Abstract
Neuroendocrine neoplasms (NENs) are rare and heterogeneous tumors with heterogeneity in morphology and molecular profile and consequently resulting in a heterogeneous biological behavior. They have a more indolent natural history compared to the classic cancer and may emerge in any site of the human body, but usually they have gastroenteropancreatic (GEP) or bronchopulmonary (BP) origin. When NENs are well differentiated, they are called neuroendocrine tumors (NETs) as opposed to poorly differentiated neuroendocrine carcinomas (NECs). They may secrete a bioactive molecule resulting in a secretory syndrome or they may not be associated with any secretory product, defining functional and non-functional NENs. The hormonal hypersecretion syndromes, the chronic symptom burden, the tumor-related inflammation, and the treatment side effects impair nutritional intake and absorption while increasing metabolic needs. The present comprehensive narrative review is summarizing established and emerging methods of nutritional and body composition assessment, and the recent evidence of interventions for sarcopenia and malnutrition in patients with NETs. Early identification and management of malnutrition and sarcopenia are fundamental steps to improve quality of life and clinical outcomes in these patients during the long natural history of these neoplasms.
1. Introduction
1.1. Background
Neuroendocrine neoplasms (NENs) are rare and heterogeneous tumors with diverse profiles in terms of morphology and molecular profile, resulting in different biological phenotypes. NENs are arising from neuroendocrine cells throughout the body, but their main origin is gastroenteropancreatic (GEP) and bronchopulmonary (BP) [1]. When NENs are well differentiated, they are called neuroendocrine tumors (NETs) as opposed to poorly differentiated neuroendocrine carcinomas (NECs). They may secrete a bioactive molecule resulting in a secretory syndrome, or they may not be associated with any secretory product, defining functional and non-functional NENs [2].
Their incidence and prevalence have increased over recent decades worldwide. Improvements in diagnostic imaging, endoscopy, histopathology, and overall disease awareness have contributed to early and more frequent detection [3,4,5]. They have a slow progression along with a more indolent natural history compared to classic cancer, but they present a metastatic potential in up to 20% of patients. As survival has improved because of earlier diagnosis and expanding systemic and locoregional treatment options, NETs are considered chronic oncologic conditions, resulting in a shift in clinical priorities toward the maintenance of health-related quality of life (QoL), functionality, and the preservation of muscle mass, the prevention of nutrition-related problems and malnutrition [6,7,8].
Within this context, deterioration of nutritional status and body composition—including malnutrition, cancer cachexia, and sarcopenia—is of particular concern in populations with NET. As the overall survival of these patients has increased, they are sustaining the cumulative effects of treatment exposures and persistent hormonal or tumor-related symptoms, which have a negative impact on physical and nutritional status [9].
1.2. Malnutrition, Cachexia, and Sarcopenia in NETs
Malnutrition generally refers to insufficient nutrient intake and absorption and, as a result, suboptimum coverage of nutritional requirements, leading to measurable changes in body mass, body composition, and physical or mental function [10]. On the other hand, cancer cachexia describes a multifactorial syndrome driven by systemic inflammation, metabolic dysregulation, anorexia, and reduced food intake, resulting in significant loss of muscle mass (with or without loss of fat mass) that cannot be fully reversed by conventional nutritional support, especially in later stages [11]. Sarcopenia, originally defined in geriatrics, denotes a progressive loss of muscle mass and strength associated with adverse outcomes [12,13]. In oncology is now frequently assessed by using imaging-based techniques evaluating muscle quantity and quality, combined with measures of muscle function or performance. Across solid tumors, sarcopenia, cachexia, and broader forms of disease-related malnutrition are consistently associated with increased treatment toxicity, impaired physical function, poorer quality of life, and reduced survival, underscoring the need to understand their determinants and management, specifically in NETs [14].
NETs increase the risk of nutrition-related complications. Hormone hypersecretion syndromes—such as carcinoid syndrome, gastrinoma, insulinoma, glucagonoma, or VIPoma—can induce chronic diarrhea, steatorrhea, vomiting, hypoglycemia, or increased catabolic activity, directly reducing nutrient absorption and increasing energy and protein requirements [15]. Disease chronicity, often spanning years to decades, exposes patients to medical and surgical procedures (including bowel or pancreatic resections), somatostatin analogs (SSAs), peptide receptor radionuclide therapy (PRRT), targeted agents, and cytotoxic chemotherapy, all of which may have a negative impact on appetite, digestion, exocrine pancreatic function, bile acid physiology, and intestinal motility [16]. As a result, many patients with NET experience a cumulative nutritional burden characterized by involutional weight loss, micronutrient deficiencies, altered body composition with muscle depletion, and fluid imbalance, often masking weight loss when traditional anthropometric indices such as body mass index (BMI) are used in isolation [17]. Although nutritional support in oncology is well established, the application of Global Leadership Initiative on Malnutrition (GLIM) malnutrition and European Working Group on Sarcopenia in Older People 2 (EWGSOP)2 sarcopenia diagnostic criteria, published data on NET-specific populations remain limited. Therefore, this review can be the basis of a more comprehensive evaluation of the applicability of these consensuses on NET specific populations. Nutrition-related dysregulations have important implications for patient-centered outcomes in NETs. Loss of skeletal muscle mass and strength contributes to declines in physical function, fatigue, and limited QoL [16,18]. Moreover, they contribute to reduced capacity to tolerate repeated cycles of systemic therapy or major surgery, potentially limiting access to optimal oncologic treatment sequences [19]. Malnutrition and sarcopenia have been linked in broader oncologic cohorts to increased postoperative complications, dose-limiting toxicities, longer hospital stays, and diminished health-related QoL, patterns that are highly relevant to patients with NETs given their prolonged survivorship and frequent multimodal therapy [20,21]. Furthermore, variable symptom burdens—such as diarrhea, abdominal pain, and early satiety—can result in inadequate oral intake, reinforcing nutritional decline, functional impairment, and psychological distress.
The present narrative review aims to synthesize current evidence on interventions targeting malnutrition and sarcopenia in adults with NETs. In the context of this review, malnutrition is defined in line with contemporary consensus frameworks as a state of inadequate nutritional intake and/or assimilation leading to altered body composition, diminished physical function, and adverse clinical outcomes, and, where available, was defined using the GLIM criteria [10] or validated oncology nutrition tools such as Nutritional Risk Screening 2002 (NRS-2002), Malnutrition Universal Screening Tool (MUST), or Patient-Generated Subjective Global Assessment (PG-SGA) [21], often in combination with clinically relevant weight loss and BMI thresholds. Sarcopenia is considered a muscle-centered phenotype characterized by low skeletal muscle mass in conjunction with reduced muscle strength and/or physical performance; in the included NET literature it was most commonly identified using EWGSOP/EWGSOP2 criteria [13] or by cross-sectional imaging (typically CT-derived skeletal muscle indices) at predefined vertebral levels, acknowledging that some studies relied primarily on quantitative imaging cut-offs without comprehensive functional assessment. Emerging interventions are defined as novel or not yet widely implemented strategies specifically targeting malnutrition, sarcopenia, or the underlying metabolic and inflammatory milieu in NETs, including but not limited to microbiome-modulating approaches, ghrelin receptor agonists, myostatin-pathway inhibitors, and digital or artificial-intelligence-supported tools designed to personalize nutritional and physical activity management beyond standard dietetic counseling and routine oncologic care. By integrating current evidence and outlining pragmatic strategies for clinical practice, this review seeks to support the development of individualized, multidisciplinary approaches to prevent, detect, and treat malnutrition and sarcopenia in patients with NETs, with the goal of enhancing QoL, treatment tolerance, and long-term survivorship.
2. Pathophysiology of Malnutrition and Sarcopenia in NETs
2.1. Tumor-Related Mechanisms
Patients with NETs increasingly present malnutrition, given the improved overall survival of these neoplasms. Thus, 5–38% of patients present with weight loss, whereas 5–12% display low BMI [9,19]. Sarcopenia is particularly frequent in patients with GEP-NET, reaching 61–87% of all affected patients [22,23]. This extraordinarily high prevalence of sarcopenia can be explained not only by classic tumor-induced catabolic effects but also due to the secretory function of these neoplasms (Figure 1).
