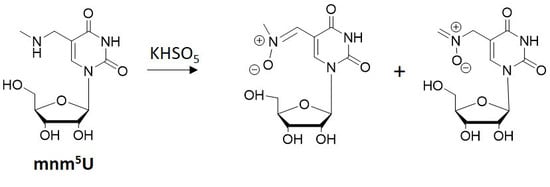
Oxidation of 5-methylaminomethyl uridine (mnm5U) by Oxone Leads to Aldonitrone Derivatives
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Oxidation Reaction
2.3. Chromatography
2.4. Mass Spectrometry
2.5. Preparation of Aldonitrone Derivatives 1 and 2
2.6. Nuclear Magnetic Resonance
2.7. Light Irradiation of Aldonitrone 1
2.8. Modeling
3. Results
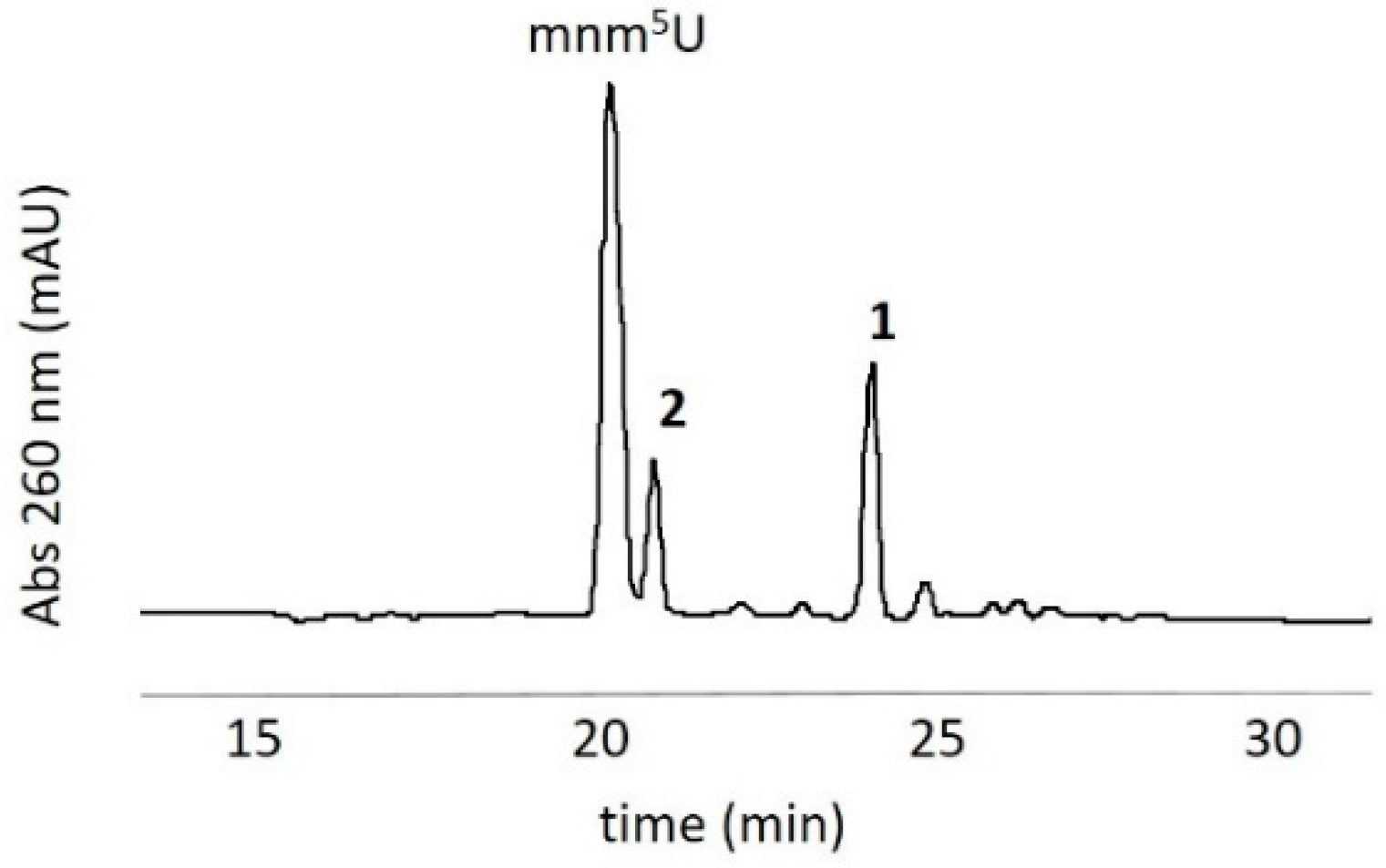
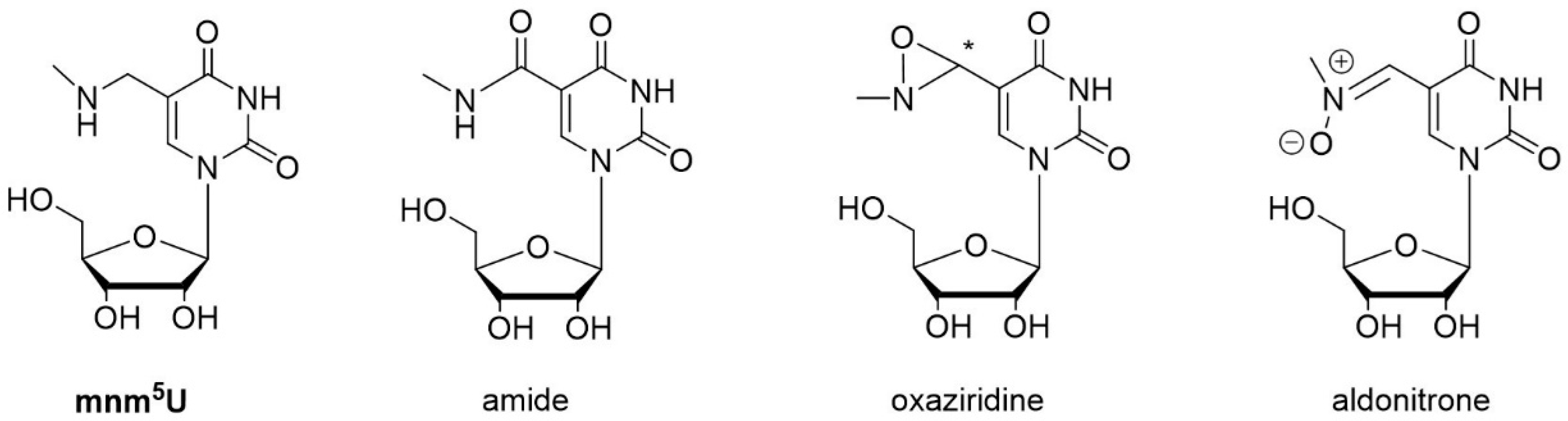
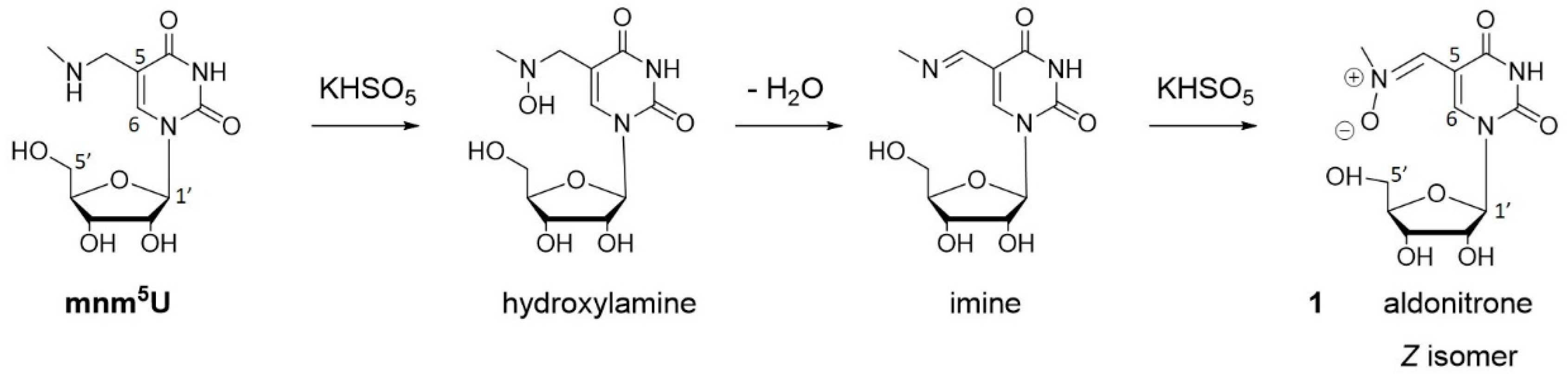
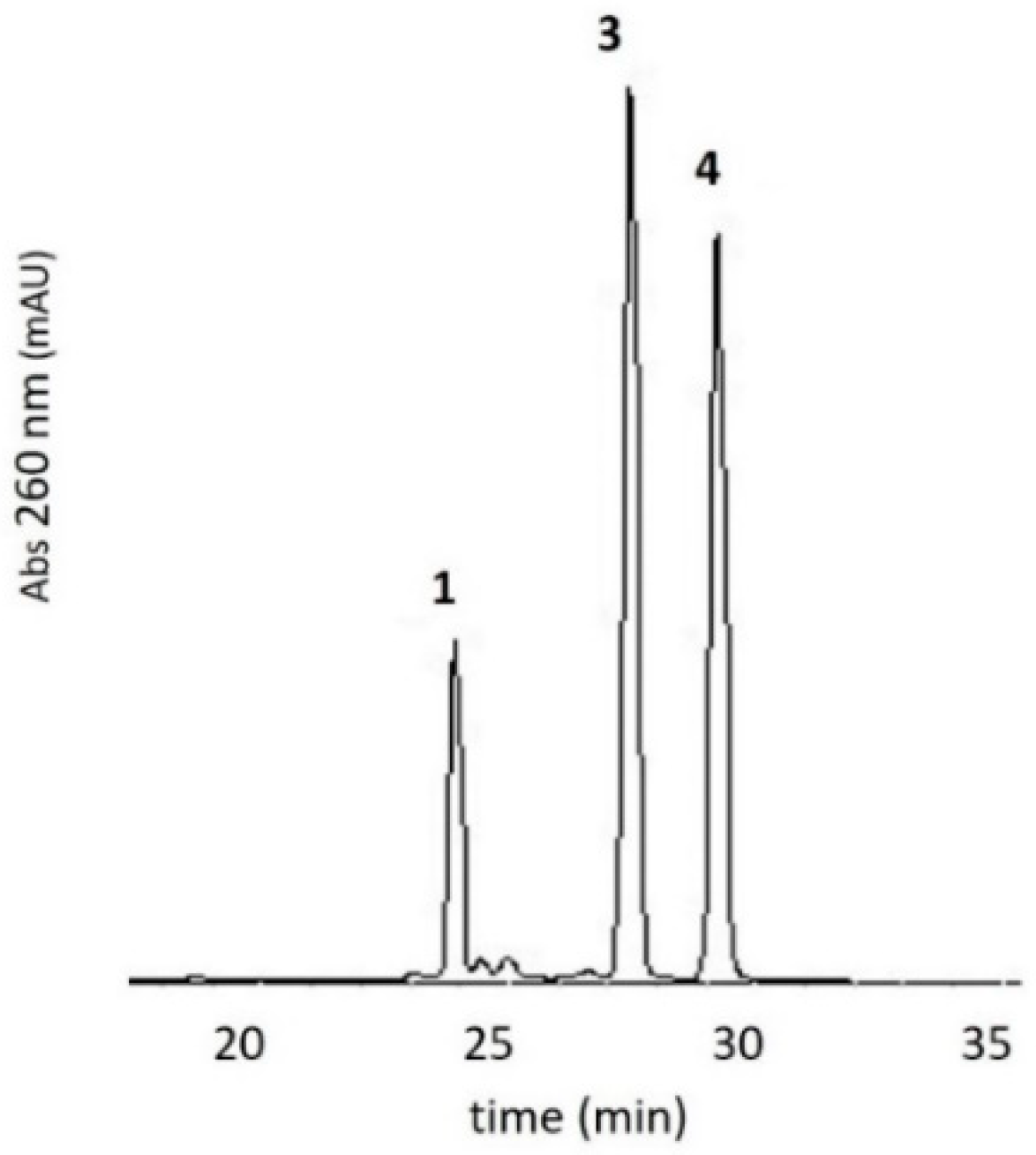
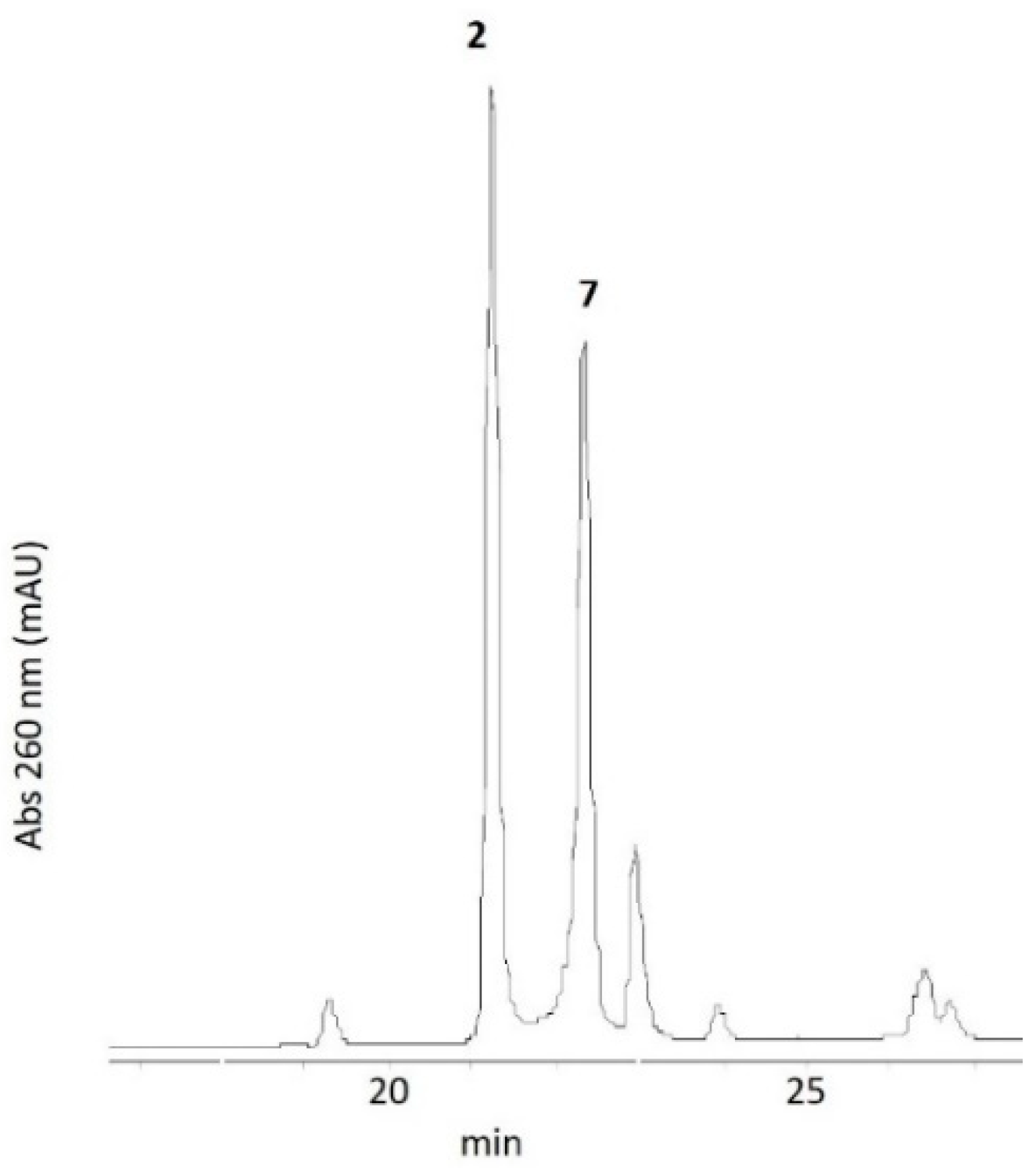
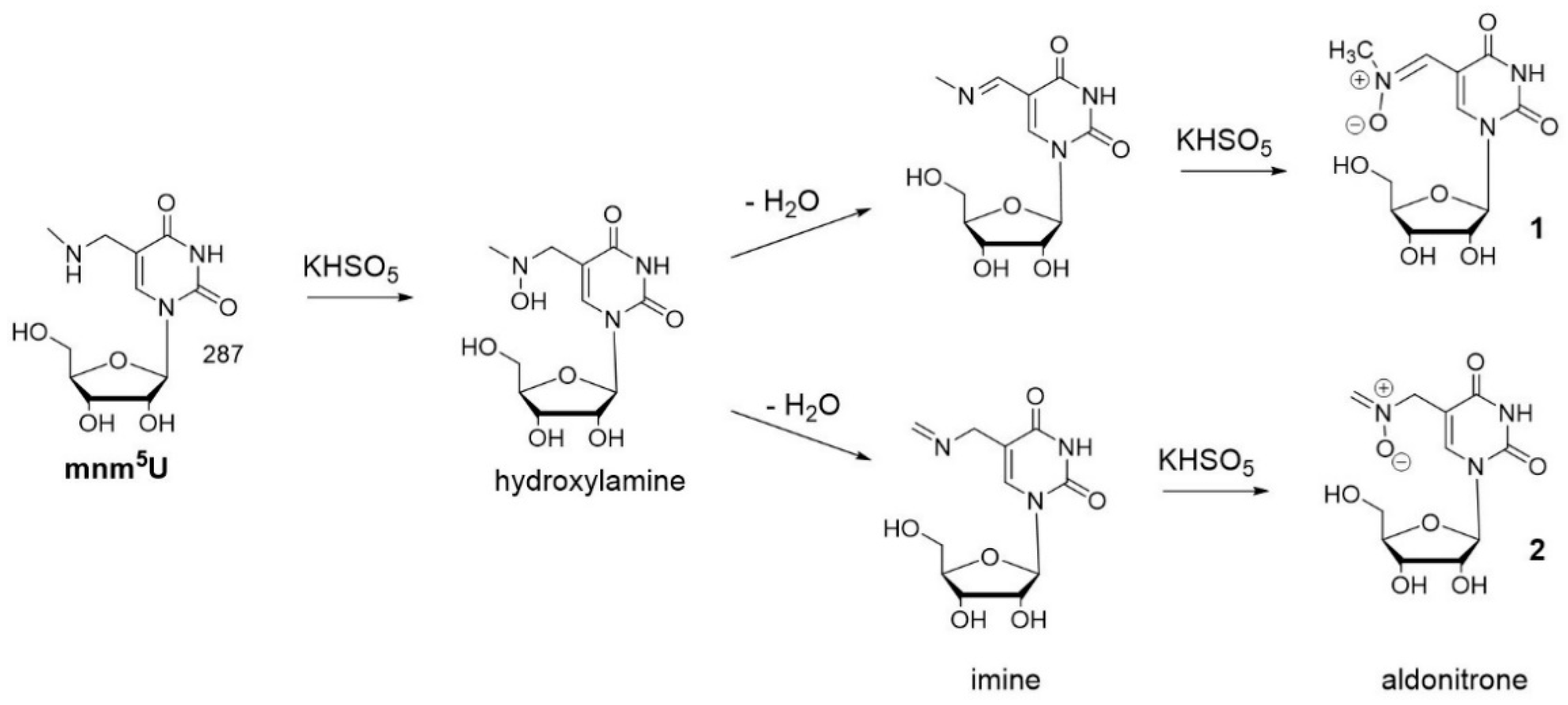
3.1. Oxidation of 5-methylaminomethyl uridine Leads to Two Oxidation Products
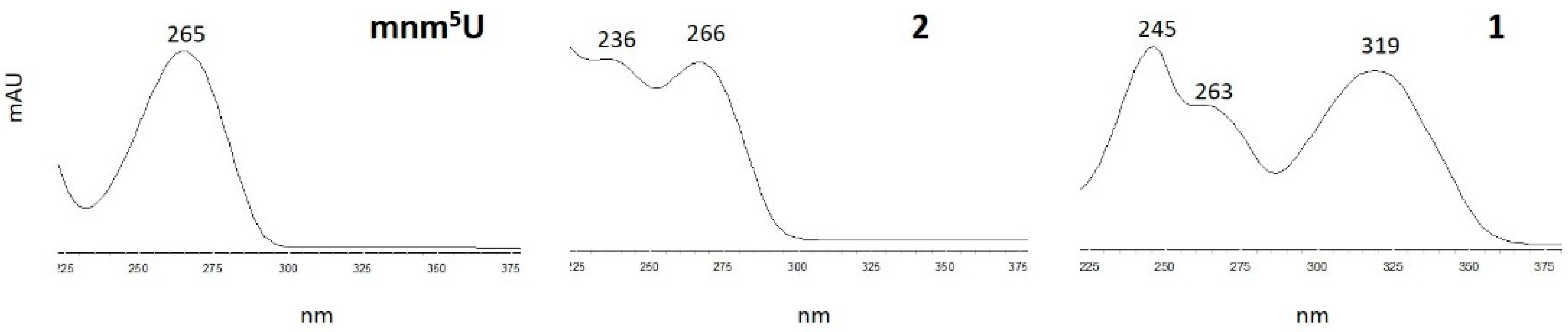
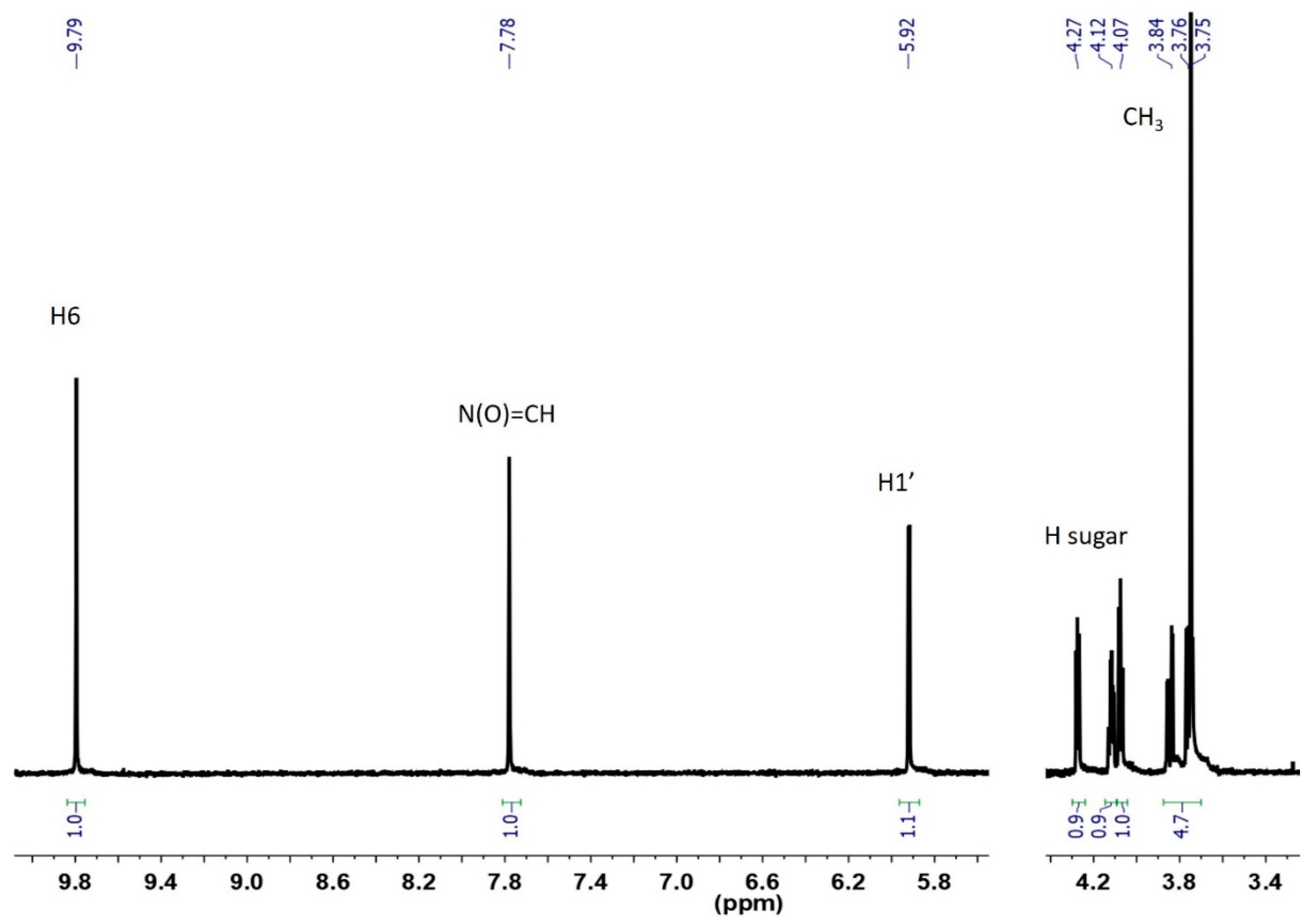
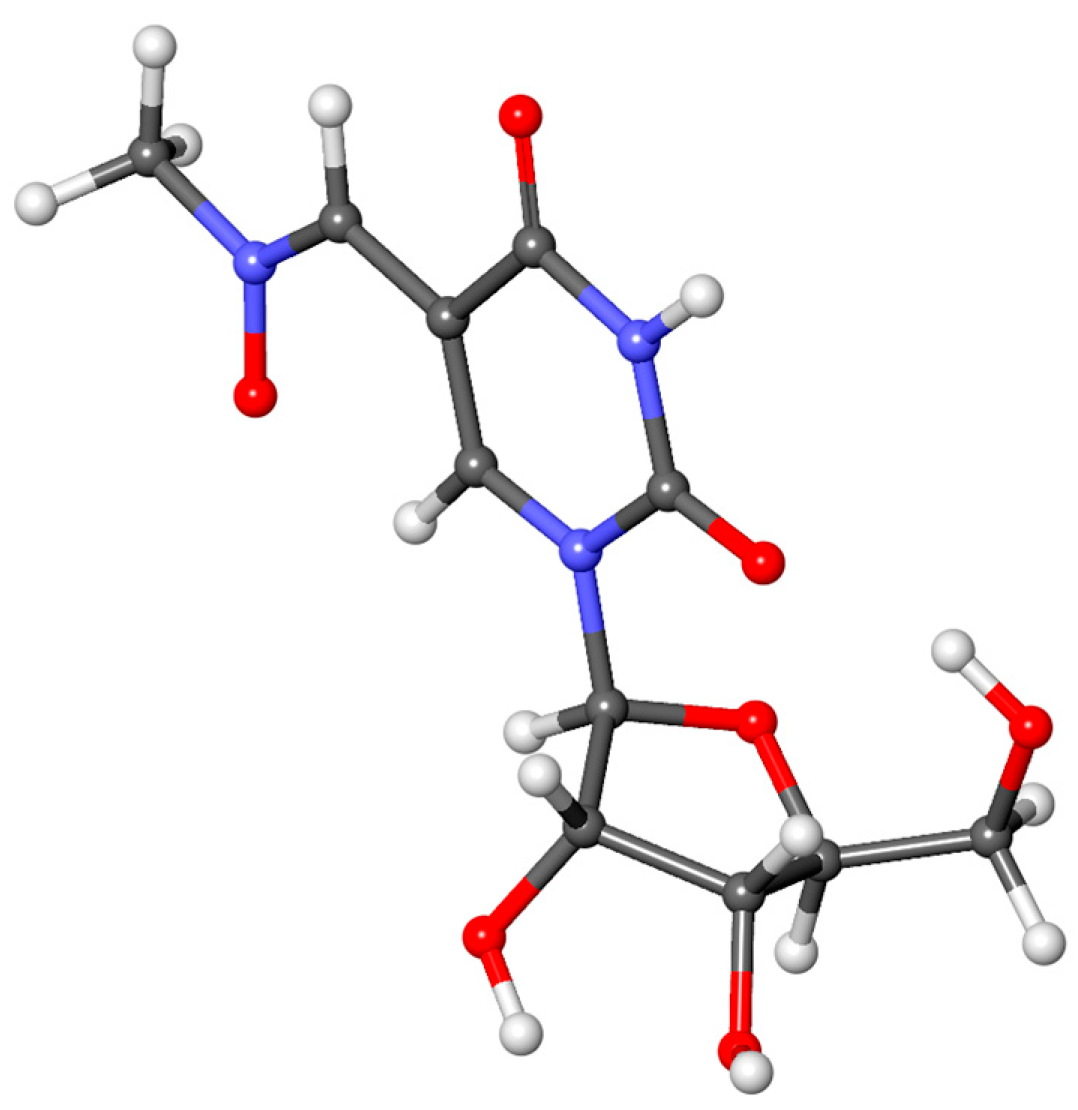
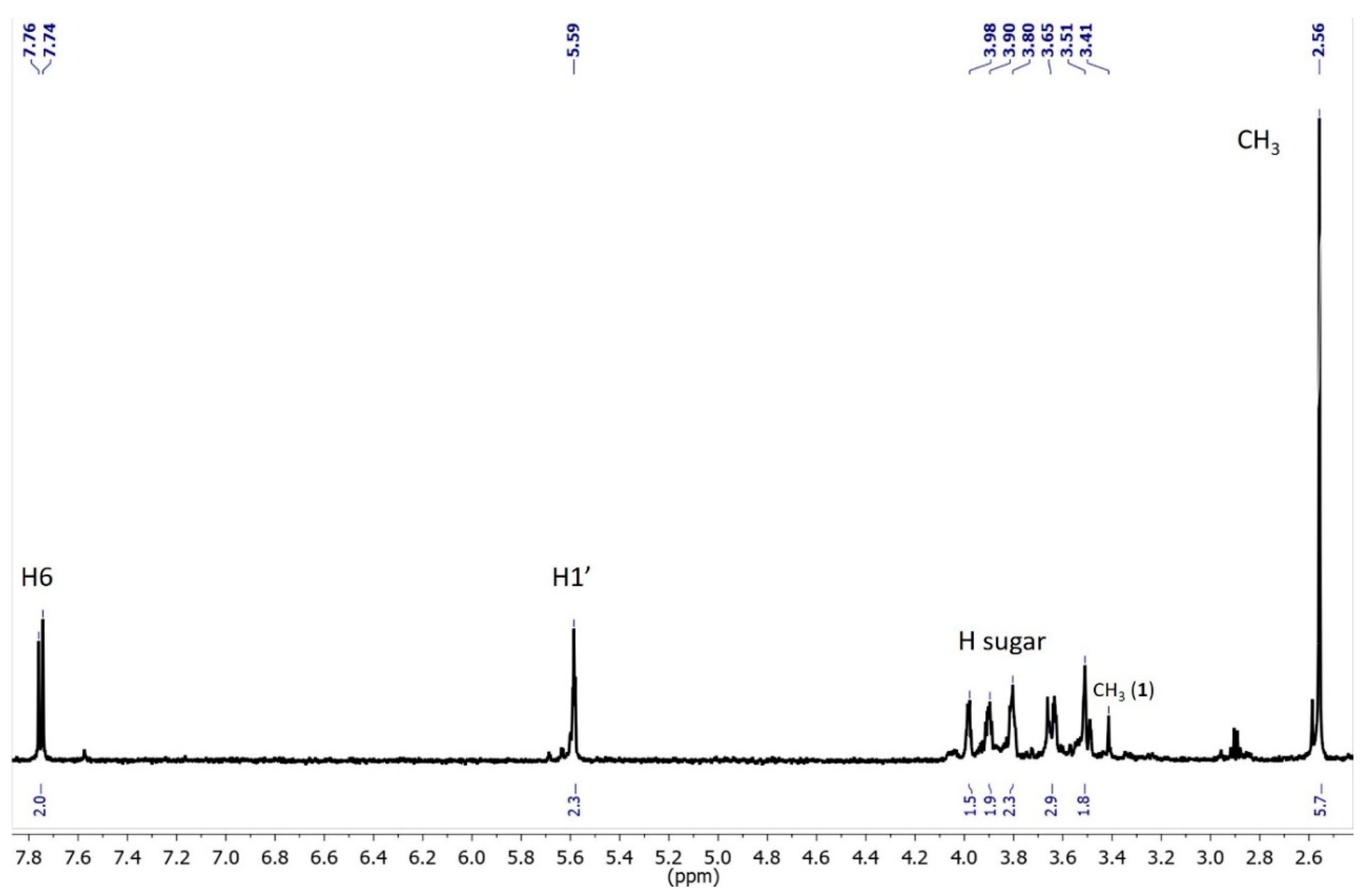
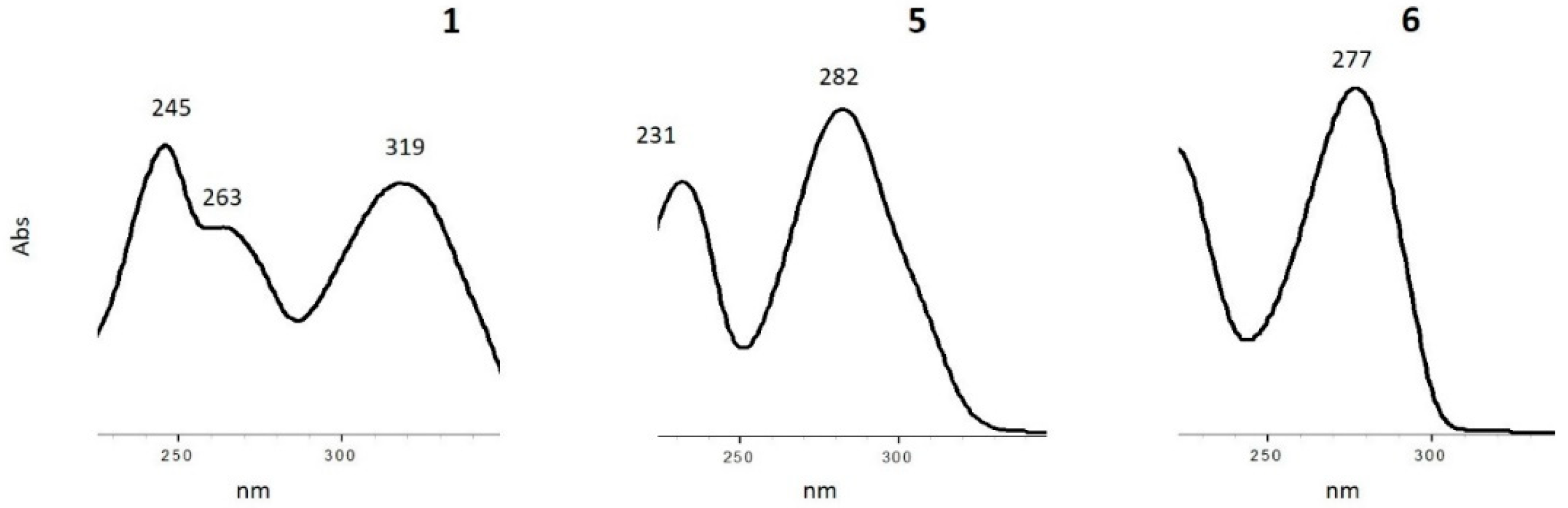
3.2. Characterization of Oxidation Product of mnm5U: Aldonitrone 1
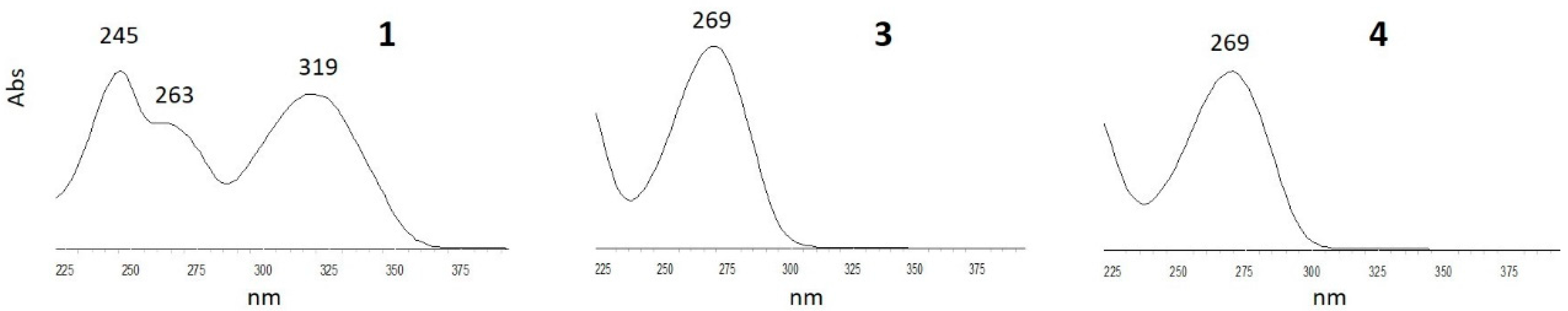
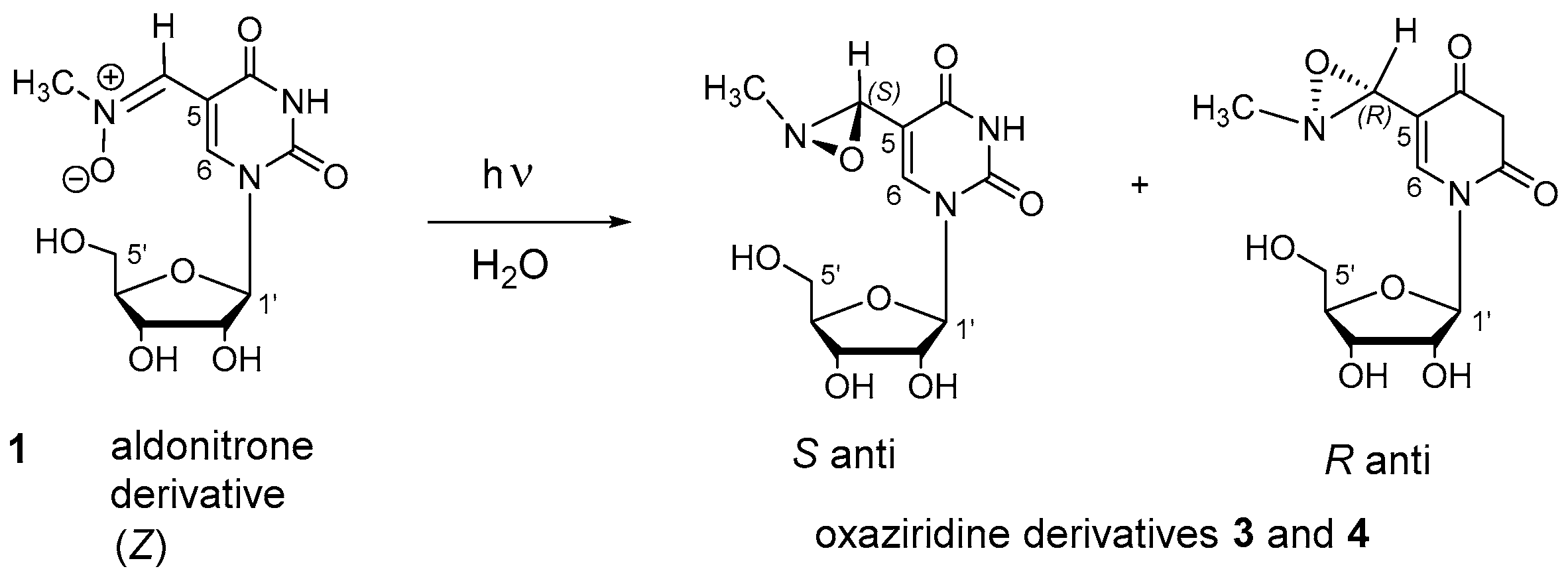
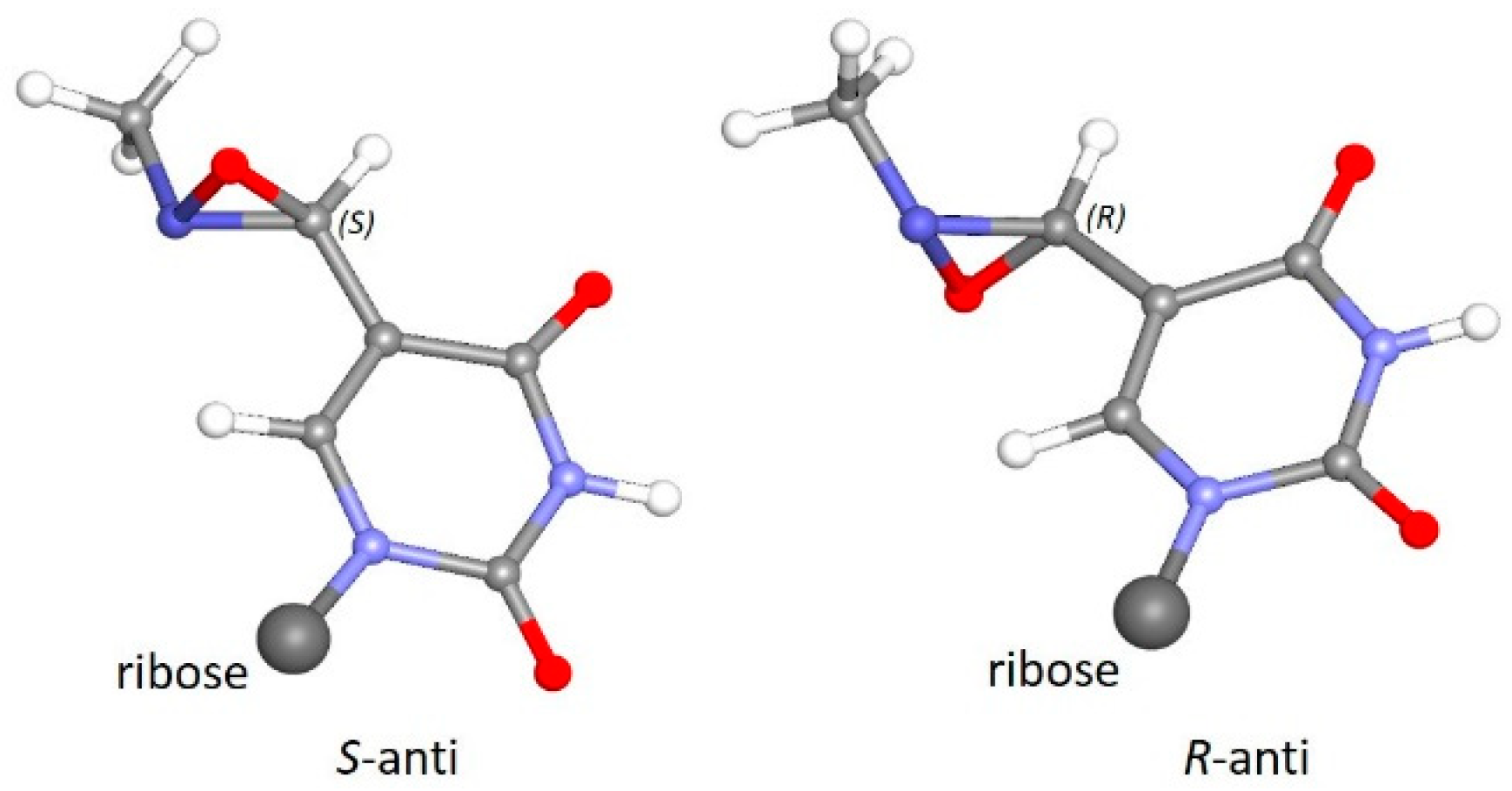
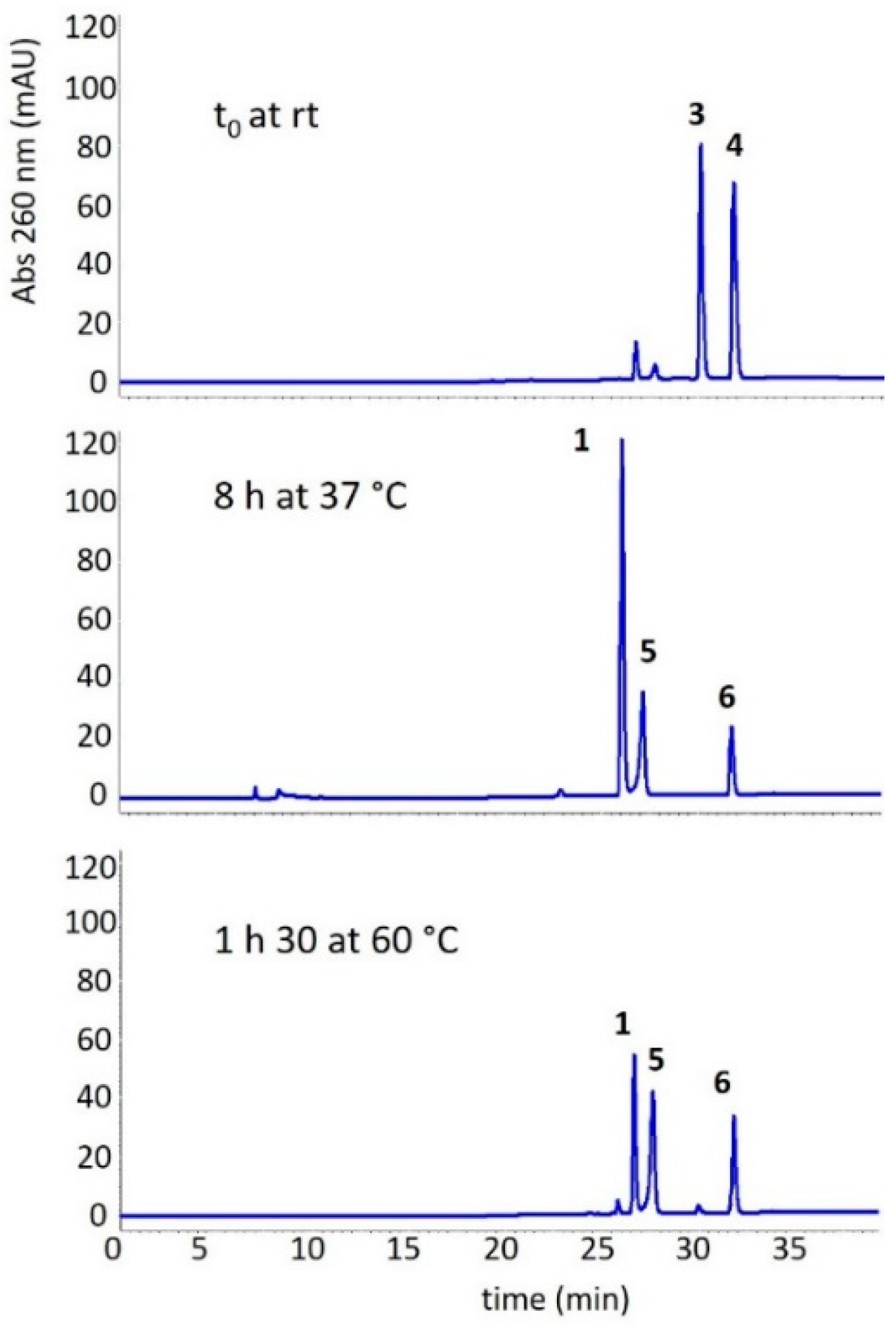
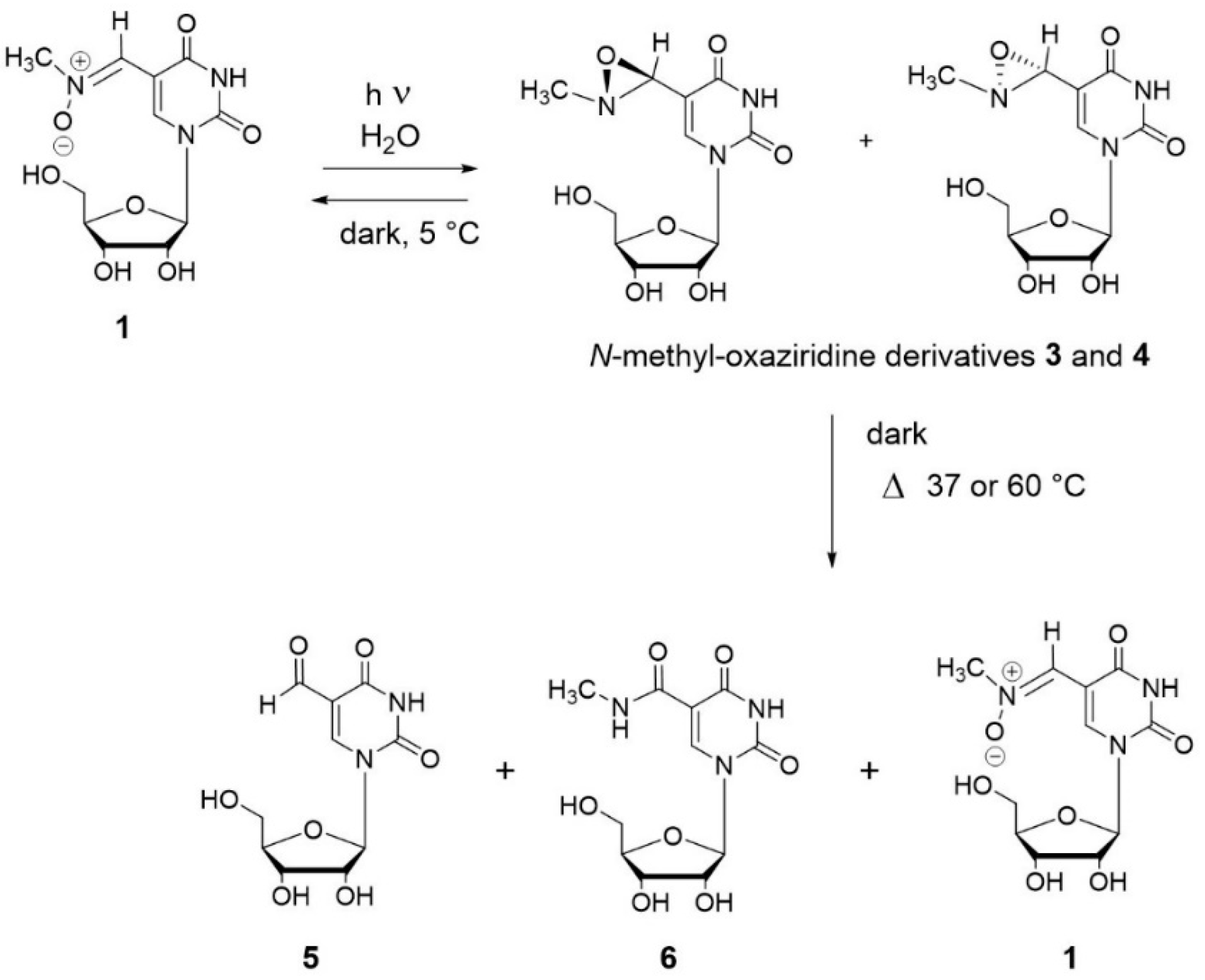
3.3. Photochemical Characterization of Aldonitrone 1
3.4. Stability of Oxaziridines 3 and 4 in the Dark
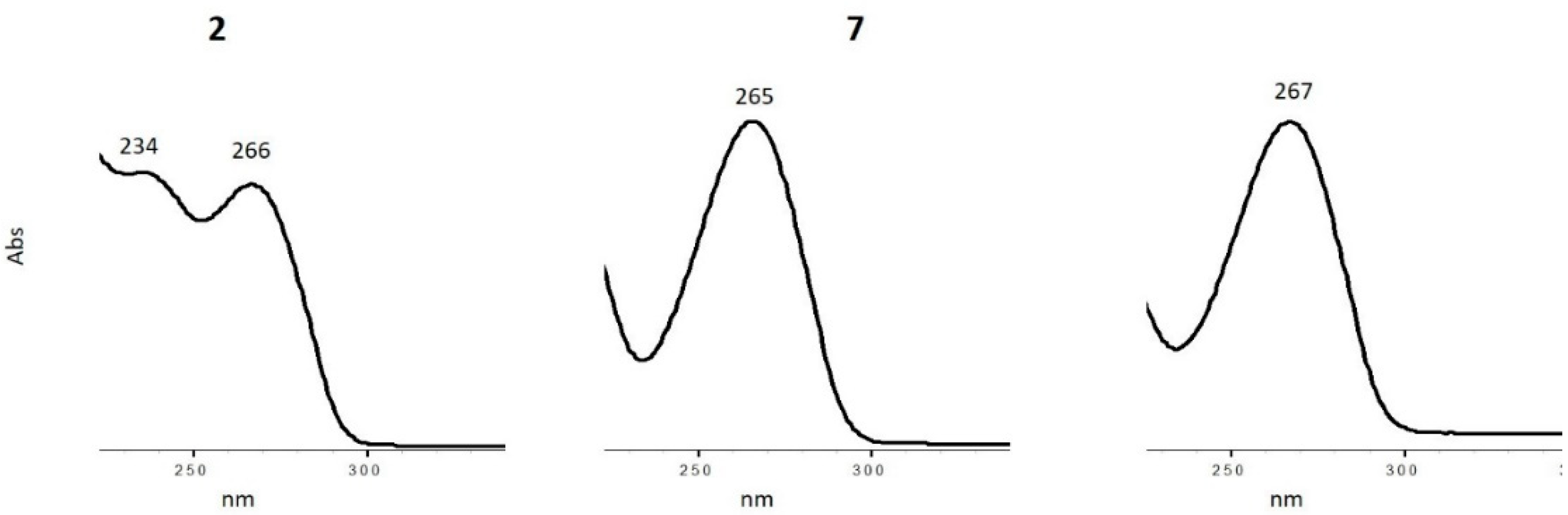
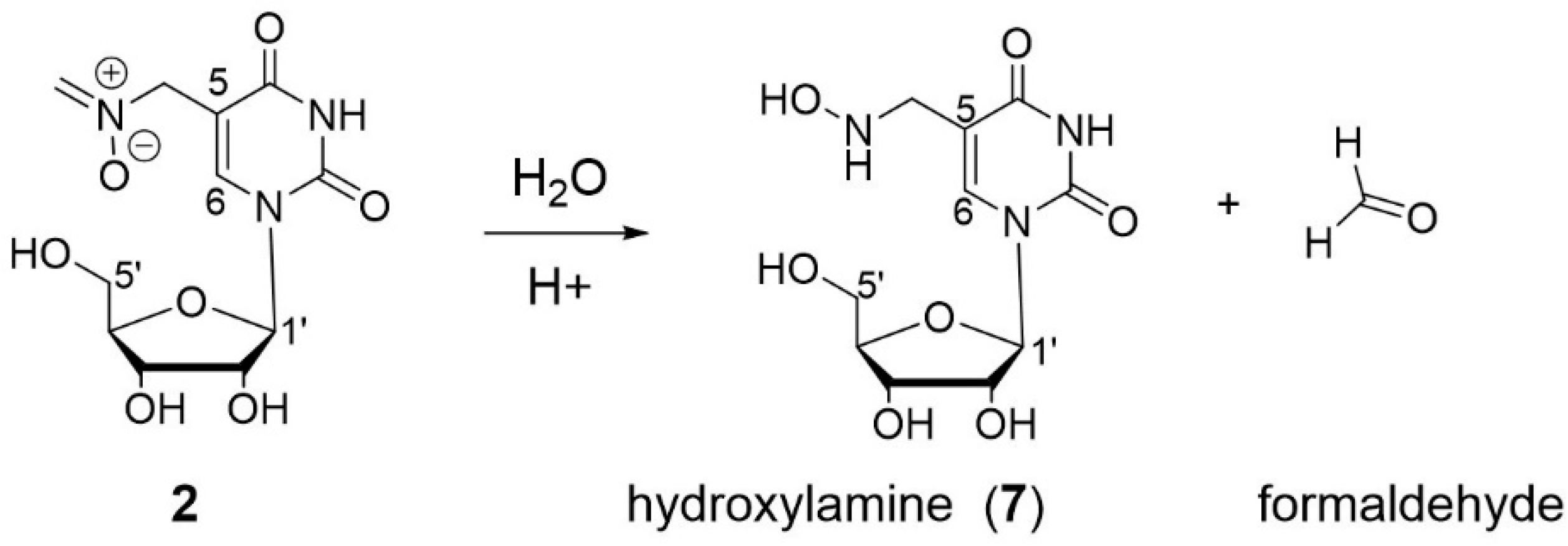
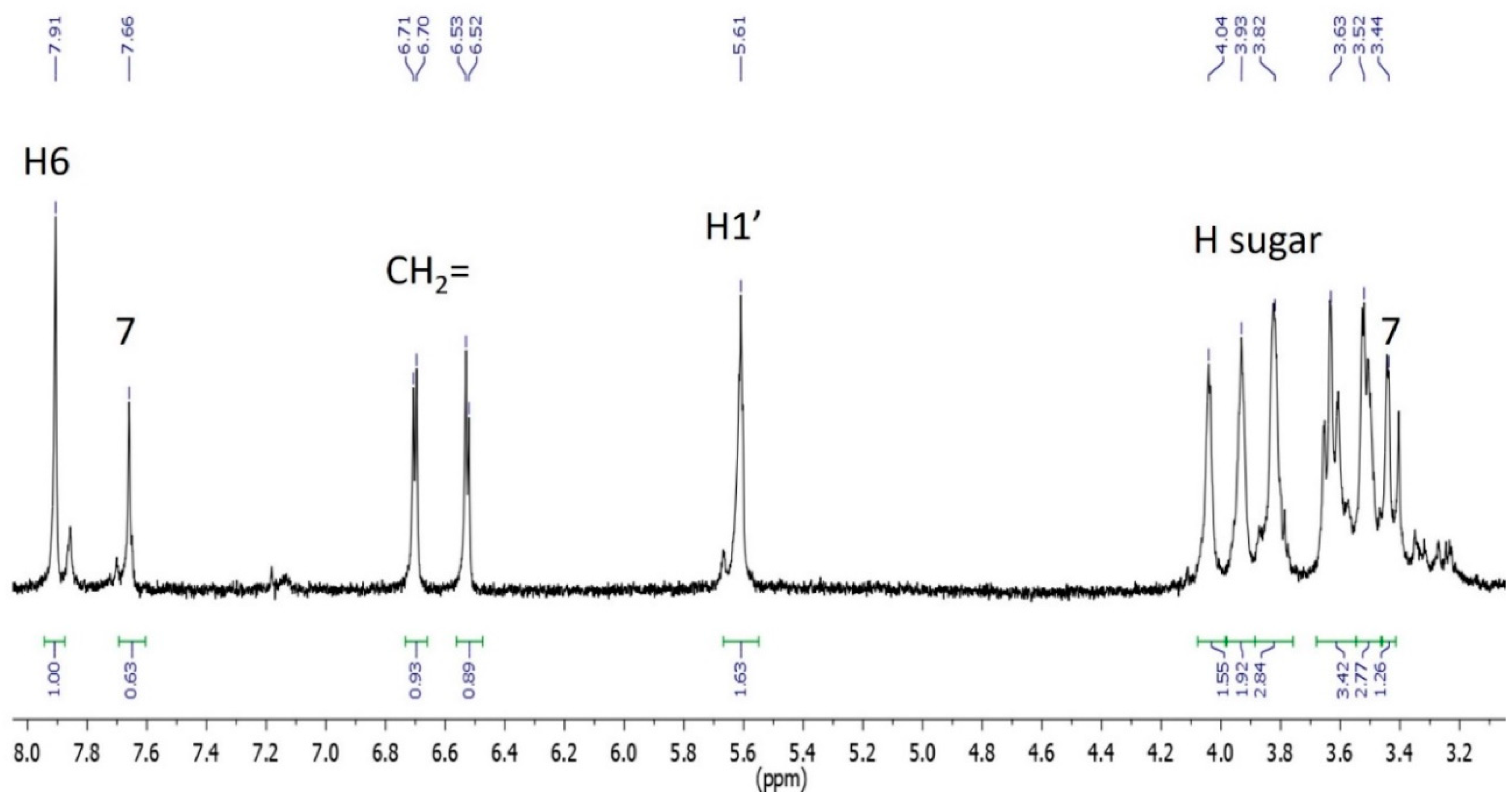
3.5. Characterization of Oxidation Product of mnm5U: Aldonitrone 2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- El Yacoubi, B.; Bailly, M.; de Crecy-Lagard, V. Biosynthesis and function of posttranscriptional modifications of transfer RNAs. Annu. Rev. Genet. 2012, 46, 69–95. [Google Scholar] [CrossRef] [PubMed]
