics technologies to clarify their specific contributions to the ferroptosis network.
4.6. Anti-Blood–Brain Barrier Damage
The blood–brain barrier (BBB) serves as the CNS’s final defense against external threats. Its structural and functional stability is vital for preserving the neuronal environment (Figure 5). However, under pathological conditions such as AIS, AD, and multiple sclerosis (MS), the BBB suffers from structural damage due to inflammatory cascades, oxidative stress imbalance, and metabolic reprogramming, with cascading increases in permeability, which exacerbates the formation of a neurotoxic microenvironment and triggers irreversible neuronal damage and dysfunction [].
4.6.1. The Regulation of Tight Junction Proteins by Active Ingredients of A. membranaceus
AST completely reversed LPS-induced BBB leakage and depressive behavior in a mouse model by down-regulating MMP-9 and upregulating Claudin-5, suggesting that it may be an adjunctive neuroprotectant for the treatment of sepsis-associated encephalopathy (SAE) []. AS-IV increases TEER and reduces sodium fluorescein extravasation, while also increasing the expression of compact proteins, such as zonula-1 and occludin, in LPS-stimulated bEnd.3 cells to protect the BBB []. Research has demonstrated that lanthanum, serving as a vascular permeability tracer during the intravenous administration of AS-IV, exclusively stains cerebral capillaries. This finding strongly suggests the BBB-protective properties of AS-IV, as it significantly inhibits the upregulation of MMP-9 and AQP4 and mitigates cerebral vasogenic edema []. CAG not only mitigated oligomeric Aβ 1-42-induced apoptosis in bEnd.3 cells and enhanced the expression of tight junction scaffolding proteins, but also facilitated the efflux of soluble Aβ across the BBB by upregulating P-glycoprotein (P-gp) and downregulating receptor for advanced glycation end-products (RAGE) expression []. Isoastragaloside I (ISOI), a cyclic alkane glycoside derived from A. membranaceus, not only restored the reduced levels of tight junction proteins in LPS-stimulated bEnd.3 cells but also reduced BBB permeability by activating the Nrf2 signaling pathway []. The combined administration of A. membranaceus and Chuanxiongzine diminishes hemorrhage and Evans blue dye extravasation in the brain tissue of I/R mice, while enhancing the expression of tight junction proteins, thereby ameliorating the ultrastructural disruption of the BBB [].
4.6.2. The Regulation of ETS1 by A. membranaceus
The transcription factor ETS1 is the central switch that triggers the endothelial-mesenchymal transition EndoMT and then disrupts the BBB. APS shows potential application in MS prevention by inhibiting the overexpression of ETS1, blocking endoMT, and stabilizing tight junctions [].
4.6.3. A. membranaceus Combats the BBB Through Other Means
A. membranaceus injection activates the BDNF/TrkB/CREB pathway, improving BBB function and reducing sepsis-induced neurological deficits []. Furthermore, Hou et al. identified that AS-IV effectively inhibits endoplasmic reticulum stress-mediated endothelial cell apoptosis, thereby protecting the BBB and reducing the cerebral infarct area in I/R rats [].
A. membranaceus and its components protect the integrity of the BBB by enhancing the expression of tight junction proteins, inhibiting ETS1, and regulating ER stress, and have shown significant effects in models such as LPS, I/R, and SAE. However, most of these results originated from short-term acute models, and their long-term protective effects in chronic BBB degenerative diseases (such as AD and MS) have not yet been verified. For instance, AS-IV enhances the expression of ZO-1 and occludin in LPS-stimulated bEnd.3 cells [], but its effect in human brain microvascular endothelial cells and its ability to penetrate the BBB remain to be confirmed. In addition, the distribution, metabolism, and potential cumulative toxicity of A. membranaceus components in the BBB destruction area have not been systematically evaluated. In the future, imaging and biomarkers should be combined to promote their translational research from basic to clinical applications.
5. Pharmacognosy of A. membranaceus
5.1. Safety
On 9 November 2023, A. membranaceus was officially recognized as an edible Chinese medicinal substance by China’s health authorities. It shows low toxicity with no reported clinical side effects. In a study by Yu’s team, rats and beagles received RAE (APS and AS) for 90 days without significant toxicity, even at doses 70 and 35 times higher than the human dose, respectively []. Xie’s team conducted three-month gavage experiments on rats using A. membranaceus, noting no fatalities or toxicity, confirming its safety. They established a NOAEL of 8800 mg/kg/day, 50 times the human clinical dose, further verifying the formulation’s safety []. In summary, A. membranaceus and its main bioactive compounds are safe at standard doses in both short- and long-term toxicity tests, showing no significant side effects. However, high doses or prolonged use may lead to toxicity, so caution is advised.
5.2. Application of A. membranaceus in the Field of Food and Medicine
5.2.1. Application of A. membranaceus in Formulated Preparations
A. membranaceus has been used in treating CNS diseases since its inclusion in Buyang Huanwu Decoction, as noted in Wang Qingren’s “Medical Forest Right and Wrong.” Modern studies have shown that this decoction enhances nerve function and prevents neuronal damage through a multi-target mechanism. It reduces oxidative stress by inhibiting ROS, lowering MDA and 8-OHdG, and boosting SOD and GSH-Px. It also restores mitochondrial function, stabilizes energy metabolism, activates the PKCε/Nrf2/HO-1 pathway, and enhances antioxidant gene expression, forming a protective loop. These effects collectively reduce cerebral I/R injury and support recovery, supporting A. membranaceus’s use in treating IS-related qi deficiency and blood stasis [].
5.2.2. Application of A. membranaceus in Health Foods
A. membranaceus is now added to health foods like drinks, pastries, and porridges, supporting the “medicine and food” philosophy (Figure 6). Patents show its use in three categories: liquid, semi-liquid, and solid, all for easy consumption (Table 2). These foods can boost the immune system and impact intestinal microbiota, which, in turn, influence both digestion and brain health through the gut–brain axis []. Thus, consuming A. membranaceus-infused health foods may indirectly benefit brain health.
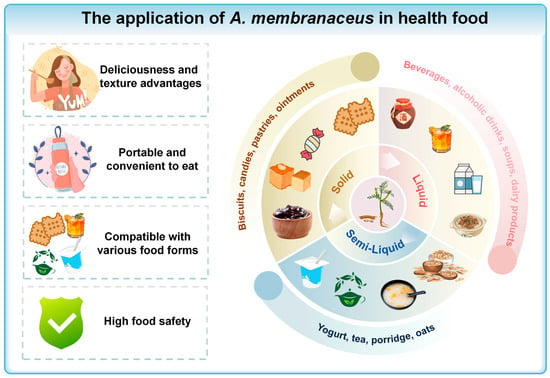
Figure 6.
The application of A. membranaceus in health food. This figure is original and was created by the authors for this publication.

Table 2.
Application of A.membranaceus in health food products and their potential relevance to CNS health.
Liquid Products
Soups are common in medicinal diets. For example, A. membranaceus chicken soup, made with black-boned chicken and goji berries, boosts qi, nourishes blood, and alleviates sub-health issues such as qi and blood deficiency and fatigue. It can also help with appetite loss in diseases like PD and act as a supportive therapy.
