Discoidin Domain Receptors in Tumor Biology and Immunology: Progression and Challenge
Abstract
1. Introduction
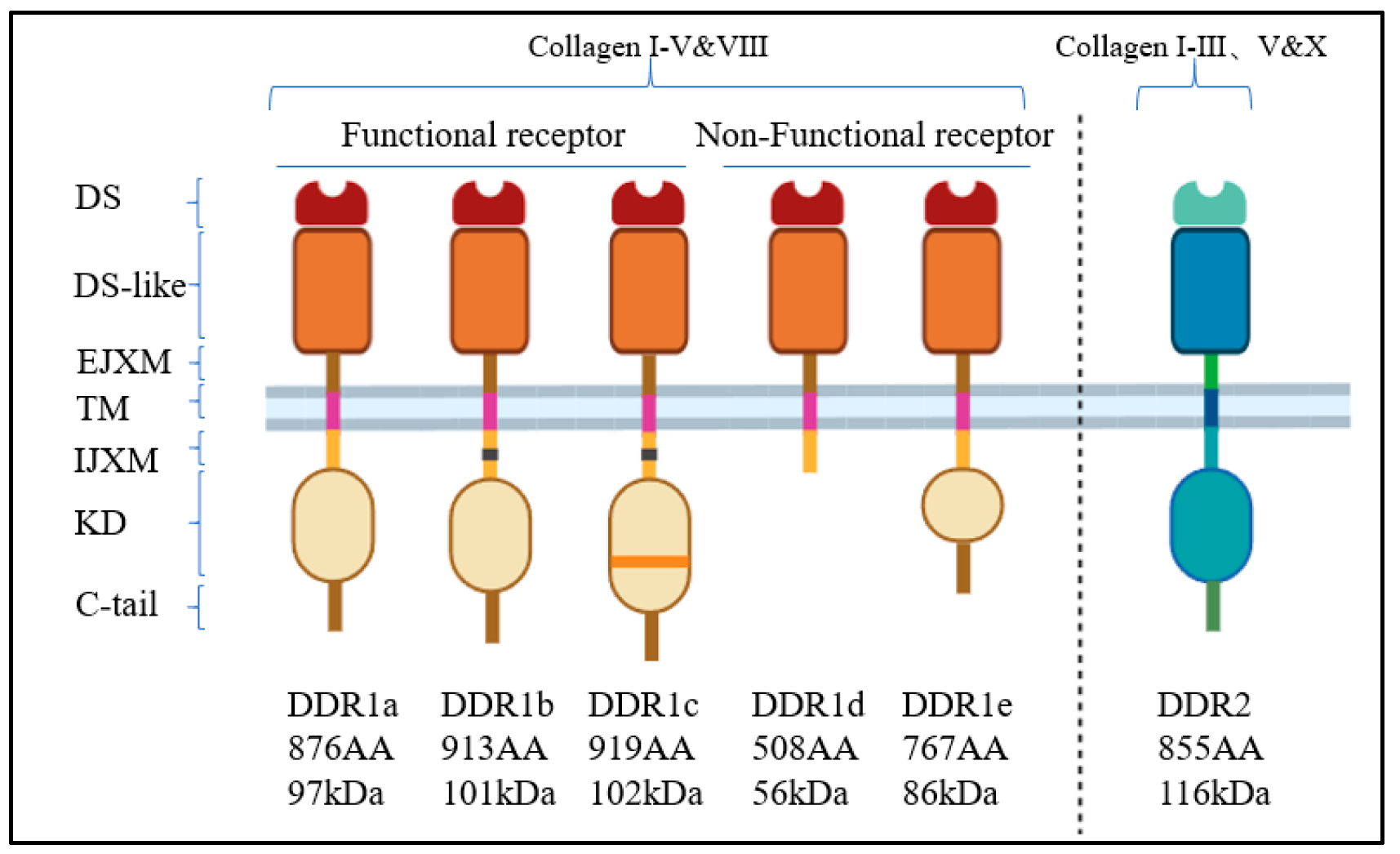
2. The Discovery, Structure, and Function of DDRs
3. The Reciprocal Regulation of DDRs and Collagen in Cancer
4. DDRs in Tumorigenesis and Progression
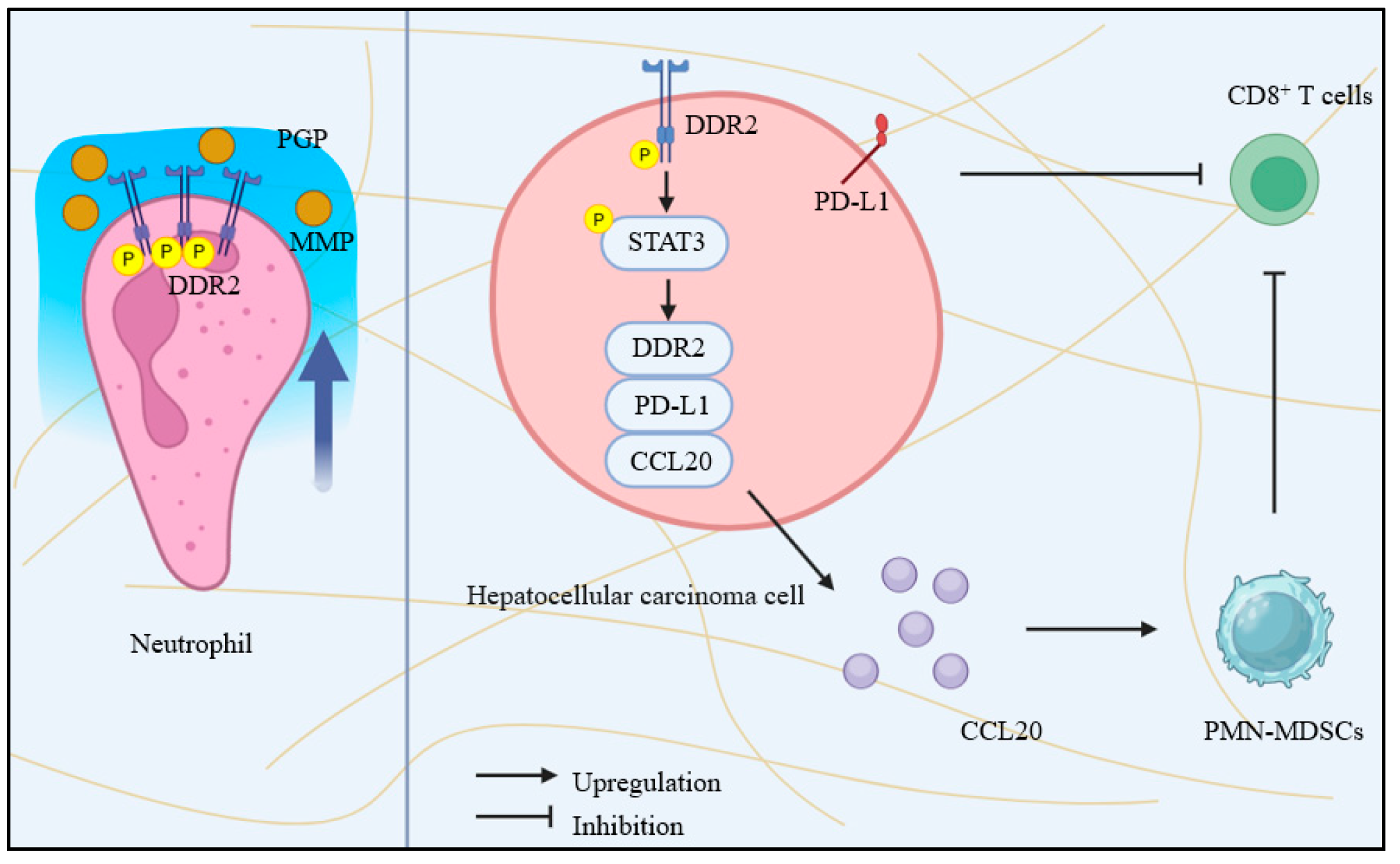
4.1. DDRs and the Tumor Immune Microenvironment
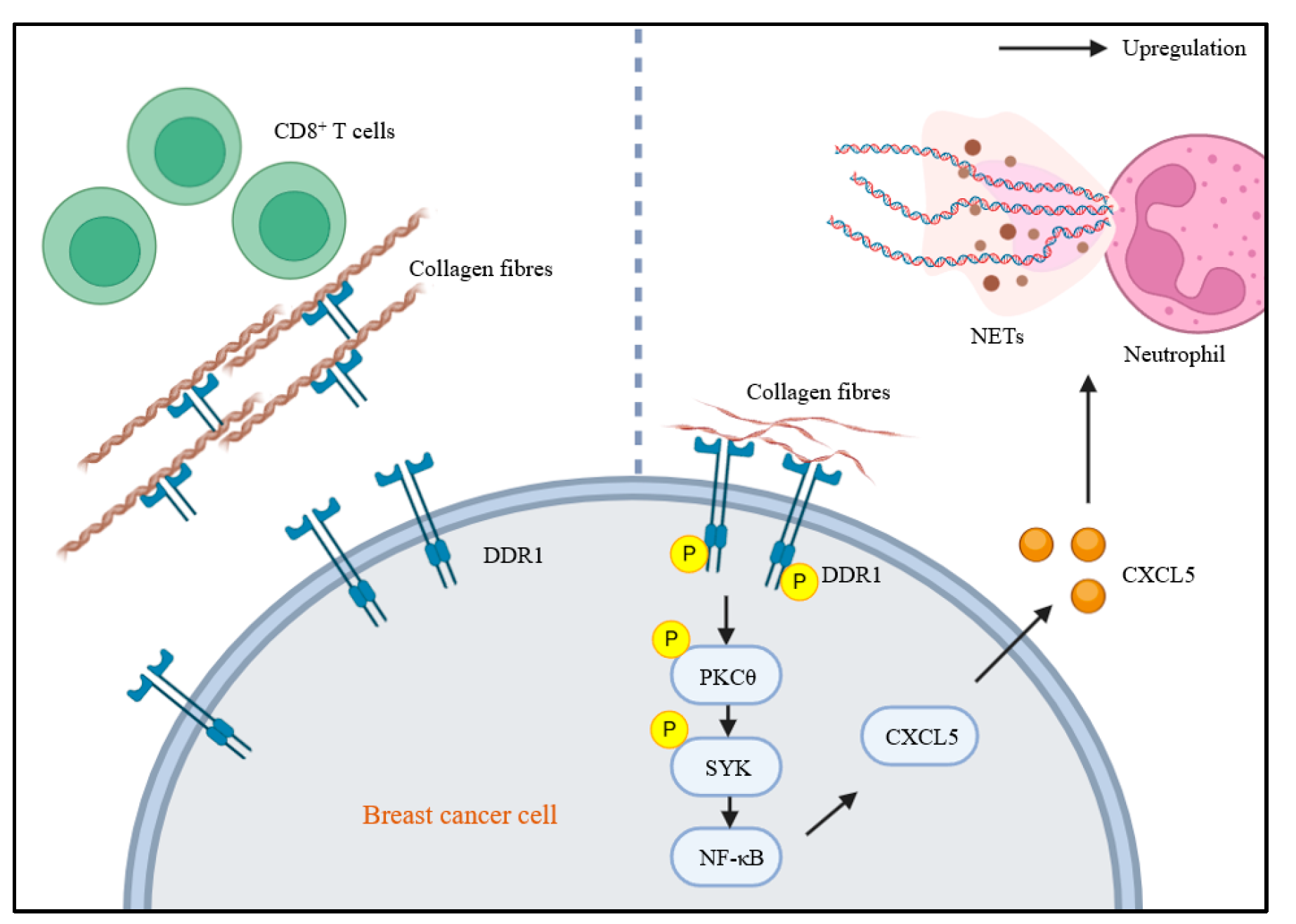
4.1.1. DDRs and T-Cells
4.1.2. DDRs and Neutrophils
4.2. DDRs in Cancer Cell Proliferation and Apoptosis
4.3. DDRs and Cancer Cell Differentiation
4.4. DDRs and Cancer Cell Metabolism Reprograming
4.5. DDRs and Tumor Metastasis
4.6. The Role of Non-Kinase Activities of DDRs in Tumors
4.7. DDRs in Tumor Therapy Resistance
4.8. DDRs Targeting Agents
5. Conclusions
Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fatherree, J.P.; Guarin, J.R.; McGinn, R.A.; Naber, S.P.; Oudin, M.J. Chemotherapy-Induced Collagen IV Drives Cancer Cell Motility through Activation of Src and Focal Adhesion Kinase. Cancer Res. 2022, 82, 2031–2044. [Google Scholar] [CrossRef] [PubMed]
- Wishart, A.L.; Conner, S.J.; Guarin, J.R.; Fatherree, J.P.; Peng, Y.; McGinn, R.A.; Crews, R.; Naber, S.P.; Hunter, M.; Greenberg, A.S.; et al. Decellularized Extracellular Matrix Scaffolds Identify Full-Length Collagen VI as a Driver of Breast Cancer Cell Invasion in Obesity and Metastasis. Sci. Adv. 2020, 6, eabc3175. [Google Scholar] [CrossRef] [PubMed]
- Bordeleau, F.; Califano, J.P.; Negrón Abril, Y.L.; Mason, B.N.; LaValley, D.J.; Shin, S.J.; Weiss, R.S.; Reinhart-King, C.A. Tissue Stiffness Regulates Serine/Arginine-Rich Protein-Mediated Splicing of the Extra Domain B-Fibronectin Isoform in Tumors. Proc. Natl. Acad. Sci. USA 2015, 112, 8314–8319. [Google Scholar] [CrossRef]
- Torrino, S.; Grasset, E.M.; Audebert, S.; Belhadj, I.; Lacoux, C.; Haynes, M.; Pisano, S.; Abélanet, S.; Brau, F.; Chan, S.Y.; et al. Mechano-Induced Cell Metabolism Promotes Microtubule Glutamylation to Force Metastasis. Cell Metab. 2021, 33, 1342–1357.e10. [Google Scholar] [CrossRef]
- Yuan, Z.; Li, Y.; Zhang, S.; Wang, X.; Dou, H.; Yu, X.; Zhang, Z.; Yang, S.; Xiao, M. Extracellular Matrix Remodeling in Tumor Progression and Immune Escape: From Mechanisms to Treatments. Mol. Cancer 2023, 22, 48. [Google Scholar] [CrossRef]
- Prakash, J.; Shaked, Y. The Interplay between Extracellular Matrix Remodeling and Cancer Therapeutics. Cancer Discov. 2024, 14, 1375–1388. [Google Scholar] [CrossRef]
- Du, Z.; Lovly, C.M. Mechanisms of Receptor Tyrosine Kinase Activation in Cancer. Mol. Cancer 2018, 17, 58. [Google Scholar] [CrossRef] [PubMed]
- Valiathan, R.R.; Marco, M.; Leitinger, B.; Kleer, C.G.; Fridman, R. Discoidin Domain Receptor Tyrosine Kinases: New Players In Cancer Progression. Cancer Metast. Rev. 2012, 31, 295–321. [Google Scholar] [CrossRef]
- Sirvent, A.; Espie, K.; Papadopoulou, E.; Naim, D.; Roche, S. New Functions of DDR1 Collagen Receptor in Tumor Dormancy, Immune Exclusion and Therapeutic Resistance. Front. Oncol. 2022, 12, 956926. [Google Scholar] [CrossRef]
- Sun, X.; Wu, B.; Chiang, H.-C.; Deng, H.; Zhang, X.; Xiong, W.; Liu, J.; Rozeboom, A.M.; Harris, B.T.; Blommaert, E.; et al. Tumour DDR1 Promotes Collagen Fibre Alignment to Instigate Immune Exclusion. Nature 2021, 599, 673–678. [Google Scholar] [CrossRef]
- Tu, M.M.; Lee, F.Y.F.; Jones, R.T.; Kimball, A.K.; Saravia, E.; Graziano, R.F.; Coleman, B.; Menard, K.; Yan, J.; Michaud, E.; et al. Targeting DDR2 Enhances Tumor Response to Anti–PD-1 Immunotherapy. Sci. Adv. 2019, 5, eaav2437. [Google Scholar] [CrossRef] [PubMed]
- Di Marco, E.; Cutuli, N.; Guerra, L.; Cancedda, R.; De Luca, M. Molecular Cloning of trkE, a Novel Trk-Related Putative Tyrosine Kinase Receptor Isolated from Normal Human Keratinocytes and Widely Expressed by Normal Human Tissues. J. Biol. Chem. 1993, 268, 24290–24295. [Google Scholar] [CrossRef] [PubMed]
- Vogel, W.; Gish, G.D.; Alves, F.; Pawson, T. The Discoidin Domain Receptor Tyrosine Kinases Are Activated by Collagen. Mol. Cell 1997, 1, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Trenker, R.; Jura, N. Receptor Tyrosine Kinase Activation: From the Ligand Perspective. Curr. Opin. Cell Biol. 2020, 63, 174–185. [Google Scholar] [CrossRef]
- Alves, F.; Vogel, W.; Mossie, K.; Millauer, B.; Höfler, H.; Ullrich, A. Distinct Structural Characteristics of Discoidin I Subfamily Receptor Tyrosine Kinases and Complementary Expression in Human Cancer. Oncogene 1995, 10, 609–618. [Google Scholar]
- Karn, T.; Holtrich, U.; Bräuninger, A.; Böhme, B.; Wolf, G.; Rübsamen-Waigmann, H.; Strebhardt, K. Structure, Expression and Chromosomal Mapping of TKT from Man and Mouse: A New Subclass of Receptor Tyrosine Kinases with a Factor VIII-like Domain. Oncogene 1993, 8, 3433–3440. [Google Scholar]
- Lai, C.; Lemke, G. Structure and Expression of the Tyro 10 Receptor Tyrosine Kinase. Oncogene 1994, 9, 877–883. [Google Scholar]
