Platinum Nanoparticles Loaded in Polydopamine-Modified Porous Coordination Network-224 with Peroxidase-Like Activity for Sensitive Glutathione Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Polydopamine-Modified PCN-224 Loaded Pt Nanoparticles
2.3. Characterization of Catalytic Activity of PCN-224-PDA-Pt
2.4. Hemolysis Experiment
2.5. Study of Catalytic Kinetics
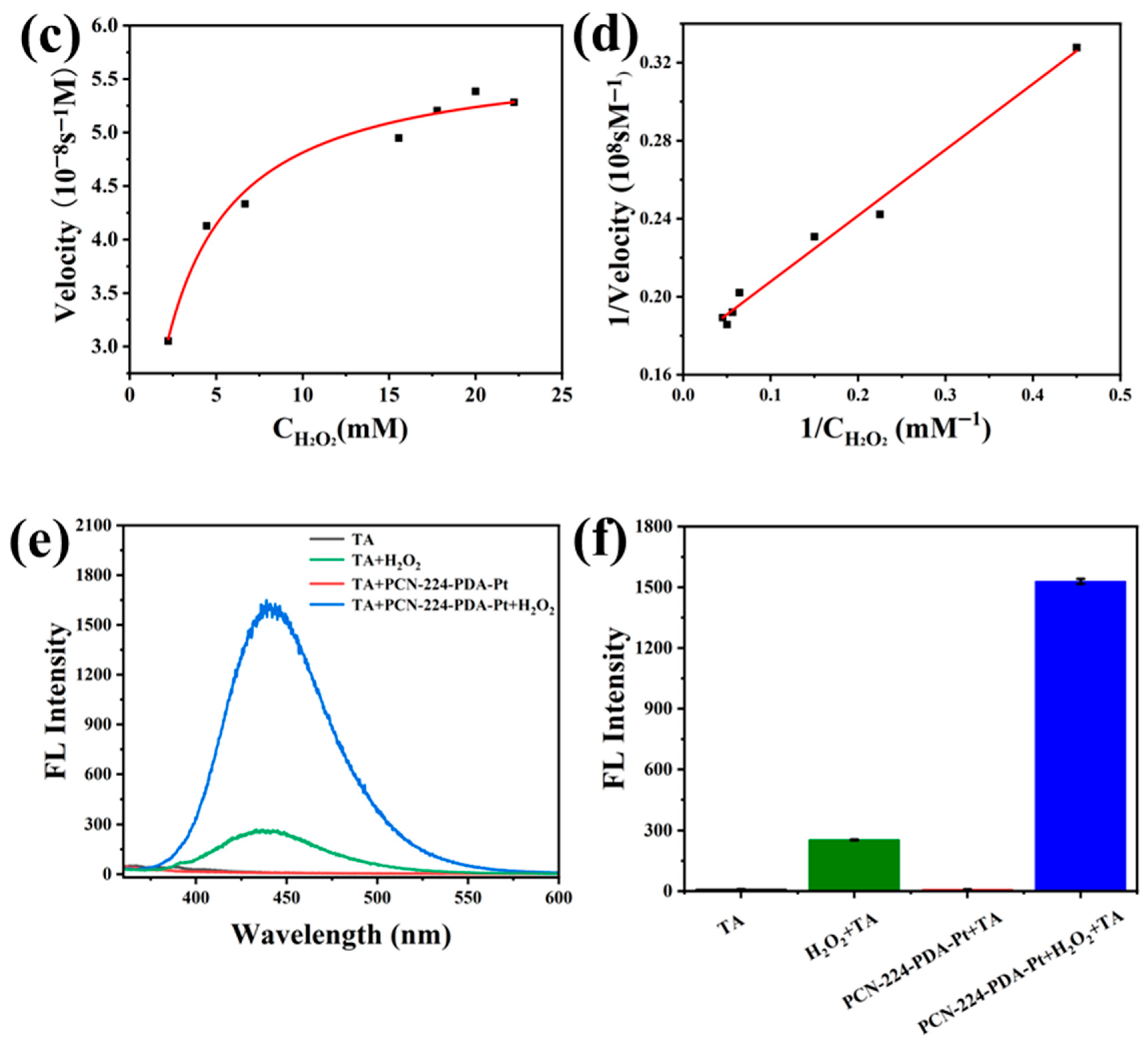
2.6. Study on the Mechanism of Peroxidase-Like Activity
2.7. Glutathione Determination
3. Results and Discussion
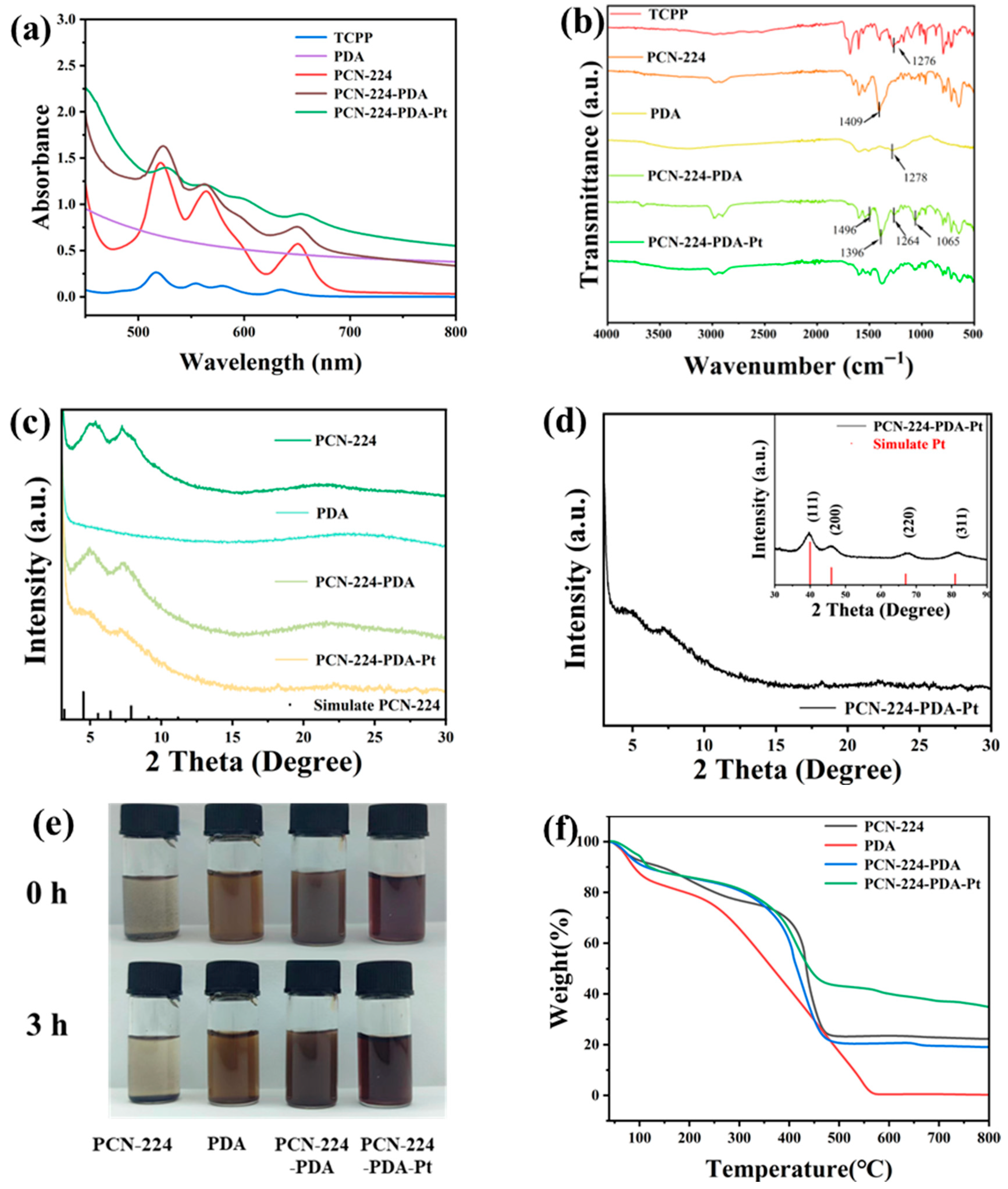
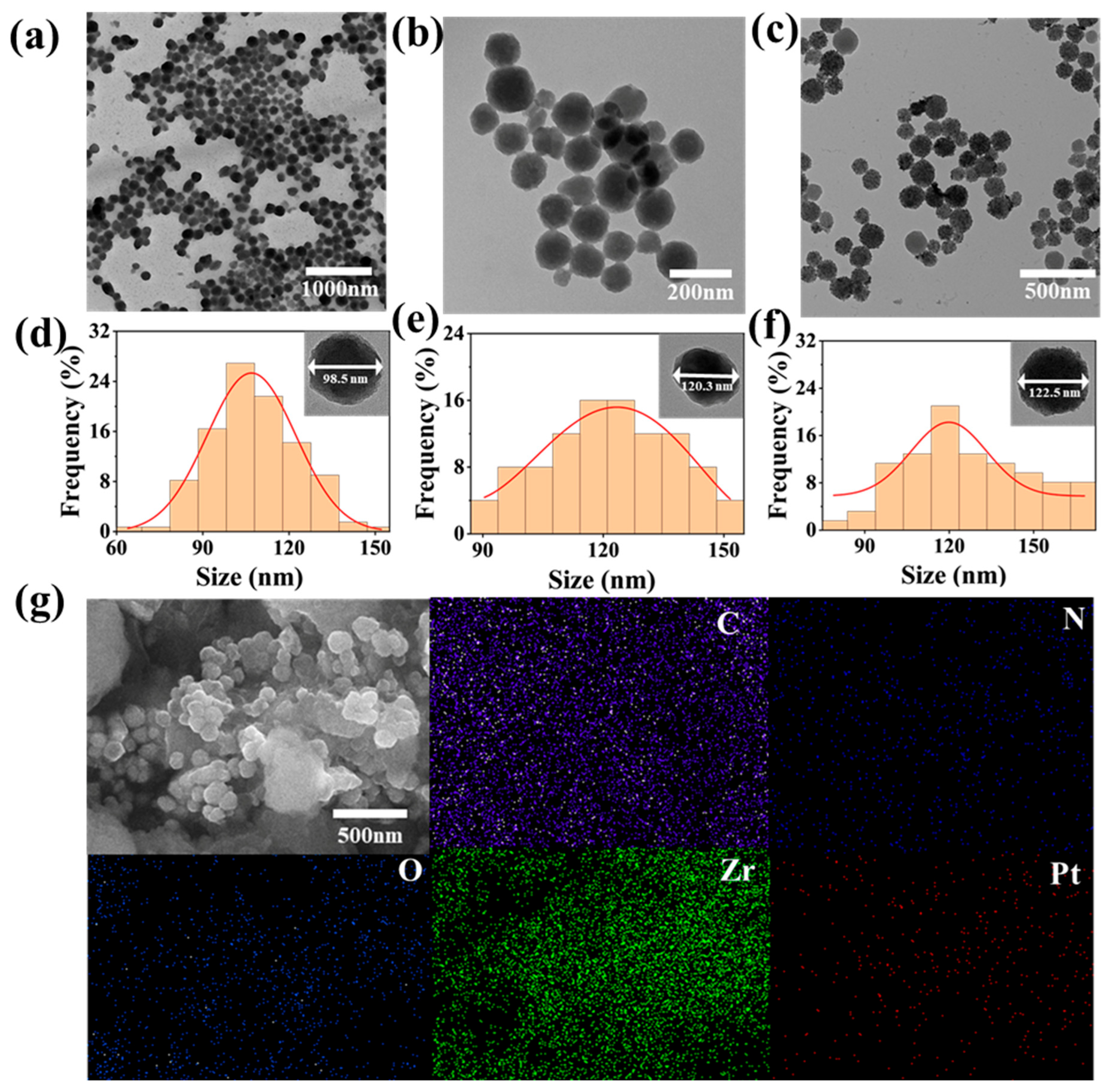
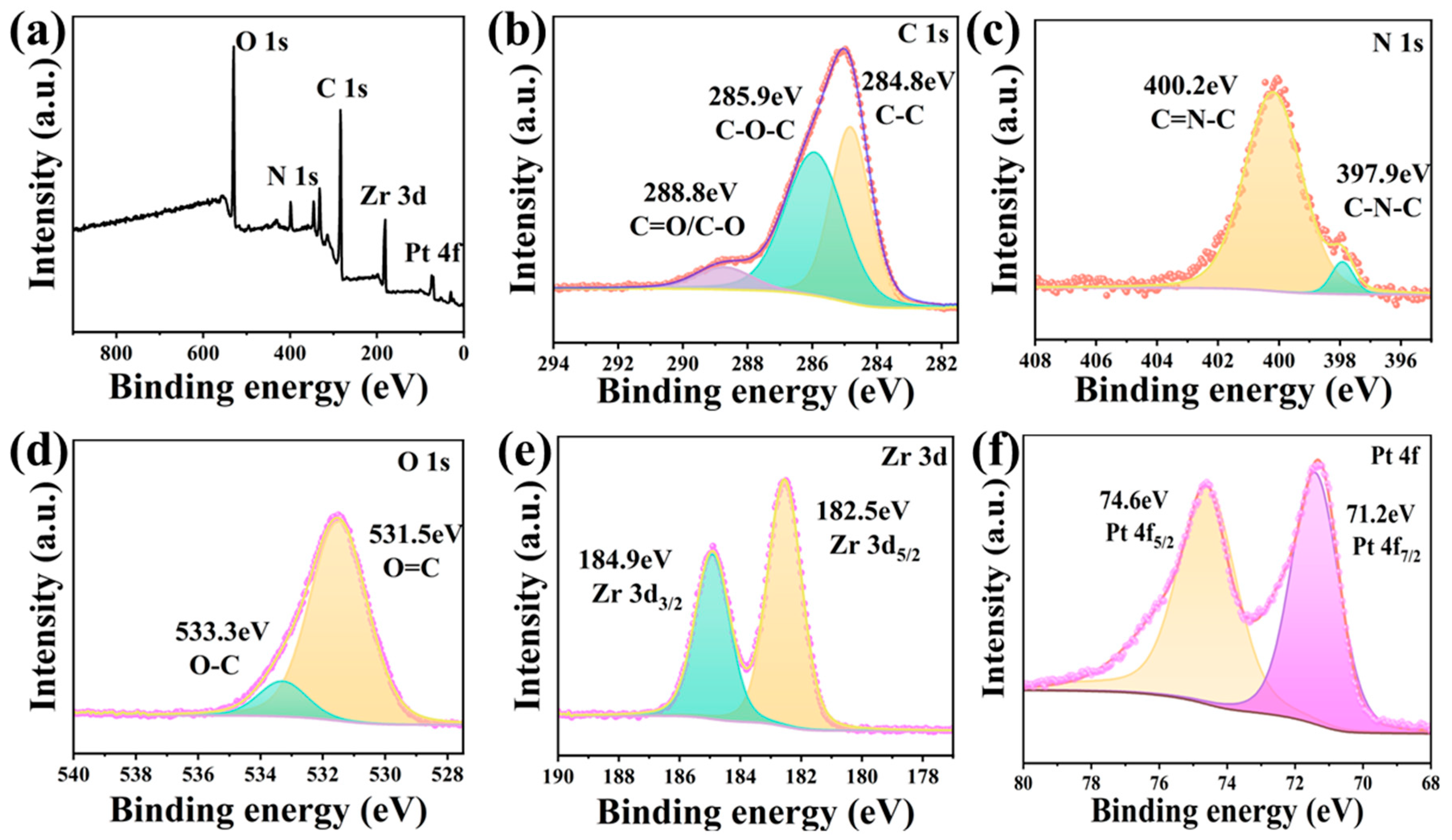
3.1. Structure Analysis of PCN-224-PDA-Pt
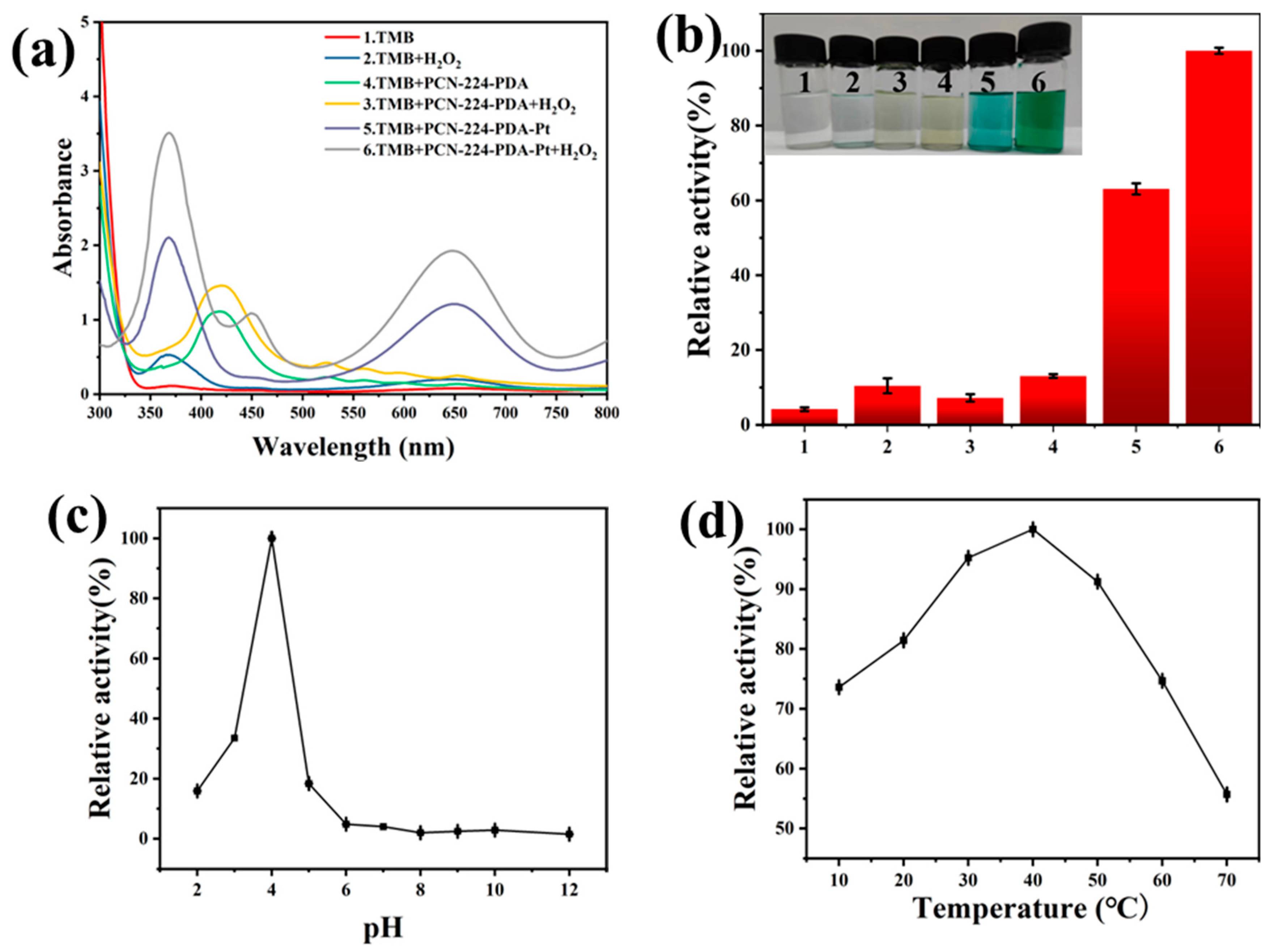
3.2. Enzyme-Like Activity Analysis of PCN-224-PDA-Pt
3.3. Hemolysis Analysis of PCN-224-PDA-Pt
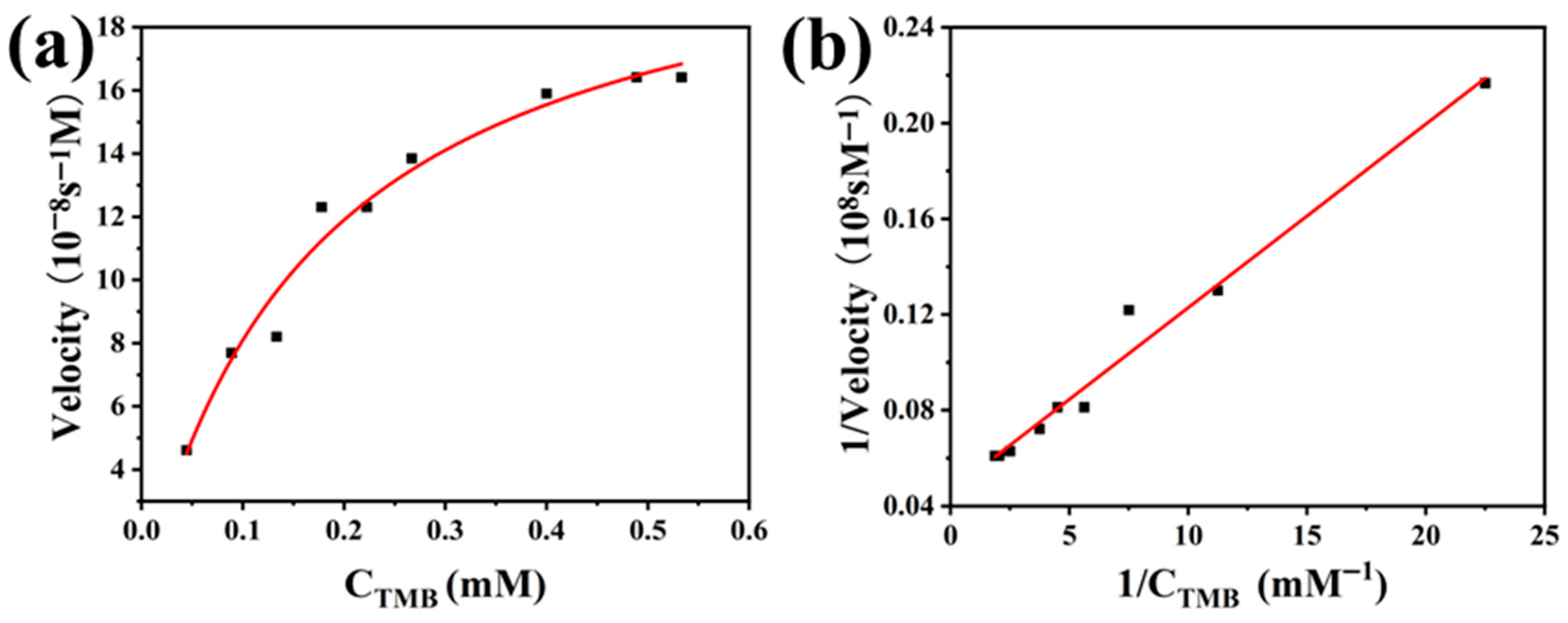
3.4. Analysis of the Catalytic Kinetics and Peroxidase-Like Activity Mechanism of PCN-224-PDA-Pt
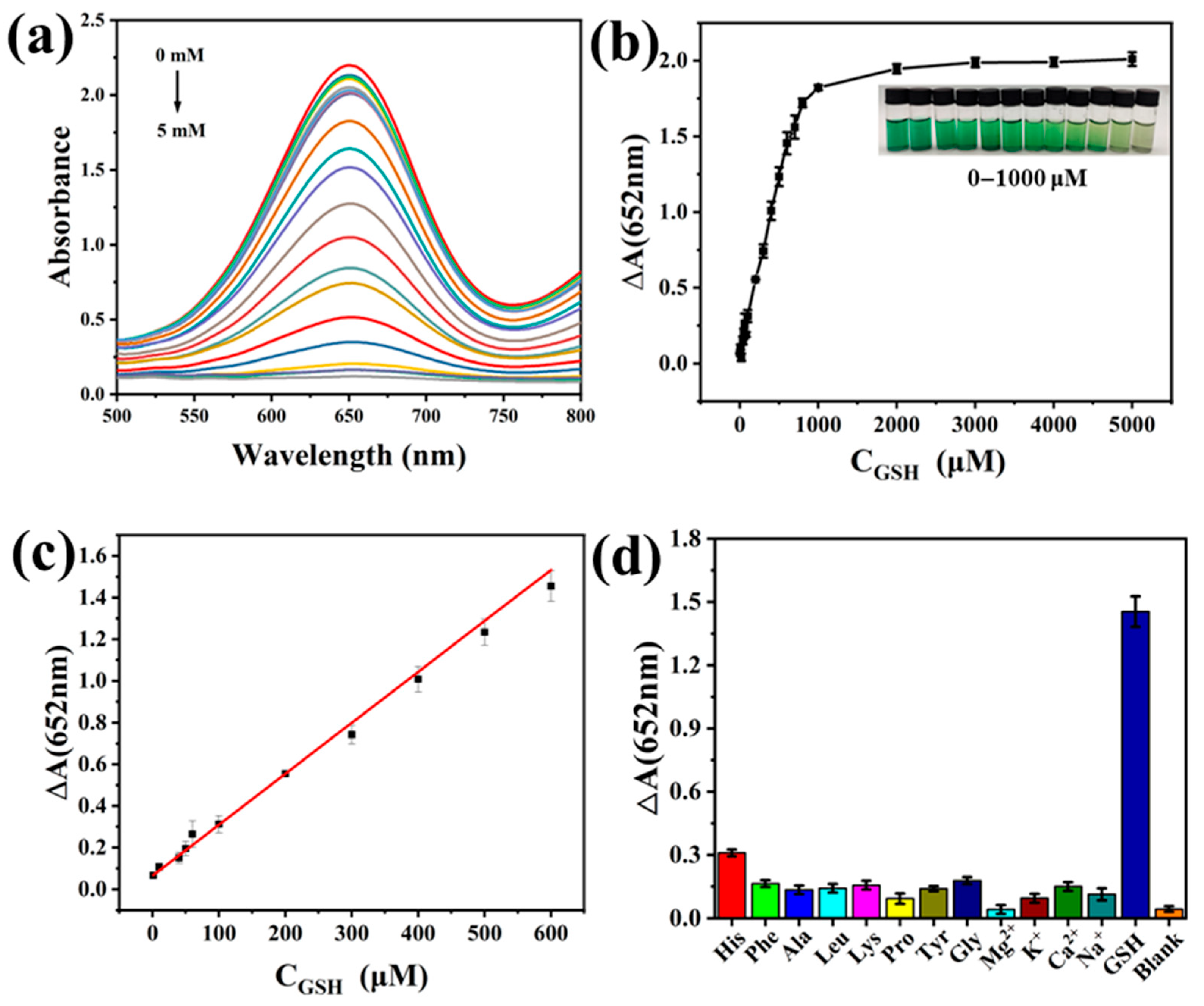
3.5. Glutathione Detection Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, S.; Li, J.; Zhou, X.; Yin, J.; Yoon, J. Recent progress on the development of glutathione (GSH) selective fluorescent and colorimetric probes. Coord. Chem. Rev. 2018, 366, 29–68. [Google Scholar] [CrossRef]
- Estrela, J.M.; Ortega, A.; Obrador, E. Glutathione in cancer biology and therapy. Crit. Rev. Clin. Lab. Sci. 2006, 43, 143–181. [Google Scholar] [CrossRef] [PubMed]
- Herzenberg, L.A.; De Rosa, S.C.; Dubs, J.G.; Roederer, M.; Anderson, M.T.; Ela, S.W.; Deresinski, S.C.; Herzenberg, L.A. Glutathione deficiency is associated with impaired survival in HIV disease. Proc. Natl. Acad. Sci. USA 1997, 94, 1967–1972. [Google Scholar] [CrossRef]
