The Effect of Electron Spin-Dependent Polarizability on Protein Activity
Abstract
1. Introduction
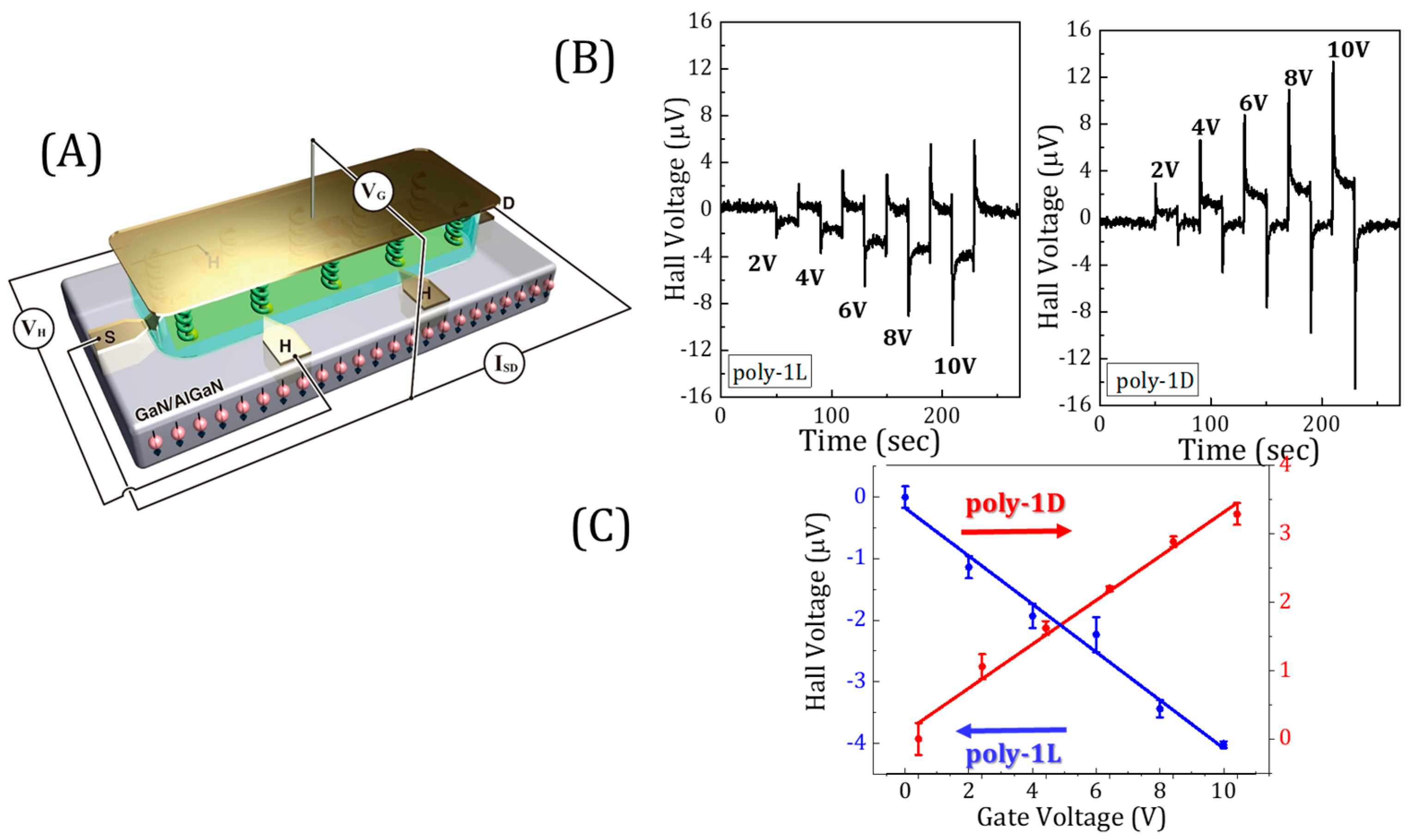
2. How to Directly Measure Spin-Dependent Polarization
3. Studying the Effect of CISS on Protein Function
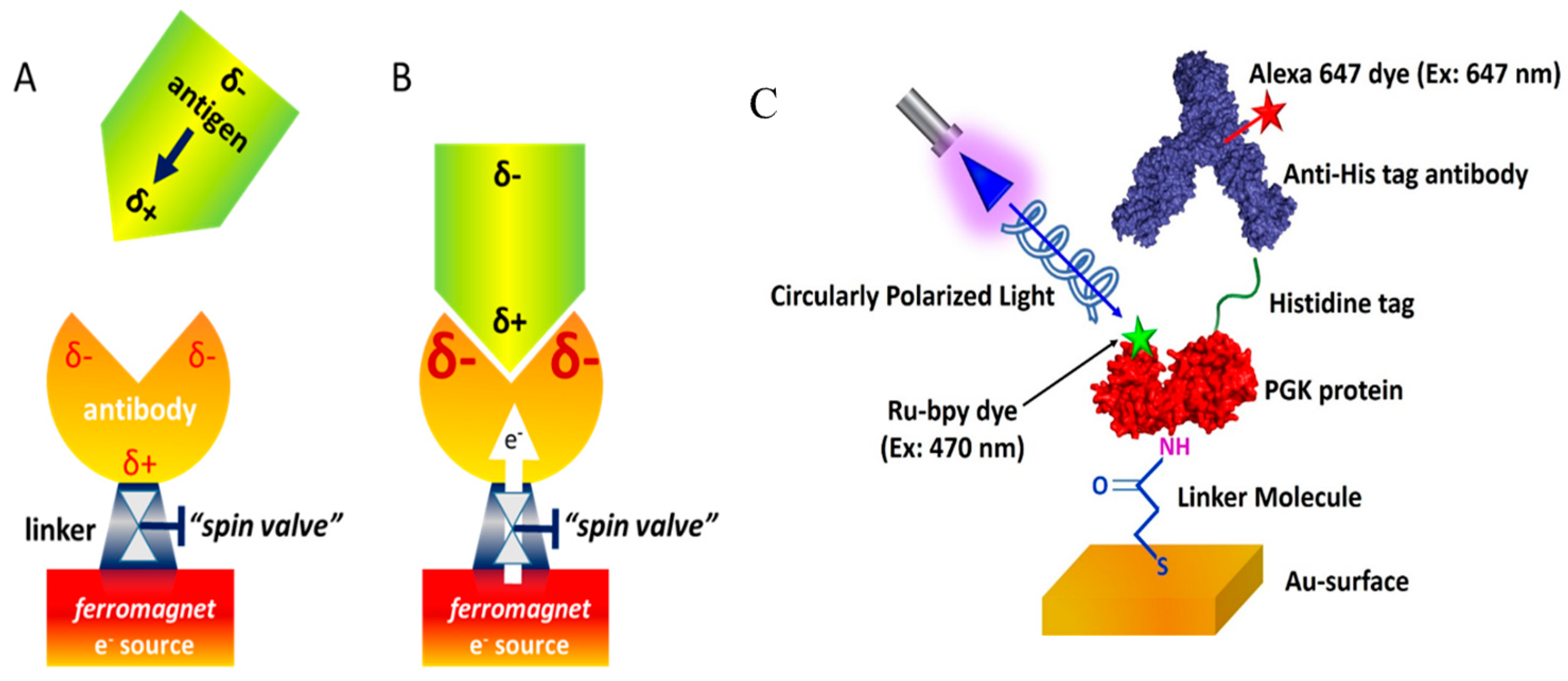
3.1. Protein–Protein Association Reactions
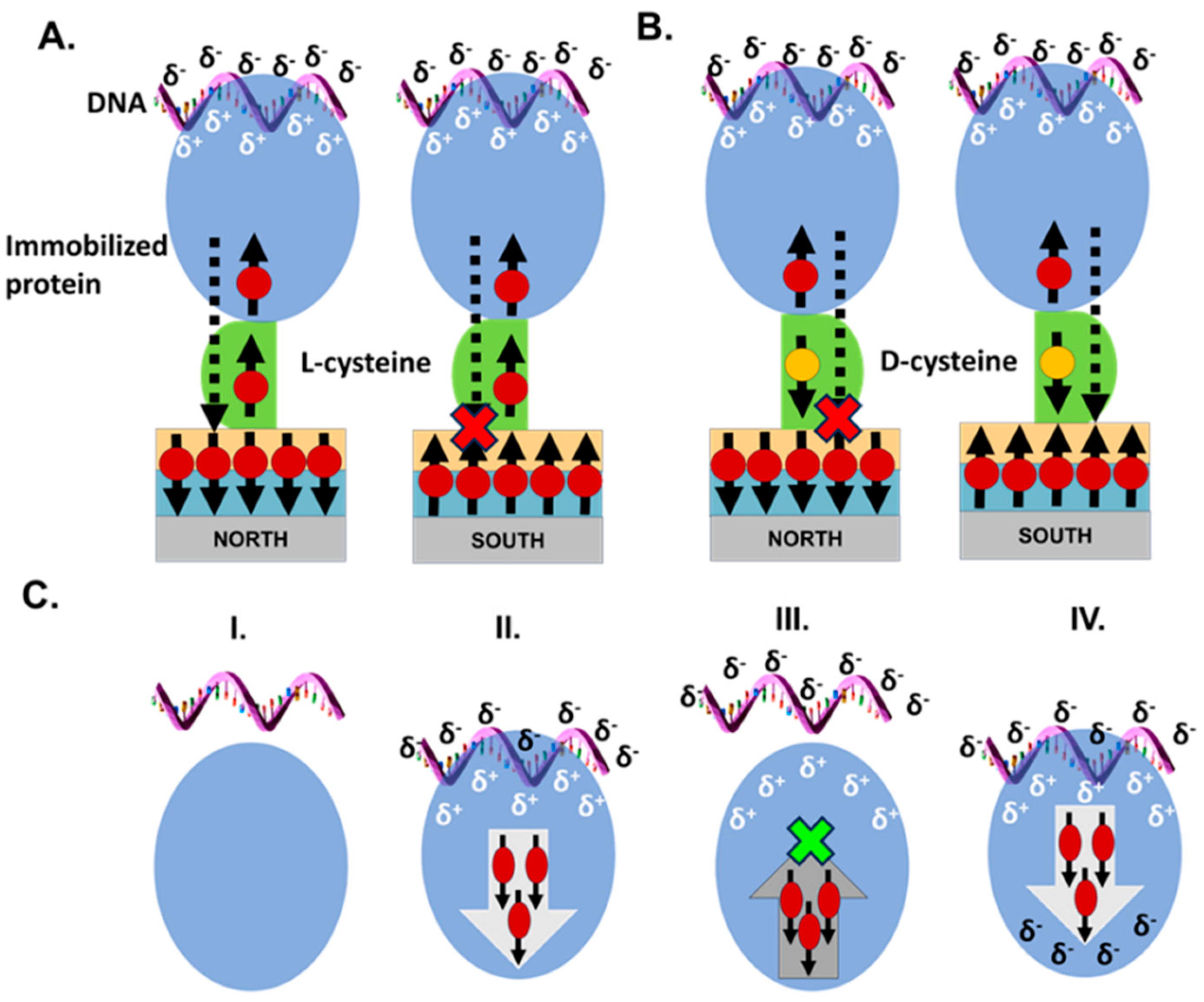
3.2. Protein–DNA Association
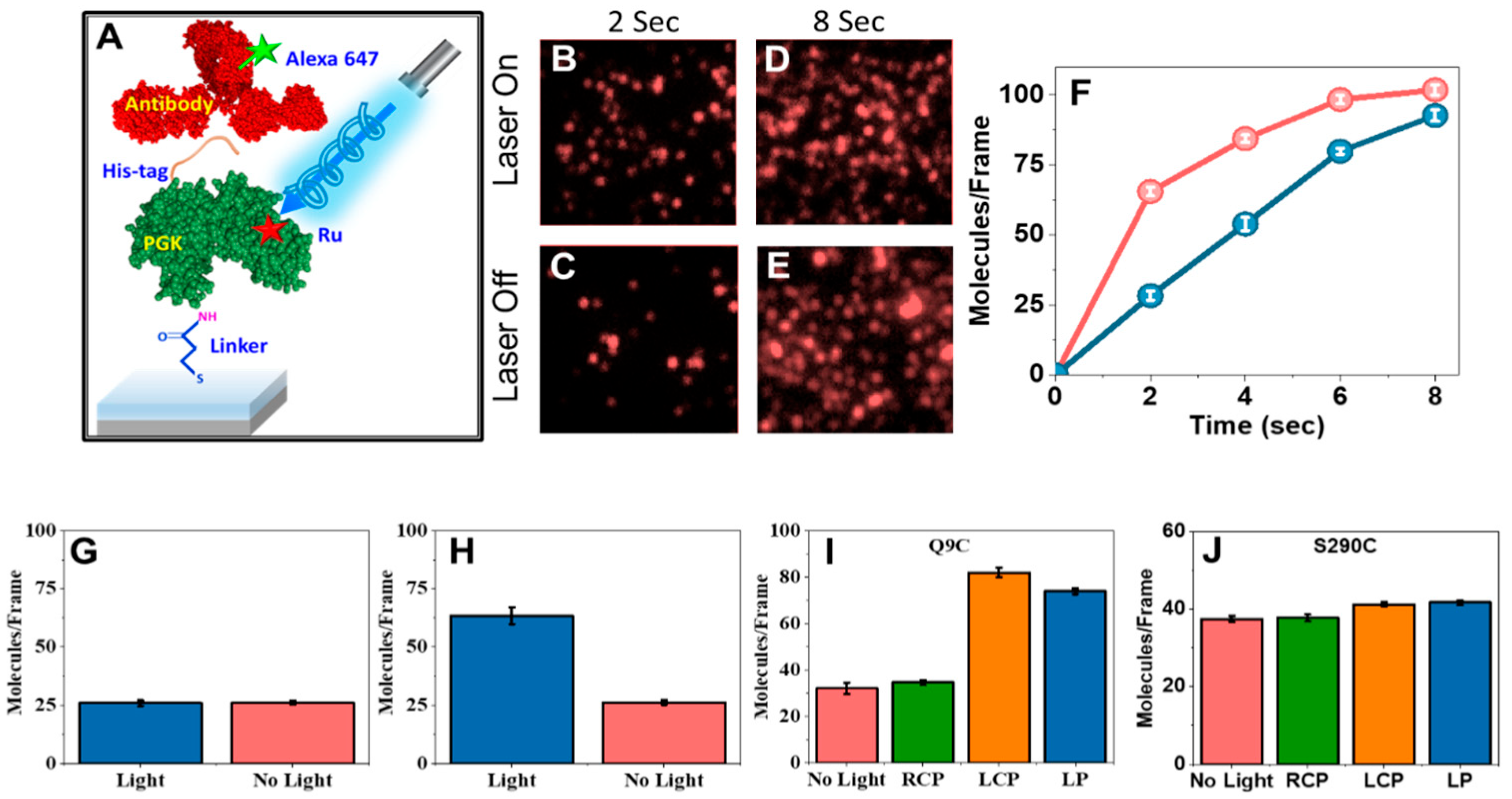
3.3. Modulation of Enzymatic Activity
4. Conclusions and Future Perspective
Funding
Acknowledgments
Conflicts of Interest
References
- Hekstra, D.R.; White, K.I.; Socolich, M.A.; Henning, R.W.; Šrajer, V.; Ranganathan, R. Electric-field-stimulated protein mechanics. Nature 2016, 540, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; He, X.; Zhang, J.Z. Accurate Calculation of Electric Fields Inside Enzymes. Methods Enzymol. 2016, 578, 45–72. [Google Scholar] [PubMed]
- Frauenfelder, H.; Sligar, S.G.; Wolynes, P.G. The Energy Landscapes and Motions of Proteins. Science 1991, 254, 1598–1603. [Google Scholar] [CrossRef] [PubMed]
- Henzler-Wildman, K.A.; Kern, D. Dynamic personalities of proteins. Nature 2007, 450, 964–972. [Google Scholar] [CrossRef]
- Frauenfelder, H.; Chen, G.; Berendzen, J.; Fenimore, P.W.; Jansson, H.; McMahon, B.H.; Stroe, I.R.; Swenson, J.; Young, R.D. A unified model of protein dynamics. Proc. Natl. Acad. Sci. USA 2009, 106, 5129–5134. [Google Scholar] [CrossRef]