- Tian, Y.; Bai, F.; Zhang, D. New Target DDR1: A “Double-Edged Sword” in Solid Tumors. Biochim. Et Biophys. Acta Rev. Cancer 2023, 1878, 188829. [Google Scholar] [CrossRef]
- Juskaite, V.; Corcoran, D.S.; Leitinger, B. Collagen Induces Activation of DDR1 through Lateral Dimer Association and Phosphorylation between Dimers. Elife 2017, 6, e25716. [Google Scholar] [CrossRef]
- Liu, A. Effect of Hypoxia on DDR1 Expression in Pituitary Adenomas. Med. Sci. Monit. 2015, 21, 2433–2438. [Google Scholar] [CrossRef] [PubMed]
- Grither, W.R.; Divine, L.M.; Meller, E.H.; Wilke, D.J.; Desai, R.A.; Loza, A.J.; Zhao, P.; Lohrey, A.; Longmore, G.D.; Fuh, K.C. TWIST1 Induces Expression of Discoidin Domain Receptor 2 to Promote Ovarian Cancer Metastasis. Oncogene 2018, 37, 1714–1729. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-G.; Tan, L.; Weisberg, E.L.; Liu, F.; Canning, P.; Choi, H.G.; Ezell, S.A.; Wu, H.; Zhao, Z.; Wang, J.; et al. Discovery of a Potent and Selective DDR1 Receptor Tyrosine Kinase Inhibitor. ACS Chem. Biol. 2013, 8, 2145–2150. [Google Scholar] [CrossRef]
- Fu, H.-L.; Valiathan, R.R.; Arkwright, R.; Sohail, A.; Mihai, C.; Kumarasiri, M.; Mahasenan, K.V.; Mobashery, S.; Huang, P.; Agarwal, G.; et al. Discoidin Domain Receptors: Unique Receptor Tyrosine Kinases in Collagen-Mediated Signaling. J. Biol. Chem. 2013, 288, 7430–7437. [Google Scholar] [CrossRef]
- Xiong, Y.-X.; Zhang, X.-C.; Zhu, J.-H.; Zhang, Y.-X.; Pan, Y.-L.; Wu, Y.; Zhao, J.-P.; Liu, J.-J.; Lu, Y.-X.; Liang, H.-F.; et al. Collagen I-DDR1 Signaling Promotes Hepatocellular Carcinoma Cell Stemness via Hippo Signaling Repression. Cell Death Differ. 2023, 30, 1648–1665. [Google Scholar] [CrossRef]
- Di Martino, J.S.; Nobre, A.R.; Mondal, C.; Taha, I.; Farias, E.F.; Fertig, E.J.; Naba, A.; Aguirre-Ghiso, J.A.; Bravo-Cordero, J.J. A Tumor-Derived Type III Collagen-Rich ECM Niche Regulates Tumor Cell Dormancy. Nat. Cancer 2022, 3, 90–107. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Yang, F.; Fu, R.; Trinh, B.; Sun, N.; Liu, J.; Kumar, A.; Baglieri, J.; Siruno, J.; Le, M.; et al. Collagenolysis-Dependent DDR1 Signalling Dictates Pancreatic Cancer Outcome. Nature 2022, 610, 366–372. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, Y.; Pan, Y.; Xiong, Y.; Zhang, Y.; Han, M.; Dong, K.; Song, J.; Liang, H.; Ding, Z.; et al. DDR1 Promotes Hepatocellular Carcinoma Metastasis through Recruiting PSD4 to ARF6. Oncogene 2022, 41, 1821–1834. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, X.; Zhu, Y.; Qiao, Y.; Gao, Y.; Chen, J.; Ge, G. Type IV Collagen A5 Chain Promotes Luminal Breast Cancer Progression through C-Myc-Driven Glycolysis. J. Mol. Cell Biol. 2023, 14, mjac068. [Google Scholar] [CrossRef]
- Yan, B.; Liu, L.; Zhao, L.; Hinz, U.; Luo, Y.; An, X.; Gladkich, J.; de la Torre, C.; Huang, Z.; Schrapel, D.; et al. Tumor and Stroma COL8A1 Secretion Induces Autocrine and Paracrine Progression Signaling in Pancreatic Ductal Adenocarcinoma. Matrix Biol. J. Int. Soc. Matrix Biol. 2022, 114, 84–107. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Xia, W.; Zhang, T.; Chen, B.; Wang, H.; Song, X.; Zhang, Z.; Xu, L.; Dong, G.; Jiang, F. Upregulated Collagen COL10A1 Remodels the Extracellular Matrix and Promotes Malignant Progression in Lung Adenocarcinoma. Front. Oncol. 2020, 10, 573534. [Google Scholar] [CrossRef]
- Zhang, K.; Corsa, C.A.; Ponik, S.M.; Prior, J.L.; Piwnica-Worms, D.; Eliceiri, K.W.; Keely, P.J.; Longmore, G.D. The Collagen Receptor Discoidin Domain Receptor 2 Stabilizes Snail1 Protein to Facilitate Breast Cancer Metastasis. Nat. Cell Biol. 2013, 15, 677–687. [Google Scholar] [CrossRef]
- Akinjiyan, F.A.; Ibitoye, Z.; Zhao, P.; Shriver, L.P.; Patti, G.J.; Longmore, G.D.; Fuh, K.C. DDR2-Regulated Arginase Activity in Ovarian Cancer-Associated Fibroblasts Promotes Collagen Production and Tumor Progression. Oncogene 2024, 43, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Chetoui, N.; El Azreq, M.-A.; Boisvert, M.; Bergeron, M.-È.; Aoudjit, F. Discoidin Domain Receptor 1 Expression in Activated T Cells Is Regulated by the ERK MAP Kinase Signaling Pathway. J. Cell. Biochem. 2011, 112, 3666–3674. [Google Scholar] [CrossRef]
- Hachehouche, L.N.; Chetoui, N.; Aoudjit, F. Implication of Discoidin Domain Receptor 1 in T Cell Migration in Three-Dimensional Collagen. Mol. Immunol. 2010, 47, 1866–1869. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, F.; Quan, B.; Yao, F.; Chen, R.; Ren, Z.; Dong, L.; Yin, X. DDR2/STAT3 Positive Feedback Loop Mediates the Immunosuppressive Microenvironment by Upregulating PD-L1 and Recruiting MDSCs in Oxaliplatin-Resistant HCC. Cell. Mol. Gastroenterol. Hepatol. 2024, 18, 101377. [Google Scholar] [CrossRef]
- Hedrick, C.C.; Malanchi, I. Neutrophils in Cancer: Heterogeneous and Multifaceted. Nat. Rev. Immunol. 2022, 22, 173–187. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Bai, Z.; Yan, S.; Li, J. Collagen-Induced DDR1 Upregulates CXCL5 to Promote Neutrophil Extracellular Traps Formation and Treg Infiltration in Breast Cancer. Int. Immunopharmacol. 2023, 120, 110235. [Google Scholar] [CrossRef]
