Massive Activation of GABAA Receptors: Rundown, Ionic and Neurodegenerative Consequences
Abstract
1. Introduction
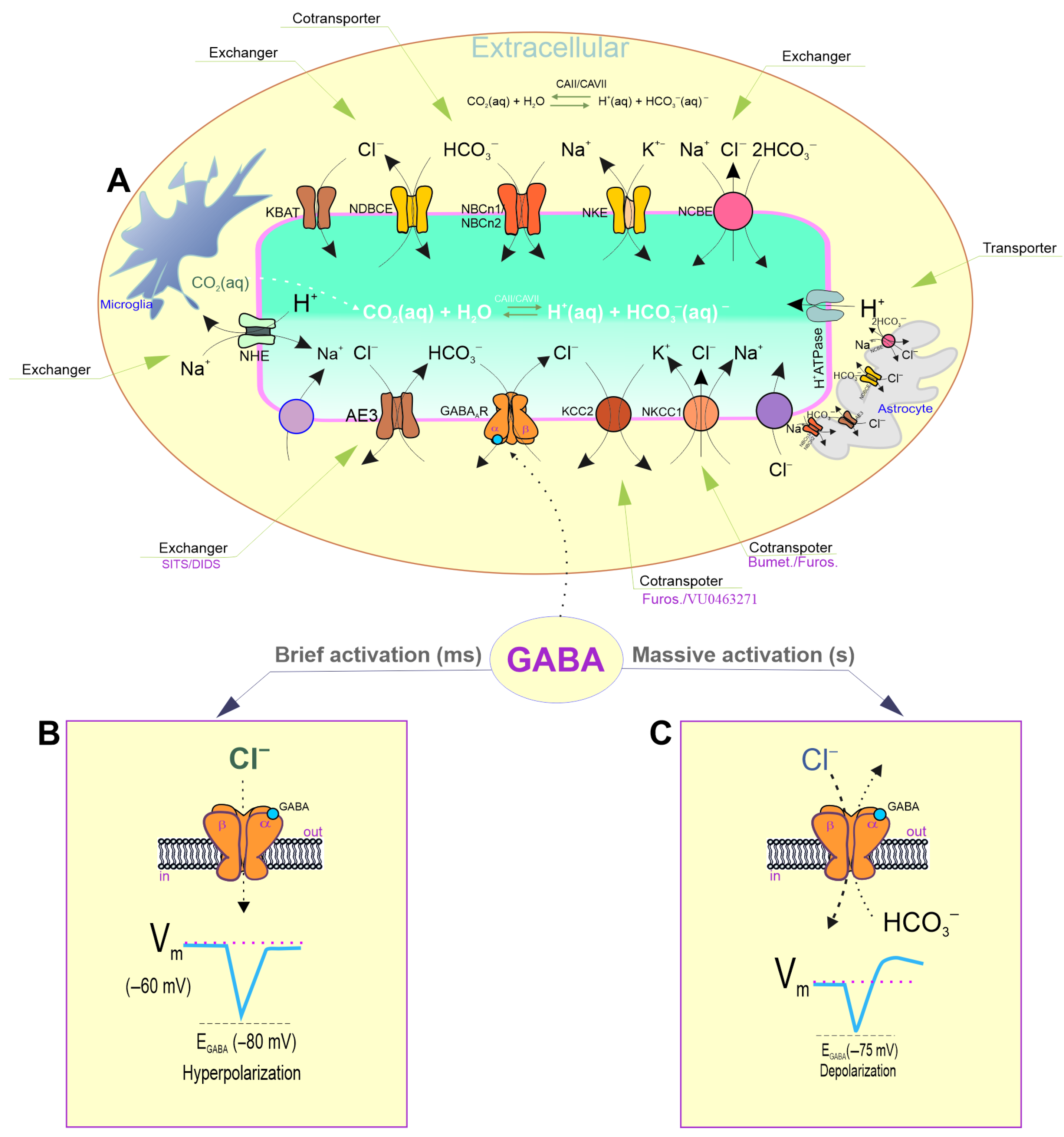
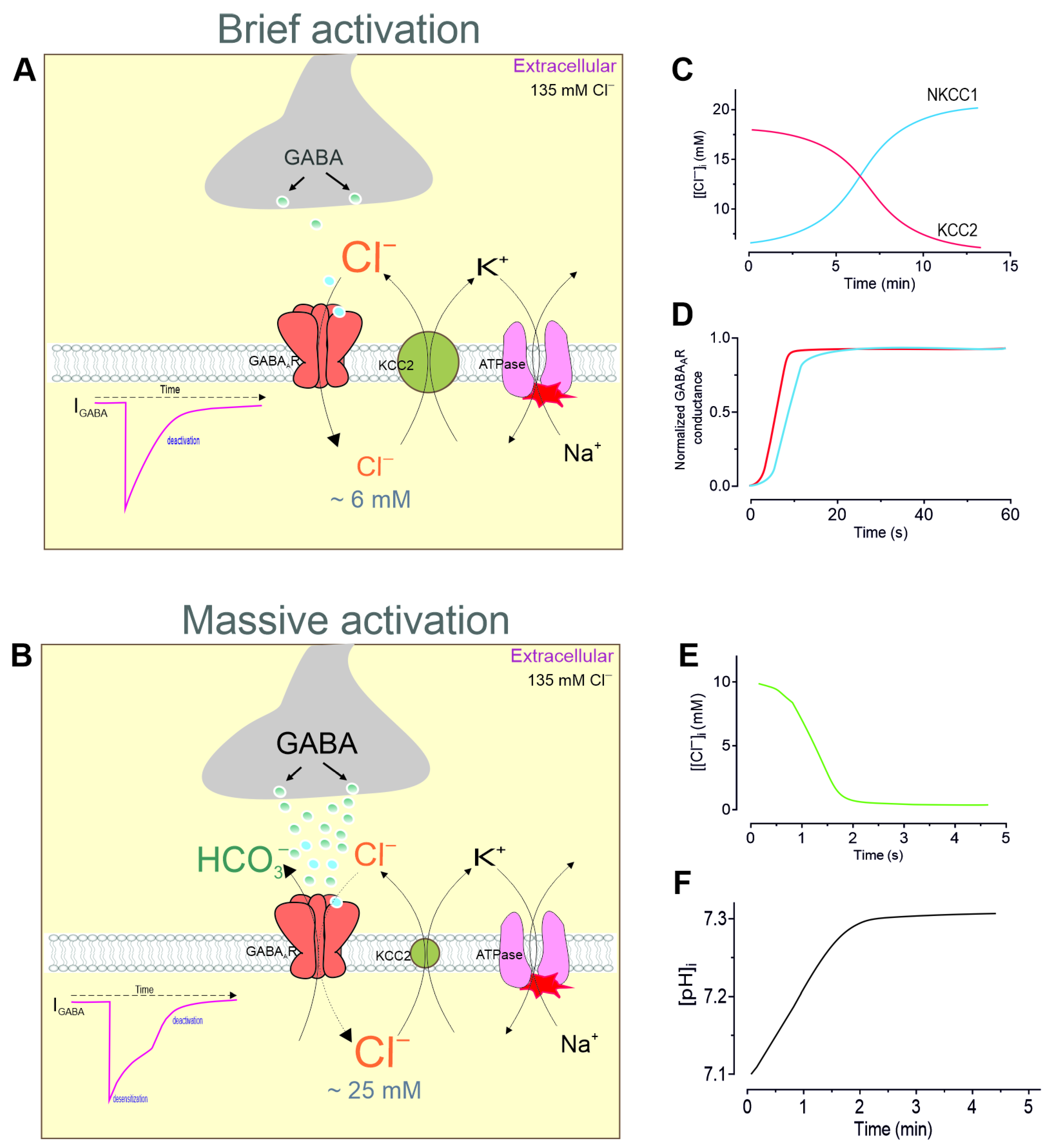
2. The “GABA Switch” from Inhibition to Excitation During Massive Activation
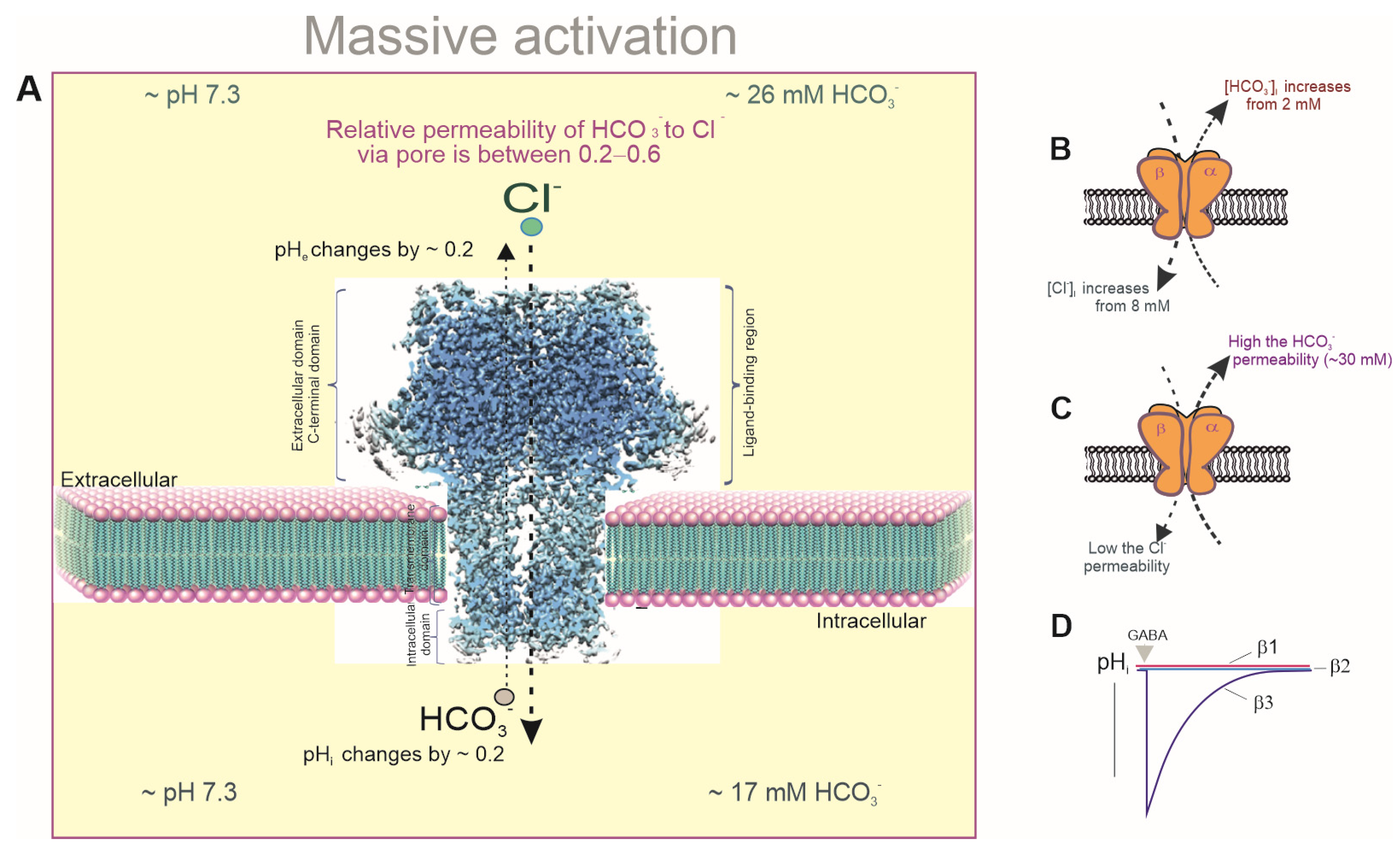
2.1. Changes in pHi During Massive Activation
2.2. Maintaining pHi/Bicarbonate Homeostasis
2.3. Changes in [Cl−]i During Massive Activation
2.4. Maintaining [Cl−]i Homeostasis
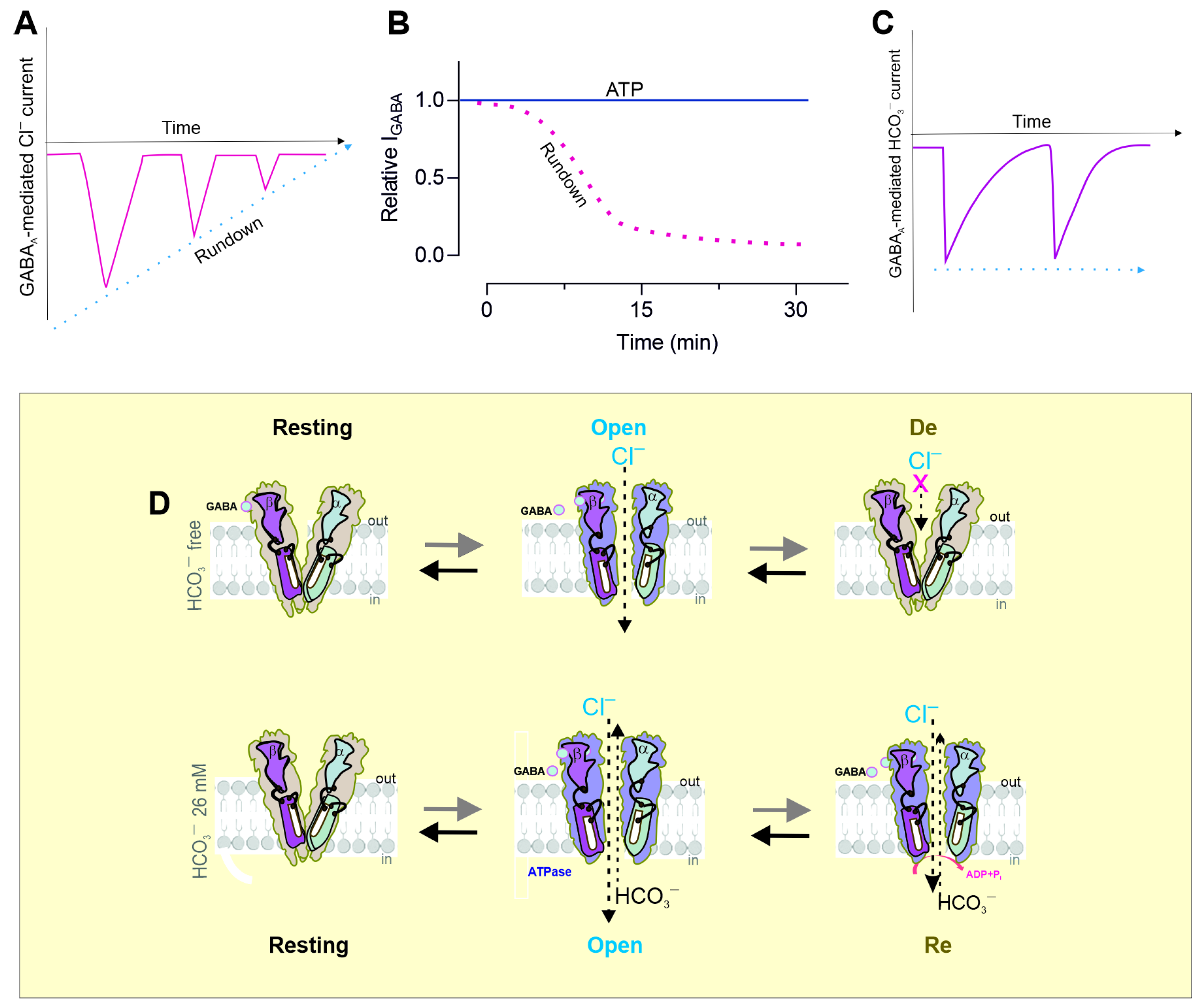
3. Conformational Changes During Massive Activation
4. Modulation of Desensitization by pH
5. Metabolic Changes During Massive Activation
6. Ionic Changes During Network/Seizure Activity
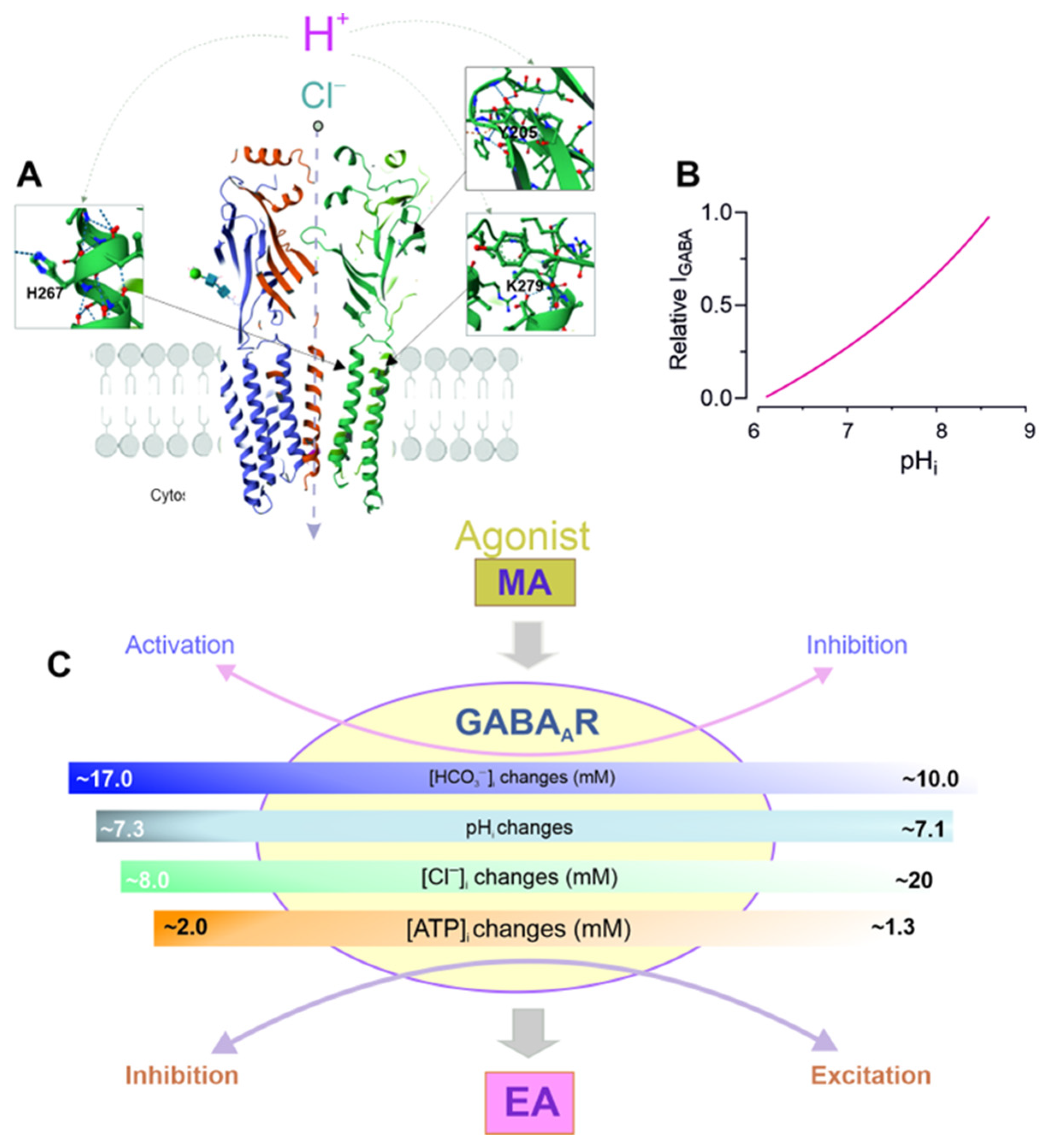
7. Role of pH in GABAAR Modulation
8. Role of β3 Subunit in pHi Changes and Seizure Activity
9. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hu, H.; Howard, R.J.; Bastolla, U.; Lindahl, E.; Delarue, M. Structural basis for allosteric transitions of a multidomain pentameric ligand-gated ion channel. Proc. Natl. Acad. Sci. USA 2020, 117, 13437–13446. [Google Scholar] [CrossRef] [PubMed]
- Petroff, J.T.; Dietzen, N.M.; Santiago-McRae, E.; Deng, B.; Washington, M.S.; Chen, L.J.; Moreland, K.T.; Deng, Z.; Rau, M.; Fitzpatricket, J.A.J.; et al. Open-channel structure of a pentameric ligand-gated ion channel reveals a mechanism of leaflet-specific phospholipid modulation. Nat. Commun. 2022, 13, 7017. [Google Scholar] [CrossRef] [PubMed]
- Gielen, M.; Corringer, P.J. The dual-gate model for pentameric ligand-gated ion channels activation and desensitization. J. Physiol. 2018, 596, 1873–1902. [Google Scholar] [CrossRef]
- Howard, R.J. Elephants in the dark: Insights and incongruities in pentameric ligand-gated ion channel models. J. Mol. Biol. 2021, 433, 167128. [Google Scholar] [CrossRef] [PubMed]
- Pless, S.A.; Sivilotti, L.G. A tale of ligands big and small: An update on how pentameric ligand-gated ion channels interact with agonists and proteins. Curr. Opin. Physiol. 2019, 2, 19–26. [Google Scholar] [CrossRef]
- Lara, C.O.; Burgos, C.F.; Moraga-Cid, G.; Carrasco, M.A.; Yévenes, G.E. Pentameric ligand-gated ion channels as pharmacological targets against chronic pain. Front. Pharmacol. 2020, 11, 167. [Google Scholar] [CrossRef]
- Goetz, T.; Arslan, A.; Wisden, W.; Wulff, P. GABA(A) receptors: Structure and function in the basal ganglia. Prog. Brain Res. 2007, 160, 21–41. [Google Scholar] [CrossRef]
- Sigel, E.; Steinmann, M.E. Structure, function, and modulation of GABA(A) receptors. J. Biol. Chem. 2012, 287, 40224–40231. [Google Scholar] [CrossRef]
- Zhu, S.; Noviello, C.M.; Teng, J.; Walsh, R.M., Jr.; Kim, J.J.; Hibbs, R.E. Structure of a human synaptic GABAA receptor. Nature 2018, 559, 67–72. [Google Scholar] [CrossRef]
- Borghese, C.M.; Wang, H.L.; McHardy, S.F.; Messing, R.O.; Trudell, J.R.; Harris, R.A.; Bertaccini, E.J. Modulation of α1β3γ2 GABAA receptors expressed in X. laevis oocytes using a propofol photoswitch tethered to the transmembrane helix. Proc. Natl. Acad. Sci. USA 2021, 118, e2008178118. [Google Scholar] [CrossRef]
- Iorio, M.T.; Vogel, F.D.; Koniuszewski, F.; Scholze, P.; Rehman, S.; Simeone, X.; Schnürch, M.; Mihovilovic, M.D.; Ernst, M. GABAA Receptor Ligands Often Interact with Binding Sites in the Transmembrane Domain and in the Extracellular Domain-Can the Promiscuity Code Be Cracked? Int. J. Mol. Sci. 2020, 21, 334. [Google Scholar] [CrossRef] [PubMed]
- Ghit, A.; Assal, D.; Al-Shami, A.S.; Hussein, D.E.E. GABAA receptors: Structure, function, pharmacology, and related disorders. J. Genet. Eng. Biotechnol. 2021, 19, 123. [Google Scholar] [CrossRef]
- Herbison, A.E.; Moenter, S.M. Depolarising and hyperpolarising actions of GABA(A) receptor activation on gonadotrophin-releasing hormone neurones: Towards an emerging consensus. J. Neuroendocrinol. 2011, 23, 557–569. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, J.V.; Richards, B.A.; Woodin, M.A. Neuronal chloride and excitability—The big impact of small changes. Curr. Opin. Neurobiol. 2017, 43, 35–42. [Google Scholar] [CrossRef]
- Staley, K.J.; Soldo, B.L.; Proctor, W.R. Ionic mechanisms of neuronal excitation by inhibitory GABAA receptors. Science 1995, 269, 977–981. [Google Scholar] [CrossRef]
- Kilb, W. When Are Depolarizing GABAergic Responses Excitatory? Front. Mol. Neurosci. 2021, 14, 747835. [Google Scholar] [CrossRef] [PubMed]
- Pressey, J.C.; de Saint-Rome, M.; Raveendran, V.A.; Woodin, M.A. Chloride transporters controlling neuronal excitability. Physiol. Rev. 2023, 103, 1095–1135. [Google Scholar] [CrossRef]
- Isomura, Y.; Sugimoto, M.; Fujiwara-Tsukamoto, Y.; Yamamoto-Muraki, S.; Yamada, J.; Fukuda, A. Synaptically activated Cl− accumulation responsible for depolarizing GABAergic responses in mature hippocampal neurons. J. Neurophysiol. 2003, 90, 2752–2756. [Google Scholar] [CrossRef]
- Jin, X.; Huguenard, J.R.; Prince, D.A. Impaired Cl− extrusion in layer V pyramidal neurons of chronically injured epileptogenic neocortex. J. Neurophysiol. 2005, 93, 2117–2126. [Google Scholar] [CrossRef]
