Abstract
Glaesserella parasuis (G. parasuis) causes vascular inflammation in piglets, resulting in vascular damage. However, the mechanism causing vascular inflammation remains unclear. Baicalin possesses an anti-inflammatory function. Tumor necrosis factor-like weak inducer of apoptosis (TWEAK) has been implicated in immunosuppression. CD163, a scavenger receptor expressed on macrophages that acts as a decoy receptor for TWEAK, plays a crucial role in the regulation of autophagy and inflammation. This research investigated the efficacy of baicalin in reducing immunosuppression elicited by G. parasuis through the regulation of CD163/TWEAK-mediated autophagy. The data demonstrated that G. parasuis altered routine blood indicators and biochemical parameters, increased cytokine production, and induced blood vessel tissue damage. G. parasuis reduced the CD3+ T cell proportion, CD3+CD4+ T cell proportion, and CD3+CD8+ T cell proportion in piglet blood. The proteomic analysis revealed that CD163 was differentially expressed in the blood vessels of challenged piglets. Baicalin was found to regulate CD163/TWEAK axis expression, inhibit Notch/Wnt signaling pathway activation, promote autophagy, and reduce NLRP3/Caspase 1 signaling pathway activation. Baicalin also decreased cytokine production and alleviated pathological tissue damage in the blood vessels of G. parasuis-challenged piglets. Taken together, this study indicates that baicalin alleviates G. parasuis-induced immunosuppression and might promote CD163/TWEAK-mediated autophagy. This finding suggests that baicalin could serve as a potential therapeutic agent to control G. parasuis infection and related vascular inflammation.
1. Introduction
Glaesserella parasuis (G. parasuis) is a Gram-negative bacterium that colonizes the pig upper respiratory tract [1]. G. parasuis is widely distributed in pig farms and is responsible for Glässer’s disease [2]. This disease can cause significant morbidity and mortality, leading to serious economic losses in piglet raising. The typical characteristics of Glässer’s disease are fibrinous polyserositis, pneumonia, arthritis, and meningitis [3]. According to the agar gel diffusion serotyping method, G. parasuis can be classified into 15 serotypes, but up to 20% of isolates cannot be typed [4]. Serovar 5 was considered the highly virulent strain [5]. The pathogenic mechanism of G. parasuis remains unclear at present, and thus, understanding the molecular mechanisms of G. parasuis infection is crucial for developing better prevention and control strategies.
G. parasuis infection in piglets can lead to immunosuppression of the host, but the immunosuppression mechanism remains unclear. CD163 is a scavenger receptor that is predominantly expressed on the surface of macrophages [6]. CD163 has important functions in hemoglobin–haptoglobin complex recognition and clearance [7]. Previous research reported that CD163 participates in various pathological processes, such as inflammation and immune responses [8]. Tumor necrosis factor-like weak inducer of apoptosis (TWEAK) is a member of the tumor necrosis factor superfamily [9]. CD163 can act as a decoy receptor for TWEAK, modulating TWEAK-induced signaling pathways [10]. The crosstalk of CD163 and TWEAK has been implicated in immunosuppression [11]. CD163-expressing macrophages could be affected by TWEAK, as they can internalize TWEAK-bound complexes and contribute to the immunosuppressive environment [12]. Studies have shown that the engagement of TWEAK with FN14 can regulate the efficacy of immune cells, such as macrophages and T cells, leading to the inhibition of immune responses and the progression of various diseases [13]. The TWEAK/Fn14 signaling pathway participates in multiple biological activities, such as angiogenesis and inflammatory responses [14,15]. However, whether CD163/TWEAK is involved in G. parasuis-induced immunosuppression has not been investigated.
Autophagy is a cellular process that involves the degradation and recycling of cellular components [16]. Recent studies have suggested that CD163 participates in modulating autophagy [17]. TWEAK regulates the expression of autophagy-related genes or proteins, leading to changes in autophagic flux [18]. However, whether CD163 interacts with TWEAK and regulates autophagy has not been explored in G. parasuis-infected piglets.
Baicalin was extracted from the root of Scutellaria baicalensis Georgi and has been reported to have important biological functions, such as antioxidant activity, antibacterial and antiviral effects, and the regulation of the immune system [19]. Baicalin has also shown great potential in the treatment of inflammatory diseases [20]. Research has reported that baicalin reduces pulmonary inflammation and injury by attenuating the TLR4/NF-κB pathway in Mycoplasma pneumoniae-infected mice [21]. Baicalin suppresses macrophage JNK-mediated adipose tissue inflammation [22]. Baicalin inhibits monosodium urate crystal-induced pyroptosis in a renal tubular epithelial cell line through Panx-1/P2 × 7 pathways [23]. In addition, baicalin reduces cell damage and affects the H6N6-induced autophagy level of RAW264.7 cells [24]. Baicalin induces the death of non-small cell lung cancer cells via MCOLN3-mediated lysosomal dysfunction and autophagy blockage [25]. Baicalin attenuates diabetic cardiomyopathy by inhibiting autophagy and cell death through SENP1/SIRT3 signaling pathway activation [25]. Thus, it is very interesting to investigate whether baicalin could regulate G. parasuis-induced autophagy.
In this study, we performed a detailed study of the efficacy of baicalin in the inhibition of G. parasuis-elicited immunosuppression by promoting CD163/TWEAK-mediated autophagy. It was reported that baicalin could regulate G. parasuis-induced autophagy, which might be regulated by CD163/TWEAK in piglets, and our results might provide new strategies to prevent G. parasuis infection in the clinic.
2. Materials and Methods
2.1. Ethics Statement
The animal study was approved by the Animal Care and Use Committee of Wuhan Polytechnic University, Hubei Province, China (WPU202308003), approval date 2 August 2023. All piglets used in this study were euthanized at the end of the experiment.
2.2. Bacterial
The serovar 5 G. parasuis SH0165 isolate used in this study is a highly virulent strain, which was isolated from the lung of a commercial pig with typical arthritis, fibrinous polyserositis, hemorrhagic pneumonia, and meningitis [26]. The SH0165 isolate was plated on TSA plates (Difco Laboratories, USA) or cultured in TSB culture (Difco Laboratories, USA). The culture was supplemented with 10 μg/mL of NAD (Biofroxx, Guangzhou, Guangzhou Saiguo biotech Co., Ltd.) and 10% fetal bovine serum (Sijiqing, Hangzhou, China) at 37 °C.
2.3. Drugs
Baicalin used in this study was obtained from Sichuan Xieli Pharmaceutical Co., Ltd. (Pengzhou, China). The drug levamisole was purchased from Beijing Solarbio Science & Technology Co., Ltd. (Beijing, China).
2.4. Animal Experiment Design 1
Twenty 30-day old naturally farrowed early-weaned (NFEW) Duroc × Landrace × Large White piglets weighing nine to ten kg were obtained from Wuhan Fenglongxin Breeding Professional Cooperative (Wuhan, China). Twenty piglets were randomly divided into control and infection groups, each comprising ten piglets. The infection group piglets were intraperitoneally infected with 1 × 108 CFU G. parasuis resuspended in 1 mL of TSB. The control group piglets were intraperitoneally challenged with an equivalent volume of TSB. All piglets from each group were monitored for three days following challenge by G. parasuis. After being challenged at 24 h, 48 h, and 72 h, blood samples were collected for routine hematological and biochemical parameter determination using commercial kits (Shanghai Kehua Bio-Engineering Co., Ltd., Shanghai, China) on a Hitec7100 automatic blood analyzer (Hitachi, Japan) [27].