Semi-Liquid Products
Semi-liquid products, renowned for their unique texture and ease of digestion, are particularly suitable for patients with CNS diseases following surgery or radiochemotherapy. A. membranaceus porridge, combining A. membranaceus for kidney and spleen health with Japonica rice for stomach benefits, helps alleviate symptoms like memory loss and joint pain in AD patients with spleen and kidney deficiencies.
Solid Products
Within the staple food category, A. membranaceus health biscuits are not only effective in alleviating hunger but also contribute to the physical well-being of patients with CNS disorders, offering health-enhancing benefits.
6. Summary
6.1. Neuroprotective Potential and Mechanisms of A. membranaceus in CNS Disorders
CNS diseases are marked by high levels of morbidity, mortality, and disability, posing a serious threat to human health. However, in current clinical practice, the efficacy of conventional drug therapy is not satisfactory and is accompanied by adverse effects. Natural Chinese medicines derived from herbs or plants offer the advantages of biological activity, safety, and accessibility to resources. They are becoming a valuable treasure trove for the development of new drugs. The significant disability burden associated with CNS diseases is further compounded by the toxicity of many current therapeutics, making the search for low-toxicity, plant-derived neuroprotective agents an urgent need in the field of neurology. A. membranaceus has been used in TCM for a long time []. Its signature active ingredients exert anti-neuroinflammation, anti-oxidative stress, anti-apoptosis, autophagy regulation, anti-ferroptosis, and anti-BBB damage effects, which deeply intervene in the core mechanism of CNS diseases. Ferroptosis, a recently identified form of programmed cell death, has garnered considerable attention in the context of CNS diseases in recent years. Contemporary research suggests that its principal mechanisms involve signaling pathways such as GPX4, NOX4, and Nrf2. A. membranaceus and its active constituents modulate ferroptosis through several mechanisms: they activate the Nrf2/HO-1 pathway, upregulate GPX4 expression, and enhance antioxidant capacity; they also inhibit NOX4 activity, reduce ROS generation, and stabilize lipid peroxidation balance. These effects have been substantiated in various CNS disease models, demonstrating notable neuroprotective properties. As previously discussed, the recent study revealed that A. membranaceus exhibits significant neuroprotective effects against a range of CNS diseases through a synergistic mechanism characterized by a “multi-component-multi-target-multi-pathway” model, as demonstrated in both in vivo and in vitro models. Notably, preclinical studies consistently demonstrate that A. membranaceus is free from both acute and long-term toxicity, ensuring high safety, and it is listed in the ‘medicine and food’ catalog []. In recent years, the idea of “medicinal food” has shifted A. membranaceus from “adjuvant treatment” to a focus on “early prevention and functional rehabilitation,” offering a new strategy for precise intervention in CNS diseases.
6.2. The Limitations Faced in the Clinical Application and Promotion of A. membranaceus
Though the neuroprotective effects of A. membranaceus in CNS diseases have been widely validated, its promotion in clinical practice still faces multiple limitations:
From Single Pathways to Integrative Multi-Omics and Human-Relevant Models. The major hurdle of unclear pathways and targets necessitates a paradigm shift from studying isolated signaling axes to a systems-level, integrative approach. Future work should employ an integrative multi-omics strategy—combining spatial transcriptomics, proteomics, and lipidomics—to profile the comprehensive effects of A. membranaceus in relevant disease models []. This data must be integrated with network pharmacology to construct a quantifiable interaction network, moving beyond descriptive mechanisms to predictive models that can identify key synergistic nodes. Furthermore, to bridge the species gap and enhance human relevance, the field should leverage advanced human stem cell-derived models, such as brain organoids and blood–brain barrier-on-a-chip systems []. These models provide a physiologically accurate platform for validating network predictions and dissecting the complex cell–cell communication mediated by A. membranaceus, ultimately translating holistic synergy into quantifiable, precise intervention strategies.
From Empirical Combination to Rational Pharmacokinetic/Pharmacodynamic (PK/PD) Studies. The role of A. membranaceus in drug combinations is unclear, with potential risks for altered efficacy or adverse reactions when co-administered with conventional neurotherapeutics. Systematic investigations into pharmacokinetic and pharmacodynamic (PK/PD) interactions are therefore essential. Research should specifically examine how A. membranaceus influences the absorption, distribution, metabolism, and excretion of CNS drugs. Utilizing in vitro models, such as Caco-2 cells and human liver microsomes, can determine whether it acts as an inhibitor or inducer of key metabolic enzymes (e.g., CYP450) or transporters (e.g., P-gp) []. This will establish a scientific basis for rational combination therapies, maximizing synergy and minimizing clinical risks, a critical step before large-scale clinical adoption.
From Preclinical Validation to Mechanism-Informed Clinical Trial Design. A significant gap exists between robust animal data and the lack of high-quality clinical evidence. Critical parameters, such as dose-exposure relationships and long-term safety in humans, remain undefined. Future research should prioritize well-designed, mechanism-informed clinical trials. Prior to large-scale trials, Phase I/IIa studies are needed to establish human pharmacokinetics and identify preliminary biomarkers of target engagement (e.g., neuroinflammation imaging, plasma neurofilament light levels) []. Subsequent multicenter, randomized controlled trials (RCTs) should focus on specific patient populations where preclinical evidence is strongest, such as using AS-IV-standardized extracts as an adjunctive therapy in early Parkinson’s disease or vascular cognitive impairment. Furthermore, long-term observational studies are required to definitively assess the risks of tolerance and other potential side effects associated with prolonged use.
As a result, to successfully transition A. membranaceus from the research laboratory setting to practical clinical use, we must gain a more detailed understanding of its underlying mechanisms of action. This involves conducting thorough scientific investigations to uncover precisely how A. membranaceus functions within the biological systems. Additionally, it is crucial to refine and optimize dosage protocols to ensure that therapeutic benefits are maximized while minimizing potential side effects. This optimization process should be based on comprehensive pharmacokinetic and pharmacodynamic studies. Furthermore, conducting high-quality, rigorous clinical trials is imperative to establish the safety and efficacy of A. membranaceus in various patient populations. These trials should be designed with robust methodologies, including randomized controlled trials, to generate reliable and statistically significant data. These concerted efforts are crucial for establishing the clinical efficacy of A. membranaceus and facilitating its translation into standard medical practice.
7. Conclusions
In summary, our review consolidates compelling evidence that A. membranaceus and its principal bioactive constituents, notably AS-IV and APS, function as a multifaceted pharmacological agent against a spectrum of CNS disorders. Its neuroprotective efficacy is underpinned by a sophisticated “multi-component, multi-target, multi-pathway” mechanism, which orchestrates the concurrent mitigation of neuroinflammation, oxidative stress, apoptosis, and ferroptosis, while also regulating autophagy and fortifying the blood–brain barrier. This polypharmacological profile positions A. membranaceus as a particularly attractive candidate for addressing the complex, intertwined pathologies of neurodegenerative and cerebrovascular diseases.
