Induction of Recombinant Lectin Expression by an Artificially Constructed Tandem Repeat Structure: A Case Study Using Bryopsis plumosa Mannose-Binding Lectin
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Native BPL2
2.2. Cloning and Construction of Recombinant BPL2
2.3. Optimization of rBPL2 Expression
2.4. Purification of rBPL2
2.5. Refolding of rBPL2
2.6. Hemagglutination Activity Assay
2.7. Analysis of Carbohydrate Specificity
2.8. Glycan Microarray
3. Results
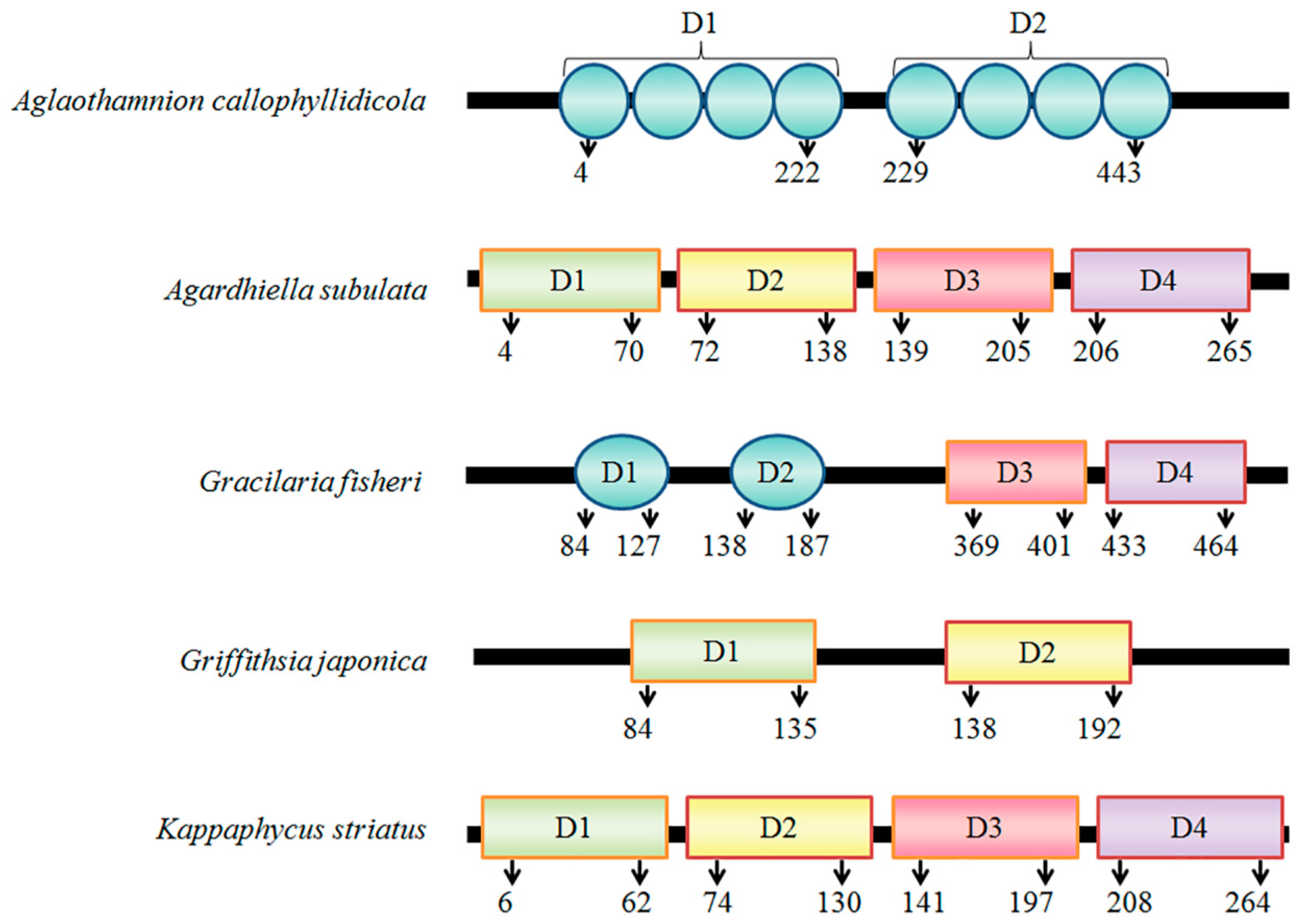
3.1. Cloning of rBPL2
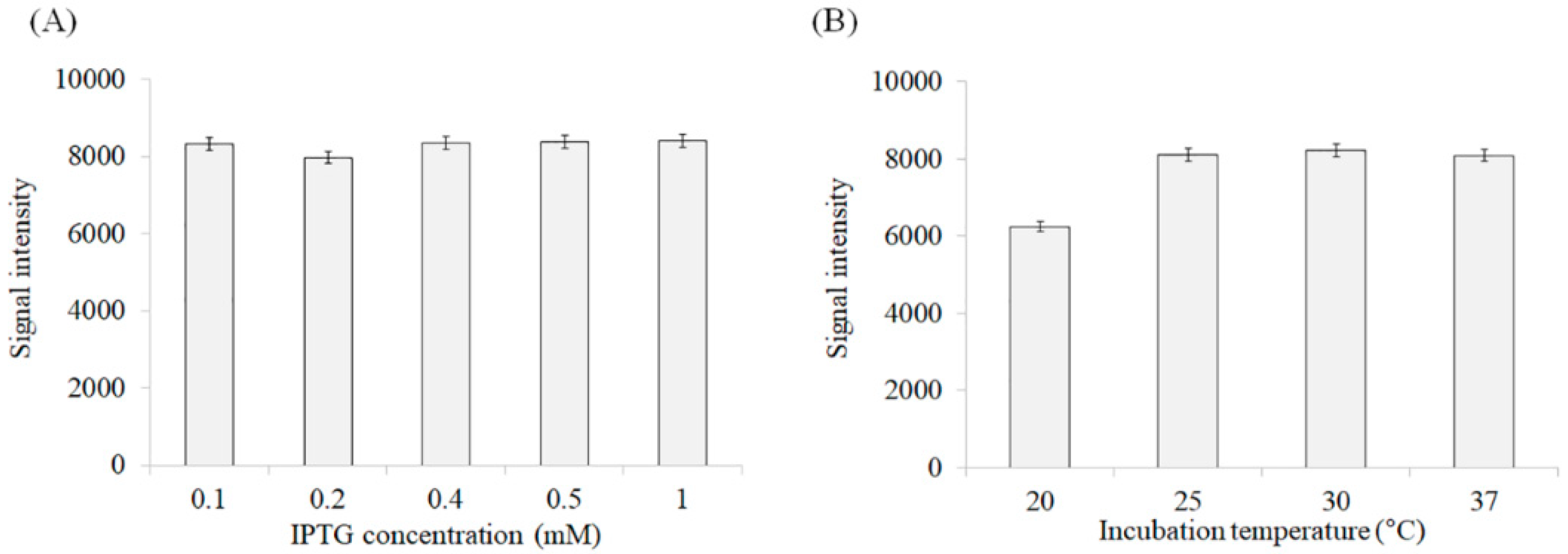
3.2. Optimization of Expression Conditions
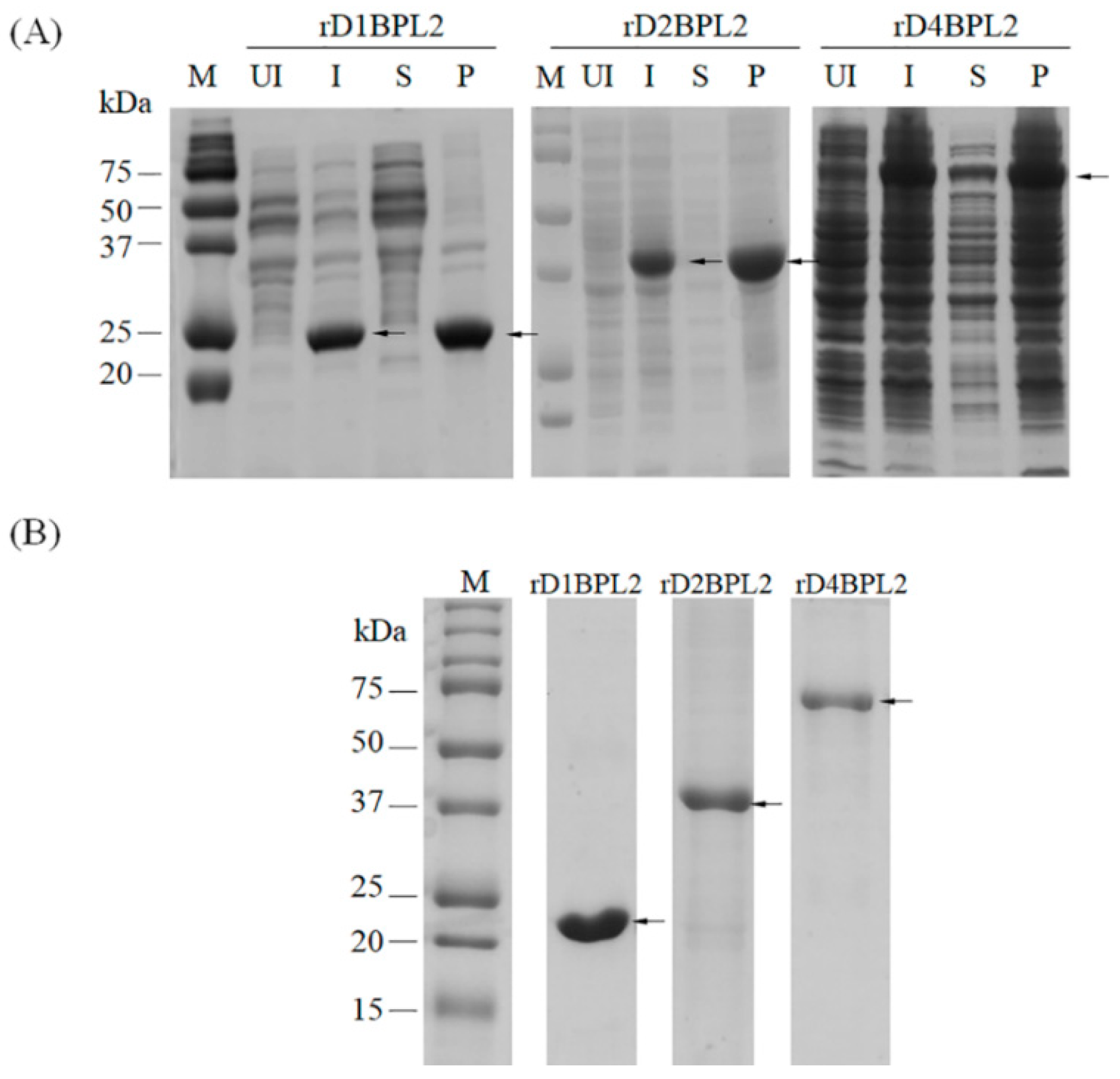
3.3. Purification of Recombinant Lectins
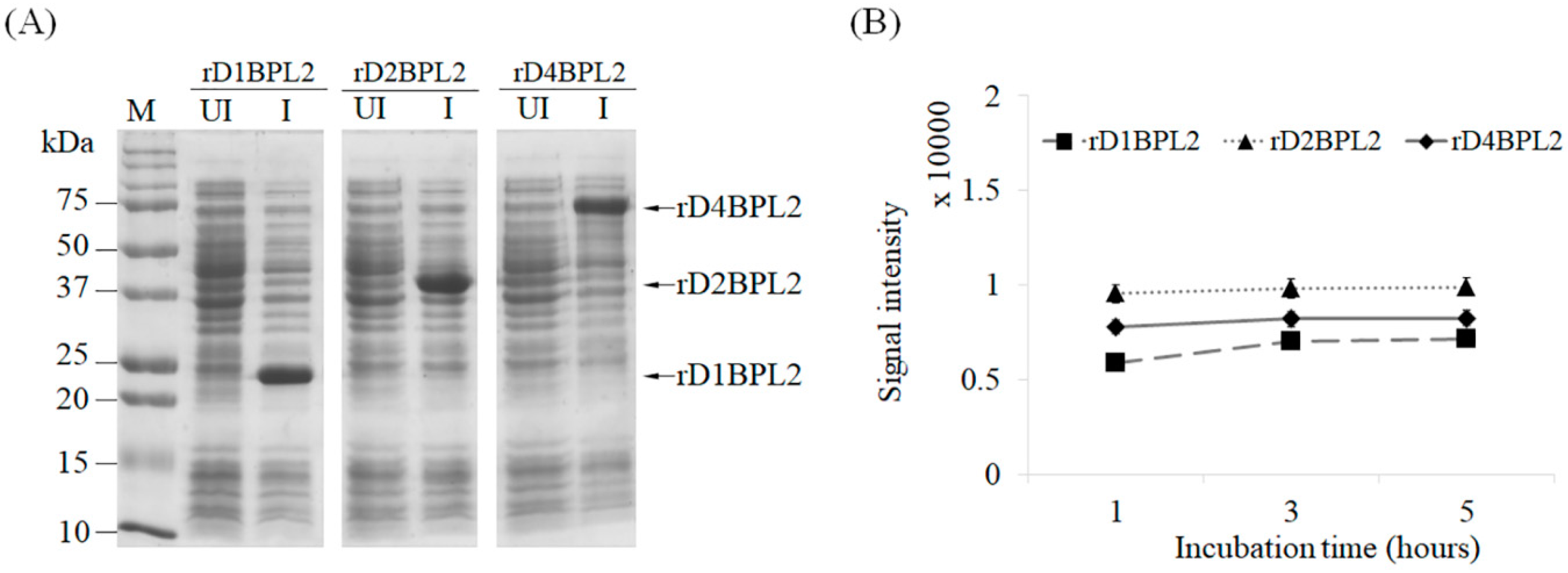
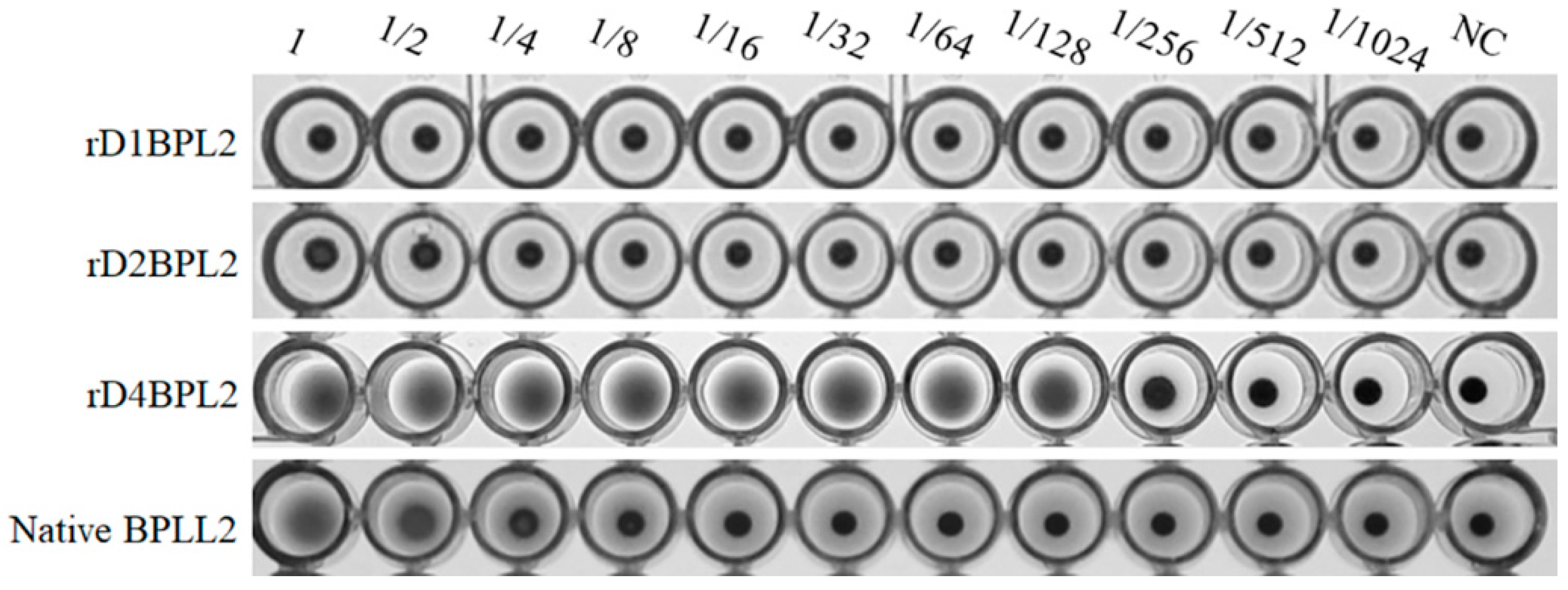
3.4. Hemagglutination Activity of Recombinant Lectins
3.5. Carbohydrate Specificity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sharon, N.; Lis, H. Lectins: Cell-agglutinating and sugar-specific proteins. Science 1972, 177, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, I.J.; Hughes, R.C.; Monsigny, M.; Ozawa, T.; Sharon, N. What should be called a lectin? Nature 1980, 285, 66. [Google Scholar] [CrossRef]
- Lis, H.; Sharon, N. Lectins as molecules and tools. Annu. Rev. Biochem. 1986, 55, 35–67. [Google Scholar] [CrossRef] [PubMed]
- Mody, R.; Joshi, S.; Chaney, W. Use of lectins as diagnostic and therapeutic tools for cancer. J. Pharmacol. Toxicol. Methods 1995, 33, 1–10. [Google Scholar] [CrossRef]