- Kan, A.S.H.; Kusay, A.S.; Mohammadi, N.A.; Lin, S.X.N.; Liao, V.W.Y.; Lesca, G.; Souci, S.; Milh, M.; Christophersen, P.; Chebib, M.; et al. Understanding paralogous epilepsy-associated GABAA receptor variants: Clinical implications, mechanisms, and potential pitfalls. Proc. Natl. Acad. Sci. USA 2024, 121, e2413011121. [Google Scholar] [CrossRef]
- Staley, K.J.; Proctor, W.R. Modulation of mammalian dendritic GABA(A) receptor function by the kinetics of Cl− and HCO3− transport. J. Physiol. 1999, 519, 693–712. [Google Scholar] [CrossRef]
- Lombardi, A.; Jedlicka, P.; Luhmann, H.J.; Kilb, W. Interactions between Membrane Resistance, GABA-A Receptor Properties, Bicarbonate Dynamics and Cl−-Transport Shape Activity-Dependent Changes of Intracellular Cl− Concentration. Int. J. Mol. Sci. 2019, 20, 1416. [Google Scholar] [CrossRef]
- Sulis Sato, S.; Artoni, P.; Landi, S.; Cozzolino, O.; Parra, R.; Pracucci, E.; Trovato, F.; Szczurkowska, J.; Luin, S.; Arosio, D.; et al. Simultaneous two-photon imaging of intracellular chloride concentration and pH in mouse pyramidal neurons in vivo. Proc. Natl. Acad. Sci. USA 2017, 114, 8770–8779. [Google Scholar] [CrossRef]
- Kim, D.Y.; Fenoglio, K.A.; Kerrigan, J.F.; Rho, J.M. Bicarbonate contributes to GABAA receptor-mediated neuronal excitation in surgically resected human hypothalamic hamartomas. Epilepsy Res. 2009, 83, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Ruffin, V.A.; Salameh, A.I.; Boron, W.F.; Parker, M.D. Intracellular pH regulation by acid-base transporters in mammalian neurons. Front. Physiol. 2014, 5, 43. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Park, H.J.; Lee, S.; Kim, Y.H.; Choi, I. The sodium-driven chloride/bicarbonate exchanger NDCBE in rat brain is upregulated by chronic metabolic acidosis. Brain Res. 2011, 1377, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Sinning, A.; Hübner, C.A. Minireview: pH and synaptic transmission. FEBS Lett. 2013, 587, 1923–1928. [Google Scholar] [CrossRef]
- Ruusuvuori, E.; Huebner, A.K.; Kirilkin, I.; Yukin, A.Y.; Blaesse, P.; Helmy, M.; Kang, H.J.; El Muayed, M.; Hennings, J.C.; Voipio, J.; et al. Neuronal carbonic anhydrase VII provides GABAergic excitatory drive to exacerbate febrile seizures. EMBO J. 2013, 32, 2275–2286. [Google Scholar] [CrossRef]
- Wilkins, M.E.; Hosie, A.M.; Smart, T.G. Proton modulation of recombinant GABA(A) receptors: Influence of GABA concentration and the beta subunit TM2-TM3 domain. J. Physiol. 2005, 567, 365–377. [Google Scholar] [CrossRef]
- Halbhuber, L.; Achtner, C.; Luhmann, H.J.; Sinning, A.; Kilb, W. Coincident Activation of Glutamate Receptors Enhances GABAA Receptor-Induced Ionic Plasticity of the Intracellular Cl−-Concentration in Dissociated Neuronal Cultures. Front. Cell. Neurosci. 2019, 13, 497. [Google Scholar] [CrossRef]
- Field, M.; Dorovykh, V.; Thomas, P.; Smart, T.G. Physiological role for GABAA receptor desensitization in the induction of long-term potentiation at inhibitory synapses. Nat. Commun. 2021, 12, 2112. [Google Scholar] [CrossRef]
- Tia, S.; Wang, J.F.; Kotchabhakdi, N.; Vicini, S. Distinct deactivation and desensitization kinetics of recombinant GABAA receptors. Neuropharmacology 1996, 35, 1375–1382. [Google Scholar] [CrossRef]
- Stelzer, A.; Kay, A.R.; Wong, R.K. GABAA-receptor function in hippocampal cells is maintained by phosphorylation factors. Science 1988, 241, 339–341. [Google Scholar] [CrossRef] [PubMed]
- Menzikov, S.A.; Zaichenko, D.M.; Moskovtsev, A.A.; Morozov, S.G.; Kubatiev, A.A. Physiological Role of ATPase for GABAA Receptor Resensitization. Int. J. Mol. Sci. 2022, 23, 5320. [Google Scholar] [CrossRef] [PubMed]
- Glykys, J.; Mody, I. Activation of GABAA receptors: Views from outside the synaptic cleft. Neuron 2007, 56, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Lambert, N.; Grover, L. The mechanism of biphasic GABA responses. Science 1995, 269, 928–929. [Google Scholar] [CrossRef]
- Peerboom, C.; Wierenga, C.J. The postnatal GABA shift: A developmental perspective. Neurosci. Biobehav. Rev. 2021, 124, 179–192. [Google Scholar] [CrossRef]
- Chavas, J.; Marty, A. Coexistence of excitatory and inhibitory GABA synapses in the cerebellar interneuron network. J. Neurosci. 2003, 23, 2019–2031. [Google Scholar] [CrossRef]
- Backus, K.H.; Kettenmann, H.; Schachner, M. Effect of benzodiazepines and pentobarbital on the GABA-induced depolarization in cultured astrocytes. Glia 1988, 1, 132–140. [Google Scholar] [CrossRef]
- Perkins, K.L.; Wong, R.K. The depolarizing GABA response. Can. J. Physiol. Pharmacol. 1997, 75, 516–519. [Google Scholar] [CrossRef]
- Perkins, K.L.; Wong, R.K. Ionic basis of the postsynaptic depolarizing GABA response in hippocampal pyramidal cells. J. Neurophysiol. 1996, 76, 3886–3894. [Google Scholar] [CrossRef] [PubMed]
- Dallwig, R.; Deitmer, J.W.; Backus, K.H. On the mechanism of GABA-induced currents in cultured rat cortical neurons. Pflugers Arch. 1999, 437, 289–297. [Google Scholar] [CrossRef]
- Zeng, X.J.; Tietz, E.I. Role of bicarbonate ion in mediating decreased synaptic conductance in benzodiazepine tolerant hippocampal CA1 pyramidal neurons. Brain Res. 2000, 868, 202–214. [Google Scholar] [CrossRef]
- Smirnov, S.; Paalasmaa, P.; Uusisaari, M.; Voipio, J.; Kaila, K. Pharmacological isolation of the synaptic and nonsynaptic components of the GABA-mediated biphasic response in rat CA1 hippocampal pyramidal cells. J. Neurosci. 1999, 19, 9252–9260. [Google Scholar] [CrossRef]