The paradigm of “homology of medicine and food” further elevates its translational potential, enabling a strategic shift from adjuvant therapy towards early prevention and long-term functional rehabilitation through health food products. However, the translation of these robust preclinical findings into clinical practice is contingent upon addressing critical gaps. Future research must pivot from descriptive mechanism elucidation to a predictive, systems-level understanding employing integrative multi-omics and human-relevant disease models. Furthermore, rigorous pharmacokinetic studies and well-designed, mechanism-informed clinical trials are imperative to definitively establish its efficacy, optimal dosing, and long-term safety in humans. By systematically navigating these challenges, A. membranaceus can be fully validated as a cornerstone in the evolving arsenal of evidence-based, natural neuroprotective strategies.
Author Contributions
Conceptualization, F.H. and J.H.; writing—original draft, J.S. and J.G.; investigation, H.Z., H.Y. and Y.Q.; writing—review and editing, J.S. and J.G.; funding acquisition, F.H. and J.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Program of the National Natural Science Foundation of China (No. 82274645).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used in this article were sourced from materials mentioned in the References Section.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AD | Alzheimer’s disease |
| AIS | Acute Ischemic Stroke |
| AMPK | Adenosine-activated protein kinase |
| APS | A. membranaceus polysaccharides |
| AS-IV | A. membranaceus IV |
| Astragalus membranaceus | A. membranaceus |
| BBB | blood–brain barrier |
| CA | Calycosin |
| CAG | Cycloastragenol |
| CaSR | calcium-sensitive receptor |
| CI | cerebral infarction |
| CIRI | cerebral ischemia–reperfusion injury |
| CNS | Central Nervous System |
| EAE | experimental autoimmune encephalomyelitis |
| EBI | stroke-triggered early brain injury |
| FMN | Formononetin |
| GSH-Px | glutathione peroxidase |
| GPX4 | Glutathione peroxidase 4 |
| HGWD | Huangqi Guizhi WuWu Tang |
| I/R | ischemia/reperfusion |
| ISOI | Isoastragaloside I |
| JNK | c-Jun N-terminal kinase |
| LPO | Lipid Peroxidation |
| LPS | Lipopolysaccharides |
| MCAO | middle cerebral artery occlusion |
| MS | multiple sclerosis |
| NF-κB | Nuclear factor-κB |
| NLRP3 | NOD-like receptor thermal protein domain-associated protein 3 |
| NOX2/4 | NADPH oxidase 2/4 |
| NOX4 | NADPH oxidase 4 |
| OGD/R | oxygen-glucose deprivation/reoxygenation |
| PD | Parkinson’s disease |
| p-ERK | phosphorylated extracellular signal-regulated kinase |
| P-gp | P-glycoprotein |
| PPARγ | peroxisome proliferator-activated receptor γ |
| PTZ | pentylenetetrazole |
| RAGE | receptor for advanced glycation end-products |
| Rg1 | Ginsenoside Rg1 |
| RNS | reactive nitrogen species |
| ROS | reactive oxygen species |
| RRS | repeated restraint stress |
| SAE | sepsis-associated encephalopathy |
| SAH | subarachnoid hemorrhage |
| SCI | spinal cord injury |
| SOD | superoxide dismutase |
| T-AOC | total antioxidant capacity |
| TBI | traumatic brain injury |
| TCM | traditional Chinese medicine |
| TFA | total flavonoids |
| TON | traumatic optic neuropathy |
References
- Feigin, V.L.; Stark, B.A.; Johnson, C.O.; Roth, G.A.; Bisignano, C.; Abady, G.G.; Abbasifard, M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abedi, V.; et al. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.Q.; Zhu, F. Trends in Prevalence Cases and Disability-Adjusted Life-Years of Parkinson’s Disease: Findings from the Global Burden of Disease Study 2019. Neuroepidemiology 2022, 56, 261–270. [Google Scholar] [CrossRef]
- Song, R.; Guo, Y.; Fu, Y.; Ren, H.; Wang, H.; Yan, H.; Ge, Y. Trends of mitochondrial changes in AD: A bibliometric study. Front. Aging Neurosci. 2023, 15, 1136400. [Google Scholar] [CrossRef]
- Wijdicks, E.F. Hepatic Encephalopathy. N. Engl. J. Med. 2016, 375, 1660–1670. [Google Scholar] [CrossRef]
- Jiang, C.; Yan, Y.; Long, T.; Xu, J.; Chang, C.; Kang, M.; Wang, X.; Chen, Y.; Qiu, J. Ferroptosis: A potential therapeutic target in cardio-cerebrovascular diseases. Mol. Cell. Biochem. 2025, 480, 4379–4399. [Google Scholar] [CrossRef]
- Li, M.; Han, B.; Zhao, H.; Xu, C.; Xu, D.; Sieniawska, E.; Lin, X.; Kai, G. Biological active ingredients of Astragali Radix and its mechanisms in treating cardiovascular and cerebrovascular diseases. Phytomedicine 2022, 98, 153918. [Google Scholar] [CrossRef]
- Shi, Y.; Shi, X.; Zhao, M.; Ma, S.; Zhang, Y. Pharmacological potential of Astragali Radix for the treatment of kidney diseases. Phytomedicine 2024, 123, 155196. [Google Scholar] [CrossRef]
- Abd Elrahim Abd Elkader, H.T.; Essawy, A.E.; Al-Shami, A.S. Astragalus species: Phytochemistry, biological actions and molecular mechanisms underlying their potential neuroprotective effects on neurological diseases. Phytochemistry 2022, 202, 113293. [Google Scholar] [CrossRef]
- Gong, A.G.W.; Duan, R.; Wang, H.Y.; Kong, X.P.; Dong, T.T.X.; Tsim, K.W.K.; Chan, K. Evaluation of the Pharmaceutical Properties and Value of Astragali Radix. Medicines 2018, 5, 46. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Huang, H.; Fan, Y.; Xu, L.; Jin, X.; Xiao, B.; Ma, C.; Fan, H.; Chai, Z. Astragaloside IV ameliorates Parkinson’s disease by inhibiting TLR4/NF-κB-dependent neuroinflammation. Int. Immunopharmacol. 2025, 160, 114972. [Google Scholar] [CrossRef] [PubMed]