- Agris, P.F.; Eruysal, E.R.; Narendran, A.; Vare, V.Y.P.; Vangaveti, S.; Ranganathan, S.V. Celebrating wobble decoding: Half a century and still much is new. RNA Biol. 2018, 15, 537–553. [Google Scholar] [CrossRef] [PubMed]
- Duechler, M.; Leszczynska, G.; Sochacka, E.; Nawrot, B. Nucleoside modifications in the regulation of gene expression: focus on tRNA. Cell. Mol. Life Sci. 2016, 73, 3075–3095. [Google Scholar] [CrossRef] [PubMed]
- Agris, P.F.; Narendran, A.; Sarachan, K.; Väre, V.Y.P.; Eruysal, E. The Importance of Being Modified: The Role of RNA Modifications in Translational Fidelity. Enzymes 2017, 41, 1–50. [Google Scholar] [PubMed]
- Motorin, Y.; Helm, M. RNA nucleotide methylation. Wiley Interdiscip. Rev. RNA 2011, 2, 611–631. [Google Scholar] [CrossRef] [PubMed]
- Carell, T.; Brandmayr, C.; Hienzsch, A.; Muller, M.; Pearson, D.; Reiter, V.; Thoma, I.; Thumbs, P.; Wagner, M. Structure and function of noncanonical nucleobases. Angew. Chem. Int. Ed. Engl. 2012, 51, 7110–7131. [Google Scholar] [CrossRef] [PubMed]
- Väre, V.Y.; Eruysal, E.R.; Narendran, A.; Sarachan, K.L.; Agris, P.F. Chemical and Conformational Diversity of Modified Nucleosides Affects tRNA Structure and Function. Biomolecules 2017, 7, 29. [Google Scholar] [CrossRef] [PubMed]
- Murphy, F.V., 4th; Ramakrishnan, V.; Malkiewicz, A.; Agris, P.F. The role of modifications in codon discrimination by tRNA(Lys)UUU. Nat. Struct. Mol. Biol. 2004, 11, 1186–1191. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.; Kawai, G.; Niimi, T.; Satoh, T.; Sekine, M.; Yamaizumi, Z.; Nishimura, S.; Miyazawa, T.; Yokoyama, S. A Modified Uridine in the 1st Position of the Anticodon of a Minor Species of Arginine Transfer-Rna, the ArgU Gene-Product, from Escherichia-Coli. Eur. J. Biochem. 1993, 216, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Rozov, A.; Demeshkina, N.; Khusainov, I.; Westhof, E.; Yusupov, M.; Yusupova, G. Novel base-pairing interactions at the tRNA wobble position crucial for accurate reading of the genetic code. Nat. Commun. 2016, 7, 10457. [Google Scholar] [CrossRef] [PubMed]