2.5. Histopathological Analysis
After being challenged for three days by G. parasuis, the piglets were euthanized, and the aortae were obtained for histopathological analysis. The aortic samples were fixed using immersion in 10% neutral buffered formalin. The 4 μm tissue sections were stained using hematoxylin and eosin [28], then the stained sections were examined with a BX43 light microscope (Olympus, Tokyo, Japan).
2.6. Flow Cytometry
To explore the effect of G. parasuis on T cell differentiation, the blood was collected at 24 h, 48 h, and 72 h after G. parasuis challenge. The collected blood was treated with RBC lysis buffer (TaKaRa, Dalian, China) to remove erythrocytes prior to staining. Following filtration to a single-cell suspension using a 200 μm filter, the samples were stained with mouse anti-porcine CD3ε-FITC (Southern Biotech, Birmingham, AL, USA), mouse anti-porcine CD4-SPRD (Southern Biotech), and mouse anti-porcine CD8a-PE (Southern Biotech). The blood samples were repeated at least three times, and the data were analyzed using CytExpert SRT software.
2.7. Proteome Analysis
The piglet aorta tissue samples from the control and the infection group were obtained and immediately put into liquid nitrogen for proteome analysis [29]. Briefly, the blood vessel samples were added to lysis buffer. After collecting supernatant, the protein concentration of the supernatant was assessed using BCA protein assay. Sequence-grade modified trypsin (Promega, Madison, WI, USA) was used to obtain the peptide mixture. The obtained peptides were analyzed using LC-MS/MS with Orbitrap Fusion™ Lumos™ Tribrid™ coupled with the EASY-nLC 1200 system (Thermo Fisher Scientific, MA, USA). Differentially expressed proteins were filtered with the standard of fold change >1.5 and Q value < 0.05. The obtained genes were mapped to the KEGG database by employing FDR ≤ 0.05 as the threshold.
2.8. Animal Experiment Design 2
This animal experiment was reported in a previous study [27]. Briefly, sixty 30-day NFEW piglets weighing nine to ten kg were randomly divided into six groups, including the control, infection, levamisole, 25 mg/kg of baicalin, 50 mg/kg of baicalin, and 100 mg/kg of baicalin groups. Before G. parasuis challenge, the levamisole, 25 mg/kg of baicalin, 50 mg/kg of baicalin, and 100 mg/kg of baicalin groups were treated via intramuscular injection with 15 mg/kg of body weight (BW) of levamisole, 25 mg/kg of BW of baicalin, 50 mg/kg of BW of baicalin, and 100 mg/kg of BW of baicalin, respectively. Two hours after administration on the first day, all treatment groups except for the control group were intraperitoneally infected with 1 × 108 CFU G. parasuis resuspended in one mL of TSB. The control group received one mL of TSB only intraperitoneally. Hence, the five groups, except the control group, used intramuscular injection with the same drugs after being challenged for six hours. Each treatment was administered for two days and was adopted twice a day. The piglets were observed for seven days following G. parasuis infection.
2.9. RT-PCR(Reverse Transcription-Polymerase Chain Reaction)
The piglet aorta tissue samples were collected for gene expression level determination by using RT-PCR [30]. Briefly, the total RNA from the blood vessels was extracted by using TRIzol reagent (Invitrogen, USA). The concentration of the isolated RNA was measured on a Qubit 2.0 fluorometer (Thermo Fisher Scientific, USA). Following reverse transcription into complementary DNA (cDNA) using reverse transcriptase (TaKaRa, Dalian, China), the RT-PCR was carried out by using a SYBR Green PCR Kit (TaKaRa) based on the manufacturer’s instructions. Each sample transcription was repeated at least three times. The internal gene adopted in this study was GAPDH. The relative expression levels of the gene were evaluated by employing the threshold cycle (CT) method. The gene relative expression fold changes were measured by employing the 2−ΔΔCT CT formula. The primers for RT-PCR used in this study are presented in Table 1.

Table 1.
Primer sequences used for qRT-PCR analysis.
2.10. Western Blot
The levels of protein expression were assessed by using Western blot [31]. Briefly, aorta total proteins were isolated using RIPA lysis buffer. The concentration of proteins was determined by using a BCA protein assay kit (Beyotime Biotechnology, China). Following transfer onto PVDF membranes (Millipore, USA), the proteins were separated by utilizing 8 to 12% concentration of SDS-PAGE. The membranes were blocked with 15% nonfat milk and incubated with anti-CD163 (#16646-1, 1:1000, Protenitech), anti-JAG1 (#WL03261, 1:1000, Wanleibio), anti-Notch1 (#A19090, 1:1500, ABclonal), anti-HES1 (#A11718,1:1000, ABclonal), anti-WNT3A (#A0642, 1:1000, ABclonal), anti-GSK-3β (#A2081, 1:2000, ABclonal), anti-RBP-J (#WL04414, 1:1000, Wanleibio), anti-c-Myc (#HA721182, 1:1000, Huaan Biotechnology), anti-P62 (#A19700, 1:40,000, ABclonal), anti-LC3B (#A19665, 1:4000, ABclonal), anti-Beclin1 (#A21191, 1:10,000, ABclonal), anti-Caspase1 (#A0964, 1:1000, ABclonal), anti-NLRP3 (#A24294, 1:1000, ABclonal), and anti-β-catenin antibodies (#51067-2, 1:2000, Protenitech), respectively, overnight at 4 °C. After washing five times with TBST, the membranes were incubated with a second antibody, HRP Goat-Anti Rabbit IgG (1:10,000, Abbkine), for 60 min under 37 °C. The band signals of proteins were detected using an enhanced chemiluminescence detection kit (ABclonal, Wuhan, China). The protein expression level data were evaluated using a FluorChemFC2 AIC system (Alpha Innotech, USA).
2.11. Detection of CD163 Expression by Immunohistochemistry
The CD163 expression level was also measured using immunohistochemistry analysis. Briefly, aorta samples were prepared from paraffin-embedded blocks. The slices were cut and incubated with anti-CD163 (#16646-1, 1:500, protenitech) immunohistochemistry (IHC) at 4 °C overnight. CD163 was detected with HRP goat anti-rabbit secondary antibody and visualized using diaminobenzidine (DAB) (Servicebio, Wuhan, China). The negative control received only the second antibody. Following counterstaining with hematoxylin (Servicebio, Wuhan, China) for three min, microscopic examination was carried out with a BX43 light microscope, and Image 1.52a software was employed to determine the optical density sum of the brown area.
2.12. Statistical Analysis
The experimental data were displayed as mean ± SD. Differences were analyzed using analysis of variance (ANOVA). A value of p < 0.05 displays statistical significance.
3. Results
3.1. G. parasuis Altered the Routine Blood Indicators and Biochemical Parameters, Increased Cytokines Production, and Induced Blood Vessel Tissue Damage
Compared to the control group, for those infected by G. parasuis for 24 h, the white blood cells (WBCs), red blood cells (RBCs), platelets (PLTs), neutrophils (Neus), and lymphocytes (Lyms) were significantly reduced in the infection group, while the monocytes (MONs) were raised in the infection group (p < 0.05), as shown in Figure 1A. At 72 h, Lym and Neu continued to reduce in the infection group compared to the control group (p < 0.05).