- Gao, S.; Liu, K.; Ji, X.; Cui, Y.; Li, R.; Ma, G.; Zhang, Y.; Wang, L. Biocompatible palladium nanoparticles prepared using vancomycin for colorimetric detection of hydroquinone. Polymers 2023, 15, 3148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, S.; Li, R.; Zhou, L.; Pei, P.; Zhao, H.; Wang, L.; Xie, D. Palladium-gold bimetallic nanoparticles stabilized by daptomycin for sensitive colorimetric detection of sulfide ions. Anal. Chim. Acta 2025, 1348, 343795. [Google Scholar] [CrossRef]
- Lai, X.; Shen, Y.; Gao, S.; Chen, Y.; Cui, Y.; Ning, D.; Ji, X.; Liu, Z.; Wang, L. The Mn-modified porphyrin metal-organic framework with enhanced oxidase-like activity for sensitively colorimetric detection of glutathione. Biosens. Bioelectron. 2022, 213, 114446. [Google Scholar] [CrossRef]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef]
- Huang, X.; Li, Z.; Yu, Z.; Deng, X.; Xin, Y. Recent advances in the synthesis, properties, and biological applications of platinum nanoclusters. J. Nanomater. 2019, 2019, 6248725. [Google Scholar] [CrossRef]
- Zhao, X.; Li, Z.; Ding, Z.; Wang, S.; Lu, Y. Ultrathin porous Pd metallene as highly efficient oxidase mimics for colorimetric analysis. J. Colloid Interface Sci. 2022, 626, 296–304. [Google Scholar] [CrossRef]
- Lou-Franco, J.; Das, B.; Elliott, C.; Cao, C. Gold nanozymes: From concept to biomedical applications. Nanomicro Lett. 2020, 13, 10. [Google Scholar] [CrossRef]
- Li, R.; Zhao, Y.; Zhang, T.; Ju, Z.; Ji, X.; Cui, Y.; Wang, L.; Xiao, H. Pd nanoparticles stabilized by bitter gourd polysaccharide with peroxidase properties for H2O2 detection. Int. J. Biol. Macromol. 2023, 233, 123513. [Google Scholar] [CrossRef]
- Li, R.; He, M.; Cui, Y.; Ji, X.; Zhang, L.; Lan, X.; Wang, L.; Han, Z.; Xiao, H. Silver-palladium bimetallic nanoparticles stabilized by elm pod polysaccharide with peroxidase-like properties for glutathione detection and photothermal anti-tumor ability. Int. J. Biol. Macromol. 2024, 264, 130673. [Google Scholar] [CrossRef]