- Wittwer, A.J.; Tsai, L.; Ching, W.M.; Stadtman, T.C. Identification and Synthesis of a Naturally-Occurring Selenonucleoside in Bacterial Transfer-RNAs: 5-[(Methylamino)Methyl]-2-Selenouridine. Biochemistry 1984, 23, 4650–4655. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, H.E.; Specht, E.; Broedbaek, K.; Henriksen, T.; Ellervik, C.; Mandrup-Poulsen, T.; Tonnesen, M.; Nielsen, P.E.; Andersen, H.U.; Weimann, A. RNA modifications by oxidation: A novel disease mechanism? Free Radic. Biol. Med. 2012, 52, 1353–1361. [Google Scholar] [CrossRef] [PubMed]
- Wurtmann, E.J.; Wolin, S.L. RNA under attack: cellular handling of RNA damage. Crit. Rev. Biochem. Mol. Biol. 2009, 44, 34–49. [Google Scholar] [CrossRef] [PubMed]
- Nawrot, B.; Sochacka, E.; Duchler, M. tRNA structural and functional changes induced by oxidative stress. Cell. Mol. Life Sci. 2011, 68, 4023–4032. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewska-Antczak, A.; Guga, P.; Nawrot, B.; Pratviel, G. Guanosine in a Single Stranded Region of Anticodon Stem-Loop tRNA Models is Prone to Oxidatively Generated Damage Resulting in Dehydroguanidinohydantoin and Spiroiminodihydantoin Lesions. Chemistry 2015, 21, 6381–6385. [Google Scholar] [CrossRef] [PubMed]
- Alenko, A.; Fleming, A.M.; Burrows, C.J. Reverse Transcription Past Products of Guanine Oxidation in RNA Leads to Insertion of A and C opposite 8-Oxo-7,8-dihydroguanine and A and G opposite 5-Guanidinohydantoin and Spiroiminodihydantoin Diastereomers. Biochemistry 2017, 56, 5053–5064. [Google Scholar] [CrossRef] [PubMed]
