Serum Catestatin Levels Correlate with Ambulatory Blood Pressure and Indices of Arterial Stiffness in Patients with Primary Hypertension
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethical Considerations
2.2. Subjects and Inclusion/Exclusion Criteria
2.3. Clinical and Laboratory Evaluations
2.4. Sample Size Calculation
2.5. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Forouzanfar, M.H.; Liu, P.; Roth, G.A.; Ng, M.; Biryukov, S.; Marczak, L.; Alexander, L.; Estep, K.; Hassen Abate, K. Global Burden of Hypertension and Systolic Blood Pressure of at Least 110 to 115 mm Hg, 1990–2015. JAMA 2017, 317, 165–182, Erratum in JAMA 2017, 317, 648. [Google Scholar] [CrossRef]
- Oparil, S.; Acelajado, M.C.; Bakris, G.L.; Berlowitz, D.R.; Cífková, R.; Dominiczak, A.F.; Grassi, G.; Jordan, J.; Poulter, N.R.; Rodgers, A.; et al. Hypertension. Nat. Rev. Dis. Primers 2018, 4, 18014. [Google Scholar] [CrossRef] [PubMed]
- Rossier, B.C.; Bochud, M.; Devuyst, O. The Hypertension Pandemic: An Evolutionary Perspective. Physiology 2017, 32, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Beevers, G.; Lip, G.Y.; O’Brien, E. ABC of hypertension: The pathophysiology of hypertension. BMJ 2001, 322, 912. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Zhao, Y.X.; Gu, N. Renal Denervation for Resistant Hypertension: Where Do We Stand? Curr. Hypertens. Rep. 2020, 22, 83. [Google Scholar] [CrossRef] [PubMed]
- Bakris, G.L.; Townsend, R.R.; Liu, M.; Cohen, S.A.; D’Agostino, R.; Flack, J.M.; Kandzari, D.E.; Katzen, B.T.; Leon, M.B.; Mauri, L.; et al. Impact of renal denervation on 24-hour ambulatory blood pressure: Results from SYMPLICITY HTN-3. J. Am. Coll. Cardiol. 2014, 64, 1071–1078. [Google Scholar] [CrossRef]
- Mitchell, G.F. Arterial stiffness and hypertension: Chicken or egg? Hypertension 2014, 64, 210–214. [Google Scholar] [CrossRef]
- Safar, M.E. Arterial stiffness as a risk factor for clinical hypertension. Nat Rev Cardiol. 2018, 15, 97–105. [Google Scholar] [CrossRef]