- Ahmadi, S.; Rahimizadeh, K.; Shafiee, A.; Rabiee, N.; Iravani, S. Nanozymes and their emerging applications in biomedicine. Process Biochem. 2023, 131, 154–174. [Google Scholar] [CrossRef]
- Fu, M.; Li, M.; Zhao, Y.; Bai, Y.; Fang, X.; Kang, X.; Yang, M.; Wei, Y.; Xu, X. A study on the high efficiency reduction of p-nitrophenol (4-NP) by a Fe(OH)3/Fe2O3@Au composite catalyst. RSC Adv. 2021, 11, 26502–26508. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Li, G.; Tang, Z. Metal-organic frameworks as emerging platform for supporting isolated single-site catalysts. Nano Today 2019, 27, 178–197. [Google Scholar] [CrossRef]
- Park, J.; Jiang, Q.; Feng, D.; Mao, L.; Zhou, H.C. Size-controlled synthesis of porphyrinic metal-organic framework and functionalization for targeted photodynamic therapy. J. Am. Chem. Soc. 2016, 138, 3518–3525. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Chung, W.C.; Wei, Z.; Gu, Z.Y.; Jiang, H.L.; Chen, Y.P.; Darensbourg, D.J.; Zhou, H.C. Construction of ultrastable porphyrin Zr metal-organic frameworks through linker elimination. J. Am. Chem. Soc. 2013, 135, 17105–17110. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, F.; Liu, C.; Wang, Z.; Kang, L.; Huang, Y.; Dong, K.; Ren, J.; Qu, X. Nanozyme decorated metal-organic frameworks for enhanced photodynamic therapy. ACS Nano 2018, 12, 651–661. [Google Scholar] [CrossRef]
- Du, P.; Niu, Q.; Chen, J.; Chen, Y.; Zhao, J.; Lu, X. “Switch-On” Fluorescence detection of glucose with high specificity and sensitivity based on silver nanoparticles supported on porphyrin metal-organic frameworks. Anal. Chem. 2020, 92, 7980–7986. [Google Scholar] [CrossRef]