- Sochacka, E.; Kraszewska, K.; Sochacki, M.; Sobczak, M.; Janicka, M.; Nawrot, B. The 2-thiouridine unit in the RNA strand is desulfured predominantly to 4-pyrimidinone nucleoside under in vitro oxidative stress conditions. Chem. Commun. (Camb.) 2011, 47, 4914–4916. [Google Scholar] [CrossRef] [PubMed]
- Bartos, P.; Ebenryter-Olbinska, K.; Sochacka, E.; Nawrot, B. The influence of the C5 substituent on the 2-thiouridine desulfuration pathway and the conformational analysis of the resulting 4-pyrimidinone products. Bioorg. Med. Chem. 2015, 23, 5587–5594. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Lan, M.D.; Qi, C.B.; Zheng, S.J.; Wei, S.Z.; Yuan, B.F.; Feng, Y.Q. Formation and determination of the oxidation products of 5-methylcytosine in RNA. Chem. Sci. 2016, 7, 5495–5502. [Google Scholar] [CrossRef] [PubMed]
- Payne, N.C.; Geissler, A.; Button, A.; Sasuclark, A.R.; Schroll, A.L.; Ruggles, E.L.; Gladyshev, V.N.; Hondal, R.J. Comparison of the redox chemistry of sulfur- and selenium-containing analogs of uracil. Free Radic. Biol. Med. 2017, 104, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Sierant, M.; Kulik, K.; Sochacka, E.; Szewczyk, R.; Sobczak, M.; Nawrot, B. Cytochrome c Catalyzes the Hydrogen Peroxide-Assisted Oxidative Desulfuration of 2-Thiouridines in Transfer RNAs. Chembiochem 2018, 19, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Bartosik, K.; Leszczynska, G. Synthesis of various substituted 5-methyluridines (xm5U) and 2-thiouridines (xm5s2U) via nucleophilic substitution of 5-pivaloyloxymethyluridine/2-thiouridine. Tetrahedron Lett. 2015, 56, 6593–6597. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum mechanical continuum solvation models. Chem. Rev. 2005, 105, 2999–3093. [Google Scholar] [CrossRef] [PubMed]