- Smulyan, H.; Lieber, A.; Safar, M.E. Hypertension, Diabetes Type II, and Their Association: Role of Arterial Stiffness. Am. J. Hypertens. 2016, 29, 5–13. [Google Scholar] [CrossRef]
- Niiranen, T.J.; Kalesan, B.; Hamburg, N.M.; Benjamin, E.J.; Mitchell, G.F.; Vasan, R.S. Relative Contributions of Arterial Stiffness and Hypertension to Cardiovascular Disease: The Framingham Heart Study. J. Am. Heart Assoc. 2016, 5, e004271. [Google Scholar] [CrossRef]
- Dumor, K.; Shoemaker-Moyle, M.; Nistala, R.; Whaley-Connell, A. Arterial Stiffness in Hypertension: An Update. Curr. Hypertens. Rep. 2018, 20, 72. [Google Scholar] [CrossRef] [PubMed]
- Bozic, J.; Kumric, M.; Ticinovic Kurir, T.; Urlic, H.; Martinovic, D.; Vilovic, M.; Tomasovic Mrcela, N.; Borovac, J.A. Catestatin as a Biomarker of Cardiovascular Diseases: A Clinical Perspective. Biomedicines 2021, 9, 1757. [Google Scholar] [CrossRef] [PubMed]
- Mahata, S.K.; Mahata, M.; Wen, G.; Wong, W.B.; Mahapatra, N.R.; Hamilton, B.A.; O’Connor, D.T. The catecholamine release-inhibitory “catestatin” fragment of chromogranin a: Naturally occurring human variants with different potencies for multiple chromaffin cell nicotinic cholinergic responses. Mol. Pharmacol. 2004, 66, 1180–1191. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Zheng, Q.Q.; Li, Y.; Chen, X.Y.; Chen, R.; Wang, X.R.; Gong, Y.S.; Fan, X.F. Role of catestatin in 2K1C-induced renal hypertension in rats and the underlying mechanism. Zhongguo Ying Yong Sheng Li Xue Za Zhi 2016, 32, 214–218. (In Chinese) [Google Scholar] [CrossRef] [PubMed]
- Biswas, N.; Gayen, J.; Mahata, M.; Su, Y.; Mahata, S.K.; O’Connor, D.T. Novel peptide isomer strategy for stable inhibition of catecholamine release: Application to hypertension. Hypertension 2012, 60, 1552–1559. [Google Scholar] [CrossRef]
