Abstract
The molecular structure and dynamics of the neuronal plasma membrane are essential for neuronal biology and function. We employed time-of-flight secondary ion mass spectrometry (ToF-SIMS) imaging to investigate the lipid composition and turnover at the plasma membrane of single human midbrain neurons. The results showed that the profile of lipid turnover was heavily influenced by the types of precursors incorporated into the membrane lipids. In addition, there was a high prevalence of phosphatidylcholines, phosphatidylserines, and ceramides in the human midbrain neurons, and a preference for incorporating stearic acid into membrane lipids compared to other precursors. These features indicate a direct link between the membrane lipids to the biological state and functions of midbrain neurons. This is among a very few studies using mass spectrometry imaging to provide an insight into the native membrane lipid organization and lipid turnover using various lipid precursors in human neurons at a single cell level, illustrating their biological relevance in neuronal functions.
1. Introduction
The midbrain is a central part of the brain that plays a key role in motor control and processing visual and auditory information []. Among different types of brain cells in the midbrain are dopaminergic, GABAergic, and glutaminergic neurons [,]. Plasma membrane lipids have been shown to influence the membrane curvature, which affects the synaptic activity and neurotransmitter secretion, signal transduction, and lipid homeostasis [,,]. The loss of neurons in the midbrain is mostly associated with the neurodegenerative disease known as Parkinson’s disease, or disorders such as restless legs syndrome, attention deficit hyperactivity disorder (ADHD), and schizophrenia [,,,]. Due to the complexity of the midbrain’s structures and functions, it is essential to further understand the molecular organization of the plasma membrane of midbrain neurons.
The plasma membrane in neurons is a highly organized and dynamic structure supporting cellular shape and structures (e.g., movement, budding, cell division, tubulation, synapses, axons, and dendrites), intracellular trafficking, extra-and intracellular signaling, exo- and endocytosis, protein interactions, vesicular trafficking, and cell differentiation, etc. [,]. To maintain cellular function, the lipid composition of the plasma membrane is dynamically adapted and renewed via a turnover process, in which old lipid molecules are replaced by newly synthesized ones. Different types of lipids and their relative compositions have significant effects during cell differentiation, cell signaling, and intracellular processes [,,,,,]. The composition and turnover of plasma membrane lipids were found to be altered in the aforementioned diseases, which implies that they are closely related to the cell status and neuronal activity [,,,,,,,,,,,,,,,,].
Despite the evident roles in neuronal functions, the lipid composition and turnover of the plasma membrane in human neuronal cells remain largely elusive. This could be explained by the limited availability of neuronal cells from humans. The development and differentiation procedures from human induced pluripotent stem cells (iPSCs) into specific neuronal cell types have been significantly improved [,], but expertise is critically needed in one’s lab for successful cell culture. In addition, single-cell analysis techniques providing specific molecular structural information of intact cellular membranes have not been established until more recently, with the emergence of gas cluster ion beams (GCIBs) using Time-of-flight secondary ion mass spectrometry (ToF-SIMS) [,,,]. Liquid chromatography mass spectrometry (LC-MS) has been the most prominent method to analyze the lipid composition and turnover of cells. However, it is constrained by a limitation to analyze the plasma membrane composition in a bulk solution of cell lysates. Other methods have been developed to selectively analyze plasma membrane lipids, such as the employment of colloidal silica beads, but they could disrupt the cells, and the specificity of the isolated plasma membrane lipids cannot be guaranteed [].
ToF-SIMS is capable of analyzing plasma membrane lipids at a single-cell level without cell disruptions. The cells can be cultured on a conductive surface (e.g., silicon wafers or indium tin oxide-coated glass), followed by quick rinsing and plunge-freezing to preserve the plasma membrane integrity [,]. Membrane lipids have also been studied using supported lipid bilayer models for chemical modeling with TOF-SIMS [,,,]. Upon ToF-SIMS analysis, a primary ion beam is rastered over the sample surface, which ejects the molecules from the sample as secondary ions. Sample molecules typically up to 3000 Da. can be detected in parallel, depending on the instrumental setup. ToF-SIMS presents some limitations, particularly sensitivity (ppm), which is a trade-off with the imaging resolution. Mass resolution is limited to around 10 000, depending on the detected mass, and sample charging may occur in low-conducting samples [,,,,]. The invention of the GCIBs has substantially increased the applications of ToF-SIMS in biology, especially research on lipid membrane structure [,,,].
We employed ToF-SIMS imaging and a shotgun approach to investigate the lipid composition and turnover of the plasma membrane in single human midbrain neurons. The major lipid composition in the native plasma membrane was identified. Different fatty acids and lipid headgroups were used as lipid precursors to examine their turnover effects. We aim to provide an insight into the biological relevance of the identified membrane lipids that support future research in neuronal functions and diseases in human cells.
2. Materials and Methods
2.1. Cell Culture and Preparation
Human neuronal progenitor cells (NPCs) were obtained from Carl Ernst’s lab at McGill University, Montreal. The use of these human cells was approved by the Research Ethics Board of the McGill University Health Center (Montreal, QC, Canada) with the ethics approval code 23-09-075 and the date of approval of 9 December 2024. NPCs were thawed rapidly in a water bath at 37 °C and transferred into a T25 flask (Fisher Scientific, Nunc™ EasYFlask™, #156340, Gothenburg, Sweden) coated with poly-D-lysine (Sigma-Aldrich, #P7280, Solna, Sweden) and laminin (Sigma-Aldrich, #L2020, Stockholm, Sweden). The NPCs were cultured until confluent in NPC medium (STEMCELL technologies, STEMDiff Neural Progenitor Basal Medium, #05834, Grenoble, France) containing 200 ng/mL sonic hedgehog (Genescript, #Z03067, Piscataway, NJ, USA).
Indium tin oxide (ITO) glasses (Bruker Nordic, #8237001, Solna, Sweden) previously coated with poly-ornithine (Sigma-Aldrich, #P3655, Solna, Sweden) and laminin were placed in a 24-well plate (Fisher Scientific, #353047, Gothenburg, Sweden) containing the cell culture medium. The NPCs were subsequently transferred onto the ITO glass slides and allowed to adhere in the incubator. After one day, the medium was replaced with midbrain differentiation medium (BrainPhys (STEMCELL technologies, #05790, Saint Egrève, France) supplemented with 2% B27 (Fisher Scientific, #17504044, Gothenburg, Sweden), 1% N2 (Fischer Scientific, #A1370701, Gothenburg, Sweden), 20 ng/mL brain-derived neurotrophic factor (BDNF, Genescript, #Z03208, Piscataway, NJ, USA), 20 ng/mL glial cell line-derived neurotrophic factor (GDNF, Genescript, #Z03387, NJ, USA), 200 nM ascorbic acid (STEMCELL technologies, Cat. #72132, Saint Egrève, France), 1 mM dibutyl cAMP (STEMCELL technologies, Cat. #100-0244, Saint Egrève, France), 1 µg/mL laminin). Half of the cell medium was replaced every 2–3 days for 2 weeks until the cells became fully matured midbrain neurons (5 weeks) and were then incubated with lipid precursors. Two biological replicates were incubated with each isotopically (13C-, or 15N-) labeled lipid precursor at a final concentration of 50 μM. The examined 13C lipid precursors were 13C-lauric acid (Eurisotop, #CLM-1586, Saint-Aubin, France), 13C-stearic acid (Eurisotop, #CLM-490-1, Saint-Aubin, France), 13C-linoleic acid (Eurisotop, #CLM-6855-0.25, Saint-Aubin, France), 13C-linolenic acid (Sigma-Aldrich, #694940, Solna, Sweden), 13C2-ethanolamine chloride (Sigma-Aldrich, #E6133-100G, Solna, Sweden), and 15N-choline chloride (Sigma-Aldrich, #609269, Solna, Sweden). The cell cultures were replaced with half of the medium containing isotopic lipid precursor every second day for 4 days. Control cell samples were also prepared under the same experimental conditions without incubation with isotopic lipid precursor. After 4 days of isotopic incubation, the cell cultures were incubated in the medium without isotopic lipid precursors for 12 h. Subsequently, the cells were quickly rinsed four times with prewarmed ammonium formate solution at 150 mM (Sigma-Aldrich, #70221-25G-F, Solna, Sweden) and once with prewarmed milliQ-water to remove excess salts from the cell medium, followed by snap freezing in cold 2-methylbutane (Sigma-Aldrich, #270342-1L, Solna, Sweden) at −185 °C. The cells were stored in liquid nitrogen until they were freeze-dried at a high vacuum of 0.05 × 10−3 mbar (Martin Christ, Alpha 1–2 LDplus, Osterode am Harz, Germany) before ToF-SIMS measurements.
2.2. Immunocytochemistry
To validate the differentiation of the NPCs into mature midbrain neurons, the cells were stained for microtubule-associated protein 2 (MAP2) and tyrosine hydroxylase (TH), which are two markers associated with mature midbrain neurons. MAP2- and TH-staining was performed on NPCs and differentiated midbrain neurons by incubating cells grown on cover slips first in a staining buffer containing PBS (Sigma-Aldrich, #P4417, Solna, Sweden), 1% bovine serum albumin (Sigma-Aldrich, #A2153, Solna, Sweden), and 0.1% Triton-X100 (Sigma-Aldrich, #T8787, Solna, Sweden) for 1 h. Next, the cells were incubated with MAP2 rabbit primary antibody (Abcam, #ab32454, Cambridge, UK) and TH mouse primary antibody (Abcam, #ab129991, Cambridge, UK) at a 1:200 dilution in the staining buffer for 1 h. The samples were then washed 3 times in PBS and followed by a similar incubation procedure with an anti-rabbit 580 nm secondary antibody (Abberior, #ST580-1002-500UG, Goettingen, Germany) for MAP2 and an anti-mouse 638 nm secondary antibody (Abberior, STRED-1001-500UG, Goettingen, Germany) for TH-labeling. After incubation, the samples were washed once with PBS, stained for 5 min in 1:1000 DAPI (nucleus staining, Sigma-Aldrich, #D9542, Solna, Sweden) diluted in PBS, washed twice with PBS before being mounted (Abberior, #MM-2011-2X15ML, Goettingen, Germany) on microscopic slides for imaging.
The samples were fluorescently imaged using the Abberior Expert Line microscope (Abberior, Goettingen, Germany) in confocal mode with a 100× UPLSAPO NA 1.4 oil immersion objective (Olympus, Tokyo, Japan). MAP2 was imaged at an excitation wavelength of 561 nm and an emission spectrum of 575–630 nm, while TH was imaged at an excitation wavelength of 640 nm and an emission spectrum of 650–763 nm. DAPI was imaged with an emission spectrum of 415–583 nm. All samples were imaged with 10% laser intensity at 1 AU, a dwell time of 15 µs, an image size of 78 × 78 µm, and a pixel size of 100 nm. MAP2 and TH were imaged using 3-line acquisitions, while DAPI was imaged with a 1-line acquisition. The image intensities of TH and MAP2 were then normalized based on the background signal at 8-bit, whilst the DAPI signal was set freely using Fiji, version 1.54p []. The verification can be observed in Figure S1, in which the midbrain neurons have a higher expression of MAP2 and TH compared to undifferentiated NPCs.
2.3. ToF-SIMS Imaging
A J105—3D Chemical Imager ToF-SIMS instrument (Ionoptika Ltd., Southampton, UK) equipped with a 40 keV (CO2)6000+ primary gas cluster ion beam (GCIB) was used for sample analysis [,]. Samples were analyzed in both positive and negative ion modes with a mass range from 100 to 1000 Da. Before imaging, a cell area of 600 × 600 µm2 was rastered for surface cleaning with 64 × 64 pixels and a primary ion dose of 3 × 1012 ion/cm2. Sequentially, the same cell area was imaged with a primary ion current of 15 pA, 696 shots/pixel, at either 128 × 128 pixels with a primary ion dose of ∼1.1 × 1013 ion/cm2, or at 256 × 256 pixels with a primary ion dose of ∼4.7 × 1013 ion/cm2. Two biological replicates were obtained for each sample condition, and 3 to 5 cell areas were imaged for each replicate (n is 6–10 for each sample condition).
2.4. Data Analysis
SIMS data was extracted using the Ionoptika Image Analyzer software, version 3.0.0 (Ionoptika Ltd., Southampton, UK) by selecting cell areas identified via the PC-headgroup ion signal (m/z 184.07) in positive mode, and FA 16:0 and FA 18:0 (m/z 255.23 and 283.25, respectively) in negative mode, examples shown in Supplementary data Figure S2. After selecting cell areas, the spectral data was exported and consecutively binned to 0.05 Da in Matlab version R2021a (The Mathworks Inc., Gothenburg, Sweden), resulting in a mass accuracy of ~65 ppm at m/z 800. Major interference peaks from the ITO glass substrate were then removed from the spectral data, and the peak intensities were normalized to the total ion intensity of all the mass peaks of the ROIs within a SIMS image.