- Spence, G.G.; Taylor, E.C.; Buchardt, O. The photochemical reactions of azoxy compounds, nitrones, and aromatic amine N-oxides. Chem. Rev. 1970, 70, 231–238. [Google Scholar] [CrossRef]
- Splitter, J.S.; Calvin, M. Preparation of Oxaziranes by Irradiation of Nitrones. J. Org. Chem. 1958, 23, 651–652. [Google Scholar] [CrossRef]
- Cicchi, S.; Corsi, M.; Goti, A. Inexpensive and environmentally friendly oxidation of hydroxylamines to nitrones with bleach. J. Org. Chem. 1999, 64, 7243–7245. [Google Scholar] [CrossRef]
- Ali, S.A.; Hashmi, S.M.A.; Siddiqui, M.N.; Wazeer, M.I.M. Regiochemistry of mercury(II) oxide oxidation of unsymmetrical N,N-disubstituted hydroxylamines. Tetrahedron 1996, 52, 14917–14928. [Google Scholar] [CrossRef]
- Soldaini, G.; Cardona, F.; Goti, A. Catalytic oxidation of imines based on methyltrioxorhenium/urea hydrogen peroxide: a mild and easy chemo- and regioselective entry to nitrones. Org. Lett. 2007, 9, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Colladon, M.; Scarso, A.; Strukul, G. Mild catalytic oxidation of secondary and tertiary amines to nitrones and N-oxides with H2O2 mediated by Pt(II) catalysts. Green Chem. 2008, 10, 793–798. [Google Scholar] [CrossRef]
- Forcato, M.; Nugent, W.A.; Licini, G. A ‘waterproof’ catalyst for the oxidation of secondary amines to nitrones with alkyl hydroperoxides. Tetrahedron Lett. 2003, 44, 49–52. [Google Scholar] [CrossRef]
- Murahashi, S.I.; Mitsui, H.; Shiota, T.; Tsuda, T.; Watanabe, S. Tungstate-Catalyzed Oxidation of Secondary-Amines to Nitrones-Alpha-Substitution of Secondary-Amines via Nitrones. J. Org. Chem. 1990, 55, 1736–1744. [Google Scholar] [CrossRef]
- Ballistreri, F.P.; Chiacchio, U.; Rescifina, A.; Tomaselli, G.A.; Toscano, R.M. One-Flask Transformation of Secondary-Amines to Nitrones by Oxidation with Hydrogen-Peroxide Mediated by Triscetylpyridinium Tetrakis Oxodiperoxotungsto-Phosphate (Pcwp)-Some Mechanistic Considerations. Tetrahedron 1992, 48, 8677–8684. [Google Scholar] [CrossRef]