- Deng, J.; Kang, Y.; Cheng, C.-C.; Li, X.; Dai, B.; Katz, M.H.; Men, T.; Kim, M.P.; Koay, E.A.; Huang, H.; et al. DDR1-Induced Neutrophil Extracellular Traps Drive Pancreatic Cancer Metastasis. JCI Insight 2021, 6, e146133. [Google Scholar] [CrossRef] [PubMed]
- Afonso, P.V.; McCann, C.P.; Kapnick, S.M.; Parent, C.A. Discoidin Domain Receptor 2 Regulates Neutrophil Chemotaxis in 3D Collagen Matrices. Blood 2013, 121, 1644–1650. [Google Scholar] [CrossRef][Green Version]
- Dean, D.C.; Feng, W.; Walker, R.L.; Thanindratarn, P.; Temple, H.T.; Trent, J.C.; Rosenberg, A.E.; Hornicek, F.J.; Duan, Z. Discoidin Domain Receptor Tyrosine Kinase 1 (DDR1) Is a Novel Therapeutic Target in Liposarcoma: A Tissue Microarray Study. Clin. Orthop. Relat. Res. 2023, 481, 2140–2153. [Google Scholar] [CrossRef]
- .Dawoud, M.M.; Salah, M.; Mohamed, A.S.E.D. Clinical Significance of Immunohistochemical Expression of DDR1 and β-Catenin in Colorectal Carcinoma. World J. Surg. Oncol. 2023, 21, 168. [Google Scholar] [CrossRef] [PubMed]
- Altaf, R.; Ilyas, U.; Ma, A.; Shi, M. Identification and Validation of Differentially Expressed Genes for Targeted Therapy in NSCLC Using Integrated Bioinformatics Analysis. Front. Oncol. 2023, 13, 1206768. [Google Scholar] [CrossRef]
- Pan, Y.; Han, M.; Zhang, X.; He, Y.; Yuan, C.; Xiong, Y.; Li, X.; Zeng, C.; Lu, K.; Zhu, H.; et al. Discoidin Domain Receptor 1 Promotes Hepatocellular Carcinoma Progression through Modulation of SLC1A5 and the mTORC1 Signaling Pathway. Cell. Oncol. 2022, 45, 163–178. [Google Scholar] [CrossRef]
- Xu, H.; Tan, M.; Hou, G.-Q.; Sang, Y.-Z.; Lin, L.; Gan, X.-C.; Cao, X.; Liu, A.-D. Blockade of DDR1/PYK2/ERK Signaling Suggesting SH2 Superbinder as a Novel Autophagy Inhibitor for Pancreatic Cancer. Cell Death Dis. 2023, 14, 811. [Google Scholar] [CrossRef] [PubMed]
- Ambrogio, C.; Gómez-López, G.; Falcone, M.; Vidal, A.; Nadal, E.; Crosetto, N.; Blasco, R.B.; Fernández-Marcos, P.J.; Sánchez-Céspedes, M.; Ren, X.; et al. Combined Inhibition of DDR1 and Notch Signaling Is a Therapeutic Strategy for KRAS-Driven Lung Adenocarcinoma. Nat. Med. 2016, 22, 270–277. [Google Scholar] [CrossRef]
- Diehl, J.N.; Klomp, J.E.; Snare, K.R.; Hibshman, P.S.; Blake, D.R.; Kaiser, Z.D.; Gilbert, T.S.K.; Baldelli, E.; Pierobon, M.; Papke, B.; et al. The KRAS-Regulated Kinome Identifies WEE1 and ERK Coinhibition as a Potential Therapeutic Strategy in KRAS-Mutant Pancreatic Cancer. J. Biol. Chem. 2021, 297, 101335. [Google Scholar] [CrossRef]
- Le, C.C.; Bennasroune, A.; Collin, G.; Hachet, C.; Lehrter, V.; Rioult, D.; Dedieu, S.; Morjani, H.; Appert-Collin, A. LRP-1 Promotes Colon Cancer Cell Proliferation in 3D Collagen Matrices by Mediating DDR1 Endocytosis. Front. Cell Dev. Biol. 2020, 8, 412. [Google Scholar] [CrossRef]
- Wu, A.; Chen, Y.; Liu, Y.; Lai, Y.; Liu, D. miR-199b-5p Inhibits Triple Negative Breast Cancer Cell Proliferation, Migration and Invasion by Targeting DDR1. Oncol. Lett. 2018, 16, 4889–4896. [Google Scholar] [CrossRef] [PubMed]
- Saby, C.; Collin, G.; Sinane, M.; Buache, E.; Van Gulick, L.; Saltel, F.; Maquoi, E.; Morjani, H. DDR1 and MT1-MMP Expression Levels Are Determinant for Triggering BIK-Mediated Apoptosis by 3D Type I Collagen Matrix in Invasive Basal-Like Breast Carcinoma Cells. Front. Pharmacol. 2019, 10, 462. [Google Scholar] [CrossRef]
- Saby, C.; Rammal, H.; Magnien, K.; Buache, E.; Brassart-Pasco, S.; Van-Gulick, L.; Jeannesson, P.; Maquoi, E.; Morjani, H. Age-Related Modifications of Type I Collagen Impair DDR1-Induced Apoptosis in Non-Invasive Breast Carcinoma Cells. Cell Adhes. Migr. 2018, 12, 335–347. [Google Scholar] [CrossRef]
- Wasinski, B.; Sohail, A.; Bonfil, R.D.; Kim, S.; Saliganan, A.; Polin, L.; Bouhamdan, M.; Kim, H.-R.C.; Prunotto, M.; Fridman, R. Discoidin Domain Receptors, DDR1b and DDR2, Promote Tumour Growth within Collagen but DDR1b Suppresses Experimental Lung Metastasis in HT1080 Xenografts. Sci. Rep. 2020, 10, 2309. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C.; Yang, W.-H.; Lin, Y.-T.; Tang, X.; Chen, P.-H.; Ding, C.-K.C.; Qu, D.C.; Alvarez, J.V.; Chi, J.-T. DDR2 Upregulation Confers Ferroptosis Susceptibility of Recurrent Breast Tumors through the Hippo Pathway. Oncogene 2021, 40, 2018–2034. [Google Scholar] [CrossRef] [PubMed]
- Zhi, D.; Zhou, K.; Liu, S.; Yu, W.; Dong, M.; Yan, C. METTL3/YTHDF1 m(6)A Axis Promotes Tumorigenesis by Enhancing DDR2 Expression in Ovarian Cancer. Pathol. Res. Pract. 2024, 253, 155047. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-W.; Lee, Y.-S.; Kim, J.S.; Lee, S.-K.; Kim, B.H.; Lee, J.A.; Lee, N.O.; Kim, S.H.; Hong, E.K. Downregulation of Discoidin Domain Receptor 2 Decreases Tumor Growth of Hepatocellular Carcinoma. J. Cancer Res. Clin. Oncol. 2015, 141, 1973–1983. [Google Scholar] [CrossRef]
