Abstract
Sex differences are increasingly being explored and reported in oncology, and glioma is no exception. As potentially meaningful sex differences are uncovered, existing gender-derived disparities mirror data generated in retrospective and prospective trials, real-world large-scale data sets, and bench work involving animals and cell lines. The resulting disparities at the data level are wide-ranging, potentially resulting in both adverse outcomes and failure to identify and exploit therapeutic benefits. We set out to analyze the literature on women’s data disparities in glioma by exploring the origins of data in this area to understand the representation of women in study samples and omics analyses. Given the current emphasis on inclusive study design and research, we wanted to explore if sex bias continues to exist in present-day data sets and how sex differences in data may impact conclusions derived from large-scale data sets, omics, biospecimen analysis, novel interventions, and standard of care management.
1. Introduction
Sex differences are increasingly being explored and reported in oncology and glioma. As potential therapy-, outcome-, and practice-altering sex differences are uncovered, existing gender and gender intersectional-derived disparities mirror the data generated in retrospective and prospective trials, real-world large-scale data sets, and bench work involving animals and cell lines. Women—especially those of child-bearing years—have been excluded from clinical trials to protect them and their fetuses from potential adverse effects and, at times, due to concerns about the inability to control for women’s variable hormonal status [1]. The impact of disparities at the data level is wide-ranging, including female patients receiving treatment based on results of studies generated by a more significant proportion of male participants. Lack of analysis aimed at sex-specific biologic differences can result in potentially unanticipated adverse events secondary to sex-specific differences in disease patterns, metabolism, and drug pharmacokinetics and clearance [2], in addition to clinical differences such as performance status and comorbidities [3], with a secondary inability to potentially capture potential benefits of interventions whose success may hinge on leveraging sex differences.
Given that male vs. female tumor incidence in glioma varies by tumor subtype, region, and age, with males exhibiting a 20–40% higher incidence of CNS (Central Nervous System) tumors in young adults [4], and with treatment received intersecting with risk factors for death, gender [5], and age [6,7], significant intersectionality is expected when analyzing disparities and unbalanced data sets. In the context of glioma, several papers have explored biological factors and sex-dependent differences between men and women and implications related to histology, sex hormones [8,9], pregnancy, menstruation, menopause, and oral contraceptives. There is, however, an ongoing lack of in-depth understanding of the physiology and metabolism that underpins sex differences in glioma, with data just emerging [3,8,10,11,12,13,14,15,16,17]. These biological differences can result in differences in the clinical outcomes of novel interventions. Currently, external validity is evolving and is often lacking for preclinical [18] and clinical data, with data sets and biospecimen repositories yet to be developed. Given this information, a complete understanding of relevant, potentially therapeutically engaging sex differences is lacking. It is therefore essential to understand relevant differences to appropriately conduct a risk assessment and design safe and effective treatments.
We analyzed the literature on women’s data disparities in glioma. To achieve this goal, we aimed to (1) explore the origins of data in this area to understand the representation of women in study samples; (2) identify if sex bias continues to exist in present-day glioblastoma clinical trials by examining how studies are representing the population that may be impacted by novel interventions and standard of care management given current guidelines for study design and analysis aiming for diversity and inclusion; and (3) determine if existing data sets allow for meaningful biospecimen and omics analyses aimed at identifying sex differences. Our hypothesis for this review was that clinical trials generating prospective data and existing retrospective data repositories mirror data in omic and biospecimen-based analyses. As a result, we wanted to determine whether gender annotation and gender-based analysis are reflected in data captured and analyzed, and how this may allow for meaningful future conclusions.
2. Examining Prospective and Retrospective Literature Regarding Male and Female Representation
A literature search was conducted on PubMed using relevant MeSH terms: glioblastomas, gliomas, genomics, and clinical trials. Papers published between 2012 and the present were reviewed and classified as retrospective and prospective studies (Table 1 and Table 2). We reviewed 47 studies, 27 retrospective (Table 1) and 17 prospective (Table 2). We collected the number of male and female participants to quantify and analyze gender distribution in these studies. With this information, we calculated the number of male and female patients studied per year (Figure 1) in both the retrospective and prospective settings. We observed female underrepresentation in glioblastoma studies, both at the retrospective and prospective level, with an average of 41% and 37% female inclusion, respectively (Table 1 and Table 2), although it should be noted that several studies report distributions that more closely emulate male vs. female tumor incidence in glioma, whereas others suffer from more flagrant imbalances. There was little increase in female representation from 2012 to the present (Figure 1). Women are not sufficiently included in mixed-sex GBM trials to reflect the disease prevalence among the general population, given the observed range of 20–75% in retrospective trials and 0–67% in prospective trials (Table 1 and Table 2). This underrepresentation is reflected in prominent glioma landmark trials, as evidenced in a recent study that independently validates sex-specific prognostic nomograms [3] based on the original NRG/RTOG 0525 and 0825 clinical trials. Even when male/female inclusion is more balanced (57.7% vs. 60.3% males (NRG/RTOG 0525) and 42.3% vs. 39.7% females (NRG/RTOG 0825), clinical features between genders may remain unbalanced. The authors found that the age at diagnosis, performance status, MGMT methylation status, the extent of resection, use of corticosteroids, and location of the tumor in the brain were significant predictors of OS for males. However, in contrast, the extent of resection was not a significant predictor of OS for females. The authors attributed this to a proportion of female patients where resection status was captured as “other” [3]. The authors also noted that the relative importance of clinical covariates in the nomogram was different between sexes with age at diagnosis, MGMT methylation status, and performance status, which was higher for males compared to females, indicating worse survival for males compared to females. Such conclusions are hypothesis-generating but remain challenging to replicate given data limitations. The question of clinical trial representation was discussed in the context of the inclusion of women in clinical trials used for drug registration [19], with the conclusion that there was no underrepresentation of women. However, it has been pointed out that underrepresentation may exist in phase 1 and 2 trials, while being addressed in phase 3 [20]. This is a concern in glioma since potential sex differences associated with clinical benefits or increased toxicity may be missed in small, unbalanced cohorts. Retrospective studies (Table 1) may provide an avenue for more balanced data sets, given that they can include larger numbers of patients than most prospective studies (Table 1 and Table 2). However, many retrospective studies also report on small numbers of patients [21,22,23,24,25,26,27,28,29,30], wherein women make up roughly a third of the cohort, and larger studies [31,32,33,34,35,36,37,38,39,40] do not report on separate analyses for men and women to identify potential sex differences. Prospective studies (Table 2) often involve novel interventions and smaller patient numbers. Examples include Sanai et al. studying AZD1775 (20 patients) [41], Geltneky et al. studying oncolytic H-1 parvovirus (18 patients) [42], Wick et al. studying BAY1436032 in IDH-mutant solid tumors (4 patients) [43], Chinnaiyan et al. studying vorinostat plus bevacizumab (19 patients) [44]. However, similar to retrospective studies in larger trials, there are limited reports on gender differences [45,46,47,48]. Nonetheless, some large retrospective cohorts did identify sex-specific differences of note (Table 3) [6,7,10,49]. This leads to further examination of data embedded in large-scale data sets, as discussed in the following section.