- Cheng, Y.; Kong, R.-M.; Hu, W.; Tian, X.; Zhang, L.; Xia, L.; Qu, F. Colorimetric-assisted photoelectrochemical sensing for dual-model detection of sialic acid via oxidation-power mediator integration. Chin. Chem. Lett. 2023, 34, 107502. [Google Scholar] [CrossRef]
- Yuan, P.; Fan, G.L.; Zhao, L.P.; Liu, L.S.; Deng, F.A.; Jiang, X.Y.; Hu, A.H.; Yu, X.Y.; Chen, A.L.; Cheng, H.; et al. Tumor targeted self-synergistic nanoplatforms for arsenic-sensitized photodynamic therapy. Acta Biomater. 2020, 117, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.-X.; Shu, W.; Wang, H.-S.; Chen, L.; Xu, J.; Zhang, F.-Z.; Lin, R.-G. Folic acid-modified metal-organic framework carries CPT and DOX for cancer treatment. J. Solid State Chem. 2022, 306, 122803. [Google Scholar] [CrossRef]
- Aguilar-Ferrer, D.; Szewczyk, J.; Coy, E. Recent developments in polydopamine-based photocatalytic nanocomposites for energy production: Physico-chemical properties and perspectives. Catal. Today 2022, 397–399, 316–349. [Google Scholar] [CrossRef]
- He, Z.; Liu, Z.; Xie, H.; Luo, P.; Li, X. An ultrasensitive lateral flow immunoassay based on metal-organic framework-decorated polydopamine for multiple sulfonylureas adulteration in functional foods. Foods 2023, 12, 539. [Google Scholar] [CrossRef]
- Gonzalez, D.H.; Kuang, X.M.; Scott, J.A.; Rocha, G.O.; Paulson, S.E. Terephthalate probe for hydroxyl radicals: Yield of 2-hydroxyterephthalic acid and transition metal interference. Anal. Lett. 2018, 51, 2488–2497. [Google Scholar] [CrossRef]
- Qi, S.-C.; Yang, Z.-H.; Zhu, R.-R.; Lu, X.-J.; Xue, D.-M.; Liu, X.-Q.; Sun, L.-B. The cascade catalysis of the porphyrinic zirconium metal–organic framework PCN-224-Cu for CO2 conversion to alcohols. J. Mater. Chem. A 2021, 9, 24510–24516. [Google Scholar] [CrossRef]