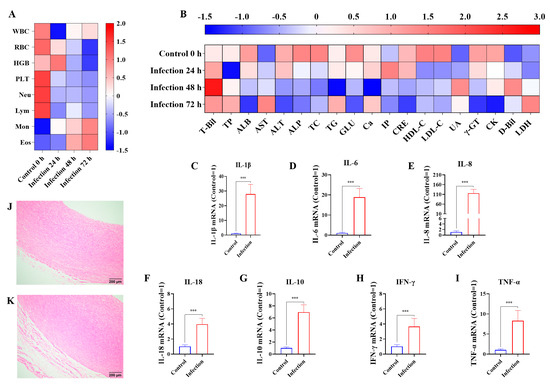
Figure 1.
The effects of G. parasuis on the routine blood indicators, biochemical parameters, cytokine production, and blood vessel tissue damage of piglets. On 24 h, 48 h, 72 h infected by G. parasuis, the blood were collected and the routine blood indicators (A) and biochemical parameters (B) were determined. At 72 h after being challenged by G. parasuis, the mRNA levels of IL-1β (C), IL-6 (D), IL-8 (E), IL-18 (F), IL-10 (G), IFN-γ (H), and TNF-α (I) in the blood vessels were assessed using RT-PCR, and the blood vessels from the control group (J) and the infection group (K) were obtained for pathological analysis. Hemoglobin: HGB; eosinophils: Eos; total protein: TP; total cholesterol: TC; triglycerides: TG; calcium: Ca; uric acid: UA; *** p < 0.001.
At 24 h, albumin (ALB), glucose (GLU), high-density lipoprotein cholesterol (HDL-C), γ-glutamyl transpeptidase (γ-GT), and creatine kinase (CK) levels were reduced, whereas total bilirubin (T-Bil), aspartate aminotransferase (AST), inorganic phosphate (IP), and direct bilirubin (D-Bil) were raised in the infection group (p < 0.05), as shown in Figure 1B. At 72 h, T-Bil, AST, D-Bil, and lactate dehydrogenase (LDH) levels were increased, and the levels of ALB, alanine aminotransferase (ALT), alkaline phosphatase (ALP), GLU, creatinine (CRE), low-density lipoprotein cholesterol (LDL-C), γ-GT, and CK were decreased in the infection group compared to the control group (p < 0.05).
At 72 h after being infected by G. parasuis, the mRNA levels of IL-1β, IL-6, IL-8, IL-10, IL-18, IFN-γ, and TNF-α in the blood vessels were upregulated in the infection group compared to the control group (p < 0.001), as shown in Figure 1C–I. Pathological analysis of vessel tissue indicated that the vessels from the infection group underwent serious tissue damage with massive inflammatory cell infiltration and severe hemorrhage, while some minor tissue damage was present in the control group, as seen in Figure 1J,K.
3.2. G. parasuis Reduced the Proportion of CD3+, CD3+CD4+, and CD3+CD8+ T Cells in the Blood of Piglets
The results showed that compared to the control group at 24 h after infection with G. parasuis, the CD3+, CD3+CD4+, and CD3+CD8+ T cell proportions were reduced in the infection group (p < 0.001), as shown in Figure 2. The CD3+, CD3+ CD4+, and CD3+ CD8+ T cell proportions increased from 48 h to 72 h.
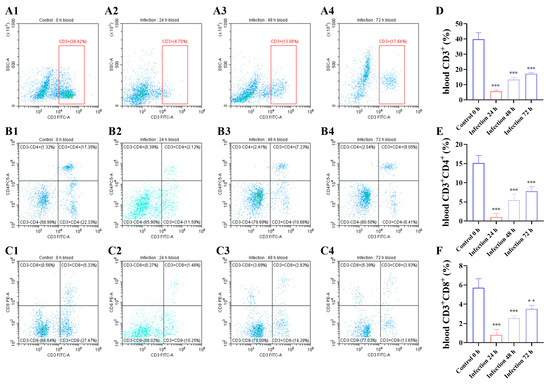
Figure 2.
Following infected by G. parasuis at 24 to 72 h, piglet bloods were collected to determine CD3+, CD3+CD4+, and CD3+CD8+ T cell proportions. (A1–A4,D): the CD3+ T cells; (B1–B4,E): the CD3+ CD4+ T cells; (C1–C4,F): the CD3+ CD8+ T cells; ** p < 0.01; *** p < 0.001.
3.3. G. parasuis Infection Dysregulated Proteins in the Blood Vessels of Piglets
Proteomics was used to evaluate the host’s immunosuppression mechanism triggered by G. parasuis in the blood vessels of piglets. The data indicated that 61,724 peptides were obtained, which corresponds to 5764 protein groups, as shown in Figure 3A. As shown in Figure 3B,C, 1358 differentially expressed proteins were identified from the blood vessels of the infection group, 760 differentially expressed proteins were upregulated, and 598 differentially expressed proteins were diminished according to the standard of |fold change| > 1.5 and Q value < 0.05. The CD163 expression level was significantly upregulated in the infection group, as shown in Figure 3B,F. Gene Ontology (GO) enrichment analysis demonstrated that the differentially expressed proteins were mainly enriched in the cellular process, metabolic process, and biological regulation from the biological processes category, as shown in Figure 3D. And the differentially expressed proteins were mainly enriched in the cellular anatomical entity and protein-containing complex in the cellular component. In the molecular function category, the differentially expressed proteins were dominantly enriched in binding, catalytic activity, and molecular function regulator, and KEGG analysis showed that the differentially expressed proteins were mainly enriched in spliceosome, necroptosis, cell adhesion molecules, Notch signaling pathway, and Wnt signaling pathway, as shown in Figure 3E.
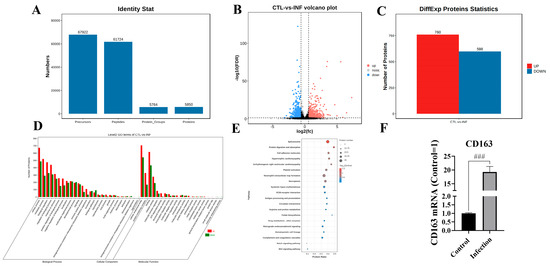
Figure 3.
Proteomic analysis of piglet blood vessels after G. parasuis challenge. (A) The quantified proteins from the proteomic analysis; (B) volcano plot of the dysregulated proteins; (C) differentially expressed proteins (DEPs) in the infection group and the control group; (D) GO enrichment analysis of the DEPs; (E) KEGG analysis of the differentially expressed proteins; (F) detection of the CD163 expression level by RT-PCR; INF: the infection group; CTL: the control group; ### p < 0.001.
3.4. Baicalin Regulated CD163/TWEAK Axis Expression in Blood Vessels from Piglets Infected by G. parasuis
When the piglets were infected with G. parasuis, the CD163 mRNA expression level was significantly increased, and the mRNA level of TWEAK was significantly decreased in the infection group (p < 0.001), as shown in Figure 4A,F. Levamisole could attenuate the CD163 mRNA expression level and alter the TWEAK mRNA expression level compared to the infection group (p < 0.001). The 25, 50 and 100 mg/kg of baicalin diminished CD163 mRNA expression and increased the TWEAK mRNA expression level from the blood vessels compared to the infection group (p < 0.001).
