Abstract
The aggregation of Tau protein into neurofibrillary tangles (NFTs), a hallmark of Alzheimer’s disease (AD), is associated with cognitive decline. Recent studies have revealed that neuronal cytoskeletal instability drives early AD pathogenesis. The physiological interaction between tau and the microtubule (MT) is crucial for maintaining axonal transport and stability. However, aberrant post-translational modifications (PTMs) in the MT binding domain—such as phosphorylation, acetylation and ubiquitination—trigger tau dissociation, causing microtubule collapse, transport deficits, and synaptic dysfunction. MT dysregulation also affects actin/cofilin-mediated dendritic spine destabilization and causes the hyperplasia of the glial intermediate filament, which exacerbates neuroinflammation and synaptic toxicity. This review systematically explores the functions of neuronal cytoskeletons, deciphers the molecular crosstalk between tau pathology and cytoskeletal remodeling, and proposes multi-target therapeutic strategies to restore cytoskeletal homeostasis, thereby providing novel perspectives for precision interventions in AD
1. Introduction
Alzheimer’s disease (AD), as the most common type of neurodegenerative disorder globally, is dramatically reshaping the landscape of public health disease burden at an alarming rate. According to the World Health Organization, the global number of AD patients is projected to rise from 55 million in 2019 to 139 million by 2050, with associated socioeconomic costs estimated to reach up to USD 2 trillion [1], thereby exceeding the healthcare coverage capacity of numerous countries. Histopathologically, AD is marked by two defining features: extracellular β-amyloid (Aβ) plaques and intracellular neurofibrillary tangles composed of hyperphosphorylated tau [2,3,4]. However, after decades of intensive research on AD and therapeutic development based on the amyloid cascade hypothesis, little progress has been made [5,6,7,8,9].
Although early Aβ deposition is thought to be an early sign of AD, a growing amount of research has shown that there is a clear spatiotemporal gap between it and cognitive loss [10,11,12,13]. Longitudinal cohort studies demonstrate that Aβ pathology accumulates years before the onset of clinical symptoms, while the spatiotemporal progression of tau pathology (Braak staging) closely parallels trajectories of cognitive decline and directly correlates with the extent of brain atrophy [14,15,16]. Additionally, recent studies have reported on three individuals who carry risk gene mutations but have escaped the clinical manifestation of AD, despite exhibiting abundant Aβ deposits in their brains and showing no significant cognitive decline. This may be attributed to the relatively low burden of tau pathology present in their neural tissue [17,18,19]. These studies suggest that tau pathology may serve as the key driver of neurodegeneration and clinical phenotypes. Indeed, the pathological toxicity of Tau extends far beyond its microtubule-dissociating effects: acting as a molecular scaffold, aberrantly phosphorylated Tau disrupts axonal transport [20,21], exacerbates neuroinflammation [22,23], induces synaptic mitochondrial dysfunction [24,25], and triggers trans neuronal degeneration through prion-like propagation [26,27,28]. These observations underscore AD-associated tau pathology as the principal executor of neurodegeneration.
However, the molecular mechanisms underlying the collapse of the cytoskeletal network associated with tau, which is a microtubule-associated protein (MAP) critical for maintaining the stability of the neuronal cytoskeleton, remain poorly understood. Neuronal complexity, exemplified by elongated axons and dynamic dendritic spines, enables functional diversity and dynamic plasticity, both of which depend on finely tuned cytoskeletal dynamics. The neuronal cytoskeleton comprises three interdependent systems: (1) Microtubules (MTs), tubular structures composed of GTPase-active tubulin [29], that mediate the transport of vesicles, lipids, and other cargo within axons. Their structural organization, polarity, and stability are tightly regulated by MAPs [30]. (2) Microfilaments (actin filaments), which are dynamic structures governing synaptic remodeling through continuous assembly/disassembly cycles and with their stability tightly controlled by actin-binding proteins (ABPs) [31,32].and (3) Neurofilaments (NFs), which are intermediate filaments providing structural support to the axons [33,34]. In AD, tau undergoes aberrant post-translational modifications (PTMs), leading to its detachment from microtubules, misfolding, and aggregation into neurofibrillary tangles (NFTs) [24,25,26,35,36,37,38,39]. Tau, a central driver of cytoskeletal collapse in AD, undergoes site-specific hyperphosphorylation, leading to its dissociation from microtubules, the disruption of cytoskeletal dynamics, and the impaired axonal transport of mitochondria and vesicles [24,25,35,36,37,38]. The liberated hyperphosphorylated Tau oligomers further propagate via prion-like mechanisms, inducing neurotoxicity through intercellular transmission [26,39].
Furthermore, the bidirectional relationship between the cytoskeletal system and tau pathology has been characterized. Abnormally post-translationally modified (PTM) tau proteins dissociate from microtubules, resulting in disrupted microtubule homeostasis. Conversely, destabilized microtubules further promote tau phosphorylation and enhance its aggregation propensity [40]. Simultaneously, actin cytoskeleton remodeling at synapses—a process tightly regulated by Rho GTPases [41,42]—is hijacked by pathological tau, leading to impaired actin polymerization and dendritic spine loss [43]. Indeed, dendritic spine and synaptic loss exhibit a stronger association with cognitive decline and may represent a structural correlate of early cognitive impairment preceding neuronal death [43]. This suggests that targeting cytoskeletal disturbances may represent a potential therapeutic strategy for early-stage AD. Given the intricate interplay between these systems, this study will systematically elucidate the bidirectional relationships between tau and the neuronal cytoskeletal network, aiming to unravel AD-related cytoskeletal dysregulation mechanisms and identify novel therapeutic targets to mitigate tau-driven cognitive decline.
2. Composition and Function of the Neuronal Cytoskeletal System
The neuronal cytoskeleton is composed of microtubules, actin filaments, and neurofilaments, along with their respective associated regulatory proteins, such as microtubule-associated proteins (MAPs) and actin-binding proteins (ABPs). Figure 1 illustrates the detailed interactions among neuronal cytoskeletal components. The dynamic assembly and disassembly of these cytoskeletal components, as well as their coordinated interactions, are essential for maintaining neuronal structural integrity and enabling morphological plasticity.
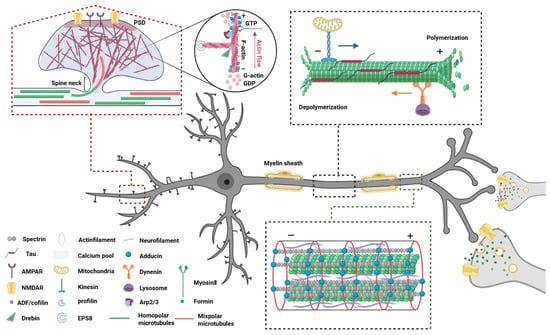
Figure 1.
Neuronal cytoskeletal architecture: composition, function, and interplay. The neuronal cytoskeleton comprises three interdependent elements—microtubules, actin filaments, and intermediate filaments—that collectively sustain structural integrity and functional plasticity. Microtubules, composed of α/β-tubulin heterodimers, establish polarized transport tracks in axons (via kinesin/dynein motors) and bidirectional networks in dendrites, dynamically regulated by tau-mediated stabilization and severing enzymes. Actin filaments, concentrated in dendritic spines and growth cones, undergo activity-dependent remodeling through calcium signaling and actin-binding proteins (e.g., cofilin severing, Arp2/3 branching), driving synaptic reorganization and axonal navigation. Intermediate filaments, including neurofilaments, provide mechanical resilience through longitudinal crosslinking, maintain axonal caliber, and stabilize transport complexes. Functionally, actin initiates pathfinding by probing microenvironments, microtubules consolidate transport pathways, and intermediate filaments reinforce structural resistance, enabling neurons to balance stability with adaptive plasticity. Abbreviations: PSD, Postsynaptic density protein; GTP, Guanosine triphosphate; GDP, Guanosine diphosphate; AMPAR, A-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptor; NMDAR, N-methyl-d-aspartate receptor; Arp2/3, Actin-related proteins 2/3 complex; EPS8, Epidermal growth factor receptor pathway Substrate 8.
2.1. Microtubule System: Architects of Neuronal Polarity and Axonal Integrity
The neuronal microtubule system comprises MTs—dynamic polymers of α/β-tubulin heterodimers—and a diverse group of MAPs that regulate their structure, dynamics, and interactions [44,45]. MTs are strategically organized to maintain structural integrity and facilitate intracellular transport in specific compartments [46], and MAPs fine-tune their assembly, organization, and interactions with other cytoskeletal and synaptic elements [47]. This system forms the architectural and functional backbone of neuronal polarity, axonal transport, and synaptic remodeling [44]. The following sections detail the molecular composition, dynamic regulation, and the compartment-specific functions of the microtubule system in neurons.
2.1.1. Microtubule
MTs are hollow cylindrical polymers composed of α/β-tubulin heterodimers, serving as a critical structural foundation for maintaining cellular morphology and mediating intracellular cargo transport. In neurons, MTs display unique spatial heterogeneity, characterized by the head-to-tail polymerization of α- and β-tubulin heterodimers, which imparts distinct polarity of microtubule ends. Specially, the β-tubulin subunit is oriented toward the fast-growing (plus) end, while the α-tubulin subunit is exposed at the slow-growing (minus) end [45]. This intrinsic polarity is especially critical in neurons: axonal MTs display uniform plus-end-out orientation toward synaptic terminals, whereas dendritic MTs exhibit mixed polarity. Such differential organization is a key determinant of neuronal polarity and underlies directional cargo trafficking and compartment-specific signaling [48,49,50]. In neuronal cargo transport, MTs mediate bidirectional transport: anterograde transport (from soma to periphery) and retrograde transport (periphery to soma) [51,52]. Anterograde transport, driven by plus-end-directed kinesins, is essential for the delivery of neurotransmitters and synaptic components to distal regions [51]. Conversely, dynein, the motor protein responsible for retrograde transport, facilitates the retrograde transmission of stimuli from axonal terminals to the soma [51]. This mechanism is crucial for neuronal survival, enabling feedback signaling via neurotrophic factors to the cell body [51,53].
Notably, MTs do not maintain a static length but instead undergo dynamic cycles of polymerization (growth) and depolymerization (shrinkage), a phenomenon termed dynamic instability [54,55,56]. The transition from polymerization to depolymerization is designated as ‘catastrophe’, while the reverse shift to growth phase is termed ‘rescue’. Axonal growth cones exploit this MT dynamism to sample extracellular environments through exploratory microtubule protrusions, facilitating navigation toward specific guidance cues [57,58]. Conversely, mature axons suppress MT dynamic instability through dense MAP coverage, ensuring stable transport tracks. This spatiotemporal regulation preserves mechanical resilience in elongated axons while maintaining the dendritic MT plasticity required for synaptic remodeling. Such homeostatic equilibrium is governed by the intrinsic GTPase activity of tubulin heterodimers [29,59] and modulated by MAPs that bind MT lattices to structurally stabilize polymerized protofilaments and regulate assembly kinetics [30,37,60].
2.1.2. Tau
Tau is the most abundant MAP in neuronal cells [61]. As an intrinsically disordered protein, tau contains four key domains: an N-terminal acidic projection domain, a proline-rich region, a microtubule-binding domain containing conserved repeats, and a C-terminal tail. In the human central nervous system, the alternative splicing of the MAPT (microtubule-associated protein tau) gene generates six tau isoforms. The differential inclusion of N-terminal exons 2 and 3 produces 0N (both excluded), 1N (exon 2 included), or 2N (both included) variants, while the alternative splicing of exon 10 in the microtubule-binding domain creates isoforms with either three (3R) or four (4R) repeat motifs, ultimately forming six combinatorial variants: 0N4R, 1N4R, 2N4R, 0N3R, 1N3R, and 2N3R [30,62,63,64]. This splicing regulation exhibits developmental and neuronal subtype specificity [65]: the shortest 0N3R isoform predominates in fetal brains, while adult brains maintain a near 1:1 ratio of 3R and 4R tau. Region-specific expression patterns emerge in the mature brain, with cerebellar neurons showing significantly reduced 0N3R tau compared to other regions, while globus pallidus neurons exhibit preferential 4R tau accumulation. Such isoform distribution heterogeneity arises from the neuron type-specific splicing factor recruitment to MAPT pre-mRNA, coordinating microtubule stabilization requirements with synaptic plasticity demands across neural circuits [66,67].
As a MAP, tau is predominantly localized to the axons of mature neurons, where it directly interacts with microtubules through its microtubule-binding repeat domains [68,69,70]. This interaction enables tau to stabilize microtubular architecture either by reinforcing structural integrity or serving as cross-bridges that interconnect microtubules with other cytoskeletal components (e.g., actin filaments and neurofilaments), thereby facilitating axonal cargo transport [71]. By binding to tubulin, tau enhances microtubule rescue events and prolongs the duration of catastrophe-to-rescue transitions while reducing the frequency of catastrophic depolymerization events by approximately 35%, thereby maintaining microtubule dynamic stability [72,73,74]. This stabilization mechanism is dependent on the presence of GDP-tubulin, as tau selectively avoids the GTP-cap at growing microtubule plus-ends, thereby modulating the extent of microtubule shortening during depolymerization phases without interfering with growth or catastrophe kinetics [75]. Furthermore, tau self-assembles via its N-terminal projection domain into large-scale cohesive complexes (termed “tau patches [76]”, “tau cohesive islands [77]”, or “tau condensates [78]”), forming an adaptive protective coating on microtubule surfaces that shields them from degradation by severing enzymes [77]. In axonal transport, at physiological levels, tau enhances kinesin-microtubule binding efficiency and promotes retrograde trafficking [76]. Conversely, however, excessive tau accumulation disrupts this balance, impeding organelle transport and causing premature cargo release by dissociating kinesin motors—without abolishing overall transport functionality [76,79,80]. In synaptic plasticity, tau dynamically shuttles between microtubules and synaptic compartments, where it interacts with actin to modulate dendritic spine morphology and volume [81,82]. Experimental studies demonstrate that tau depletion reduces dendritic spine density by over 50% in primary neurons [83]. Furthermore, tau is essential for the formation of postsynaptic densities and dendritic spine maturation, as its knockout diminishes the sensitivity of newborn granule neurons to neurogenic regulators [84]. Mechanistically, tau directly binds to the postsynaptic scaffolding protein postsynaptic density protein-95 (PSD-95), stabilizing the synaptic localization of NMDA receptors, thereby regulating long-term potentiation (LTP) and spatial memory formation [85,86]. The dynamic modulation of Tau orchestrates neuronal structural and functional homeostasis while critically supporting adaptive plasticity in response to synaptic and environmental demands.
2.1.3. Other MAPs
The MT network is regulated by a diverse array of MAPs that act in concert with tau to maintain cytoskeletal architecture, compartmental identity, and neuronal plasticity. While tau is the principal MT stabilizer in axons, other MAPs—including the MAP1 family, MAP2 and doublecortin (DCX) and so on—exhibit compartment-specific roles that synergize with tau to maintain cytoskeletal integrity, spatial organization, and adaptive plasticity.
Within the MAP1 family, MAP1A and MAP1B are highly expressed in neurons, exhibiting distinct structural and functional specializations [87,88,89]. MAP1A, predominantly localized to dendrites of mature neurons, contains three microtubule-binding domains (MTBDs)—two on its heavy chain and one on its light chain. In contrast, MAP1B, enriched in embryonic axonal projections, harbors two MTBDs distributed across its heavy and light chains [90,91]. Both isoforms directly bind MTs via their MTBDs to promote assembly and stability, while also interacting with actin filaments—a dual-binding capability critical for establishing CNS developmental networks and adult dendritic arborization, as well as postsynaptic structure formation.
MAP2, another neuron-specific MAP, is composed of an N-terminal projection domain, a central proline-rich region, and a C-terminal MTBD homologous to tau [92]. Unlike tau, which suppresses MT dynamics, MAP2 stabilizes MTs by reducing the frequency of catastrophic depolymerization events during polymerization, thereby facilitating MT growth without inhibiting overall dynamic turnover. This stabilization is particularly relevant in dendritic spines, which are primarily actin-based but transiently intersected by dynamic MT. MAP2 enhances synaptic plasticity by anchoring these transient MTs in dendrites [93,94,95]. Additionally, MAP2 regulates axonal protein transport by modulating kinesin and dynein activity, enabling selective cargo sorting [96,97].