Lipid class abundance was detected using ChiToolbox in Matlab with the code adapted from previous literature [,] to detect the 90 most highly abundant lipids (~5% of the total peak number), 45 of the most abundant peaks in each ion mode.
Next, the lipid profiles of the cellular plasma membrane after incubation with different isotopic lipid precursors, including 13C2-ethanolamine and 15N-choline, 13C-linoleic acid and 13C-linolenic acid, 13C-linoleic acid and 13C-stearic acid, 13C-linolenic acid and 13C-stearic acid, and 13C-lauric acid and 13C-stearic acid, were compared. Lipid turnover was evaluated via the isotopic incorporation based on the changes in the peak intensity across the entire mass spectrum of the treated samples compared to those of the control. This was obtained using principal components analysis (PCA) in SIMCA, version 17.0.0.24543 (Sartorius, Goettingen, Germany) to select the mass peaks contributing to the distinct differences between the isotopically incubated samples and the control. The selected peaks were then analyzed statistically using GraphPad Prism, version 10.3.0 (GraphPad Software, MA, USA). All comparisons were performed using a multiple Mann–Whitney test with a p-value of ≤0.05.
The significant mass peaks were sequentially assigned using the LMSD database LIPIDMAPS® (https://www.lipidmaps.org/, accessed on 9 July 2024) and with peaks detected in positive mode in the typical ion forms of [M+H]+, [M+H–H2O]+, [M+Na]+, or [M+K]+, while typical ions of [M−H]− were detected in negative mode. A peak assignment was given with an accuracy of no higher than 100 ppm. Detection of ceramides, phosphatidylglycerol, and phosphatidylethanolamine was based on previous studies [,,,,,,,,,,,,] as well as lipid fragment generation [,,,,,,,,,]. The lipid assignment list can be found in the Supplementary Materials, Tables S1 and S2.
3. Results and Discussion
3.1. Lipid Abundancy in Neuronal Plasma Membrane
Human midbrain neurons were first analyzed to detect the native abundance of major lipid components in the plasma membrane. Figure 1A,B displays the abundance of the 45 most common lipids detected in each ion mode. This includes 35 different lipids in positive mode, where some lipids were detected in different ionization forms such as molecular ions, salt adducts, and fragments, and 44 unique compounds in negative mode. The number of lipids in each lipid class is shown in Figure 1C.
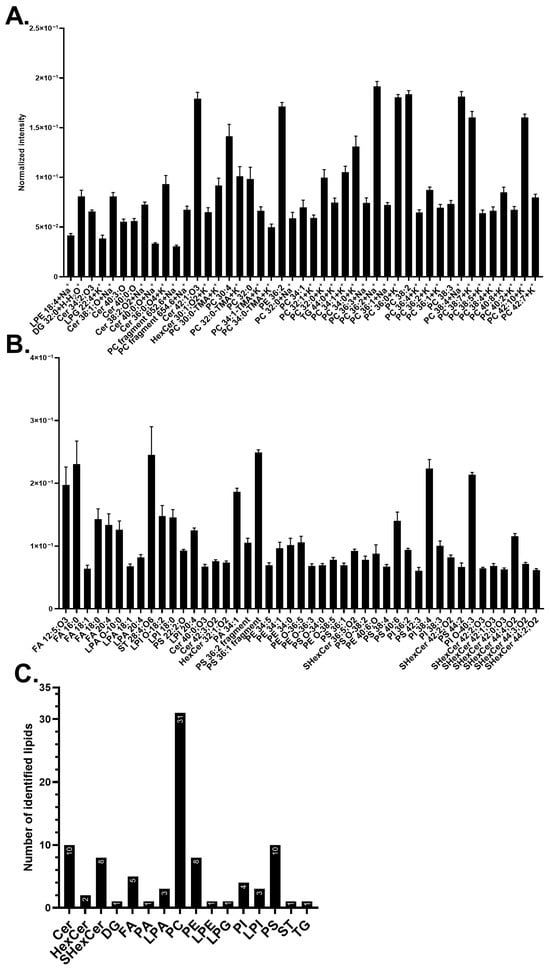
Figure 1.
Major membrane lipid compositions of human midbrain neurons. (A) The 45 most abundant lipids detected in positive mode. (B) The 45 most abundant lipids detected in negative mode. The lipid names on the x-axis from left to right are in ascending mass/charge (m/z). Error bars are in the standard error of the mean (SEM). (C) Summary of the abundant lipid classes detected in positive (A) and negative (B) modes. The analysis was performed on five biological replicates with 20 measurements in total. The lipid classes included: ceramide (Cer), diacylglycerol (DG), fatty acid (FA), hexosylceramide (HexCer), lysophosphatidic acid (LPA), lysophosphatidylethanolamine (LPE), lysophosphatidylglycerol (LPG), lysophosphatidylinositol (LPI), phosphatidic acid (PA), phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylinositol (PI), and phosphatidylserine (PS), sulfatide (SHexCer), sterol (ST), and triacylglycerol (TG). Lipids with no annotated ionization were detected as [M+H]+ in (A) and [M–H]− in (B).
Phosphatidylcholine (PC) species were found to be the most abundant lipid (Figure 1A,C); their salt adducts and fragments have the total carbon numbers ranging from C32 to C42 and 0 to 8 double bonds. Phosphatidylserine (PS) species are the second most common lipid class (Figure 1B,C) along with ceramides (Cers). PSs have carbon chains ranging from C22 to C44 and 0 to 6 double bonds, whilst Cers are composed of carbon chains from C34 to C42 with 0 to 3 double bonds. Among those, compounds [PC 38:2+H]+ at m/z 814.63, [PC 36:2+Na]+ at m/z 808.58, and [PC 38:4+Na]+ at m/z 832.58 are the three most abundant lipids in positive mode (Figure 1A) whereas PS fragment [PS 36:1–87-H]− at m/z 701.51, sterol (ST) [ST 28:4;O6-H]− at m/z 473.29, and fatty acid (FA) [FA 16:0-H]− at m/z 255.23 are the three most abundant in negative mode (Figure 1B). The occurrence of different lipid classes amongst highly abundant lipids is summarized in Figure 1C, where the three major membrane components are PCs (~33%), PSs (~11%), and Cers (~11%), followed by sulfatides (SHexCer) and phosphatidylethanolamine (PE) (each ~9%). Example images of control cells can be found in Figure S2 for PC-headgroup [C5H15PNO4]+ at m/z 184.07 and [FA 18:0-H]− at m/z 283.26.
We identified 90 highly abundant lipids at the plasma membrane of human midbrain neurons, among which the major components are PCs (~33%), PSs (~11%), and Cers (~11%), followed by SHexCer and PE (each ~9%) (Figure 1). Mammalian neurons and brain tissue have been found with a high abundance of PCs and STs, followed by PEs, PSs, and Cers, according to the previous literature [,,,,,,]; however, detailed information on native plasma membrane lipids of human neurons has been scarcely addressed. The midbrain region of the human and mouse brain was reported to contain PCs, PSs, PEs, and Cers [,]. In addition, we previously discovered Cers, PSs, PCs, and DGs as major lipid components of the plasma membrane of human one-week-old differentiating NPCs []. The results of these studies are aligned with the data of our current study in several aspects. The differences between our current data and our previous study are likely due to the different cell stages of human neuronal cells [,,,,,,,,].
PCs are known to be amongst the most common lipids in neurons. Certain PC species detected here have previously been identified as highly abundant in neurons, such as PC 34:1, PC 36:1, PC 34:0, and PC 32:0, and their salt adducted compounds. These have been known to associate with important neuronal functions such as dopamine transport [,,,,]. In addition, PSs were amongst the second most common lipids found in this study, and they are known to be prominent in neurons, and appear to increase during neuronal maturation []. They regulate mechanisms such as the prevention of neuronal apoptosis, maintenance of the cell membrane, storage of vital FAs, cell signaling, neurotransmission, and synapse formation. PSs also affect the progression of the midbrain disorder Parkinson’s disease, where certain PS species increase disease progression, whilst others can alleviate it. The varying effect of PSs on the midbrain could possibly relate to a strong PS-dependent cellular biology of neurons, thus explaining the high amount observed here [,,,,,].
Cers were found to be more abundant (~11%) than expected (~1% of whole cell lipid content) [,]; however, our previous study on differentiating NPCs also found Cers abundant (~24%) []. The higher amount of Cers detected in our studies compared to others could possibly be due to the differences in methodologies, analysis of the plasma membrane specifically, or cell types. Here, we employed ToF-SIMS to image intact plasma membrane lipids, while mass spectrometry bulk analysis of isolated cell membranes was used in other studies [,,,]. Cers are considered to regulate cell survival and cell death as well as to affect neuronal signaling, promotion of neurites and axons, metabolism, neuronal extracellular interactions, and synaptic transmission. Cers have also been observed to increase in midbrain dopaminergic neuronal maturation, which could explain the higher Cer content in our study [,,]. Decreased levels of Cers and certain Cer species have been associated with Parkinson’s disease [,].
Another major lipid component is PEs, which is supported by other studies [,,]. In the mouse midbrain, PEs appeared to be the second most common lipid among all phospholipids []. PEs are needed in FA storage, cell membrane curvature, ferroptosis indicator for phagocytosis, synaptic signaling, prevention of oxidative stress, and support in protein folding. The synthesis of PE, PC, and PS shares pathways that could, thus. relate to the high amount of these three lipids [,,,,]. In Parkinson’s disease, ethanolamine was shown to protect dopaminergic neurons of C. elegans from alpha-synuclein, a pathological hallmark of the disease [,]. PE was also reported to decrease in the midbrain, especially in the substantia nigra pars compacta, of early Parkinson’s patients.
Finally, SHexCers were found as an abundant component, which is well correlated with the high abundance of Cers, since SHexCer synthesis is reliant on Cer synthesis [,]. However, it is difficult to relate our results to others because of the variation in the reported values. This variation is possibly due to the differences in the used cell type, organism, or analytical method, for example, in tissue isolation method tends to produce a high value because of a high amount of SHexCers in the myelin in the tissues [,,,,]. SHexCers are important for immune regulation, extracellular interactions, membrane proteins, and maintenance of the action potential in the brain. SHexCers have also been associated with Parkinson’s disease [,]. Overall, the abundance of the above-discussed lipids could be related to the biology of mature midbrain neurons.
3.2. Effects of Lipid Head Groups on Neuronal Membrane Lipid Turnover—Ethanolamine Versus Choline
We further examined the lipid turnover of the plasma membrane in the midbrain neurons to identify how cells incorporate different precursors according to their specific biological demand and condition. In this section, isotopically labeled lipid headgroups, 13C2-ethanolamine or 15N-choline, were incubated in the cell cultures for 4 days before being removed from the cell medium for another 12 h. The change in lipid abundance in the isotopically incubated cells was then assessed by comparing the abundance of the respective lipids in control cells.
The lipids contributing to the significant differences between the treated samples and the control are shown in Figure 2. Example ion images of several representative lipids are presented in Figure S3. The effects of 15N-choline incubation were mainly observed in positive mode (Figure 2A). Neurons incubated with 15N-choline incorporated the isotopic headgroup primarily into lysophosphatidylcholines (LPCs) and PCs, resulting in a decrease in the non-isotopic PC-headgroup species [C5H15PNO4]+ at m/z 184.07 (Figure S3A,C,D) and an increase in intensity of 15N-PC headgroup at m/z 185.07 (Figure 2A and Figure S3E,G,H). Other lipids, although having no choline headgroup in the structures, such as [PE 20:0+Na]+ at m/z 547.32, hexosylceramide (HexCer) [HexCer 32:2;O3+Na]+ at m/z 708.5, and [Cer 38:0;O-H]− at m/z 578.59, also increased their turnover. In contrast, a few lipids containing no PC-headgroup were found decreased in their abundance, particularly [Cer 34:1;O4+H]+ at m/z 570.5 (Figure 2A), diacylglycerol (DG) [DG 34:3+H-H2O]+ at m/z 573.49, [FA 16:0-H]− at m/z 255.23, [FA 18:0-H]− at m/z 283.26, [PS 20:0;O-H]− at m/z 582.3, and several phosphatidylinositols (PIs) and lysophosphatidylinositols (LPIs) (Figure 2B).
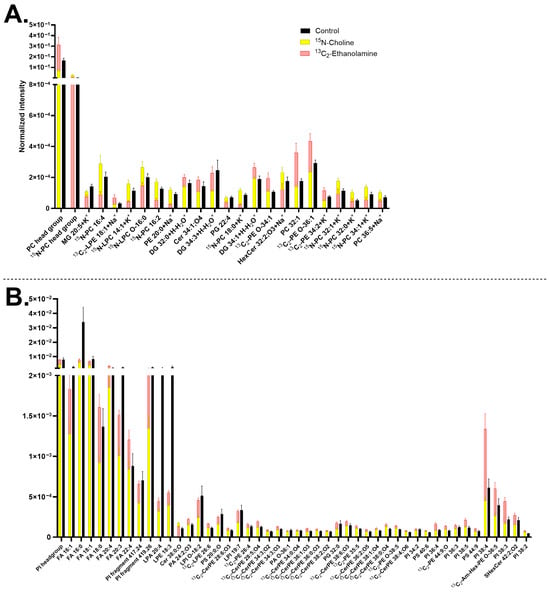
Figure 2.