- Bonnet, U.; Bingmann, D. GABAA-responses of CA3 neurones: Contribution of bicarbonate and of Cl−-extrusion mechanisms. Neuroreport 1995, 6, 700–704. [Google Scholar] [CrossRef] [PubMed]
- Grover, L.M.; Lambert, N.A.; Schwartzkroin, P.A.; Teyler, T.J. Role of HCO3− ions in depolarizing GABAA receptor-mediated responses in pyramidal cells of rat hippocampus. J. Neurophysiol. 1993, 69, 1541–1555. [Google Scholar] [CrossRef]
- Branchereau, P.; Cattaert, D.; Delpy, A.; Allain, A.E.; Martin, E.; Meyrand, P. Depolarizing GABA/glycine synaptic events switch from excitation to inhibition during frequency increases. Sci. Rep. 2016, 6, 21753. [Google Scholar] [CrossRef] [PubMed]
- Mason, M.J.; Mattsson, K.; Pasternack, M.; Voipio, J.; Kaila, K. Postsynaptic fall in intracellular pH and increase in surface pH caused by efflux of formate and acetate anions through GABA-gated channels in crayfish muscle fibres. Neuroscience 1990, 34, 359–368. [Google Scholar] [CrossRef]
- Kulik, A.; Nishimaru, H.; Ballanyi, K. Role of bicarbonate and chloride in GABA- and glycine-induced depolarization and [Ca2+]i rise in fetal rat motoneurons in situ. J. Neurosci. 2000, 20, 7905–7913. [Google Scholar] [CrossRef]
- Chen, J.C.; Chesler, M.A. Bicarbonate-dependent increase in extracellular pH mediated by GABAA receptors in turtle cerebellum. Neurosci. Lett. 1990, 116, 130–135. [Google Scholar] [CrossRef]
- Chesler, M.; Chen, J.C. Alkaline extracellular pH shifts generated by two transmitter-dependent mechanisms. Can. J. Physiol. Pharmacol. 1992, 70, 286–292. [Google Scholar] [CrossRef]
- Lückermann, M.; Trapp, S.; Ballanyi, K. GABA- and glycine-mediated fall of intracellular pH in rat medullary neurons in situ. J. Neurophysiol. 1997, 77, 1844–1852. [Google Scholar] [CrossRef][Green Version]
- Gulledge, A.T.; Stuart, G.J. Excitatory actions of GABA in the cortex. Neuron 2003, 37, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.F.; Xie, M.J.; Zhou, M. Bicarbonate efflux via GABA(A) receptors depolarizes membrane potential and inhibits two-pore domain potassium channels of astrocytes in rat hippocampal slices. Glia 2012, 60, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Kaila, K.; Voipio, J.; Paalasmaa, P.; Pasternack, M.; Deisz, R.A. The role of bicarbonate in GABAA receptor-mediated IPSPs of rat neocortical neurones. J. Physiol. 1993, 464, 273–289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Jian, L.; Yao, D.; Rao, B.; Xia, Y.; Hu, K.; Li, S.; Shen, Y.; Cao, M.; Qin, A.; et al. The structural basis of the pH-homeostasis mediated by the Cl−/HCO3− exchanger, AE2. Nat. Commun. 2023, 14, 1812. [Google Scholar] [CrossRef]
- Schwiening, C.J.; Boron, W.F. Regulation of intracellular pH in pyramidal neurones from the rat hippocampus by Na+-dependent Cl−-HCO3− exchange. J. Physiol. 1994, 475, 59–67. [Google Scholar] [CrossRef]
- Bouyer, P.; Bradley, S.R.; Zhao, J.; Wang, W.; Richerson, G.B.; Boron, W.F. Effect of extracellular acid-base disturbances on the intracellular pH of neurones cultured from rat medullary raphe or hippocampus. J. Physiol. 2004, 559, 85–101. [Google Scholar] [CrossRef]
- Bouyer, P.G.; Salameh, A.I.; Zhou, Y.; Kolba, T.N.; Boron, W.F. Effects of extracellular metabolic acidosis and out-of-equilibrium CO2/HCO3− solutions on intracellular pH in cultured rat hippocampal neurons. Front. Physiol. 2024, 15, 1434359. [Google Scholar] [CrossRef]
- Hamm, L.L.; Nakhoul, N.; Hering-Smith, K.S. Acid-Base Homeostasis. Clin. J. Am. Soc. Nephrol. 2015, 10, 2232–2242. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, X.; Sun, S.X. Hydrogen, Bicarbonate, and Their Associated Exchangers in Cell Volume Regulation. Front. Cell Dev. Biol. 2021, 9, 683686. [Google Scholar] [CrossRef]
- Casey, J.R.; Sly, W.S.; Shah, G.N.; Alvarez, B.V. Bicarbonate homeostasis in excitable tissues: Role of AE3 Cl−/HCO3− exchanger and carbonic anhydrase XIV interaction. Am. J. Physiol. Cell Physiol. 2009, 297, 1091–1102. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, J.; Chen, L.M. Structure and Function of SLC4 Family Transporters. Front. Physiol. 2015, 6, 355. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Z.; Yano, H.; Nagashima, K.; Seino, S. The Na+-driven Cl−/HCO3− exchanger. Cloning, tissue distribution, and functional characterization. J. Biol. Chem. 2000, 275, 35486–35490. [Google Scholar] [CrossRef] [PubMed]
- Deitmer, J.W.; Theparambil, S.M.; Ruminot, I.; Becker, H.M. The role of membrane acid/base transporters and carbonic anhydrases for cellular pH and metabolic processes. Front. Neurosci. 2015, 8, 430. [Google Scholar] [CrossRef] [PubMed]
- Shcheynikov, N.; Son, A.; Hong, J.H.; Yamazaki, O.; Ohana, E.; Kurtz, I.; Shin, D.M.; Muallem, S. Intracellular Cl− as a signaling ion that potently regulates Na+/HCO3− transporters. Proc. Natl. Acad. Sci. USA 2015, 112, 329–337. [Google Scholar] [CrossRef]
- Hübner, C.A.; Holthoff, K. Anion transport and GABA signaling. Front. Cell. Neurosci. 2013, 7, 177. [Google Scholar] [CrossRef]
- Roos, A.; Boron, W.F. Intracellular pH. Physiol. Rev. 1981, 61, 296–434. [Google Scholar] [CrossRef]