DCX, a MAP essential for neuronal migration, consists of an N-terminal tandem repeat of doublecortin-like domains (DC domains), a linker region, and a serine/proline-rich C-terminal tail [98]. DCX stabilizes MTs through both its DC domains and C-terminal region [99], which also mediates crosslinking with actin [100]. Cryo-electron microscopy (cryo-EM) reveals that DCX binds MTs at the interface of four α/β-tubulin dimers, reinforcing longitudinal MT stability and lateral interactions [101,102]. DCX further modulates MT stiffness and curvature [103,104], enhances nucleation rates, and directs the formation of anterogradely polarized MT bundles that drive the leading process extension during neuronal migration, while simultaneously suppressing catastrophe frequency [104].
Under AD conditions, aberrant modifications of tau disrupt its electrostatic binding to MTs, leading to detachment and subsequent MT destabilization [105]. This loss of MT stability compromises the polarized MT network critical for axonal identity, impairing kinesin/dynein-mediated transport of organelles (e.g., mitochondria) and synaptic vesicles. The resulting deficits in axonal transport induce the mislocalization of organelles and subsequent synaptic dysfunction [76,80]. Concurrently, the MT disarray drives ectopic dendritic tau accumulation and synapse loss [83]. A self-amplifying cycle ensues: MT fragmentation releases additional tau for hyperphosphorylation, while impaired mitochondrial transport elevates oxidative stress, further oxidizing tau and exacerbating MT instability. Consequently, the microtubule-dependent functions of tau in maintaining neuronal polarity and axonal integrity collapse, driving neurodegeneration through cytoskeletal failure and pathological propagation.
2.2. Actin Filament System: Spatiotemporal Regulator of Synaptic Dynamics
In neurons, actin exists as globular monomers (G-actin) that polymerize into filamentous actin (F-actin), forming essential components of the axonal cytoskeleton [106]. The intrinsic dynamicity of F-actin underlies its critical roles in neural development and axonal regeneration [107]. During brain development, actin constitutes 7–8% of total cellular proteins, stabilizing at approximately 5% in adulthood [107,108,109]. Actin-mediated functions—including synaptic plasticity, axonal guidance, vesicle trafficking, and mechanosensing—rely on the precise spatiotemporal regulation of F-actin assembly [110]. F-actin assembly proceeds through three sequential phases: nucleation (the rate-limiting step), elongation, and steady-state equilibrium, where net F-actin mass remains constant [31]. This dynamic process is regulated by ATP availability, G-actin monomer concentration relative to the critical concentration (Cc), and interactions with actin-binding protein (ABP) [111]. Actin monomers (G-actin) initially assemble into asymmetric filamentous structures (F-actin). During polymerization, ATP-bound actin subunits are preferentially incorporated at the barbed (plus) ends of growing filaments, whereas ADP-actin subunits undergo gradual dissociation from pointed (minus) ends. This polarized assembly-disassembly cycle drives “treadmilling,” a dynamic state in which filaments maintain constant length while subunits continuously cycle through [111,112].
2.2.1. Axon: Growth Cone and Terminal Axonization
Actin plays a central role in axon guidance and terminal axonization by orchestrating the dynamic remodeling of the growth cone, the motile tip of elongating axons. Driven by dynamic remodeling of actin and ABPs, the growth cone undergoes continuous extension or retraction to detect attractive or repulsive surface-bound guidance cues, enabling precise navigation through complex neural microenvironments toward specific target tissues for synaptic connection [113]. At the leading edge of the growth cone, G-actin undergoes rapid polymerization into F-actin networks, driven by nucleation factors like the Arp2/3 complex (generating branched filaments) and formins (producing linear filaments) [31,114,115]. These newly formed F-actin structures push the plasma membrane forward, forming transient protrusions that explore the microenvironment. Concurrently, myosin II, an actin-associated motor protein, generates contractile forces through ATP hydrolysis, pulling the F-actin network rearward in a process termed retrograde F-actin flow [116,117]. This retrograde flow is further modulated by the depolymerization of actin filaments at their “minus” ends, facilitated by proteins such as CAP (cyclase-associated protein) and ADF/cofilin, which sever and disassemble older filaments, recycling G-actin for reuse in polymerization [50,118,119,120,121,122]. Specifically, the actin-mediated steering of growth cones relies on the precise spatiotemporal regulation of cytoskeletal dynamics by attractive and repulsive signaling cues. Under attractive guidance signals such as Netrin, the activation of surface receptors (e.g., DCC) triggers downstream signaling cascades that promote the activation of small GTPases, including Rac1 and Cdc42. These GTPases orchestrate localized actin remodeling by recruiting nucleation-promoting factors such as the Arp2/3 complex (driving branched actin networks) and Formin (assembling linear filaments), thereby inducing protrusive structures like filopodia and lamellipodia [123,124]. Concurrently, attractive signaling suppresses RhoA pathway activity, attenuating myosin II-mediated contractility to permit sustained actin network expansion in the direction of the chemoattractant [125,126]. This polarized actin polymerization is further amplified by calcium influx, which activates proteases like calpain to modulate cofilin activity [127]. Enhanced cofilin-mediated severing and depolymerization at filament minus ends facilitates actin monomer recycling, establishing a polarized actin treadmilling flow that reinforces directional protrusion. This attraction mechanism further involves microtubule extension into nascent protrusions, which stabilizes new pathways and synergizes with cell adhesion molecules (e.g., integrins) to translate mechanical traction into persistent axonal guidance. In contrast, repulsive guidance signals, such as Slit or Semaphorin, bind to their cognate receptors (e.g., Robo or Neuropilin) to activate the RhoA-ROCK signaling axis, which suppresses Rac1/Cdc42 activity. RhoA phosphorylates LIM kinase, thereby inactivating cofilin and reducing actin depolymerization to stabilize preexisting filamentous networks. Concurrently, ROCK kinase enhances myosin II phosphorylation, promoting actomyosin crosslinking and generating hypercontractile stress fibers that drive the localized retraction of protrusions [128]. Notably, attractive and repulsive signals often coexist spatially and temporally. Growth cones integrate these opposing gradients to dynamically balance actin polymerization-depolymerization rates through bidirectional regulatory mechanisms. This spatiotemporal coordination ensures precise neuronal navigation in complex microenvironments, establishing the foundation for proper neural circuit formation [119].
2.2.2. Dendrites: Morphological Maintenance and Synaptic Plasticity
Dendrites, the primary sites for neuronal signal reception and integration, are densely populated with dendritic spines. A spine is structurally composed of a bulbous head (containing the postsynaptic density, PSD) connected to the dendritic shaft via a slender neck [129,130]. Studies demonstrate that spine head volume is proportional to PSD density, presynaptic structures, AMPA receptor abundance (mediating excitatory synaptic transmission), and the amplitude of receptor-mediated currents [131,132,133]. Thus, spine head morphology is closely correlated with synaptic activity. The electrical resistance of the spine neck generates a voltage gradient between the spine and dendritic shaft, leading to the localized amplification of excitatory postsynaptic potentials (EPSPs) within the spine head [134]. Furthermore, neck geometry governs Ca2+ influx through N-methyl-D-aspartate receptors (NMDARs); narrower necks result in higher compartmentalized [Ca2+]i within the spine head. This spatial restriction preferentially enables smaller spines to serve as hotspots for long-term potentiation (LTP) induction [135]. Consequently, dendritic spine morphology critically determines synaptic strength and plasticity [136].
Actin filaments and ABPs exhibit prominent accumulation within dendritic spine heads, particularly enriched in the PSD macromolecular complex. The actin cytoskeleton fundamentally participates in spine morphogenesis during neuronal development and maintains activity-dependent structural plasticity-sustaining spine enlargement during synaptic potentiation while facilitating shrinkage upon activity reduction [137,138,139]. The spine actin cytoskeleton comprises a hybrid architecture of branched lattice networks and cross-linked linear filaments that extend from the spine base through the neck into the head compartment, ultimately terminating near the PSD [140,141]. In dendrite shafts, F-actin cooperates with βII-spectrin to form periodic meshwork structures that maintain cylindrical morphology and mechanical resilience, whereas spine actin undergoes rapid spatiotemporal reorganization to mediate activity-induced head expansion/contraction [142,143,144]. This dynamic remodeling is precisely orchestrated by ABPs: The Arp2/3 complex nucleates branched actin networks, facilitating spine head enlargement to accommodate AMPA receptor recruitment [145,146]. Formins promote linear actin filament elongation, stabilizing the spine neck [113], while cofilin severs aged ADP-actin filaments, releasing G-actin monomers for repolymerization at growing filament ends, thereby sustaining cytoskeletal turnover [138,147]. This regulatory cascade integrates Rho GTPase signaling pathways—Rac1 activates Arp2/3-mediated branching for spinogenesis, whereas RhoA-ROCK signaling enhances actomyosin contractility to drive spine retraction [148,149,150,151]. The canonical models of synaptic plasticity—LTP and long-term depression (LTD)—are fundamentally driven by actin cytoskeletal dynamics. LTP induction triggers calcium influx that activates Ca2+/calmodulin-dependent protein kinase II (CaMKII), which phosphorylates Rac1-specific guanine nucleotide exchange factors (GEFs) to enhance Arp2/3-mediated actin polymerization. This process drives spine head expansion and AMPA receptor enrichment through cytoskeletal restructuring [151,152]. Conversely, LTD-associated calcium signaling activates calcineurin, leading to dephosphorylation and the activation of cofilin. Enhanced cofilin severing activity promotes actin depolymerization, spine shrinkage, and AMPA receptor internalization [153,154]. Actin filaments additionally interact directly with PSD scaffolding proteins (e.g., PSD-95, Shank) to anchor NMDA receptors and coordinate their coupling with downstream signaling effectors (e.g., Ras, ERK), thereby translating synaptic activity into transcriptional regulation [155,156,157]. Pathological dysregulation of actin dynamics—manifested as aberrant G-actin-F-actin equilibrium or impaired nucleotide cycling—disrupts synaptic homeostasis, contributing to neurodevelopmental disorders and neurodegenerative diseases.
In AD, pathological tau directly binds and sequesters the actin-severing protein cofilin, blocking its rephosphorylation and prolonging its active state [158]. This drives the excessive cleavage of F-actin, forming stable actin rods that impair dendritic spine plasticity by obstructing AMPA receptor trafficking and spine remodeling [159,160]. Concurrently, tau’s dissociation from microtubules destabilizes spectrin-actin networks at the PSD, leading to AMPAR internalization and synaptic weakening [161]. Tau oligomers further co-aggregate with F-actin via hydrophobic interactions, forming insoluble complexes that exacerbate neuroinflammation and synaptic loss [162]. Mitochondrial transport deficits caused by tau-induced microtubule collapse deprive synapses of ATP, crippling actin dynamics (e.g., Arp2/3-mediated branching). Failed cross-talk between microtubule and actin networks (via MAP2/drebrin) disrupts synaptic anchoring and promotes tau oligomer propagation through actin-dependent endocytosis [163]. Collectively, these mechanisms erode synaptic resilience, driving AD-like neurodegenerative pathology.
2.3. Neurofilaments: Neuronal Crosslinking Network
Neurofilaments (NFs) are specialized intermediate filaments in neurons, with type IV intermediate filaments predominantly expressed in central neurons [164]. These filaments primarily consist of neurofilament light (NfL, ~68 kDa), neurofilament medium (NfM, ~150 kDa), neurofilament heavy (NfH, ~200 kDa), and α-internexin (~66 kDa) [165,166,167]. All neurofilament subunits share a conserved tripartite structural organization [168,169,170]: (1) a short, hydrophilic amino acid-rich head domain at the N-terminus; (2) a central hydrophobic α-helical rod domain facilitating coiled-coil dimer formation [170]; and (3) a highly variable C-terminal tail domain. The length of the glutamate- and lysine-rich C-terminal domains determines the subunit-specific molecular weights. Notably, NfL possesses a short tail containing multiple glutamate-rich segments (“E-segments”), while NfM and NfH exhibit extended tails with numerous E-segments [33,165]. Upon extensive phosphorylation, these C-terminal domains extend radially from the filament core to form sidearms, which regulate interfilament spacing by maintaining minimum distances between adjacent NFs and between NFs and MT [171,172,173]. This phosphorylation-dependent sidearm extension creates electrostatic repulsion forces that prevent pathological aggregation while enabling dynamic mechanical coupling within the neuronal cytoskeletal network.
Neurofilaments, similar to microtubules and microfilaments, undergo a multi-step assembly process; however, this process is distinct in its independence from ATP synthesis or hydrolysis, relying predominantly on ionic strength, pH, and temperature [174]. The assembly initiates with the formation of dimers via interactions between the central α-helical rod domains of neurofilament monomers. These dimers subsequently organize into staggered antiparallel tetramers through lateral associations. Subsequently, eight tetramers (comprising 32 monomers) assemble laterally to form cylindrical structures termed unit-length filaments (ULFs), measuring approximately 60 nm in length and 16 nm in diameter. The maturation of neurofilaments involves the elongation and extension of ULFs, driven by the post-translational modifications of the N-terminal domains within the tail regions of neurofilament subunits [33,175,176]. These modifications facilitate structural reorganization, ultimately yielding mature neurofilaments with a uniform diameter of 10 nm. In proximal axonal regions, NF subunits undergo active transport through direct binding to kinesin/dynein motor proteins. In contrast, distal axonal compartments predominantly exhibit the translocation of shorter NF species. During axonal transport, NF proteins exist in multiple assembly states—including oligomeric complexes, protofilaments, and short filaments—indicating that NF assembly occurs progressively during transit [177]. When NF concentrations reach a critical threshold, the axonal NF pool initiates the establishment of a stationary NF network, beginning from proximal axonal segments. The local regulation of NF precursor incorporation into this network enables neurons to spatially control NF cytoskeletal dimensions along the axon, a process modulated by interactions with myelinating glia and other axonal microenvironmental demands [178]. This dynamic assembly–transport coupling ensures the precise calibration of axonal caliber and mechanical properties essential for neuronal function.
NFs serve as critical cytoskeletal components that maintain neuronal structural stability while undergoing dynamic reorganization during axonal and dendritic morphogenesis. Experimental evidence from NF gene knockout mouse models demonstrates significant axonal caliber reduction, whereas the co-overexpression of NfL/NfM or NfL/NfH rescues or increases axonal diameter, implicating NFs in radial growth regulation [179,180]. This process is mechanistically governed by the phosphorylation states of NF subunit C-terminal domains. The phosphorylation of C-terminal residues enhances a molecular negative charge, generating electrostatic repulsion between adjacent NFs to increase interfilament spacing and axonal diameter [181]. Such spatial regulation optimizes organelle distribution (e.g., mitochondria, vesicles) and molecular motor transport efficiency (e.g., kinesin, dynein) by maintaining a compartmentalized cytoskeletal architecture. The axonal diameter critically determines the conduction velocity of action potentials in myelinated axons through its geometric relationship with myelination parameters. The length of internodal segments (between the successive nodes of Ranvier) and myelin sheath thickness exhibit direct proportionality to axonal caliber. Optimal nerve conduction is achieved when internodal lengths approximate 100 times the axonal diameter, coupled with a g-ratio (axonal diameter-to-total fiber diameter ratio) between 0.6 and 0.7 NfL—null animal models exhibit reduced conduction velocities proportional to axonal caliber diminution and NF depletion [182], accompanied by aberrant electrophysiological phenotypes such as diminished resting membrane potentials, impaired current modulation, and sensory/auditory deficits [183,184]. Under pathological conditions, disrupted NF homeostasis leads to aberrant filament aggregation and impaired axoplasmic transport. Proteolytic NF degradation products (e.g., NfL) released into cerebrospinal fluid (CSF) serve as early diagnostic biomarkers for neurodegenerative diseases, reflecting cytoskeletal disintegration prior to overt clinical symptom onset [176]. This NF-dependent regulation of axonal geometry and transport underscores their dual role in structural integrity and functional optimization, with dysregulation contributing to both developmental and degenerative neurological pathologies.