- Mahapatra, N.R.; O’Connor, D.T.; Vaingankar, S.M.; Hikim, A.P.; Mahata, M.; Ray, S.; Staite, E.; Wu, H.; Gu, Y.; Dalton, N.; et al. Hypertension from targeted ablation of chromogranin A can be rescued by the human ortholog. J. Clin. Investig. 2005, 115, 1942–1952. [Google Scholar] [CrossRef]
- Mahata, S.K.; Mahapatra, N.R.; Mahata, M.; Wang, T.C.; Kennedy, B.P.; Ziegler, M.G.; O’Connor, D.T. Catecholamine secretory vesicle stimulus-transcription coupling in vivo. Demonstration by a novel transgenic promoter/photoprotein reporter and inhibition of secretion and transcription by the chromogranin A fragment catestatin. J. Biol. Chem. 2003, 278, 32058–32067. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104, Erratum in Eur. Heart J. 2019, 40, 475. [Google Scholar] [CrossRef]
- O’Connor, D.T.; Kailasam, M.T.; Kennedy, B.P.; Ziegler, M.G.; Yanaihara, N.; Parmer, R.J. Early decline in the catecholamine release-inhibitory peptide catestatin in humans at genetic risk of hypertension. J. Hypertens. 2002, 20, 1335–1345. [Google Scholar] [CrossRef]
- Simac, P.; Perkovic, D.; Bozic, I.; Matijas, M.; Gugo, K.; Martinovic, D.; Bozic, J. Serum catestatin levels in patients with rheumatoid arthritis. Sci. Rep. 2022, 12, 3812. [Google Scholar] [CrossRef]
- Simunovic, M.; Supe-Domic, D.; Karin, Z.; Degoricija, M.; Paradzik, M.; Bozic, J.; Unic, I.; Skrabic, V. Serum catestatin concentrations are decreased in obese children and adolescents. Pediatr. Diabetes 2019, 20, 549–555. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, D.T.; Zhu, G.; Rao, F.; Taupenot, L.; Fung, M.M.; Das, M.; Mahata, S.K.; Mahata, M.; Wang, L.; Zhang, K.; et al. Heritability and genome-wide linkage in US and australian twins identify novel genomic regions controlling chromogranin a: Implications for secretion and blood pressure. Circulation 2008, 118, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Durakoğlugil, M.E.; Ayaz, T.; Kocaman, S.A.; Kırbaş, A.; Durakoğlugil, T.; Erdoğan, T.; Çetin, M.; Şahin, O.Z.; Çiçek, Y. The relationship of plasma catestatin concentrations with metabolic and vascular parameters in untreated hypertensive patients: Influence on high-density lipoprotein cholesterol. Anatol. J. Cardiol. 