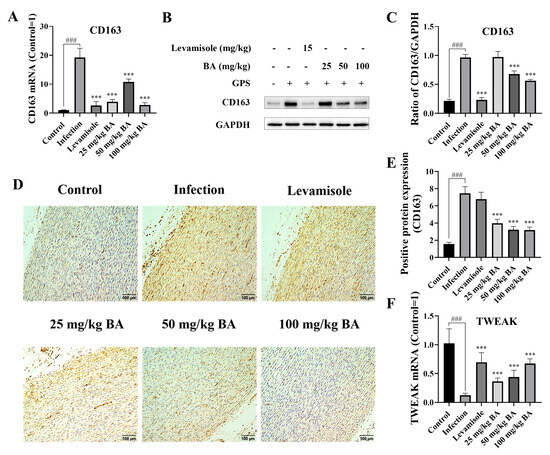
Figure 4.
Determination of the function of baicalin on CD163/TWEAK expression in the blood vessels. When the piglets were challenged by G. parasuis, the CD163 expression level was assessed using RT-PCR (A), Western blot (B,C), and immunohistochemistry (D,E). The TWEAK expression level was determined using RT-PCR (F). BA: baicalin; ### p < 0.001 versus controls; *** significance at p < 0.001. Original images can be found in Supplementary Materials.
The CD163 protein expression level was determined using Western blot analysis. The data indicated that G. parasuis triggered upregulated CD163 protein expression compared to the control group (p < 0.001), as shown in Figure 4B,C. Levamisole and 50 to 100 mg/kg of baicalin inhibited the CD163 protein expression level compared to the infection group (p < 0.001).
The protein expression level of CD163 was also measured via immunohistochemistry. The results demonstrated that the CD163 protein expression level was raised in the infection group compared to the control group (p < 0.001), as shown in Figure 4D,E, while the protein expression level of CD163 was decreased by 25 to 100 mg/kg of baicalin compared to the infection group (p < 0.001).
3.5. Baicalin Inhibited Notch/Wnt Signaling Pathways Activation in the Blood Vessels from Piglets Challenged by G. parasuis
Previous research reported that CD163 could regulate Notch signaling pathway activation [10]. Notch 1 is a key receptor in the Notch 1 signaling pathway. After G. parasuis infection of piglets, the mRNA expression level of Notch 1 from the blood vessels was observed to decrease compared to the control group (p < 0.001), as shown in Figure 5A. The RBP-J could interact with Notch 1 and adjust the expression of downstream target genes. The blood vessels from the infection group showed a decrease in RBP-J mRNA expression compared to the control group (p < 0.001), as shown in Figure 5E. JAG1, a ligand in the Notch signaling pathway, was found to be downregulated at mRNA levels after G. parasuis infection (p < 0.001), as shown in Figure 5I, which could further enhance the Notch pathway activation. HES1, as the downstream target gene of the Notch signaling pathway, was found to show a decrease in mRNA expression from the blood vessels of the G. parasuis-challenged piglets (p < 0.001), as shown in Figure 5M, which indicated that the Notch pathway actively regulated its target genes. The levels of Notch 1, RBP-J, JAG1, and HES1 mRNA expression were significantly raised in the vessels from the levamisole group compared to the infection group (p < 0.001), as shown in Figure 5A,E,I,M. The use of 25 to 100 mg/kg of baicalin upregulated Notch 1, RBP-J, JAG1, and HES1 mRNA expression levels from the blood vessels compared to the infection group (p < 0.01). In the infection group, Notch 1, RBP-J, JAG1, and HES1 protein levels were significantly inhibited compared to the control group, while 25 to 50 mg/kg of baicalin increased the protein expression levels of Notch 1, RBP-J, JAG1, and HES1 compared to the infection group (p < 0.05), as shown in Figure 5B,F,J,N.
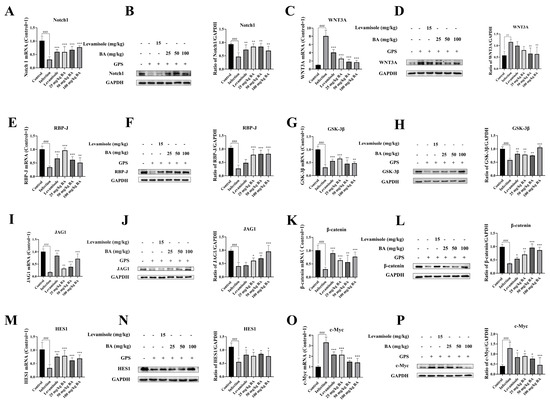
Figure 5.
The effects of baicalin on Notch/Wnt signaling pathways’ activation in piglets’ blood vessels. Notch pathway-related molecule expression levels (Notch 1, RBP-J, JAG1, and HES1) and the expression levels of Wnt pathway-related molecules (WNT3A, GSK-3β, β-catenin, and c-Myc) were assessed using RT-PCR and Western blot. (A): Notch 1, (E): RBP-J, (I): JAG1, (M): HES1, (C): WNT3A, (G): GSK-3β, (K): β-catenin, (O): c-Myc at mRNA level; (B): Notch 1, (F): RBP-J, (J): JAG1, (N): HES1, (D): WNT3A, (H): GSK-3β, (L): β-catenin, (P): c-Myc at protein level; BA: baicalin; ## p < 0.01 versus controls; ### p < 0.001 versus controls; * significance at p < 0.05; ** significance at p < 0.01; *** significance at p < 0.001. Original images can be found in Supplementary Materials.
Compared to the control group, the WNT3A mRNA expression level was raised in the infection group (p < 0.001), as shown in Figure 5C. β-catenin is an effector of the Wnt signaling pathway. In the infection group, the mRNA expression level of β-catenin was significantly upregulated in the blood vessels compared to the control group (p < 0.001), as shown in Figure 5K. GSK-3β is a kinase that phosphorylates β-catenin, marking it for degradation. The expression of GSK-3β was found to be downregulated after infection (p < 0.001), as shown in Figure 5G. The mRNA expression level of c-Myc, as a well-known downstream target gene in the Wnt signaling pathway, was increased from the infection group (p < 0.001), as shown in Figure 5O. The mRNA levels of GSK-3β and β-catenin were significantly increased, and WNT3A and c-Myc mRNA levels were significantly downregulated in the levamisole group compared to the infection group (p < 0.001), as shown in Figure 5C,G,K,O. The 25 to 100 mg/kg of baicalin could enhance GSK-3β and β-catenin mRNA expressions and attenuate WNT3A and c-Myc mNRA expression levels compared to the infection group (p < 0.01).
The protein expressions of WNT3A, GSK-3β, β-catenin, and c-Myc were also assessed using Western blot. The data indicated that G. parasuis upregulated WNT3A and c-Myc protein levels and downregulated the GSK-3β and β-catenin protein levels in the infection group (p < 0.01), as shown in Figure 5D,H,L,P. Treatment with 25 mg/kg–50 mg/kg of baicalin raised the protein levels of GSK-3β and β-catenin and inhibited the protein levels of WNT3A and c-Myc compared to the infection group (p < 0.05).
3.6. Baicalin Promoted Autophagy in the Blood Vessels of G. parasuis-Infected Piglets
Following infection with G. parasuis, the protein levels of Beclin1 and LC3B in the blood vessels of piglets were decreased significantly compared to the control group (p < 0.001), as shown in Figure 6A–D. In contrast, the protein expression level of P62 was increased compared to the control group (p < 0.001), as shown in Figure 6E,F. When the piglets were treated with levamisole, the protein level of LC3B was upregulated compared to the infection group (p < 0.001), as shown in Figure 6C,D. Overall, 50 to 100 mg/kg baicalin could reduce p62 protein levels and promote Beclin1 and LC3B protein expression compared to the infection group (p < 0.05), as shown in Figure 6A–D.