- Milyaeva, O.Y.; Bykov, A.G.; Campbell, R.A.; Loglio, G.; Miller, R.; Noskov, B.A. The dynamic properties of PDA-laccase films at the air-water interface. Colloids Surf. A Physicochem. Eng. Asp. 2020, 599, 124930. [Google Scholar] [CrossRef]
- Tao, Y.; Sun, Y.; Shi, K.; Pei, P.; Ge, F.; Yang, K.; Liu, T. Versatile labeling of multiple radionuclides onto a nanoscale metal-organic framework for tumor imaging and radioisotope therapy. Biomater. Sci. 2021, 9, 2947–2954. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Cao, Y.; Han, J.; Xia, Y.; He, Z.; Sun, L.; Liang, J. A novel fluorescence sensor based on Zn porphyrin MOFs for the detection of bisphenol A with highly selectivity and sensitivity. Food Control 2022, 132, 108551. [Google Scholar] [CrossRef]
- Sohrabi, S.; Dehghanpour, S.; Ghalkhani, M. A cobalt porphyrin-based metal organic framework/multi-walled carbon nanotube composite electrocatalyst for oxygen reduction and evolution reactions. J. Mater. Sci. 2018, 53, 3624–3639. [Google Scholar] [CrossRef]
- Zhao, F.Y.; Ji, Y.L.; Weng, X.D.; Mi, Y.F.; Ye, C.C.; An, Q.F.; Gao, C.J. High-flux positively charged nanocomposite nanofiltration membranes filled with poly(dopamine) modified multiwall carbon nanotubes. ACS Appl. Mater. Interfaces 2016, 8, 6693–6700. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.A. Growth of uniform nanoparticles of platinum by an economical approach at relatively low temperature. Sci. Iran. 2012, 19, 964–966. [Google Scholar] [CrossRef]
- Fu, M.; Chai, B.; Yan, J.; Wang, C.; Fan, G.; Song, G.; Xu, F. Facile preparation of MIL-88B-Fe metal–organic framework with high peroxidase-like activity for colorimetric detection of hydrogen peroxide in milk and beer. Appl. Phys. A 2021, 127, 928. [Google Scholar] [CrossRef]
- Wan, H.; Wang, Y.; Chen, J.; Meng, H.M.; Li, Z. 2D Co-MOF nanosheet-based nanozyme with ultrahigh peroxidase catalytic activity for detection of biomolecules in human serum samples. Mikrochim. Acta 2021, 188, 130. [Google Scholar] [CrossRef]