- Dan, X.; Ng, T.B.; Wong, J.H.; Chan, Y.S.; Cheung, R.C.F.; Chan, W.Y. A hemagglutinin isolated from Northeast China black beans induced mitochondrial dysfunction and apoptosis in colorectal cancer cells. Biochim. Biophys. Acta 2016, 1863, 2201–2211. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.H.; Yoon, M.; West, J.A.; Klochkova, T.A.; Kim, S.-H. Possible surface carbohydrates involved in signaling during conjugation process in Zygnema cruciatum monitored with fluorescein isothiocyanate-lectins (Zygnemataceae, Chlorophyta). Phycol. Res. 2007, 55, 135–142. [Google Scholar] [CrossRef]
- Hashim, O.H.; Jayapalan, J.J.; Lee, C.-S. Lectins: An effective tool for screening of potential cancer biomarkers. PeerJ 2017, 5, e3784. [Google Scholar] [CrossRef] [PubMed]
- Tateno, H.; Toyota, M.; Saito, S.; Onuma, Y.; Ito, Y.; Hiemori, K.; Fukumura, M.; Matsushima, A.; Nakanishi, M.; Ohnuma, K.; et al. Glycome diagnosis of human induced pluripotent stem cells using lectin microarray. J. Biol. Chem. 2011, 286, 20345–20353. [Google Scholar] [CrossRef] [PubMed]
- Gabius, H.J.; André, S.; Kaltner, H.; Siebert, H.C. The sugar code: functional lectinomics. Biochim. Biophys. Acta 2002, 1572, 165–177. [Google Scholar] [CrossRef]
- Gabius, H.J.; Wu, A.M. The emerging functionality of endogenous lectins: A primer to the concept and a case study on galectins including medical implications. Chang. Gung. Med. J. 2006, 29, 37–62. [Google Scholar] [PubMed]