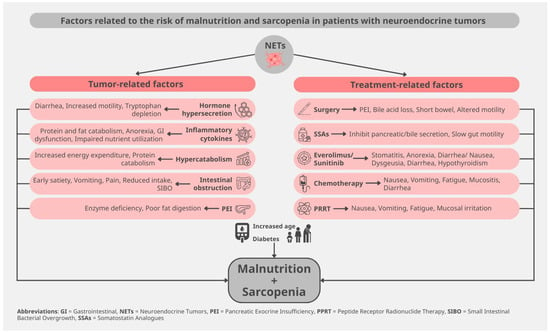
Figure 1.
Risk factors for malnutrition and sarcopenia in patients with neuroendocrine tumors.
When serotonin and othe bioactive substances (histamine, bradykinin, kallikrein) are secreted by NET cells, are causing carcinoid syndrome with diarrhea or bronchospasm, flushing, and carcinoid heart disease. Each of these symptoms can lead to malnutrition. Muscle wasting is now being acknowledged as one of the metabolic consequences of carcinoid syndrome due to uncontrolled diarrhea, which also causes electrolyte loss, dehydration, fatigue, and thereby also reduced appetite [24]. Bronchospasm leads to fatigue and difficulty eating during dyspnea episodes, but also to increased energy expenditure. Similarly, carcinoid heart disease also causes anorexia due to fatigue but also due to right cardiac failure, with hepatic congestion and ascites [16]. Flushing, also dependent on specific food consumption, leads to food avoidance and thereby to reduced appetite [25]. As the amino acid tryptophan is the main precursor for serotonin production in carcinoid syndrome, the synthesis of the water-soluble vitamin B3 (niacin) from the same precursor is impaired in these patients, leading to niacin deficiency, with dermatitis, chronic diarrhea, and dementia [25,26,27].
Gastrin hypersecretion in Zollinger-Ellison syndrome causes peptic ulcers and steatorrhea, resulting in impaired nutrient uptake, whereas watery diarrhea syndrome related to VIPoma causes, among others, hypokalemia, with muscle weakness, metabolic acidosis, and hypochlorhydria, thus, mineral loss, leading to bone demineralization [16,17,18,19,20,21,22,23,24,25,26,27,28]. On the other hand, glucagonomas, besides diarrhea, cause zinc deficiency with glossitis, stomatitis, impaired food intake, diabetes with catabolic stress, increased hepatic gluconeogenesis, lipolysis, and muscle wasting, thus leading to cachexia [15,29]. In line with these observations, somatostatinomas present with diabetes, diarrhea, and hypochloridria, resulting in muscle wasting and sarcopenia. Finally, unlike all previous functioning NETs, insulinoma-related malnutrition is not linked to weight loss. In fact, the inappropriate weight gain upon hypoglycemia symptoms suggests a metabolic impairment, with increased fat mass [30]. Taken together, all these hormones affect overall nutritional status in NETs, contributing to malabsorption. In addition, the released hormones and cytokines exert direct effects on muscle turnover, leading to muscle wasting, altogether attenuating sarcopenia.
It has been demonstrated that the expression levels of ghrelin-O-acyltransferase, the activating enzyme of ghrelin, were significantly higher in patients with NET presenting with weight loss, whereas the ghrelin levels and the two splice variants of its receptor [growth hormone secretagogue receptor 1-a (GHSR1a) and growth hormone secretagogue receptor 1-b (GHSR1b)] also present altered expression in NETs. Ghrelin is an orexigenic gut hormone that antagonizes muscle catabolism occurring in cancer cachexia but also has a role in processes related to tumor proliferation and progression [23].
Still, not only functioning NETs but also hormonally inactive tumors are associated with malnutrition. The presence of malignancy is associated with nonspecific symptoms such as fatigue, low appetite, nausea, and weight loss. In parallel, tumor inflammation, acknowledged as one of the contributing factors in cachexia, also exerts its effects in patients with NETs. Inflammation can either promote or suppress neuroendocrine carcinogenesis, with contrasting results in tumor progression vs. nutritional status [17]. It has been observed that patients with NEC suffer much more frequently from malnutrition in comparison to patients with NETs, possibly in the context of a hypermetabolic state [19,22,31]. Furthermore, depending on the tumor localization, mass effects can affect food intake and/or absorption. In this context, gastric NETs cause nausea, vomiting, early satiety, pain, and/or obstruction, whereas pancreatic NETs can cause altered glucose metabolism, impaired pancreatic exocrine function, biliary obstruction, and thereby malabsorption [27]. In the case of small intestine neoplasms tumor mass can cause bowel obstruction and diarrhea, and, in particular, the mesenteric metastases can lead to desmoplastic reaction with fibrosis and ischemia, causing postprandial abdominal pain, malabsorption, and sarcopenia [17].
Micronutrient deficiencies are a major challenge in patients with NETs. As previously mentioned, vitamin B3 biosynthesis is impaired in carcinoid syndrome due to the common precursor with serotonin. Hypochlorhydria in gastric NETs, of autoimmune origin or upon treated Zollinger-Ellison syndrome, or in patients under SSA therapy, can lead to B12 deficiency, but B12 malabsorption can also be observed in patients with small intestine neoplasms or postoperatively. Vitamin D deficiency is also frequent in patients with NETs, particularly in those suffering from diarrhea or steatorrhea [9,15,17]. Possibly, this vitamin D deficiency is contributing to the increased prevalence of osteopenia and osteoporosis in these patients (41% and 10%, respectively), while higher levels of urinary 5-hydroxy-indoleacetic acid (5-HIAA), the metabolite of serotonin, could also play a role [32].
2.2. Treatment-Related Mechanisms
Beyond surgical and pharmacologic effects on digestion and absorption, accumulating evidence suggests that alterations of the gut microbiota may contribute to malnutrition and metabolic dysregulation in NETs. Patients with small bowel NETs appear particularly prone to small intestinal bacterial overgrowth (SIBO), driven by anatomical changes, impaired motility, use of proton pump inhibitors, and recurrent abdominal surgery, which may lead to bile acid deconjugation, carbohydrate malabsorption, steatorrhea, and micronutrient deficiencies. These microbiota-related mechanisms can amplify existing hormone-mediated diarrhea and malabsorption, thereby worsening weight loss and sarcopenia, and highlight the potential role of targeted diagnosis and management of SIBO (for example, with breath testing and evidence-based antibiotic or dietary strategies) as part of a comprehensive nutritional approach in selected NET patients [15].
Several different treatment modalities used in the therapeutic armamentarium of NETs can present as a side effect the aggravation of malnutrition and sarcopenia [33]. Previous surgeries in the gastroenteropancreatic tract can frequently cause alterations of the digestive tract, with subsequent reduced nutrition uptake. Gastrectomy or removal of the terminal ileum can lead to vitamin B12 deficiency, while small bowel resection can additionally cause bile acid malabsorption [34]. Furthermore, short bowel syndrome, but also bacterial overgrowth, can be present after both small intestine resection and the Whipple procedure. Whipple procedure, pylorus-preserving pancreatoduodenectomy, or distal pancreatectomy can also induce pancreatic exocrine insufficiency, causing steatorrhea and malabsorption of fat-soluble vitamins [9,22], but also impaired glucose metabolism [27].
Therapeutic administration of SSAs is often used to control gastrointestinal problems in patients with NETs [35]. SSA therapy can also be associated with abdominal pain, nausea, pancreatic exocrine insufficiency, and suboptimal nutritional absorption, and negative effects on nutritional status [27,36]. Moreover, due to their mechanism of action, SSAs may contribute to malnutrition and, consequently, to sarcopenia, highlighting the need for careful assessment and management of muscle mass and nutritional status in these patients.