- Weng, W.; Lin, B.; Zheng, J.; Sun, Y.; Li, Z.; Chen, X.; Wang, Y.; Pan, X. Novel application of cycloastragenol target microglia for the treatment of Alzheimer’s disease: Evidence from single-cell analysis, network pharmacology and experimental assessment. Phytomedicine 2025, 139, 156502. [Google Scholar] [CrossRef]
- Zheng, Y.; Ren, W.; Zhang, L.; Zhang, Y.; Liu, D.; Liu, Y. A Review of the Pharmacological Action of Astragalus Polysaccharide. Front. Pharmacol. 2020, 11, 349. [Google Scholar] [CrossRef]
- Liu, X.; Ma, J.; Ding, G.; Gong, Q.; Wang, Y.; Yu, H.; Cheng, X. Microglia Polarization from M1 toward M2 Phenotype Is Promoted by Astragalus Polysaccharides Mediated through Inhibition of miR-155 in Experimental Autoimmune Encephalomyelitis. Oxidative Med. Cell. Longev. 2021, 2021, 5753452. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Gan, H.; Jin, H.; Fang, Y.; Yang, Y.; Zhang, J.; Hu, X.; Chu, L. Astragaloside IV promotes microglia/macrophages M2 polarization and enhances neurogenesis and angiogenesis through PPARγ pathway after cerebral ischemia/reperfusion injury in rats. Int. Immunopharmacol. 2021, 92, 107335. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Mu, B.; Guo, M.; Liu, C.; Meng, T.; Yan, Y.; Song, L.; Yu, J.; Kumar, G.; Ma, C. Astragaloside IV inhibits experimental autoimmune encephalomyelitis by modulating the polarization of both microglia/macrophages and astrocytes. Folia. Neuropathol. 2023, 61, 273–290. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Shi, H.; Jin, X.; Guo, S.; Zhou, X.; Gao, W. Mechanism of astragaloside IV regulating NLRP3 through LOC102555978 to attenuate cerebral ischemia reperfusion induced microglia pyroptosis. Int. Immunopharmacol. 2024, 131, 111862. [Google Scholar] [CrossRef]
- Song, M.T.; Ruan, J.; Zhang, R.Y.; Deng, J.; Ma, Z.Q.; Ma, S.P. Astragaloside IV ameliorates neuroinflammation-induced depressive-like behaviors in mice via the PPARγ/NF-κB/NLRP3 inflammasome axis. Acta. Pharmacol. Sin. 2018, 39, 1559–1570. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, G.; Lang, J.; Sun, B.; Feng, S.; Li, D.; Sun, G. Astragaloside IV ameliorated neuroinflammation and improved neurological functions in mice exposed to traumatic brain injury by modulating the PERK-eIF2α-ATF4 signaling pathway. J. Investig. Med. 2024, 72, 747–762. [Google Scholar] [CrossRef]
- Thakur, A.; Wang, X.; Siedlak, S.L.; Perry, G.; Smith, M.A.; Zhu, X. c-Jun phosphorylation in Alzheimer disease. J. Neurosci. Res. 2007, 85, 1668–1673. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Wu, W.Y.; Gong, H.L.; Li, W.Z.; Yin, Y.Y. Astragalosides attenuate learning and memory impairment in rats following ischemia—Reperfusion injury. Mol. Med. Rep. 2014, 9, 1319–1324. [Google Scholar] [CrossRef]
- Lin, J.; Pan, X.; Huang, C.; Gu, M.; Chen, X.; Zheng, X.; Shao, Z.; Hu, S.; Wang, B.; Lin, H.; et al. Dual regulation of microglia and neurons by Astragaloside IV-mediated mTORC1 suppression promotes functional recovery after acute spinal cord injury. J. Cell. Mol. Med. 2020, 24, 671–685. [Google Scholar] [CrossRef]
- Ma, T.; Du, J.; Zhang, Y.; Wang, Y.; Wang, B.; Zhang, T. GPX4-independent ferroptosis—A new strategy in disease’s therapy. Cell Death Discov. 2022, 8, 434. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.M.; Guo, Z.; Wang, J.; Ma, H.Y.; Qin, X.Y.; Hu, Y.; Lan, R. Astragalin alleviates lipopolysaccharide-induced depressive-like behavior in mice by preserving blood-brain barrier integrity and suppressing neuroinflammation. Free. Radic. Biol. Med. 2025, 232, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Xie, L.; Liu, Y.; Liu, T.; Yang, P.; Hu, J.; Peng, Z.; Luo, K.; Du, M.; Chen, C. Astragalus polysaccharide (APS) exerts protective effect against acute ischemic stroke (AIS) through enhancing M2 micoglia polarization by regulating adenosine triphosphate (ATP)/ purinergic receptor (P2X7R) axis. Bioengineered 2022, 13, 4468–4480. [Google Scholar] [CrossRef]
- Li, H.L.; Jin, J.M.; Yang, C.; Wang, P.; Huang, F.; Wu, H.; Zhang, B.B.; Shi, H.L.; Wu, X.J. Isoastragaloside I suppresses LPS-induced tight junction disruption and monocyte adhesion on bEnd.3 cells via an activating Nrf2 antioxidant defense system. RSC Adv. 2018, 8, 464–471. [Google Scholar] [CrossRef]
- Li, H.; Wang, P.; Huang, F.; Jin, J.; Wu, H.; Zhang, B.; Wang, Z.; Shi, H.; Wu, X. Astragaloside IV protects blood-brain barrier integrity from LPS-induced disruption via activating Nrf2 antioxidant signaling pathway in mice. Toxicol. Appl. Pharmacol. 2018, 340, 58–66. [Google Scholar] [CrossRef]
- Li, M.; Huan, Y.; Jiang, T.; He, Y.; Gao, Z. Rehabilitation training enhanced the therapeutic effect of calycosin on neurological function recovery of rats following spinal cord injury. J. Chem. Neuroanat. 2024, 136, 102384. [Google Scholar] [CrossRef]
- Lei, P.; Walker, T.; Ayton, S. Neuroferroptosis in health and diseases. Nat. Rev. Neurosci. 2025, 26, 497–511. [Google Scholar] [CrossRef]
- Hou, B.; Liu, R.; Wu, Y.; Huang, S. Astragaloside IV Reduces Cerebral Ischemia/Reperfusion-Induced Blood-Brain Barrier Permeability in Rats by Inhibiting ER Stress-Mediated Apoptosis. Evid.-Based Complement Altern. 2020, 2020, 9087873. [Google Scholar] [CrossRef] [PubMed]
- Senapati, P.K.; Mahapatra, K.K.; Singh, A.; Bhutia, S.K. mTOR inhibitors in targeting autophagy and autophagy-associated signaling for cancer cell death and therapy. BBA Rev. Cancer 2025, 1880, 189342. [Google Scholar] [CrossRef]
- Rathod, S.S.; Agrawal, Y.O.; Nakhate, K.T.; Meeran, M.F.N.; Ojha, S.; Goyal, S.N. Neuroinflammation in the Central Nervous System: Exploring the Evolving Influence of Endocannabinoid System. Biomedicines 2023, 11, 2642. [Google Scholar] [CrossRef] [PubMed]