- Hsu, K.L.; Pilobello, K.T.; Mahal, L.K. Analyzing the dynamic bacterial glycome with a lectin microarray approach. Nat. Chem. Biol. 2006, 2, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.-J.; Han, J.-W.; Kim, G.H.; Han, J.W. Functional expression and characterization of the recombinant N-acetyl-glucosamine/N-acetyl-galactosamine-specific marine algal lectin BPL3. Mar. Drugs. 2018, 16, 13. [Google Scholar] [CrossRef] [PubMed]
- Han, J.W.; Jung, M.G.; Shim, E.Y.; Shim, J.B.; Kim, Y.M.; Kim, G.H. Functional recombinants designed from a fetuin/asialofetuin-specific marine algal lectin, rhodobindin. Mar. Drugs 2015, 13, 2183–2195. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Alarcón, D.; Blanco-Labra, A.; García-Gasca, T. Expression of lectins in heterologous systems. Int. J. Mol. Sci. 2018, 19, E616. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.; Teixeira, J.A.; Domingues, L. Recombinant production of plant lectins in microbial systems for biomedical application-the frutalin case study. Front. Plant Sci. 2014, 5, 390. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Van Eijk, M.; Guo, H.; van Dijk, A.; Bleijerveld, O.B.; Verheije, M.H.; Wang, G.; Haagsman, H.P.; Veldhuizen, E.J. Expression and characterization of recombinant chicken mannose binding lectin. Immunobiology 2017, 222, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.S.; Walia, A.K. Lectins from red algae and their biomedical potential. J. Appl. Phycol. 2018, 30, 1833–1858. [Google Scholar] [CrossRef]