In AD, pathological tau co-aggregates with NF subunits (NFM/NFH) via electrostatic/hydrophobic interactions, forming insoluble complexes that destabilize NF side-arm crosslinking, leading to axonal spheroids and transport obstruction [176]. Concurrently, the hyperactivity of shared kinases drives the aberrant phosphorylation of tau and NF C-terminal tails, impairing NF-microtubule coupling and organelle trafficking [185,186]. This dual pathology—tau-NF co-aggregation and phosphorylation imbalance—compromises axonal mechanical strength, disrupts mitochondrial/synaptic vesicle transport, destabilizes PSD scaffolds, and accelerates AMPAR lateral diffusion and synaptic weakening. The collapse of NF-microtubule synergy exacerbates neuronal polarity loss, as mislocalized NFs in dendrites disrupt spine stability and synaptic input–output balance [187,188,189]. NF dysregulation drives axonal degeneration, synaptic network disintegration, and pathological propagation, positioning NFs as active mediators in AD.
3. Early AD-Associated Tau Pathology and Cytoskeletal Dysregulation: Post-Translational Modifications in MTBD
AD is characterized by dual pathological hallmarks—extracellular Aβ plaques and intracellular tau aggregates—each contributing to neurodegeneration through distinct but converging mechanisms [3]. Among them, tau pathology, involving complex post-translational modifications (PTMs) and the trans-synaptic propagation of tau aggregates, has been shown to correlate more strongly with cognitive decline and disease progression [14,15,16]. Under physiological conditions, tau binds and stabilizes microtubules, ensuring axonal transport integrity and supporting synaptic plasticity [190,191,192]. In AD, however, aberrant PTMs disrupt tau’s normal function, promoting its misfolding, aggregation, and eventual formation of neurofibrillary tangles (NFTs), which contribute to cytoskeletal destabilization and neuronal loss [193,194].
Tau is subject to a wide array of PTMs, including phosphorylation, acetylation, ubiquitination, methylation, glycosylation, nitration, and truncation, spanning approximately 100 residues [193,194]. However, not all of these modifications exhibit pathophysiological relevance in AD pathogenesis [195]. Increasing evidence indicates that PTMs within the MT-binding domain (MTBD) (encompasses two hexapeptide motifs, 244-368aa) of tau protein critically modulate its pathogenic properties and self-propagating seeding capacity [65,196,197,198,199]. For instance, acetylation within the MTBD is elevated during early-to-moderate Braak stages, preceding NFT formation [65,199], while seeding-competent tau species are predominantly hyperphosphorylated oligomers [200]. Importantly, many of these pathological modifications arise during the pre-tangle stage—prior to significant cytoskeletal disruption or neuronal death—suggesting a potential therapeutic window for targeted intervention. In this context, early-stage tau PTMs within the MTBD may represent critical molecular switches that trigger conformational changes, promote cytoskeletal disassembly, and initiate tau propagation. Therefore, we focus here on three major PTMs within the MTBD—acetylation, phosphorylation, and ubiquitination—emphasizing specific modification sites that are most strongly associated with early tau misfolding and cytoskeletal dysfunction in AD (Figure 2).
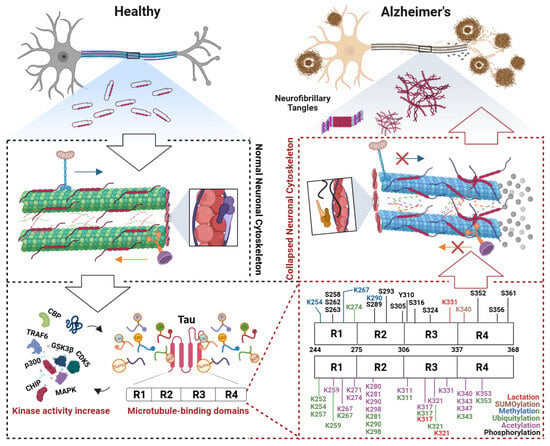
Figure 2.
Post-translational modifications of the tau microtubule-binding domain induce cytoskeletal collapse in Alzheimer’s disease. In early Alzheimer’s disease (AD), the microtubule-binding domain (MTBD) of tau undergoes aberrant post-translational modifications (PTMs). These modifications expose hydrophobic regions in tau, leading to its dissociation from microtubules and subsequent microtubule depolymerization. The collapse of axonal transport tracks disrupts the trafficking of mitochondria and synaptic vesicles, triggering synaptic energy crises and neurotransmitter release deficits. Concurrent actin cytoskeletal dysregulation—such as cofilin-mediated hyperactivation—exacerbates dendritic spine atrophy and synaptic plasticity loss. Dissociated tau undergoes conformational changes, forming filamentous aggregates that further assemble into oligomers capable of prion-like propagation, spreading pathology trans-synaptically. Microtubule destabilization activates stress-responsive pathways, which drive further tau hyperphosphorylation, forming a self-reinforcing vicious cycle. This cascade culminates in neuronal death and progressive cognitive decline, which are hallmarks of AD progression. Abbreviations: CBP, CREB-binding protein; TRAF6, TNF receptor associated factor 6; GSK3β, Glycogen synthase kinase-3 beta; CDK5, Cyclin-dependent Kinase 5; p300, E1A-binding protein; CHIP, C-terminus of Hsc70-Interacting protein; MAPK, Mitogen-activated protein kinase.
3.1. Acetylation
Tau acetylation has been extensively mapped through radioisotope labeling and immunoaffinity-based high-resolution mass spectrometry, revealing the presence of hundreds of acetylation sites across the protein [201]. This reversible modification is catalyzed by lysine acetyltransferases (KATs), which transfer acetyl groups (-COCH3) from acetyl-CoA to the ε-amino group of lysine residues, neutralizing their positive charge and altering protein charge distribution and conformational dynamics. Conversely, lysine deacetylases (KDACs) hydrolyze acetyl groups to restore lysine protonation. While initially characterized in histones, acetylation is now widely recognized as a major regulatory mechanism for numerous non-histone proteins, including tau [202].
In AD, tau acetylation is primarily mediated by p300/CBP acetyltransferases and reversed by HDAC6 and NAD+-dependent deacetylase SIRT1 [196,203]. Intriguingly, tau also exhibits intrinsic acetyltransferase activity, enabling it to undergo self-acetylation in the presence of acetyl-CoA. Pathologically, tau acetylation in AD occurs predominantly within its MTBD—a region critical for regulating tau’s pathogenic aggregation and prion-like seeding capacity [204,205]. This spatial preference for MTBD aggregation aligns with the observation that acetylation facilitates core tau protofilament formation [206,207]. Analyses of size-fractionated tau phase revealed that a minimal PTM repertoire localized to the MTBD governs seeding activity. High-density MTBD modifications exhibited positive correlations with seeding potency and fibril dimensions, with acetylation and ubiquitination (see below) being uniquely associated with seeding-competent tau [193]. These findings underscore the pivotal role of tau acetylation in generating pathogenic tau conformers.
In the human AD brain—but not in non-demented controls—specific acetylation sites within the MTBD of tau, including K274, K280, K281, and K311, are consistently hyperacetylated. These sites have been identified as key modulators of tau aggregation and neurotoxicity [208,209]. Experimental models demonstrate that transgenic Drosophila overexpressing human tau with an acetyl-mimetic K280Q mutation exhibit severe neurotoxicity, potentially linked to exacerbated phosphorylation at the S262 residue [210]. Similarly, transgenic mice expressing acetyl-mimetic K274Q/K281Q tau mutants show disrupted activity-dependent postsynaptic actin remodeling and impaired AMPA receptor insertion, recapitulating AD-associated memory deficits and hippocampal long-term potentiation (LTP) impairment [198]. Clinicopathological studies also reveal Braak stage-dependent dynamics, with acetyl-K274 levels significantly elevated in late-stage AD (Braak V/VI) compared to early stages (Braak I/II), particularly in neurons harboring tau inclusions [211]. Furthermore, brains from mild dementia AD cases (Clinical Dementia Rating, CDR = 0.5) exhibit higher acetyl-K281 levels than non-demented controls, while seeding-competent tau oligomers are selectively enriched in acetylated K281, K343, and K353 [193]. Primary cortical neurons exposed to tau seeds demonstrate increased acetylation at K280 and K369, correlating with enhanced aggregation propensity [212].
Given the critical role of acetylation in tau aggregation and propagation, further elucidation of its pathophysiological mechanisms is essential. Under physiological conditions, tau binds to microtubules and stabilizes the cytoskeleton, ensuring efficient axonal transport. However, lysine acetylation neutralizes the positive charge of tau, disrupting electrostatic interactions with negatively charged microtubule surfaces. This leads to tau-microtubule dissociation, resulting in compromised MT stability, axonal transport collapse [197,213,214], and the disruption of critical cargo trafficking—including neurotrophic factors and mitochondria—which collectively trigger neuronal metabolic dysfunction and synaptic failure [76,198]. The dissociated tau undergoes conformational changes, exposing hydrophobic regions that promote oligomerization and NFT formation. Acetylated tau further disrupts AMPA receptor localization at postsynaptic membranes and LTP [215], a defect that has been shown to be rescued by enhancing actin polymerization. Similarly, acetylated tau-induced destabilization and mislocalization at the axon initial segment can be rectified by microtubule-stabilizing agents such as Taxol D [211]. These findings underscore that acetylated tau-induced pathology not only compromises microtubule integrity but also disrupts actin-mediated synaptic plasticity, collectively destabilizing cytoskeletal homeostasis. However, emerging evidence suggests that cytoskeletal stabilizers may serve as a promising therapeutic strategy to counteract tau acetylation-driven pathology during the early stage of AD.
3.2. Phosphorylation
Phosphorylation, a critical PTM, dynamically regulates protein structure, function, and interactions by adding phosphate groups (-PO43−) to specific amino acid residues, including serine (S), threonine (T), or tyrosine residues [216]. Among all PTMs of tau, phosphorylation is the most prevalent, with at least 45 out of 85 potential S/T residues identified as phosphorylated in PHF-tau [202]. Unlike other PTMs that are regulated by a limited number of enzymes, tau phosphorylation involves a vast array of kinases and phosphatases, providing a high degree of regulatory complexity.
Tau phosphorylation is tightly regulated by a balance between kinases and phosphatases. On one hand, a broad array of kinases promotes the addition of phosphate groups to specific tau residues. These include glycogen synthase kinase-3β (GSK-3β), mitogen-activated protein kinase (MAPK), c-Jun N-terminal kinase (JNK), tau-tubulin kinase 1/2 (TTBK1/2), dual-specificity tyrosine-phosphorylation-regulated kinase 1A/2 (DYRK1A/2), microtubule affinity-regulating kinase (MARK), phosphorylase kinase (phK), protein kinase A (PKA), protein kinase B (PKB), protein kinase C (PKC), Ca2+/calmodulin-dependent protein kinase II (CaMKII), and casein kinase 1/2 (CK1/2) [217]. On the other hand, phosphatases counteract these effects by removing phosphate groups, thereby maintaining physiological tau phosphorylation levels. Major phosphatases include protein phosphatase 1 (PP1), PP2A, PP2B, PP5, and phosphatase and tensin homolog (PTEN) [218]. This enzymatic interplay allows for the precise and dynamic regulation of tau function under both physiological and pathological conditions.
Under physiological conditions, the moderate phosphorylation of tau does not impair its microtubule-binding affinity. In fact, it contributes to the regulation of neuronal cytoskeletal dynamics and axonal plasticity [219,220]. In contrast, in AD, tau becomes abnormally hyperphosphorylated, leading to its dissociation from microtubules and subsequent aggregation into NFTs. Consequently, identifying phosphorylation sites that reduce tau’s microtubule-binding affinity during early AD stages is critical. Given the pivotal role of MTBD-associated PTMs in early AD pathogenesis, elucidating phosphorylation events within the MTBD holds significant mechanistic and therapeutic relevance.
Phosphorylation at specific residues, including S258, S262, S324, and S256, has been demonstrated to alter microtubule stability, with S262 emerging as the most critical site [221,222]. In the temporal cortex of AD patients, phospho-S262 (p-S262) immunoreactivity is predominantly localized to non-fibrillar punctate structures within neurons at the pre-tangle stage, where dendritic morphology remains intact without observable folding or curvature [223]. The inhibition of S262 phosphorylation rescues early synaptic toxicity in hippocampal neurons, underscoring its pathogenic role in initial synaptic dysfunction [224]. Notably, phosphorylation at S262 has also been identified in high-molecular-weight (HMW) tau oligomers associated with seeding competence, suggesting its involvement in the prion-like propagation of tau pathology [193]. Intriguingly, the neurotoxicity induced by the acetyl-mimetic tau K280Q mutation in Drosophila models may be mediated through enhanced S262 phosphorylation [210], while the inactivation of K280 acetylation in SH-SY5Y cells reduces phosphorylation levels. Furthermore, phosphorylation within the MTBD activates tau’s intrinsic acetyltransferase activity, elevating acetylation levels and establishing a feedforward loop of PTM cross-talk [203]. Hyperphosphorylated tau also disrupts interactions with the ubiquitin ligase CHIP, impairing proteasomal degradation and promoting pathological aggregation [196]. These findings collectively reveal an intricate interplay between phosphorylation, acetylation, and ubiquitination within the MTBD, highlighting the need for integrated analyses to unravel the multi-layered mechanisms driving AD-associated tau pathology. Such complexity underscores the importance of adopting a systems-level approach to identify convergent therapeutic targets capable of disrupting this pathogenic PTM network.
The phosphorylation of the tau protein induces conformational changes that significantly weaken its electrostatic interactions with microtubules, reducing MT affinity and destabilizing MT networks through decreased polymerization rates [221,225]. Elevated tau phosphorylation promotes dissociation from microtubules, leading to its aberrant dendritic mislocalization—a process that compromises axonal microtubule integrity and promotes actin polymerization, induing synaptic dysfunction prior to widespread NFT formation in AD brains [81,226]. Dissociated hyperphosphorylated tau undergoes misfolding and oligomerization in the cytosol, and these soluble tau oligomers propagate via prion-like mechanisms across neurons while disrupting microtubule-associated motor proteins (e.g., kinesin, dynein), thereby amplifying cargo transport deficits [227]. The limited clinical efficacy of kinase-targeted therapies suggests that restoring cytoskeletal integrity—rather than solely inhibiting phosphorylation—may represent a critical intervention point to halt disease progression.
3.3. Ubiquitylation
Protein ubiquitination is executed through a hierarchical three-step enzymatic cascade: the E1 ubiquitin-activating enzyme activates ubiquitin via ATP hydrolysis to form a thioester intermediate, followed by ubiquitin transfer to the E2 conjugating enzyme, and finally, the E3 ligase facilitates covalent attachment of ubiquitin to substrate lysine residues. Ubiquitination can occur either as mono-ubiquitination or as polyubiquitination, with the latter forming chains through lysine linkages (e.g., K6, K11, K27, K29, K33, K48, K63). Notably, K48-linked chains predominantly targets substrates for 26S proteasomal degradation, whereas K63-linked chains regulate non-degradative processes such as DNA repair, inflammation, and membrane trafficking [228,229].
In AD, tau is hyperubiquitinated, with 28 identified ubiquitination sites in human AD brains—the highest number reported for any single protein [230]. Key E3 ligases are implicated in tau ubiquitination, including CHIP (C-terminus of HSC70-Interacting Protein), TRAF6 (TNF receptor-associated factor 6), and axotrophin/MARCH7. These enzymes often function in conjunction with molecular chaperones HSP70/90 to recognize misfolded or hypermodified (e.g., phosphorylated, acetylated) tau, thereby targeting it for degradation via the ubiquitin–proteasome system [231]. In mouse models, endogenous tau contains approximately 15 putative ubiquitination sites, predominantly localized to the MTBD [205]. Strikingly, seeding-competent tau soluble oligomers display a unique ubiquitination signature [193].