- Cherry, J.D.; Olschowka, J.A.; O’Banion, M.K. Neuroinflammation and M2 microglia: The good, the bad, and the inflamed. J. Neuroinflamm. 2014, 11, 98. [Google Scholar] [CrossRef]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef]
- Ruan, J.; Shi, Z.; Cao, X.; Dang, Z.; Zhang, Q.; Zhang, W.; Wu, L.; Zhang, Y.; Wang, T. Research Progress on Anti-Inflammatory Effects and Related Mechanisms of Astragalin. Int. J. Mol. Sci. 2024, 25, 4476. [Google Scholar] [CrossRef] [PubMed]
- Haftcheshmeh, S.M.; Abedi, M.; Mashayekhi, K.; Mousavi, M.J.; Navashenaq, J.G.; Mohammadi, A.; Momtazi-Borojeni, A.A. Berberine as a natural modulator of inflammatory signaling pathways in the immune system: Focus on NF-κB, JAK/STAT, and MAPK signaling pathways. Phytother. Res. 2022, 36, 1216–1230. [Google Scholar] [CrossRef] [PubMed]
- Tak, P.P.; Firestein, G.S. NF-kappaB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef]
- Coll, R.C.; Schroder, K.; Pelegrín, P. NLRP3 and pyroptosis blockers for treating inflammatory diseases. Trends Pharmacol. Sci. 2022, 43, 653–668. [Google Scholar] [CrossRef]
- Kodi, T.; Sankhe, R.; Gopinathan, A.; Nandakumar, K.; Kishore, A. New Insights on NLRP3 Inflammasome: Mechanisms of Activation, Inhibition, and Epigenetic Regulation. J. Neuroimmune Pharmacol. 2024, 19, 7. [Google Scholar] [CrossRef]
- Mao, H.; Zhao, X.; Sun, S.C. NF-κB in inflammation and cancer. Cell. Mol. Immunol. 2025, 22, 811–839. [Google Scholar] [CrossRef]
- Yu, J.; Guo, M.; Li, Y.; Zhang, H.; Chai, Z.; Wang, Q.; Yan, Y.; Yu, J.; Liu, C.; Zhang, G.; et al. Astragaloside IV protects neurons from microglia-mediated cell damage through promoting microglia polarization. Folia. Neuropathol. 2019, 57, 170–181. [Google Scholar] [CrossRef]
- Kong, H.; Xu, T.; Wang, S.; Zhang, Z.; Li, M.; Qu, S.; Li, Q.; Gao, P.; Cong, Z. The molecular mechanism of polysaccharides in combating major depressive disorder: A comprehensive review. Int. J. Biol. Macromol. 2024, 259, 129067. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.; Kouadir, M.; Song, H.; Shi, F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 2019, 10, 128. [Google Scholar] [CrossRef]
- Hou, Y.; Yan, Z.; Wan, H.; Yang, J.; Ding, Z.; He, Y. A Combination of Astragaloside IV and Hydroxysafflor Yellow A Attenuates Cerebral Ischemia-Reperfusion Injury via NF-κB/NLRP3/Caspase-1/GSDMD Pathway. Brain Sci. 2024, 14, 781. [Google Scholar] [CrossRef]
- Souza, D.G.; Almeida, R.F.; Souza, D.O.; Zimmer, E.R. The astrocyte biochemistry. Semin. Cell Dev. Biol. 2019, 95, 142–150. [Google Scholar] [CrossRef]
- Tujula, I.; Hyvärinen, T.; Lotila, J.; Rogal, J.; Voulgaris, D.; Sukki, L.; Tornberg, K.; Korpela, K.; Jäntti, H.; Malm, T.; et al. Modeling neuroinflammatory interactions between microglia and astrocytes in a human iPSC-based coculture platform. Cell Commun. Signal 2025, 23, 298. [Google Scholar] [CrossRef]
- Wang, T.; Sun, Q.; Yang, J.; Wang, G.; Zhao, F.; Chen, Y.; Jin, Y. Reactive astrocytes induced by 2-chloroethanol modulate microglia polarization through IL-1β, TNF-α, and iNOS upregulation. Food Chem. Toxicol. 2021, 157, 112550. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, Y.; Du, Y.; Wan, Q.; Xu, Y.; Wu, J. Astragaloside IV attenuates penicillin-induced epilepsy via inhibiting activation of the MAPK signaling pathway. Mol. Med. Rep. 2018, 17, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.L.; Xie, X.H.; Ding, J.H.; Du, R.H.; Hu, G. Astragaloside IV inhibits astrocyte senescence: Implication in Parkinson’s disease. J. Neuroinflamm. 2020, 17, 105. [Google Scholar] [CrossRef]
- Li, W.T.; Shao, C.Y.; Huang, P.; Yu, D.; Yang, J.H.; Wan, H.T.; He, Y. Optimization, characterization of Astragalus polysaccharides, and evaluation of anti-inflammation effect in primary cultured astrocytes via HMGB1/RAGE/NF-κB/NLRP3 signal pathway. Ind. Crop. Prod. 2023, 197, 116594. [Google Scholar] [CrossRef]
- Xiao, Y.; Cheng, Y.; Liu, W.J.; Liu, K.; Wang, Y.; Xu, F.; Wang, D.M.; Yang, Y. Effects of neutrophil fate on inflammation. Inflamm. Res. 2023, 72, 2237–2248. [Google Scholar] [CrossRef] [PubMed]
- Hagemann, N.; Mohamud Yusuf, A.; Martiny, C.; Zhang, X.; Kleinschnitz, C.; Gunzer, M.; Kolesnick, R.; Gulbins, E.; Hermann, D.M. Homozygous Smpd1 deficiency aggravates brain ischemia/reperfusion injury by mechanisms involving polymorphonuclear neutrophils, whereas heterozygous Smpd1 deficiency protects against mild focal cerebral ischemia. Basic. Res. Cardiol. 2020, 115, 64. [Google Scholar] [CrossRef]
- Li, M.; Qu, Y.Z.; Zhao, Z.W.; Wu, S.X.; Liu, Y.Y.; Wei, X.Y.; Gao, L.; Gao, G.D. Astragaloside IV protects against focal cerebral ischemia/reperfusion injury correlating to suppression of neutrophils adhesion-related molecules. Neurochem. Int. 2012, 60, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.F.; Shi, M.M.; Luo, M.Y.; Liu, D.D.; Guo, D.M.; Ling, C.; Zhong, X.L.; Xu, Y.; Cao, W.Y. Targeting PERK mediated endoplasmic reticulum stress attenuates neuroinflammation and alleviates lipopolysaccharide-induced depressive-like behavior in male mice. Int. Immunopharmacol. 2022, 111, 109092. [Google Scholar] [CrossRef]
- Li, H.L.; Shao, L.H.; Chen, X.; Wang, M.; Qin, Q.J.; Yang, Y.L.; Zhang, G.R.; Hai, Y.; Tian, Y.H. Anti-inflammatory and DNA Repair Effects of Astragaloside IV on PC12 Cells Damaged by Lipopolysaccharide. Curr. Med. Sci. 2024, 44, 854–863. [Google Scholar] [CrossRef]
- Chen, G.; Jiang, N.; Zheng, J.; Hu, H.; Yang, H.; Lin, A.; Hu, B.; Liu, H. Structural characterization and anti-inflammatory activity of polysaccharides from Astragalus membranaceus. Int. J. Biol. Macromol. 2023, 241, 124386. [Google Scholar] [CrossRef]