- Shim, E.; Shim, J.; Klochkova, T.A.; Han, J.W.; Kim, G.H. Purification of a sex-specific lectin involved in gamete binding of Aglaothamnion callophyllidicola (Rhodophyta). J. Phycol. 2012, 48, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Michelow, I.C.; Lear, C.; Scully, C.; Prugar, L.I.; Longley, C.B.; Yantosca, L.M.; Ji, X. Karpel, M.; Brudner, M.; Takahashi, K.; et al. High-dose mannose-binding lectin therapy for Ebola virus infection. J. Infect. Dis. 2011, 203, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Han, J.W.; Jung, M.G.; Kim, M.J.; Yoon, K.S.; Lee, K.P.; Kim, G.H. Purification and characterization of a d-mannose specific lectin from the green marine alga, Bryopsis plumosa. Phycol. Res. 2010, 58, 143–150. [Google Scholar] [CrossRef]
- Han, J.W.; Yoon, K.S.; Klochkova, T.A.; Hwang, M.S.; Kim, G.H. Purification and characterization of a lectin, BPL-3, from the marine green alga Bryopsis plumosa. J. Appl. Phycol. 2011, 23, 745–753. [Google Scholar] [CrossRef]
- Hosono, M.; Ishikawa, K.; Mineki, R.; Murayama, K.; Numata, C.; Ogawa, Y.; Takayanagi, Y.; Nitta, K. Tandem repeat structure of rhamnose-binding lectin from catfish (Silurus asotus) eggs. Biochim. Biophys. Acta 1999, 1472, 668–675. [Google Scholar] [CrossRef]