- Ruusuvuori, E.; Li, H.; Huttu, K.; Palva, J.M.; Smirnov, S.; Rivera, C.; Kaila, K.; Voipio, J. Carbonic anhydrase isoform VII acts as a molecular switch in the development of synchronous gamma-frequency firing of hippocampal CA1 pyramidal cells. J. Neurosci. 2004, 24, 2699–2707. [Google Scholar] [CrossRef]
- Pasternack, M.; Smirnov, S.; Kaila, K. Proton modulation of functionally distinct GABAA receptors in acutely isolated pyramidal neurons of rat hippocampus. Neuropharmacology 1996, 35, 1279–1288. [Google Scholar] [CrossRef]
- Deitmer, J.W.; Theparambil, S.M.; Ruminot, I.; Noor, S.I.; Becker, H.M. Energy Dynamics in the Brain: Contributions of Astrocytes to Metabolism and pH Homeostasis. Front. Neurosci. 2019, 13, 1301. [Google Scholar] [CrossRef] [PubMed]
- Blumenfeld, H. What is a seizure network? Long-range network consequences of focal seizures. Adv. Exp. Med. Biol. 2014, 813, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Menzikov, S.A.; Zaichenko, D.M.; Moskovtsev, A.A.; Morozov, S.G.; Kubatiev, A.A. Zinc Inhibits the GABAAR/ATPase during Postnatal Rat Development: The Role of Cysteine Residue. Int. J. Mol. Sci. 2023, 24, 2764. [Google Scholar] [CrossRef] [PubMed]
- Halverson, H.E.; Kim, J.; Freeman, J.H. Dynamic Changes in Local Activity and Network Interactions among the Anterior Cingulate, Amygdala, and Cerebellum during Associative Learning. J. Neurosci. 2023, 43, 8385–8402. [Google Scholar] [CrossRef]
- Ferrini, F.; Perez-Sanchez, J.; Ferland, S.; Lorenzo, L.E.; Godin, A.G.; Plasencia-Fernandez, I.; Cottet, M.; Castonguay, A.; Wang, F.; Salio, C.; et al. Differential chloride homeostasis in the spinal dorsal horn locally shapes synaptic metaplasticity and modality-specific sensitization. Nat. Commun. 2020, 11, 3935. [Google Scholar] [CrossRef]
- Schulte, J.T.; Wierenga, C.J.; Bruining, H. Chloride transporters and GABA polarity in developmental, neurological and psychiatric conditions. Neurosci. Biobehav. Rev. 2018, 90, 260–271. [Google Scholar] [CrossRef]
- Schmidt, T.; Ghaffarian, N.; Philippot, C.; Seifert, G.; Steinhäuser, C.; Pape, H.C.; Blaesse, P. Differential regulation of chloride homeostasis and GABAergic transmission in the thalamus. Sci. Rep. 2018, 8, 13929. [Google Scholar] [CrossRef]
- Hartmann, A.M.; Tesch, D.; Nothwang, H.G.; Bininda-Emonds, O.R. Evolution of the cation chloride cotransporter family: Ancient origins, gene losses, and subfunctionalization through duplication. Mol. Biol. Evol. 2014, 31, 434–447. [Google Scholar] [CrossRef]
- Hartmann, A.M.; Nothwang, H.G. Molecular and evolutionary insights into the structural organization of cation chloride cotransporters. Front. Cell. Neurosci. 2015, 8, 470. [Google Scholar] [CrossRef]
- Chamma, I.; Chevy, Q.; Poncer, J.C.; Lévi, S. Role of the neuronal K-Cl co-transporter KCC2 in inhibitory and excitatory neurotransmission. Front. Cell. Neurosci. 2012, 21, 5. [Google Scholar] [CrossRef]
- Khirug, S.; Huttu, K.; Ludwig, A.; Smirnov, S.; Voipio, J.; Rivera, C.; Kaila, K.; Khiroug, L. Distinct properties of functional KCC2 expression in immature mouse hippocampal neurons in culture and in acute slices. Eur. J. Neurosci. 2005, 21, 899–904. [Google Scholar] [CrossRef]
- He, Y.; Ji, W.; Huang, H. Effect of the cation-chloride cotransporter inhibitor furosemide in a rat model of postoperative pain. Pain Med. 2011, 12, 1427–1434. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kakazu, Y.; Uchida, S.; Nakagawa, T.; Akaike, N.; Nabekura, J. Reversibility and cation selectivity of the K+-Cl− cotransport in rat central neurons. J. Neurophysiol. 2000, 84, 281–288. [Google Scholar] [CrossRef]
- Yelhekar, T.D.; Druzin, M.; Johansson, S. Contribution of Resting Conductance, GABAA-Receptor Mediated Miniature Synaptic Currents and Neurosteroid to Chloride Homeostasis in Central Neurons. eNeuro 2017, 4, ENEURO.0019-17.2017. [Google Scholar] [CrossRef] [PubMed]
- McMoneagle, E.; Zhou, J.; Zhang, S.; Huang, W.; Josiah, S.S.; Ding, K.; Wang, Y.; Zhang, J. Neuronal K+-Cl− cotransporter KCC2 as a promising drug target for epilepsy treatment. Acta Pharmacol. Sin. 2024, 45, 1–22. [Google Scholar] [CrossRef]
- Prael, F.J., III; Kim, K.; Du, Y.; Spitznagel, B.D.; Sulikowski, G.A.; Delpire, E.; Weaver, C.D. Discovery of Small Molecule KCC2 Potentiators Which Attenuate In Vitro Seizure-Like Activity in Cultured Neurons. Front. Cell Dev. Biol. 2022, 10, 912812. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.C.; Lytle, C.; Zhu, T.T.; Payne, J.A.; Benz, E., Jr.; Forbush, B. Molecular cloning and functional expression of the bumetanide-sensitive Na-K-Cl cotransporter. Proc. Natl. Acad. Sci. USA 1994, 91, 2201–2205. [Google Scholar] [CrossRef]
- Wang, C.; Shimizu-Okabe, C.; Watanabe, K.; Okabe, A.; Matsuzaki, H.; Ogawa, T.; Mori, N.; Fukuda, A.; Sato, K. Developmental changes in KCC1, KCC2, and NKCC1 mRNA expressions in the rat brain. Brain Res. Dev. Brain Res. 2002, 139, 59–66. [Google Scholar] [CrossRef]
- Williams, J.R.; Payne, J.A. Cation transport by the neuronal K+-Cl− cotransporter KCC2: Thermodynamics and kinetics of alternate transport modes. Am. J. Physiol. Cell Physiol. 2004, 287, C919–C931. [Google Scholar] [CrossRef]
- Zhu, L.; Lovinger, D.; Delpire, E. Cortical neurons lacking KCC2 expression show impaired regulation of intracellular chloride. J. Neurophysiol. 2005, 93, 1557–1568. [Google Scholar] [CrossRef]