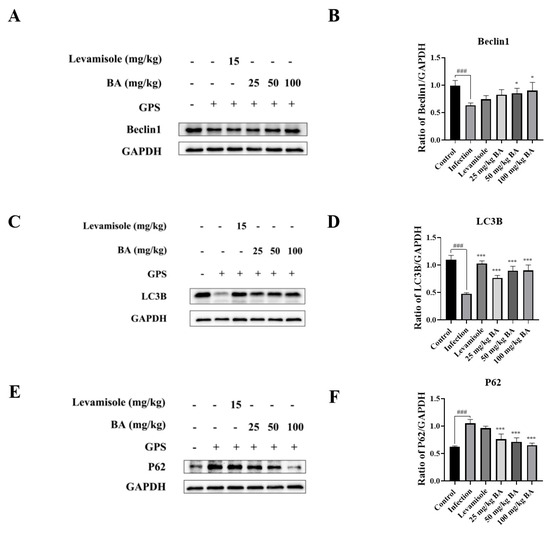
Figure 6.
The function of baicalin on autophagy in the blood vessels of G. parasuis-infected piglets. Beclin1 (A,B), LC3B (C,D), and p62 (E,F) expression levels were assessed using Western blot. BA: baicalin; ### p < 0.001 versus control; * significance present at p < 0.05; *** significance present at p < 0.001. Original images can be found in Supplementary Materials.
3.7. Baicalin Inhibited NLRP3/Caspase 1 Signaling Pathway Activation in the Blood Vessels of G. parasuis-Infected Piglets
As displayed in Figure 7A,D, the mRNA expression levels of NLRP3 and Caspase 1 in the infection group were raised compared to the control group (p < 0.001). Compared to the infection group, levamisole and 25 to 100 mg/kg of baicalin attenuated NLRP3 and Caspase 1 mRNA expression levels (p < 0.001). At the protein levels, NLRP3 and Caspase 1 were raised from the infection group compared to the control group (p < 0.01), as shown in Figure 7B,C,E,F. Compared to the infection group, treatment with levamisole and 25 mg/kg–50 mg/kg of baicalin reduced the NLRP3 and Caspase 1 expression levels (p < 0.05).
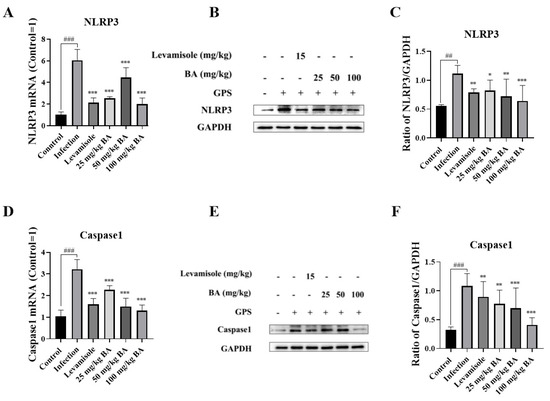
Figure 7.
The function of baicalin on NLRP3/Caspase 1 signaling pathway activation in the blood vessels of G. parasuis-infected piglets. The NLRP3 and Caspase 1 expression levels were measured using RT-PCR method and Western blot. (A): NLRP3 mRNA level; (B,C): NLRP3 protein level; (D): Caspase 1 mRNA level; (E,F): Caspase 1 protein level; BA: baicalin; ## p < 0.01 versus control; ### p < 0.001 versus control; * significance present at p < 0.05; ** significance present at p < 0.01; *** significance present at p < 0.001. Original images can be found in Supplementary Materials.
3.8. Baicalin Decreased Cytokine Production and Diminished Pathological Tissue Damage in Blood Vessels of G. parasuis-Infected Piglets
The data showed that G. parasuis significantly induced the mRNA expression levels of IL-1β, IL-18, and TNF-α in the blood vessels (p < 0.001), as shown in Figure 8A–C. When the piglets were administered levamisole or baicalin, the mRNA expression levels of IL-1β, IL-18, and TNF-α in the blood vessels were weakened compared to the infection group (p < 0.001).
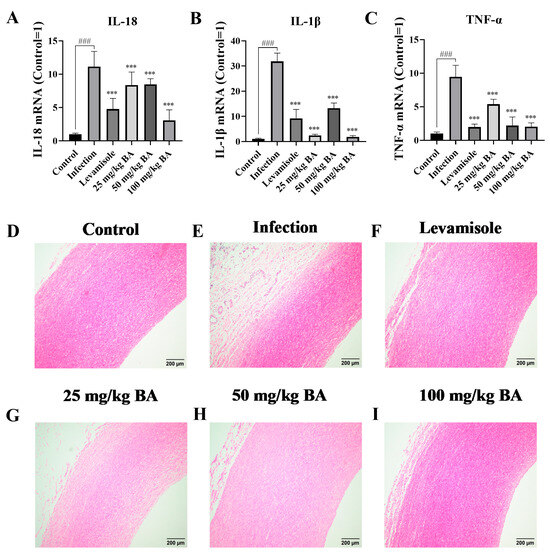
Figure 8.
The effect of baicalin on cytokine production and pathological tissue injury in the blood vessels of G. parasuis-infected piglets. IL-18 (A), IL-1β (B), and TNF-α (C) expression levels were determined by RT-PCR. The pathological tissue damage assessment in blood vessels was carried out by histopathological analysis. (D): the control group; (E): the infection group; (F): the levamisole group; (G): 25 mg/kg of baicalin group; (H): 50 mg/kg of baicalin group; (I): 100 mg/kg of baicalin group; BA: baicalin; ### p < 0.001 versus control; *** significance present at p < 0.001.
The alleviating effect of baicalin on blood vessel damage was also measured. The data indicated that in the infection group, inflammatory cell infiltration and hemorrhage were detected in the blood vessels (Figure 8E), while there was little injury displayed in the control group (Figure 8D). Only some minor damage was seen in the levamisole group or baicalin groups, as shown in Figure 8F–I.
4. Discussion
G. parasuis is a significant pathogen in the pig industry, causing Glässer’s disease with significant clinical signs [32]. In this study, G. parasuis infection in piglets led to a significant decrease in the proportion of CD3+, CD3+CD4, and CD3+CD8+ T cells in the blood. This indicates that G. parasuis could elicit immunosuppression in the host, which has also been reported in previous work [27,29]. It has been reported that human immunodeficiency virus (HIV) primarily attacks CD4+ T cells, CD3+ T cells, and CD8+ T cells, resulting in a sharp decline in numbers and leading to immunosuppression [33]. The imbalance and abnormal function of CD4+ and CD8+ T cells have important functions in the regulation of the development of systemic lupus erythematosus [34]. In the tumor microenvironment, the distribution and function of CD3+, CD4+, and CD8+ T cells also change [35], and tumor cells might suppress T cell activity through immune checkpoint molecule production and immunosuppressive cytokine secretion [36]. It was therefore hypothesized that G. parasuis could directly affect the host’s immune response’s contribution to the progression of immunosuppression. The mechanism underlying immunosuppression remains unclear. However, it is crucial to understand for developing effective prevention and control strategies.