- Sala, M.; Allain, N.; Moreau, M.; Jabouille, A.; Henriet, E.; Abou-Hammoud, A.; Uguen, A.; Di-Tommaso, S.; Dourthe, C.; Raymond, A.-A.; et al. Discoidin Domain Receptor 2 Orchestrates Melanoma Resistance Combining Phenotype Switching and Proliferation. Oncogene 2022, 41, 2571–2586. [Google Scholar] [CrossRef]
- Sun, M.; Shen, Z. Knockdown of Long Non-Coding RNA (lncRNA) Colon Cancer-Associated Transcript-1 (CCAT1) Suppresses Oral Squamous Cell Carcinoma Proliferation, Invasion, and Migration by Inhibiting the Discoidin Domain Receptor 2 (DDR2)/ERK/AKT Axis. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020, 26, e920020-1–e920020-10. [Google Scholar] [CrossRef]
- Deng, L.; Liu, G.; Zheng, C.; Zhang, L.; Kang, Y.; Yang, F. Circ-LAMP1 Promotes T-Cell Lymphoblastic Lymphoma Progression via Acting as a ceRNA for miR-615-5p to Regulate DDR2 Expression. Gene 2019, 701, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Qiu, S.; Liu, X.; Guo, F.; Zhai, J.; Li, Z.; Deng, L.; Ge, L.; Qian, H.; Yang, L.; et al. Extracellular Matrix-Derived Mechanical Force Governs Breast Cancer Cell Stemness and Quiescence Transition through Integrin-DDR Signaling. Signal Transduct. Target. Ther. 2023, 8, 247. [Google Scholar] [CrossRef] [PubMed]
- Wall, S.J.; Werner, E.; Werb, Z.; DeClerck, Y.A. Discoidin Domain Receptor 2 Mediates Tumor Cell Cycle Arrest Induced by Fibrillar Collagen. J. Biol. Chem. 2005, 280, 40187–40194. [Google Scholar] [CrossRef]
- Yeh, Y.-C.; Lin, H.-H.; Tang, M.-J. Dichotomy of the Function of DDR1 in Cells and Disease Progression. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 2019, 1866, 118473. [Google Scholar] [CrossRef]
- Vella, V.; Nicolosi, M.L.; Cantafio, P.; Massimino, M.; Lappano, R.; Vigneri, P.; Ciuni, R.; Gangemi, P.; Morrione, A.; Malaguarnera, R.; et al. DDR1 Regulates Thyroid Cancer Cell Differentiation via IGF-2/IR-A Autocrine Signaling Loop. Endocr.-Relat. Cancer 2019, 26, 197–214. [Google Scholar] [CrossRef]
- Ge, C.; Li, Y.; Wu, F.; Ma, P.; Franceschi, R.T. Synthetic Peptides Activating Discoidin Domain Receptor 2 and Collagen-Binding Integrins Cooperate to Stimulate Osteoblast Differentiation of Skeletal Progenitor Cells. Acta Biomater. 2023, 166, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Mossmann, D.; Müller, C.; Park, S.; Ryback, B.; Colombi, M.; Ritter, N.; Weißenberger, D.; Dazert, E.; Coto-Llerena, M.; Nuciforo, S.; et al. Arginine Reprograms Metabolism in Liver Cancer via RBM39. Cell 2023, 186, 5068–5083.e23. [Google Scholar] [CrossRef]
- Xiong, B.; Xie, Z.; Song, F.; Chen, H.; Wang, X.; Jin, Z.; Han, T.; Li, Y.; Zhang, D. DDR1 Promotes LoVo Cell Proliferation by Regulating Energy Metabolism. Acta Biochim. Et Biophys. Sin. 2022, 54, 615–624. [Google Scholar] [CrossRef]
- Vella, V.; Giuliano, M.; Nicolosi, M.L.; Majorana, M.G.; Marć, M.A.; Muoio, M.G.; Morrione, A.; Maggiolini, M.; Lappano, R.; De Francesco, E.M.; et al. DDR1 Affects Metabolic Reprogramming in Breast Cancer Cells by Cross-Talking to the Insulin/IGF System. Biomolecules 2021, 11, 926. [Google Scholar] [CrossRef]
- Schab, A.M.; Greenwade, M.M.; Stock, E.; Lomonosova, E.; Cho, K.; Grither, W.R.; Noia, H.; Wilke, D.; Mullen, M.M.; Hagemann, A.R.; et al. Stromal DDR2 Promotes Ovarian Cancer Metastasis through Regulation of Metabolism and Secretion of Extracellular Matrix Proteins. Mol. Cancer Res. MCR 2023, 21, 1234–1248. [Google Scholar] [CrossRef]
- Mitchell, A.V.; Wu, J.; Meng, F.; Dong, L.; Block, C.J.; Song, W.; Zhang, B.; Li, J.; Wu, G. DDR2 Coordinates EMT and Metabolic Reprogramming as a Shared Effector of FOXQ1 and SNAI1. Cancer Res. Commun. 2022, 2, 1388–1403. [Google Scholar] [CrossRef] [PubMed]
- Castaneda; den Hollander, P.; Kuburich, N.A.; Rosen, J.M.; Mani, S.A. Mechanisms of Cancer Metastasis. Semin. Cancer Biol. 2022, 87, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Hong, W.; Wei, X. The Molecular Mechanisms and Therapeutic Strategies of EMT in Tumor Progression and Metastasis. J. Hematol. Oncol. 2022, 15, 129. [Google Scholar] [CrossRef] [PubMed]
- Azizi, R.; Salemi, Z.; Fallahian, F.; Aghaei, M. Inhibition of Didscoidin Domain Receptor 1 Reduces Epithelial-Mesenchymal Transition and Induce Cell-Cycle Arrest and Apoptosis in Prostate Cancer Cell Lines. J. Cell. Physiol. 2019, 234, 19539–19552. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhao, S.; Luo, L.; Xiang, Q.; Zhu, Z.; Wang, J.; Liu, Y.; Luo, J. miR-199b-5p-DDR1-ERK Signalling Axis Suppresses Prostate Cancer Metastasis via Inhibiting Epithelial-Mesenchymal Transition. Br. J. Cancer 2021, 124, 982–994. [Google Scholar] [CrossRef]
- Goddard, E.T.; Bozic, I.; Riddell, S.R.; Ghajar, C.M. Dormant Tumour Cells, Their Niches and the Influence of Immunity. Nat. Cell Biol. 2018, 20, 1240–1249. [Google Scholar] [CrossRef]
- Recasens, A.; Munoz, L. Targeting Cancer Cell Dormancy. Trends Pharmacol. Sci. 2019, 40, 128–141. [Google Scholar] [CrossRef]