- Saini, P.; Banerjee, M.; Chattopadhyay, A. Computational Investigation of the Photochemical Reaction Path of Some Synthesized and Experimentally Analyzed Small-Chain Conjugated Nitrones. J. Phys. Chem. A 2016, 120, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Williamson, K.S.; Michaelis, D.J.; Yoon, T.P. Advances in the chemistry of oxaziridines. Chem. Rev. 2014, 114, 8016–8036. [Google Scholar] [CrossRef] [PubMed]
- Boyd, D.R.; Coulter, P.B.; Mcguckin, M.R.; Sharma, N.D.; Jennings, W.B.; Wilson, V.E. Imines and Derivatives. Part 24. Nitrone Synthesis by Imine Oxidation Using Either a Peroxyacid or Dimethyldioxirane. J. Chem. Soc. Perkin Trans. 1 1990, 1, 301–306. [Google Scholar] [CrossRef]
- Davis, F.A.; Sheppard, A.C. Applications of Oxaziridines in Organic-Synthesis. Tetrahedron 1989, 45, 5703–5742. [Google Scholar] [CrossRef]
- Gella, C.; Ferrer, E.; Alibes, R.; Busque, F.; de March, P.; Figueredo, M.; Font, J. A metal-free general procedure for oxidation of secondary amines to nitrones. J. Org. Chem. 2009, 74, 6365–6367. [Google Scholar] [CrossRef] [PubMed]
- Hood, T.S.; Huehls, C.B.; Yang, J. A modular approach to alpha,beta-unsaturated N-aryl ketonitrones. Tetrahedron Lett. 2012, 53, 4679–4682. [Google Scholar] [CrossRef]
- Gober, C.M.; Joullie, M.M. From Roquefortine C to Roquefortine L: Formation of a Complex Nitrone with Simple Oxidizing Agents. Isr. J. Chem. 2017, 57, 303–308. [Google Scholar] [CrossRef]
- Splitter, J.S.; Calvin, M. Oxaziridines. I. The irradiation products of several nitrones. J. Org. Chem. 1965, 30, 3427–3436. [Google Scholar] [CrossRef]