Therapy with the mechanistic target of rapamycin (mTOR) inhibitor everolimus contributes to impaired glucose metabolism [37]. Furthermore, the mTOR pathway plays a pivotal role in activating skeletal muscle synthesis and the regulation of lipid and carbohydrate metabolism, with respective effects of its inhibitor on sarcopenia [38]. On the other hand, systemic chemotherapy can cause stomatitis, nausea, vomiting, diarrhea, all leading to malabsorption [9]. Peptide receptor radionuclide therapy can also cause fatigue, nausea and vomiting, but also pain and a carcinoid syndrome flare in the weeks following each cycle, together with diarrhea and, in particular, steatorrhea due to pancreatic insufficiency, leading to a transient reduction in food intake and subsequent weight loss [27,39]. Moreover, patients with small intestine NETs present an alteration of their gut microbiota, possibly affecting both malnutrition and carcinogenesis, rendering this a possible therapeutic target for the improvement of malnutrition, but also of prognosis [31].
2.3. Host-Related Factors
Survival in patients with NETs is significantly prolonged due to both better natural history of the disease per se, compared to classic cancer, but also due to new treatment modalities [40], so that the population with NETs is progressively aging. In this context, older adults with NETs are more susceptible to sarcopenia. In addition, the presence of comorbidities such as diabetes mellitus can aggravate this phenotype, whereas side effects of the various treatments mentioned before act additively and can complicate nutritional status [17,36].
3. Study Selection and Quality Appraisal
Given the narrative design and the heterogeneity of available data, a structured appraisal of methodological quality was undertaken for the main observational and interventional studies. Non-randomized observational cohorts examining malnutrition, sarcopenia, and outcomes in NETs were evaluated using domains adapted from ROBINS-I [41], focusing on confounding, selection of participants, classification and measurement of exposures/outcomes, missing data, and selective reporting. Across these studies, the overall risk of bias was generally judged as moderate to serious, primarily due to limited adjustment for key confounders (e.g., tumor burden, performance status, treatment line), single-center designs, and relatively small sample sizes, which may inflate or obscure associations between nutritional status, body composition, and clinical endpoints.
For interventional evidence (dietary, exercise, multimodal, and emerging pharmacologic or microbiome-targeted approaches), the certainty of evidence was appraised using a GRADE-informed approach at the level of each intervention category rather than per individual trial [42]. Most available data in NETs derive from small, often non-randomized or single-arm studies, frequently with short follow-up and reliance on surrogate outcomes such as changes in weight, skeletal muscle indices, or symptom scores, leading to an overall low to very low certainty of evidence for effects on hard outcomes, including survival, treatment tolerance, and long-term QoL. Consequently, while the direction of effect for early nutritional support and multimodal strategies is broadly consistent with findings in mixed-cancer cohorts, conclusions in NET-specific populations should be considered hypothesis-generating, and clinical recommendations must be interpreted with caution and individualized within multidisciplinary care.
4. Epidemiology and Clinical Significance
4.1. Prevalence of Nutrition-Related Problems
Malnutrition and sarcopenia are highly prevalent among patients with GEPNETs, with recent cohort studies using nutritional assessment and cross-sectional imaging reporting that nearly half of newly diagnosed or surgically treated patients meet the diagnostic criteria for sarcopenia or computed tomography (CT)-derived skeletal muscle indices [22,43]. With regard to GEPNENs, a substantial proportion of patients also fulfill the GLIM-malnutrition criteria, and malnutrition frequently coexists with sarcopenia and metabolic comorbidities such as diabetes, suggesting a complex interplay between tumor biology, hormonal syndromes, and host factors [17]. Although data directly applying GLIM and EWGSOP2 criteria for malnutrition and sarcopenia, respectively, in NET-specific populations remain limited, extrapolation from broader oncology cohorts indicates that these frameworks capture a clinically relevant burden of disease-related malnutrition that is likely underdiagnosed if only simple anthropometrics are used [44].
The co-existence of malnutrition and sarcopenia has important prognostic implications for patients with NETs. In patients with GEPNET, CT-defined sarcopenia has been associated with reduced overall survival and shorter progression-free survival, even after adjustment for conventional prognosticators [45]. More specifically, malnutrition roughly doubles mortality risk and increases postoperative complications [38], while sarcopenia identified using EWGSOP2 or imaging-based criteria is consistently linked to worse survival, higher treatment toxicity, and more frequent hospitalizations, patterns that are highly relevant to patients with NETs, given their long disease trajectories [46].
4.2. Nutritional Status, Prognosis, and Treatment Tolerance
Nutritional status and body composition have a significant impact on treatment tolerance and QoL in NETs. Based on an observational study in patients with advanced GEP-NENs, malnutrition and sarcopenia correlate with fatigue, gastrointestinal symptoms, and impaired health-related QoL scores, while symptom-directed therapies that improve diarrhea control can stabilize weight and reduce nutritional decline in patients with carcinoid syndrome [16,47]. According to the study of Ranallo et al., in NETs treated with targeted agents such as everolimus, baseline low muscle mass and the phenotype of sarcopenia have been associated with higher rates of dose reductions or interruptions, underlining the potential role of sarcopenia as a predictor of treatment-related toxicity [38]. These findings underpin the importance of early detection of nutrition-related problems and functionality deterioration, to treat early and sufficiently therapy-limiting complications over the course of chronic NET management [19].
An additional consideration is the temporal relationship between the onset of malnutrition or sarcopenia and the initiation of systemic therapy in NETs. Baseline malnutrition and low skeletal muscle mass at the time of diagnosis or before treatment have been associated in broader oncologic cohorts with higher postoperative morbidity, increased treatment toxicity, and poorer survival, suggesting that nutritional risk present at the outset may confer a different prognostic profile than deterioration that develops later under therapy [15]. In NET populations, observational data similarly indicate that patients often enter PRRT, targeted therapies, or cytotoxic regimens with pre-existing weight loss, muscle depletion, and micronutrient deficiencies, which may already compromise treatment tolerance and functional reserve. This underscores the need to embed systematic nutrition and body composition assessment into the pre-treatment work-up and to distinguish early, potentially modifiable deficits from end-stage cachectic trajectories when interpreting outcomes and designing interventions [16,17].
5. Assessment and Diagnosis
5.1. Nutritional Screening—Assessment
Nutritional screening is the first step for identifying patients at risk of malnutrition. Validated nutritional screening tools such as the NRS-2002, the MUST, and the PG-SGA are widely recommended in oncology and have been shown in mixed-cancer cohorts to correlate with GLIM-diagnostic criteria of malnutrition. More specifically, NRS-2002 is demonstrating particularly good agreement, and PG-SGA offers high sensitivity for detecting malnutrition [10,44]. In clinical practice, these tools can be used at baseline and at regular intervals to early identify patients who would require more detailed nutritional assessment, including the evaluation of dietary intake, the nutrition-related symptoms, changes in body weight, and in functional status [21].
5.2. Body Composition Analysis
Objective assessment of body composition is pivotal for the diagnosis of sarcopenia and the quantitative and qualitative estimation of body mass changes in patients with NETs [39]. The evaluation of analysis of muscle mass based on the analysis of images of CT, at the third lumbar vertebra on routine staging or restaging scans, is considered a reference method for quantifying muscle mass and identifying sarcopenia in oncology [48,49], and has been successfully applied in patients with NET to demonstrate high prevalence muscle mass deterioration and to explore associations with survival and treatment outcomes, with reduced overall survival (OS)/progression-free survival (PFS) (Hazard Ratio, HR) 1.8–2.5 across cohorts) [22,34,45]. Magnetic resonance imaging (MRI) offers similar capabilities with superior soft-tissue contrast and radiation exposure, but it is less standardized for body composition analysis; dual-energy X-ray absorptiometry (DXA) and bioelectrical impedance analysis (BIA) are usually more accessible in a clinical setting, but the estimation of lean body mass and fat mass may be affected by the fluid status of the patients. Apart from that, based on the study by Kroll et al., body composition analysis based on Computed Tomography (CT) scans can significantly reduce the cost as regular staging exams can be used for the analysis, and at the same time limit the radiation exposure from DXA, providing a significant improvement of QoL in patients with GEPNET [50].