The mass spectrometry analysis of immunopurified soluble PHF-tau from AD brains identified three ubiquitination sites: K254, K353, and K311 [232]. An additional lysine residue, K290, located within the MTBD, was found to be ubiquitinated in AD mouse models, while K317 and K257 were confirmed in soluble tau oligomers from AD patients [193]. The temporal analysis of ubiquitination and tau oligomerization revealed that the progressive accumulation of soluble tau oligomers coincides with the ubiquitin-mediated maturation of these aggregates into insoluble filaments [207,233]. Furthermore, acetylation competitively occupies overlapping lysine residues (e.g., K280/K281) that are also targets for ubiquitination, thereby delaying tau degradation via the ubiquitin–proteasome system and promoting its pathological accumulation, hyperphosphorylation, and aggregation [196]. However, the role of ubiquitination in tau aggregation remains debated: while ubiquitin is a component of tau aggregates in AD brains, early-stage tau oligomers (“pro-tangles”) lack ubiquitin immunoreactivity, indicating that ubiquitination does not initiate oligomerization [234,235]. Instead, ubiquitination in soluble oligomers likely arises as a secondary response to aberrant phosphorylation. Evidence from AD NFTs and in vitro studies demonstrates that tau phosphorylation precedes ubiquitination, with PHF assembly occurring prior to ubiquitin attachment [236]. This temporal hierarchy suggests a molecular mechanism: aberrant phosphorylation induces conformational changes in tau, triggering oligomerization and subsequent ubiquitination as part of the cellular quality control system to target misfolded tau for proteasomal degradation. However, during persistent oligomerization, proteasomal overload and dysfunction allow insoluble aggregates to evade clearance, ultimately maturing into PHF [237].
3.4. Other PTMs
Beyond phosphorylation, acetylation, and ubiquitination, several additional PTMs within the MTBD of tau have been identified, although their roles in early AD remain less defined.
Methylation at residues such as K254, K290, and K267 has been detected in PHF-tau, potentially impairing ubiquitin-mediated degradation and contributing to tau accumulation [238]. Notably, many methylation sites overlap with acetylation targets [196], and methylation of K267 in PHF-tau has been associated with enhanced phosphorylation at S262 [238], suggesting cross-regulatory mechanisms between these PTMs. However, methylated tau predominantly co-localizes with NFTs in late-stage AD [238], raising questions about its early pathogenic relevance.
SUMOylating at K340 on hyperphosphorylated tau has been shown to inhibit ubiquitination and subsequent proteasomal degradation [239], with transgenic mouse models expressing SUMOylated tau exhibiting presynaptic dysfunction and reduced dendritic spine density [240]. Intriguingly, SUMOylation appears to be selective for microtubule-dissociated tau, suggesting that it may mark tau at a specific point along its pathological trajectory [241].
Lactylation, a recently discovered PTM driven by elevated lactate levels in AD brains, has been detected at K317, K321, and K331. Lactylation promotes tau phosphorylation, suppresses ubiquitination, and indirectly facilitates tau aggregation, thereby linking altered brain metabolism to tau pathology [242].
Collectively, PTMs represent critical mechanisms regulating protein function, subcellular localization, interactome dynamics, and turnover in both health and disease [190,192]. The transition of tau from an intrinsically disordered state to β-sheet-rich pathogenic aggregates is governed by PTM-induced conformational changes. For instance, aberrant PTMs neutralize the positive charge of lysine residues in tau, disrupting its electrostatic interaction with negatively charged microtubule surfaces. This results in tau dissociation from microtubules, leading to microtubule destabilization and axonal transport deficits. The liberated tau undergoes conformational shifts, with exposed hydrophobic motifs driving its adoption of pathological folds such as the “paperclip” conformation (N-terminal domain occluding the MTBD) or β-sheet oligomers stabilized by MTBD-localized hydrophobic domains (e.g., PHF6* [VQIINK] and PHF6 [VQIVYK]) [214,226,227,237,242]). These oligomers are further stabilized by prion-like protofilament architectures, enabling trans-synaptic propagation via heparan sulfate proteoglycans (HSPGs) on neuronal surfaces. HSPGs bind tau’s polybasic regions, mediate endocytosis, and seed aggregation in recipient cells [243,244]. Concurrently, cytoskeletal dysfunction arises as tau aggregates sequester actin-binding proteins (e.g., cofilin), stabilizing pathological actin rods and impairing synaptic plasticity [158,245], while interactions with neurofilaments form perinuclear inclusions that obstruct organelle transport and induce oxidative stress via disrupted mitochondrial dynamics [176]. Thus, restoring cytoskeletal balance and blocking this dual “loss-of-function” (microtubule destabilization) and “gain-of-toxic-function” (prion-like spread) cascade represents a promising therapeutic strategy for early-stage interventions in AD.
4. Perspectives
Current evidence identifies the phosphorylation and acetylation of the tau MTBD as pivotal PTMs driving microtubule destabilization in early AD. These PTMs initiate cytoskeletal collapse, synaptic dysfunction, and neurodegeneration by impairing microtubule integrity and axonal transport, while later-stage modifications (e.g., methylation, SUMOylation) may amplify toxicity or reflect compensatory responses to proteostatic stress. This remarkable mechanistic convergence underscores the therapeutic imperative to target cytoskeletal stabilization in AD. By preserving microtubule networks and maintaining actin cytoskeletal plasticity, these interventions prevent the collapse of axonal integrity caused by pathological tau dissociation, while enhanced actin dynamics support dendritic spine remodeling and AMPA receptor trafficking—processes critical for synaptic strength and LTP. This dual cytoskeletal stabilization strategy mitigates activity-dependent synaptic loss and axonal transport deficits, key hallmarks of early AD, while disrupting the self-perpetuating cycle of tau mislocalization, oligomerization, and prion-like propagation. These therapeutic approaches aim to restore homeostatic equilibrium between cytoskeletal stability and structural adaptability, thereby addressing the root pathological drivers of neurodegeneration rather than merely alleviating downstream consequences.
Preclinical studies Refs. [246,247,248,249,250,251,252,253] have validated microtubule-stabilizing agents (e.g., EpoD [246,247,248], NAP [249,250], and CNDR-51997 [251]) and actin modulators (e.g., fasudil [252]) in mitigating AD-associated tau pathology and restoring synaptic plasticity, with early-phase clinical trials demonstrating feasibility (Table 1). However, the therapeutic potential of cytoskeletal-stabilizing agents in early-stage AD has been demonstrated in preclinical studies. Despite these advances, translating cytoskeletal-targeted therapies into clinical success faces several key challenges: (1) model limitations: current transgenic animal models inadequately recapitulate the chronic progression and multifaceted microenvironment of human AD, contributing to discrepancies between preclinical efficacy (e.g., microtubule stabilizers in mice) and clinical trial outcomes; (2) pharmacokinetics: most cytoskeletal modulators—both MT-stabilizing agents and cofilin inhibitors—exhibit limited blood–brain barrier penetration, necessitating innovative delivery systems such as brain-targeted nanoparticles or small-molecule analogs engineered for enhanced bioavailability; (3) biomarker deficiency: the lack of reliable biomarkers to dynamically monitor microtubule stability or axonal transport restoration hinders optimal dose adjustment and therapeutic response evaluation; (4) multifactorial pathology: the multifactorial nature of AD pathology—encompassing tau aggregation, Aβ toxicity, and neuroinflammation—demands combinatorial approaches (e.g., MT stabilizers with anti-Aβ antibodies) to address intersecting pathogenic cascades; (5) timing of the intervention: clinical trials predominantly target late-stage AD patients, when cytoskeletal damage is irreversible, highlighting the need for early intervention during pre-tangle stages. However, the lack of robust biomarkers for identifying early cytoskeletal dysfunction in living patients remains a major barrier. Notably, while T181 and T217 are not located within tau’s MTBD, their PTMs, particularly phosphorylation, hold significant diagnostic value. Phosphorylated T181 and T217 exhibit abnormal elevations in CSF earlier than tau-PET positivity, providing a sensitive window for detecting prodromal AD. Emerging assays for plasma p-tau217 further underscore its potential as a minimally invasive tool for early diagnosis and therapeutic monitoring, potentially offering a critical time window for cytoskeletal-targeted interventions. To maximize clinical benefits, the interdisciplinary integration of advanced disease models, biomarker innovation (e.g., tau-PET tracers), and the precision targeting of cytoskeletal homeostasis in preclinical AD is essential—a strategy poised to disrupt tau-driven neurodegeneration at its roots.

Table 1.
Therapeutic interventions targeting cytoskeletal homeostasis in AD-associated tau pathology.
5. Conclusions
Preclinical studies of cytoskeletal stabilizers underscore their ability to reverse tau-induced axonal atrophy and synaptic loss, while early clinical trials affirm their tolerability. However, challenges in drug development highlight the complexity of translating cytoskeletal therapies, though interdisciplinary strategies hold promise for overcoming current limitations. Future success will require harmonizing mechanistic insights from improved disease models with clinical innovations to achieve the precision targeting of cytoskeletal homeostasis in prodromal AD.
Author Contributions
Conceptualization and funding acquisition, J.X.; writing—original draft preparation, G.J.; writing—review and editing, G.J. and G.X.; Investigation, X.L.; supervision oversight, J.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (Project No. 82271446 and 82471442 to J.X.), Major Project of Science and Technology Innovation of Hubei Province (2024BCA003).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
All figures were created using BioRender (https://app.biorender.com/, accessed on 25 April 2025). We extend our gratitude to the creators of BioRender for providing this platform.
Conflicts of Interest
The authors declare no competing interests.
References
- World Health Organization. Global Action Plan on the Public Health Response to Dementia 2017–2025; World Health Organization: Geneva, Switzerland, 2017; Available online: https://iris.who.int/handle/10665/259615 (accessed on 6 February 2025).
- Selkoe, D.J. The molecular pathology of Alzheimer’s disease. Neuron 1991, 6, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Qiu, Y.; Zhao, C.; Zhou, Z.; Bao, J.; Qian, W. The Role of Amyloid-Beta and Tau in the Early Pathogenesis of Alzheimer’s Disease. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2021, 27, e933084. [Google Scholar] [CrossRef] [PubMed]
- Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Available online: https://pubmed.ncbi.nlm.nih.gov/33302541/ (accessed on 26 March 2025).
- Sperling, R.A.; Donohue, M.C.; Raman, R.; Rafii, M.S.; Johnson, K.; Masters, C.L.; van Dyck, C.H.; Iwatsubo, T.; Marshall, G.A.; Yaari, R.; et al. Trial of Solanezumab in Preclinical Alzheimer’s Disease. N. Engl. J. Med. 2023, 389, 1096–1107. [Google Scholar] [CrossRef] [PubMed]
- Heidebrink, J.L.; Paulson, H.L. Lessons Learned from Approval of Aducanumab for Alzheimer’s Disease. Annu. Rev. Med. 2024, 75, 99–111. [Google Scholar] [CrossRef]
- Fillit, H.; Green, A. Aducanumab and the FDA—Where are we now? Nat. Rev. Neurol. 2021, 17, 129–130. [Google Scholar] [CrossRef]
- Lecanemab in Early Alzheimer’s Disease. Available online: https://pubmed.ncbi.nlm.nih.gov/36449413/ (accessed on 26 March 2025).
- Lecanemab: First Approval. Available online: https://pubmed.ncbi.nlm.nih.gov/36856953/ (accessed on 26 March 2025).
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef]
- Aisen, P.S.; Cummings, J.; Doody, R.; Kramer, L.; Salloway, S.; Selkoe, D.J.; Sims, J.; Sperling, R.A.; Vellas, B. The Future of Anti-Amyloid Trials. J. Prev. Alzheimers Dis. 2020, 7, 146–151. [Google Scholar] [CrossRef]
- Ackley, S.F.; Zimmerman, S.C.; Brenowitz, W.D.; Tchetgen Tchetgen, E.J.; Gold, A.L.; Manly, J.J.; Mayeda, E.R.; Filshtein, T.J.; Power, M.C.; Elahi, F.M.; et al. Effect of reductions in amyloid levels on cognitive change in randomized trials: Instrumental variable meta-analysis. BMJ 2021, 372, n156. [Google Scholar] [CrossRef]
- Lee, V.M.; Goedert, M.; Trojanowski, J.Q. Neurodegenerative tauopathies. Annu. Rev. Neurosci. 2001, 24, 1121–1159. [Google Scholar] [CrossRef]
- Hanseeuw, B.J.; Betensky, R.A.; Jacobs, H.I.L.; Schultz, A.P.; Sepulcre, J.; Becker, J.A.; Cosio, D.M.O.; Farrell, M.; Quiroz, Y.T.; Mormino, E.C.; et al. Association of Amyloid and Tau with Cognition in Preclinical Alzheimer Disease: A Longitudinal Study. JAMA Neurol. 2019, 76, 915–924. [Google Scholar] [CrossRef]
- Ossenkoppele, R.; Schonhaut, D.R.; Schöll, M.; Lockhart, S.N.; Ayakta, N.; Baker, S.L.; O’Neil, J.P.; Janabi, M.; Lazaris, A.; Cantwell, A.; et al. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer’s disease. Brain J. Neurol. 2016, 139 Pt 5, 1551–1567. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Götz, J. Tau-based therapies in neurodegeneration: Opportunities and challenges. Nat. Rev. Drug Discov. 2017, 16, 863–883. [Google Scholar] [CrossRef] [PubMed]
- Llibre-Guerra, J.J.; Fernandez, M.V.; Joseph-Mathurin, N.; Bian, S.; Carter, K.; Li, Y.; Aschenbrenner, A.J.; Pottier, C.; Sigurdson, W.; McDade, E.; et al. Longitudinal analysis of a dominantly inherited Alzheimer disease mutation carrier protected from dementia. Nat. Med. 2025, 31, 1267–1275. [Google Scholar] [CrossRef] [PubMed]
- Resilience to Autosomal Dominant Alzheimer’s Disease in a Reelin-COLBOS Heterozygous Man. Available online: https://pubmed.ncbi.nlm.nih.gov/37188781/ (accessed on 26 March 2025).
- Resistance to Autosomal Dominant Alzheimer’s Disease in an APOE3 Christchurch Homozygote: A Case Report. Available online: https://pubmed.ncbi.nlm.nih.gov/31686034/ (accessed on 26 March 2025).
- Christensen, K.R.; Combs, B.; Richards, C.; Grabinski, T.; Alhadidy, M.M.; Kanaan, N.M. Phosphomimetics at Ser199/Ser202/Thr205 in Tau Impairs Axonal Transport in Rat Hippocampal Neurons. Mol. Neurobiol. 2023, 60, 3423–3438. [Google Scholar] [CrossRef]
- Alzheimer’s Disease: Phenotypic Approaches Using Disease Models and the Targeting of Tau Protein. Available online: https://pubmed.ncbi.nlm.nih.gov/32116063/ (accessed on 26 March 2025).
- Tau Induces Inflammasome Activation and Microgliosis Through Acetylating NLRP3. Available online: https://pubmed.ncbi.nlm.nih.gov/38488468/ (accessed on 26 March 2025).
- Aggregated Tau Activates NLRP3-ASC Inflammasome Exacerbating Exogenously Seeded and Non-Exogenously Seeded Tau Pathology In Vivo. Available online: https://pubmed.ncbi.nlm.nih.gov/30721409/ (accessed on 26 March 2025).
- Kshirsagar, S.; Sawant, N.; Morton, H.; Reddy, A.P.; Reddy, P.H. Mitophagy enhancers against phosphorylated Tau-induced mitochondrial and synaptic toxicities in Alzheimer disease. Pharmacol. Res. 2021, 174, 105973. [Google Scholar] [CrossRef]
- Guha, S.; Johnson, G.V.W.; Nehrke, K. The Crosstalk Between Pathological Tau Phosphorylation and Mitochondrial Dysfunction as a Key to Understanding and Treating Alzheimer’s Disease. Mol. Neurobiol. 2020, 57, 5103–5120. [Google Scholar] [CrossRef]
- Glynn, C.; Rodriguez, J.A.; Hyman, B.T. The structural line between prion and “prion-like”: Insights from prion protein and tau. Curr. Opin. Neurobiol. 2024, 86, 102857. [Google Scholar] [CrossRef]
- Li, L.; Shi, R.; Gu, J.; Tung, Y.C.; Zhou, Y.; Zhou, D.; Wu, R.; Chu, D.; Jin, N.; Deng, K.; et al. Alzheimer’s disease brain contains tau fractions with differential prion-like activities. Acta Neuropathol. Commun. 2021, 9, 28. [Google Scholar] [CrossRef]
- Lymphocyte-Activation Gene 3 Facilitates Pathological Tau Neuron-to-Neuron Transmission. Available online: https://pubmed.ncbi.nlm.nih.gov/38327094/ (accessed on 26 March 2025).