- Zhao, X.L.; Liu, J.L.; Xie, F.T.; Yang, T.; Hu, R.; Yang, Y.H. Iodide-enhanced Co/Fe-MOFs nanozyme for sensitively colorimetric detection of H2S. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 262, 120117. [Google Scholar] [CrossRef]
- Dementjev, A.P.; de Graaf, A.; van de Sanden, M.C.M.; Maslakov, K.I.; Naumkin, A.V.; Serov, A.A. X-Ray photoelectron spectroscopy reference data for identification of the C3N4 phase in carbon–nitrogen films. Diam. Relat. Mater. 2000, 9, 1904–1907. [Google Scholar] [CrossRef]
- Dong, W.; Li, Z.; Wen, W.; Liu, B.; Wen, G. Novel CdS/MOF cathodic photoelectrochemical (PEC) platform for the detection of doxorubicin hydrochloride and gentamicin sulfate. ACS Appl. Mater. Interfaces 2021, 13, 57497–57504. [Google Scholar] [CrossRef]
- Li, J.; Jiao, L.; Xu, W.; Yan, H.; Chen, G.; Wu, Y.; Hu, L.; Gu, W. Cobalt oxyhydroxide nanosheets integrating with metal indicator enable sensitive detection of glutathione. Sens. Actuators B Chem. 2021, 329, 129247. [Google Scholar] [CrossRef]
- Liu, J.; Meng, L.; Fei, Z.; Dyson, P.J.; Jing, X.; Liu, X. MnO2 nanosheets as an artificial enzyme to mimic oxidase for rapid and sensitive detection of glutathione. Biosens. Bioelectron. 2017, 90, 69–74. [Google Scholar] [CrossRef]
- Gu, J.; Hu, D.; Wang, W.; Zhang, Q.; Meng, Z.; Jia, X.; Xi, K. Carbon dot cluster as an efficient “off-on” fluorescent probe to detect Au(III) and glutathione. Biosens. Bioelectron. 2015, 68, 27–33. [Google Scholar] [CrossRef]