- Warshel, A.; Levitt, M. Theoretical studies of enzymic reaction—Dielectric, electrostatic and steric stabilization of carbonium-ion in reaction of lysozyme. J. Mol. Biol. 1976, 103, 227–249. [Google Scholar] [CrossRef]
- Warshel, A.; Kato, M.; Pisliakov, A.V. Polarizable Force Fields: History, Test Cases, and Prospects. J. Chem. Theory Comp. 2007, 3, 2034–2045. [Google Scholar] [CrossRef]
- Jing, Z.; Liu, C.; Cheng, S.Y.; Qi, R.; Walker, B.D.; Piquemal, J.-P.; Ren, P. Polarizable Force Fields for Biomolecular Simulations: Recent Advances and Applications. Ann. Rev. Biophys. 2019, 48, 371–394. [Google Scholar] [CrossRef]
- Lemkul, J.A.; Huang, J.; Roux, B.; MacKerell, A.D., Jr. An Empirical Polarizable Force Field Based on the Classical Drude Oscillator Model: Development History and Recent Applications. Chem. Rev. 2016, 116, 4983–5013. [Google Scholar] [CrossRef]
- Sato, T.; Ohnuki, J.; Takano, M. Dielectric Allostery of Protein: Response of Myosin to ATP Binding. J. Phys. Chem. B 2016, 120, 13047–13055. [Google Scholar] [CrossRef]
- Gunasekaran, K.; Ma, B.; Nussinov, R. Is allostery an intrinsic property of all dynamic proteins? Proteins 2004, 57, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Motlagh, H.N.; Wrabl, J.O.; Hilser, V.J. The ensemble nature of allostery. Nature 2014, 508, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, R.A.; Gerick, F.; Thornton, J.M. The structural basis of allosteric regulation in proteins. FEBS Lett. 2009, 583, 1692–1698. [Google Scholar] [CrossRef]