- Weisenberg, R.C. Microtubule formation in vitro in solutions containing low calcium concentrations. Science 1972, 177, 1104–1105. [Google Scholar] [CrossRef]
- Muñoz-Lasso, D.C.; Romá-Mateo, C.; Pallardó, F.V.; Gonzalez-Cabo, P. Much More Than a Scaffold: Cytoskeletal Proteins in Neurological Disorders. Cells 2020, 9, 358. [Google Scholar] [CrossRef]
- Actin and Actin-Binding Proteins: Masters of Dendritic Spine Formation, Morphology, and Function. Available online: https://pubmed.ncbi.nlm.nih.gov/20717495/ (accessed on 26 March 2025).
- Jung, M.; Kim, D.; Mun, J.Y. Direct Visualization of Actin Filaments and Actin-Binding Proteins in Neuronal Cells. Front. Cell Dev. Biol. 2020, 8, 588556. [Google Scholar] [CrossRef] [PubMed]
- Yuan, A.; Rao, M.V.; Veeranna; Nixon, R.A. Neurofilaments and Neurofilament Proteins in Health and Disease. Cold Spring Harb. Perspect. Biol. 2017, 9, a018309. [Google Scholar] [CrossRef]
- Ding, E.A.; Kumar, S. Neurofilament Biophysics: From Structure to Biomechanics. Mol. Biol. Cell 2024, 35, re1. [Google Scholar] [CrossRef] [PubMed]
- Acosta, D.M.; Mancinelli, C.; Bracken, C.; Eliezer, D. Post-translational modifications within tau paired helical filament nucleating motifs perturb microtubule interactions and oligomer formation. J. Biol. Chem. 2022, 298, 101442. [Google Scholar] [CrossRef]
- Lee, V.M. Disruption of the cytoskeleton in Alzheimer’s disease. Curr. Opin. Neurobiol. 1995, 5, 663–668. [Google Scholar] [CrossRef]
- Guo, T.; Noble, W.; Hanger, D.P. Roles of tau protein in health and disease. Acta Neuropathol. 2017, 133, 665–704. [Google Scholar] [CrossRef]
- Grundke-Iqbal, I.; Iqbal, K.; Tung, Y.C.; Quinlan, M.; Wisniewski, H.M.; Binder, L.I. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc. Natl. Acad. Sci. USA 1986, 83, 4913–4917. [Google Scholar] [CrossRef]
- Ballatore, C.; Brunden, K.R.; Huryn, D.M.; Trojanowski, J.Q.; Lee, V.M.Y.; Smith, A.B. Microtubule stabilizing agents as potential treatment for Alzheimer’s disease and related neurodegenerative tauopathies. J. Med. Chem. 2012, 55, 8979–8996. [Google Scholar] [CrossRef]
- Miyasaka, T.; Sato, S.; Tatebayashi, Y.; Takashima, A. Microtubule destruction induces tau liberation and its subsequent phosphorylation. FEBS Lett. 2010, 584, 3227–3232. [Google Scholar] [CrossRef]
- Tashiro, A.; Minden, A.; Yuste, R. Regulation of dendritic spine morphology by the rho family of small GTPases: Antagonistic roles of Rac and Rho. Cereb. Cortex 2000, 10, 927–938. [Google Scholar] [CrossRef]
- Hall, A. Rho GTPases and the actin cytoskeleton. Science 1998, 279, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Tzioras, M.; McGeachan, R.I.; Durrant, C.S.; Spires-Jones, T.L. Synaptic degeneration in Alzheimer disease. Nat. Rev. Neurol. 2023, 19, 19–38. [Google Scholar] [CrossRef] [PubMed]
- Kelliher, M.T.; Saunders, H.A.; Wildonger, J. Microtubule control of functional architecture in neurons. Curr. Opin. Neurobiol. 2019, 57, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Conde, C.; Cáceres, A. Microtubule assembly, organization and dynamics in axons and dendrites. Nat. Rev. Neurosci. 2009, 10, 319–332. [Google Scholar] [CrossRef]
- Microtubules in Neurons as Information Carriers. Available online: https://pubmed.ncbi.nlm.nih.gov/24266899/ (accessed on 23 April 2025).
- Mohan, R.; John, A. Microtubule-associated proteins as direct crosslinkers of actin filaments and microtubules. IUBMB Life 2015, 67, 395–403. [Google Scholar] [CrossRef]
- Dogterom, M.; Koenderink, G.H. Actin-microtubule crosstalk in cell biology. Nat. Rev. Mol. Cell Biol. 2019, 20, 38–54. [Google Scholar] [CrossRef]
- Kapitein, L.C.; Hoogenraad, C.C. Building the Neuronal Microtubule Cytoskeleton. Neuron 2015, 87, 492–506. [Google Scholar] [CrossRef]
- Actin Dynamics in Growth Cone Motility and Navigation. Available online: https://pubmed.ncbi.nlm.nih.gov/24164353/ (accessed on 26 March 2025).
- Nicolini, G.; Monfrini, M.; Scuteri, A. Axonal Transport Impairment in Chemotherapy-Induced Peripheral Neuropathy. Toxics 2015, 3, 322–341. [Google Scholar] [CrossRef]
- Chevalier-Larsen, E.; Holzbaur, E.L.F. Axonal transport and neurodegenerative disease. Biochim. Biophys. Acta. 2006, 1762, 1094–1108. [Google Scholar] [CrossRef]
- Gibbs, K.L.; Greensmith, L.; Schiavo, G. Regulation of Axonal Transport by Protein Kinases. Trends Biochem. Sci. 2015, 40, 597–610. [Google Scholar] [CrossRef]
- Desai, A.; Mitchison, T.J. Microtubule polymerization dynamics. Annu. Rev. Cell Dev. Biol. 1997, 13, 83–117. [Google Scholar] [CrossRef] [PubMed]
- Burbank, K.S.; Mitchison, T.J. Microtubule dynamic instability. Curr. Biol. 2006, 16, R516–R517. [Google Scholar] [CrossRef] [PubMed]
- Goodson, H.V.; Jonasson, E.M. Microtubules and Microtubule-Associated Proteins. Cold Spring Harb. Perspect. Biol. 2018, 10, a022608. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, M.; Mitchison, T. Beyond self-assembly: From microtubules to morphogenesis. Cell 1986, 45, 329–342. [Google Scholar] [CrossRef]
- Gundersen, G.G.; Bretscher, A. Microtubule asymmetry. Science 2003, 300, 2040–2041. [Google Scholar] [CrossRef]
- Shelanski, M.L.; Gaskin, F.; Cantor, C.R. Microtubule assembly in the absence of added nucleotides. Proc. Natl. Acad. Sci. USA 1973, 70, 765–768. [Google Scholar] [CrossRef]
- Hancock, W.O. Bidirectional cargo transport: Moving beyond tug of war. Nat. Rev. Mol. Cell Biol. 2014, 15, 615–628. [Google Scholar] [CrossRef]
- Zempel, H.; Mandelkow, E. Lost after translation: Missorting of Tau protein and consequences for Alzheimer disease. Trends Neurosci. 2014, 37, 721–732. [Google Scholar] [CrossRef]
- Multiple Isoforms of Human Microtubule-Associated Protein Tau: Sequences and Localization in Neurofibrillary Tangles of Alzheimer’s Disease. Available online: https://pubmed.ncbi.nlm.nih.gov/2484340/ (accessed on 26 March 2025).
- Llorens-Martin, M.; Teixeira, C.M.; Fuster-Matanzo, A.; Jurado-Arjona, J.; Borrell, V.; Soriano, E.; Avila, J.; Hernández, F. Tau isoform with three microtubule binding domains is a marker of new axons generated from the subgranular zone in the hippocampal dentate gyrus: Implications for Alzheimer’s disease. J. Alzheimers Dis. 2012, 29, 921–930. [Google Scholar] [CrossRef]
- Tau Isoform Regulation is Region- and Cell-Specific in Mouse Brain. Available online: https://pubmed.ncbi.nlm.nih.gov/18925637/ (accessed on 26 March 2025).
- Tau in Physiology and Pathology. Available online: https://pubmed.ncbi.nlm.nih.gov/26631930/ (accessed on 26 March 2025).
- Tau Protein and Adult Hippocampal Neurogenesis. Available online: https://pubmed.ncbi.nlm.nih.gov/22787440/ (accessed on 26 March 2025).
- Avila, J.; Jiménez, J.S.; Sayas, C.L.; Bolós, M.; Zabala, J.C.; Rivas, G.; Hernández, F. Tau Structures. Front. Aging Neurosci. 2016, 8, 262. [Google Scholar] [CrossRef]
- Mukrasch, M.D.; von Bergen, M.; Biernat, J.; Fischer, D.; Griesinger, C.; Mandelkow, E.; Zweckstetter, M. The “jaws” of the tau-microtubule interaction. J. Biol. Chem. 2007, 282, 12230–12239. [Google Scholar] [CrossRef] [PubMed]
- Sillen, A.; Barbier, P.; Landrieu, I.; Lefebvre, S.; Wieruszeski, J.M.; Leroy, A.; Peyrot, V.; Lippens, G. NMR investigation of the interaction between the neuronal protein tau and the microtubules. Biochemistry 2007, 46, 3055–3064. [Google Scholar] [CrossRef] [PubMed]
- Kadavath, H.; Hofele, R.V.; Biernat, J.; Kumar, S.; Tepper, K.; Urlaub, H.; Mandelkow, E.; Zweckstetter, M. Tau stabilizes microtubules by binding at the interface between tubulin heterodimers. Proc. Natl. Acad. Sci. USA 2015, 112, 7501–7506. [Google Scholar] [CrossRef] [PubMed]
- Gustke, N.; Trinczek, B.; Biernat, J.; Mandelkow, E.M.; Mandelkow, E. Domains of tau protein and interactions with microtubules. Biochemistry 1994, 33, 9511–9522. [Google Scholar] [CrossRef]
- Bunker, J.M.; Wilson, L.; Jordan, M.A.; Feinstein, S.C. Modulation of microtubule dynamics by tau in living cells: Implications for development and neurodegeneration. Mol. Biol. Cell. 2004, 15, 2720–2728. [Google Scholar] [CrossRef]
- FTDP-17 Mutations Compromise the Ability of Tau to Regulate Microtubule Dynamics in Cells. Available online: https://pubmed.ncbi.nlm.nih.gov/16495230/ (accessed on 26 March 2025).
- Breuzard, G.; Hubert, P.; Nouar, R.; De Bessa, T.; Devred, F.; Barbier, P.; Sturgis, J.N.; Peyrot, V. Molecular mechanisms of Tau binding to microtubules and its role in microtubule dynamics in live cells. J. Cell Sci. 2013, 126 Pt 13, 2810–2819. [Google Scholar] [CrossRef]
- Castle, B.T.; McKibben, K.M.; Rhoades, E.; Odde, D.J. Tau Avoids the GTP Cap at Growing Microtubule Plus-Ends. iScience 2020, 23, 101782. [Google Scholar] [CrossRef]
- Dixit, R.; Ross, J.L.; Goldman, Y.E.; Holzbaur, E.L.F. Differential regulation of dynein and kinesin motor proteins by tau. Science 2008, 319, 1086–1089. [Google Scholar] [CrossRef]
- Siahaan, V.; Krattenmacher, J.; Hyman, A.A.; Diez, S.; Hernández-Vega, A.; Lansky, Z.; Braun, M. Kinetically distinct phases of tau on microtubules regulate kinesin motors and severing enzymes. Nat. Cell Biol. 2019, 21, 1086–1092. [Google Scholar] [CrossRef]
- Microtubules Gate Tau Condensation to Spatially Regulate Microtubule Functions. Available online: https://pubmed.ncbi.nlm.nih.gov/31481790/ (accessed on 26 March 2025).
- Overexpression of Tau Protein Inhibits Kinesin-Dependent Trafficking of Vesicles, Mitochondria, and Endoplasmic Reticulum: Implications for Alzheimer’s Disease. Available online: https://pubmed.ncbi.nlm.nih.gov/9813097/ (accessed on 26 March 2025).
- Vershinin, M.; Carter, B.C.; Razafsky, D.S.; King, S.J.; Gross, S.P. Multiple-motor based transport and its regulation by Tau. Proc. Natl. Acad. Sci. USA 2007, 104, 87–92. [Google Scholar] [CrossRef]
- Dendritic Function of Tau Mediates Amyloid-Beta Toxicity in Alzheimer’s Disease Mouse Models. Available online: https://pubmed.ncbi.nlm.nih.gov/20655099/ (accessed on 26 March 2025).
- Activity-Dependent Tau Protein Translocation to Excitatory Synapse is Disrupted by Exposure to Amyloid-Beta Oligomers. Available online: https://pubmed.ncbi.nlm.nih.gov/24760868/ (accessed on 26 March 2025).
- Chen, Q.; Zhou, Z.; Zhang, L.; Wang, Y.; Zhang, Y.W.; Zhong, M.; Xu, S.C.; Chen, C.H.; Li, L.; Yu, Z.P. Tau protein is involved in morphological plasticity in hippocampal neurons in response to BDNF. Neurochem. Int. 2012, 60, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Novel Function of Tau in Regulating the Effects of External Stimuli on Adult Hippocampal Neurogenesis. Available online: https://pubmed.ncbi.nlm.nih.gov/27198172/ (accessed on 26 March 2025).
- Interaction Between the Guanylate Kinase Domain of PSD-95 and the Proline-Rich Region and Microtubule Binding Repeats 2 and 3 of Tau. Available online: https://pubmed.ncbi.nlm.nih.gov/33794133/ (accessed on 26 March 2025).
- Interaction of Endogenous Tau Protein with Synaptic Proteins Is Regulated by N-Methyl-D-Aspartate Receptor-Dependent Tau Phosphorylation. Available online: https://pubmed.ncbi.nlm.nih.gov/22833681/ (accessed on 26 March 2025).
- The MAP1 Family of Microtubule-Associated Proteins. Available online: https://pubmed.ncbi.nlm.nih.gov/16938900/ (accessed on 27 March 2025).
- Bloom, G.S.; Luca, F.C.; Vallee, R.B. Microtubule-associated protein 1B: Identification of a major component of the neuronal cytoskeleton. Proc. Natl. Acad. Sci. USA 1985, 82, 5404–5408. [Google Scholar] [CrossRef] [PubMed]
- Microtubule-Associated Proteins (MAPs): A Monoclonal Antibody to MAP 1 Decorates Microtubules In Vitro but Stains Stress Fibers and not Microtubules In Vivo. Available online: https://pubmed.ncbi.nlm.nih.gov/3883359/ (accessed on 27 March 2025).
- Tögel, M.; Wiche, G.; Propst, F. Novel features of the light chain of microtubule-associated protein MAP1B: Microtubule stabilization, self interaction, actin filament binding, and regulation by the heavy chain. J. Cell Biol. 1998, 143, 695–707. [Google Scholar] [CrossRef] [PubMed]
- Orbán-Németh, Z.; Simader, H.; Badurek, S.; Tranciková, A.; Propst, F. Microtubule-associated protein 1S, a short and ubiquitously expressed member of the microtubule-associated protein 1 family. J. Biol. Chem. 2005, 280, 2257–2265. [Google Scholar] [CrossRef]
- Kwei, S.L.; Clement, A.; Faissner, A.; Brandt, R. Differential interactions of MAP2, tau and MAP5 during axogenesis in culture. Neuroreport 1998, 9, 1035–1040. [Google Scholar] [CrossRef]
- Kaech, S.; Fischer, M.; Doll, T.; Matus, A. Isoform specificity in the relationship of actin to dendritic spines. J. Neurosci. Off. J. Soc. Neurosci. 1997, 17, 9565–9572. [Google Scholar] [CrossRef]
- Shirao, T.; González-Billault, C. Actin filaments and microtubules in dendritic spines. J. Neurochem. 2013, 126, 155–164. [Google Scholar] [CrossRef]
- Microtubule-Associated Protein 2 Mediates Induction of Long-Term Potentiation in Hippocampal Neurons. Available online: https://pubmed.ncbi.nlm.nih.gov/32237183/ (accessed on 27 March 2025).