- Doyon, N.; Vinay, L.; Prescott, S.A.; De Koninck, Y. Chloride Regulation: A Dynamic Equilibrium Crucial for Synaptic Inhibition. Neuron 2016, 89, 1157–1172. [Google Scholar] [CrossRef]
- DeFazio, R.A.; Keros, S.; Quick, M.W.; Hablitz, J.J. Potassium-coupled chloride cotransport controls intracellular chloride in rat neocortical pyramidal neurons. J. Neurosci. 2000, 20, 8069–8076. [Google Scholar] [CrossRef]
- Sun, D.; Murali, S.G. Stimulation of Na+-K+-2Cl− cotransporter in neuronal cells by excitatory neurotransmitter glutamate. Am. J. Physiol. 1998, 275, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Achilles, K.; Okabe, A.; Ikeda, M.; Shimizu-Okabe, C.; Yamada, J.; Fukuda, A.; Luhmann, H.J.; Kilb, W. Kinetic properties of Cl− uptake mediated by Na+-dependent K+-2Cl cotransport in immature rat neocortical neurons. J. Neurosci. 2007, 27, 8616–8627. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Islas, C.; Chub, N.; Wenner, P. NKCC1 and AE3 appear to accumulate chloride in embryonic motoneurons. J. Neurophysiol. 2009, 101, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, U.; Druzin, M.; Johansson, S. Cl− concentration changes and desensitization of GABA(A) and glycine receptors. J. Gen. Physiol. 2011, 138, 609–626. [Google Scholar] [CrossRef]
- Chen, M.; Wang, J.; Jiang, J.; Zheng, X.; Justice, N.J.; Wang, K.; Ran, X.; Li, Y.; Huo, Q.; Zhang, J.; et al. APP modulates KCC2 expression and function in hippocampal GABAergic inhibition. eLife 2017, 6, e20142. [Google Scholar] [CrossRef]
- Deisz, R.A.; Lehmann, T.N.; Horn, P.; Dehnicke, C.; Nitsch, R. Components of neuronal chloride transport in rat and human neocortex. J. Physiol. 2011, 589, 1317–1347. [Google Scholar] [CrossRef]
- Brickley, S.G.; Cull-Candy, S.G.; Farrant, M. Single-channel properties of synaptic and extrasynaptic GABAA receptors suggest differential targeting of receptor subtypes. J. Neurosci. 1999, 19, 2960–2973. [Google Scholar] [CrossRef]
- Sallard, E.; Letourneur, D.; Legendre, P. Electrophysiology of ionotropic GABA receptors. Cell. Mol. Life Sci. 2021, 78, 5341–5370. [Google Scholar] [CrossRef]
- Bianchi, M.T.; Macdonald, R.L. Slow phases of GABA(A) receptor desensitization: Structural determinants and possible relevance for synaptic function. J. Physiol. 2002, 544, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Gielen, M.; Barilone, N.; Corringer, P.J. The desensitization pathway of GABAA receptors, one subunit at a time. Nat. Commun. 2020, 11, 5369. [Google Scholar] [CrossRef]
- Böhm, S.K.; Khitin, L.M.; Grady, E.F.; Aponte, G.; Payan, D.G.; Bunnett, N.W. Mechanisms of desensitization and resensitization of proteinase-activated receptor-2. J. Biol. Chem. 1996, 271, 22003–22016. [Google Scholar] [CrossRef]
- Kaila, K.; Saarikoski, J.; Voipio, J. Mechanism of action of GABA on intracellular pH and on surface pH in crayfish muscle fibres. J. Physiol. 1990, 427, 241–260. [Google Scholar] [CrossRef] [PubMed]
- Barberis, A.; Mozrzymas, J.W.; Ortinski, P.I.; Vicini, S. Desensitization and binding properties determine distinct alpha1beta2gamma2 and alpha3beta2gamma2 GABA(A) receptor-channel kinetic behavior. Eur. J. Neurosci. 2007, 5, 2726–2740. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Ghansah, E.; Chen, Y.; Ye, J.; Weiss, D.S. Desensitization mechanism of GABA receptors revealed by single oocyte binding and receptor function. J. Neurosci. 2002, 22, 7982–7990. [Google Scholar] [CrossRef]
- Terejko, K.; Michałowski, M.A.; Iżykowska, I.; Dominik, A.; Brzóstowicz, A.; Mozrzymas, J.W. Mutations at the M2 and M3 Transmembrane Helices of the GABAARs α1 and β2 Subunits Affect Primarily Late Gating Transitions Including Opening/Closing and Desensitization. ACS Chem. Neurosci. 2021, 12, 2421–2436. [Google Scholar] [CrossRef]
- Huntsman, M.M.; Huguenard, J.R. Fast IPSCs in rat thalamic reticular nucleus require the GABAA receptor beta1 subunit. J. Physiol. 2006, 572, 459–475. [Google Scholar] [CrossRef]
- Rotaru, D.C.; Olezene, C.; Miyamae, T.; Povysheva, N.V.; Zaitsev, A.V.; Lewis, D.A.; Gonzalez-Burgos, G. Functional properties of GABA synaptic inputs onto GABA neurons in monkey prefrontal cortex. J. Neurophysiol. 2015, 113, 1850–1861. [Google Scholar] [CrossRef][Green Version]
- Jones, M.V.; Westbrook, G.L. The impact of receptor desensitization on fast synaptic transmission. Trends Neurosci. 1996, 19, 96–101. [Google Scholar] [CrossRef]
- Pierce, S.R.; Germann, A.L.; Evers, A.S.; Steinbach, J.H.; Akk, G. Reduced Activation of the Synaptic-Type GABAA Receptor Following Prolonged Exposure to Low Concentrations of Agonists: Relationship between Tonic Activity and Desensitization. Mol. Pharmacol. 2020, 98, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Gravielle, M.C.C. Regulation of GABAA receptors by prolonged exposure to endogenous and exogenous ligands. Neurochem. Int. 2018, 118, 96–104. [Google Scholar] [CrossRef]
- Kang, Y.; Saito, M.; Toyoda, H. Molecular and Regulatory Mechanisms of Desensitization and Resensitization of GABAA Receptors with a Special Reference to Propofol/Barbiturate. Int. J. Mol. Sci. 2020, 21, 563. [Google Scholar] [CrossRef]