- Monod, J.; Wyman, J.; Changeux, J.P. On the nature of allosteric transitions: A plausible model. J. Mol. Biol. 1965, 12, 88–118. [Google Scholar] [CrossRef]
- Koshland, D.E., Jr.; Némethy, G.; Filmer, D. Comparison of experimental binding data and theoretical models in proteins containing subunits. Biochemistry 1966, 5, 365–385. [Google Scholar] [CrossRef]
- Cooper, A.; Dryden, D.T. Allostery without conformational change. A plausible model. Eur. Biophys. J. 1984, 11, 103–109. [Google Scholar] [CrossRef]
- Warshel, A.; Sharma, P.K.; Kato, M.; Xiang, Y.; Liu, H.; Olsson, M.H.M. Electrostatic Basis for Enzyme Catalysis. Chem. Rev. 2006, 106, 3210–3235. [Google Scholar] [CrossRef]
- Fried, S.D.; Boxer, S.G. Electric Fields and Enzyme Catalysis. Annu. Rev. Biochem. 2017, 86, 387–415. [Google Scholar] [CrossRef]
- Dinpajooh, M.; Martin, D.R.; Matyushov, D.V. Polarizability of the active site of cytochrome c reduces the activation barrier for electron transfer. Sci. Rep. 2016, 6, 28152. [Google Scholar] [CrossRef]
- Gupta, R.; Balo, A.; Garg, R.; Mondal, A.K.; Ghosh, K.B.; Mondal, P.C. The chirality-induced spin selectivity effect in asymmetric spin transport: From solution to device applications. Chem. Sci. 2024, 15, 18751–18771. [Google Scholar] [CrossRef]
- Bloom, B.; Paltiel, Y.; Naaman, R.; Waldeck, D. Chiral Induced Spin Selectivity. Chem. Rev. 2024, 124, 1950–1991. [Google Scholar] [CrossRef]