2015, 15, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, X.; Yang, C.; Su, X.; Yang, W.; Dai, Y.; Han, H.; Jiang, J.; Lu, L.; Wang, H.; et al. Decreased circulating catestatin levels are associated with coronary artery disease: The emerging anti-inflammatory role. Atherosclerosis 2019, 281, 78–88. [Google Scholar] [CrossRef]
- Meng, L.; Ye, X.J.; Ding, W.H.; Yang, Y.; Di, B.B.; Liu, L.; Huo, Y. Plasma catecholamine release-inhibitory peptide catestatin in patients with essential hypertension. J. Cardiovasc. Med. 2011, 12, 643–647. [Google Scholar] [CrossRef]
- Schillaci, G.; De Vuono, S.; Pucci, G. An endogenous brake on the sympathetic nervous system: The emerging role of catestatin in hypertension. J. Cardiovasc. Med. 2011, 12, 609–612. [Google Scholar] [CrossRef]
- Tüten, N.; Güralp, O.; Gök, K.; Hamzaoglu, K.; Oner, Y.O.; Makul, M.; Bulut, H.; Irmak, K.; Tüten, A.; Malik, E. Serum catestatin level is increased in women with preeclampsia. J. Obstet. Gynaecol. 2022, 42, 55–60. [Google Scholar] [CrossRef]
- Gubareva, E.; Gubareva, I. Catestatin in patients with essential hypertension of different cardiovascular risk. Eur. Heart J. 2021, 42, ehab724.2280. [Google Scholar] [CrossRef]
- Grassi, G.; Cattaneo, B.M.; Seravalle, G.; Lanfranchi, A.; Pozzi, M.; Morganti, A.; Carugo, S.; Mancia, G. Effects of chronic ACE inhibition on sympathetic nerve traffic and baroreflex control of circulation in heart failure. Circulation 1997, 96, 1173–1179. [Google Scholar] [CrossRef]
- Azevedo, E.R.; Mak, S.; Floras, J.S.; Parker, J.D. Acute effects of angiotensin-converting enzyme inhibition versus angiotensin II receptor blockade on cardiac sympathetic activity in patients with heart failure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 313, R410–R417. [Google Scholar] [CrossRef]