Major lipids with significantly different turnover in human midbrain neurons following 13C2-ethanolamine or 15N-choline incubation. (A) Lipids detected in positive mode and (B) lipids detected in negative mode. The 13C2-ethanolamine (pink) and 15N-choline (yellow) bars are superimposed, and the control (black) is interleaved. Lipids are arranged in each chart from left to right in ascending mass/charge (m/z). Error bars represent standard error of mean (SEM). The data of 13C2-ethanolamine and 15N-choline are significantly different from each other by a multiple Mann–Whitney test with a p-value of ≤0.05. Cells treated with either 15N-choline or 13C2-ethanolamine each had two biological replicates with seven to nine measurements per ion mode. The control had five biological replicates with 20 measurements per ion mode. 1-(1Z-alkenyl),2-acylglycerophosphoethanolamine glycan (Am-Hex-PE), ceramide (Cer), ceramide phosphoethanolamine (CerPE), diacylglycerol (DG), fatty acid (FA), hexosylceramide (HexCer), lysophosphatidic acid (LPA), lysohosphatidylcholine (LPC), lysophosphatidylethanolamine (LPE), lysophosphatidylinositol (LPI), monoacylglycerol (MG), phosphatidic acid (PA), phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylglycerol (PG), phosphatidylinositol (PI), phosphatidylserine (PS), and sulfatide (SHexCer). Lipids with no annotated ionization were detected as [M+H]+ in (A) and [M–H]− in (B).
Midbrain neurons incubated with 13C2-ethanolamine appeared to have a higher number of lipids with increasing turnover compared to 15N-choline-incubated cells. The 13C2-ethanolamine headgroup was predominantly incorporated into ceramide phosphoethanolamines (CerPEs), followed by PEs, lysophosphatidylethanolamines (LPEs, Figure S3J,L), and PSs, all of which were mainly observed in the negative mode (Figure 2B).
Several other lipids containing no ethanolamine headgroup had an increased abundance such as PC-headgroup [C5H15PNO4]+ at m/z 184.07, [PC 32:1+H]+ at m/z 732.55 (Figure 2A), phosphatidic acid (PA) [PA 24:2;O3-H]− at m/z 579.29, [SHexCer 42:2;O2-H]− at m/z 888.62, and a few PI species (Figure 2B). [PS 40:6-H]− at m/z 834.53 (Figure 2B) further increased its abundance following both 13C2-ethanolamine and 15N-choline treatment. In contrast, two lipids were found to decrease in turnover following 13C2-ethanolamine incubation, particularly a salt adduct of [HexCer 32:2;O3+Na]+ at m/z 708.5 and phosphatidylglycerol (PG) [PG 22:4+H]+ at m/z 575.3 (Figure 2A). In addition, both the isotopic incubations exhibited an opposite trend regarding the change in the abundance of [13C2-LPE 26:6-H]− at m/z 582.34 (Figure 2B and Figure S3G–I), whereas they showed a similar decreasing trend in other lipids, such as monoacylglycerol (MG) [MG 20:5+K]+ at m/z 415.22 (Figure 2A), [FA 16:0-H]− at m/z 255.23, lysophosphatidic acid (LPA) [LPA 20:4-H]− at m/z 457.24, and [LPE 18:3-H]− at m/z 474.26 (Figure 2B).
Comparing the results with high abundant lipids shown in Figure 1, several lipids were observed to overlap with the results of Figure 2, such as salt adducted PCs [15N-PC 32:0+K]+ at m/z 773.53, [15N-PC 32:1+K]+ at m/z 771.51, [15N-PC 34:1+K]+ at m/z 799.54 (Figure 2A), [13C2-PE O-38:5-H]− at m/z 752.54, [PI 38:3-H]− at m/z 887.57, [PI 38:4-H]− at m/z 885.55, [PS 40:6-H]− at m/z 834.53, and [SHex Cer 42:2;O2-H]− at m/z 888.62 (Figure 2B).
PCs and PEs are among the major constituents of the neuronal plasma membrane. The difference in their lipid headgroup structures results in distinct molecular shapes. PCs containing a choline headgroup adopt a cylindrical shape, whereas PEs with an ethanolamine headgroup possess a conical shape [,]. These structural differences influence membrane properties, particularly fluidity and the dynamic formation of membrane curvature, which are critical for various neuronal processes, including exocytosis during neurotransmission [,,]. Here, we investigated how these lipid headgroups contribute to the dynamic turnover of lipids within the neuronal plasma membrane. It was observed that the 15N-choline-incubated cells exhibit a high amount of turnover in many PCs, as expected []. However, 15N-choline appeared to incorporate into a smaller number of lipids and to a lower extent compared to 13C2-ethanolamine (Figure 2), which is contradictory to the higher abundance of PCs in neuronal plasma membrane (Figure 1). This may indicate a longer lifetime and, thus, slower turnover of choline-containing lipids compared to the ethanolamine-containing ones. Such an observation was also reported in rat brains and other cell types where PEs had a higher turnover than PCs [,]. Alternatively, the incubation with 13C2-ethanolamine might exert an overstimulating effect on the synthesis of ethanolamine-containing lipids.
Upon the cell incubation with 13C2-ethanolamine, many CerPEs were found to increase in abundance. This is a noteworthy result since CerPEs are thought to be less common than sphingomyelins (SMs) in mammalian cells [,]. SMs have a choline headgroup, but in our study, SMs were not found to increase in turnover upon 15N-choline incubation. Instead, 13C-ethanolamine incubation appeared to promote the synthesis and turnover of CerPEs, which contain ethanolamine headgroups in their structures. In addition, our findings are supported by several studies where CerPEs were detected in rat brain tissues [], in rat brain microsomes and synaptic membrane [], human NPCs [], and in human and mouse brains using mass spectrometry imaging [,]. They were shown to have different properties and functions than SMs, and to be related to brain aging and Parkinson’s disease [,,]. The total CerPE abundance in the plasma membrane is likely not high enough to be observed as common membrane lipids, since they were not detected within the 90 most abundant lipids. Our data illustrate the possibility of using specific isotopic precursor enrichment and ToF-SIMS imaging to explore the uncharted lipid organization and turnover aspects in neuronal plasma membrane.
3.3. Effects of the Unsaturation Level on Neuronal Membrane Lipid Turnover—Linolenic Acid Versus Linoleic Acid
The effects of the lipid precursor number and position of double bonds on neuronal membrane lipid turnover were examined by comparing the cell incubation with 13C-FA 18:3 omega-3 (13C-linolenic acid) and 13C-FA 18:2 omega-6 (13C-linoleic acid).
The two 13C-FAs exhibited different trends regarding the effects on the lipid turnover. Particularly, 13C-linoleic acid was incorporated into many lipids, resulting in their higher abundance, such as DGs, PCs, and PSs, whereas 13C-linolenic acid maintained the abundance of most of these lipids similar to the control (Figure 3). 13C-linolenic acid increased the turnover of fewer lipids than its counterpart, including [13C-PE 34:3+H]+ at m/z 715.51, [13C-PG 34:0+Na]+ at 774.53 (Figure 3A), [13C-FA 18:3;O-H]− at m/z 294.21, and [13C-PS 44:2-H]− at m/z 899.65 (Figure 3B). Nevertheless, both 13C-FA incubations were found to have the same effects on several lipid compounds. For example, the turnover of [13C-FA 16:0-H]− at 256.23, [13C-LPA 16:1-H]− at m/z 408.22, and [13C-PS 44:12-H]− at m/z 879.5 increased, whereas FAs, [PI 38:4-H]− at m/z 885.55, and [PS 44:8-H]− at m/z 886.56 decreased by both isotopic incubations (Figure 3B). The increased abundance of [13C-FA 18:3;O-H]− at m/z 294.21 in 13C-linolenic acid incubated neurons compared to that of the 13C-linoleic acid incubated ones is shown in Figure S4A–C, whilst a higher abundance of [13C-PS 44:12-H]− at m/z 879.5 in 13C-linoleic acid incubated cells is demonstrated in Figure S4D–F.
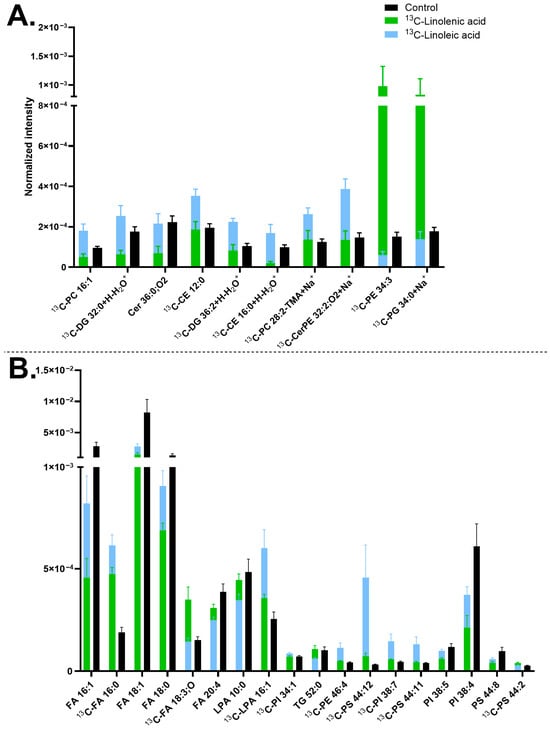
Figure 3.
Lipid abundancy in mature human midbrain neurons upon 13C-linolenic acid and 13C-linoleic acid incubation. (A) Lipids detected in positive mode and (B) lipids detected in negative mode. Error bars represent standard error of mean (SEM). The 13C-linolenic acid (green) and 13C-linoleic acid (blue) bars are superimposed, and the control (black) is interleaved. All bars of 13C-linolenic acid and 13C-linoleic acid are significantly different from each other by a multiple Mann–Whitney test with a p-value of ≤0.05. Lipids are arranged from left to right in ascending mass per charge (m/z). Cells treated with either 13C-linolenic acid or with 13C-linoleic acid each had two biological replicates with seven to eight measurements per mode. The control had five biological replicates with 20 measurements in total per mode. Cholesterol ester (CE), ceramide (Cer), ceramide phosphoethanolamine (CerPE), diacylglycerol (DG), fatty acid (FA), lysophosphatidic acid (LPA), phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylglycerol (PG), phosphatidylinositol (PI), phosphatidylserine (PS), and triglyceride (TG). Lipids with no annotated ionization were detected as [M+H+] in (A) and [M-H−] in (B).
Linolenic acid (one of the three main types of omega-3 FAs) and linoleic acid (a type of omega-6 FAs) are essential FAs for brain function and development [,]. Omega-3 improves cell membrane fluidity and neurotransmission to promote cognition and protection against neurodegeneration [,], as well as preventing neural apoptosis by reducing the production of reactive oxygen species (ROS). On the other hand, while omega-6 is critical for brain development, an excess of it could induce brain inflammation and neurodegenerative diseases by promoting ROS accumulation []. Many studies have focused on the balance of omega FAs, where a balance of 3:1 of omega-6 to omega-3 has been linked to neurodevelopment and brain function, whilst a large imbalance appeared to cause negative effects [,]. Therefore, a balance between omega-3 and omega-6 is essential to health, neuronal function, cognition, learning, and memory []. The two FAs have quite similar molecular structures (C18 carbon chain), except for the number and position of the double bonds. We explored the effect of 13C-linolenic acid and 13C-linoleic acid on the neuronal membrane lipids and found that the incorporation of 13C-linoleic acid occurred in more lipids than 13C-linolenic acid. Both incubations decreased several non-isotopic FAs, such as [FA 16:1-H]−, [FA 18:1-H]−, and [FA 18:0-H]−. This could be due to a high amount of the isotopic precursors leading to their preferred incorporation into the cells.
Our results of a higher incorporation of 13C-linoleic acid than 13C-linolenic acid differ from previous studies using ToF-SIMS on the plasma membrane of PC12 cells [,], where a higher incorporation of 13C-linolenic acid was found. However, other studies on neural tissues and cells have reported similar results to our study [,,,,,,]. Omega-6 intake was shown to play a role in inducing dopaminergic neurogenesis in the midbrain, indicating the relevance of our results for mature midbrain neurons []. 13C-linoleic acid incubated midbrain neurons had a high incorporation into DGs, PCs, and PSs. Prominent incorporation and reliance of omega-6 FAs, including linoleic acid in DGs, PCs, and PSs, has been previously observed in brain cells and other cell types [,,,,,,]. In neurons, DGs in the plasma membrane have been shown to be important for neuronal synaptic formation and function []. In addition, omega-6 FAs have previously been found to incorporate into many phospholipids, which supports our observation. On the other hand, omega-3 FAs are more common in PEs or PSs [,,,,], but we did not observe such a trend in these compounds here. Omega-3 FAs have been associated with differentiation [], which could explain the lower incorporation of 13C-linolenic acid in mature neurons.