- Kubota, T.; Yamakawa, M.; Mori, Y. The electronic spectra of nitrones and the solvent effect on them. Bull. Soc. Chim. Jpn. 1963, 36, 1552–1563. [Google Scholar] [CrossRef]
- Prakash, P.; Gravel, E.; Nguyen, D.V.; Namboothiri, I.N.N.; Doris, E. Direct and Co-catalytic Oxidation of Hydroxylamines to Nitrones Promoted by Rhodium Nanoparticles Supported on Carbon Nanotubes. ChemCatChem 2017, 9, 2091–2094. [Google Scholar] [CrossRef]
- Sivasubramanian, S.; Mohan, P.; Thirumalaikumar, M.; Muthusubramanian, S. Synthesis and Separation of the E-Isomer and Z-Isomer of Simple Aldonitrones. J. Chem. Soc., Perkin Trans. 1 1994, 23, 3353–3354. [Google Scholar] [CrossRef]
- Jerina, D.M.; Boyd, D.R.; Paolillo, L.; Becker, E.D. Stereospecific chemical shifts and coupling constants in 15N-oxaziridines. Tetrahedron Lett. 1970, 11, 1483–1484. [Google Scholar] [CrossRef]
- Saini, P.; Chattopadhyay, A. Spectroscopic features of the low-lying singlet states of some N-alkyl retinylnitrone model systems and their involvement in oxaziridine formation. RSC Adv. 2014, 4, 20466–20478. [Google Scholar] [CrossRef]
- Bjorgo, J.; Boyd, D.R.; Campbell, R.M.; Neill, D.C. Photoracemization at a Chiral Pyramidal Nitrogen Center. J. Chem. Soc. Chem. Comm. 1976, 5, 162–163. [Google Scholar] [CrossRef]
- Splitter, J.S.; Su, T.M.; Ono, H.; Calvin, M. Orbital symmetry control in the nitrone-oxaziridine system. Nitrone photostationary states. J. Am. Chem. Soc. 1971, 93, 4075–4076. [Google Scholar] [CrossRef]
- Hamer, J.; Macaluso, A. Nitrones. Chem. Rev. 1964, 64, 473–495. [Google Scholar] [CrossRef]
- Koyano, K.; Suzuki, H. The NMR spectra and molecular geometry of nitrones. Tetrahedron Lett. 1968, 9, 1859–1864. [Google Scholar] [CrossRef]
- Emmons, W.D. The Preparation and Properties of Oxaziranes. J. Am. Chem. Soc. 1957, 79, 5739–5754. [Google Scholar] [CrossRef]