- Seitz, A.; Kojima, H.; Oiwa, K.; Mandelkow, E.M.; Song, Y.H.; Mandelkow, E. Single-molecule investigation of the interference between kinesin, tau and MAP2c. EMBO J. 2002, 21, 4896–4905. [Google Scholar] [CrossRef]
- More than a Marker: Potential Pathogenic Functions of MAP2. Available online: https://pubmed.ncbi.nlm.nih.gov/36187353/ (accessed on 27 March 2025).
- Burger, D.; Stihle, M.; Sharma, A.; Di Lello, P.; Benz, J.; D’Arcy, B.; Debulpaep, M.; Fry, D.; Huber, W.; Kremer, T.; et al. Crystal Structures of the Human Doublecortin C- and N-terminal Domains in Complex with Specific Antibodies. J. Biol. Chem. 2016, 291, 16292–16306. [Google Scholar] [CrossRef]
- Taylor, K.R.; Holzer, A.K.; Bazan, J.F.; Walsh, C.A.; Gleeson, J.G. Patient mutations in doublecortin define a repeated tubulin-binding domain. J. Biol. Chem. 2000, 275, 34442–34450. [Google Scholar] [CrossRef]
- Fu, X.; Brown, K.J.; Yap, C.C.; Winckler, B.; Jaiswal, J.K.; Liu, J.S. Doublecortin (Dcx) family proteins regulate filamentous actin structure in developing neurons. J. Neurosci. Off. J. Soc. Neurosci. 2013, 33, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Fourniol, F.J.; Sindelar, C.V.; Amigues, B.; Clare, D.K.; Thomas, G.; Perderiset, M.; Francis, F.; Houdusse, A.; Moores, C.A. Template-free 13-protofilament microtubule-MAP assembly visualized at 8 A resolution. J. Cell Biol. 2010, 191, 463–470. [Google Scholar] [CrossRef]
- Liu, J.S.; Schubert, C.R.; Fu, X.; Fourniol, F.J.; Jaiswal, J.K.; Houdusse, A.; Stultz, C.M.; Moores, C.A.; Walsh, C.A. Molecular basis for specific regulation of neuronal kinesin-3 motors by doublecortin family proteins. Mol. Cell 2012, 47, 707–721. [Google Scholar] [CrossRef] [PubMed]
- Moores, C.A.; Perderiset, M.; Francis, F.; Chelly, J.; Houdusse, A.; Milligan, R.A. Mechanism of microtubule stabilization by doublecortin. Mol. Cell 2004, 14, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, A.; van Haren, J.; Ribeiro, S.A.; Wittmann, T. Doublecortin Is Excluded from Growing Microtubule Ends and Recognizes the GDP-Microtubule Lattice. Curr. Biol. 2016, 26, 1549–1555. [Google Scholar] [CrossRef]
- Brandt, R.; Bakota, L. Microtubule dynamics and the neurodegenerative triad of Alzheimer’s disease: The hidden connection. J. Neurochem. 2017, 143, 409–417. [Google Scholar] [CrossRef]
- Huxley, H.E. Electron microscope studies on the structure of natural and synthetic protein filaments from striated muscle. J. Mol. Biol. 1963, 7, 281–308. [Google Scholar] [CrossRef]
- Santerre, R.F.; Rich, A. Actin accumulation in developing chick brain and other tissues. Dev. Biol. 1976, 54, 1–12. [Google Scholar] [CrossRef]
- Clark, S.E.; Moss, D.J.; Bray, D. Actin polymerization and synthesis in cultured neurones. Exp. Cell Res. 1983, 147, 303–314. [Google Scholar] [CrossRef]
- Letourneau, P.C. Actin in axons: Stable scaffolds and dynamic filaments. Results Probl. Cell Differ. 2009, 48, 65–90. [Google Scholar] [CrossRef]
- Control of Synapse Structure and Function by Actin and Its Regulators. Available online: https://pubmed.ncbi.nlm.nih.gov/35203254/ (accessed on 27 March 2025).
- Carlier, M.F. Actin polymerization and ATP hydrolysis. Adv. Biophys. 1990, 26, 51–73. [Google Scholar] [CrossRef] [PubMed]
- Pollard, T.D.; Cooper, J.A. Actin, a central player in cell shape and movement. Science 2009, 326, 1208–1212. [Google Scholar] [CrossRef] [PubMed]
- Lowery, L.A.; Van Vactor, D. The trip of the tip: Understanding the growth cone machinery. Nat. Rev. Mol. Cell Biol. 2009, 10, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Pring, M.; Evangelista, M.; Boone, C.; Yang, C.; Zigmond, S.H. Mechanism of formin-induced nucleation of actin filaments. Biochemistry 2003, 42, 486–496. [Google Scholar] [CrossRef]
- Paul, A.S.; Pollard, T.D. Review of the mechanism of processive actin filament elongation by formins. Cell Motil. Cytoskelet. 2009, 66, 606–617. [Google Scholar] [CrossRef]
- Craig, E.M.; Van Goor, D.; Forscher, P.; Mogilner, A. Membrane tension, myosin force, and actin turnover maintain actin treadmill in the nerve growth cone. Biophys. J. 2012, 102, 1503–1513. [Google Scholar] [CrossRef]
- Mechanochemical Regulation of Growth Cone Motility. Available online: https://pubmed.ncbi.nlm.nih.gov/26217175/ (accessed on 27 March 2025).
- Omotade, O.F.; Pollitt, S.L.; Zheng, J.Q. Actin-based growth cone motility and guidance. Mol. Cell Neurosci. 2017, 84, 4–10. [Google Scholar] [CrossRef]
- Schneider, F.; Metz, I.; Rust, M.B. Regulation of actin filament assembly and disassembly in growth cone motility and axon guidance. Brain Res. Bull. 2023, 192, 21–35. [Google Scholar] [CrossRef]
- Lin, C.H.; Forscher, P. Growth cone advance is inversely proportional to retrograde F-actin flow. Neuron 1995, 14, 763–771. [Google Scholar] [CrossRef]
- Craig, E.M.; Stricker, J.; Gardel, M.; Mogilner, A. Model for adhesion clutch explains biphasic relationship between actin flow and traction at the cell leading edge. Phys. Biol. 2015, 12, 035002. [Google Scholar] [CrossRef]
- Nichol, R.H.; Hagen, K.M.; Lumbard, D.C.; Dent, E.W.; Gómez, T.M. Guidance of Axons by Local Coupling of Retrograde Flow to Point Contact Adhesions. J. Neurosci. Off. J. Soc. Neurosci. 2016, 36, 2267–2282. [Google Scholar] [CrossRef] [PubMed]
- Schneider, F.; Duong, T.A.; Rust, M.B. Neuron Replating, a Powerful and Versatile Approach to Study Early Aspects of Neuron Differentiation. eNeuro 2021, 8, ENEURO.0536-20.2021. [Google Scholar] [CrossRef] [PubMed]
- Attention-Deficit/Hyperactivity Disorder-Like Phenotype in a Mouse Model with Impaired Actin Dynamics. Available online: https://pubmed.ncbi.nlm.nih.gov/24768258/ (accessed on 27 March 2025).
- Medeiros, N.A.; Burnette, D.T.; Forscher, P. Myosin II functions in actin-bundle turnover in neuronal growth cones. Nat. Cell Biol. 2006, 8, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Myosin 1b Promotes Axon Formation by Regulating Actin Wave Propagation and Growth Cone Dynamics. Available online: https://pubmed.ncbi.nlm.nih.gov/29588377/ (accessed on 27 March 2025).
- Bamburg, J.R.; Bernstein, B.W.; Davis, R.C.; Flynn, K.C.; Goldsbury, C.; Jensen, J.R.; Maloney, M.T.; Marsden, I.T.; Minamide, L.S.; Pak, C.W.; et al. ADF/Cofilin-actin rods in neurodegenerative diseases. Curr. Alzheimer Res. 2010, 7, 241–250. [Google Scholar] [CrossRef]
- Cai, R.; Wang, Y.; Huang, Z.; Zou, Q.; Pu, Y.; Yu, C.; Cai, Z. Role of RhoA/ROCK signaling in Alzheimer’s disease. Behav. Brain Res. 2021, 414, 113481. [Google Scholar] [CrossRef]
- Harris, K.M.; Jensen, F.E.; Tsao, B. Three-dimensional structure of dendritic spines and synapses in rat hippocampus (CA1) at postnatal day 15 and adult ages: Implications for the maturation of synaptic physiology and long-term potentiation. J. Neurosci. Off. J. Soc. Neurosci. 1992, 12, 2685–2705. [Google Scholar] [CrossRef]
- Izeddin, I.; Specht, C.G.; Lelek, M.; Darzacq, X.; Triller, A.; Zimmer, C.; Dahan, M. Super-resolution dynamic imaging of dendritic spines using a low-affinity photoconvertible actin probe. PLoS ONE 2011, 6, e15611. [Google Scholar] [CrossRef]
- Harris, K.M.; Stevens, J.K. Dendritic spines of CA 1 pyramidal cells in the rat hippocampus: Serial electron microscopy with reference to their biophysical characteristics. J. Neurosci. Off. J. Soc. Neurosci. 1989, 9, 2982–2997. [Google Scholar] [CrossRef]
- Dendritic Spine Geometry is Critical for AMPA Receptor Expression in Hippocampal CA1 Pyramidal Neurons. Available online: https://pubmed.ncbi.nlm.nih.gov/11687814/ (accessed on 27 March 2025).
- Takumi, Y.; Ramírez-León, V.; Laake, P.; Rinvik, E.; Ottersen, O.P. Different modes of expression of AMPA and NMDA receptors in hippocampal synapses. Nat. Neurosci. 1999, 2, 618–624. [Google Scholar] [CrossRef]
- Noguchi, J.; Matsuzaki, M.; Ellis-Davies, G.C.R.; Kasai, H. Spine-neck geometry determines NMDA receptor-dependent Ca2+ signaling in dendrites. Neuron 2005, 46, 609–622. [Google Scholar] [CrossRef]
- Lamprecht, R. Actin Cytoskeleton Role in the Maintenance of Neuronal Morphology and Long-Term Memory. Cells 2021, 10, 1795. [Google Scholar] [CrossRef] [PubMed]
- Gray, E.G. Axo-somatic and axo-dendritic synapses of the cerebral cortex: An electron microscope study. J. Anat. 1959, 93 Pt 4, 420–433. [Google Scholar] [PubMed]
- Matsuzaki, M.; Honkura, N.; Ellis-Davies, G.C.R.; Kasai, H. Structural basis of long-term potentiation in single dendritic spines. Nature 2004, 429, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Homma, K.J.; Poo, M.M. Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron 2004, 44, 749–757. [Google Scholar] [CrossRef]
- Hotulainen, P.; Hoogenraad, C.C. Actin in dendritic spines: Connecting dynamics to function. J. Cell Biol. 2010, 189, 619–629. [Google Scholar] [CrossRef]
- Landis, D.M.; Reese, T.S. Cytoplasmic organization in cerebellar dendritic spines. J. Cell Biol. 1983, 97, 1169–1178. [Google Scholar] [CrossRef]
- Fifková, E.; Delay, R.J. Cytoplasmic actin in neuronal processes as a possible mediator of synaptic plasticity. J. Cell Biol. 1982, 95, 345–350. [Google Scholar] [CrossRef]
- Actin, Spectrin, and Associated Proteins Form a Periodic Cytoskeletal Structure in Axons. Available online: https://pubmed.ncbi.nlm.nih.gov/23239625/ (accessed on 27 March 2025).
- D’Este, E.; Kamin, D.; Göttfert, F.; El-Hady, A.; Hell, S.W. STED nanoscopy reveals the ubiquity of subcortical cytoskeleton periodicity in living neurons. Cell Rep. 2015, 10, 1246–1251. [Google Scholar] [CrossRef]
- Periodic F-Actin Structures Shape the Neck of Dendritic Spines. Available online: https://pubmed.ncbi.nlm.nih.gov/27841352/ (accessed on 27 March 2025).
- Blanchoin, L.; Amann, K.J.; Higgs, H.N.; Marchand, J.B.; Kaiser, D.A.; Pollard, T.D. Direct observation of dendritic actin filament networks nucleated by Arp2/3 complex and WASP/Scar proteins. Nature 2000, 404, 1007–1011. [Google Scholar] [CrossRef]
- Kim, I.H.; Racz, B.; Wang, H.; Burianek, L.; Weinberg, R.; Yasuda, R.; Wetsel, W.C.; Soderling, S.H. Disruption of Arp2/3 results in asymmetric structural plasticity of dendritic spines and progressive synaptic and behavioral abnormalities. J. Neurosci. Off. J. Soc. Neurosci. 2013, 33, 6081–6092. [Google Scholar] [CrossRef]
- Defining Mechanisms of Actin Polymerization and Depolymerization During Dendritic Spine Morphogenesis. Available online: https://pubmed.ncbi.nlm.nih.gov/19380880/ (accessed on 27 March 2025).
- Fleming, I.N.; Elliott, C.M.; Buchanan, F.G.; Downes, C.P.; Exton, J.H. Ca2+/calmodulin-dependent protein kinase II regulates Tiam1 by reversible protein phosphorylation. J. Biol. Chem. 1999, 274, 12753–12758. [Google Scholar] [CrossRef] [PubMed]
- RICS, a Novel GTPase-Activating Protein for Cdc42 and Rac1, is Involved in the Beta-Catenin-N-Cadherin and N-Methyl-D-Aspartate Receptor Signaling. Available online: https://pubmed.ncbi.nlm.nih.gov/12531901/ (accessed on 27 March 2025).
- The Rac1-GEF Tiam1 Couples the NMDA Receptor to the Activity-Dependent Development of Dendritic Arbors and Spines. Available online: https://pubmed.ncbi.nlm.nih.gov/15721239/ (accessed on 27 March 2025).
- Xie, Z.; Srivastava, D.P.; Photowala, H.; Kai, L.; Cahill, M.E.; Woolfrey, K.M.; Shum, C.Y.; Surmeier, D.J.; Penzes, P. Kalirin-7 controls activity-dependent structural and functional plasticity of dendritic spines. Neuron 2007, 56, 640–656. [Google Scholar] [CrossRef] [PubMed]
- Local, Persistent Activation of Rho GTPases During Plasticity of Single Dendritic Spines. Available online: https://pubmed.ncbi.nlm.nih.gov/21423166/ (accessed on 27 March 2025).
- Lai, F.P.L.; Szczodrak, M.; Block, J.; Faix, J.; Breitsprecher, D.; Mannherz, H.G.; Stradal, T.E.; Dunn, G.A.; Small, J.V.; Rottner, K. Arp2/3 complex interactions and actin network turnover in lamellipodia. EMBO J. 2008, 27, 982–992. [Google Scholar] [CrossRef]
- Ghosh, M.; Song, X.; Mouneimne, G.; Sidani, M.; Lawrence, D.S.; Condeelis, J.S. Cofilin promotes actin polymerization and defines the direction of cell motility. Science 2004, 304, 743–746. [Google Scholar] [CrossRef]
- Sheng, M.; Hoogenraad, C.C. The postsynaptic architecture of excitatory synapses: A more quantitative view. Annu. Rev. Biochem. 2007, 76, 823–847. [Google Scholar] [CrossRef]
- Han, K.; Holder, J.L.; Schaaf, C.P.; Lu, H.; Chen, H.; Kang, H.; Tang, J.; Wu, Z.; Hao, S.; Cheung, S.W.; et al. SHANK3 overexpression causes manic-like behaviour with unique pharmacogenetic properties. Nature 2013, 503, 72–77. [Google Scholar] [CrossRef]
- Chazeau, A.; Mehidi, A.; Nair, D.; Gautier, J.J.; Leduc, C.; Chamma, I.; Kage, F.; Kechkar, A.; Thoumine, O.; Rottner, K.; et al. Nanoscale segregation of actin nucleation and elongation factors determines dendritic spine protrusion. EMBO J. 2014, 33, 2745–2764. [Google Scholar] [CrossRef]
- Role of Cofilin in Alzheimer’s Disease. Available online: https://pubmed.ncbi.nlm.nih.gov/33324642/ (accessed on 23 May 2025).