The two omega FAs share a lipid bioconversion pathway of elongation and unsaturation; however, our data indicate a distinctly molecular difference in membrane lipid turnover induced by these two FAs, which is consistent with previous observations [,]. This could be the key factor contributing to the difference in their neurological effects.
3.4. Effects of Carbon Chain Saturation of Lipid Precursor on Neuronal Membrane Lipid Turnover—Linoleic Acid and Linolenic Acid Versus Stearic Acid
We next examined the neuronal membrane lipid turnover, comparing cell incubations with 13C−linoleic acid, 13C−linolenic acid, and 13C-stearic acid. These FAs have the same carbon chain (C18) but differ in their degree of unsaturation. A three-component PCA was initially performed to compare the three sample groups, which showed some overlap. Therefore, a two-component PCA was used to better highlight the similarities and differences among the samples.
It was observed that 13C-stearic acid is generally incorporated into a larger number of neuronal membrane lipids than the 13C-linoleic acid precursor (Figure 4). Particularly, all the 13C-PCs (correspondingly the PC headgroup at m/z 184.07) and [13C-PI fragment] at m/z 420.26 increased their concentrations in the 13C-stearic acid incubated samples. Several 13C-LPIs also increased, such as [13C-LPI O-18:2-H]− at m/z 582.31 and [13C-LPI 18:0-H]− at m/z 600.32, which is likely corresponding to the elevated amount of [13C-PI fragment] at m/z 420.26. In addition, the 13C-stearic acid precursor [13C-FA 18:0-H]− at m/z 284.26 was observed. An example of the high PC incorporation is shown in Figure S5 via the PC-headgroup [C5H15PNO4]+ at m/z 184.07 and [13C-PC 30:1+K]+ at m/z 743.48 (Figure 4A and Figure S5D–F). The incorporation of [13C-PI fragment] at m/z 420.26 is shown in Figure S5G–I.
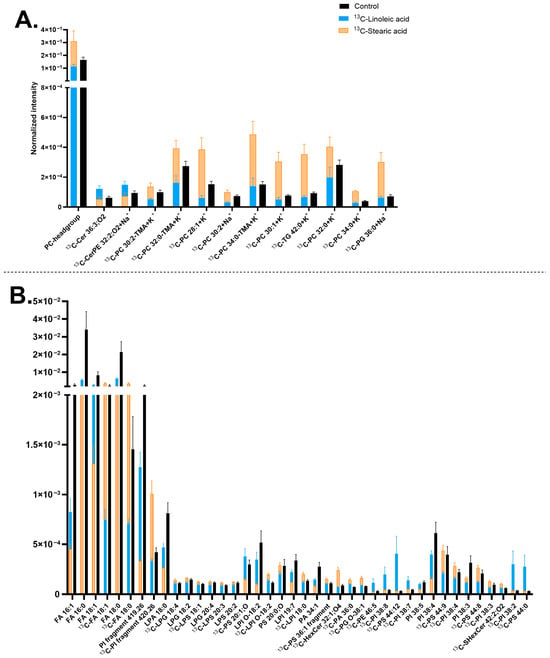
Figure 4.
Lipid abundancy in mature human midbrain neurons upon 13C-linoleic acid or 13C-stearic acid incubation. (A) Lipids detected in positive mode; (B) Lipids detected in negative mode. Error bars represent standard error of mean (SEM). The 13C-stearic acid (orange) and 13C-linoleic acid (blue) bars are superimposed, and the control (black) is interleaved. All bars of 13C-stearic acid and 13C-linoleic acid are significantly different from each other by a multiple Mann–Whitney test with a p-value of ≤0.05. Lipids are arranged (A,B) from left to right in ascending mass per charge (m/z). 13C-linoleoic acid incubated samples had two biological replicates with four measurements per ion mode. 13C-stearic acid incubated samples had two biological replicates with seven to eight measurements per ion mode, and the control had five biological replicates with 20 measurements per ion mode. Ceramide (Cer), ceramide phosphoethanolamine (CerPE), fatty acid (FA), hexosylceramide (HexCer), lysophosphatidic acid (LPA), lysophosphatidylglycerol (LPG), lysophosphatidylinositol (LPI), lysophosphatidylserine (LPS), phosphatidic acid (PA), phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylglycerol (PG), phosphatidylinositol (PI), phosphatidylserine (PS), sulfatide (SHexCer), and triglyceride (TG). Lipids with no annotated ionization were detected as [M+H]+ in (A) and [M-H]− in (B).
13C-linoleic acid is incorporated primarily into PIs and PSs, mostly with high double bonds, such as [13C-PI 38:8-H]− at m/z 878.49, [13C-PI 38:7-H]− at m/z 880.5, [13C-PI 38:2-H]− at m/z 890.58, [13C-PS 44:12-H]− at m/z 879.5, except for [13C-PS 44:0-H]− at m/z 903.69 (Figure 4B). The ion images of [13C-PI 38:7-H]− at m/z 880.5 are shown in Figure S5J–L. In addition, the two isotopic precursors showed an opposite effect on [13C-FA 18:0-H]− at m/z 284.26 (13C-stearic acid) (Figure 4B) and several 13C-PCs (Figure 4A), which have higher abundance with 13C-stearic acid but lower abundance with 13C-linoleic acid. Finally, both precursors exerted similar negative effects on the turnover of a few FAs and PIs, particularly non-isotopic lipid compounds [FA 16:0-H]− at m/z 255.23, [FA 16:1-H]− at m/z 253.22, [FA 18:0-H]− at m/z 283.26, [PI 38:4-H]− at m/z 885.55, and [PI 38:3-H]− at 887.57 (Figure 2B).
Compared with the results in Figure 3, similar trends were observed for several lipids. For example, [13C-CerPE 32:2;O2+Na]+ at m/z 654.46, [13C-PI 38:7-H]− at m/z 880.5, and [13C-PS 44:12-H]− at m/z 879.5 showed increased abundance whereas [FA 18:0-H]− at m/z 283.26, [FA 18:1-H]− at m/z 281.25, [PI 38:4-H]− at m/z 885.55, and [PI 38:5-H]− at m/z 883.53 showed decreased abundance. Additionally, comparison with Figure 1 reveals that many of significantly turned-over lipids identified in Figure 4 are major components of neuronal membrane lipids, including PCs (Figure 4A), FAs ([FA 16:0-H]− at m/z 255.23, [FA 18:0-H]− at m/z 283.26, and [FA 18:1-H]− at m/z 281.25), LPIs, and PIs (Figure 4B).
Next, lipid turnover of midbrain neurons was compared between 13C-stearic acid and 13C-linolenic acid incubations. Upon 13C-stearic acid treatment, PCs and several PIs significantly increased their abundance compared to the control and 13C-linolenic samples, such as [13C-PC 34:1+K]+ at m/z 799.54 (Figure 5A and Figure S6A–C) and 13C-PI fragment [C21H40O6P]− at m/z 420.26 (Figure 5A and Figure S6D–F). The precursor also induced higher abundance of [13C-FA 18:1-H]− at m/z 282.25 and [13C-FA 18:0-H]− at m/z 284.26 (precursor itself) (Figure S6G–I). On the other hand, 13C-linolenic acid incubation exhibited an increased amount of Cers and some PSs, such as [13C-Cer 34:1;O2+K]+ at m/z 577.48 (Figure 5A and Figure S6J–L), and [13C-PS 44:13-H]− at m/z 877.48 (Figure 5B and Figure S6M–O). Among these lipids, some contained a high double bond number, for example [13C-PS 44:13-H]− at m/z 877.48 and [13C-PS 44:14-H]− at m/z 875.47. In addition, both incubations increased the amount of [13C-FA 20:4-H]− at m/z 304.23 and [13C-LPA 16:1-H]− at m/z 408.22, with 13C-linolenic acid showing a greater extent. In contrast, they decreased the level of [FA 16:0-H]− at m/z 255.23, [FA 20:4-H]− at m/z 303.23, and [LPI O-18:2-H]− at m/z 581.31 (Figure 5B).
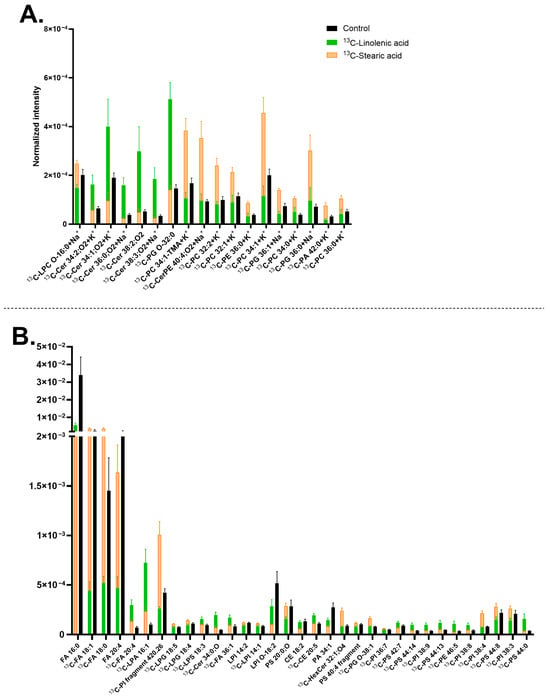
Figure 5.
Lipids abundance in mature human midbrain neurons upon 13C-linolenic acid or 13C-stearic acid incubation. (A) Lipids detected in positive mode; (B) Lipids detected in negative mode. Error bars represent standard error of mean (SEM). The 13C-stearic acid (orange) and 13C-linolenic acid (green) bars are superimposed, and the control (black) is interleaved. All bars of 13C-stearic acid and 13C-linolenic acid are significantly different from each other by a multiple Mann–Whitney test with a p-value of ≤0.05. Lipids are arranged (A,B) from left to right in ascending mass per charge (m/z). 13C-linolenic acid and 13C-stearic acid incubated samples had two biological replicates with seven to eight measurements per ion mode. The control had five biological replicates with 20 measurements per ion mode. Cholesterol ester (CE), ceramide (Cer), ceramide phosphoethanolamine (CerPE), fatty acid (FA), hexosylceramide (HexCer), lysophosphatidic acid (LPA), lysophosphatidylglycerol (LPG), lysophosphatidylinositol (LPI), lysophosphatidylserine (LPS), phosphatidic acid (PA), phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylglycerol (PG), phosphatidylinositol (PI), and phosphatidylserine (PS). Lipids with no annotated ionization were detected as [M+H]+ in (A) and [M–H]− in (B).
In relation to Figure 3, which compares 13C-linolenic acid and 13C-linoleic acid, some lipids were common, such as [13C-LPA 16:1-H]− at m/z 408.22, having higher abundance with 13C-linolenic acid (Figure 3B and Figure 5B). On the other hand, [13C-PI 38:4-H]− at m/z 886.55 and [13C-PS 44:8-H]− at m/z 887.56 showed higher abundance with 13C-stearic acid. In addition, comparing Figure 4 and Figure 5, similar lipids were observed, for example, [FA 16:0-H]− at m/z 255.23 with lower abundance, whereas [13C-FA 18:1-H]− at m/z 282.25, [13C-FA 18:0-H]− at m/z 284.26, and PCs having higher abundance with 13C-stearic acid.
The difference in lipid turnover compared between 13C-linoleic acid, 13C-linolenic acid, and 13C-stearic acid incubation (Figure 4 and Figure 5) revealed that the largest number of lipids have the isotopic incorporation with 13C-stearic acid, particularly all the 13C-PCs, 13C-PI fragment 4.26 [C21H40O6P]−, and several 13C-LPIs. On the other hand, fewer lipids were found to turn over by 13C-linoleic acid, particularly PIs and PSs with high double bonds, such as [13C-PI 38:8-H]− at m/z 878.49, [13C-PI 38:7-H]− at m/z 880.5, [13C-PI 38:2-H]− at m/z 890.58, and [13C-PS 44:12-H]− at m/z 879.5. 13C-linolenic acid was also incorporated into a few lipids, primarily Cers and PSs, for example [13C-Cer 38:2;O2+H]+ at m/z 593.57, [13C-Cer 34:1,O2+K]+ at m/z 577.48, [13C-PS 42:7-H]− at m/z 861.54, and [13C-PS 44:13-H]− at m/z 877.48. Cells incubated with 13C-stearic acid increased the abundance of lighter lipids (C28-C42 with 13C-stearic acid, C30-C46 for 13C-linoleic and 13C-linolenic acids) with fewer double bonds (0–8 for 13C-stearic acid, 0–14 for 13C-linoleic and 13C-linolenic acid). This is expected due to their molecular structures, and unsaturated FAs tend to produce cellular lipids that are elongated and unsaturated [,]. PSs and PIs have been found to contain omega-3 FAs and omega-6 FAs in the brain [,,,,]. A low omega-3 intake also resulted in a decrease in PS lipids in the brain [,,]. These lipids are known to affect cellular signaling, synaptic membrane fusion, and regulation of glutamate receptors, which are located in midbrain neurons. The higher turnover of 13C-stearic acid is consistent with our previous report in human differentiating NPCs with 13C-stearic acid []. The lower plasma membrane lipid turnover of 13C-linoleic acid compared to 13C-stearic acid could be explained by other findings that over half the amount of linoleic acid entering the brain is used in energy production [,]. In addition, it was found that the in vivo turnover of omega FAs is considerably slow and may take up to several weeks [,].