- Ungai-Salánki, R.; Haty, E.; Gerecsei, T.; Francz, B.; Béres, B.; Sztilkovics, M.; Székács, I.; Szabó, B.; Horvath, R. Single-Cell Adhesion Strength and Contact Density Drops in the M Phase of Cancer Cells. Sci. Rep. 2021, 11, 18500. [Google Scholar] [CrossRef]
- Eswaramoorthy, R.; Wang, C.-K.; Chen, W.-C.; Tang, M.-J.; Ho, M.-L.; Hwang, C.-C.; Wang, H.-M.; Wang, C.-Z. DDR1 regulates the stabilization of cell surface E-cadherin and E-cadherin-mediated cell aggregation. J. Cell. Physiol. 2010, 224, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.-C.; Wu, C.-C.; Wang, Y.-K.; Tang, M.-J. DDR1 Triggers Epithelial Cell Differentiation by Promoting Cell Adhesion through Stabilization of E-Cadherin. Mol. Biol. Cell 2011, 22, 940–953. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Cheng, H.; Wang, L.; Xu, W.; Wang, J.; Han, Q.; Lee, J.-H.; Du, L.; Lyu, J. Discoidin Domain Receptor 1 Promotes Lung Adenocarcinoma Migration via the AKT/Snail Signaling Axis. Mol. Biol. Rep. 2022, 49, 7275–7286. [Google Scholar] [CrossRef]
- Dai, W.; Liu, S.; Wang, S.; Zhao, L.; Yang, X.; Zhou, J.; Wang, Y.; Zhang, J.; Zhang, P.; Ding, K.; et al. Activation of Transmembrane Receptor Tyrosine Kinase DDR1-STAT3 Cascade by Extracellular Matrix Remodeling Promotes Liver Metastatic Colonization in Uveal Melanoma. Signal Transduct. Target. Ther. 2021, 6, 176. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Kurtova, A.V.; Xiao, J.; Nikolos, F.; Hayashi, K.; Tramel, Z.; Jain, A.; Chen, F.; Chokshi, M.; Lee, C.; et al. Collagen-Rich Airway Smooth Muscle Cells Are a Metastatic Niche for Tumor Colonization in the Lung. Nat. Commun. 2019, 10, 2131. [Google Scholar] [CrossRef] [PubMed]
- Jeitany, M.; Leroy, C.; Tosti, P.; Lafitte, M.; Le Guet, J.; Simon, V.; Bonenfant, D.; Robert, B.; Grillet, F.; Mollevi, C.; et al. Inhibition of DDR1-BCR Signalling by Nilotinib as a New Therapeutic Strategy for Metastatic Colorectal Cancer. EMBO Mol. Med. 2018, 10, e7918. [Google Scholar] [CrossRef]
- Yang, J.-C.; Zhang, Y.; He, S.-J.; Li, M.-M.; Cai, X.-L.; Wang, H.; Xu, L.-M.; Cao, J. TM4SF1 Promotes Metastasis of Pancreatic Cancer via Regulating the Expression of DDR1. Sci. Rep. 2017, 7, 45895. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, X.; Bao, Y.; Mo, J.; Du, H.; Hu, J.; Zhang, X. E2F1 Silencing Inhibits Migration and Invasion of Osteosarcoma Cells via Regulating DDR1 Expression. Int. J. Oncol. 2017, 51, 1639–1650. [Google Scholar] [CrossRef]
- Yuge, R.; Kitadai, Y.; Takigawa, H.; Naito, T.; Oue, N.; Yasui, W.; Tanaka, S.; Chayama, K. Silencing of Discoidin Domain Receptor-1 (DDR1) Concurrently Inhibits Multiple Steps of Metastasis Cascade in Gastric Cancer. Transl. Oncol. 2018, 11, 575–584. [Google Scholar] [CrossRef]
- Baltes, F.; Caspers, J.; Henze, S.; Schlesinger, M.; Bendas, G. Targeting Discoidin Domain Receptor 1 (DDR1) Signaling and Its Crosstalk with β(1)-Integrin Emerges as a Key Factor for Breast Cancer Chemosensitization upon Collagen Type 1 Binding. Int. J. Mol. Sci. 2020, 21, 4956. [Google Scholar] [CrossRef]
- Wang, Y.-G.; Xu, L.; Jia, R.-R.; Wu, Q.; Wang, T.; Wei, J.; Ma, J.-L.; Shi, M.; Li, Z.-S. DDR2 Induces Gastric Cancer Cell Activities via Activating mTORC2 Signaling and Is Associated with Clinicopathological Characteristics of Gastric Cancer. Dig. Dis. Sci. 2016, 61, 2272–2283. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; You, E.; Jeong, J.; Ko, P.; Kim, J.-W.; Rhee, S. DDR2 Controls the Epithelial-Mesenchymal-Transition-Related Gene Expression via c-Myb Acetylation upon Matrix Stiffening. Sci. Rep. 2017, 7, 6847. [Google Scholar] [CrossRef]
- Ren, L.; Ren, Q.; Wang, J.; He, Y.; Deng, H.; Wang, X.; Liu, C. miR-199a-3p Promotes Gastric Cancer Progression by Promoting Its Stemness Potential via DDR2 Mediation. Cell. Signal. 2023, 106, 110636. [Google Scholar] [CrossRef]
- Wu, C.; Ying, J.; Dai, M.; Peng, J.; Zhang, D. Co-Expression of DDR2 and IFITM1 Promotes Breast Cancer Cell Proliferation, Migration and Invasion and Inhibits Apoptosis. J. Cancer Res. Clin. Oncol. 2022, 148, 3385–3398. [Google Scholar] [CrossRef]
- Chen, J.; Wang, S.; Zhang, Z.; Richards, C.I.; Xu, R. Heat Shock Protein 47 (HSP47) Binds to Discoidin Domain-Containing Receptor 2 (DDR2) and Regulates Its Protein Stability. J. Biol. Chem. 2019, 294, 16846–16854. [Google Scholar] [CrossRef] [PubMed]
- Bayer, S.V.; Grither, W.R.; Brenot, A.; Hwang, P.Y.; Barcus, C.E.; Ernst, M.; Pence, P.; Walter, C.; Pathak, A.; Longmore, G.D. DDR2 Controls Breast Tumor Stiffness and Metastasis by Regulating Integrin Mediated Mechanotransduction in CAFs. Elife 2019, 8, e45508. [Google Scholar] [CrossRef]
- Akinjiyan, F.A.; Dave, R.M.; Alpert, E.; Longmore, G.D.; Fuh, K.C. DDR2 Expression in Cancer-Associated Fibroblasts Promotes Ovarian Cancer Tumor Invasion and Metastasis through Periostin-ITGB1. Cancers 2022, 14, 3482. [Google Scholar] [CrossRef]
- Majkowska, I.; Shitomi, Y.; Ito, N.; Gray, N.S.; Itoh, Y. Discoidin Domain Receptor 2 Mediates Collagen-Induced Activation of Membrane-Type 1 Matrix Metalloproteinase in Human Fibroblasts. J. Biol. Chem. 2017, 292, 6633–6643. [Google Scholar] [CrossRef]