- Missault, L.H.; De Buyzere, M.L.; De Bacquer, D.D.; Duprez, D.D.; Clement, D.L. Relationship between left ventricular mass and blood pressure in treated hypertension. J. Hum. Hypertens. 2002, 16, 61–66. [Google Scholar] [CrossRef][Green Version]
- Kennedy, B.P.; Mahata, S.K.; O’Connor, D.T.; Ziegler, M.G. Mechanism of cardiovascular actions of the chromogranin A fragment catestatin in vivo. Peptides 1998, 19, 1241–1248. [Google Scholar] [CrossRef]
- Mahapatra, N.R. Catestatin is a novel endogenous peptide that regulates cardiac function and blood pressure. Cardiovasc. Res. 2008, 80, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Fung, M.M.; Salem, R.M.; Mehtani, P.; Thomas, B.; Lu, C.F.; Perez, B.; Rao, F.; Stridsberg, M.; Ziegler, M.G.; Mahata, S.K.; et al. Direct vasoactive effects of the chromogranin A (CHGA) peptide catestatin in humans in vivo. Clin. Exp. Hypertens. 2010, 32, 278–287. [Google Scholar] [CrossRef]
- Mahata, S.K.; Mahata, M.; Parmer, R.J.; O’Connor, D.T. Desensitization of catecholamine release: The novel catecholamine release-inhibitory peptide catestatin (chromogranin A344-364) acts at the receptor to prevent nicotinic cholinergic tolerance. J. Biol. Chem. 1999, 274, 2920–2928. [Google Scholar] [CrossRef]
- Zivkovic, P.M.; Matetic, A.; Tadin Hadjina, I.; Rusic, D.; Vilovic, M.; Supe-Domic, D.; Borovac, J.A.; Mudnic, I.; Tonkic, A.; Bozic, J. Serum Catestatin Levels and Arterial Stiffness Parameters Are Increased in Patients with Inflammatory Bowel Disease. J. Clin. Med. 2020, 9, 628. [Google Scholar] [CrossRef] [PubMed]
- Nardone, M.; Incognito, A.V.; Millar, P.J. Evidence for Pressure-Independent Sympathetic Modulation of Central Pulse Wave Velocity. J. Am. Heart Assoc. 2018, 7, e007971. [Google Scholar] [CrossRef]
- Dinenno, F.A.; Jones, P.P.; Seals, D.R.; Tanaka, H. Age-associated arterial wall thickening is related to elevations in sympathetic activity in healthy humans. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H1205–H1210. [Google Scholar] [CrossRef]