The 13C-stearic acid incubated cells showed an increased abundance of [13C-FA 18:0-H]− at m/z 284.26, indicating a high accumulation of the isotopic precursor after the incubation. Other studies have shown a similar trend of non-isotopic stearic acid and the lipids containing the stearic acid chain in neuronal cells and the brain [,,,,,,]. The strong preference for stearic acid observed here is likely attributed to its high turnover and abundance in the brain, along with its frequent incorporation into PCs [,,,]. In addition, in our previous study on human NPCs, a high incorporation of 13C-stearic acid was shown to occur mainly into Cers and DGs, and a few PCs. Thus, the lipid turnover is different between the two cell stages with 13C-stearic acid, and this could be elaborated that Cers and DGs have a higher abundance during neuronal differentiation [,,]. Our data show that stearic acid is a preferred precursor for PCs. In the mouse midbrain, PCs containing a FA 18:0 chain are amongst the common lipids, following PSs, PIs, and SHexCers [], and they also incorporate 13C-stearic acid. The results suggest that 13C-stearic acid is one of the most efficiently incorporated lipid precursors in human neuronal cells.
3.5. Effect of Carbon Chain Length in Neuronal Membrane Lipid Turnover—Stearic Acid Versus Lauric Acid
In this section, we studied the role of carbon chain length on the membrane lipid turnover by comparing 13C-stearic acid (FA 18:0) and 13C-lauric acid incubations (FA 12:0), often referred to as medium- and short-chain FAs, respectively. Incubation with 13C-stearic acid resulted in the turnover of a larger number of lipids than 13C-lauric acid (Figure 6). Both precursors were incorporated into many PCs, PGs, and several Cers; however, 13C-lauric acid appears to be favored by lipids with shorter carbon chains (from C28 to C32), whereas 13C-stearic acid is preferred by compounds with longer carbon chains (from C30 to C38). Both were also incorporated mainly into lipids with a low unsaturation level (0 and 1 double bond in the carbon chain).
Midbrain neurons treated with 13C-stearic acid generally decreased the turnover of non-isotopic lipids, for example [PC 16:4+H]+ at m/z 502.26, [HexCer 30:2;O2+K]+ at m/z 680.45, [PC 34:1-TMA+K]+ at m/z 739.47, [PC 34:1+H]+ at m/z 760.59 (Figure 6A), [FA 18:1-H]− at m/z 281.25, and [FA 18:0-H]− at m/z 283.26 (Figure 6B), but increased the concentration of many isotopic counterparts of these compounds. In addition, 13C-stearic acid increased several Cers (e.g., [13C-Cer 38:1;O+Na]+ at m/z 601.57), CerPEs (e.g., [13C-CerPE 40:4;O2+Na]+ at m/z 762.56), and PIs (e.g., [13C-LPI O-18:2-H]− at m/z 582.31, 13C-PI fragment 420.26 [C21H40O6P]−(Figure S7M–O), notably the PIs only showed increase trend with 13C-stearic acid. This indicates that PI turnover seems connected to only 13C-stearic acid, not 13C-lauric acid. Ion images of [LPC 16:0+K]+ at m/z 535.3, [13C-PS 36:0-H]− at m/z 791.56 and several other lipids are shown in Figure S7. Finally, these detected lipids were within the highly abundant lipids in human neuronal plasma membrane shown in Figure 1, such as FAs, PCs, LPIs, Cers, HexCers, and PSs.
Both precursors were found to incorporate into many lipids, including PCs, PGs, and several Cers, most of them with a low number of double bonds (0 or 1 double bond). In addition, 13C-lauric acid was favored for lipids with shorter carbon chains than 13C-stearic acid (range of C28-C32 and C30-C38, respectively). This is expected due to the saturation state and length of the carbon chain of these precursors. The comparison between 13C-lauric acid and 13C-stearic acid has also been performed previously in our lab on human differentiating NPCs using ToF-SIMS with a (CO2)6000+ GCIB, which showed that 13C-lauric acid and 13C-stearic acid were incorporated mainly into Cers and DGs, but not PCs []. The shift in lipid turnover, with increased incorporation into PCs by 13C-lauric acid and 13C-stearic acid in this study, showed a similar trend compared to the results for 13C-stearic acid and 13C-linoleic acid. Lipid turnover consistently shifting from Cers and DGs to PCs may reflect the transition from NPCs to mature midbrain neurons. Overall, 13C-lauric acid induced fewer lipids to increase turnover than 13C-stearic acid, possibly because the brain favors longer-chain FAs. In addition, shorter-chain FAs are more likely to be used for energy metabolism compared to longer-chain FAs [,,,,].
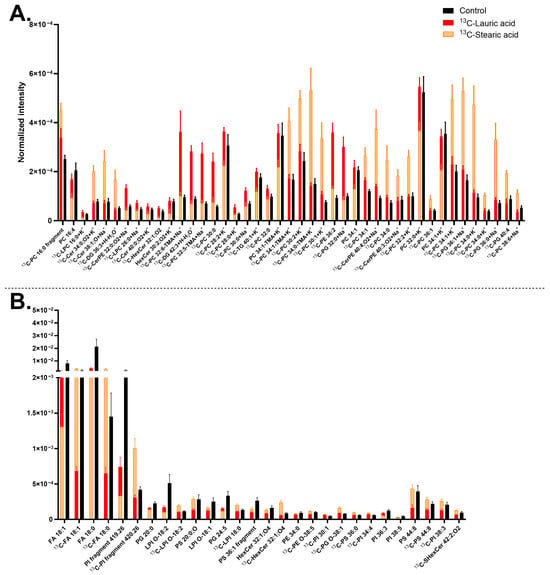
Figure 6.
Lipid abundancy in mature human midbrain neurons upon 13C-lauric acid or 13C-stearic acid incubation. (A) Lipids detected in positive mode; (B) Lipids detected in negative mode. Error bars represent standard error of mean (SEM). The 13C-stearic acid (orange) and 13C-lauric acid (red) bars are superimposed, and the control (black) is interleaved. All bars of 13C-stearic acid and 13C-lauric acid are significantly different from each other by a multiple Mann–Whitney test with a p-value of ≤0.05. Lipids are arranged from left to right in ascending mass per charge (m/z). 13C-stearic acid incubated samples had two biological replicates with six to eight measurements per ion mode. 13C-lauric acid incubated samples had two biological replicates with five to eight measurements per mode, and the control had five biological replicates with 20 measurements per mode. Ceramide (Cer), ceramide phosphoethanolamine (CerPE), diacylglycerol (DG), fatty acid (FA), hexosylceramide (HexCer), lysophosphatidic acid (LPA), lysohosphatidylcholine (LPC), lysophosphatidylinositol (LPI), phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylglycerol (PG), phosphatidylinositol (PI), phosphatidylserine (PS), sulfatide (SHexCer), and triglyceride (TG). Lipids with no ionization annotated were detected as [M+H]+ in (A) and [M–H]− in (B).
4. Conclusions
In this study, we aimed to characterize the molecular organization and dynamic turnover of the major plasma membrane lipids in human midbrain neurons to advance understanding of neuronal biology in relation to membrane lipids. Using ToF-SIMS imaging and the shotgun approach, we identified the predominant neuronal plasma membrane lipids and compared the molecular turnover patterns of the membrane lipids using different lipid precursors, generating a comprehensive dataset with new insights into the membrane composition of human midbrain neurons.
The lipid profile and turnover pattern, which are strikingly characterized by a high prevalence of PCs, PSs, Cers, and a preference for utilizing stearic acid as a precursor, could be directly linked to the biological state and functions of midbrain neurons. In addition, the lipid turnover alters drastically depending on the types of precursors, indicating a significant role of these exogenous molecules in mediating the molecular structure of neuronal membranes, and, hence, influencing neuronal functions. This may have a significant implication for future medical intervention. These findings provide a groundwork for research on lipid turnover and metabolism across neuronal developmental stages, different neuronal cell types, and under varying precursors or physiological conditions. Our work demonstrates the potential of ToF-SIMS imaging to investigate neuronal plasma membrane lipid abundance and turnover, elucidating the dynamic molecular structure of the neuronal plasma membrane and its link to neuronal functions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biom15121650/s1, Figure S1: Immunocytochemistry images of neural progenitor cells (NPCs) and differentiated mature midbrain neurons; Figure S2: Example ToF-SIMS images of mature human midbrain neurons samples; Figure S3: Comparison of plasma membrane lipid turnover in mature human midbrain neurons incubated with 15N-choline and 13C2-ethanolamine headgroup precursors; Figure S4: Comparison of plasma membrane lipid turnover in mature human midbrain neurons incubated with 13C-linoleic acid and 13C-linolenic acid; Figure S5: Comparison of plasma membrane lipid turnover in mature human midbrain neurons incubated with 13C-linoleic acid and 13C-stearic acid; Figure S6: Comparison of plasma membrane lipid turnover in mature human midbrain neurons incubated with 13C-linolenic acid and 13C-stearic acid; Figure S7: Comparison of plasma membrane lipid turnover in mature human midbrain neurons incubated with 13C-lauric acid and 13C-stearic acid; Table S1: Summary of identified lipids in positive mode; Table S2: Summary of identified lipids in negative mode.