Table 1.
Retrospective studies in glioma illustrating the number of patients included in the study with % female inclusion and generated conclusions.

Table 2.
Prospective studies in glioma illustrating the number of patients included in the study with % female inclusion and generated conclusions.
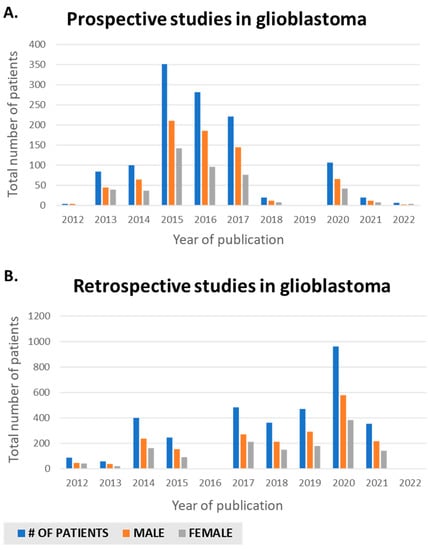
Figure 1.
Sex differences publications in glioblastoma in PubMed from 2012 to present. (A) Retrospective. (B) Prospective.

Table 3.
Studies aimed at sex-specific outcome differences in glioma illustrating the number of patients included in the study with % female inclusion and generated conclusions.
3. Large-Scale Data Sets and Male/Female Representation
Large-scale data sets may originate in registries or trials investigating specific therapeutic interventions. Exploration of sex differences in large-scale data is increasingly highly relevant, particularly with the improved capability of obtaining vast data sets from a relatively small number of samples, as with genomic, transcriptomic, and proteomic panels. Notable large databases include SEER [66], TCGA [67], CGGA [68], and several other evolving repositories (Table 4). These repositories are instrumental in advancing the field. However, they require orientation to provide detail for features selected for inclusion in analyses, as they exhibit significant heterogeneity in the proportion of histologies, number and type of features captured, and gender makeup (Table 4 and Figure 2). These aspects can result in nontransferable results when not corrected for and examined through a clinical lens. In a recent systematic review investigating validation of preclinical models employing 14 studies published between 2017 and 2020 using TCGA RNA microarray data, at least five biomarkers with discrepancies were identified, with only 29.4% of studies employing sex as a covariate, identical to MGMT methylation status [18]. Data sharing and collaborative data curation of multi-institutional data sets remain challenging. As a result, it is expected that data originating in large-scale registries compared to databases originating from clinical trial data may produce different conclusions, partly due to varying gender distribution and the impact of sex differences in molecular and management features. However, it should also be noted that other variables are significant in addition to gender, including comorbidities and molecular features. In a recent study aimed at generating sex-specific nomograms based on two significant large glioma trials, most patients included in the analysis had no comorbidities (45.9%), and patients with unknown methylation status were excluded from the analysis [3]. These aspects have intersectionality with gender and impact outcomes with significant confounders, many of which may be impossible to define given available data. Large-scale omics registries may also have missing values for molecular features, which diminishes the numbers available for analysis (Figure 2 panel E and F as of TCGA), and lack capture of comorbidities or performance status, which impact survival.

Table 4.
Large scale multi-channel data repositories with gender capture parameters [67,68].
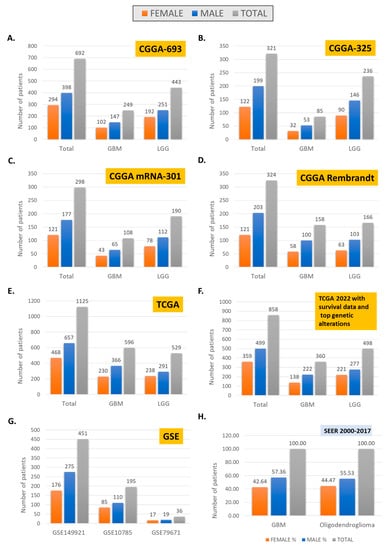
Figure 2.
Gender distribution in large-scale omic data sets, CGGA (A–D), TCGA (E,F), GSE (G), and SEER (H).
For example, data on all patients with GBM reported to the Swedish National Quality Registry for Primary Brain Tumors revealed that women had worse preoperative performance than males. For women with radical surgery, overall survival was improved. However, a survival advantage for women was no longer statistically significant in multivariate analysis, including of sex, age, surgery, and performance status [10]. Intersectionality with age is revealed in several studies, including in a recent analysis of brainstem tumors from the Surveillance, Epidemiology, and End Results (SEER) database between 2004 and 2018 [6], which revealed that, in younger patients, females had a higher age-adjusted mortality rate compared to males, with the reverse trend noted in older patients. A similar trend was identified for gliomas in CBTRUS (data from the NPCR and SEER) from 2000 to 2017 [7]. The significant intersectionality observed in large-scale data sets may be unavoidable. Hence, computational analyses must involve mitigation strategies to develop transferrable conclusions [69]. The question is perhaps not whether the numbers of women and men are comparable to each other, but instead (1) whether they reflect real-world data as exemplified by SEER (Figure 2H), and (2) have parallel analyses been carried out to identify sex differences if present.