- Okamoto, M.; Tsutsui, S.; Tasumi, S.; Suetake, S.; Kikuchi, K.; Suzuki, Y. Tandem repeat L-rhamnose-binding lectin from the skin mucus of ponyfish, Leiognathus nuchalis. Biochem. Biophys. Res. Commun. 2005, 333, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Hori, K.; Sato, Y.; Ito, K.; Fujiwara, Y.; Iwamoto, Y.; Makino, H.; Kawakubo, A. Strict specificity for high-mannose type N-glycans and primary structure of a red alga Eucheuma serra lectin. Glycobiology 2007, 17, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Moulaei, T.; Alexandre, K.B.; Shenoy, S.R.; Meyerson, J.R.; Krumpe, L.R.; Constantine, B.; Wilson, J.; Buckheit, R.W.Jr.; McMahon, J.B.; Subramaniam, S.; et al. Griffithsin tandemers: Flexible and potent lectin inhibitors of the human immunodeficiency virus. Retrovirology 2015, 12, 6. [Google Scholar] [CrossRef] [PubMed]
- Hori, K. Novel Polypeptide, Polynucleotide Encoding the Polypeptide and Use the Polypeptide and Polynucleotide. EP1860184A1, 28 November 2007. [Google Scholar]
- Glick, B.R.; Whitney, G.K. Factors affecting the expression of foreign proteins in Escherichia coli. J. Ind. Microbiol. 1987, 1, 277–282. [Google Scholar] [CrossRef]
- Berrow, N.S.; Büssow, K.; Coutard, B.; Diprose, J.; Ekberg, M.; Folkers, G.E.; Levy, N.; Lieu, V.; Owens, R.J.; Peleg, Y.; et al. Recombinant protein expression and solubility screening in Escherichia coli: A comparative study. Acta Crystallogr. D Biol. Crystallogr. 2006, 62, 1218–1226. [Google Scholar] [CrossRef] [PubMed]