- Chen, L.; Wang, H.; Vicini, S.; Olsen, R.W. The γ-aminobutyric acid type A (GABAA) receptor-associated protein (GABARAP) promotes GABAA receptor clustering and modulates the channel kinetics. Proc. Natl. Acad. Sci. USA 2000, 97, 11557–11562. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, H.; Saito, M.; Sato, H.; Tanaka, T.; Ogawa, T.; Yatani, H.; Kawano, T.; Kanematsu, T.; Hirata, M.; Kang, Y. Enhanced desensitization followed by unusual resensitization in GABAA receptors in phospholipase C-related catalytically inactive protein-1/2 double-knockout mice. Pflugers Arch. 2015, 467, 267–284. [Google Scholar] [CrossRef]
- Mozrzymas, J.W.; Cherubini, E. Changes in intracellular calcium concentration affect desensitization of GABAA receptors in acutely dissociated P2-P6 rat hippocampal neurons. J. Neurophysiol. 1998, 79, 1321–1328. [Google Scholar] [CrossRef] [PubMed]
- Pytel, M.; Mercik, K.; Mozrzymas, J.W. The voltage dependence of GABAA receptor gating depends on extracellular pH. Neuroreport 2005, 16, 1951–1954. [Google Scholar] [CrossRef][Green Version]
- Mozrzymas, J.W.; Zarnowska, E.D.; Pytel, M.; Mercik, K. Modulation of GABA(A) receptors by hydrogen ions reveals synaptic GABA transient and a crucial role of the desensitization process. J. Neurosci. 2003, 23, 7981–7992. [Google Scholar] [CrossRef]
- Mercik, K.; Pytel, M.; Cherubini, E.; Mozrzymas, J.W. Effect of extracellular pH on recombinant alpha1beta2gamma2 and alpha1beta2 GABAA receptors. Neuropharmacology 2006, 51, 305–314. [Google Scholar] [CrossRef]
- Gyenes, M.; Farrant, M.; Farb, D.H. “Run-down” of gamma-aminobutyric acid A receptor function during whole-cell recording: A possible role for phosphorylation. Mol. Pharmacol. 1988, 34, 719–723. [Google Scholar] [CrossRef]
- Shirasaki, T.; Aibara, K.; Akaike, N. Direct modulation of GABAA receptor by intracellular ATP in dissociated nucleus tractus solitarii neurones of rat. J. Physiol. 1992, 449, 551–572. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, D.J.; Simmons, M.A. gamma-Aminobutyric acid A receptor function is modulated by cyclic GMP. Brain Res. Bull. 1995, 37, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Amico, C.; Cupello, A.; Fossati, C.; Robello, M. Involvement of phosphatase activities in the run-down of GABA(A) receptor function in rat cerebellar granule cells in culture. Neuroscience 1998, 84, 529–535. [Google Scholar] [CrossRef]
- Harata, N.; Wu, J.; Ishibashi, H.; Ono, K.; Akaike, N. Run-down of the GABAA response under experimental ischaemia in acutely dissociated CA1 pyramidal neurones of the rat. J. Physiol. 1997, 500, 673–688. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Yang, K.; Zheng, C.; Liu, Q.; Chang, Y.; Kerrigan, J.F.; Wu, J. Functional rundown of gamma-aminobutyric acid(A) receptors in human hypothalamic hamartomas. Ann. Neurol. 2011, 69, 664–672. [Google Scholar] [CrossRef]
- Huang, R.Q.; Dillon, G.H. Maintenance of recombinant type A gamma-aminobutyric acid receptor function: Role of protein tyrosine phosphorylation and calcineurin. J. Pharmacol. Exp. Ther. 1998, 286, 243–255. [Google Scholar] [CrossRef]
- Rapallino, M.V.; Cupello, A.; Hydén, H. An electrogenic ionic pump derived from an ionotropic receptor: Assessment of a candidate. Cell. Mol. Neurobiol. 1999, 19, 681–690. [Google Scholar] [CrossRef]
- Katchman, A.N.; Vicini, S.; Hershkowitz, N. Mechanism of early anoxia-induced suppression of the GABAA-mediated inhibitory postsynaptic current. J. Neurophysiol. 1994, 71, 1128–1138. [Google Scholar] [CrossRef]
- Lehnertz, K.; Bröhl, T.; Wrede, R.V. Epileptic-network-based prediction and control of seizures in humans. Neurobiol. Dis. 2023, 181, 106098. [Google Scholar] [CrossRef]
- Khambhati, A.N.; Chang, E.F.; Baud, M.O.; Rao, V.R. Hippocampal network activity forecasts epileptic seizures. Nat. Med. 2024, 30, 2787–2790. [Google Scholar] [CrossRef]
- Pedersen, M.; Pardoe, H.; Mito, R.; Sethi, M.; Vaughan, D.N.; Carney, P.W.; Jackson, G.D. Brain network changes after the first seizure: An insight into medication response? Brain Commun. 2024, 6, fcae328. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, P.N.; Wait, S.D.; Lekovic, G.P.; Rekate, H.L.; Kerrigan, J.F. Firing behavior and network activity of single neurons in human epileptic hypothalamic hamartoma. Front. Neurol. 2013, 4, 210. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara-Tsukamoto, Y.; Isomura, Y.; Takada, M. Comparable GABAergic mechanisms of hippocampal seizurelike activity in posttetanic and low-Mg2+ conditions. J. Neurophysiol. 2006, 95, 2013–2019. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Gao, M.; Shen, J.X.; Qiu, S.F.; Kerrigan, J.F. Mechanisms of intrinsic epileptogenesis in human gelastic seizures with hypothalamic hamartoma. CNS Neurosci. Ther. 2015, 21, 104–111. [Google Scholar] [CrossRef]
- Giacopelli, G.; Tegolo, D.; Migliore, M. The role of network connectivity on epileptiform activity. Sci. Rep. 2021, 11, 20792. [Google Scholar] [CrossRef]
- Sinha, N.; Joshi, R.B.; Sandhu, M.R.S.; Netoff, T.I.; Zaveri, H.P.; Lehnertz, K. Perspectives on Understanding Aberrant Brain Networks in Epilepsy. Front. Netw. Physiol. 2022, 2, 868092. [Google Scholar] [CrossRef]
- Cifelli, P.; Palma, E.; Roseti, C.; Verlengia, G.; Simonato, M. Changes in the sensitivity of GABAA current rundown to drug treatments in a model of temporal lobe epilepsy. Front. Cell. Neurosci. 2013, 7, 108. [Google Scholar] [CrossRef]