4. Omics and Biospecimen Analysis
Biospecimen analysis is key to defining sex differences in malignancy. In neuro-oncology, this is evident given the growth in publications wherein sex differences are explored using omic approaches. Retrospective and prospective studies have previously identified potential prognostic and predictive factors grounded in sex differences. Sex differences have been noted in anti-epileptic management [70], chemotherapy [12,71], and immunotherapy [11,17] (Table 3). Looking ahead at the potential future results of omic analyses will therefore involve looking back at current and previous trials wherein a biospecimen was/is being collected to examine where future conclusions may originate from (Table 5) and how this bedside data connects to bench data, including tissue culture and animal studies. However, data from ongoing studies (Table 5) is years away, and it is unclear how existing data sets originating from completed studies may be combined to elicit robust conclusions. Data from tissue culture and animal studies may help fill this void as clinical and omic data sets grow. However, bench data underreporting biological sex in cell lines and biospecimens remains a significant barrier [72], and the functional characterization of brain tumor cells concerning sex differences is evolving [14]. In preclinical data studies published in AJP-Cell Physiology in 2013 [72], 75% of articles did not specify the sex of the cells employed, with the remaining 20% male and 5% female; in addition, biological sex remains underreported in biospecimen studies [18,73]. When screening for novel anti-cancer drugs in male- and female-derived human cell lines, higher toxicity levels were identified in male-derived cells, emphasizing the importance of annotating cell origin [72]. In a recent analysis aimed at the characterization of brain tumor-initiating cells for glioblastoma (GBM) preclinical models, two male-derived GBM cell lines (QNS108 and QNS120) and one female-derived GBM cell line (QNS315) were found to grow more rapidly in female mice brains with one male-derived GBM cell line (QNS108) exhibiting decreased survival in female mice in comparison with male mice [14]. Of note, commonly employed glioblastoma cell lines U87 (used in over 2000 studies) and U251 (used in over 2000 studies) are male-derived cell lines [74,75]. Because of ongoing concerns with genetic drift and long-term culture, patient-derived cultures are being proposed [76]. The GBM Patient Derived Xenograft (PDX) database represents a tumor patient-derived xenograft model repository with multi-omic characterizations [77]. In parallel, evidence is mounting about biological differences based on sex in genomic data. A recent systematic review and meta-analysis lent further evidence about existing sexual dimorphism of the immune system as related to clinical outcomes in glioblastoma immunotherapy. Upon analyzing genomic data and clinical trials looking at the effect of sex on the immune system and GBM outcome following immunotherapy, the authors identified that females exhibited enriched immunological signatures on gene set enrichment analysis, which correlated with survival advantage as compared to males, particularly as related to vaccine-based immunotherapy [17]. An increasing quantity of data is emerging on the relationship between tumor mutational burden (TMB) and its role as a prognostic marker, and gender, with male and female patients exhibiting differential TMB in some cancers concerning prognosis [78]. Currently, however, this relationship in glioma is not well established.

Table 5.
Glioma trials aimed at biomarker identification with estimated enrollment exceeding 100 participants [80].
Nonetheless, as the depth and richness of data increases, the number of cases with available information decreases, and sex differences may become more challenging to tease out, as evidenced by data sets (Figure 2B–D, and Table 4). Molecular classification is also lacking or uneven in many data sets. This likely reflects more profound disparities, as recently discussed by Wang et al., in bioethical implications of current practices of molecular diagnostics in neuropathology [79].
5. Conclusions
In this review, we aimed to explore the origins of data in this area to understand the representation of women in study samples, and to identify if uneven sex distribution continues to exist in present-day glioblastoma clinical trials and large-scale data, given current guidelines for study design and analysis aiming for diversity and inclusion. Further, the goal was to determine if existing data sets allow for meaningful biospecimen collection and omics analyses to identify sex differences. Having explored the literary landscape in glioma from 2012 to the present, we identified that data sets do suffer from uneven male/female distribution, lagging in representation and data analysis, which impacts conclusions being generated. The existing limitations of data sets, both preclinical and clinical, currently used to generate biospecimens for omic analysis, and the lack of transparency in cohort selection, results in many analyses being inconsistent and challenging to validate. The difficulties in data acquisition and analysis are twofold: (1) health disparities and underrepresentation of women in existing and emerging data sets; and (2) potentially smaller and difficult to characterize connections at the genomic and proteomic level, particularly when analyzed in combined data sets lacking statistical power. This is further complicated by the intersectionality of social and economic differences between sexes, combined with distinct side effects that women may experience due to treatments (e.g., thrombocytopenia secondary to temozolomide), which are more nuanced and challenging to robustly capture. With unequal distributions in data sets, both prospective and retrospective, there are concerns that understanding treatment and outcome-altering differences may continue to be delayed or missed. This can lead to novel treatments generating conclusions based on data from a more significant proportion of male participants and healthier participants able to obtain treatment in clinical trials. In the long term, this can undermine optimization (dosage-dependent or biological effect-dependent factors) of management for both sexes. Seeds being sown for future analyses must ensure adequate inclusive specimens in glioma capable of detecting and generating testable hypotheses to capitalize on sex differences that carry clinically and therapeutically meaningful conclusions for improved therapies. The ability to achieve inclusiveness has the potential to drive the understanding of cancer biology beyond glioma and improve the overall outcome for patients. Strategic study design and analysis must reach beyond gender inclusion in trials and biospecimen-driven analysis to parallel separate omic characterization of male and female cells, animals, and patients to harness potentially meaningful sex differences. Sex and gender aspects must be considered when investigating novel agents, especially pharmacokinetic and pharmacodynamic aspects, in addition to health care delivery and public health initiatives. Enumerating the molecular bases for sex differences in GBM is likely to reveal fundamental modulators of cancer risk and outcomes and guide specific components of precision medicine approaches to cancer treatments. At this time, data on sex differences in glioma are just emerging and, given that significant mechanistic and physiologic questions remain unanswered, there is a lack of in-depth understanding regarding the underlying biologic role for sex differences being observed with data undergoing evolution in this area. Guidelines for data capture and analysis must ensure that studies are appropriately designed to detect sex differences to conduct a parallel but separate analysis of male and female cells, animals, and patients to allow for the possibility of building representative data sets of all subtypes. Participants in clinical trials must accurately reflect the demographic profile of those who will likely receive treatment in the future. Sex bias in clinical trials and retrospective data sets lead to treatments with understudied efficacy in the neglected sex. Citing similarities in disease characteristics and incidence as reasoning for observed uneven gender distribution is no longer sufficient to overcome barriers to in-depth analyses, from bench to bedside and back.
Author Contributions
Conceptualization, A.K. and M.D.R.; methodology, A.K.; investigation, M.D.R., H.K., E.T. and A.K.; data curation, M.D.R., H.K., E.T. and A.K.; writing—original draft preparation, M.D.R. and A.K.; writing—review and editing, M.D.R., A.K., E.T., H.K., U.S., M.S., Y.Z. and K.C.; visualization, M.D.R., A.K. and H.K., supervision, A.K. and K.C.; project administration, A.K.; funding acquisition, A.K. and K.C. All authors have read and agreed to the published version of the manuscript.