- Perkins, M.J. Spin trapping. Adv. Phys. Org. Chem. 1980, 17, 1–64. [Google Scholar]
- Davies, M.J.; Hawkins, C.L. EPR spin trapping of protein radicals. Free Radic. Biol. Med. 2004, 36, 1072–1086. [Google Scholar] [CrossRef] [PubMed]
- Marfey, P.; Robinson, E. The genetic toxicology of hydroxylamines. Mutat. Res. 1981, 86, 155–191. [Google Scholar] [CrossRef]
- Davis, F.A.; Jenkins, R., Jr.; Yocklovich, S.G. 2-arenesulfonyl-3-aryloxaziridine: a new class of aprotic oxidizing agents (oxidation of organic sulfur compounds). Tetrahedron Lett. 1978, 52, 5171–5174. [Google Scholar] [CrossRef]
- Lin, S.; Yang, X.; Jia, S.; Weeks, A.M.; Hornsby, M.; Lee, P.S.; Nichiporuk, R.V.; Iavarone, A.T.; Wells, J.A.; Toste, F.D.; et al. Redox-based reagents for chemoselective methionine bioconjugation. Science 2017, 355, 597–602. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Q.; Vu Ngoc, B.T.; Leszczynska, G.; Stigliani, J.-L.; Pratviel, G. Oxidation of 5-methylaminomethyl uridine (mnm5U) by Oxone Leads to Aldonitrone Derivatives. Biomolecules 2018, 8, 145. https://doi.org/10.3390/biom8040145
Zhou Q, Vu Ngoc BT, Leszczynska G, Stigliani J-L, Pratviel G. Oxidation of 5-methylaminomethyl uridine (mnm5U) by Oxone Leads to Aldonitrone Derivatives. Biomolecules. 2018; 8(4):145. https://doi.org/10.3390/biom8040145
Chicago/Turabian StyleZhou, Qishun, Bao Tram Vu Ngoc, Grazyna Leszczynska, Jean-Luc Stigliani, and Geneviève Pratviel. 2018. "Oxidation of 5-methylaminomethyl uridine (mnm5U) by Oxone Leads to Aldonitrone Derivatives" Biomolecules 8, no. 4: 145. https://doi.org/10.3390/biom8040145
APA StyleZhou, Q., Vu Ngoc, B. T., Leszczynska, G., Stigliani, J.-L., & Pratviel, G. (2018). Oxidation of 5-methylaminomethyl uridine (mnm5U) by Oxone Leads to Aldonitrone Derivatives. Biomolecules, 8(4), 145. https://doi.org/10.3390/biom8040145