Author Contributions
Conceptualization, N.T.N.P., A.A.L., and E.B.; methodology, N.T.N.P., A.A.L., E.B., and C.E.; formal analysis, E.B.; investigation, N.T.N.P., E.B., and J.S.F.; resources, N.T.N.P. and C.E.; data curation, E.B.; writing—original draft preparation, E.B.; writing—review and editing, N.T.N.P., E.B., J.S.F., A.A.L., and C.E.; supervision, N.T.N.P.; project administration, N.T.N.P.; funding acquisition, N.T.N.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Hasselblad Foundation 2020 and the Swedish Research Council (VR 2020-00815, VR StG 2023–04579) for T.N.N.P, and the Swedish Research Council (2022-04498) for J.S.F.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the research Ethics Board of the McGill University Health Center, Montreal, Canada (23-09-075, 9 December 2024).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Acknowledgments
Thank you to Simon Uzoni at the Fletcher lab, University of Gothenburg, for assistance with implementing the ChiToolbox data analysis code in Matlab. The ToF-SIMS measurements were performed using the 3D Chemical Analyzer J105 at the University of Gothenburg, Department of Chemistry and Molecular Biology, Sweden.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ruchalski, K.; Hathout, G.M. A Medley of Midbrain Maladies: A Brief Review of Midbrain Anatomy and Syndromology for Radiologists. Radiol. Res. Pract. 2012, 2012, 258524. [Google Scholar] [CrossRef] [PubMed]
- Hegarty, S.V.; Sullivan, A.M.; O’Keeffe, G.W. Midbrain Dopaminergic Neurons: A Review of the Molecular Circuitry That Regulates Their Development. Dev. Biol. 2013, 379, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.; Root, D.H. Glutamate Neurons within the Midbrain Dopamine Regions. Neuroscience 2014, 282, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Ingólfsson, H.I.; Carpenter, T.S.; Bhatia, H.; Bremer, P.T.; Marrink, S.J.; Lightstone, F.C. Computational Lipidomics of the Neuronal Plasma Membrane. Biophys. J. 2017, 113, 2271–2280. [Google Scholar] [CrossRef]
- Samhan-Arias, A.K.; Poejo, J.; Marques-da-Silva, D.; Martínez-Costa, O.H.; Gutierrez-Merino, C. Are There Lipid Membrane-Domain Subtypes in Neurons with Different Roles in Calcium Signaling? Molecules 2023, 28, 7909. [Google Scholar] [CrossRef]
- Duménieu, M.; Oulé, M.; Kreutz, M.R.; Lopez-Rojas, J. The Segregated Expression of Voltage-Gated Potassium and Sodium Channels in Neuronal Membranes: Functional Implications and Regulatory Mechanisms. Front. Cell. Neurosci. 2017, 11, 115. [Google Scholar] [CrossRef]
- Antelmi, E.; Rocchi, L.; Latorre, A.; Belvisi, D.; Magrinelli, F.; Bhatia, K.P.; Tinazzi, M. Restless Legs Syndrome: Known Knowns and Known Unknowns. Brain Sci. 2022, 12, 118. [Google Scholar] [CrossRef]
- Sonnenschein, S.F.; Gomes, F.V.; Grace, A.A. Dysregulation of Midbrain Dopamine System and the Pathophysiology of Schizophrenia. Front. Psychiatry 2020, 11, 613. [Google Scholar] [CrossRef]
- Triarhou, L.C. Dopamine and Parkinson’s Disease. In Madame Curie Bioscience Database; Landes Bioscience: Austin, TX, USA, 2013. [Google Scholar]
- Ernst, M.; Zametkin, A.J.; Matochik, J.A.; Pascualvaca, D.; Jons, P.H.; Cohen, R.M. High Midbrain [18F]DOPA Accumulation in Children with Attention Deficit Hyperactivity Disorder. Am. J. Psychiatry 1999, 156, 1209–1215. [Google Scholar] [CrossRef]
- Van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane Lipids: Where They Are and How They Behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef]
- Agüi-Gonzalez, P.; Guobin, B.; Gomes De Castro, M.A.; Rizzoli, S.O.; Phan, N.T.N. Secondary Ion Mass Spectrometry Imaging Reveals Changes in the Lipid Structure of the Plasma Membranes of Hippocampal Neurons Following Drugs Affecting Neuronal Activity. ACS Chem. Neurosci. 2021, 12, 1542–1551. [Google Scholar] [CrossRef]
- Egawa, J.; Pearn, M.L.; Lemkuil, B.P.; Patel, P.M.; Head, B.P. Membrane Lipid Rafts and Neurobiology: Age—Related Changes in Membrane Lipids and Loss of Neuronal Function. J. Physiol. 2016, 594, 4565–4579. [Google Scholar] [CrossRef]
- Miranda, A.M.; Bravo, F.V.; Chan, R.B.; Sousa, N.; Di Paolo, G.; Oliveira, T.G. Differential Lipid Composition and Regulation along the Hippocampal Longitudinal Axis. Transl. Psychiatry 2019, 9, 144. [Google Scholar] [CrossRef]
- Tracey, T.J.; Steyn, F.J.; Wolvetang, E.J.; Ngo, S.T. Neuronal Lipid Metabolism: Multiple Pathways Driving Functional Outcomes in Health and Disease. Front. Mol. Neurosci. 2018, 11, 10. [Google Scholar] [CrossRef]
- Philipsen, M.H.; Phan, N.T.N.; Fletcher, J.S.; Ewing, A.G. Interplay between Cocaine, Drug Removal, and Methylphenidate Reversal on Phospholipid Alterations in Drosophila Brain Determined by Imaging Mass Spectrometry. ACS Chem. Neurosci. 2020, 11, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, B.G.; Mandad, S.; Truckenbrodt, S.; Kröhnert, K.; Schäfer, C.; Rammner, B.; Koo, S.J.; Claßen, G.A.; Krauss, M.; Haucke, V.; et al. Composition of Isolated Synaptic Boutons Reveals the Amounts of Vesicle Trafficking Proteins. Science 2014, 344, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.G.; Park, S.S.; Park, J.H.; Lee, S.B. Dysregulated Plasma Membrane Turnover Underlying Dendritic Pathology in Neurodegenerative Diseases. Front. Cell. Neurosci. 2020, 14, 556461. [Google Scholar] [CrossRef] [PubMed]
- Porcellati, G.; Goracci, G.; Arienti, G. Lipid Turnover. In Handbook of Neurochemistry; Springer: Boston, MA, USA, 1983; pp. 277–294. [Google Scholar] [CrossRef]
- Schmitt, A.; Maras, A.; Petroianu, G.; Braus, D.F.; Scheuer, L.; Gattaz, W.F. Effects of Antipsychotic Treatment on Membrane Phospholipid Metabolism in Schizophrenia. J. Neural Transm. 2001, 108, 1081–1091. [Google Scholar] [CrossRef]
- Jensen, J.E.; Miller, J.; Williamson, P.C.; Neufeld, R.W.J.; Menon, R.S.; Malla, A.; Manchanda, R.; Schaefer, B.; Densmore, M.; Drost, D.J. Grey and White Matter Differences in Brain Energy Metabolism in First Episode Schizophrenia: 31P-MRS Chemical Shift Imaging at 4 Tesla. Psychiatry Res. Neuroimaging 2006, 146, 127–135. [Google Scholar] [CrossRef]
- Tessier, C.; Sweers, K.; Frajerman, A.; Bergaoui, H.; Ferreri, F.; Delva, C.; Lapidus, N.; Lamaziere, A.; Roiser, J.P.; De Hert, M.; et al. Membrane Lipidomics in Schizophrenia Patients: A Correlational Study with Clinical and Cognitive Manifestations. Transl. Psychiatry 2016, 6, e906. [Google Scholar] [CrossRef]
- Nuss, P.; Tessier, C.; Ferreri, F.; De Hert, M.; Peuskens, J.; Trugnan, G.; Masliah, J.; Wolf, C. Abnormal Transbilayer Distribution of Phospholipids in Red Blood Cell Membranes in Schizophrenia. Psychiatry Res. 2009, 169, 91–96. [Google Scholar] [CrossRef]
- Horrobin, D.F.; Glen, A.I.M.; Vaddadi, K. The Membrane Hypothesis of Schizophrenia. Schizophr. Res. 1994, 13, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Horrobin, D.F.; Bennett, C.N. The Membrane Phospholipid Concept of Schizophrenia. In Search for the Causes of Schizophrenia; Springer: Darmstadt, Germany, 1999; pp. 261–277. [Google Scholar] [CrossRef]
- Hannestad, J.K.; Rocha, S.; Agnarsson, B.; Zhdanov, V.P.; Wittung-Stafshede, P.; Höök, F. Single-Vesicle Imaging Reveals Lipid-Selective and Stepwise Membrane Disruption by Monomeric α-Synuclein. Proc. Natl. Acad. Sci. USA 2020, 117, 14178–14186. [Google Scholar] [CrossRef] [PubMed]
- Kaur, U.; Lee, J.C. Unroofing Site-Specific α-Synuclein Lipid Interactions at the Plasma Membrane. Proc. Natl. Acad. Sci. USA 2020, 117, 18977–18983. [Google Scholar] [CrossRef] [PubMed]
- Plotegher, N.; Bubacco, L.; Greggio, E.; Civiero, L. Ceramides in Parkinson’s Disease: From Recent Evidence to New Hypotheses. Front. Neurosci. 2019, 13, 330. [Google Scholar] [CrossRef]
- Alecu, I.; Bennett, S.A.L. Dysregulated Lipid Metabolism and Its Role in α-Synucleinopathy in Parkinson’s Disease. Front. Neurosci. 2019, 13, 328. [Google Scholar] [CrossRef]
- Schulte, E.C.; Altmaier, E.; Berger, H.S.; Do, K.T.; Kastenmüller, G.; Wahl, S.; Adamski, J.; Peters, A.; Krumsiek, J.; Suhre, K.; et al. Alterations in Lipid and Inositol Metabolisms in Two Dopaminergic Disorders. PLoS ONE 2016, 11, e0147129. [Google Scholar] [CrossRef]
- Haynes, P.R.; Pyfrom, E.S.; Li, Y.; Stein, C.; Cuddapah, V.A.; Jacobs, J.A.; Yue, Z.; Sehgal, A. A Neuron–Glia Lipid Metabolic Cycle Couples Daily Sleep to Mitochondrial Homeostasis. Nat. Neurosci. 2024, 27, 666–678. [Google Scholar] [CrossRef]
- Ugur, C.; Uneri, O.S.; Goker, Z.; Sekmen, E.; Aydemir, H.; Solmaz, E. The Assessment of Serum Lipid Profiles of Children with Attention Deficit Hyperactivity Disorder. Psychiatry Res. 2018, 264, 231–235. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.; Wucher, A.; Winograd, N. Molecular Depth Profiling with Argon Gas Cluster Ion Beams. J. Phys. Chem. C 2015, 119, 15316–15324. [Google Scholar] [CrossRef]
- Angerer, T.B.; Blenkinsopp, P.; Fletcher, J.S. High Energy Gas Cluster Ions for Organic and Biological Analysis by Time-of-Flight Secondary Ion Mass Spectrometry. Int. J. Mass Spectrom. 2015, 377, 591–598. [Google Scholar] [CrossRef]
- Tian, H.; Maciążek, D.; Postawa, Z.; Garrison, B.J.; Winograd, N. CO2 Cluster Ion Beam, an Alternative Projectile for Secondary Ion Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2016, 27, 1476–1482. [Google Scholar] [CrossRef]
- Tian, H.; Sheraz Née Rabbani, S.; Vickerman, J.C.; Winograd, N. Multiomics Imaging Using High-Energy Water Gas Cluster Ion Beam Secondary Ion Mass Spectrometry [(H2O)n-GCIB-SIMS] of Frozen-Hydrated Cells and Tissue. Anal. Chem. 2021, 93, 7808–7814. [Google Scholar] [CrossRef]
- Ogiso, H.; Taniguchi, M.; Okazaki, T. Analysis of Lipid-Composition Changes in Plasma Membrane Microdomains. J. Lipid Res. 2015, 56, 1594–1605. [Google Scholar] [CrossRef]
- Agüi-Gonzalez, P.; Jähne, S.; Phan, N.T.N. SIMS Imaging in Neurobiology and Cell Biology. J. Anal. At. Spectrom. 2019, 34, 1355–1368. [Google Scholar] [CrossRef]
- Philipsen, M.H.; Sämfors, S.; Malmberg, P.; Ewing, A.G. Relative Quantification of Deuterated Omega-3 and -6 Fatty Acids and Their Lipid Turnover in PC12 Cell Membranes Using TOF-SIMS. J. Lipid Res. 2018, 59, 2098–2107. [Google Scholar] [CrossRef]
- Troiano, J.M.; Olenick, L.L.; Kuech, T.R.; Melby, E.S.; Hu, D.; Lohse, S.E.; Mensch, A.C.; Dogangun, M.; Vartanian, A.M.; Torelli, M.D.; et al. Direct probes of 4 nm diameter gold nanoparticles interacting with supported lipid bilayers. J. Phys. Chem. C. 2015, 119, 534–546. [Google Scholar] [CrossRef]
- Vaezian, B.; Anderton, C.R.; Kraft, M.L. Discriminating and Imaging Different Phosphatidylcholine Species within Phase-Separated Model Membranes by Principal Component Analysis of TOF-Secondary Ion Mass Spectrometry Images. Anal. Chem. 2010, 82, 10006–10014. [Google Scholar] [CrossRef] [PubMed]
- Prinz, C.; Höök, F.; Malm, J.; Sjövall, P. Structural Effects in the Analysis of Supported Lipid Bilayers by Time-of-Flight Secondary Ion Mass Spectrometry. Langmuir 2007, 23, 8035–8041. [Google Scholar] [CrossRef] [PubMed]
- Kunze, A.; Sjövall, P.; Kasemo, B.; Svedhem, S. In Situ Preparation and Modification of Supported Lipid Layers by Lipid Transfer from Vesicles Studied by QCM-D and TOF-SIMS. J. Am. Chem. Soc. 2009, 131, 2450–2451. [Google Scholar] [CrossRef] [PubMed]
- Passarelli, M.K.; Winograd, N. Lipid Imaging with Time-of-Flight Secondary Ion Mass Spectrometry (ToF-SIMS). Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2011, 1811, 976–990. [Google Scholar] [CrossRef]
- Munem, M.; Zaar, O.; Dimovska Nilsson, K.; Neittaanmäki, N.; Paoli, J.; Fletcher, J.S. Chemical Imaging of Aggressive Basal Cell Carcinoma Using Time-of-Flight Secondary Ion Mass Spectrometry. Biointerphases 2018, 13, 03B402. [Google Scholar] [CrossRef]
- Dimovska Nilsson, K.; Neittaanmäki, N.; Zaar, O.; Angerer, T.B.; Paoli, J.; Fletcher, J.S. TOF-SIMS Imaging Reveals Tumor Heterogeneity and Inflammatory Response Markers in the Microenvironment of Basal Cell Carcinoma. Biointerphases 2020, 15, 041012. [Google Scholar] [CrossRef]
- Siljeström, S.; Parenteau, M.N.; Jahnke, L.L.; Cady, S.L. A Comparative ToF-SIMS and GC–MS Analysis of Phototrophic Communities Collected from an Alkaline Silica-Depositing Hot Spring. Org. Geochem. 2017, 109, 14–30. [Google Scholar] [CrossRef]
- Yang, K.; Han, X. Lipidomics: Techniques, Applications, and Outcomes Related to Biomedical Sciences. Trends Biochem. Sci. 2016, 41, 954–969. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Fletcher, J.S.; Rabbani, S.; Henderson, A.; Blenkinsopp, P.; Thompson, S.P.; Lockyer, N.P.; Vickerman, J.C. A New Dynamic in Mass Spectral Imaging of Single Biological Cells. Anal. Chem. 2008, 80, 9058–9064. [Google Scholar] [CrossRef]
- Angerer, T.B.; Dowlatshahi Pour, M.; Malmberg, P.; Fletcher, J.S. Improved Molecular Imaging in Rodent Brain with Time-of-Flight-Secondary Ion Mass Spectrometry Using Gas Cluster Ion Beams and Reactive Vapor Exposure. Anal. Chem. 2015, 87, 4305–4313. [Google Scholar] [CrossRef]
- Fransson, A.; Dimovska Nilsson, K.; Henderson, A.; Farewell, A.; Fletcher, J.S. PCA, PC-CVA, and Random Forest of GCIB-SIMS Data for the Elucidation of Bacterial Envelope Differences in Antibiotic Resistance Research. Anal. Chem. 2024, 96, 14168–14177. [Google Scholar] [CrossRef] [PubMed]
- Henderson, A. ChiToolbox. 2024. Available online: https://zenodo.org/records/10932517 (accessed on 19 September 2024).