- Ma, S.; Lee, H.; Moon, J. Chirality-Induced Spin Selectivity Enables New Breakthrough in Electrochemical and Photoelectrochemical Reactions. Adv. Mater. 2024, 36, 2405685. [Google Scholar] [CrossRef] [PubMed]
- Eckvahl, H.J.; Tcyrulnikov, N.A.; Chiesa, A.; Bradley, J.M.; Young, R.M.; Carretta, S.; Krzyaniak, M.D.; Wasielewski, M.R. Direct observation of chirality-induced spin selectivity in electron donor–acceptor molecules. Science 2023, 382, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Carmeli, I.; Kumar, K.S.; Hieflero, O.; Carmeli, C.; Naaman, R. Spin Selectivity in Electron Transfer in Photosystem I. Angew. Chem. 2014, 53, 8953–8958. [Google Scholar] [CrossRef] [PubMed]
- Preeyanka, N.; Zhu, Q.; Das, T.-K.; Naaman, R. The Importance of Spin-Polarized Charge Reorganization in the Catalytic Activity of D-Glucose Oxidase. PhysChemPhys 2024, 25, e202400033. [Google Scholar] [CrossRef]
- Mishra, S.; Pirbadian, S.; Mondal, A.K.; El-Naggar, M.Y.; Naaman, R. Spin-Dependent Electron Transport through Bacterial Cell Surface Multiheme Electron Conduits. J. Am. Chem. Soc. 2019, 141, 19198–19202. [Google Scholar] [CrossRef]
- Mitrofanov, A.; Urazhdin, S. Energy and momentum conservation in spin transfer. Phys. Rev. B 2020, 102, 184402. [Google Scholar] [CrossRef]
- Andrews, D.L. On the conveyance of angular momentum in electronic energy transfer. Phys. Chem. Chem. Phys. 2010, 12, 7409–7417. [Google Scholar] [CrossRef]