- Sanskriti, I.; Upadhyay, K.K. Cysteine, homocysteine and glutathione guided hierarchical self-assemblies of spherical silver nanoparticles paving the way for their naked eye discrimination in human serum. New J. Chem. 2017, 41, 4316–4321. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, Z.; Ren, C.; Liu, G.; Chen, X. A novel colorimetric determination of reduced glutathione in A549 cells based on Fe3O4 magnetic nanoparticles as peroxidase mimetics. Analyst 2012, 137, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Hormozi Jangi, S.R.; Akhond, M.; Absalan, G. A novel selective and sensitive multinanozyme colorimetric method for glutathione detection by using an indamine polymer. Anal. Chim. Acta 2020, 1127, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.-M.; Cai, Q.-Y.; Ding, D.-C.; Ge, J.; Hu, Y.-L.; Yang, J.; Zhang, L.; Li, Z.-H. A facile label-free colorimetric method for highly sensitive glutathione detection by using manganese dioxide nanosheets. Sens. Actuators B Chem. 2017, 242, 355–361. [Google Scholar] [CrossRef]
- Zhong, Q.; Chen, Y.; Su, A.; Wang, Y. Synthesis of catalytically active carbon quantum dots and its application for colorimetric detection of glutathione. Sens. Actuators B Chem. 2018, 273, 1098–1102. [Google Scholar] [CrossRef]
- Shang, H.; Zhang, X.; Ding, M.; Zhang, A.; Wang, C. A smartphone-assisted colorimetric and photothermal probe for glutathione detection based on enhanced oxidase-mimic CoFeCe three-atom nanozyme in food. Food Chem. 2023, 423, 136296. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, B.; Lang, L.-M.; Liu, G.-X.; Xia, X.-H. Bioinspired fabrication of two-dimensional metal–organic framework-based nanozyme for sensitive colorimetric detection of glutathione. ACS Appl. Nano Mater. 2022, 5, 18761–18769. [Google Scholar] [CrossRef]
- Bian, B.; Liu, Q.; Yu, S. Peroxidase mimetic activity of porphyrin modified ZnFe2O4/reduced graphene oxide and its application for colorimetric detection of H2O2 and glutathione. Colloids Surf. B Biointerfaces 2019, 181, 567–575. [Google Scholar] [CrossRef]