- Dai, X.; Liu, Y.; Meng, F.; Li, Q.; Wu, F.; Yuan, J.; Chen, H.; Lv, H.; Zhou, Y.; Chang, Y. Amplification of oxidative damage using near-infrared II-mediated photothermal/thermocatalytic effects for periodontitis treatment. Acta. Biomater. 2023, 171, 519–531. [Google Scholar] [CrossRef]
- Simonian, N.A.; Coyle, J.T. Oxidative stress in neurodegenerative diseases. Annu. Rev. Pharmacol. Toxicol. 1996, 36, 83–106. [Google Scholar] [CrossRef] [PubMed]
- Teleanu, D.M.; Niculescu, A.G.; Lungu, I.I.; Radu, C.I.; Vladâcenco, O.; Roza, E.; Costăchescu, B.; Grumezescu, A.M.; Teleanu, R.I. An Overview of Oxidative Stress, Neuroinflammation, and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 5938. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, K.; Sandoval, H.; Yamamoto, S.; Jaiswal, M.; Sanz, E.; Li, Z.; Hui, J.; Graham, B.H.; Quintana, A.; et al. Glial lipid droplets and ROS induced by mitochondrial defects promote neurodegeneration. Cell 2015, 160, 177–190. [Google Scholar] [CrossRef]
- Liu, J.; Han, X.; Zhang, T.; Tian, K.; Li, Z.; Luo, F. Reactive oxygen species (ROS) scavenging biomaterials for anti-inflammatory diseases: From mechanism to therapy. J. Hematol. Oncol. 2023, 16, 116. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Yao, J.; Peng, T.; Shao, C.; Liu, Y.; Lin, H.; Liu, Y. The Antioxidant Action of Astragali radix: Its Active Components and Molecular Basis. Molecules 2024, 29, 1691. [Google Scholar] [CrossRef]
- Shao, A.; Guo, S.; Tu, S.; Ammar, A.B.; Tang, J.; Hong, Y.; Wu, H.; Zhang, J. Astragaloside IV alleviates early brain injury following experimental subarachnoid hemorrhage in rats. Int. J. Med. Sci. 2014, 11, 1073–1081. [Google Scholar] [CrossRef]
- He, Y.; Du, M.; Gao, Y.; Liu, H.; Wang, H.; Wu, X.; Wang, Z. Astragaloside IV attenuates experimental autoimmune encephalomyelitis of mice by counteracting oxidative stress at multiple levels. PLoS ONE 2013, 8, e76495. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Wu, L.; Wang, J.L.; Yang, J.D.; Zhang, J.; Zhang, J.; Li, L.H.; Xia, Y.; Yao, L.B.; Qin, H.Z.; et al. Astragaloside IV prevents MPP⁺-induced SH-SY5Y cell death via the inhibition of Bax-mediated pathways and ROS production. Mol. Cell Biochem. 2012, 364, 209–216. [Google Scholar] [CrossRef]
- He, Y.X.; Du, M.; Shi, H.L.; Huang, F.; Liu, H.S.; Wu, H.; Zhang, B.B.; Dou, W.; Wu, X.J.; Wang, Z.T. Astragalosides from Radix Astragali benefits experimental autoimmune encephalomyelitis in C57BL /6 mice at multiple levels. BMC Complem. Altern. Med. 2014, 14, 313. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Kan, P.; Yao, X.; Yang, P.; Wang, J.; Xiang, L.; Zhu, Y. Astragaloside IV reversed the autophagy and oxidative stress induced by the intestinal microbiota of AIS in mice. J. Microbiol. 2018, 56, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Biala, A.K.; Dhingra, R.; Kirshenbaum, L.A. Mitochondrial dynamics: Orchestrating the journey to advanced age. J. Mol. Cell. Cardiol. 2015, 83, 37–43. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Lu, M.H.; Yuan, D.J.; Xu, D.E.; Yao, P.P.; Ji, W.L.; Chen, H.; Liu, W.L.; Yan, C.X.; Xia, Y.Y.; et al. Mitochondrial Dysfunction in Neural Injury. Front. Neurosci. 2019, 13, 30. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Jia, N.; Wang, W.; Jin, H.; Xu, J.; Hu, H. Protective effects of astragaloside IV against amyloid beta1-42 neurotoxicity by inhibiting the mitochondrial permeability transition pore opening. PLoS ONE 2014, 9, e98866. [Google Scholar] [CrossRef]
- Aldarmaa, J.; Liu, Z.; Long, J.; Mo, X.; Ma, J.; Liu, J. Anti-convulsant effect and mechanism of Astragalus mongholicus extract in vitro and in vivo: Protection against oxidative damage and mitochondrial dysfunction. Neurochem. Res. 2010, 35, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Bahar, E.; Kim, J.Y.; Yoon, H. Quercetin Attenuates Manganese-Induced Neuroinflammation by Alleviating Oxidative Stress through Regulation of Apoptosis, iNOS/NF-κB and HO-1/Nrf2 Pathways. Int. J. Mol. Sci. 2017, 18, 1989. [Google Scholar] [CrossRef] [PubMed]
- Silva-Islas, C.A.; Maldonado, P.D. Canonical and non-canonical mechanisms of Nrf2 activation. Pharmacol. Res. 2018, 134, 92–99. [Google Scholar] [CrossRef]
- Gu, D.M.; Lu, P.H.; Zhang, K.; Wang, X.; Sun, M.; Chen, G.Q.; Wang, Q. EGFR mediates astragaloside IV-induced Nrf2 activation to protect cortical neurons against in vitro ischemia/reperfusion damages. Biochem. Biophys. Res. Commun. 2015, 457, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.P.; Qiu, Y.Y.; Wang, B.; Ding, H.; Tang, Y.H.; Zeng, R.; Deng, C.Q. Effects of Astragaloside IV combined with the active components of Panax notoginseng on oxidative stress injury and nuclear factor-erythroid 2-related factor 2/heme oxygenase-1 signaling pathway after cerebral ischemia-reperfusion in mice. Pharmacogn. Mag. 2014, 10, 402–409. [Google Scholar] [CrossRef]
- Wang, N.; Huang, Y.; Lin, L.; Jiang, X.; Wu, J.; Liao, J.; Zhang, J.; Li, Z. Neuroprotective Effects of Formononetin on Traumatic Brain Injury Through miR-155 Modulation of the Nrf2/HO-1 Signalling Pathway. Neurochem. Res. 2025, 50, 351. [Google Scholar] [CrossRef]
- Lv, Z.; Shen, J.; Gao, X.; Ruan, Y.; Ling, J.; Sun, R.; Dai, J.; Fan, H.; Cheng, X.; Cao, P. Herbal formula Huangqi Guizhi Wuwu decoction attenuates paclitaxel-related neurotoxicity via inhibition of inflammation and oxidative stress. Chin. Med. 2021, 16, 76. [Google Scholar] [CrossRef]
- Castillo Ferrer, C.; Berthenet, K.; Ichim, G. Apoptosis—Fueling the oncogenic fire. FEBS J. 2021, 288, 4445–4463. [Google Scholar] [CrossRef]