Funding
Funding provided in part by NCI NIH intramural program (ZID BC 010990).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data supporting reported results is public and can be found in alphabetical order at: CGGA (Chinese Glioma Genome Atlas) (http://www.cgga.org.cn/, accessed on 25 July 2022); ClinicalTrials.gov. (https://clinicaltrials.gov/ct2/results/, accessed on 25 July 2022); PubMed (https://pubmed.ncbi.nlm.nih.gov//, accessed on 25 July 2022); SEER (The Surveillance, Epidemiology, and End Results)(SEER) Program. (https://seer.cancer.gov//, accessed on 25 July 2022); TCGA (The Cancer Genome Atlas Program) (https://portal.gdc.cancer.gov//, accessed on 25 July 2022).
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| CBTRUS | Central Brain Tumor Registry of the United States |
| CGGA | Chinese Glioma Genome Atlas +CNS Central Nervous System |
| GBM | Glioblastoma Multiforme |
| MGMT | Management Science Replication Project. |
| NPCR | National Program of Cancer Registries |
| PDX | Patient-Derived Xenograft |
| SEER | Surveillance, Epidemiology, and End Results |
| TCGA | The Cancer Genome Atlas |
| TCIA | The Cancer Imaging Archive |
| TMB | Tumor Mutational burden |
References
- Paulsen, E. Recognizing, Addressing Unintended Gender Bias in Patient Care. Duke Health Ref. Physicians 2020, 8, 2021. [Google Scholar]
- Feldman, S.; Ammar, W.; Lo, K.; Trepman, E.; Van Zuylen, M.; Etzioni, O. Quantifying Sex Bias in Clinical Studies at Scale With Automated Data Extraction. JAMA Netw. Open 2019, 2, e196700. [Google Scholar] [CrossRef] [PubMed]
- Patil, N.; Somasundaram, E.; Waite, K.A.; Lathia, J.D.; Machtay, M.; Gilbert, M.R.; Connor, J.R.; Rubin, J.B.; Berens, M.E.; Buerki, R.A.; et al. Independently validated sex-specific nomograms for predicting survival in patients with newly diagnosed glioblastoma: NRG Oncology RTOG 0525 and 0825. J. Neuro-Oncol. 2021, 155, 363–372. [Google Scholar] [CrossRef]
- Sorajja, N.; Moore, K.J.; Sample, J.M.; Hubbard, A.K.; Williams, L.A. Global variation in young adult central nervous system tumor incidence by region, age, and sex from 1988 to 2012. Cancer Epidemiol. 2022, 78, 102151. [Google Scholar] [CrossRef]
- Moore, K.J.; Moertel, C.L.; Williams, L.A. Young adult males have worse survival than females that is largely independent of treatment received for many types of central nervous system tumors: A National Cancer Database analysis. Cancer 2022, 128, 1616–1625. [Google Scholar] [CrossRef] [PubMed]
- Tomita, Y.; Tanaka, Y.; Takata, N.; A Hibler, E.; Hashizume, R.; Becher, J.O. Fifteen-year trends and differences in mortality rates across sex, age, and race/ethnicity in patients with brainstem tumors. Neuro-Oncol. Adv. 2021, 3, vdab137. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-M.; Cioffi, G.; Patil, N.; A Waite, K.; Lanese, R.; Ostrom, Q.T.; Kruchko, C.; E Berens, M.; Connor, J.R.; Lathia, J.D.; et al. Importance of the intersection of age and sex to understand variation in incidence and survival for primary malignant gliomas. Neuro-Oncology 2021, 24, 302–310. [Google Scholar] [CrossRef]
- Lee, J.; Troike, K.; Fodor, R.; Lathia, J.D. Unexplored functions of sex hormones in glioblastoma cancer stem cells. Endocrinology 2022, 163, bqac002. [Google Scholar] [CrossRef]
- Mondragón, J.A.; Serrano, Y.; Torres, A.; Orozco, M.; Segovia, J.; Manjarrez, G.; Romano, M.C. Glioblastoma cells express crucial enzymes involved in androgen synthesis: 3β-hydroxysteroid dehydrogenase, 17-20α-hydroxylase, 17β-hydroxysteroid dehydrogenase and 5α-reductase. Endocrinol. Diabetes Metab. 2021, 4, e00289. [Google Scholar] [CrossRef]
- Tavelin, B.; Malmström, A. Sex Differences in Glioblastoma—Findings from the Swedish National Quality Registry for Primary Brain Tumors between 1999–2018. J. Clin. Med. 2022, 11, 486. [Google Scholar] [CrossRef]
- Lee, J.; Kay, K.; Troike, K.; Ahluwalia, M.S.; Lathia, J.D. Sex Differences in Glioblastoma Immunotherapy Response. NeuroMolecular Med. 2021, 24, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Marcu, L.G. Gender and Sex-Related Differences in Normal Tissue Effects Induced by Platinum Compounds. Pharmaceuticals 2022, 15, 255. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, B.; Oertelt-Prigione, S.; Adjei, A.; Borchmann, S.; Haanen, J.; Letsch, A.; Mir, O.; Quaas, A.; Verhoeven, R.; Wagner, A. Investigation of sex and gender differences in oncology gains momentum: ESMO announces the launch of a Gender Medicine Task Force. Ann. Oncol. 2022, 33, 126–128. [Google Scholar] [CrossRef]
- Garcia, C.A.; Bhargav, A.G.; Brooks, M.; Suárez-Meade, P.; Mondal, S.K.; Zarco, N.; ReFaey, K.; Jentoft, M.; Middlebrooks, E.H.; Snuderl, M.; et al. Functional Characterization of Brain Tumor-Initiating Cells and Establishment of GBM Preclinical Models that Incorporate Heterogeneity, Therapy, and Sex Differences. Mol. Cancer Ther. 2021, 20, 2585–2597. [Google Scholar] [CrossRef]