- Gao, H.; Chakraborty, G.; Zhang, Z.; Akalay, I.; Gadiya, M.; Gao, Y.; Sinha, S.; Hu, J.; Jiang, C.; Akram, M.; et al. Multi-Organ Site Metastatic Reactivation Mediated by Non-Canonical Discoidin Domain Receptor 1 Signaling. Cell 2016, 166, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.; Kwak, D.; Kim, K.K. Inhibition of Discoidin Domain Receptor 2 Reveals Kinase-Dependent and Kinase-Independent Functions in Regulating Fibroblast Activity. Am. J. Physiol. Lung Cell. Mol. Physiol. 2023, 325, L342–L351. [Google Scholar] [CrossRef]
- Barcus, C.E.; Hwang, P.Y.; Morikis, V.; Brenot, A.; Pence, P.; Clarke, M.; Longmore, G.D. Tyrosine Kinase-Independent Actions of DDR2 in Tumor Cells and Cancer-Associated Fibroblasts Influence Tumor Invasion, Migration and Metastasis. J. Cell Sci. 2021, 134, jcs258431. [Google Scholar] [CrossRef] [PubMed]
- Modulating Undruggable Targets to Overcome Cancer Therapy Resistance-PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/35168144/ (accessed on 19 August 2024).
- Shariati, M.; Evans, K.W.; Zheng, X.; Bristow, C.A.; Ng, P.K.-S.; Rizvi, Y.Q.; Tapia, C.; Yang, F.; Carugo, A.; Heffernan, T.P.; et al. Combined Inhibition of DDR1 and CDK4/6 Induces Synergistic Effects in ER-Positive, HER2-Negative Breast Cancer with PIK3CA/AKT1 Mutations. Oncogene 2021, 40, 4425–4439. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhao, C.; Yao, R.; Sui, A.; Sun, L.; Liu, X.; Wu, S.; Su, Z.; Li, T.; Liu, S.; et al. 3D Culture Enhances Chemoresistance of ALL Jurkat Cell Line by Increasing DDR1 Expression. Exp. Ther. Med. 2019, 17, 1593–1600. [Google Scholar] [CrossRef]
- Ongusaha, P.P.; Kim, J.; Fang, L.; Wong, T.W.; Yancopoulos, G.D.; Aaronson, S.A.; Lee, S.W. P53 Induction and Activation of DDR1 Kinase Counteract P53-Mediated Apoptosis and Influence P53 Regulation through a Positive Feedback Loop. EMBO J. 2003, 22, 1289–1301. [Google Scholar] [CrossRef]
- Berestjuk, I.; Lecacheur, M.; Carminati, A.; Diazzi, S.; Rovera, C.; Prod’homme, V.; Ohanna, M.; Popovic, A.; Mallavialle, A.; Larbret, F.; et al. Targeting Discoidin Domain Receptors DDR1 and DDR2 Overcomes Matrix-Mediated Tumor Cell Adaptation and Tolerance to BRAF-Targeted Therapy in Melanoma. EMBO Mol. Med. 2022, 14, e11814. [Google Scholar] [CrossRef]
- Xiong, B.; Song, F.-X.; Chen, H.-L.; Wang, X.-J.; Jin, Z.-X.; Han, T.-Y.; Li, Y.; Zhang, D.-K. Discoidin Domain Receptor 1a (DDR1a) Confers 5-Fluorouracil Cytotoxicity in LoVo Cell via PI3K/AKT/Bcl-2 Pathway. Bioengineered 2022, 13, 9805–9814. [Google Scholar] [CrossRef]
- Miao, L.; Wang, Y.; Zhu, S.; Shi, M.; Li, Y.; Ding, J.; Yang, J.; Ye, Q.; Cai, H.; Zhang, D.; et al. Identification of Novel Driver Mutations of the Discoidin Domain Receptor 2 (DDR2) Gene in Squamous Cell Lung Cancer of Chinese Patients. BMC Cancer 2014, 14, 369. [Google Scholar] [CrossRef]
- Liu, A.; Zhang, S.; Wang, M.; Zhang, L.; Xu, S.; Nasimian, A.; Li, S.; Zhao, S.; Cao, X.; Tian, J.; et al. DDR1/2 Enhance KIT Activation and Imatinib Resistance of Primary and Secondary KIT Mutants in Gastrointestinal Stromal Tumors. Mol. Carcinog. 2024, 63, 75–93. [Google Scholar] [CrossRef]
- Nallanthighal, S.; Rada, M.; Heiserman, J.P.; Cha, J.; Sage, J.; Zhou, B.; Yang, W.; Hu, Y.; Korgaonkar, C.; Hanos, C.T.; et al. Inhibition of Collagen XI Alpha 1-Induced Fatty Acid Oxidation Triggers Apoptotic Cell Death in Cisplatin-Resistant Ovarian Cancer. Cell Death Dis. 2020, 11, 258. [Google Scholar] [CrossRef] [PubMed]
- Nokin, M.-J.; Darbo, E.; Travert, C.; Drogat, B.; Lacouture, A.; San José, S.; Cabrera, N.; Turcq, B.; Prouzet-Mauleon, V.; Falcone, M.; et al. Inhibition of DDR1 Enhances in Vivo Chemosensitivity in KRAS-Mutant Lung Adenocarcinoma. JCI Insight 2020, 5, 137869. [Google Scholar] [CrossRef]
- Lu, Q.-P.; Chen, W.-D.; Peng, J.-R.; Xu, Y.-D.; Cai, Q.; Feng, G.-K.; Ding, K.; Zhu, X.-F.; Guan, Z. Antitumor Activity of 7RH, a Discoidin Domain Receptor 1 Inhibitor, Alone or in Combination with Dasatinib Exhibits Antitumor Effects in Nasopharyngeal Carcinoma Cells. Oncol. Lett. 2016, 12, 3598–3608. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Hu, H.; Song, Y.; Shi, Y.; Hu, D.; Shen, W.; Ning, B. DDR1 Is a Novel Biomarker and Potential Therapeutic Target for the Combination Treatment of Liver Hepatocellular Carcinoma. Cancer Control 2024, 31, 10732748241286257. [Google Scholar] [CrossRef]
- Reger De Moura, C.; Battistella, M.; Sohail, A.; Caudron, A.; Feugeas, J.P.; Podgorniak, M.; Pages, C.; Mazouz Dorval, S.; Marco, O.; Menashi, S.; et al. Discoidin Domain Receptors: A Promising Target in Melanoma. Pigment Cell Melanoma Res. 2019, 32, 697–707. [Google Scholar] [CrossRef]
- Hur, H.; Ham, I.-H.; Lee, D.; Jin, H.; Aguilera, K.Y.; Oh, H.J.; Han, S.-U.; Kwon, J.E.; Kim, Y.-B.; Ding, K.; et al. Discoidin Domain Receptor 1 Activity Drives an Aggressive Phenotype in Gastric Carcinoma. BMC Cancer 2017, 17, 87. [Google Scholar] [CrossRef]