Our previous study showed that the PD-1/PD-L1 axis triggered immunosuppression via the PI3K/Akt/mTOR signaling pathway [29]. Whether there are any other molecules that can induce host immunosuppression needs to be explored. It was found that CD163 was significantly upregulated in the vessels of piglets infected by G. parasuis through vessel proteomics analysis. CD163 is a scavenger receptor predominantly expressed on macrophages [7]. CD163 acts as a decoy receptor for TWEAK [37], which is considered a multifunctional pro-inflammatory cytokine and is significantly expressed on macrophages [38]. The CD163/TWEAK axis is involved in various pathological processes of disease. The lipid droplet (LD)-laden macrophages (LLMs) were present with immunosuppressive phenotypes, accompanied by extensive expression of CD163, and reduced the antitumor activities of CD8+ T cells [39]. The CD163+ macrophages are thought to counteract tumor immunity by enhancing immunosuppressive mechanisms [40]. In this study, the CD163 expression level was increased, while the mRNA level of TWEAK was decreased in the infection group, suggesting that CD163 and TWEAK may be involved in G. parasuis-induced immunosuppression. Baicalin regulated the expression of the CD163/TWEAK axis, indicating that baicalin may alleviate G. parasuis-induced immunosuppression by modulating the CD163/TWEAK axis. Further studies are needed to elucidate the detailed mechanism of action of baicalin on the CD163/TWEAK axis, and other pig breeds or ages will also be used to study the function of baicalin on the regulation of CD163/TWEAK.
The CD163/TWEAK axis was reported to be one of the chief regulators of Notch signaling during the inflammation process [41]. The Notch signaling pathway was thought to be related to dextran sodium sulfate-induced colitis in mice [42]. Blocking the Notch signaling pathway reduced PRRSV infection both in vitro and in vivo [43], so the Notch signaling pathway can act as a potential novel therapeutic target for kidney and liver diseases [44,45]. The Wnt signaling has important roles in liver regeneration, tumorigenesis, and cardiovascular disease [46,47]; thus, regulation of Wnt signaling has become an attractive method to control liver disease and cardiovascular disease. However, the mechanism by which the CD163/TWEAK axis might regulate Notch and Wnt signaling pathways in response to G. parasuis infection remains largely unexplored. This study notably observed a significant regulation of key components of the Notch and Wnt signaling pathway. The use of levamisole and baicalin provided further insights into the modulation of these pathways. Levamisole, which is known to have immunomodulatory effects, significantly regulated the expression of key Notch and Wnt pathway components. These findings suggest that levamisole and baicalin might have therapeutic potential in modulating the Notch and Wnt signaling pathways in response to G. parasuis challenges.
Autophagy, a critical cellular process involving the degradation and recycling of cellular components, plays an important role in pathological conditions [48]. The crosstalk between autophagy and Wnt/Notch signaling controls cardiac differentiation [49]. Notch and Wnt signaling pathways can interact with autophagy and affect the survival and death of tumor cells [50]. Autophagy was involved in mouse kidney development and podocyte differentiation, which was regulated by Notch signaling [51]. Wnt signaling activation regulated autophagy due to a dietary magnesium deficiency in injury-induced osteoarthritis [52]. This study found that when piglets were infected with G. parasuis, the Notch and Wnt signaling pathways were altered, and baicalin could regulate Notch/Wnt signaling and autophagy, suggesting that Notch and Wnt signaling pathways may regulate autophagy in G. parasuis infection.
5. Conclusions
In conclusion, our study showed that baicalin regulated CD163/TWEAK axis expression, inhibited Notch/Wnt signaling pathways’ activation, promoted autophagy, reduced NLRP3/Caspase 1 signaling pathway activation, decreased cytokine production, and alleviated pathological tissue damage in blood vessels. This study provides initial evidence that the CD163/TWEAK axis could induce host immunosuppression, and baicalin promotes autophagy in piglets challenged by G. parasuis. Understanding the role of autophagy in G. parasuis infection could offer a new view of the mechanism of disease and potential treatment targets to control G. parasuis infection in clinical settings. We will focus on exploring the relationship between autophagy and G. parasuis-induced immunosuppression in our future studies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biom15050722/s1, File S1: original western blot images.
Author Contributions
S.F. conceived and designed the experiments; S.F., R.L., J.L., Y.F., Q.D., S.L., and Y.S. performed the experiments; S.F., R.L., J.L., Y.F., L.G., J.H., and Y.Q. analyzed the data; S.F. authored the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Key Research and Development Plan of Hubei Province, China (2023BBB069), the National Natural Science Foundation of China (grant No. 32273067), and Hubei Provincial Natural Science Funding (2023AFB746).
Institutional Review Board Statement
The animal study was approved by the Animal Care and Use Committee of Wuhan Polytechnic University, Hubei Province, China (WPU202308003), approval date 2 August 2023.All piglets used in this study were euthanized at the end of the experiment.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gong, X.; Cui, Q.; Zhang, W.; Shi, Y.; Zhang, P.; Zhang, C.; Hu, G.; Sahin, O.; Wang, L.; Shen, Z.; et al. Genomic insight into the diversity of Glaesserella parasuis isolates from 19 countries. mSphere 2024, 9, e0023124. [Google Scholar] [CrossRef]