- Hallaert, G.; Pinson, H.; Broecke, C.V.D.; Van Roost, D.; Kalala, J.; Boterberg, T. Sex-based survival differences in IDH-wildtype glioblastoma: Results from a retrospective cohort study. J. Clin. Neurosci. 2021, 91, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Carrano, A.; Juarez, J.; Incontri, D.; Ibarra, A.; Cazares, H.G. Sex-Specific Differences in Glioblastoma. Cells 2021, 10, 1783. [Google Scholar] [CrossRef]
- Shireman, J.M.; Ammanuel, S.; Eickhoff, J.C.; Dey, M. Sexual dimorphism of the immune system predicts clinical outcomes in glioblastoma immunotherapy: A systematic review and meta-analysis. Neuro-Oncol. Adv. 2022, 4, vdac082. [Google Scholar] [CrossRef]
- Fitt, B.; Loy, G.; Christopher, E.; Brennan, P.M.; Poon, M.T.C. Analytic approaches to clinical validation of results from preclinical models of glioblastoma: A systematic review. PLoS ONE 2022, 17, e0264740. [Google Scholar] [CrossRef]
- Labots, G.; Jones, A.; de Visser, S.J.; Rissmann, R.; Burggraaf, J. Gender differences in clinical registration trials: Is there a real problem? Br. J. Clin. Pharmacol. 2018, 84, 700–707. [Google Scholar] [CrossRef]
- Gispen-de Wied, C.; de Boer, A. Commentary on ‘Gender differences in clinical registration trials; is there a real problem?’ by Labots et al. Br. J. Clin. Pharmacol. 2018, 84, 1639–1640. [Google Scholar] [CrossRef]
- Mata, D.A.; Benhamida, J.K.; Lin, A.L.; Vanderbilt, C.M.; Yang, S.-R.; Villafania, L.B.; Ferguson, D.C.; Jonsson, P.; Miller, A.M.; Tabar, V.; et al. Genetic and epigenetic landscape of IDH-wildtype glioblastomas with FGFR3-TACC3 fusions. Acta Neuropathol. Commun. 2020, 8, 186. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Yoo, H.; Chang, J.H.; Kim, C.-Y.; Chung, D.S.; Kim, S.H.; Park, S.-H.; Lee, Y.S.; Yang, S.H. Procarbazine and CCNU Chemotherapy for Recurrent Glioblastoma with MGMT Promoter Methylation. J. Korean Med. Sci. 2018, 33, e167. [Google Scholar] [CrossRef] [PubMed]
- Burgenske, D.M.; Yang, J.; A Decker, P.; Kollmeyer, T.M.; Kosel, M.L.; Mladek, A.C.; A Caron, A.; A Vaubel, R.; Gupta, S.K.; Kitange, G.J.; et al. Molecular profiling of long-term IDH-wildtype glioblastoma survivors. Neuro-Oncology 2019, 21, 1458–1469. [Google Scholar] [CrossRef]
- Håvik, A.B.; Brandal, P.; Honne, H.; Dahlback, H.-S.S.; Scheie, D.; Hektoen, M.; Meling, T.R.; Helseth, E.; Heim, S.; Lothe, R.A.; et al. MGMT promoter methylation in gliomas-assessment by pyrosequencing and quantitative methylation-specific PCR. J. Transl. Med. 2012, 10, 36. [Google Scholar] [CrossRef]
- Wick, W.; Steinbach, J.P.; Platten, M.; Hartmann, C.; Wenz, F.; Von Deimling, A.; Shei, P.; Moreau-Donnet, V.; Stoffregen, C.; Combs, S.E. Enzastaurin before and concomitant with radiation therapy, followed by enzastaurin maintenance therapy, in patients with newly diagnosed glioblastoma without MGMT promoter hypermethylation. Neuro-Oncology 2013, 15, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Galia, A.; Calogero, A.; Condorelli, R.A.; Fraggetta, F.; La Corte, A.; Ridolfo, F.; Bosco, P.; Castiglione, R.; Salemi, M. PARP-1 protein expression in glioblastoma multiforme. Eur. J. Histochem. 2012, 56, e9. [Google Scholar] [CrossRef] [PubMed]
- Song, A.; Bar-Ad, V.; Martinez, N.; Glass, J.; Andrews, D.W.; Judy, K.; Evans, J.J.; Farrell, C.J.; Werner-Wasik, M.; Chervoneva, I.; et al. Initial experience with scalp sparing radiation with concurrent temozolomide and tumor treatment fields (SPARE) for patients with newly diagnosed glioblastoma. J. Neuro-Oncol. 2020, 147, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Kaley, T.; Touat, M.; Subbiah, V.; Hollebecque, A.; Rodon, J.; Lockhart, A.C.; Keedy, V.; Bielle, F.; Hofheinz, R.D.; Joly, F.; et al. BRAF Inhibition in BRAF(V600)-Mutant Gliomas: Results From the VE-BASKET Study. J. Clin. Oncol. 2018, 36, 3477–3484. [Google Scholar] [CrossRef]
- Nishii, R.; Higashi, T.; Kagawa, S.; Arimoto, M.; Kishibe, Y.; Takahashi, M.; Yamada, S.; Saiki, M.; Arakawa, Y.; Yamauchi, H.; et al. Differential Diagnosis between Low-Grade and High-Grade Astrocytoma Using System A Amino Acid Transport PET Imaging with C-11-MeAIB: A Comparison Study with C-11-Methionine PET Imaging. Contrast Media Mol. Imaging 2018, 2018, 1292746. [Google Scholar] [CrossRef]
- Schaff, L.R.; Yan, D.; Thyparambil, S.; Tian, Y.; Cecchi, F.; Rosenblum, M.; Reiner, A.S.; Panageas, K.S.; Hembrough, T.; Lin, A.L. Characterization of MGMT and EGFR protein expression in glioblastoma and association with survival. J. Neuro-Oncol. 2019, 146, 163–170. [Google Scholar] [CrossRef]
- Tanguturi, S.K.; Trippa, L.; Ramkissoon, S.H.; Pelton, K.; Knoff, D.; Sandak, D.; Lindeman, N.I.; Ligon, A.H.; Beroukhim, R.; Parmigiani, G.; et al. Leveraging molecular datasets for biomarker-based clinical trial design in glioblastoma. Neuro-Oncology 2017, 19, 908–917. [Google Scholar] [CrossRef] [PubMed]