- Chaves, R.P.; Silva, S.R.; Silva, J.P.F.A.; Carneiro, R.F.; Sousa, B.L.; Abreu, J.O.; Carvalho, F.C.T.; Rocha, C.R.C.; Farias, W.R.L.; Sousa, O.V.; et al. Meristiella echinocarpa lectin (MEL): A new member of the OAAH-lectin family. J. Appl. Phycol. 2018, 30, 2629–2638. [Google Scholar] [CrossRef]
- Pellegrini, M. Tandem repeats in proteins: prediction algorithms and biological role. Front. Bioeng. Biotechnol. 2015, 3, 143. [Google Scholar] [CrossRef] [PubMed]
- Andrade, M.A.; Perez-Iratxeta, C.; Ponting, C.P. Protein repeats: structures, functions, and evolution. J. Struct. Biol. 2001, 134, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, N.; Chen, J.; Regan, L. All repeats are not equal: A module-based approach to guide repeat protein design. J. Mol. Biol. 2013, 425, 1826–1838. [Google Scholar] [CrossRef] [PubMed]
- Kusui, K.; Takasaki, S. Structural study of N-linked sugar chains of sheep erythrocyte membrane glycoproteins. Glycoconj. J. 1998, 15, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, H.; Cleve, H. Solubilization and comparative analysis of mammalian erythrocyte membrane glycoproteins. Biochem. Biophys. Res. Commun. 1972, 47, 459–464. [Google Scholar] [CrossRef]

| Protein | Horse Erythrocytes | Sheep Erythrocytes |
|---|---|---|
| rD1BPL2 | ND | ND |
| rD2BPL2 | ND | ND |
| rD4BPL2 | 4.2 μg/mL | ND |
| Native BPL2 | 160 μg/mL | 0.3 μg/mL |
| Substance | Minimum Inhibitory Concentration | |
|---|---|---|
| rD4BPL2 | Native BPL2 | |