- Yang, S.H.; Baek, H.A.; Lee, H.J.; Park, H.S.; Jang, K.Y.; Kang, M.J.; Lee, D.G.; Lee, Y.C.; Moon, W.S.; Chung, M.J. Discoidin Domain Receptor 1 Is Associated with Poor Prognosis of Non-Small Cell Lung Carcinomas. Oncol. Rep. 2010, 24, 311–319. [Google Scholar] [CrossRef]
- Toy, K.A.; Valiathan, R.R.; Núñez, F.; Kidwell, K.M.; Gonzalez, M.E.; Fridman, R.; Kleer, C.G. Tyrosine Kinase Discoidin Domain Receptors DDR1 and DDR2 Are Coordinately Deregulated in Triple-Negative Breast Cancer. Breast Cancer Res. Treat. 2015, 150, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lu, W.; Zhang, S.; Zhu, C.; Ren, T.; Zhu, T.; Zhao, H.; Liu, Y.; Su, J. Overexpression of DDR2 Contributes to Cell Invasion and Migration in Head and Neck Squamous Cell Carcinoma. Cancer Biol. Ther. 2014, 15, 612–622. [Google Scholar] [CrossRef]
- Fan, Y.; Xu, Z.; Fan, J.; Huang, L.; Ye, M.; Shi, K.; Huang, Z.; Liu, Y.; He, L.; Huang, J.; et al. Prognostic Significance of Discoidin Domain Receptor 2 (DDR2) Expression in Ovarian Cancer. Am. J. Transl. Res. 2016, 8, 2845–2850. [Google Scholar]
- Villalba, M.; Redin, E.; Exposito, F.; Pajares, M.J.; Sainz, C.; Hervas, D.; Guruceaga, E.; Diaz-Lagares, A.; Cirauqui, C.; Redrado, M.; et al. Identification of a Novel Synthetic Lethal Vulnerability in Non-Small Cell Lung Cancer by Co-Targeting TMPRSS4 and DDR1. Sci. Rep. 2019, 9, 15400. [Google Scholar] [CrossRef]
- von Mässenhausen, A.; Sanders, C.; Brägelmann, J.; Konantz, M.; Queisser, A.; Vogel, W.; Kristiansen, G.; Duensing, S.; Schröck, A.; Bootz, F.; et al. Targeting DDR2 in Head and Neck Squamous Cell Carcinoma with Dasatinib. Int. J. Cancer 2016, 139, 2359–2369. [Google Scholar] [CrossRef] [PubMed]
- Shyam Sunder, S.; Sharma, U.C.; Pokharel, S. Adverse Effects of Tyrosine Kinase Inhibitors in Cancer Therapy: Pathophysiology, Mechanisms and Clinical Management. Signal Transduct. Target. Ther. 2023, 8, 262. [Google Scholar] [CrossRef]
- Ko, S.; Jung, K.H.; Yoon, Y.-C.; Han, B.S.; Park, M.S.; Lee, Y.J.; Kim, S.E.; Cho, Y.J.; Lee, P.; Lim, J.H.; et al. A Novel DDR1 Inhibitor Enhances the Anticancer Activity of Gemcitabine in Pancreatic Cancer. Am. J. Cancer Res. 2022, 12, 4326–4342. [Google Scholar]
- Wang, X.; Lu, Y.; Chen, S.; Zhu, Z.; Fu, Y.; Zhang, J.; He, J.; Huang, L.; Luo, L.; Guo, W.; et al. Discovery of a Prominent Dual-Target DDR1/EGFR Inhibitor Aimed DDR1/EGFR-Positive NSCLC. Bioorg. Chem. 2024, 149, 107500. [Google Scholar] [CrossRef]
- Gao, M.; Duan, L.; Luo, J.; Zhang, L.; Lu, X.; Zhang, Y.; Zhang, Z.; Tu, Z.; Xu, Y.; Ren, X.; et al. Discovery and Optimization of 3-(2-(Pyrazolo[1,5-a]Pyrimidin-6-Yl)Ethynyl)Benzamides as Novel Selective and Orally Bioavailable Discoidin Domain Receptor 1 (DDR1) Inhibitors. J. Med. Chem. 2013, 56, 3281–3295. [Google Scholar] [CrossRef] [PubMed]
- Elkamhawy, A.; Lu, Q.; Nada, H.; Woo, J.; Quan, G.; Lee, K. The Journey of DDR1 and DDR2 Kinase Inhibitors as Rising Stars in the Fight Against Cancer. Int. J. Mol. Sci. 2021, 22, 6535. [Google Scholar] [CrossRef] [PubMed]
- Mo, C.; Zhang, Z.; Li, Y.; Huang, M.; Zou, J.; Luo, J.; Tu, Z.-C.; Xu, Y.; Ren, X.; Ding, K.; et al. Design and Optimization of 3′-(Imidazo[1,2-a]Pyrazin-3-Yl)-[1,1′-Biphenyl]-3-Carboxamides as Selective DDR1 Inhibitors. ACS Med. Chem. Lett. 2020, 11, 379–384. [Google Scholar] [CrossRef]
- Wu, D.; Ding, Z.; Lu, T.; Chen, Y.; Zhang, F.; Lu, S. DDR1-Targeted Therapies: Current Limitations and Future Potential. Drug Discov. Today 2024, 29, 103975. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhao, L.; Yang, L.; Chai, M.; Liu, Z.; Ma, N.; Wang, Y.; Wu, Q.; Guo, J.; Zhou, F.; et al. Discovery of LLC355 as an Autophagy-Tethering Compound for the Degradation of Discoidin Domain Receptor 1. J. Med. Chem. 2024, 67, 8043–8059. [Google Scholar] [CrossRef]
- Ruzi, Z.; Bozorov, K.; Nie, L.; Zhao, J.; Akber Aisa, H. Discovery of Novel (E)-1-Methyl-9-(3-Methylbenzylidene)-6,7,8,9-Tetrahydropyrazolo[3,4-d]Pyrido[1,2-a]Pyrimidin-4(1H)-One as DDR2 Kinase Inhibitor: Synthesis, Molecular Docking, and Anticancer Properties. Bioorg. Chem. 2023, 135, 106506. [Google Scholar] [CrossRef]
- Grither, W.R.; Longmore, G.D. Inhibition of Tumor-Microenvironment Interaction and Tumor Invasion by Small-Molecule Allosteric Inhibitor of DDR2 Extracellular Domain. Proc. Natl. Acad. Sci. USA 2018, 115, E7786–E7794. [Google Scholar] [CrossRef]
- Siddiqui, K.; Kim, G.W.; Lee, D.H.; Shin, H.R.; Yang, E.G.; Lee, N.T.; Yang, B.-S. Actinomycin D Identified as an Inhibitor of Discoidin Domain Receptor 2 Interaction with Collagen through an Insect Cell Based Screening of a Drug Compound Library. Biol. Pharm. Bull. 2009, 32, 136–141. [Google Scholar] [CrossRef]
- A First-in-Human Study of PRTH-101 Monotherapy +/− Pembrolizumab in Subjects with Advanced Malignancies. Available online: https://trialsearch.who.int/Trial2.aspx?TrialID=NCT05753722 (accessed on 11 May 2025).