In the available studies on NET populations, sarcopenia was predominantly quantified using cross-sectional imaging, yet substantial methodological heterogeneity exists in how skeletal muscle was assessed and classified. Investigators have applied different vertebral landmarks (most commonly the third lumbar vertebra, but in some cohorts the third cervical or other levels), variable segmentation protocols, and a range of cut-off values for skeletal muscle index or muscle attenuation, while some reports relied solely on muscle quantity without incorporating strength or performance measures as recommended by EWGSOP2 [13]. This variability complicates direct comparison of sarcopenia prevalence and effect estimates across studies and may dilute the apparent strength of associations between sarcopenia, treatment tolerance, and survival; therefore, the prognostic inferences presented in this review should be interpreted as hypothesis-generating and viewed in the context of these methodological differences. Harmonization of CT-based protocols and incorporation of functional testing in future NET-specific research will be crucial to refine risk stratification and to support more robust, generalizable conclusions. Table 1 summarizes the nutritional screening tools, the assessment tools, and the diagnostic criteria commonly used in populations with NETs.

Table 1.
Nutritional screening and assessment tools and diagnostic criteria for malnutrition and Sarcopenia in NETs.
5.3. Biomarkers
Biomarkers can be used as a complementary measure for clinical and imaging assessment, reflecting systemic inflammation, visceral protein status, and anabolic-catabolic balance, all of which influence nutritional and muscle health. Serum albumin, although affected by factors beyond nutrition, remains a widely used marker associated with prognosis and perioperative risk, while C-reactive protein (CRP) and composite indices incorporating CRP and albumin are used to evaluate the inflammatory component of cancer cachexia. Apart from that, CRP is used as an aetiologic criterion for malnutrition diagnosis based on GLIM criteria [10,51].
Within the GLIM framework, biomarkers such as serum albumin and CRP are often considered in the context of inflammation and disease burden, yet their interpretation in NET populations requires caution. Albumin is a negative acute-phase reactant strongly influenced by systemic inflammation, hepatic function, fluid status, and overall disease severity, and therefore, low concentrations may reflect catabolic and inflammatory activity rather than isolated nutritional depletion, especially in patients with advanced tumors or treatment-related toxicity [20]. Similarly, CRP is endorsed as an etiologic indicator of inflammation in GLIM, but in NETs, it may be chronically elevated due to tumor-related inflammatory pathways, infections, or therapy complications, complicating its attribution to malnutrition per se and potentially leading to overestimation of the inflammatory component of GLIM-defined malnutrition [10,51]. In this context, biomarker changes should be integrated with clinical assessment, body composition measures, and functional outcomes, and future NET-specific studies are needed to validate GLIM-based biomarker thresholds against hard endpoints such as survival, treatment tolerance, and quality of life to refine their use in this setting.
Additional markers such as insulin-like growth factor 1 (IGF-1), vitamin D, and muscle-derived cytokines and myokines have been investigated as potential indicators of muscle mass and function in patients with cancer, but their roles in NET-specific settings are not yet firmly established and are primarily used in research rather than routine care [52,53].
6. Nutritional and Metabolic Interventions
Interventions aiming to improve nutritional status and body composition in patients with NETs require a multidisciplinary approach, addressing changes in dietary intake and behavior, physical activity, artificial nutritional support, and pharmacological agents. This multimodal strategy focuses on the management of malnutrition and sarcopenia, thereby improving QoL and clinical outcomes [54].
In clinical practice, the timing of nutritional and metabolic interventions appears crucial. Evidence from mixed-cancer populations suggests that supportive care initiated before or at the very beginning of systemic therapy is more likely to stabilize weight, maintain function, and reduce dose-limiting toxicities than interventions introduced only at advanced or refractory stages, when catabolic drive and symptom burden are pronounced [17]. For patients with NETs, this supports a strategy of proactive, early screening and individualized intervention planning around key treatment milestones—such as planned surgery, PRRT, or commencement of targeted agents—rather than a reactive approach triggered only by severe weight loss or overt functional decline [15]. To facilitate the early intervention, nutritional screening should be implemented at diagnosis or before the initiation of major treatments (surgery/ PRRT) and repeated every 3 months during active therapy or every 6 months in stable follow-up [17,21,55]
6.1. Dietary Interventions
Several factors, namely the tumor-induced metabolic alterations and malabsorption, change energy and nutrient needs in patients with NET. As there are no specific guidelines for patients with NETs regarding the recommended energy and protein intake, the guidelines of the European Society of Clinical Nutrition and Metabolism for patients with cancer can be used [21,55]. Energy targets should be set at 25–30 kcal/kg/day, while protein intake should be increased to reach 1.2–1.5 g/kg/day (or 1.5–2.0 g/kg in sarcopenia/severe malnutrition), adjusted for diarrhea/renal function [21,55].
Dietary modification plays a key role in symptom control for patients with carcinoid syndrome, though evidence remains largely observational [24,25]. Common triggers include high histamine/tyramine foods and specific beverages that may precipitate flushing, diarrhea, or abdominal discomfort via biogenic amine release or vasoactive effects [27]. Rather than universal avoidance—which increases the risk of malnutrition—current expert guidance recommends individualized testing via 1–2-week food-symptom diaries followed by selective trigger reduction under dietitian supervision, ensuring maintenance of energy targets. In Table 2, the common nutritional triggers for carcinoid syndrome and dietary alternatives are presented.

Table 2.
Common nutritional triggers for carcinoid syndrome.
Dietary counseling should be provided on an individual basis to identify and treat nutrition-related symptoms that compromise nutritional status early in the course of the disease. Nutrition modification may involve the number of meals, adjustments in fat and fiber intake, and increases in energy and protein, to facilitate improved tolerance and the preservation of body weight and muscle reserves [15,21,55]. Pancreatic enzyme replacement therapy (PERT) can effectively manage exocrine pancreatic insufficiency, which is common in patients with NETs or postoperatively in patients who have undergone pancreatic surgery. PERT improves fat absorption by reducing steatorrhea and helps in the restoration of nutritional status and muscle mass. To achieve these results, the management of PERT doses should be based not solely on the improvement of gastrointestinal symptoms but on the achievement of nutritional intake goals [56].
For patients not covering their nutritional needs through their diet, food fortification and oral nutritional supplements (ONS) can be considered, especially during treatments [55,57]. For the ONS studies in patients with cancer have shown that high protein supplements containing omega-3 fatty acids can improve nutritional and inflammatory markers, resulting in shorter length of stay and overall improved clinical outcomes [58]. Importantly, the evidence base for ONS in NETs is almost entirely extrapolated from broader oncology cohorts, as NET-specific ONS trials are currently lacking. Recent meta-analyses and systematic reviews in mixed-cancer settings indicate that high-protein, energy-dense ONS can improve or stabilize body weight and, in some studies, selected QoL domains, particularly in patients at nutritional risk or undergoing active treatment. However, these benefits are not uniform across all outcome measures: changes in serum albumin, CRP, and other inflammatory or visceral protein biomarkers are often modest or inconsistent, and improvements in comprehensive tools such as the PG-SGA may vary by population and study design. Consequently, ONS in NETs should be viewed as a pragmatic component of a broader, individualized nutrition strategy aimed at supporting intake, symptom control, and functional status, rather than as a stand-alone intervention expected to normalize laboratory markers in isolation.
Enteral nutrition can also be considered in advanced disease stages and severe malnutrition. It can cover the needs of patients, not tolerating oral nutrition. For those with severe malabsorption or bowel dysfunction, parenteral nutrition, supplemental or total parenteral nutrition, can also be provided [55]. More specifically, in patients failing to achieve <60% requirements despite counseling/ONS for >7–10 days, enteral nutrition support should be considered, if gut function). If enteral nutrition is contraindicated or not tolerated or is covering less than 50% of the patient’s needs, supplemental or total parenteral nutrition should be considered, especially in patients with short bowel or severe steatorrhea [17,55]
Special care should be taken for patients with malabsorption and hormone hypersecretion. In these patients, vitamin and trace element deficiencies are common, especially B complex, vitamin D, and the other fat-soluble vitamins [9].