| Material | Linear Range (μM) | Detection Limit (μM) | Reference |
|---|---|---|---|
| PCN-224-PDA-Pt | 1–600 | 0.306 | This work |
| CoOOH | 33–1300 | 13.70 | [38] |
| MnO2 | 1–25 | 0.30 | [39] |
| Au(III)/CDC | 1–300 | 3.65 | [40] |
| Ag NP | 0–400 | 4.11 | [41] |
| Fe3O4 NPs | 3–30 | 3 | [42] |
| MnO2-nanozymes | 0.16–36.93 | 0.005 | [43] |
| MnO2 nanosheets | 0.5–10 | 0.1 | [44] |
| CQDs | 0.05–20 | 0.016 | [45] |
| CoFeCe-MOFs | 7–70 | 6.8 | [46] |
| 2D Cu-TCPP(Fe) MOFs | 0.1–60 | 0.0908 | [47] |
| Por-ZnFe2O4/rGO | 2–40 | 0.76 | [48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, S.; Gao, M.; Zhen, C.; Cui, Y.; Ji, X.; Li, R.; Wang, L. Platinum Nanoparticles Loaded in Polydopamine-Modified Porous Coordination Network-224 with Peroxidase-Like Activity for Sensitive Glutathione Detection. Biomolecules 2025, 15, 1002. https://doi.org/10.3390/biom15071002
Gao S, Gao M, Zhen C, Cui Y, Ji X, Li R, Wang L. Platinum Nanoparticles Loaded in Polydopamine-Modified Porous Coordination Network-224 with Peroxidase-Like Activity for Sensitive Glutathione Detection. Biomolecules. 2025; 15(7):1002. https://doi.org/10.3390/biom15071002
Chicago/Turabian StyleGao, Shoubei, Mingyue Gao, Chenran Zhen, Yanshuai Cui, Xianbing Ji, Ruyu Li, and Longgang Wang. 2025. "Platinum Nanoparticles Loaded in Polydopamine-Modified Porous Coordination Network-224 with Peroxidase-Like Activity for Sensitive Glutathione Detection" Biomolecules 15, no. 7: 1002. https://doi.org/10.3390/biom15071002
APA StyleGao, S., Gao, M., Zhen, C., Cui, Y., Ji, X., Li, R., & Wang, L. (2025). Platinum Nanoparticles Loaded in Polydopamine-Modified Porous Coordination Network-224 with Peroxidase-Like Activity for Sensitive Glutathione Detection. Biomolecules, 15(7), 1002. https://doi.org/10.3390/biom15071002