- Taletrectinib in Previously Treated Metastatic CDH1-Mutated Invasive Lobular Cancer (ILC). Available online: https://clinicaltrials.gov/study/NCT06214793 (accessed on 11 May 2025).
- Yan, S.B.; Peek, V.L.; Ajamie, R.; Buchanan, S.G.; Graff, J.R.; Heidler, S.A.; Hui, Y.-H.; Huss, K.L.; Konicek, B.W.; Manro, J.R.; et al. LY2801653 Is an Orally Bioavailable Multi-Kinase Inhibitor with Potent Activity against MET, MST1R, and Other Oncoproteins, and Displays Anti-Tumor Activities in Mouse Xenograft Models. Invest. New Drugs 2013, 31, 833–844. [Google Scholar] [CrossRef]
- Adapting Treatment to the Tumor Molecular Alterations for Patients with Advanced Solid Tumors: MyOwnSpecificTreatment (MOST Plus). Available online: https://clinicaltrials.gov/study/NCT02029001 (accessed on 13 May 2025).
- Egan, J.B.; Barrett, M.T.; Champion, M.D.; Middha, S.; Lenkiewicz, E.; Evers, L.; Francis, P.; Schmidt, J.; Shi, C.-X.; Van Wier, S.; et al. Whole Genome Analyses of a Well-Differentiated Liposarcoma Reveals Novel SYT1 and DDR2 Rearrangements. PLoS ONE 2014, 9, e87113. [Google Scholar] [CrossRef]
- Dasatinib in Advanced Squamous Cell Lung Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT01491633 (accessed on 11 May 2025).
- Testing Dasatinib as a Potential Targeted Treatment in Cancers with DDR2 Genetic Changes (MATCH-Subprotocol X). Available online: https://clinicaltrials.gov/ct2/show/NCT04439305 (accessed on 11 May 2025).
- Trial of Dasatinib in Patients with Advanced Cancers Harboring DDR2 Mutation or Inactivating B-RAF Mutation. Available online: https://www.clinicaltrials.gov/ct2/show/NCT01514864 (accessed on 11 May 2025).
- First-in-Human Phase 1/1b Study to Evaluate Sitravatinib in Patients with Advanced Solid Tumors. Available online: https://clinicaltrials.gov/ct2/show/NCT02219711 (accessed on 11 May 2025).
- Targeted Therapy Directed by Genetic Testing in Treating Patients with Advanced Refractory Solid Tumors, Lymphomas, or Multiple Myeloma (The MATCH Screening Trial). Available online: https://clinicaltrials.gov/ct2/show/NCT02465060 (accessed on 11 May 2025).
- Pembrolizumab with Sitravatinib in Recurrent Endometrial Cancer and Other Solid Tumors with Deficient Mismatch Repair System. Available online: https://clinicaltrials.gov/study/NCT05419817 (accessed on 11 May 2025).
- Yoshimori, A.; Asawa, Y.; Kawasaki, E.; Tasaka, T.; Matsuda, S.; Sekikawa, T.; Tanabe, S.; Neya, M.; Natsugari, H.; Kanai, C. Design and Synthesis of DDR1 Inhibitors with a Desired Pharmacophore Using Deep Generative Models. Chemmedchem 2021, 16, 955–958. [Google Scholar] [CrossRef] [PubMed]
| DDR Subtype | Cancer Type | Clinical Association | Key Finding | Reference |
|---|---|---|---|---|
| DDR1 | Colorectal Carcinoma | Overall Survival | High DDR1 correlates with reduced overall survival | [41] |
| DDR1 | Liver Hepatocellular Carcinoma | Chemoresistance | DDR1 inhibition sensitizes tumors to sorafenib | [107] |
| DDR1 | Melanoma | Prognosis | High DDR1 correlates with poor prognosis | [108] |
| DDR1 | Gastric Carcinoma | Invasiveness | DDR1 promotes invasiveness | [109] |
| DDR1 | Non-small Cell Lung Carcinoma | Metastasis | High DDR1 correlates with lymph node metastasis | [110] |
| DDR2 | Breast Cancer | Tumor Grade & Prognosis | High DDR2 correlates with high tumor grade and reduced overall survival | [111] |
| DDR2 | Gastric Carcinoma | Invasiveness | DDR2 overexpression correlates with lymph node metastasis | [85] |
| DDR2 | Head and Neck Squamous Cell Carcinoma | Tumor Pathologic Stage | High DDR2 correlates with tumor pathologic stage and lymph node metastasis | [112] |
| DDR2 | Ovarian Cancer | Prognosis | High DDR2 correlates with reduced overall survival and metastasis | [113] |
| Drug Name | Investigational Solid Tumor Indications | Target | Study Phase | Reference |
|---|---|---|---|---|
| 7 rh | Gastric Carcinoma | DDR1 antagonists | Preclinical | [109] |
| PRTH-101 | Advanced or Metastatic Solid Tumors | DDR1 antagonists | Phase I clinical (NCT05753722) | [127] |
| Taletrectinib | Breast Cancer; Metastatic Breast Cancer | DDR1 antagonists | Phase II clinical (NCT06214793) | [128] |
| LY2801653 | Breast cancer | DDR-1/2 antagonists | Preclinical | [129] |
| Nilotinib | Malignant Solid Neoplasms | DDR-1/2 antagonists | Phase II clinical (NCT02029001) | [130] |
| Dasatinib | Liposarcoma | DDR2 antagonists | Preclinical | [131] |
| Dasatinib | Squamous Cell Lung Cancer | DDR2 antagonists | Phase II clinical (NCT01491633) | [132] |
| Dasatinib | Advanced Lymphoma; Advanced Malignant Solid Neoplasm; Hematopoietic and Lymphoid Cell Neoplasm Refractory Lymphoma; Refractory Malignant Solid Neoplasm; Refractory Plasma Cell Myeloma | DDR2 antagonists | Phase II clinical (NCT04439305) | [133] |
| Dasatinib | Carcinoma; Non-small Cell Lung Carcinoma | DDR2 antagonists | Phase II clinical (NCT01514864) | [134] |
| Sitravatinib | Advanced Cancer | DDR2 antagonists | Phase I clinical (NCT02219711) | [135] |
| Dasatinib | Subprotocol X (DDR2 S768R, I638F, or L239R mutation) | DDR2 antagonists | Phase II clinical (NCT02465060) | [136] |
| Pembrolizumab With Sitravatinib | Recurrent Endometrial Cancer; Solid Tumors | DDR2 antagonists | Phase II clinical (NCT05419817) | [137] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Chen, W.; Zhu, H.; Tsai, H.-i. Discoidin Domain Receptors in Tumor Biology and Immunology: Progression and Challenge. Biomolecules 2025, 15, 832. https://doi.org/10.3390/biom15060832
Zhang H, Chen W, Zhu H, Tsai H-i. Discoidin Domain Receptors in Tumor Biology and Immunology: Progression and Challenge. Biomolecules. 2025; 15(6):832. https://doi.org/10.3390/biom15060832
Chicago/Turabian StyleZhang, Heng, Wenlong Chen, Haitao Zhu, and Hsiang-i Tsai. 2025. "Discoidin Domain Receptors in Tumor Biology and Immunology: Progression and Challenge" Biomolecules 15, no. 6: 832. https://doi.org/10.3390/biom15060832
APA StyleZhang, H., Chen, W., Zhu, H., & Tsai, H.-i. (2025). Discoidin Domain Receptors in Tumor Biology and Immunology: Progression and Challenge. Biomolecules, 15(6), 832. https://doi.org/10.3390/biom15060832