- Sjövall, P.; Gregoire, S.; Wargniez, W.; Skedung, L.; Luengo, G.S. 3D Molecular Imaging of Stratum Corneum by Mass Spectrometry Suggests Distinct Distribution of Cholesteryl Esters Compared to Other Skin Lipids. Int. J. Mol. Sci. 2022, 23, 13799. [Google Scholar] [CrossRef] [PubMed]
- Heim, C.; Sjövall, P.; Lausmaa, J.; Leefmann, T.; Thiel, V. Spectral Characterisation of Eight Glycerolipids and Their Detection in Natural Samples Using Time-of-Flight Secondary Ion Mass Spectrometry. Rapid Commun. Mass Spectrom. 2009, 23, 2741–2753. [Google Scholar] [CrossRef] [PubMed]
- Adams, K.J.; DeBord, J.D.; Fernandez-Lima, F. Lipid Specific Molecular Ion Emission as a Function of the Primary Ion Characteristics in TOF-SIMS. J. Vac. Sci. Technol. B 2016, 34, 051084. [Google Scholar] [CrossRef]
- Keating, E.; Waring, A.J.; Walther, F.J.; Possmayer, F.; Veldhuizen, R.A.W.; Petersen, N.O. A ToF-SIMS Study of the Lateral Organization of Lipids and Proteins in Pulmonary Surfactant Systems. Biochim. Biophys. Acta (BBA)—Biomembr. 2011, 1808, 614–621. [Google Scholar] [CrossRef]
- Ren, J.; Li, H.-W.; Chen, L.; Zhang, M.; Liu, Y.-X.; Zhang, B.-W.; Xu, R.; Miao, Y.-Y.; Xu, X.-M.; Hua, X.; et al. Mass Spectrometry Imaging-Based Single-Cell Lipidomics Profiles Metabolic Signatures of Heart Failure. Research 2023, 6, 0019. [Google Scholar] [CrossRef]
- Cappuccio, G.; Khalil, S.M.; Osenberg, S.; Li, F.; Maletic-Savatic, M. Mass Spectrometry Imaging as an Emerging Tool for Studying Metabolism in Human Brain Organoids. Front. Mol. Biosci. 2023, 10, 1181965. [Google Scholar] [CrossRef]
- Sjövall, P.; Skedung, L.; Gregoire, S.; Biganska, O.; Clément, F.; Luengo, G.S. Imaging the Distribution of Skin Lipids and Topically Applied Compounds in Human Skin Using Mass Spectrometry. Sci. Rep. 2018, 8, 16683. [Google Scholar] [CrossRef]
- Starr, N.J.; Khan, M.H.; Edney, M.K.; Trindade, G.F.; Kern, S.; Pirkl, A.; Kleine-Boymann, M.; Elms, C.; O’Mahony, M.M.; Bell, M.; et al. Elucidating the Molecular Landscape of the Stratum Corneum. Proc. Natl. Acad. Sci. USA 2022, 119, e2114380119. [Google Scholar] [CrossRef]
- Slijkhuis, N.; Towers, M.; Mirzaian, M.; Korteland, S.A.; Heijs, B.; van Gaalen, K.; Nieuwenhuizen, I.; Nigg, A.; van der Heiden, K.; de Rijke, Y.B.; et al. Identifying Lipid Traces of Atherogenic Mechanisms in Human Carotid Plaque. Atherosclerosis 2023, 385, 117340. [Google Scholar] [CrossRef] [PubMed]
- Benabdellah, F.; Seyer, A.; Quinton, L.; Touboul, D.; Brunelle, A.; Laprévote, O. Mass Spectrometry Imaging of Rat Brain Sections: Nanomolar Sensitivity with MALDI versus Nanometer Resolution by TOF-SIMS. Anal. Bioanal. Chem. 2010, 396, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.B.; Lu, J.G.; Jiang, Z.H.; Zhang, W.; Li, W.J.; Qian, Z.M.; Bai, L.P. In Situ Chemical Profiling and Imaging of Cultured and Natural Cordyceps Sinensis by TOF-SIMS. Front. Chem. 2022, 10, 862007. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Zou, L.; Lin, Q.; Ong, C.N. Use of Liquid Chromatography/Tandem Mass Spectrometry and Online Databases for Identification of Phosphocholines and Lysophosphatidylcholines in Human Red Blood Cells. Rapid Commun. Mass Spectrom. 2009, 23, 3243–3254. [Google Scholar] [CrossRef]
- CHEBI:131441—Phosphatidylcholine 16:2. Available online: https://www.ebi.ac.uk/chebi/CHEBI:131441 (accessed on 29 October 2025).
- Konjevod, M.; Nedic Erjavec, G.; Nikolac Perkovic, M.; Sáiz, J.; Tudor, L.; Uzun, S.; Kozumplik, O.; Svob Strac, D.; Zarkovic, N.; Pivac, N. Metabolomics in Posttraumatic Stress Disorder: Untargeted Metabolomic Analysis of Plasma Samples from Croatian War Veterans. Free Radic. Biol. Med. 2021, 162, 636–641. [Google Scholar] [CrossRef]
- Philipsen, M.H.; Phan, N.T.N.; Fletcher, J.S.; Malmberg, P.; Ewing, A.G. Mass Spectrometry Imaging Shows Cocaine and Methylphenidate Have Opposite Effects on Major Lipids in Drosophila Brain. ACS Chem. Neurosci. 2018, 9, 1462–1468. [Google Scholar] [CrossRef]
- Hsu, F.F.; Turk, J. Characterization of Phosphatidylinositol, Phosphatidylinositol-4-Phosphate, and Phosphatidylinositol-4,5-Bisphosphate by Electrospray Ionization Tandem Mass Spectrometry: A Mechanistic Study. J. Am. Soc. Mass Spectrom. 2000, 11, 986–999. [Google Scholar] [CrossRef]
- Saud, Z.; Tyrrell, V.J.; Zaragkoulias, A.; Protty, M.B.; Statkute, E.; Rubina, A.; Bentley, K.; White, D.A.; Dos Santos Rodrigues, P.; Murphy, R.C.; et al. The SARS-CoV2 Envelope Differs from Host Cells, Exposes Procoagulant Lipids, and Is Disrupted in Vivo by Oral Rinses. J. Lipid Res. 2022, 63, 100208. [Google Scholar] [CrossRef]
- Jackson, S.N.; Wang, H.Y.J.; Woods, A.S. In Situ Structural Characterization of Glycerophospholipids and Sulfatides in Brain Tissue Using MALDI-MS/MS. J. Am. Soc. Mass Spectrom. 2007, 18, 17–26. [Google Scholar] [CrossRef]
- Bakker, B.; Eijkel, G.B.; Heeren, R.M.A.; Karperien, M.; Post, J.N.; Cillero-Pastor, B. Oxygen-Dependent Lipid Profiles of Three-Dimensional Cultured Human Chondrocytes Revealed by MALDI-MSI. Anal. Chem. 2017, 89, 9438–9444. [Google Scholar] [CrossRef]
- Gopalan, A.B.; van Uden, L.; Sprenger, R.R.; Marx, N.F.N.; Bogetofte, H.; Neveu, P.A.; Meyer, M.; Noh, K.M.; Diz-Muñoz, A.; Ejsing, C.S. Lipotype Acquisition during Neural Development Is Not Recapitulated in Stem Cell–Derived Neurons. Life Sci. Alliance 2024, 7, e202402622. [Google Scholar] [CrossRef] [PubMed]
- Marlet, F.R.; Muñoz, S.S.; Sotiraki, N.; Eliasen, J.N.; Woessmann, J.; Weicher, J.; Dreier, J.E.; Schoof, E.M.; Kohlmeier, K.A.; Maeda, K.; et al. Lipid Levels Correlate with Neuronal and Dopaminergic Markers during the Differentiation of SH-SY5Y Cells. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2024, 1870, 167212. [Google Scholar] [CrossRef] [PubMed]
- Osetrova, M.; Tkachev, A.; Mair, W.; Guijarro Larraz, P.; Efimova, O.; Kurochkin, I.; Stekolshchikova, E.; Anikanov, N.; Foo, J.C.; Cazenave-Gassiot, A.; et al. Lipidome Atlas of the Adult Human Brain. Nat. Commun. 2024, 15, 4455. [Google Scholar] [CrossRef] [PubMed]
- Symons, J.L.; Cho, K.J.; Chang, J.T.; Du, G.; Waxham, M.N.; Hancock, J.F.; Levental, I.; Levental, K.R. Lipidomic Atlas of Mammalian Cell Membranes Reveals Hierarchical Variation Induced by Culture Conditions, Subcellular Membranes, and Cell Lineages. Soft Matter 2021, 17, 288–297. [Google Scholar] [CrossRef]
- Fitzner, D.; Bader, J.M.; Penkert, H.; Bergner, C.G.; Su, M.; Weil, M.T.; Surma, M.A.; Mann, M.; Klose, C.; Simons, M. Cell-Type- and Brain-Region-Resolved Mouse Brain Lipidome. Cell Rep. 2020, 32, 108132. [Google Scholar] [CrossRef]
- Bhaduri, A.; Neumann, E.K.; Kriegstein, A.R.; Sweedler, J.V. Identification of Lipid Heterogeneity and Diversity in the Developing Human Brain. JACS Au 2021, 1, 2261–2270. [Google Scholar] [CrossRef]
- Guschina, I.; Millership, S.; O’Donnell, V.; Ninkina, N.; Harwood, J.; Buchman, V. Lipid Classes and Fatty Acid Patterns Are Altered in the Brain of γ-Synuclein Null Mutant Mice. Lipids 2010, 46, 121–130. [Google Scholar] [CrossRef]
- Berlin, E.; Lork, A.A.; Bornecrantz, M.; Ernst, C.; Phan, N.T.N. Lipid Organization and Turnover in the Plasma Membrane of Human Differentiating Neural Progenitor Cells Revealed by Time-of-Flight Secondary Ion Mass Spectrometry Imaging. Talanta 2024, 272, 125762. [Google Scholar] [CrossRef]
- Choi, J.; Yin, T.; Shinozaki, K.; Lampe, J.W.; Stevens, J.F.; Becker, L.B.; Kim, J. Comprehensive Analysis of Phospholipids in the Brain, Heart, Kidney, and Liver: Brain Phospholipids Are Least Enriched with Polyunsaturated Fatty Acids. Mol. Cell. Biochem. 2017, 442, 187–201. [Google Scholar] [CrossRef]
- Setou, M.; Kurabe, N. Mass Microscopy: High-Resolution Imaging Mass Spectrometry. J. Electron Microsc. 2011, 60, 47–56. [Google Scholar] [CrossRef]
- Kuge, H.; Akahori, K.; Yagyu, K.I.; Honke, K. Functional Compartmentalization of the Plasma Membrane of Neurons by a Unique Acyl Chain Composition of Phospholipids. J. Biol. Chem. 2014, 289, 26783–26793. [Google Scholar] [CrossRef] [PubMed]
- Guschina, I.A.; Ninkina, N.; Roman, A.; Pokrovskiy, M.V.; Buchman, V.L. Triple-Knockout, Synuclein-Free Mice Display Compromised Lipid Pattern. Molecules 2021, 26, 3078. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y. Novel Metabolism of Docosahexaenoic Acid in Neural Cells. J. Biol. Chem. 2007, 282, 18661–18665. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Khan, I.; Chaudhary, M.N.; Ali, K.; Mushtaq, A.; Jiang, B.; Zheng, L.; Pan, Y.; Hu, J.; Zou, X. Phosphatidylserine: A Comprehensive Overview of Synthesis, Metabolism, and Nutrition. Chem. Phys. Lipids 2024, 264, 105422. [Google Scholar] [CrossRef]
- Ma, X.; Li, X.; Wang, W.; Zhang, M.; Yang, B.; Miao, Z. Phosphatidylserine, Inflammation, and Central Nervous System Diseases. Front. Aging Neurosci. 2022, 14, 975176. [Google Scholar] [CrossRef]