- Das, T.K.; Tassinari, F.; Naaman, R.; Fransson, J. The Temperature-Dependent Chiral-Induced Spin Selectivity Effect: Experiments and Theory. J. Phys. Chem. C 2022, 126, 3257–3264. [Google Scholar] [CrossRef]
- Smolinsky, E.Z.B.; Neubauer, A.; Kumar, A.; Yochelis, S.; Capua, E.; Carmieli, R.; Paltiel, Y.; Naaman, R.; Michaeli, K. Electric Field Controlled Magnetization in GaAs/AlGaAs Heterostructures-chiral Organic Molecules Hybrids. J. Phys. Chem. Lett. 2019, 10, 1139–1145. [Google Scholar] [CrossRef]
- Mishra, S.; Mondal, A.K.; Smolinsky, E.Z.B.; Naaman, R.; Maeda, K.; Nishimura, T.; Taniguchi, T.; Yoshida, T.; Takayama, K.; Yashima, E. Spin Filtering Along Chiral Polymers. Angew. Chem. 2020, 59, 14671–14676. [Google Scholar] [CrossRef] [PubMed]
- Beratan, D.N.; Onuchic, J.N.; Winkler, J.R.; Gray, H.B. Electron-tunneling pathways in proteins. Science 1992, 258, 1740–1741. [Google Scholar] [CrossRef] [PubMed]
- Banerjee-Ghosh, K.; Ghosh, S.; Mazal, H.; Riven, I.; Haran, G.; Naaman, R. Long-range charge reorganization as an allosteric control signal in proteins. J. Am. Chem. Soc. 2020, 142, 20456–20462. [Google Scholar] [CrossRef]
- Vyas, P.; Santra, K.; Preeyanka, N.; Gupta, A.; Weil-Ktorza, O.; Zhu, Q.; Metanis, N.; Fransson, J.; Longo, L.M.; Naaman, R. Role of Electron Spin, Chirality, and Charge Dynamics in Promoting the Persistence of Nascent Nucleic Acid-Peptide Complexes. J. Phys. Chem. B 2025, 129, 3978–3987. [Google Scholar] [CrossRef]