- Kang, D.E.; Woo, J.A. Cofilin, a Master Node Regulating Cytoskeletal Pathogenesis in Alzheimer’s Disease. J. Alzheimers Dis. 2019, 72, S131–S144. [Google Scholar] [CrossRef]
- Chevy, Q.; Heubl, M.; Goutierre, M.; Backer, S.; Moutkine, I.; Eugène, E.; Bloch-Gallego, E.; Lévi, S.; Poncer, J.C. KCC2 Gates Activity-Driven AMPA Receptor Traffic through Cofilin Phosphorylation. J. Neurosci. Off. J. Soc. Neurosci. 2015, 35, 15772–15786. [Google Scholar] [CrossRef]
- Frost, N.A.; Kerr, J.M.; Lu, H.E.; Blanpied, T.A. A network of networks: Cytoskeletal control of compartmentalized function within dendritic spines. Curr. Opin. Neurobiol. 2010, 20, 578–587. [Google Scholar] [CrossRef]
- Cabrales Fontela, Y.; Kadavath, H.; Biernat, J.; Riedel, D.; Mandelkow, E.; Zweckstetter, M. Multivalent cross-linking of actin filaments and microtubules through the microtubule-associated protein Tau. Nat. Commun. 2017, 8, 1981. [Google Scholar] [CrossRef] [PubMed]
- Quintanilla, R.A.; Tapia-Monsalves, C.; Vergara, E.H.; Pérez, M.J.; Aranguiz, A. Truncated Tau Induces Mitochondrial Transport Failure Through the Impairment of TRAK2 Protein and Bioenergetics Decline in Neuronal Cells. Front. Cell Neurosci. 2020, 14, 175. [Google Scholar] [CrossRef] [PubMed]
- Gafson, A.R.; Barthélemy, N.R.; Bomont, P.; Carare, R.O.; Durham, H.D.; Julien, J.P.; Kuhle, J.; Leppert, D.; Nixon, R.A.; Weller, R.O.; et al. Neurofilaments: Neurobiological foundations for biomarker applications. Brain J. Neurol. 2020, 143, 1975–1998. [Google Scholar] [CrossRef]
- Bomont, P. The dazzling rise of neurofilaments: Physiological functions and roles as biomarkers. Curr. Opin. Cell Biol. 2021, 68, 181–191. [Google Scholar] [CrossRef]
- Yuan, A.; Nixon, R.A. Neurofilament Proteins as Biomarkers to Monitor Neurological Diseases and the Efficacy of Therapies. Front. Neurosci. 2021, 15, 689938. [Google Scholar] [CrossRef]
- Neurofilament Light Chain as a Biomarker for Diagnosis of Multiple Sclerosis. Available online: https://pubmed.ncbi.nlm.nih.gov/34602928/ (accessed on 27 March 2025).
- Verde, F.; Otto, M.; Silani, V. Neurofilament Light Chain as Biomarker for Amyotrophic Lateral Sclerosis and Frontotemporal Dementia. Front. Neurosci. 2021, 15, 679199. [Google Scholar] [CrossRef]
- Neurofilaments as Biomarkers in Neurological Disorders—Towards Clinical Application. Available online: https://pubmed.ncbi.nlm.nih.gov/38609644/ (accessed on 27 March 2025).
- Heckler, I.; Venkataraman, I. Phosphorylated neurofilament heavy chain: A potential diagnostic biomarker in amyotrophic lateral sclerosis. J. Neurophysiol. 2022, 127, 737–745. [Google Scholar] [CrossRef]
- Chen, J.; Nakata, T.; Zhang, Z.; Hirokawa, N. The C-terminal tail domain of neurofilament protein-H (NF-H) forms the crossbridges and regulates neurofilament bundle formation. J. Cell Sci. 2000, 113 Pt 21, 3861–3869. [Google Scholar] [CrossRef]
- Rao, M.V.; Garcia, M.L.; Miyazaki, Y.; Gotow, T.; Yuan, A.; Mattina, S.; Ward, C.M.; Calcutt, N.A.; Uchiyama, Y.; Nixon, R.A.; et al. Gene replacement in mice reveals that the heavily phosphorylated tail of neurofilament heavy subunit does not affect axonal caliber or the transit of cargoes in slow axonal transport. J. Cell Biol. 2002, 158, 681–693. [Google Scholar] [CrossRef]
- Rao, M.V.; Campbell, J.; Yuan, A.; Kumar, A.; Gotow, T.; Uchiyama, Y.; Nixon, R.A. The neurofilament middle molecular mass subunit carboxyl-terminal tail domains is essential for the radial growth and cytoskeletal architecture of axons but not for regulating neurofilament transport rate. J. Cell Biol. 2003, 163, 1021–1031. [Google Scholar] [CrossRef]
- Angelides, K.J.; Smith, K.E.; Takeda, M. Assembly and exchange of intermediate filament proteins of neurons: Neurofilaments are dynamic structures. J. Cell Biol. 1989, 108, 1495–1506. [Google Scholar] [CrossRef] [PubMed]
- van Asperen, J.V.; Kotaich, F.; Caillol, D.; Bomont, P. Neurofilaments: Novel findings and future challenges. Curr. Opin. Cell Biol. 2024, 87, 102326. [Google Scholar] [CrossRef] [PubMed]
- Devarakonda, S.S.; Basha, S.; Pithakumar, A.; Mukunda, D.C.; Rodrigues, J.; Ameera, K.; Biswas, S.; Pai, A.R.; Belurkar, S.; Mahato, K.K. Molecular mechanisms of neurofilament alterations and its application in assessing neurodegenerative disorders. Ageing Res. Rev. 2024, 102, 102566. [Google Scholar] [CrossRef] [PubMed]
- Yuan, A.; Sasaki, T.; Rao, M.V.; Kumar, A.; Kanumuri, V.; Dunlop, D.S.; Liem, R.K.; Nixon, R.A. Neurofilaments form a highly stable stationary cytoskeleton after reaching a critical level in axons. J. Neurosci. Off. J. Soc. Neurosci. 2009, 29, 11316–11329. [Google Scholar] [CrossRef]
- Local Control of Neurofilament Accumulation During Radial Growth of Myelinating Axons In Vivo. Selective Role of Site-Specific Phosphorylation. Available online: https://pubmed.ncbi.nlm.nih.gov/11086003/ (accessed on 27 March 2025).
- Ohara, O.; Gahara, Y.; Miyake, T.; Teraoka, H.; Kitamura, T. Neurofilament deficiency in quail caused by nonsense mutation in neurofilament-L gene. J. Cell Biol. 1993, 121, 387–395. [Google Scholar] [CrossRef]
- Yamasaki, H.; Itakura, C.; Mizutani, M. Hereditary hypotrophic axonopathy with neurofilament deficiency in a mutant strain of the Japanese quail. Acta Neuropathol. 1991, 82, 427–434. [Google Scholar] [CrossRef]
- Dale, J.M.; Garcia, M.L. Neurofilament Phosphorylation during Development and Disease: Which Came First, the Phosphorylation or the Accumulation? J. Amino Acids 2012, 2012, 382107. [Google Scholar] [CrossRef]
- Reduced Diameter and Conduction Velocity of Myelinated Fibers in the Sciatic Nerve of a Neurofilament-Deficient Mutant Quail. Available online: https://pubmed.ncbi.nlm.nih.gov/8510825/ (accessed on 27 March 2025).
- Electrophysiological Properties of Axons in Mice Lacking Neurofilament Subunit Genes: Disparity Between Conduction Velocity and Axon Diameter in Absence of NF-H. Available online: https://pubmed.ncbi.nlm.nih.gov/11121527/ (accessed on 27 March 2025).
- Kriz, J.; Meier, J.; Julien, J.P.; Padjen, A.L. Altered ionic conductances in axons of transgenic mouse expressing the human neurofilament heavy gene: A mouse model of amyotrophic lateral sclerosis. Exp. Neurol. 2000, 163, 414–421. [Google Scholar] [CrossRef]
- Sternberger, N.H.; Sternberger, L.A.; Ulrich, J. Aberrant neurofilament phosphorylation in Alzheimer disease. Proc. Natl. Acad. Sci. USA 1985, 82, 4274–4276. [Google Scholar] [CrossRef]
- Gold, B.G.; Austin, D.R. Regulation of aberrant neurofilament phosphorylation in neuronal perikarya. III. Alterations following single and continuous beta, beta’-iminodipropionitrile administrations. Brain Res. 1991, 563, 151–162. [Google Scholar] [CrossRef]
- Leterrier, J.F.; Janmey, P.A.; Eyer, J. Microtubule-independent regulation of neurofilament interactions in vitro by neurofilament-bound ATPase activities. Biochem. Biophys. Res. Commun. 2009, 384, 37–42. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhu, Q.; Lindenbaum, M.; Levavasseur, F.; Jacomy, H.; Julien, J.P. Disruption of the NF-H gene increases axonal microtubule content and velocity of neurofilament transport: Relief of axonopathy resulting from the toxin beta, beta’-iminodipropionitrile. J. Cell Biol. 1998, 143, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.V.; Flanagan, L.A.; Janmey, P.A.; Leterrier, J.F. Bidirectional translocation of neurofilaments along microtubules mediated in part by dynein/dynactin. Mol. Biol. Cell 2000, 11, 3495–3508. [Google Scholar] [CrossRef]
- Mann, M.; Jensen, O.N. Proteomic analysis of post-translational modifications. Nat. Biotechnol. 2003, 21, 255–261. [Google Scholar] [CrossRef]
- Seo, J.; Lee, K.J. Post-translational modifications and their biological functions: Proteomic analysis and systematic approaches. J. Biochem. Mol. Biol. 2004, 37, 35–44. [Google Scholar] [CrossRef]
- Walsh, C.T.; Garneau-Tsodikova, S.; Gatto, G.J. Protein posttranslational modifications: The chemistry of proteome diversifications. Angew. Chem. Int. Ed. Engl. 2005, 44, 7342–7372. [Google Scholar] [CrossRef]
- Wesseling, H.; Mair, W.; Kumar, M.; Schlaffner, C.N.; Tang, S.; Beerepoot, P.; Fatou, B.; Guise, A.J.; Cheng, L.; Takeda, S.; et al. Tau PTM Profiles Identify Patient Heterogeneity and Stages of Alzheimer’s Disease. Cell 2020, 183, 1699–1713.e13. [Google Scholar] [CrossRef]
- Haj-Yahya, M.; Lashuel, H.A. Protein Semisynthesis Provides Access to Tau Disease-Associated Post-translational Modifications (PTMs) and Paves the Way to Deciphering the Tau PTM Code in Health and Diseased States. J. Am. Chem. Soc. 2018, 140, 6611–6621. [Google Scholar] [CrossRef]
- Singh, S.A.; Winter, D.; Kirchner, M.; Chauhan, R.; Ahmed, S.; Ozlu, N.; Tzur, A.; Steen, J.A.; Steen, H. Co-regulation proteomics reveals substrates and mechanisms of APC/C-dependent degradation. EMBO J. 2014, 33, 385–399. [Google Scholar] [CrossRef]
- Acetylation of Tau Inhibits its Degradation and Contributes to Tauopathy. Available online: https://pubmed.ncbi.nlm.nih.gov/20869593/ (accessed on 27 March 2025).
- Cohen, T.J.; Guo, J.L.; Hurtado, D.E.; Kwong, L.K.; Mills, I.P.; Trojanowski, J.Q.; Lee, V.M. The acetylation of tau inhibits its function and promotes pathological tau aggregation. Nat. Commun. 2011, 2, 252. [Google Scholar] [CrossRef]
- Tracy, T.E.; Sohn, P.D.; Minami, S.S.; Wang, C.; Min, S.W.; Li, Y.; Zhou, Y.; Le, D.; Lo, I.; Ponnusamy, R.; et al. Acetylated Tau Obstructs KIBRA-Mediated Signaling in Synaptic Plasticity and Promotes Tauopathy-Related Memory Loss. Neuron 2016, 90, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Mukrasch, M.D.; Biernat, J.; von Bergen, M.; Griesinger, C.; Mandelkow, E.; Zweckstetter, M. Sites of tau important for aggregation populate β-structure and bind to microtubules and polyanions. J. Biol. Chem. 2005, 280, 24978–24986. [Google Scholar] [CrossRef] [PubMed]
- Dujardin, S.; Commins, C.; Lathuiliere, A.; Beerepoot, P.; Fernandes, A.R.; Kamath, T.V.; De Los Santos, M.B.; Klickstein, N.; Corjuc, D.L.; Corjuc, B.T.; et al. Author Correction: Tau molecular diversity contributes to clinical heterogeneity in Alzheimer’s disease. Nat. Med. 2021, 27, 356. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.C.; Sprung, R.; Chen, Y.; Xu, Y.; Ball, H.; Pei, J.; Cheng, T.; Kho, Y.; Xiao, H.; Xiao, L.; et al. Substrate and functional diversity of lysine acetylation revealed by a proteomics survey. Mol. Cell 2006, 23, 607–618. [Google Scholar] [CrossRef]
- The Growing Landscape of Lysine Acetylation Links Metabolism and Cell Signalling. Available online: https://pubmed.ncbi.nlm.nih.gov/25053359/ (accessed on 27 March 2025).
- Cohen, T.J.; Friedmann, D.; Hwang, A.W.; Marmorstein, R.; Lee, V.M.Y. The microtubule-associated tau protein has intrinsic acetyltransferase activity. Nat. Struct. Mol. Biol. 2013, 20, 756–762. [Google Scholar] [CrossRef]
- Goode, B.L.; Feinstein, S.C. Identification of a novel microtubule binding and assembly domain in the developmentally regulated inter-repeat region of tau. J. Cell Biol. 1994, 124, 769–782. [Google Scholar] [CrossRef]
- Morris, M.; Knudsen, G.M.; Maeda, S.; Trinidad, J.C.; Ioanoviciu, A.; Burlingame, A.L.; Mucke, L. Tau post-translational modifications in wild-type and human amyloid precursor protein transgenic mice. Nat. Neurosci. 2015, 18, 1183–1189. [Google Scholar] [CrossRef]
- Cryo-EM Structures of Tau Filaments from Alzheimer’s Disease. Available online: https://pubmed.ncbi.nlm.nih.gov/28678775/ (accessed on 27 March 2025).
- Arakhamia, T.; Lee, C.E.; Carlomagno, Y.; Duong, D.M.; Kundinger, S.R.; Wang, K.; Williams, D.; DeTure, M.; Dickson, D.W.; Cook, C.N.; et al. Posttranslational Modifications Mediate the Structural Diversity of Tauopathy Strains. Cell 2020, 180, 633–644.e12. [Google Scholar] [CrossRef]
- Li, W.; Lee, V.M.Y. Characterization of two VQIXXK motifs for tau fibrillization in vitro. Biochemistry 2006, 45, 15692–15701. [Google Scholar] [CrossRef]
- Seidler, P.M.; Boyer, D.R.; Rodriguez, J.A.; Sawaya, M.R.; Cascio, D.; Murray, K.; Gonen, T.; Eisenberg, D.S. Structure-based inhibitors of tau aggregation. Nat. Chem. 2018, 10, 170–176. [Google Scholar] [CrossRef]
- Acetylation Mimic of Lysine 280 Exacerbates Human Tau Neurotoxicity In Vivo. Available online: https://pubmed.ncbi.nlm.nih.gov/26940749/ (accessed on 27 March 2025).