- Palmiero, P.; Zito, A.; Maiello, M.; Cameli, M.; Modesti, P.A.; Muiesan, M.L.; Novo, S.; Saba, P.S.; Scicchitano, P.; Pedrinelli, R.; et al. Left ventricular diastolic function in hypertension: Methodological considerations and clinical implications. J. Clin. Med. Res. 2015, 7, 137–144. [Google Scholar] [CrossRef]
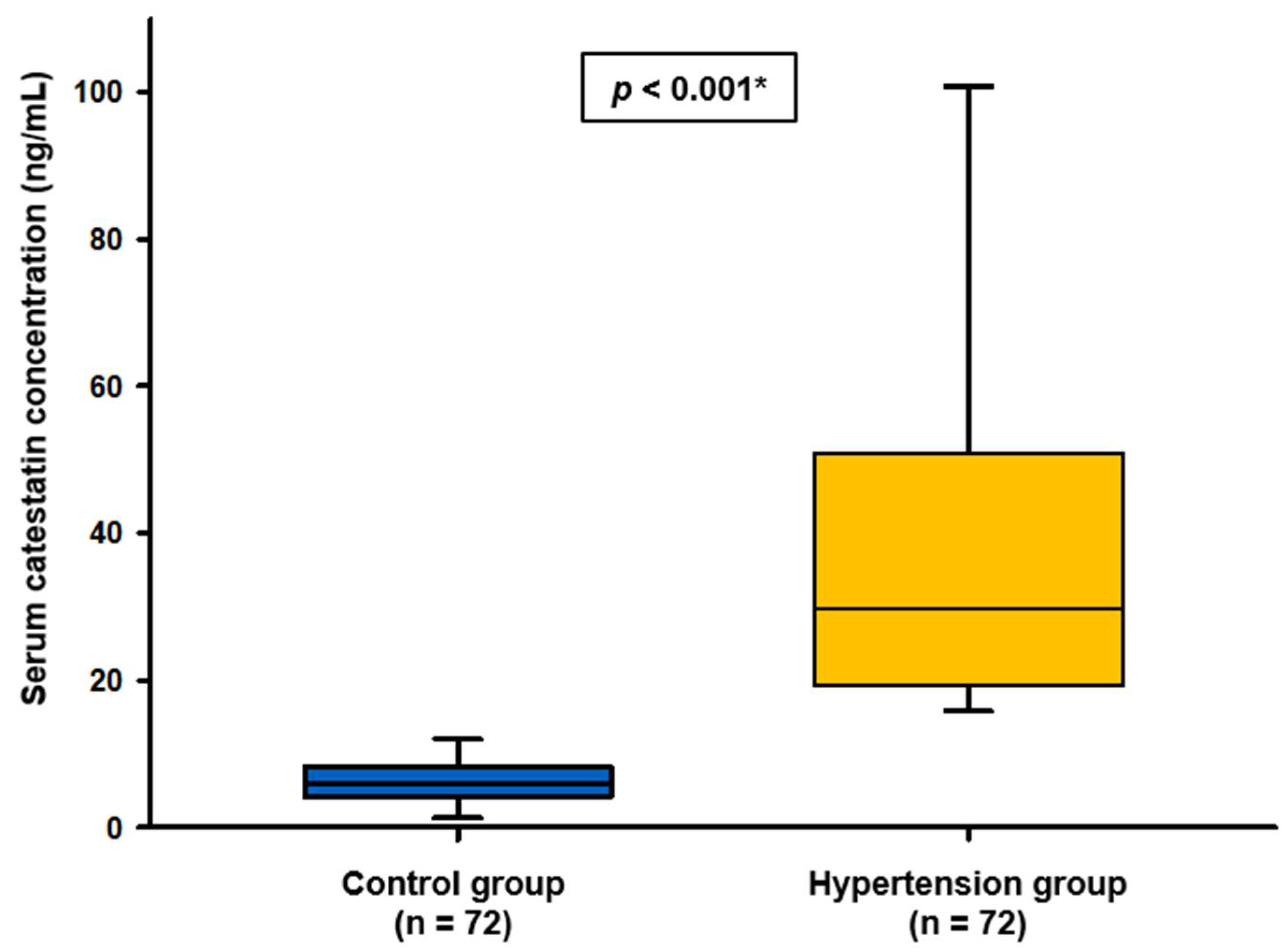
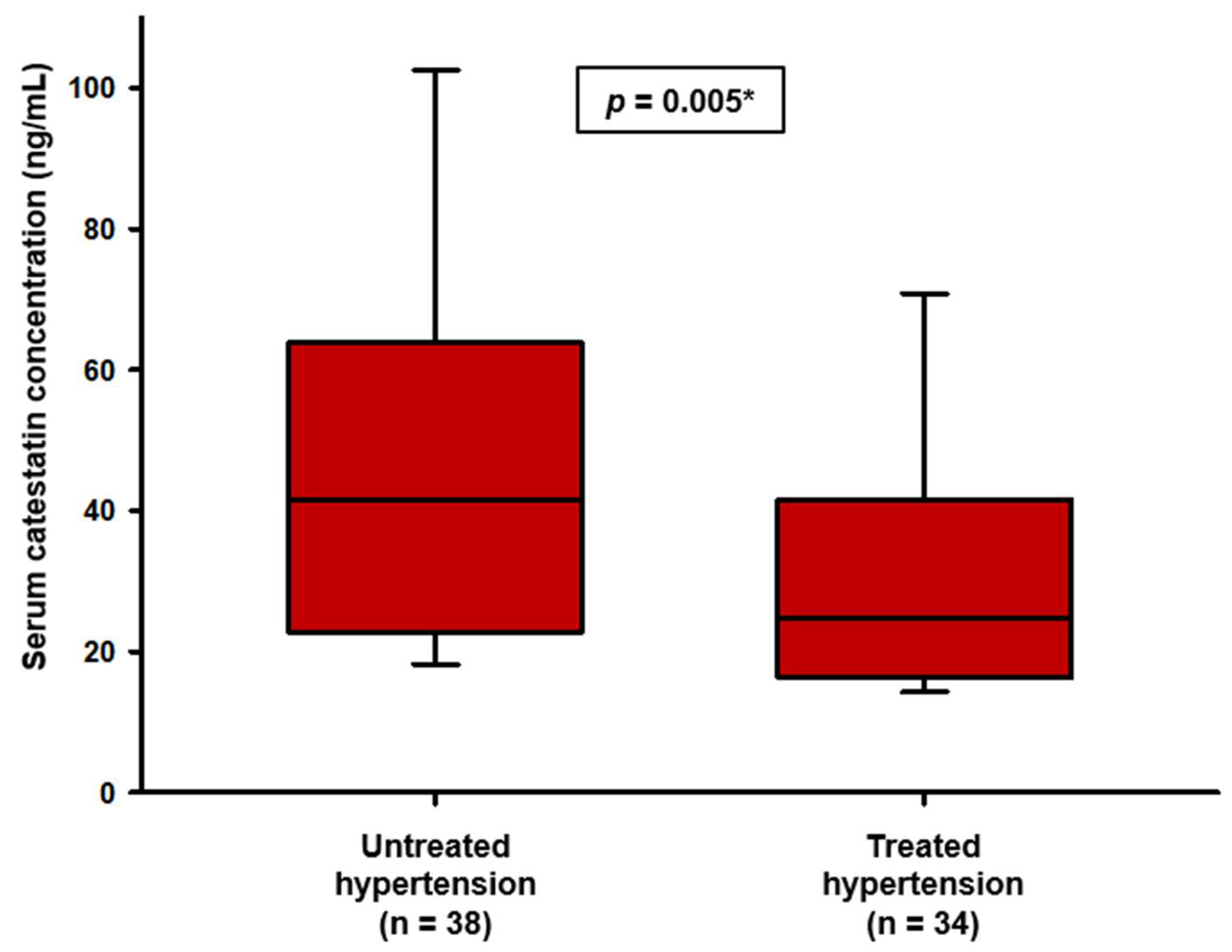

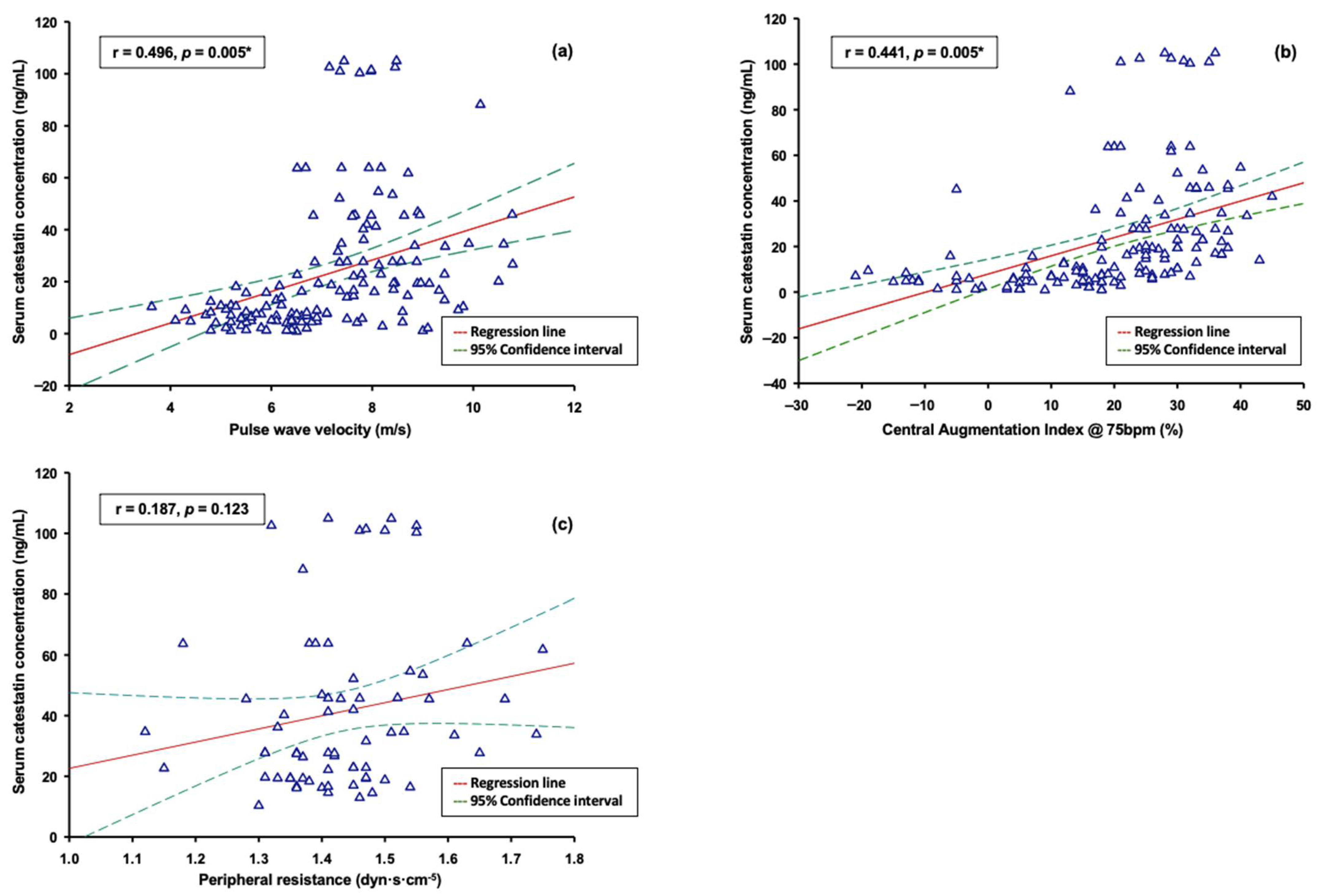
| Characteristic | Control Group (n = 72) | Hypertension Group (n = 72) | p |
|---|---|---|---|
| Age, years | 57.2 ± 9.2 | 54.6 ± 8.8 | 0.652 * |
| Male sex, n (%) | 41 (56.9%) | 45 (62.5%) | 0.498 ** |
| Body mass index, kg/m2 | 26.6 ± 2.8 | 27.2 ± 2.6 | 0.772 * |
| Waist-to-hip ratio | 0.95 ± 0.31 | 0.98 ± 0.36 | 0.879 * |
| Dyslipidemia, n (%) | 43 (59.7%) | 48 (66.7%) | 0.388 ** |
| Family history of CV disease, n (%) | 35 (48.6%) | 30 (41.7%) | 0.402 ** |
| Time since diagnosis, years | N/A | 4 (2–6) | N/A |
| Ambulatory blood pressure | |||
| SBP, mmHg | 121.7 ± 9.2 | 134.7 ± 13.0 | <0.001 * |
| DBP, mmHg | 78.4 ± 6.8 | 84.3 ± 10.3 | <0.001 * |
| MAP, mmHg | 91.5 ± 6.9 | 104.5 ± 10.8 | <0.001 * |
| Pulse wave velocity, m/s | 6.37 ± 1.43 | 8.05 ± 1.17 | <0.001 * |
| End-organ damage 1, n (%) | 2 (2.8%) | 4 (5.6%) | 0.681 *** |
| Fasting blood glucose, mmol/L | 5.1 ± 0.7 | 5.2 ± 0.6 | 0.083 * |
| Cholesterol, mmol/L | 5.24 ± 1.19 | 5.61 ± 0.97 | 0.045 * |
| LDL, mmol/L | 3.27 ± 1.07 | 3.43 ± 0.86 | 0.315 * |
| HDL, mmol/L | 1.41 ± 0.32 | 1.51 ± 0.45 | 0.151 * |
| Triglycerides, mmol/L | 1.22 ± 0.63 | 1.54 ± 0.90 | 0.014 * |
| Parameter | Untreated Group (n = 38) | Treated Group (n = 34) | p |
|---|---|---|---|
| Average SBP, mmHg | 135.4 ± 13.5 | 133.9 ± 12.6 | 0.632 * |
| Average DBP, mmHg | 85.9 ± 11.0 | 82.3 ± 9.0 | 0.144 * |
| Average MAP, mmHg | 105.8 ± 11.5 | 102.9 ± 9.9 | 0.265 * |
| Awake SBP, mmHg | 140.6 ± 13.1 | 137.8 ± 12.3 | 0.368 * |
| Awake DBP, mmHg | 90.4 ± 10.9 | 85.9 ± 8.7 | 0.264 * |