- González-Fernández, A.; Mencía-Ares, O.; García-Iglesias, M.J.; Petrocchi-Rilo, M.; Miguélez-Pérez, R.; Perelló-Jiménez, A.; Herencia-Lagunar, E.; Acebes-Fernández, V.; Gutiérrez-Martín, C.B.; Martínez-Martínez, S. TbpB-based oral mucosal vaccine provides heterologous protection against Glässer’s disease caused by different serovars of Spanish field isolates of Glaesserella parasuis. Porcine Health Manag. 2024, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yu, B.; Su, F.; Ye, S.; Xu, L.; Yuan, X.; Wu, S.; Zhang, H.; Li, J. Ribosomal protein L32 contributes to the growth, antibiotic resistance and virulence of Glaesserella parasuis. Front. Vet. Sci. 2024, 11, 1361023. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhao, Q.; Wei, H.; Wen, X.; Cao, S.; Huang, X.; Wu, R.; Yan, Q.; Huang, Y.; Wen, Y. Prevalence and seroepidemiology of Haemophilus parasuis in Sichuan province, China. PeerJ 2017, 5, e3379. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Jia, Y.C.; Zhang, X.L.; Zhou, Y.Y.; Guo, Y.; Yin, R.L.; Yuan, J.; Wang, L.X.; Guo, Z.B.; Wang, J.Y.; et al. Virulence assessment of four Glaesserella parasuis strains isolated in Liaoning province of China. Res. Vet. Sci. 2023, 158, 226–234. [Google Scholar] [CrossRef]
- Siwan, E.; Wong, J.; Brooks, B.A.; Shinko, D.; Baker, C.J.; Deshpande, N.; McLennan, S.V.; Twigg, S.M.; Min, D. Deep Immune and RNA Profiling Revealed Distinct Circulating CD163+ Monocytes in Diabetes-Related Complications. Int. J. Mol. Sci. 2024, 25, 10094. [Google Scholar] [CrossRef]
- Schleh, M.W.; Ameka, M.; Rodriguez, A.; Hasty, A.H. Deficiency of the hemoglobin-haptoglobin receptor, CD163, worsens insulin sensitivity in obese male mice. Diabetes 2024, 73, 1990–2002. [Google Scholar] [CrossRef]
- Gobejishvili, L.; Vatsalya, V.; Avila, D.V.; Feygin, Y.B.; McClain, C.J.; Mokshagundam, S.; Barve, S. Association of Circulating Markers of Microbial Translocation and Hepatic Inflammation with Liver Injury in Patients with Type 2 Diabetes. Biomedicines 2024, 12, 1227. [Google Scholar] [CrossRef]
- Zhu, Y.J.; Chen, H.L.; Huang, J.K.; Cai, X.J.; Zhan, B.L. TWEAK increases angiogenesis to promote diabetic skin wound healing by regulating Fn14/EGFR signaling. J. Cosmet. Dermatol. 2024, 23, 4230–4238. [Google Scholar] [CrossRef]
- Akahori, H.; Karmali, V.; Polavarapu, R.; Lyle, A.N.; Weiss, D.; Shin, E.; Husain, A.; Naqvi, N.; Van Dam, R.; Habib, A.; et al. CD163 interacts with TWEAK to regulate tissue regeneration after ischaemic injury. Nat. Commun. 2015, 6, 7792. [Google Scholar] [CrossRef]
- Kowal-Bielecka, O.; Bielecki, M.; Guiducci, S.; Trzcinska-Butkiewicz, B.; Michalska-Jakubus, M.; Matucci-Cerinic, M.; Brzosko, M.; Krasowska, D.; Chyczewski, L.; Kowal, K. High serum sCD163/sTWEAK ratio is associated with lower risk of digital ulcers but more severe skin disease in patients with systemic sclerosis. Arthritis Res. Ther. 2013, 15, R69. [Google Scholar] [CrossRef]
- Gorvel, L.; Olive, D. Tumor associated macrophage in HPV(+) tumors: Between immunosuppression and inflammation. Semin. Immunol. 2023, 65, 101671. [Google Scholar] [PubMed]
- Campbell, S.; Michaelson, J.; Burkly, L.; Putterman, C. The role of TWEAK/Fn14 in the pathogenesis of inflammation and systemic autoimmunity. Front. Biosci. 2004, 9, 2273–2284. [Google Scholar] [CrossRef]
- Winkles, J.A. The TWEAK-Fn14 cytokine-receptor axis: Discovery, biology and therapeutic targeting. Nat. Rev. Drug Discov. 2008, 7, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, L.; Dave, M.; Menghini, P.; Xin, W.; Arseneau, K.O.; Pizarro, T.T.; Cominelli, F. Protective Role for TWEAK/Fn14 in Regulating Acute Intestinal Inflammation and Colitis-Associated Tumorigenesis. Cancer Res. 2016, 76, 6533–6542. [Google Scholar] [CrossRef]
- Shan, C.; Wang, Y.; Wang, Y. The Crosstalk between Autophagy and Nrf2 Signaling in Cancer: From Biology to Clinical Applications. Int. J. Biol. Sci. 2024, 20, 6181–6206. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Lindstrom, A.; Kim, E.J.; Hwang, C.I.; Hall, M.L.; Lin, T.Y.; Li, Y. SEMA3C Supports Pancreatic Cancer Progression by Regulating the Autophagy Process and Tumor Immune Microenvironment. Front. Oncol. 2022, 12, 890154. [Google Scholar]
- Carr, D.; Lau, R.; Hnatykiw, A.D.; Ward, G.C.D.; Daneshmand, M.; Cabrita, M.A.; Pratt, M.A.C. cIAP2 Is an Independent Signaling and Survival Factor during Mammary Lactational Involution and Tumorigenesis. J. Mammary Gland. Biol. Neoplasia 2018, 23, 109–123. [Google Scholar] [CrossRef]
- Zhang, Q.; Guo, S.; Wang, H. The Protective Role of Baicalin in the Regulation of NLRP3 Inflammasome in Different Diseases. Cell Biochem. Biophys. 2024. [Google Scholar] [CrossRef]
- Wen, Y.; Wang, Y.; Zhao, C.; Zhao, B.; Wang, J. The Pharmacological Efficacy of Baicalin in Inflammatory Diseases. Int. J. Mol. Sci. 2023, 24, 9317. [Google Scholar]
- Song, D.; Wei, W.; Zhang, J.; Zhang, L.; Huo, J.; Wang, W. The mechanism of baicalin in improving pulmonary inflammatory response and injury and regulating intestinal flora in Mycoplasma pneumoniae pneumonia mice. Cell Signal 2025, 126, 111530. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Yu, M.; Xu, M.; Ji, X.; Zong, X.; Zhang, Z.; Shang, W.; Zhang, L.; Fang, P. Baicalin suppresses macrophage JNK-mediated adipose tissue inflammation to mitigate insulin resistance in obesity. J. Ethnopharmacol. 2024, 332, 118355. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Liu, Z.; Wang, Y.; Li, X.; Yu, X.; Li, Y.; Yu, Z.; Qiu, Y.; Mei, Z.; Xu, L. Baicalin inhibits monosodium urate crystal-induced pyroptosis in renal tubular epithelial cell line through Panx-1/P2 × 7 pathways: Molecular docking, molecular dynamics, and in vitro experiments. Chem. Biol. Drug Des. 2024, 103, e14522. [Google Scholar] [CrossRef]
- Yang, X.; Li, J.; Shan, C.; Song, X.; Yang, J.; Xu, H.; Ou, D. Baicalin reduced injury of and autophagy-related gene expression in RAW264.7 cells infected with H6N6 avian influenza virus. Heliyon 2024, 10, e32645. [Google Scholar] [CrossRef]
- Dong, X.; Liu, X.; Lin, D.; Zhang, L.; Wu, Y.; Chang, Y.; Jin, M.; Huang, G. Baicalin induces cell death of non-small cell lung cancer cells via MCOLN3-mediated lysosomal dysfunction and autophagy blockage. Phytomedicine 2024, 133, 155872. [Google Scholar] [CrossRef]
- Fu, S.; Liu, S.; Li, J.; Dong, Q.; Fu, Y.; Luo, R.; Sun, Y.; Tian, X.; Liu, W.; Zong, B.; et al. Baicalin and probenecid protect against Glaesserella parasuis challenge in a piglet model. Vet. Res. 2024, 55, 96. [Google Scholar] [CrossRef]
- Fu, S.; Li, J.; You, J.; Liu, S.; Dong, Q.; Fu, Y.; Luo, R.; Sun, Y.; Tian, X.; Liu, W.; et al. Correction: Baicalin attenuates PD-1/PD-L1 axis-induced immunosuppression in piglets challenged with Glaesserella parasuis by inhibiting the PI3K/Akt/mTOR and RAS/MEK/ERK signalling pathways. Vet. Res. 2024, 55, 127. [Google Scholar] [CrossRef]