- Sohn, P.D.; Tracy, T.E.; Son, H.I.; Zhou, Y.; Leite, R.E.; Miller, B.L.; Seeley, W.W.; Grinberg, L.T.; Gan, L. Acetylated tau destabilizes the cytoskeleton in the axon initial segment and is mislocalized to the somatodendritic compartment. Mol. Neurodegener. 2016, 11, 47. [Google Scholar] [CrossRef] [PubMed]
- Tseng, J.H.; Ajit, A.; Tabassum, Z.; Patel, N.; Tian, X.; Chen, Y.; Prevatte, A.W.; Ling, K.; Rigo, F.; Meeker, R.B.; et al. Tau seeds are subject to aberrant modifications resulting in distinct signatures. Cell Rep. 2021, 35, 109037. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Ma, B.; Nussinov, R.; Wei, G. Structural Insight into Tau Protein’s Paradox of Intrinsically Disordered Behavior, Self-Acetylation Activity, and Aggregation. J. Phys. Chem. Lett. 2014, 5, 3026–3031. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Dakkak, D.; Rodriguez-Martin, T.; Noble, W.; Hanger, D.P. A pathogenic tau fragment compromises microtubules, disrupts insulin signaling and induces the unfolded protein response. Acta Neuropathol. Commun. 2019, 7, 2. [Google Scholar] [CrossRef]
- Tracy, T.E.; Gan, L. Acetylated tau in Alzheimer’s disease: An instigator of synaptic dysfunction underlying memory loss: Increased levels of acetylated tau blocks the postsynaptic signaling required for plasticity and promotes memory deficits associated with tauopathy. BioEssays News Rev. Mol. Cell Dev. Biol. 2017, 39, 1600224. [Google Scholar] [CrossRef]
- Bilbrough, T.; Piemontese, E.; Seitz, O. Dissecting the role of protein phosphorylation: A chemical biology toolbox. Chem. Soc. Rev. 2022, 51, 5691–5730. [Google Scholar] [CrossRef]
- Martin, L.; Latypova, X.; Wilson, C.M.; Magnaudeix, A.; Perrin, M.L.; Yardin, C.; Terro, F. Tau protein kinases: Involvement in Alzheimer’s disease. Ageing Res. Rev. 2013, 12, 289–309. [Google Scholar] [CrossRef]
- Tau Protein Phosphatases in Alzheimer’s Disease: The Leading Role of PP2A. Available online: https://pubmed.ncbi.nlm.nih.gov/22771380/ (accessed on 27 March 2025).
- Mechanisms of Tau-Induced Neurodegeneration. Available online: https://pubmed.ncbi.nlm.nih.gov/19184068/ (accessed on 27 March 2025).
- Stoothoff, W.H.; Johnson, G.V.W. Tau phosphorylation: Physiological and pathological consequences. Biochim. Biophys. Acta 2005, 1739, 280–297. [Google Scholar] [CrossRef]
- Brotzakis, Z.F.; Lindstedt, P.R.; Taylor, R.J.; Rinauro, D.J.; Gallagher, N.C.T.; Bernardes, G.J.L.; Vendruscolo, M. A Structural Ensemble of a Tau-Microtubule Complex Reveals Regulatory Tau Phosphorylation and Acetylation Mechanisms. ACS Cent. Sci. 2021, 7, 1986–1995. [Google Scholar] [CrossRef]
- An Acetylation-Phosphorylation Switch that Regulates Tau Aggregation Propensity and Function. Available online: https://pubmed.ncbi.nlm.nih.gov/28760828/ (accessed on 27 March 2025).
- Augustinack, J.C.; Schneider, A.; Mandelkow, E.M.; Hyman, B.T. Specific tau phosphorylation sites correlate with severity of neuronal cytopathology in Alzheimer’s disease. Acta Neuropathol. 2002, 103, 26–35. [Google Scholar] [CrossRef]
- Mairet-Coello, G.; Courchet, J.; Pieraut, S.; Courchet, V.; Maximov, A.; Polleux, F. The CAMKK2-AMPK kinase pathway mediates the synaptotoxic effects of Aβ oligomers through Tau phosphorylation. Neuron 2013, 78, 94–108. [Google Scholar] [CrossRef]
- Drewes, G.; Trinczek, B.; Illenberger, S.; Biernat, J.; Schmitt-Ulms, G.; Meyer, H.E.; Mandelkow, E.M.; Mandelkow, E. Microtubule-associated protein/microtubule affinity-regulating kinase (p110mark). A novel protein kinase that regulates tau-microtubule interactions and dynamic instability by phosphorylation at the Alzheimer-specific site serine 262. J. Biol. Chem. 1995, 270, 7679–7688. [Google Scholar] [CrossRef] [PubMed]
- Moreno, H.; Morfini, G.; Buitrago, L.; Ujlaki, G.; Choi, S.; Yu, E.; Moreira, J.E.; Avila, J.; Brady, S.T.; Pant, H.; et al. Tau pathology-mediated presynaptic dysfunction. Neuroscience 2016, 325, 30–38. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Durairajan, S.S.K.; Selvarasu, K.; Singh, A.K.; Patnaik, S.; Iyaswamy, A.; Jaiswal, Y.; Williams, L.L.; Huang, J.D. Unraveling the interplay of kinesin-1, tau, and microtubules in neurodegeneration associated with Alzheimer’s disease. Front. Cell Neurosci. 2024, 18, 1432002. [Google Scholar] [CrossRef]
- Schulman, B.A.; Harper, J.W. Ubiquitin-like protein activation by E1 enzymes: The apex for downstream signalling pathways. Nat. Rev. Mol. Cell Biol. 2009, 10, 319–331. [Google Scholar] [CrossRef]
- Beck, D.B.; Werner, A.; Kastner, D.L.; Aksentijevich, I. Disorders of ubiquitylation: Unchained inflammation. Nat. Rev. Rheumatol. 2022, 18, 435–447. [Google Scholar] [CrossRef]
- Abreha, M.H.; Dammer, E.B.; Ping, L.; Zhang, T.; Duong, D.M.; Gearing, M.; Lah, J.J.; Levey, A.I.; Seyfried, N.T. Quantitative Analysis of the Brain Ubiquitylome in Alzheimer’s Disease. Proteomics 2018, 18, e1800108. [Google Scholar] [CrossRef]
- Flach, K.; Ramminger, E.; Hilbrich, I.; Arsalan-Werner, A.; Albrecht, F.; Herrmann, L.; Goedert, M.; Arendt, T.; Holzer, M. Axotrophin/MARCH7 acts as an E3 ubiquitin ligase and ubiquitinates tau protein in vitro impairing microtubule binding. Biochim. Biophys. Acta 2014, 1842, 1527–1538. [Google Scholar] [CrossRef]
- Alzheimer Disease-Specific Conformation of Hyperphosphorylated Paired Helical Filament-Tau is Polyubiquitinated Through Lys-48, Lys-11, and Lys-6 Ubiquitin Conjugation. Available online: https://pubmed.ncbi.nlm.nih.gov/16443603/ (accessed on 27 March 2025).
- Park, S.; Lee, J.H.; Jeon, J.H.; Lee, M.J. Degradation or aggregation: The ramifications of post-translational modifications on tau. BMB Rep. 2018, 51, 265–273. [Google Scholar] [CrossRef]
- Bancher, C.; Grundke-Iqbal, I.; Iqbal, K.; Fried, V.A.; Smith, H.T.; Wisniewski, H.M. Abnormal phosphorylation of tau precedes ubiquitination in neurofibrillary pathology of Alzheimer disease. Brain Res. 1991, 539, 11–18. [Google Scholar] [CrossRef]
- García-Sierra, F.; Jarero-Basulto, J.J.; Kristofikova, Z.; Majer, E.; Binder, L.I.; Ripova, D. Ubiquitin is associated with early truncation of tau protein at aspartic acid421 during the maturation of neurofibrillary tangles in Alzheimer’s disease. Brain Pathol. 2012, 22, 240–250. [Google Scholar] [CrossRef]
- Alquezar, C.; Arya, S.; Kao, A.W. Tau Post-translational Modifications: Dynamic Transformers of Tau Function, Degradation, and Aggregation. Front. Neurol. 2020, 11, 595532. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jiang, Y.; Wang, J.Z.; Liu, R.; Wang, X. Tau Ubiquitination in Alzheimer’s Disease. Front. Neurol. 2021, 12, 786353. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.N.; Funk, K.E.; Wan, Y.; Liao, Z.; Davies, P.; Kuret, J.; Yang, A.J. Dual modification of Alzheimer’s disease PHF-tau protein by lysine methylation and ubiquitylation: A mass spectrometry approach. Acta Neuropathol. 2012, 123, 105–117. [Google Scholar] [CrossRef]
- Luo, H.B.; Xia, Y.Y.; Shu, X.J.; Liu, Z.C.; Feng, Y.; Liu, X.H.; Yu, G.; Yin, G.; Xiong, Y.S.; Zeng, K.; et al. SUMOylation at K340 inhibits tau degradation through deregulating its phosphorylation and ubiquitination. Proc. Natl. Acad. Sci. USA 2014, 111, 16586–16591. [Google Scholar] [CrossRef]
- Matsuzaki, S.; Lee, L.; Knock, E.; Srikumar, T.; Sakurai, M.; Hazrati, L.N.; Katayama, T.; Staniszewski, A.; Raught, B.; Arancio, O.; et al. SUMO1 Affects Synaptic Function, Spine Density and Memory. Sci. Rep. 2015, 5, 10730. [Google Scholar] [CrossRef]
- Dorval, V.; Fraser, P.E. Small ubiquitin-like modifier (SUMO) modification of natively unfolded proteins tau and alpha-synuclein. J. Biol. Chem. 2006, 281, 9919–9924. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Rekowski, M.J.; Wang, N. Lactylation of tau in human Alzheimer’s disease brains. Alzheimers Dement. J. Alzheimers Assoc. 2025, 21, e14481. [Google Scholar] [CrossRef]
- Distinct Regulation of Tau Monomer and Aggregate Uptake and Intracellular Accumulation in Human Neurons. Available online: https://pubmed.ncbi.nlm.nih.gov/39736627/ (accessed on 23 May 2025).
- Falkon, K.F.; Danford, L.; Gutierrez Kuri, E.; Esquinca-Moreno, P.; Peña Señeriz, Y.L.; Smith, S.; Wickline, J.L.; Louwrier, A.; McPhail, J.A.; Sayre, N.L.; et al. Microglia internalize tau monomers and fibrils using distinct receptors but similar mechanisms. Alzheimers Dement. J. Alzheimers Assoc. 2025, 21, e14418. [Google Scholar] [CrossRef]
- Woo, J.A.A.; Liu, T.; Fang, C.C.; Cazzaro, S.; Kee, T.; LePochat, P.; Yrigoin, K.; Penn, C.; Zhao, X.; Wang, X.; et al. Activated cofilin exacerbates tau pathology by impairing tau-mediated microtubule dynamics. Commun. Biol. 2019, 2, 112. [Google Scholar] [CrossRef]
- Lee, V.M.; Daughenbaugh, R.; Trojanowski, J.Q. Microtubule stabilizing drugs for the treatment of Alzheimer’s disease. Neurobiol. Aging 1994, 15 (Suppl. 2), S87–S89. [Google Scholar] [CrossRef] [PubMed]
- Brunden, K.R.; Zhang, B.; Carroll, J.; Yao, Y.; Potuzak, J.S.; Hogan, A.M.; Iba, M.; James, M.J.; Xie, S.X.; Ballatore, C.; et al. Epothilone D improves microtubule density, axonal integrity, and cognition in a transgenic mouse model of tauopathy. J. Neurosci. Off. J. Soc. Neurosci. 2010, 30, 13861–13866. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Carroll, J.; Trojanowski, J.Q.; Yao, Y.; Iba, M.; Potuzak, J.S.; Hogan, A.M.; Xie, S.X.; Ballatore, C.; Smith, A.B.; et al. The microtubule-stabilizing agent, epothilone D, reduces axonal dysfunction, neurotoxicity, cognitive deficits, and Alzheimer-like pathology in an interventional study with aged tau transgenic mice. J. Neurosci. Off. J. Soc. Neurosci. 2012, 32, 3601–3611. [Google Scholar] [CrossRef] [PubMed]
- Gozes, I.; Stewart, A.; Morimoto, B.; Fox, A.; Sutherland, K.; Schmeche, D. Addressing Alzheimer’s disease tangles: From NAP to AL-108. Curr. Alzheimer Res. 2009, 6, 455–460. [Google Scholar] [CrossRef]
- Morimoto, B.H.; Schmechel, D.; Hirman, J.; Blackwell, A.; Keith, J.; Gold, M.; AL-108-211 Study Investigators. A double-blind, placebo-controlled, ascending-dose, randomized study to evaluate the safety, tolerability and effects on cognition of AL-108 after 12 weeks of intranasal administration in subjects with mild cognitive impairment. Dement. Geriatr. Cogn. Disord. 2013, 35, 325–336. [Google Scholar] [CrossRef]
- Yao, Y.; Muench, M.; Alle, T.; Zhang, B.; Lucero, B.; Perez-Tremble, R.; McGrosso, D.; Newman, M.; Gonzalez, D.J.; Lee, V.M.; et al. A small-molecule microtubule-stabilizing agent safely reduces Aβ plaque and tau pathology in transgenic mouse models of Alzheimer’s disease. Alzheimers Dement. J. Alzheimers Assoc. 2024, 20, 4540–4558. [Google Scholar] [CrossRef]
- Rush, T.; Martinez-Hernandez, J.; Dollmeyer, M.; Frandemiche, M.L.; Borel, E.; Boisseau, S.; Jacquier-Sarlin, M.; Buisson, A. Synaptotoxicity in Alzheimer’s Disease Involved a Dysregulation of Actin Cytoskeleton Dynamics through Cofilin 1 Phosphorylation. J. Neurosci. Off. J. Soc. Neurosci. 2018, 38, 10349–10361. [Google Scholar] [CrossRef]
- Heredia, L.; Helguera, P.; de Olmos, S.; Kedikian, G.; Solá Vigo, F.; LaFerla, F.; Staufenbiel, M.; de Olmos, J.; Busciglio, J.; Cáceres, A.; et al. Phosphorylation of actin-depolymerizing factor/cofilin by LIM-kinase mediates amyloid beta-induced degeneration: A potential mechanism of neuronal dystrophy in Alzheimer’s disease. J. Neurosci. Off. J. Soc. Neurosci. 2006, 26, 6533–6542. [Google Scholar] [CrossRef]
- Galushkin, A.; Gozes, I. Intranasal NAP (Davunetide): Neuroprotection and circadian rhythmicity. Adv. Drug Deliv. Rev. 2025, 220, 115573. [Google Scholar] [CrossRef]
- Al-Kuraishy, H.M.; Sulaiman, G.M.; Mohammed, H.A.; Al-Gareeb, A.I.; Albuhadily, A.K.; Mohammed, S.G. Role of RhoA-ROCK signaling inhibitor fasudil in Alzheimer disease. Behav. Brain Res. 2025, 484, 115524. [Google Scholar] [CrossRef]
- Singh, Y.P.; Kumar, H. Recent Advances in Medicinal Chemistry of Memantine Against Alzheimer’s Disease. Chem. Biol. Drug Des. 2024, 104, e14638. [Google Scholar] [CrossRef] [PubMed]
- Tsai, R.M.; Miller, Z.; Koestler, M.; Rojas, J.C.; Ljubenkov, P.A.; Rosen, H.J.; Rabinovici, G.D.; Fagan, A.M.; Cobigo, Y.; Brown, J.A.; et al. Reactions to Multiple Ascending Doses of the Microtubule Stabilizer TPI-287 in Patients with Alzheimer Disease, Progressive Supranuclear Palsy, and Corticobasal Syndrome: A Randomized Clinical Trial. JAMA Neurol. 2020, 77, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Kim, H.J.; Yang, J.; Chae, S.; Lee, W.; Chung, S.; Kim, J.; Choi, H.; Song, H.; Lee, C.K.; et al. Acetylation changes tau interactome to degrade tau in Alzheimer’s disease animal and organoid models. Aging Cell 2020, 19, e13081. [Google Scholar] [CrossRef] [PubMed]
- Edland, S.D.; Llibre-Guerra, J.J. Semorinemab in Mild-to-Moderate Alzheimer Disease: A Glimmer of Hope Though Cautions Remain. Neurology 2023, 101, 593–594. [Google Scholar] [CrossRef]
- Teng, E.; Manser, P.T.; Pickthorn, K.; Brunstein, F.; Blendstrup, M.; Sanabria Bohorquez, S.; Wildsmith, K.R.; Toth, B.; Dolton, M.; Ramakrishnan, V.; et al. Safety and Efficacy of Semorinemab in Individuals With Prodromal to Mild Alzheimer Disease: A Randomized Clinical Trial. JAMA Neurol. 2022, 79, 758–767. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).