| Awake MAP, mmHg | 110.6 ± 11.0 | 106.6 ± 9.5 | 0.113 * |
| Nocturnal SBP, mmHg | 126.9 ± 17.2 | 127.5 ± 15.4 | 0.884 * |
| Nocturnal DBP, mmHg | 78.5 ± 13.9 | 76.3 ± 11.1 | 0.482 * |
| Nocturnal MAP, mmHg | 97.9 ± 14.7 | 96.9 ± 12.3 | 0.772 * |
| Dipper, n (%) | 22 (58%) | 16 (47%) | 0.361 ** |
| Time since diagnosis, years | 3 (2–4) | 5 (4.3–7.8) | <0.001 *** |
| Hypertension stage | |||
| Mild, n (%) | 29 (76.3%) | 30 (88.2%) | 0.192 ** |
| Moderate, n (%) | 9 (23.7%) | 4 (11.8%) | |
| Pulse wave velocity, m/s | 7.98 ± 1.22 | 8.13 ± 1.13 | 0.604 * |
| Peripheral resistance, dyn·s·cm−5 | 1.44 ± 0.11 | 1.42 ± 0.13 | 0.488 * |
| cAIx@75bpm, % | 30.1 ± 6.3 | 28.7 ± 7.9 | 0.424 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumric, M.; Vrdoljak, J.; Dujic, G.; Supe-Domic, D.; Ticinovic Kurir, T.; Dujic, Z.; Bozic, J. Serum Catestatin Levels Correlate with Ambulatory Blood Pressure and Indices of Arterial Stiffness in Patients with Primary Hypertension. Biomolecules 2022, 12, 1204. https://doi.org/10.3390/biom12091204
Kumric M, Vrdoljak J, Dujic G, Supe-Domic D, Ticinovic Kurir T, Dujic Z, Bozic J. Serum Catestatin Levels Correlate with Ambulatory Blood Pressure and Indices of Arterial Stiffness in Patients with Primary Hypertension. Biomolecules. 2022; 12(9):1204. https://doi.org/10.3390/biom12091204
Chicago/Turabian StyleKumric, Marko, Josip Vrdoljak, Goran Dujic, Daniela Supe-Domic, Tina Ticinovic Kurir, Zeljko Dujic, and Josko Bozic. 2022. "Serum Catestatin Levels Correlate with Ambulatory Blood Pressure and Indices of Arterial Stiffness in Patients with Primary Hypertension" Biomolecules 12, no. 9: 1204. https://doi.org/10.3390/biom12091204
APA StyleKumric, M., Vrdoljak, J., Dujic, G., Supe-Domic, D., Ticinovic Kurir, T., Dujic, Z., & Bozic, J. (2022). Serum Catestatin Levels Correlate with Ambulatory Blood Pressure and Indices of Arterial Stiffness in Patients with Primary Hypertension. Biomolecules, 12(9), 1204. https://doi.org/10.3390/biom12091204