6.2. Exercise and Physical Activity
As the main therapeutic aim is the preservation or improvement of muscle mass and function, resistance and combined training are generally recommended, with evidence in broader oncology and geriatric sarcopenia populations showing that such programs can stimulate muscle protein synthesis, enhance mitochondrial function, and improve physical performance [59]. Nevertheless, the strength of evidence supporting exercise interventions specifically in patients with NETs remains limited. Existing data largely derive from small randomized or quasi-experimental trials and observational studies in gastrointestinal or endocrine cancers that include but do not focus exclusively on NETs, as well as expert consensus statements such as the recent ENETS position paper on nutritional support [17]. To date, randomized controlled trials designed explicitly for NET cohorts and powered to evaluate functional outcomes, body composition, and treatment tolerance are sparse, so current recommendations for structured, personalized exercise in NETs should be regarded as extrapolated from related populations and prioritized as a key area for future research [60].
The ENETS position statement on nutritional support in neuroendocrine neoplasms explicitly recommends integrating structured physical activity and resistance exercise into multimodal care for NET patients, with tailoring to symptom burden, comorbidities, and treatment phase [17]. In addition, NET nutrition resources and expert guidance advise incorporating regular walking and simple resistance exercises to counteract sarcopenia and treatment-related fatigue, while emphasizing the need to individualize session duration and intensity in those with carcinoid-related diarrhea, PRRT-related fatigue, or advanced disease [61].
6.3. Multimodal Interventions
Multimodal interventions combine nutrition, exercise, and anti-inflammatory treatment to treat cancer cachexia and sarcopenia efficiently. This type of intervention has been described in mixed cancer Randomized Controlled Studies (RCTs), such as the Multimodal-Exercise, Nutrition and Anti-inflammatory medication for Cachexia (MENAC) trial. Based on the rationale of this trial, the effects of the combination of interventions aim to improve muscle mass, functionality, nutritional status, and treatment tolerance [62]. It should be stressed, though that studies on patients with NETs remain limited. Therefore, the application of this type of intervention in patients with NETs should be adopted to tumor-specific factors, including the chronicity of symptoms and the hormonal syndromes, and should be applied by a multidisciplinary team of oncologists, endocrinologists, surgeons, gastroenterologists, nutritionists, nurses, psychologists, physiotherapists, and exercise specialists [17]. The steps of nutrition screening, assessment, and intervention are summarized in Figure 2.
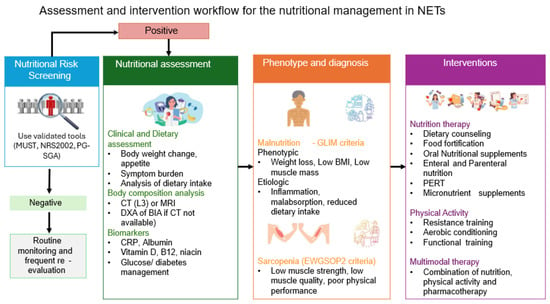
Figure 2.
Nutritional screening, assessment, and management of patients with NETs. Footnote: NRS2002: Nutritional Risk Screening 2002, MUST: Malnutrition Universal Screening Tool, CT: Computed tomography, BIA: Bioelectrical Impedance Analysis, DXA: Dual-energy X-ray Absorptiometry, BMI: Body mass index, NETs: Neuroendocrine tumors, CRP: C-reactive protein, PERT: Pancreatic Enzyme Replacement Therapy, GLIM: Global Leadership Initiative on Malnutrition, EWGSOP2: European Working Group on Sarcopenia in Older People 2, L3: 3rd lumbar disc.
7. Emerging and Experimental Interventions
Even if nutritional intervention is the cornerstone of malnutrition and sarcopenia treatment, nowadays, new therapeutic approaches emerge. For the purposes of this narrative review, “emerging interventions” are defined as novel or not yet routinely implemented strategies that specifically target malnutrition, sarcopenia, or their underlying metabolic and inflammatory pathways in NETs. These approaches go beyond standard nutritional counseling, symptom-directed pharmacotherapy and conventional exercise prescriptions, and include microbiome-modulating strategies (such as probiotics, prebiotics, or dietary patterns designed to favorably alter the gut–muscle axis), orexigenic and anabolic agents (e.g., ghrelin receptor agonists and myostatin-pathway inhibitors), as well as digital health and artificial-intelligence–enabled tools that individualize dietary, activity and body-composition monitoring. Given the very limited number of NET-specific trials, most of these interventions are currently supported by early-phase data or extrapolation from broader oncology and geriatric populations and should therefore be considered exploratory.
Among emerging and experimental interventions, modulation of the gut microbiota represents a particularly promising but still exploratory field [63]. The gut–muscle axis, mediated in part by microbial metabolites such as short-chain fatty acids, has been implicated in the regulation of energy balance and muscle homeostasis, and dysbiosis has been described in patients with small bowel NETs, especially those with SIBO and bile acid malabsorption [15,22]. Pilot data from non-NET cancer and geriatric populations suggest that targeted dietary patterns, probiotics, prebiotics, and, in selected cases, antibiotics can influence symptoms, inflammatory markers, and body composition [64,65], but high-quality NET-specific trials are lacking [15,17]. Accordingly, microbiome-directed strategies in NET-related malnutrition and sarcopenia should currently be considered investigational adjuncts within multidisciplinary care, ideally implemented in the context of clinical studies.
The gut microbes-muscle axis is an important regulator of muscle homeostasis, and particularly microbial metabolites like short-chain fatty acids, such as acetate, propionate, and butyrate, exert effects on host energy balance [66]. Different exercise modalities (endurance, moderate or high intensity, resistance, and aerobics) have respective effects on microbiome and thereby can act as therapeutic modulators of the gut-muscle axis, combating muscle wasting in sarcopenia [66]. As previously mentioned, patients with NETs display altered ghrelin expression. Ghrelin receptor agonists have been shown to stimulate appetite, improve body composition, and reverse muscle mass decline [67]. Similarly, myostatin inhibitors have shown promising effects in enhancing strength in preclinical mouse models, and although clinical studies have not substantiated these observations so far, these compounds could possibly serve as a therapeutic option for a range of muscle-wasting disorders [68,69]. Finally, the application of digital technologies such as machine learning and artificial intelligence in nutrition-related diseases, such as diabetes, obesity, but also sarcopenia, could be applied for the management of patients, and also predict outcomes based on a personalized approach [70,71].
Overall, the current evidence base for emerging interventions in NET-related malnutrition and sarcopenia remains sparse and heterogeneous, with small sample sizes, short follow-up, and frequent reliance on surrogate endpoints such as changes in body composition rather than hard clinical outcomes. When appraised using structured certainty-of-evidence frameworks, most data on microbiome modulation, ghrelin agonists, myostatin inhibition, and digital/AI-supported interventions would be classified as low to very low certainty, underscoring the need for adequately powered, NET-focused trials with standardized nutritional and functional endpoints. Until such data becomes available, these strategies should be implemented cautiously, ideally within research protocols or specialized multidisciplinary programs, and always in conjunction with established nutritional and exercise interventions rather than as stand-alone therapies.
8. Conclusions
Malnutrition and sarcopenia are very common in patients with NETs. The complex interplay of tumor biology, the hormone-mediated metabolic alterations, the chronic treatment effects, and the symptom burden has a negative impact on nutritional intake, increasing malabsorption and overall metabolic needs. Despite the limited data on patients with NETs, emerging evidence supports the need for a multidisciplinary approach incorporating individualized nutritional support, pharmacological therapy, exercise-based rehabilitation, and integrated multimodal care. Early identification of malnutrition risk and sarcopenia through validated screening and body composition assessment tools is paramount to optimize outcomes, preserve physical function, and improve QoL. Future research should focus on tailored interventions that address the unique pathophysiological and clinical features of patients with NETs. Multidisciplinary collaboration among oncologists, endocrinologists, nutritionists, nurses, physiotherapists, and exercise specialists is essential to provide personalized, evidence-based nutritional and metabolic care for these patients.