- Sakimoto, Y.; Oo, P.M.; Goshima, M.; Kanehisa, I.; Tsukada, Y.; Mitsushima, D. Significance of GABAA Receptor for Cognitive Function and Hippocampal Pathology. Int. J. Mol. Sci. 2021, 22, 12456. [Google Scholar] [CrossRef] [PubMed]
- Mazzuferi, M.; Palma, E.; Martinello, K.; Maiolino, F.; Roseti, C.; Fucile, S.; Fabene, P.F.; Schio, F.; Pellitteri, M.; Sperk, G.; et al. Enhancement of GABA(A)-current run-down in the hippocampus occurs at the first spontaneous seizure in a model of temporal lobe epilepsy. Proc. Natl. Acad. Sci. USA 2010, 107, 3180–3185. [Google Scholar] [CrossRef]
- Galanopoulou, A.S. GABA(A) receptors in normal development and seizures: Friends or foes? Curr. Neuropharmacol. 2008, 6, 1–20. [Google Scholar] [CrossRef]
- Kim, Y.S.; Yoon, B.E. Altered GABAergic Signaling in Brain Disease at Various Stages of Life. Exp. Neurobiol. 2017, 26, 122–131. [Google Scholar] [CrossRef]
- Fujiwara-Tsukamoto, Y.; Isomura, Y.; Imanishi, M.; Fukai, T.; Takada, M. Distinct types of ionic modulation of GABA actions in pyramidal cells and interneurons during electrical induction of hippocampal seizure-like network activity. Eur. J. Neurosci. 2007, 25, 2713–2725. [Google Scholar] [CrossRef]
- Lillis, K.P.; Kramer, M.A.; Mertz, J.; Staley, K.J.; White, J.A. Pyramidal cells accumulate chloride at seizure onset. Neurobiol. Dis. 2012, 47, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Ellender, T.J.; Raimondo, J.V.; Irkle, A.; Lamsa, K.P.; Akerman, C.J. Excitatory effects of parvalbumin-expressing interneurons maintain hippocampal epileptiform activity via synchronous afterdischarges. J. Neurosci. 2014, 34, 15208–15222. [Google Scholar] [CrossRef] [PubMed]
- D’Antuono, M.; Louvel, J.; Köhling, R.; Mattia, D.; Bernasconi, A.; Olivier, A.; Turak, B.; Devaux, A.; Pumain, R.; Avoli, M. GABAA receptor-dependent synchronization leads to ictogenesis in the human dysplastic cortex. Brain 2004, 127, 1626–1640. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.Q.; Saggau, P.; Stringer, J.L. Activity-dependent intracellular acidification correlates with the duration of seizure activity. J. Neurosci. 2000, 20, 1290–1296. [Google Scholar] [CrossRef]
- Raimondo, J.V.; Joyce, B.; Kay, L.; Schlagheck, T.; Newey, S.E.; Srinivas, S.; Akerman, C.J. A genetically-encoded chloride and pH sensor for dissociating ion dynamics in the nervous system. Front. Cell. Neurosci. 2013, 7, 202. [Google Scholar] [CrossRef]
- Perez Velazquez, J.L. Bicarbonate-dependent depolarizing potentials in pyramidal cells and interneurons during epileptiform activity. Eur. J. Neurosci. 2003, 18, 1337–1342. [Google Scholar] [CrossRef]
- Magheru, C.; Magheru, S.; Coltau, M.; Hoza, A.; Moldovan, C.; Sachelarie, L.; Gradinaru, I.; Hurjui, L.L.; Marc, F.; Farcas, D.M. Antiepileptic Drugs and Their Dual Mechanism of Action on Carbonic Anhydrase. J. Clin. Med. 2022, 11, 2614. [Google Scholar] [CrossRef]
- Poggetti, V.; Salerno, S.; Baglini, E.; Barresi, E.; Da Settimo, F.; Taliani, S. Carbonic Anhydrase Activators for Neurodegeneration: An Overview. Molecules 2022, 27, 2544. [Google Scholar] [CrossRef]
- Liu, R.; Wang, J.; Liang, S.; Zhang, G.; Yang, X. Role of NKCC1 and KCC2 in Epilepsy: From Expression to Function. Front. Neurol. 2020, 10, 1407. [Google Scholar] [CrossRef] [PubMed]
- Ciccone, L.; Cerri, C.; Nencetti, S.; Orlandini, E. Carbonic Anhydrase Inhibitors and Epilepsy: State of the Art and Future Perspectives. Molecules 2021, 26, 6380. [Google Scholar] [CrossRef] [PubMed]
- Pracucci, E.; Graham, R.T.; Alberio, L.; Nardi, G.; Cozzolino, O.; Pillai, V.; Pasquini, G.; Saieva, L.; Walsh, D.; Landi, S.; et al. Daily rhythm in cortical chloride homeostasis underpins functional changes in visual cortex excitability. Nat. Commun. 2023, 14, 7108. [Google Scholar] [CrossRef]
- Huberfeld, G.; Wittner, L.; Clemenceau, S.; Baulac, M.; Kaila, K.; Miles, R.; Rivera, C. Perturbed chloride homeostasis and GABAergic signaling in human temporal lobe epilepsy. J. Neurosci. 2007, 27, 9866–9873. [Google Scholar] [CrossRef] [PubMed]
- Briggs, S.W.; Galanopoulou, A.S. Altered GABA signaling in early life epilepsies. Neural. Plast. 2011, 2011, 527605. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Chen, Z. Double-edged GABAergic synaptic transmission in seizures: The importance of chloride plasticity. Brain Res. 2018, 1701, 126–136. [Google Scholar] [CrossRef]
- Elverson, K.; Freeman, S.; Manson, F.; Warwicker, J. Computational Investigation of Mechanisms for pH Modulation of Human Chloride Channels. Molecules 2023, 28, 5753. [Google Scholar] [CrossRef]
- Călin, A.; Waseem, T.; Raimondo, J.V.; Newey, S.E.; Akerman, C.J. A genetically targeted ion sensor reveals distinct seizure-related chloride and pH dynamics in GABAergic interneuron populations. iScience 2023, 26, 106363. [Google Scholar] [CrossRef]
- Benarroch, E.E. GABAA receptor heterogeneity, function, and implications for epilepsy. Neurology 2007, 20, 612–614. [Google Scholar] [CrossRef]
- Boron, W.F. Intracellular pH Regulation. In Membrane Transport Processes in Organized Systems; Andreoli, T.E., Hoffman, J.F., Fanestil, D.D., Schultz, S.G., Eds.; Springer: Boston, MA, USA, 1987. [Google Scholar] [CrossRef]