- Jang, B.-S.; Kim, I.A. A Radiosensitivity Gene Signature and PD-L1 Status Predict Clinical Outcome of Patients with Glioblastoma Multiforme in The Cancer Genome Atlas Dataset. Cancer Res. Treat. 2020, 52, 530–542. [Google Scholar] [CrossRef] [PubMed]
- Nowosielski, M.; Ellingson, B.M.; Chinot, O.L.; Garcia, J.; Revil, C.; Radbruch, A.; Nishikawa, R.; Mason, W.P.; Henriksson, R.; Saran, F.; et al. Radiologic progression of glioblastoma under therapy—an exploratory analysis of AVAglio. Neuro-Oncology 2017, 20, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Patrizz, A.; Dono, A.; Zhu, P.; Tandon, N.; Ballester, L.Y.; Esquenazi, Y. Tumor recurrence or treatment-related changes following chemoradiation in patients with glioblastoma: Does pathology predict outcomes? J. Neuro-Oncol. 2021, 152, 163–172. [Google Scholar] [CrossRef]
- Malmström, A.; Poulsen, H.S.; Grønberg, B.H.; Stragliotto, G.; Hansen, S.; Asklund, T.; Holmlund, B.; Lysiak, M.; Dowsett, J.; Kristensen, B.W.; et al. Postoperative neoadjuvant temozolomide before radiotherapy versus standard radiotherapy in patients 60 years or younger with anaplastic astrocytoma or glioblastoma: A randomized trial. Acta Oncol. 2017, 56, 1776–1785. [Google Scholar] [CrossRef]
- E Piccioni, D.; Achrol, A.S.; A Kiedrowski, L.; Banks, K.; Boucher, N.; Barkhoudarian, G.; Kelly, D.F.; Juarez, T.; Lanman, R.B.; Raymond, V.M.; et al. Analysis of cell-free circulating tumor DNA in 419 patients with glioblastoma and other primary brain tumors. CNS Oncol. 2019, 8, CNS34. [Google Scholar] [CrossRef]
- Etcheverry, A.; Aubry, M.; Idbaih, A.; Vauleon, E.; Marie, Y.; Menei, P.; Boniface, R.; Figarella-Branger, M.; Karayan-Tapon, L.; Quillien, V.; et al. DGKI Methylation Status Modulates the Prognostic Value of MGMT in Glioblastoma Patients Treated with Combined Radio-Chemotherapy with Temozolomide. PLoS ONE 2014, 9, e104455. [Google Scholar] [CrossRef]
- Weller, M.; Tabatabai, G.; Kästner, B.; Felsberg, J.; Steinbach, J.P.; Wick, A.; Schnell, O.; Hau, P.; Herrlinger, U.; Sabel, M.C.; et al. MGMT Promoter Methylation Is a Strong Prognostic Biomarker for Benefit from Dose-Intensified Temozolomide Rechallenge in Progressive Glioblastoma: The Director Trial. Clin. Cancer Res. 2015, 21, 2057–2064. [Google Scholar] [CrossRef]
- Faria, G.M.; Soares, I.D.P.; Salazar, M.D.; Amorim, M.R.; Pessoa, B.L.; Da Fonseca, C.O.; Quirico-Santos, T. Intranasal perillyl alcohol therapy improves survival of patients with recurrent glioblastoma harboring mutant variant for MTHFR rs1801133 polymorphism. BMC Cancer 2020, 20, 294. [Google Scholar] [CrossRef]
- Egaña, L.; Auzmendi-Iriarte, J.; Andermatten, J.; Villanua, J.; Ruiz, I.; Elua-Pinin, A.; Aldaz, P.; Querejeta, A.; Sarasqueta, C.; Zubia, F.; et al. Methylation of MGMT promoter does not predict response to temozolomide in patients with glioblastoma in Donostia Hospital. Sci. Rep. 2020, 10, 18445. [Google Scholar] [CrossRef]
- Sanai, N.; Li, J.; Boerner, J.; Stark, K.; Wu, J.; Kim, S.; Derogatis, A.; Mehta, S.; Dhruv, H.D.; Heilbrun, L.K.; et al. Phase 0 Trial of AZD1775 in First-Recurrence Glioblastoma Patients. Clin. Cancer Res. 2018, 24, 3820–3828. [Google Scholar] [CrossRef] [PubMed]
- Geletneky, K.; Hajda, J.; Angelova, A.L.; Leuchs, B.; Capper, D.; Bartsch, A.J.; Neumann, J.-O.; Schöning, T.; Hüsing, J.; Beelte, B.; et al. Oncolytic H-1 Parvovirus Shows Safety and Signs of Immunogenic Activity in a First Phase I/IIa Glioblastoma Trial. Mol. Ther. 2017, 25, 2620–2634. [Google Scholar] [CrossRef]
- Wick, A.; Bähr, O.; Schuler, M.; Rohrberg, K.S.; Chawla, S.P.; Janku, F.; Schiff, D.; Heinemann, V.; Narita, Y.; Lenz, H.-J.; et al. Phase I Assessment of Safety and Therapeutic Activity of BAY1436032 in Patients with IDH1-Mutant Solid Tumors. Clin. Cancer Res. 2021, 27, 2723–2733. [Google Scholar] [CrossRef]
- Chinnaiyan, P.; Chowdhary, S.; Potthast, L.; Prabhu, A.; Tsai, Y.-Y.; Sarcar, B.; Kahali, S.; Brem, S.; Yu, H.M.; Rojiani, A.; et al. Phase I trial of vorinostat combined with bevacizumab and CPT-11 in recurrent glioblastoma. Neuro-Oncology 2011, 14, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Cloughesy, T.; Finocchiaro, G.; Belda-Iniesta, C.; Recht, L.; Brandes, A.A.; Pineda, E.; Mikkelsen, T.; Chinot, O.L.; Balana, C.; Macdonald, D.R.; et al. Randomized, Double-Blind, Placebo-Controlled, Multicenter Phase II Study of Onartuzumab Plus Bevacizumab Versus Placebo Plus Bevacizumab in Patients With Recurrent Glioblastoma: Efficacy, Safety, and Hepatocyte Growth Factor and O6-Methylguanine–DNA Methyltransferase Biomarker Analyses. J. Clin. Oncol. 2017, 35, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Maraka, S.; Groves, M.D.; Mammoser, A.G.; Melguizo-Gavilanes, I.; Conrad, C.A.; Tremont-Lukats, I.W.; Loghin, M.E.; Brien, B.J.O.; Puduvalli, V.K.; Sulman, E.P.; et al. Phase 1 lead-in to a phase 2 factorial study of temozolomide plus memantine, mefloquine, and metformin as postradiation adjuvant therapy for newly diagnosed glioblastoma. Cancer 2018, 125, 424–433. [Google Scholar] [CrossRef]