- Crosson, S.; Rajagopal, S.; Moffat, K. The LOV Domain Family: Photoresponsive Signaling Modules Coupled to Diverse Output Domains. Biochemistry 2003, 42, 2–10. [Google Scholar] [CrossRef]
- Rubinson, K.A. Why Proteins are Big: Length Scale Effects on Equilibria and Kinetics. Protein J. 2019, 38, 95–119. [Google Scholar] [CrossRef]
- Zhang, J.; Maslov, S.; Shakhnovich, E.I. Constraints imposed by non-functional protein–protein interactions on gene expression and proteome size. Mol. Syst. Biol. 2008, 4, 210. [Google Scholar] [CrossRef]
- Tóth-Petróczy, A.; Tawfik, D.S. The robustness and innovability of protein folds. Curr. Opin. Struct. Biol. 2014, 26, 131–138. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haran, G.; Naaman, R. The Effect of Electron Spin-Dependent Polarizability on Protein Activity. Biomolecules 2025, 15, 830. https://doi.org/10.3390/biom15060830
Haran G, Naaman R. The Effect of Electron Spin-Dependent Polarizability on Protein Activity. Biomolecules. 2025; 15(6):830. https://doi.org/10.3390/biom15060830
Chicago/Turabian StyleHaran, Gilad, and Ron Naaman. 2025. "The Effect of Electron Spin-Dependent Polarizability on Protein Activity" Biomolecules 15, no. 6: 830. https://doi.org/10.3390/biom15060830
APA StyleHaran, G., & Naaman, R. (2025). The Effect of Electron Spin-Dependent Polarizability on Protein Activity. Biomolecules, 15(6), 830. https://doi.org/10.3390/biom15060830