Author Contributions
Conceptualization, K.A.P. and K.I.A.; writing—original draft preparation, K.A.P. and K.I.A.; writing—review and editing, A.G.P., G.M., A.S. and O.V.; data curation, K.A.P., A.S. and O.V.; visualization, K.A.P. and K.I.A.; supervision, K.I.A., G.M. and A.G.P.; project administration, K.I.A. and A.G.P. All authors have read and agreed to the published version of the manuscript.
Funding
This study received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| NENs | Neuroendocrine neoplasms |
| GEP | Gastroenteropancreatic |
| BP | Bronchopulmonary |
| NETs | Neuroendocrine tumors |
| NECs | Neuroendocrine carcinomas |
| QoL | Quality of life |
| PRRT | Peptide receptor radionuclide therapy |
| BMI | Body mass index |
| GHSR1a | Growth hormone secretagogue receptor 1-a |
| GHSR1b | Growth hormone secretagogue receptor 1-b |
| GI | Gastrointestinal |
| SIBO | Small intestine bacterial overgrowth |
| PEI | Pancreatic exocrine insufficiency |
| 5-HIAA | 5-hydroxy-indoleacetic acid |
| SSAs | Somatostatin analogs |
| mTOR | Mechanistic target of rapamycin |
| CT | Computed tomography |
| GLIM | Global leadership initiative on malnutrition |
| EWGSOP2 | European Working Group on Sarcopenia in Older People 2 |
| NRS-2002 | Nutritional Risk Screening 2002 |
| MUST | Malnutrition Universal Screening Tool |
| PG-SGA | Patient-Generated Subjective Global Assessment |
| MRI | Magnetic resonance imaging |
| DEXA | Dual-energy X-ray absorptiometry |
| BIA | Bioelectrical impedance analysis |
| CRP | C-reactive protein |
| IGF-1 | Insulin-like growth factor 1 |
| PERT | Pancreatic enzyme replacement therapy |
| ONS | Oral nutritional supplements |
| MENAC | Multimodal-Exercise, Nutrition and Anti-inflammatory Medication for Cachexia |
References
- Cives, M.; Strosberg, J.R. Gastroenteropancreatic Neuroendocrine Tumors. CA A Cancer J. Clin. 2018, 68, 471–487. [Google Scholar] [CrossRef] [PubMed]
- Pavel, M.; Öberg, K.; Falconi, M.; Krenning, E.P.; Sundin, A.; Perren, A.; Berruti, A. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2020, 31, 844–860. [Google Scholar] [CrossRef] [PubMed]
- Ramage, J.; Srirajaskanthan, R. Epidemiology and Health Impacts of Neuroendocrine Tumours. Neuroendocrinology 2025, 1, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Dasari, A.; Wallace, K.; Halperin, D.M.; Maxwell, J.; Kunz, P.; Singh, S.; Chasen, B.; Yao, J.C. Epidemiology of Neuroendocrine Neoplasms in the US. JAMA Netw. Open 2025, 8, e2515798. [Google Scholar] [CrossRef]
- Fraenkel, M.; Faggiano, A.; Valk, G.D. Epidemiology of Neuroendocrine Tumors. Front. Horm. Res. 2015, 44, 1–23. [Google Scholar]
- Uhlig, J.; Nie, J.; Gibson, J.; Cecchini, M.; Stein, S.; Lacy, J.; Kunz, P.; Kim, H.S. Epidemiology, treatment and outcomes of gastroenteropancreatic neuroendocrine neoplasms. Sci. Rep. 2024, 14, 30536. [Google Scholar] [CrossRef]
- Yao, J.C.; Hassan, M.; Phan, A.; Dagohoy, C.; Leary, C.; Mares, J.E.; Abdalla, E.K.; Fleming, J.B.; Vauthey, J.N.; Rashid, A.; et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 3063–3072. [Google Scholar] [CrossRef]
- Hofland, J.; Kaltsas, G.; de Herder, W.W. Advances in the Diagnosis and Management of Well-Differentiated Neuroendocrine Neoplasms. Endocr. Rev. 2020, 41, 371–403. [Google Scholar] [CrossRef]
- Clement, D.; Tesselaar, M.E.T.; van Leerdam, M.E.; Srirajaskanthan, R.; Ramage, J.K. Nutritional and vitamin status in patients with neuroendocrine neoplasms. World J. Gastroenterol. 2019, 25, 1171–1184. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L.; Correia, M.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 38, 1–9. [Google Scholar] [CrossRef]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet. Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Shachar, S.S.; Williams, G.R.; Muss, H.B.; Nishijima, T.F. Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review. Eur. J. Cancer (Oxf. Engl. 1990) 2016, 57, 58–67. [Google Scholar] [CrossRef]
- Massironi, S.; Panzuto, F.; Zilli, A.; Rinzivillo, M.; Ciliberto, A.; Romano, E.; Danese, S.; Laviano, A. Nutritional aspects in neuroendocrine neoplasms. bridging the gap between dietary interventions and cancer care strategies: A scoping review. J. Endocrinol. Investig. 2025, 48, 269–281. [Google Scholar] [CrossRef]
- del Olmo-García, M.; Hernandez-Rienda, L.; Garcia-Carbonero, R.; Hernando, J.; Custodio, A.; Anton-Pascual, B.; Gomez, M.; Palma Milla, S.; Suarez, L.; Bellver, M.; et al. Nutritional status and quality of life of patients with advanced gastroenteropancreatic neuroendocrine neoplasms in Spain: The NUTRIGETNE (GETNE-S2109) study. Oncologist 2025, 30, oyae343. [Google Scholar] [CrossRef]
- Grozinsky-Glasberg, S.; Hofland, J.; Alband, S.; de Lima, Y.C.; Croitoru, A.; Geilvoet, W.; Igaz, P.; Kos-Kudła, B.; Krejs, G.J.; Laviano, A.; et al. Controversies in NEN: An ENETS position statement on nutritional support in neuroendocrine neoplasms. J. Neuroendocrinol. 2025, 37, e70062. [Google Scholar] [CrossRef]
- Alexandraki, K.I.; Kaltsas, G.; Grozinsky-Glasberg, S.; Oleinikov, K.; Kos-Kudła, B.; Kogut, A.; Srirajaskanthan, R.; Pizanias, M.; Poulia, K.A.; Ferreira, C.; et al. The effect of prophylactic surgery in survival and HRQoL in appendiceal NEN. Endocrine 2020, 70, 178–186. [Google Scholar] [CrossRef]
- Maasberg, S.; Knappe-Drzikova, B.; Vonderbeck, D.; Jann, H.; Weylandt, K.H.; Grieser, C.; Pascher, A.; Schefold, J.C.; Pavel, M.; Wiedenmann, B.; et al. Malnutrition Predicts Clinical Outcome in Patients with Neuroendocrine Neoplasia. Neuroendocrinology 2017, 104, 11–25. [Google Scholar] [CrossRef]
- Baracos, V.E.; Martin, L.; Korc, M.; Guttridge, D.C.; Fearon, K.C.H. Cancer-associated cachexia. Nat. Rev. Dis. Primers 2018, 4, 17105. [Google Scholar] [CrossRef]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef] [PubMed]
- Clement, D.; Brown, S.; Leerdam, M.V.; Tesselaar, M.; Ramage, J.; Srirajaskanthan, R. Sarcopenia and Neuroendocrine Neoplasms. Curr. Oncol. Rep. 2024, 26, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Martínez, Y.; Alzas Teomiro, C.; León Idougourram, S.; Molina Puertas, M.J.; Calañas Continente, A.; Serrano Blanch, R.; Castaño, J.P.; Gálvez Moreno, M.; Gahete, M.D.; Luque, R.M.; et al. Sarcopenia and Ghrelin System in the Clinical Outcome and Prognosis of Gastroenteropancreatic Neuroendocrine Neoplasms. Cancers 2021, 14, 111. [Google Scholar] [CrossRef] [PubMed]