- Nabors, L.B.; Fink, K.L.; Mikkelsen, T.; Grujicic, D.; Tarnawski, R.; Nam, D.H.; Mazurkiewicz, M.; Salacz, M.; Ashby, L.; Zagonel, V.; et al. Two cilengitide regimens in combination with standard treatment for patients with newly diagnosed glioblastoma and unmethylated MGMT gene promoter: Results of the open-label, controlled, randomized phase II CORE study. Neuro-Oncology 2015, 17, 708–717. [Google Scholar] [CrossRef]
- Herrlinger, U.; Schäfer, N.; Steinbach, J.P.; Weyerbrock, A.; Hau, P.; Goldbrunner, R.; Friedrich, F.; Rohde, V.; Ringel, F.; Schlegel, U.; et al. Bevacizumab Plus Irinotecan Versus Temozolomide in Newly Diagnosed O6-Methylguanine–DNA Methyltransferase Nonmethylated Glioblastoma: The Randomized GLARIUS Trial. J. Clin. Oncol. 2016, 34, 1611–1619. [Google Scholar] [CrossRef]
- Tewari, S.; Tom, M.C.; Park, D.Y.; Wei, W.; Chao, S.T.; Jennifer, S.Y.; Suh, J.H.; Kilic, S.; Peereboom, D.M.; Stevens, G.H.; et al. Sex-Specific Differences in Low Grade Glioma Presentation and Outcome. Int. J. Radiat. Oncol. Biol. Phys. 2022, in press. [Google Scholar]
- Montemurro, N.; Fanelli, G.N.; Scatena, C.; Ortenzi, V.; Pasqualetti, F.; Mazzanti, C.M.; Morganti, R.; Paiar, F.; Naccarato, A.G.; Perrini, P. Surgical outcome and molecular pattern characterization of recurrent glioblastoma multiforme: A single-center retrospective series. Clin. Neurol. Neurosurg. 2021, 207, 106735. [Google Scholar] [CrossRef]
- Fontanilles, M.; Marguet, F.; Ruminy, P.; Basset, C.; Noel, A.; Beaussire, L.; Viennot, M.; Viailly, P.-J.; Cassinari, K.; Chambon, P.; et al. Simultaneous detection of EGFR amplification and EGFRvIII variant using digital PCR-based method in glioblastoma. Acta Neuropathol. Commun. 2020, 8, 52. [Google Scholar] [CrossRef] [PubMed]
- Massey, S.C.; White, H.; Whitmire, P.; Doyle, T.; Johnston, S.K.; Singleton, K.W.; Jackson, P.R.; Hawkins-Daarud, A.; Bendok, B.R.; Porter, A.B.; et al. Image-based metric of invasiveness predicts response to adjuvant temozolomide for primary glioblastoma. PLoS ONE 2020, 15, e0230492. [Google Scholar] [CrossRef]
- Beije, N.; Kraan, J.; Taal, W.; Van Der Holt, B.; Oosterkamp, H.M.; Walenkamp, A.M.; Beerepoot, L.; Hanse, M.; E Van Linde, M.; Otten, A.; et al. Prognostic value and kinetics of circulating endothelial cells in patients with recurrent glioblastoma randomised to bevacizumab plus lomustine, bevacizumab single agent or lomustine single agent. A report from the Dutch Neuro-Oncology Group BELOB trial. Br. J. Cancer 2015, 113, 226–231. [Google Scholar] [CrossRef][Green Version]
- Mohan, R.; Liu, A.Y.; Brown, P.D.; Mahajan, A.; Dinh, J.; Chung, C.; McAvoy, S.; McAleer, M.F.; Lin, S.H.; Li, J.; et al. Proton therapy reduces the likelihood of high-grade radiation-induced lymphopenia in glioblastoma patients: Phase II randomized study of protons vs photons. Neuro-Oncology 2020, 23, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Xiong, J.; Pan, M.; Shi, W.; Li, J.; Zhu, H.; Gong, X.; Li, C.; Mei, G.; Liu, X.; et al. Safety and efficacy of Hypofractionated stereotactic radiosurgery for high-grade Gliomas at first recurrence: A single-center experience. BMC Cancer 2021, 21, 123. [Google Scholar] [CrossRef]
- Biau, J.; Chautard, E.; de Schlichting, E.; Dupic, G.; Pereira, B.; Fogli, A.; Müller-Barthélémy, M.; Dalloz, P.; Khalil, T.; Dillies, A.F.; et al. Radiotherapy plus temozolomide in elderly patients with glioblastoma: A “real-life” report. Radiat. Oncol. 2017, 12, 197. [Google Scholar] [CrossRef]
- Omuro, A.; Beal, K.; Gutin, P.; Karimi, S.; Correa, D.D.; Kaley, T.J.; DeAngelis, L.M.; Chan, T.A.; Gavrilovic, I.T.; Nolan, C.; et al. Phase II Study of Bevacizumab, Temozolomide, and Hypofractionated Stereotactic Radiotherapy for Newly Diagnosed Glioblastoma. Clin. Cancer Res. 2014, 20, 5023–5031. [Google Scholar] [CrossRef]
- Miller, K.E.; Cassady, K.A.; Roth, J.C.; Clements, J.; Schieffer, K.M.; Leraas, K.; Miller, A.R.; Prasad, N.; Leavenworth, J.W.; Aban, I.B.; et al. Immune Activity and Response Differences of Oncolytic Viral Therapy in Recurrent Glioblastoma: Gene Expression Analyses of a Phase IB Study. Clin. Cancer Res. 2022, 28, 498–506. [Google Scholar] [CrossRef]
- A Thomas, A.; E Abrey, L.; Terziev, R.; Raizer, J.; Martinez, N.; Forsyth, P.; Paleologos, N.; Matasar, M.; Sauter, C.S.; Moskowitz, C.; et al. Multicenter phase II study of temozolomide and myeloablative chemotherapy with autologous stem cell transplant for newly diagnosed anaplastic oligodendroglioma. Neuro-Oncology 2017, 19, 1380–1390. [Google Scholar] [CrossRef][Green Version]
- Norden, A.D.; Lesser, G.J.; Drappatz, J.; Ligon, K.L.; Hammond, S.N.; Lee, E.Q.; Reardon, D.R.; Fadul, C.E.; Plotkin, S.R.; Batchelor, T.T.; et al. Phase 2 study of dose-intense temozolomide in recurrent glioblastoma. Neuro-Oncology 2013, 15, 930–935. [Google Scholar] [CrossRef]