- Huang, B.; Li, F.; You, D.; Deng, L.; Xu, T.; Lai, S.; Ai, Y.; Huang, J.; Zhou, Y.; Ge, L.; et al. Porcine reproductive and respiratory syndrome virus infects the reproductive system of male piglets and impairs development of the blood-testis barrier. Virulence 2024, 15, 2384564. [Google Scholar] [CrossRef]
- Li, J.; Liu, S.; Dong, Q.; Fu, Y.; Sun, Y.; Luo, R.; Tian, X.; Guo, L.; Liu, W.; Qiu, Y.; et al. PD-1/PD-L1 axis induced host immunosuppression via PI3K/Akt/mTOR signalling pathway in piglets infected by Glaesserella parasuis. BMC Vet. Res. 2024, 20, 141. [Google Scholar] [CrossRef]
- Fu, S.; Yin, R.; Zuo, S.; Liu, J.; Zhang, Y.; Guo, L.; Qiu, Y.; Ye, C.; Liu, Y.; Wu, Z.; et al. The effects of baicalin on piglets challenged with Glaesserella parasuis. Vet. Res. 2020, 51, 102. [Google Scholar] [CrossRef]
- Hu, X.; Zheng, Y.; Fang, M.; Liang, Z.; Wen, C.; Lin, J.; Lin, Z.; Chen, S. Knockdown of the long noncoding RNA VSIG2-1:1 promotes the angiogenic ability of human pulmonary microvascular endothelial cells by activating the VEGF/PI3K/AKT pathway. Respir. Res. 2024, 25, 412. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zhu, N.; Liu, J.; Wen, S.; Xu, Y.; Xu, X.; Cai, X. The role of cytolethal distending toxin in Glaesserella parasuis JS0135 strain infection: Cytotoxicity, phagocytic resistance and pathogenicity. Vet. Microbiol. 2024, 295, 110168. [Google Scholar] [CrossRef]
- Shenton, J.M.; Teranishi, M.; Abu-Asab, M.S.; Yager, J.A.; Uetrecht, J.P. Characterization of a potential animal model of an idiosyncratic drug reaction: Nevirapine-induced skin rash in the rat. Chem. Res. Toxicol. 2003, 16, 1078–1089. [Google Scholar] [CrossRef]
- Yuan, S.; Zeng, Y.; Li, J.; Wang, C.; Li, W.; He, Z.; Ye, J.; Li, F.; Chen, Y.; Lin, X.; et al. Phenotypical changes and clinical significance of CD4(+)/CD8(+) T cells in SLE. Lupus Sci. Med. 2022, 9, e000660. [Google Scholar] [CrossRef]
- Nagatani, Y.; Kiyota, N.; Imamura, Y.; Koyama, T.; Funakoshi, Y.; Komatsu, M.; Itoh, T.; Teshima, M.; Nibu, K.I.; Sakai, K.; et al. Different characteristics of the tumor immune microenvironment among subtypes of salivary gland cancer. Asia Pac. J. Clin. Oncol. 2024, 20, 779–788. [Google Scholar] [CrossRef]
- Wu, Y.; Xiao, Y.; Ding, Y.; Ran, R.; Wei, K.; Tao, S.; Mao, H.; Wang, J.; Pang, S.; Shi, J.; et al. Colorectal cancer cell-derived exosomal miRNA-372-5p induces immune escape from colorectal cancer via PTEN/AKT/NF-κB/PD-L1 pathway. Int. Immunopharmacol. 2024, 143, 113261. [Google Scholar] [CrossRef]
- Qian, J.K.; Ma, Y.; Huang, X.; Li, X.R.; Xu, Y.F.; Liu, Z.Y.; Gu, Y.; Shen, K.; Tian, L.J.; Wang, Y.T.; et al. The CD163/TWEAK/Fn14 axis: A potential therapeutic target for alleviating inflammatory bone loss. J. Orthop. Translat 2024, 49, 82–95. [Google Scholar] [CrossRef]
- Liu, M.; Ren, Y.; Zhou, Z.; Yang, J.; Shi, X.; Cai, Y.; Arreola, A.X.; Luo, W.; Fung, K.M.; Xu, C.; et al. The crosstalk between macrophages and cancer cells potentiates pancreatic cancer cachexia. Cancer Cell 2024, 42, 885–903.e884. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, W.; Qiao, S.; Zou, H.; Yu, X.J.; Yang, Y.; Li, Z.; Wang, J.; Chen, M.S.; Xu, J.; et al. Lipid droplet accumulation mediates macrophage survival and Treg recruitment via the CCL20/CCR6 axis in human hepatocellular carcinoma. Cell Mol. Immunol. 2024, 21, 1120–1130. [Google Scholar] [CrossRef] [PubMed]
- Davidsson, S.; Huotilainen, S.; Carlsson, J.; Sundqvist, P. Soluble Levels of CD163, PD-L1, and IL-10 in Renal Cell Carcinoma Patients. Diagnostics 2022, 12, 336. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, W.; Atkinson, S.D.; Kelly, C. The TWEAK/Fn14/CD163 axis-implications for metabolic disease. Rev. Endocr. Metab. Disord. 2022, 23, 449–462. [Google Scholar] [CrossRef]
- Xu, L.; Liu, W.; Huang, X.; Sun, T.; Mei, L.; Liu, M.; Ren, Z.; Wang, M.; Zheng, H.; Wang, Q.; et al. Sinomenine hydrochloride improves DSS-induced colitis in mice through inhibition of the Notch signaling pathway. BMC Gastroenterol. 2024, 24, 451. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.; Li, Y.; Li, D.; Dou, M.; Fu, C.; Chen, T.; Cui, X.; Zhang, Q.; Yang, P.; Hou, Y.; et al. SRCAP is involved in porcine reproductive and respiratory syndrome virus activated Notch signaling pathway. J. Virol. 2024, 98, e0121624. [Google Scholar] [CrossRef]
- Adams, J.M.; Jafar-Nejad, H. The Roles of Notch Signaling in Liver Development and Disease. Biomolecules 2019, 9, 608. [Google Scholar] [CrossRef]
- Mukherjee, M.; Fogarty, E.; Janga, M.; Surendran, K. Notch Signaling in Kidney Development, Maintenance, and Disease. Biomolecules 2019, 9, 692. [Google Scholar] [CrossRef] [PubMed]
- Zou, G.; Park, J.I. Wnt signaling in liver regeneration, disease, and cancer. Clin. Mol. Hepatol. 2023, 29, 33–50. [Google Scholar] [CrossRef]
- Akoumianakis, I.; Polkinghorne, M.; Antoniades, C. Non-canonical WNT signalling in cardiovascular disease: Mechanisms and therapeutic implications. Nat. Rev. Cardiol. 2022, 19, 783–797. [Google Scholar] [CrossRef]
- Ren, S.Y.; Xu, X. Role of autophagy in metabolic syndrome-associated heart disease. Biochim. Biophys. Acta 2015, 1852, 225–231. [Google Scholar] [CrossRef]
- Kolahdouzmohammadi, M.; Kolahdouz-Mohammadi, R.; Tabatabaei, S.A.; Franco, B.; Totonchi, M. Revisiting the Role of Autophagy in Cardiac Differentiation: A Comprehensive Review of Interplay with Other Signaling Pathways. Genes 2023, 14, 1328. [Google Scholar] [CrossRef]
- Carballo, G.B.; Ribeiro, J.H.; Lopes, G.P.F.; Ferrer, V.P.; Dezonne, R.S.; Pereira, C.M.; Spohr, T. GANT-61 Induces Autophagy and Apoptosis in Glioblastoma Cells despite their heterogeneity. Cell Mol. Neurobiol. 2021, 41, 1227–1244. [Google Scholar] [CrossRef]
- Zhang, C.; Li, W.; Wen, J.; Yang, Z. Autophagy is involved in mouse kidney development and podocyte differentiation regulated by Notch signalling. J. Cell Mol. Med. 2017, 21, 1315–1328. [Google Scholar] [CrossRef]
- Bai, R.; Miao, M.Z.; Li, H.; Wang, Y.; Hou, R.; He, K.; Wu, X.; Jin, H.; Zeng, C.; Cui, Y.; et al. Increased Wnt/β-catenin signaling contributes to autophagy inhibition resulting from a dietary magnesium deficiency in injury-induced osteoarthritis. Arthritis Res. Ther. 2022, 24, 165. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).