- Clement, D.; Ramage, J.; Srirajaskanthan, R. Update on Pathophysiology, Treatment, and Complications of Carcinoid Syndrome. J. Oncol. 2020, 2020, 8341426. [Google Scholar] [CrossRef]
- Artale, S.; Barzaghi, S.; Grillo, N.; Maggi, C.; Lepori, S.; Butti, C.; Bovio, A.; Barbarini, L.; Colombo, A.; Zanlorenzi, L.; et al. Role of Diet in the Management of Carcinoid Syndrome: Clinical Recommendations for Nutrition in Patients with Neuroendocrine Tumors. Nutr. Cancer 2022, 74, 2–11. [Google Scholar] [CrossRef]
- Altieri, B.; Barrea, L.; Modica, R.; Muscogiuri, G.; Savastano, S.; Colao, A.; Faggiano, A. Nutrition and neuroendocrine tumors: An update of the literature. Rev. Endocr. Metab. Disord. 2018, 19, 159–167. [Google Scholar] [CrossRef]
- Laing, E.; Kiss, N.; Michael, M.; Krishnasamy, M. Nutritional Complications and the Management of Patients with Gastroenteropancreatic Neuroendocrine Tumors. Neuroendocrinology 2020, 110, 430–442. [Google Scholar] [CrossRef]
- Angelousi, A.; Koffas, A.; Grozinsky-Glasberg, S.; Gertner, J.; Kassi, E.; Alexandraki, K.; Caplin, M.E.; Kaltsas, G.; Toumpanakis, C. Diagnostic and Management Challenges in Vasoactive Intestinal Peptide Secreting Tumors: A Series of 15 Patients. Pancreas 2019, 48, 934–942. [Google Scholar] [CrossRef]
- Alexandraki, K.I.; Kaltsas, G.A.; Grozinsky-Glasberg, S. Emerging therapies for advanced insulinomas and glucagonomas. Endocr. Relat. Cancer 2023, 30, e230020. [Google Scholar] [CrossRef]
- de Herder, W.W.; Hofland, J. Insulinoma. In Endotext; Copyright © 2000–2025; Feingold, K.R., Ahmed, S.F., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Barrea, L.; Verde, L.; Annunziata, G.; Camajani, E.; Caprio, M.; Sojat, A.S.; Marina, L.V.; Guarnotta, V.; Colao, A.; Muscogiuri, G. Role of Mediterranean diet in endocrine diseases: A joint overview by the endocrinologist and the nutritionist. J. Endocrinol. Investig. 2024, 47, 17–33. [Google Scholar] [CrossRef]
- Aktypis, C.; Yavropoulou, M.P.; Efstathopoulos, E.; Polichroniadi, D.; Poulia, K.A.; Papatheodoridis, G.; Kaltsas, G. Bone and muscle mass characteristics in patients with gastroenteropancreatic neuroendocrine neoplasms. Endocrine 2025, 88, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Alexandraki, K.I.; Daskalakis, K.; Tsoli, M.; Grossman, A.B.; Kaltsas, G.A. Endocrinological Toxicity Secondary to Treatment of Gastroenteropancreatic Neuroendocrine Neoplasms (GEP-NENs). Trends Endocrinol. Metab. TEM 2020, 31, 239–255. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.B.; Xue, Z.; Lu, J.; He, Q.L.; Zheng, Z.F.; Xu, B.B.; Xie, J.W.; Li, P.; Xu, Y.; Lin, J.X.; et al. Effect of sarcopenia on short- and long-term outcomes in patients with gastric neuroendocrine neoplasms after radical gastrectomy: Results from a large, two-institution series. BMC Cancer 2020, 20, 1002. [Google Scholar] [CrossRef] [PubMed]
- Alexandraki, K.I.; Angelousi, A.; Chatzellis, E.; Chrisoulidou, A.; Kalogeris, N.; Kanakis, G.; Savvidis, C.; Vassiliadi, D.; Spyroglou, A.; Kostopoulos, G.; et al. The Role of Somatostatin Analogues in the Control of Diarrhea and Flushing as Markers of Carcinoid Syndrome: A Systematic Review and Meta-Analysis. J. Pers. Med. 2023, 13, 304. [Google Scholar] [CrossRef]
- Romano, E.; Polici, M.; Marasco, M.; Lerose, F.; Dell’Unto, E.; Nardacci, S.; Zerunian, M.; Iannicelli, E.; Rinzivillo, M.; Laghi, A.; et al. Sarcopenia in Patients with Advanced Gastrointestinal Well-Differentiated Neuroendocrine Tumors. Nutrients 2024, 16, 2224. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Barrea, L.; Cantone, M.C.; Guarnotta, V.; Mazzilli, R.; Verde, L.; Vetrani, C.; Colao, A.; Faggiano, A. Neuroendocrine Tumors: A Comprehensive Review on Nutritional Approaches. Cancers 2022, 14, 4402. [Google Scholar] [CrossRef]
- Ranallo, N.; Iamurri, A.P.; Foca, F.; Liverani, C.; De Vita, A.; Mercatali, L.; Calabrese, C.; Spadazzi, C.; Fabbri, C.; Cavaliere, D.; et al. Prognostic and Predictive Role of Body Composition in Metastatic Neuroendocrine Tumor Patients Treated with Everolimus: A Real-World Data Analysis. Cancers 2022, 14, 3231. [Google Scholar] [CrossRef]
- Chan, D.L.; Clarke, S.J.; Engel, A.; Diakos, C.I.; Pavlakis, N.; Roach, P.J.; Bailey, D.L.; Bauer, J.; Findlay, M. Computed tomography (CT)-defined sarcopenia and myosteatosis are prevalent in patients with neuroendocrine neoplasms (NENs) treated with peptide receptor radionuclide therapy (PRRT). Eur. J. Clin. Nutr. 2022, 76, 143–149. [Google Scholar] [CrossRef]
- Spyroglou, A.; Bramis, K.; Alexandraki, K. Neuroendocrine neoplasms: Evolving and future treatments. Curr. Opin. Endocr. Metab. Res. 2021, 19, 15–21. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ (Clin. Res. Ed.) 2016, 355, i4919. [Google Scholar] [CrossRef]
- Morgan, R.L.; Thayer, K.A.; Santesso, N.; Holloway, A.C.; Blain, R.; Eftim, S.E.; Goldstone, A.E.; Ross, P.; Ansari, M.; Akl, E.A.; et al. A risk of bias instrument for non-randomized studies of exposures: A users’ guide to its application in the context of GRADE. Environ. Int. 2019, 122, 168–184. [Google Scholar] [CrossRef] [PubMed]
- Perelmuter, M.; Buch, A.; Rabinowich, A.; Izkhakov, E.; Greenman, Y.; Wolf, I.; Geva, R.; Osher, E. Clinical implications of measuring muscle mass by computed tomography in neuroendocrine tumor patients. Endocr. Abstr. 2025, 110, 491. [Google Scholar] [CrossRef]
- Zhang, Z.; Wan, Z.; Zhu, Y.; Zhang, L.; Zhang, L.; Wan, H. Prevalence of malnutrition comparing NRS2002, MUST, and PG-SGA with the GLIM criteria in adults with cancer: A multi-center study. Nutrition 2021, 83, 111072. [Google Scholar] [CrossRef] [PubMed]
- Clement, D.; Leerdam, M.E.V.; de Jong, S.; Weickert, M.O.; Ramage, J.K.; Tesselaar, M.E.T.; Srirajaskanthan, R. Prevalence of Sarcopenia and Impact on Survival in Patients with Metastatic Gastroenteropancreatic Neuroendocrine Tumours. Cancers 2023, 15, 782. [Google Scholar] [CrossRef]
- Celik, E.; Suzan, V.; Samanci, N.S.; Suzan, A.A.; Karadag, M.; Sahin, S.; Aslan, M.S.; Yavuzer, H.; Demirci, N.S.; Doventas, A.; et al. Sarcopenia assessment by new EWGSOP2 criteria for predicting chemotherapy dose-limiting toxicity in patients with gastrointestinal tract tumors. Eur. Geriatr. Med. 2022, 13, 267–274. [Google Scholar] [CrossRef]