- Reshi, L.; Wu, H.C.; Wu, J.L.; Wang, H.V.; Hong, J.R. GSIV serine/threonine kinase can induce apoptotic cell death via p53 and pro-apoptotic gene Bax upregulation in fish cells. Apoptosis 2016, 21, 443–458. [Google Scholar] [CrossRef]
- Zhao, X.; Fang, K.; Liu, X.; Yao, R.; Wang, M.; Li, F.; Hao, S.; He, J.; Wang, Y.; Fan, M.; et al. QSER1 preserves the suppressive status of the pro-apoptotic genes to prevent apoptosis. Cell Death Differ. 2023, 30, 779–793. [Google Scholar] [CrossRef]
- Soliman, D.H.; Nafie, M.S. Design, synthesis, and docking studies of novel pyrazole-based scaffolds and their evaluation as VEGFR2 inhibitors in the treatment of prostate cancer. RSC Adv. 2023, 13, 20443–20456. [Google Scholar] [CrossRef]
- Hoffman, B.; Liebermann, D.A. The proto-oncogene c-myc and apoptosis. Oncogene 1998, 17, 3351–3357. [Google Scholar] [CrossRef]
- Araya, L.E.; Soni, I.V.; Hardy, J.A.; Julien, O. Deorphanizing Caspase-3 and Caspase-9 Substrates In and Out of Apoptosis with Deep Substrate Profiling. ACS Chem. Biol. 2021, 16, 2280–2296. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Chen, X.L.; Zheng, J.Y.; Zhou, J.W.; Ma, Z.L. Astragaloside IV protects new born rats from anesthesia-induced apoptosis in the developing brain. Exp. Ther. Med. 2016, 12, 1829–1835. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Zhou, H.; Fang, Y.; Li, C.; He, Y.; Yu, L.; Wan, H.; Yang, J. Astragaloside IV alleviates ischemia reperfusion-induced apoptosis by inhibiting the activation of key factors in death receptor pathway and mitochondrial pathway. J. Ethnopharmacol. 2020, 248, 112319. [Google Scholar] [CrossRef]
- Du, S.J.; Zhang, Y.; Zhao, Y.M.; Dong, Y.J.; Tang, J.L.; Zhou, X.H.; Gao, W.J. Astragaloside IV attenuates cerebral ischemia—Reperfusion injury in rats through the inhibition of calcium-sensing receptor-mediated apoptosis. Int. J. Mol. Med. 2021, 47, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, K.M.; Alshehri, S.A.; Almarwani, W.A.; Aljohani, K.K.; Albalawi, A.Z.; Alatawi, A.S.; Al-Atwi, S.M.; Alhwyty, L.S.; Hassan, H.M.; Al-Gayyar, M.M.H. Effects of Cycloastragenol on Alzheimer’s Disease in Rats by Reducing Oxidative Stress, Inflammation, and Apoptosis. Curr. Alzheimer Res. 2024, 21, 141–154. [Google Scholar] [CrossRef]
- Wang, S.; Yang, Y.; Lin, J.; Zhang, W.; Yang, C.; Zhang, R.; Zhou, C.; Zhang, L.; Wang, X.; Liu, J.; et al. Astragalin actives autophagy and inhibits apoptosis of astrocytes in AD mice via down-regulating Fas/Fasl-VDAC1 pathway. Free. Radic. Biol. Med. 2025, 232, 72–85. [Google Scholar] [CrossRef]
- Wang, X.; Xu, W.; Chen, H.; Li, W.; Li, W.; Zhu, G. Astragaloside IV prevents Aβ(1-42) oligomers-induced memory impairment and hippocampal cell apoptosis by promoting PPARγ/BDNF signaling pathway. Brain Res. 2020, 1747, 147041. [Google Scholar] [CrossRef]
- Debnath, J.; Gammoh, N.; Ryan, K.M. Autophagy and autophagy-related pathways in cancer. Nat. Rev. Mol. Cell Biol. 2023, 24, 560–575. [Google Scholar] [CrossRef]
- Xu, H.M.; Hu, F. The role of autophagy and mitophagy in cancers. Arch. Physiol. Biochem. 2022, 128, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Kapil, L.; Kumar, V.; Kaur, S.; Sharma, D.; Singh, C.; Singh, A. Role of Autophagy and Mitophagy in Neurodegenerative Disorders. CNS Neurol. Disord. Drug Targets 2024, 23, 367–383. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Zhou, X.; Lu, J.H.; Yue, Z. Autophagy deficiency in neurodevelopmental disorders. Cell Biosci. 2021, 11, 214. [Google Scholar] [CrossRef]
- Lizama, B.N.; Chu, C.T. Neuronal autophagy and mitophagy in Parkinson’s disease. Mol. Aspects. Med. 2021, 82, 100972. [Google Scholar] [CrossRef]
- Wang, S.; Li, H.; Yuan, M.; Fan, H.; Cai, Z. Role of AMPK in autophagy. Front. Physiol. 2022, 13, 1015500. [Google Scholar] [CrossRef]
- Jang, M.; Park, R.; Kim, H.; Namkoong, S.; Jo, D.; Huh, Y.H.; Jang, I.S.; Lee, J.I.; Park, J. AMPK contributes to autophagosome maturation and lysosomal fusion. Sci. Rep. 2018, 8, 12637. [Google Scholar] [CrossRef]
- Deleyto-Seldas, N.; Efeyan, A. The mTOR-Autophagy Axis and the Control of Metabolism. Front. Cell. Dev. Biol. 2021, 9, 655731. [Google Scholar] [CrossRef]
- Yang, C.Z.; Wang, S.H.; Zhang, R.H.; Lin, J.H.; Tian, Y.H.; Yang, Y.Q.; Liu, J.; Ma, Y.X. Neuroprotective effect of astragalin via activating PI3K/Akt-mTOR-mediated autophagy on APP/PS1 mice. Cell Death Discov. 2023, 9, 15. [Google Scholar] [CrossRef]
- Hao, H.; Yang, J.J.; Zhu, J.G. Astragaloside IV ameliorates cerebral ischemic damage by restraining adenosine monophosphate-activated protein kinase/mTOR-triggered autophagic process and apoptotic activity in neurons. Mater. Res. Express 2023, 13, 1265–1273. [Google Scholar] [CrossRef]
- Sun, W.; Chao, G.; Wu, Q.; Xia, Y.; Shang, M.; Wei, Q.; Zhou, J.; Liao, L. Astragaloside IV improves the survival rates of retinal ganglion cells in traumatic optic neuropathy by regulating autophagy mediated by the AMPK-MTOR-ULK signaling pathway. Mol. Vis. 2025, 31, 99–112. [Google Scholar]
- Alves, F.; Lane, D.; Nguyen, T.P.M.; Bush, A.I.; Ayton, S. In defence of ferroptosis. Signal Transduct. Target. Ther. 2025, 10, 2. [Google Scholar] [CrossRef]
- Hadian, K.; Stockwell, B.R. SnapShot: Ferroptosis. Cell 2020, 181, 1188-1188.e1. [Google Scholar] [CrossRef]