| d-Glucose | NI | NI |
| d-Galactose | NI | NI |
| d-Mannose | NI | 62.5 mM |
| N-Acetyl-d-glucosamine | NI | NI |
| N-Acetyl-d-galactosamine | NI | NI |
| l-fucose | NI | NI |
| Maltose | NI | NI |
| Lactose | NI | NI |
| Fructose | NI | NI |
| Fetuin | 12.2 μg/mL | 195.3 μg/mL |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, H.-J.; Han, J.-W.; Jeon, H.; Han, J.W. Induction of Recombinant Lectin Expression by an Artificially Constructed Tandem Repeat Structure: A Case Study Using Bryopsis plumosa Mannose-Binding Lectin. Biomolecules 2018, 8, 146. https://doi.org/10.3390/biom8040146
Hwang H-J, Han J-W, Jeon H, Han JW. Induction of Recombinant Lectin Expression by an Artificially Constructed Tandem Repeat Structure: A Case Study Using Bryopsis plumosa Mannose-Binding Lectin. Biomolecules. 2018; 8(4):146. https://doi.org/10.3390/biom8040146
Chicago/Turabian StyleHwang, Hyun-Ju, Jin-Woo Han, Hancheol Jeon, and Jong Won Han. 2018. "Induction of Recombinant Lectin Expression by an Artificially Constructed Tandem Repeat Structure: A Case Study Using Bryopsis plumosa Mannose-Binding Lectin" Biomolecules 8, no. 4: 146. https://doi.org/10.3390/biom8040146
APA StyleHwang, H.-J., Han, J.-W., Jeon, H., & Han, J. W. (2018). Induction of Recombinant Lectin Expression by an Artificially Constructed Tandem Repeat Structure: A Case Study Using Bryopsis plumosa Mannose-Binding Lectin. Biomolecules, 8(4), 146. https://doi.org/10.3390/biom8040146