- Han, S.J.; Rolston, J.D.; Molinaro, A.M.; Clarke, J.L.; Prados, M.D.; Chang, S.M.; Berger, M.S.; DeSilva, A.; Butowski, N.A. Phase II trial of 7 days on/7 days off temozolomide for recurrent high-grade glioma. Neuro-Oncology 2014, 16, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Wick, W.; Gorlia, T.; Bady, P.; Platten, M.; Van Den Bent, M.J.; Taphoorn, M.J.; Steuve, J.; Brandes, A.A.; Hamou, M.-F.; Wick, A.; et al. Phase II Study of Radiotherapy and Temsirolimus versus Radiochemotherapy with Temozolomide in Patients with Newly Diagnosed Glioblastoma without MGMT Promoter Hypermethylation (EORTC 26082). Clin. Cancer Res. 2016, 22, 4797–4806. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, G.; Rumiato, E.; Bertorelle, R.; Saggioro, D.; Farina, P.; della Puppa, A.; Zustovich, F.; Berti, F.; Sacchetto, V.; Marcato, R.; et al. Clinical and Genetic Factors Associated With Severe Hematological Toxicity in Glioblastoma Patients During Radiation Plus Temozolomide Treatment: A Prospective Study. Am. J. Clin. Oncol. 2015, 38, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Pitz, M.W.; Eisenhauer, E.A.; MacNeil, M.V.; Thiessen, B.; Easaw, J.C.; Macdonald, D.R.; Eisenstat, D.D.; Kakumanu, A.S.; Salim, M.; Chalchal, H.; et al. Phase II study of PX-866 in recurrent glioblastoma. Neuro-Oncology 2015, 17, 1270–1274. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wen, P.Y.; Abrey, L.E.; Fadul, C.E.; Drappatz, J.; Salem, N.; Supko, J.G.; Hochberg, F. A phase II trial of oral gimatecan for recurrent glioblastoma. J. Neuro-Oncol. 2012, 111, 347–353. [Google Scholar] [CrossRef] [PubMed]
- SEER. The Surveillance, Epidemiology, and End Results (SEER) Program. 2022. Available online: https://seer.cancer.gov/ (accessed on 7 July 2022).
- TCGA. The Cancer Genome Atlas Program. 2022. Available online: https://portal.gdc.cancer.gov/ (accessed on 7 July 2022).
- CGGA. Chinese Glioma Genoma Atlas. 2022. Available online: http://www.cgga.org.cn/ (accessed on 7 July 2022).
- Tasci, E.; Zhuge, Y.; Camphausen, K.; Krauze, A.V. Bias and Class Imbalance in Oncologic Data—Towards Inclusive and Transferrable AI in Large Scale Oncology Data Sets. Cancers 2022, 14, 2897. [Google Scholar] [CrossRef]
- Cucchiara, F.; Luci, G.; Giannini, N.; Giorgi, F.S.; Orlandi, P.; Banchi, M.; Di Paolo, A.; Pasqualetti, F.; Danesi, R.; Bocci, G. Association of plasma levetiracetam concentration, MGMT methylation and sex with survival of chemoradiotherapy-treated glioblastoma patients. Pharmacol. Res. 2022, 181, 106290. [Google Scholar] [CrossRef]
- Smits, A.; Lysiak, M.; Magnusson, A.; Rosell, J.; Söderkvist, P.; Malmström, A. Sex Disparities in MGMT Promoter Methylation and Survival in Glioblastoma: Further Evidence from Clinical Cohorts. J. Clin. Med. 2021, 10, 556. [Google Scholar] [CrossRef]
- James, B.D.; Guerin, P.; Allen, J.B. Let’s Talk About Sex—Biological Sex Is Underreported in Biomaterial Studies. Adv. Healthc. Mater. 2020, 10, e2001034. [Google Scholar] [CrossRef]
- Shah, K.; McCormack, C.E.; Bradbury, N.A. Do you know the sex of your cells? Am. J. Physiol. Cell Physiol. 2014, 306, C3–C18. [Google Scholar] [CrossRef]
- Torsvik, A.; Stieber, D.; Enger, P.; Golebiewska, A.; Molven, A.; Svendsen, A.; Westermark, B.; Niclou, S.P.; Olsen, T.K.; Enger, M.C.; et al. U-251 revisited: Genetic drift and phenotypic consequences of long-term cultures of glioblastoma cells. Cancer Med. 2014, 3, 812–824. [Google Scholar] [CrossRef] [PubMed]
- Haddad, A.F.; Young, J.S.; Amara, D.; Berger, M.S.; Raleigh, D.R.; Aghi, M.K.; A Butowski, N. Mouse models of glioblastoma for the evaluation of novel therapeutic strategies. Neuro-Oncol. Adv. 2021, 3, vdab100. [Google Scholar] [CrossRef] [PubMed]
- da Hora, C.C.; Schweiger, M.W.; Wurdinger, T.; Tannous, B.A. Patient-Derived Glioma Models: From Patients to Dish to Animals. Cells 2019, 8, 1177. [Google Scholar] [CrossRef] [PubMed]
- Vaubel, R.A.; Tian, S.; Remonde, D.; Schroeder, M.A.; Mladek, A.C.; Kitange, G.J.; Caron, A.; Kollmeyer, T.M.; Grove, R.; Peng, S.; et al. Genomic and Phenotypic Characterization of a Broad Panel of Patient-Derived Xenografts Reflects the Diversity of Glioblastoma. Clin. Cancer Res. 2020, 26, 1094–1104. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Bai, L.; Lin, H.; Dong, L.; Zhang, R.; Cheng, X.; Liu, Z.; Ouyang, Y.; Ding, K. Multiomics analysis of tumor mutational burden across cancer types. Comput. Struct. Biotechnol. J. 2021, 19, 5637–5646. [Google Scholar] [CrossRef]
- Wang, W.; Howard, D.; Giglio, P.; Thomas, D.; Otero, J.J. Bioethical implications of current state practices of molecular diagnostics in neuropathology. Neuro-Oncology 2022, 24, 853–854. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. 2022. Available online: https://clinicaltrials.gov/ct2/results?term=biospecimen&cond=glioma&Search=Apply&recrs=a&age_v=&gndr=&type=&rslt= (accessed on 7 July 2022).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).