- Huang, M.Y.; Yu, G.R. Cycloastragenol inhibits Aβ(1-42)-induced blood-brain barrier disruption and enhances soluble Aβ efflux in vitro. J. Asian. Nat. Prod. Res. 2021, 23, 556–569. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.T.; Wang, G.T.; Chen, Y.J.; Yuan, W.K.; Cai, J.; Feng, A.P.; Fang, J.; Xu, Q.; Wu, X.J. Total flavonoids of Astragalus membranaceus protect against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity in mice by inhibiting ferroptosis through SLC7A11/GPX-4 signaling pathway. Food Sci. Hum. Wellness 2024, 13, 414–420. [Google Scholar] [CrossRef]
- Zhang, C.; Shi, Z.; Xu, Q.; He, J.; Chen, L.; Lu, Z.; Huan, Q.; Wang, Y.; Cui, G. Astragaloside IV alleviates stroke-triggered early brain injury by modulating neuroinflammation and ferroptosis via the Nrf2/HO-1 signaling pathway. Acta. Cir. Bras. 2023, 38, e380723. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, Z.; Ai, P.; Zhang, C.; Chen, J.; Wang, Y. Astragaloside IV attenuates ferroptosis after subarachnoid hemorrhage via Nrf2/HO-1 signaling pathway. Front. Pharmacol. 2022, 13, 924826. [Google Scholar] [CrossRef]
- Mai, L.; Liu, J.; Wu, H.; Wang, H.; Lin, Z.; Rao, S.; Sun, W.; Tan, A.; Lin, Y.; Chen, B. Enhanced inhibition of neuronal ferroptosis and regulation of microglial polarization with multifunctional traditional Chinese medicine active ingredients-based selenium nanoparticles for treating spinal cord injury. Mater. Today Bio. 2025, 32, 101758. [Google Scholar] [CrossRef]
- Wang, M.; Li, M.; Jiang, Y.; Wang, S.; Yang, X.; Naseem, A.; Algradi, A.M.; Hao, Z.; Guan, W.; Chen, Q.; et al. Saponins from Astragalus membranaceus (Fisch.) Bge Alleviated Neuronal Ferroptosis in Alzheimer’s Disease by Regulating the NOX4/Nrf2 Signaling Pathway. J. Agric. Food Chem. 2025, 73, 7725–7740. [Google Scholar] [CrossRef] [PubMed]
- Patabendige, A.; Janigro, D. The role of the blood-brain barrier during neurological disease and infection. Biochem. Soc. Trans. 2023, 51, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ma, R.N.; Li, L.H.; Qu, Y.Z.; Gao, G.D. Astragaloside IV reduces cerebral edema post-ischemia/reperfusion correlating the suppression of MMP-9 and AQP4. Eur. J. Pharmacol. 2013, 715, 189–195. [Google Scholar] [CrossRef]
- Cai, J.; Pan, R.; Jia, X.; Li, Y.; Hou, Z.; Huang, R.Y.; Chen, X.; Huang, S.; Yang, G.Y.; Sun, J.; et al. The combination of astragalus membranaceus and ligustrazine ameliorates micro-haemorrhage by maintaining blood-brain barrier integrity in cerebrally ischaemic rats. J. Ethnopharmacol. 2014, 158 Pt A, 301–309. [Google Scholar] [CrossRef]
- Lu, Q.; Ma, J.; Zhao, Y.; Ding, G.; Wang, Y.; Qiao, X.; Cheng, X. Disruption of blood-brain barrier and endothelial-to-mesenchymal transition are attenuated by Astragalus polysaccharides mediated through upregulation of ETS1 expression in experimental autoimmune encephalomyelitis. Biomed. Pharmacother. 2024, 180, 117521. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Wan, G.; Jiang, R.; Zou, L.; Wan, D.; Zhu, H.; Feng, S. Astragalus injection ameliorates lipopolysaccharide-induced cognitive decline via relieving acute neuroinflammation and BBB damage and upregulating the BDNF-CREB pathway in mice. Pharm. Biol. 2022, 60, 825–839. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.Y.; Ouyang, H.T.; Yang, J.Y.; Huang, X.L.; Yang, T.; Duan, J.P.; Cheng, J.P.; Chen, Y.X.; Yang, Y.J.; Qiong, P. Subchronic toxicity studies of Radix Astragali extract in rats and dogs. J. Ethnopharmacol. 2007, 110, 352–355. [Google Scholar] [CrossRef]
- Xie, J.H.; Chen, Z.W.; Pan, Y.W.; Luo, D.M.; Su, Z.R.; Chen, H.M.; Qin, Z.; Huang, S.Q.; Lei, G. Evaluation of safety of modified-Danggui Buxue Tang in rodents:immunological, toxicity and hormonal aspects. J. Ethnopharmacol. 2016, 183, 59–70. [Google Scholar] [CrossRef]
- Yin, M.; Liu, Z.; Wang, J.; Gao, W. Buyang Huanwu decoction alleviates oxidative injury of cerebral ischemia-reperfusion through PKCε/Nrf2 signaling pathway. J. Ethnopharmacol. 2023, 303, 115953. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, Y.; Chen, C.; Cao, Y.; Wang, Q.; Tang, C. Gut microbiota: A new window for the prevention and treatment of neuropsychiatric disease. J. Cent. Nerv. Syst. Dis. 2025, 17, 11795735251322450. [Google Scholar] [CrossRef]
- Liu, H.X.; Li, Y.C.; Su, R.B.; Liu, C.X.; Wen, S.Y. Astragalus injection inhibits the growth of osteosarcoma by activating cytotoxic T lymphocyte and targeting CTSL. J. Ethnopharmacol. 2025, 345, 119607. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.D.; Liu, D.N.; Shang, Y.F.; Zhang, W.F.; Xu, S.; Feng, D.H.; Wang, Y.H. Neuroimmune modulators derived from natural products: Mechanisms and potential therapies. Pharmacol. Ther. 2025, 269, 108830. [Google Scholar] [CrossRef]
- Williams, C.G.; Lee, H.J.; Asatsuma, T.; Vento-Tormo, R.; Haque, A. An introduction to spatial transcriptomics for biomedical research. Genome. Med. 2022, 14, 68. [Google Scholar] [CrossRef]
- Park, J.; Wetzel, I.; Marriott, I.; Dréau, D.; D’Avanzo, C.; Kim, D.Y.; Tanzi, R.E.; Cho, H. A 3D human triculture system modeling neurodegeneration and neuroinflammation in Alzheimer’s disease. Nat. Neurosci. 2018, 21, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Zamek-Gliszczynski, M.J.; Sangha, V.; Shen, H.; Feng, B.; Wittwer, M.B.; Varma, M.V.S.; Liang, X.; Sugiyama, Y.; Zhang, L.; Bendayan, R. Transporters in Drug Development: International Transporter Consortium Update on Emerging Transporters of Clinical Importance. Clin. Pharmacol. Ther. 2022, 112, 485–500. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.; Teunissen, C.E.; Otto, M.; Piehl, F.; Sormani, M.P.; Gattringer, T.; Barro, C.; Kappos, L.; Comabella, M.; Fazekas, F.; et al. Neurofilaments as biomarkers in neurological disorders. Nat. Rev. Neurol. 2018, 14, 577–589. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).