- Srirajaskanthan, R.; Pavel, M.; Kulke, M.; Clement, D.; Houchard, A.; Keeber, L.; Weickert, M.O. Weight Maintenance up to 48 Weeks in Patients With Carcinoid Syndrome Treated With Telotristat Ethyl: Pooled Data From the Open-Label Extensions of the Phase III Clinical Trials TELESTAR and TELECAST. Clin. Ther. 2021, 43, 1779–1785. [Google Scholar] [CrossRef]
- Papadopoulos, E.; Irving, B.A.; Brown, J.C.; Heymsfield, S.B.; Sattar, S.; Alibhai, S.M.H.; Williams, G.R.; Dunne, R.F. Sarcopenia and Cachexia in Older Patients with Cancer: Pathophysiology, Diagnosis, Impact on Outcomes, and Management Strategies. Drugs Aging 2025, 42, 1113–1142. [Google Scholar] [CrossRef]
- Borys, K.; Haubold, J.; Keyl, J.; Bali, M.A.; De Angelis, R.; Boni, K.B.; Coquelet, N.; Kohnke, J.; Baldini, G.; Kroll, L.; et al. Leveraging Sarcopenia index by automated CT body composition analysis for pan cancer prognostic stratification. NPJ Digit. Med. 2025, 8, 611. [Google Scholar] [CrossRef]
- Kroll, L.; Mathew, A.; Baldini, G.; Hosch, R.; Koitka, S.; Kleesiek, J.; Rischpler, C.; Haubold, J.; Fuhrer, D.; Nensa, F.; et al. CT-derived body composition analysis could possibly replace DXA and BIA to monitor NET-patients. Sci. Rep. 2022, 12, 13419. [Google Scholar] [CrossRef]
- Jensen, G.L.; Cederholm, T.; Ballesteros-Pomar, M.D.; Blaauw, R.; Correia, M.; Cuerda, C.; Evans, D.C.; Fukushima, R.; Gautier, J.B.O.; Gonzalez, M.C.; et al. Guidance for assessment of the inflammation etiologic criterion for the GLIM diagnosis of malnutrition: A modified Delphi approach. JPEN. J. Parenter. Enter. Nutr. 2024, 48, 145–154. [Google Scholar] [CrossRef]
- Park, S.Y.; Hwang, B.O.; Song, N.Y. The role of myokines in cancer: Crosstalk between skeletal muscle and tumor. BMB Rep. 2023, 56, 365–373. [Google Scholar] [CrossRef] [PubMed]
- de Castro, G.S.; Correia-Lima, J.; Simoes, E.; Orsso, C.E.; Xiao, J.; Gama, L.R.; Gomes, S.P.; Gonçalves, D.C.; Costa, R.G.F.; Radloff, K.; et al. Myokines in treatment-naïve patients with cancer-associated cachexia. Clin. Nutr. 2021, 40, 2443–2455. [Google Scholar] [CrossRef] [PubMed]
- Zandee, W.T.; Merola, E.; Poczkaj, K.; de Mestier, L.; Klümpen, H.J.; Geboes, K.; de Herder, W.W.; Munir, A. Evaluation of multidisciplinary team decisions in neuroendocrine neoplasms: Impact of expert centres. Eur. J. Cancer Care 2022, 31, e13639. [Google Scholar] [CrossRef] [PubMed]
- Muscaritoli, M.; Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN practical guideline: Clinical Nutrition in cancer. Clin. Nutr. 2021, 40, 2898–2913. [Google Scholar] [CrossRef]
- Kadaj-Lipka, R.; Monica, M.; Stożek-Tutro, A.; Ryś, P.; Rydzewska, G. Pancreatic Enzyme Replacement Therapy in Pancreatic Exocrine Insufficiency-Real-World’s Dosing and Effectiveness: A Systematic Review. Dig. Dis. Sci. 2025, 70, 2270–2284. [Google Scholar] [CrossRef]
- Orsso, C.E.; Caretero, A.; Poltronieri, T.S.; Arends, J.; de van der Schueren, M.A.; Kiss, N.; Laviano, A.; Prado, C.M. Effects of high-protein supplementation during cancer therapy: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2024, 120, 1311–1324. [Google Scholar] [CrossRef]
- Delsoglio, M.; Capener, R.; Smith, T.R.; Donald, M.; Hubbard, G.P.; Stratton, R.J. High-protein oral nutritional supplement use in patients with cancer reduces complications and length of hospital stay: A systematic review and meta-analysis. Front. Nutr. 2025, 12, 1654637. [Google Scholar] [CrossRef]
- Heywood, R.; McCarthy, A.L.; Skinner, T.L. Efficacy of Exercise Interventions in Patients With Advanced Cancer: A Systematic Review. Arch. Phys. Med. Rehabil. 2018, 99, 2595–2620. [Google Scholar] [CrossRef]
- Cormie, P.; Atkinson, M.; Bucci, L.; Cust, A.; Eakin, E.; Hayes, S.; McCarthy, S.; Murnane, A.; Patchell, S.; Adams, D. Clinical Oncology Society of Australia position statement on exercise in cancer care. Med. J. Aust. 2018, 209, 184–187. [Google Scholar] [CrossRef]
- Neuroendocrine Tumor Research Foundation. Exercise While Living with a Neuroendocrine Tumor. Available online: https://netrf.org/old-for-patients/living-with-nets/exercise/ (accessed on 12 December 2025).
- Solheim, T.S.; Laird, B.J.A.; Balstad, T.R.; Bye, A.; Stene, G.; Baracos, V.; Strasser, F.; Griffiths, G.; Maddocks, M.; Fallon, M.; et al. Cancer cachexia: Rationale for the MENAC (Multimodal-Exercise, Nutrition and Anti-inflammatory medication for Cachexia) trial. BMJ Support. Palliat. Care 2018, 8, 258–265. [Google Scholar] [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Agirman, G.; Hsiao, E.Y. SnapShot: The microbiota-gut-brain axis. Cell 2021, 184, 2524–2524.e1. [Google Scholar] [CrossRef] [PubMed]
- Bindels, L.B.; Delzenne, N.M.; Cani, P.D.; Walter, J. Towards a more comprehensive concept for prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 303–310. [Google Scholar] [CrossRef]
- Xu, Y.; He, B. The gut-muscle axis: A comprehensive review of the interplay between physical activity and gut microbiota in the prevention and treatment of muscle wasting disorders. Front. Microbiol. 2025, 16, 1695448. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Geng, J.; Bao, J.; Tang, Y.; Liu, M.; Yu, H.; Han, Y.; Huang, W.; Zhou, S. Two ghrelin receptor agonists for adults with malnutrition: A systematic review and meta-analysis. Nutr. J. 2016, 15, 97. [Google Scholar] [CrossRef] [PubMed]
- Wetzlich, B.; Nyakundi, B.B.; Yang, J. Therapeutic applications and challenges in myostatin inhibition for enhanced skeletal muscle mass and functions. Mol. Cell. Biochem. 2025, 480, 1535–1553. [Google Scholar] [CrossRef]
- Smith, R.C.; Lin, B.K. Myostatin inhibitors as therapies for muscle wasting associated with cancer and other disorders. Curr. Opin. Support. Palliat. Care 2013, 7, 352–360. [Google Scholar] [CrossRef]
- Salinari, A.; Machì, M.; Armas Diaz, Y.; Cianciosi, D.; Qi, Z.; Yang, B.; Ferreiro Cotorruelo, M.S.; Villar, S.G.; Dzul Lopez, L.A.; Battino, M.; et al. The Application of Digital Technologies and Artificial Intelligence in Healthcare: An Overview on Nutrition Assessment. Diseases 2023, 11, 97. [Google Scholar] [CrossRef]
- Castano, J.P.; Dattani, M.T.; Grozinsky-Glasberg, S.; Karavitaki, N.; Pavel, M.E.; Andoniadou, C.; Alexandraki, K.; Capatina, C.; Cerbone, M.; Ferone, D.; et al. EndoCompass project: Research roadmap for pituitary and neuroendocrine tumor endocrinology. Eur. J. Endocrinol. 2025, 193, ii84–ii96. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).