- Huang, R.Q.; Dillon, G.H. Effect of extracellular pH on GABA-activated current in rat recombinant receptors and thin hypothalamic slices. J. Neurophysiol. 1999, 82, 1233–1243. [Google Scholar] [CrossRef]
- Pasternack, M.; Bountra, C.; Voipio, J.; Kaila, K. Influence of extracellular and intracellular pH on GABA-gated chloride conductance in crayfish muscle fibres. Neuroscience 1992, 47, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Limon, A.; Delbruck, E.; Yassine, A.; Pandya, D.; Myers, R.M.; Barchas, J.D.; Lee, F.; Schatzberg; Watson, S.J.; Akil, H.; et al. Electrophysiological evaluation of extracellular spermine and alkaline pH on synaptic human GABAA receptors. Transl. Psychiatry 2019, 9, 218. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.Q.; Chen, Z.; Dillon, G.H. Molecular basis for modulation of recombinant alpha1beta2gamma2 GABAA receptors by protons. J. Neurophysiol. 2004, 92, 883–894. [Google Scholar] [CrossRef]
- Chen, Z.L.; Huang, R.Q. Extracellular pH modulates GABAergic neurotransmission in rat hypothalamus. Neuroscience 2014, 271, 64–76. [Google Scholar] [CrossRef]
- Wang, M.D.; Rahman, M.; Zhu, D. Protons inhibit Cl− conductance by direct or allosteric interaction with the GABA-binding site in the rat recombinant alpha1beta2gamma2L and alpha1beta2 GABAA receptor. Eur. J. Pharmacol. 2005, 528, 1–6. [Google Scholar] [CrossRef]
- Zhai, I.J.; Peoples, R.W.; Li, C. Proton inhibition of GABA-activated current in rat primary sensory neurons. Pflugers Arch. 1998, 435, 539–545. [Google Scholar] [CrossRef]
- Kisiel, M.; Jatczak-Śliwa, M.; Mozrzymas, J.W. Protons modulate gating of recombinant α1β2γ2 GABAA receptor by affecting desensitization and opening transitions. Neuropharmacology 2019, 146, 300–315. [Google Scholar] [CrossRef]
- Krishek, B.J.; Smart, T.G. Proton sensitivity of rat cerebellar granule cell GABAA receptors: Dependence on neuronal development. J. Physiol. 2001, 530, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Robello, M.; Baldelli, P.; Cupello, A. Modulation by extracellular pH of the activty of GABAA receptors on rat cerebellum granule cells. Neuroscience 1994, 61, 833–837. [Google Scholar] [CrossRef]
- Zhou, C.; Xiao, C.; Deng, C.; Ye, J.H. Extracellular proton modulates GABAergic synaptic transmission in rat hippocampal CA3 neurons. Brain Res. 2007, 1145, 213–220. [Google Scholar] [CrossRef]
- Wilkins, M.E.; Hosie, A.M.; Smart, T.G. Identification of a beta subunit TM2 residue mediating proton modulation of GABA type A receptors. J. Neurosci. 2002, 22, 5328–5333. [Google Scholar] [CrossRef] [PubMed]
- Garifulina, A.; Friesacher, T.; Stadler, M.; Zangerl-Plessl, E.M.; Ernst, M.; Stary-Weinzinger, A.; Willam, A.; Hering, S. β subunits of GABAA receptors form proton-gated chloride channels: Insights into the molecular basis. Commun. Biol. 2022, 5, 784. [Google Scholar] [CrossRef] [PubMed]
- Connolly, C.N.; Wooltorton, J.R.; Smart, T.G.; Moss, S.J. Subcellular localization of gamma-aminobutyric acid type A receptors is determined by receptor beta subunits. Proc. Natl. Acad. Sci. USA 1996, 93, 9899–9904. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.L.; Timmermann, D.B.; Johansen, T.H.; Schousboe, A.; Varming, T.; Ahring, P.K. The beta subunit determines the ion selectivity of the GABAA receptor. J. Biol. Chem. 2002, 277, 41438–41447. [Google Scholar] [CrossRef]
- Nguyen, Q.A.; Nicoll, R.A. The GABAA Receptor β Subunit is Required for Inhibitory Transmission. Neuron 2018, 98, 718–725. [Google Scholar] [CrossRef]
- Hernandez, C.C.; Zhang, Y.; Hu, N.; Shen, D.; Shen, W.; Liu, X.; Kong, W.; Jiang, Y.; Macdonald, R.L. GABA A Receptor Coupling Junction and Pore GABRB3 Mutations are Linked to Early-Onset Epileptic Encephalopathy. Sci. Rep. 2017, 7, 15903. [Google Scholar] [CrossRef]
- Hörtnagl, H.; Tasan, R.O.; Wieselthaler, A.; Kirchmair, E.; Sieghart, W.; Sperk, G. Patterns of mRNA and protein expression for 12 GABAA receptor subunits in the mouse brain. Neuroscience 2013, 236, 345–372. [Google Scholar] [CrossRef]
- Lin, S.X.N.; Ahring, P.K.; Keramidas, A.; Liao, V.W.Y.; Møller, R.S.; Chebib, M.; Absalom, N.L. Correlations of receptor desensitization of gain-of-function GABRB3 variants with clinical severity. Brain 2024, 147, 224–239. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menzikov, S.A.; Zaichenko, D.M.; Moskovtsev, A.A.; Morozov, S.G.; Kubatiev, A.A. Massive Activation of GABAA Receptors: Rundown, Ionic and Neurodegenerative Consequences. Biomolecules 2025, 15, 1003. https://doi.org/10.3390/biom15071003
Menzikov SA, Zaichenko DM, Moskovtsev AA, Morozov SG, Kubatiev AA. Massive Activation of GABAA Receptors: Rundown, Ionic and Neurodegenerative Consequences. Biomolecules. 2025; 15(7):1003. https://doi.org/10.3390/biom15071003
Chicago/Turabian StyleMenzikov, Sergey A., Danila M. Zaichenko, Aleksey A. Moskovtsev, Sergey G. Morozov, and Aslan A. Kubatiev. 2025. "Massive Activation of GABAA Receptors: Rundown, Ionic and Neurodegenerative Consequences" Biomolecules 15, no. 7: 1003. https://doi.org/10.3390/biom15071003
APA StyleMenzikov, S. A., Zaichenko, D. M., Moskovtsev, A. A., Morozov, S. G., & Kubatiev, A. A. (2025). Massive Activation of GABAA Receptors: Rundown, Ionic and Neurodegenerative Consequences. Biomolecules, 15(7), 1003. https://doi.org/10.3390/biom15071003