- Dou, T.; Kurouski, D. Phosphatidylcholine and Phosphatidylserine Uniquely Modify the Secondary Structure of α-Synuclein Oligomers Formed in Their Presence at the Early Stages of Protein Aggregation. ACS Chem. Neurosci. 2022, 13, 2380–2385. [Google Scholar] [CrossRef]
- Ali, A.; Zhaliazka, K.; Dou, T.; Holman, A.P.; Kurouski, D. The Toxicities of A30P and A53T α-Synuclein Fibrils Can Be Uniquely Altered by the Length and Saturation of Fatty Acids in Phosphatidylserine. J. Biol. Chem. 2023, 299, 105383. [Google Scholar] [CrossRef]
- Custodia, A.; Aramburu-Núñez, M.; Correa-Paz, C.; Posado-Fernández, A.; Gómez-Larrauri, A.; Castillo, J.; Gómez-Muñoz, A.; Sobrino, T.; Ouro, A. Ceramide Metabolism and Parkinson’s Disease—Therapeutic Targets. Biomolecules 2021, 11, 945. [Google Scholar] [CrossRef]
- Abbott, S.K.; Li, H.; Muñoz, S.S.; Knoch, B.; Batterham, M.; Murphy, K.E.; Halliday, G.M.; Garner, B. Altered Ceramide Acyl Chain Length and Ceramide Synthase Gene Expression in Parkinson’s Disease. Mov. Disord. 2014, 29, 518–526. [Google Scholar] [CrossRef]
- Patel, D.; Witt, S.N. Ethanolamine and Phosphatidylethanolamine: Partners in Health and Disease. Oxidative Med. Cell. Longev. 2017, 2017, 4829180. [Google Scholar] [CrossRef]
- Luo, X.; Gong, H.B.; Gao, H.Y.; Wu, Y.P.; Sun, W.Y.; Li, Z.Q.; Wang, G.; Liu, B.; Liang, L.; Kurihara, H.; et al. Oxygenated Phosphatidylethanolamine Navigates Phagocytosis of Ferroptotic Cells by Interacting with TLR2. Cell Death Differ. 2021, 28, 1971–1989. [Google Scholar] [CrossRef] [PubMed]
- St Germain, M.; Iraji, R.; Bakovic, M. Phosphatidylethanolamine Homeostasis under Conditions of Impaired CDP-Ethanolamine Pathway or Phosphatidylserine Decarboxylation. Front. Nutr. 2023, 9, 1094273. [Google Scholar] [CrossRef] [PubMed]
- Blomqvist, M.; Zetterberg, H.; Blennow, K.; Månsson, J.E. Sulfatide in Health and Disease. The Evaluation of Sulfatide in Cerebrospinal Fluid as a Possible Biomarker for Neurodegeneration. Mol. Cell. Neurosci. 2021, 116, 103670. [Google Scholar] [CrossRef]
- Takahashi, T.; Suzuki, T. Role of Sulfatide in Normal and Pathological Cells and Tissues. J. Lipid Res. 2012, 53, 1437–1450. [Google Scholar] [CrossRef]
- Berntson, Z.; Hansson, E.; Rönnbäck, L.; Fredman, P. Intracellular Sulfatide Expression in a Subpopulation of Astrocytes in Primary Cultures. J. Neurosci. Res. 1998, 52, 559–568. [Google Scholar] [CrossRef]
- Pernber, Z.; Molander-Melin, M.; Berthold, C.H.; Hansson, E.; Fredman, P. Expression of the Myelin and Oligodendrocyte Progenitor Marker Sulfatide in Neurons and Astrocytes of Adult Rat Brain. J. Neurosci. Res. 2002, 69, 86–93. [Google Scholar] [CrossRef]
- Cockcroft, S. Mammalian Lipids: Structure, Synthesis and Function. Essays Biochem. 2021, 65, 813–845. [Google Scholar] [CrossRef]
- Uchiyama, Y.; Maxson, M.M.; Sawada, T.; Nakano, A.; Ewing, A.G. Phospholipid Mediated Plasticity in Exocytosis Observed in PC12 Cells. Brain Res. 2007, 1151, 46–54. [Google Scholar] [CrossRef]
- Rosenberger, T.A.; Oki, J.; Purdon, A.D.; Rapoport, S.I.; Murphy, E.J. Rapid Synthesis and Turnover of Brain Microsomal Ether Phospholipids in the Adult Rat. J. Lipid Res. 2002, 43, 59–68. [Google Scholar] [CrossRef]
- Xu, Z.; Byers, D.M.; Palmer, F.B.S.C.; Spence, M.W.; Cook, H.W. Limited Metabolic Interaction of Serine with Ethanolamine and Choline in the Turnover of Phosphatidylserine, Phosphatidylethanolamine and Plasmalogens in Cultured Glioma Cells. Biochim. Biophys. Acta (BBA)—Lipids Lipid Metab. 1993, 1168, 167–174. [Google Scholar] [CrossRef]
- van Echten-Deckert, G.; Herget, T. Sphingolipid Metabolism in Neural Cells. Biochim. Biophys. Acta (BBA)—Biomembr. 2006, 1758, 1978–1994. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Koshibu, K.; Rytz, A.; Giuffrida, F.; Sultan, S.; Patin, A.; Gaudin, M.; Tomezyk, A.; Steiner, P.; Schneider, N. Early Life to Adult Brain Lipidome Dynamic: A Temporospatial Study Investigating Dietary Polar Lipid Supplementation Efficacy. Front. Nutr. 2022, 9, 898655. [Google Scholar] [CrossRef]
- Malgat, M.; Maurice, A.; Baraud, J. Sphingomyelin and Ceramide-Phosphoethanolamine Synthesis by Microsomes and Plasma Membranes from Rat Liver and Brain. J. Lipid Res. 1987, 27, 251–260. [Google Scholar] [CrossRef]
- Michno, W.; Bowman, A.; Jha, D.; Minta, K.; Ge, J.; Koutarapu, S.; Zetterberg, H.; Blennow, K.; Lashley, T.; Heeren, R.M.A.; et al. Spatial Neurolipidomics at the Single Amyloid-β Plaque Level in Postmortem Human Alzheimer’s Disease Brain. ACS Chem. Neurosci. 2024, 15, 877–888. [Google Scholar] [CrossRef]
- Beger, A.W.; Dudzik, B.; Woltjer, R.L.; Wood, P.L. Human Brain Lipidomics: Pilot Analysis of the Basal Ganglia Sphingolipidome in Parkinson’s Disease and Lewy Body Disease. Metabolites 2022, 12, 187. [Google Scholar] [CrossRef]
- Térová, B.; Heczko, R.; Slotte, J.P. On the Importance of the Phosphocholine Methyl Groups for Sphingomyelin/Cholesterol Interactions in Membranes: A Study with Ceramide Phosphoethanolamine. Biophys. J. 2005, 88, 2661–2669. [Google Scholar] [CrossRef]
- Chen, T.C.; Hsu, W.L.; Wu, C.Y.; Lai, Y.R.; Chao, H.R.; Chen, C.H.; Tsai, M.H. Effect of Omega-6 Linoleic Acid on Neurobehavioral Development in Caenorhabditis Elegans. Prostaglandins Leukot. Essent. Fat. Acids 2023, 191, 102557. [Google Scholar] [CrossRef]
- Dighriri, I.M.; Alsubaie, A.M.; Hakami, F.M.; Hamithi, D.M.; Alshekh, M.M.; Khobrani, F.A.; Dalak, F.E.; Hakami, A.A.; Alsueaadi, E.H.; Alsaawi, L.S.; et al. Effects of Omega-3 Polyunsaturated Fatty Acids on Brain Functions: A Systematic Review. Cureus 2022, 14, e30091. [Google Scholar] [CrossRef]
- Heras-Sandoval, D.; Pedraza-Chaverri, J.; Pérez-Rojas, J.M. Role of Docosahexaenoic Acid in the Modulation of Glial Cells in Alzheimer’s Disease. J. Neuroinflammation 2016, 13, 61. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Fan, H.; He, J.; Wang, L.; Tian, Z.; Wang, C. Protective Effects of Omega-3 Fatty Acids against Alzheimer’s Disease in Rat Brain Endothelial Cells. Brain Behav. 2018, 8, e01037. [Google Scholar] [CrossRef] [PubMed]
- Belkind-Gerson, J.; Carreón-Rodríguez, A.; Contreras-Ochoa, C.O.; Estrada-Mondaca, S.; Parra-Cabrera, M.S. Fatty Acids and Neurodevelopment. J. Pediatr. Gastroenterol. Nutr. 2008, 47, S7–S9. [Google Scholar] [CrossRef] [PubMed]
- Dec, K.; Alsaqati, M.; Morgan, J.; Deshpande, S.; Wood, J.; Hall, J.; Harwood, A.J. A High Ratio of Linoleic Acid (n-6 PUFA) to Alpha-Linolenic Acid (n-3 PUFA) Adversely Affects Early Stage of Human Neuronal Differentiation and Electrophysiological Activity of Glutamatergic Neurons in Vitro. Front. Cell Dev. Biol. 2023, 11, 1166808. [Google Scholar] [CrossRef]
- Sheppard, K.W.; Cheatham, C.L. Omega-6 to Omega-3 Fatty Acid Ratio and Higher-Order Cognitive Functions in 7- to 9-y-Olds: A Cross-Sectional Study. Am. J. Clin. Nutr. 2013, 98, 659–667. [Google Scholar] [CrossRef]
- Gu, C.; Philipsen, M.H.; Ewing, A.G. Omega-3 and -6 Fatty Acids Alter the Membrane Lipid Composition and Vesicle Size to Regulate Exocytosis and Storage of Catecholamines. ACS Chem. Neurosci. 2024, 15, 816–826. [Google Scholar] [CrossRef]
- Ordóñez-Gutiérrez, L.; Fábrias, G.; Casas, J.; Wandosell, F. Diets with Higher ω-6/ω-3 Ratios Show Differences in Ceramides and Fatty Acid Levels Accompanied by Increased Amyloid-Beta in the Brains of Male APP/PS1 Transgenic Mice. Int. J. Mol. Sci. 2021, 22, 10907. [Google Scholar] [CrossRef]
- Rapoport, S.I.; Chang, M.C.J.; Spector, A.A. Delivery and Turnover of Plasma-Derived Essential PUFAs in Mammalian Brain. J. Lipid Res. 2001, 42, 678–685. [Google Scholar] [CrossRef]
- Novak, E.M.; Dyer, R.A.; Innis, S.M. High Dietary ω-6 Fatty Acids Contribute to Reduced Docosahexaenoic Acid in the Developing Brain and Inhibit Secondary Neurite Growth. Brain Res. 2008, 1237, 136–145. [Google Scholar] [CrossRef]
- Levental, K.R.; Malmberg, E.; Symons, J.L.; Fan, Y.Y.; Chapkin, R.S.; Ernst, R.; Levental, I. Lipidomic and Biophysical Homeostasis of Mammalian Membranes Counteracts Dietary Lipid Perturbations to Maintain Cellular Fitness. Nat. Commun. 2020, 11, 1339. [Google Scholar] [CrossRef]
- Carson, R.H.; Lewis, C.R.; Erickson, M.N.; Zagieboylo, A.P.; Naylor, B.C.; Li, K.W.; Farnsworth, P.B.; Price, J.C. Imaging Regiospecific Lipid Turnover in Mouse Brain with Desorption Electrospray Ionization Mass Spectrometry. J. Lipid Res. 2017, 58, 1884–1892. [Google Scholar] [CrossRef] [PubMed]
- Axelsen, P.H.; Murphy, R.C. Quantitative Analysis of Phospholipids Containing Arachidonate and Docosahexaenoate Chains in Microdissected Regions of Mouse Brain. J. Lipid Res. 2010, 51, 660–671. [Google Scholar] [CrossRef] [PubMed]
- Little, S.J.; Lynch, M.A.; Manku, M.; Nicolaou, A. Docosahexaenoic Acid-Induced Changes in Phospholipids in Cortex of Young and Aged Rats: A Lipidomic Analysis. Prostaglandins Leukot. Essent. Fat. Acids 2007, 77, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Sakayori, N.; Katakura, M.; Hamazaki, K.; Higuchi, O.; Fujii, K.; Fukabori, R.; Iguchi, Y.; Setogawa, S.; Takao, K.; Miyazawa, T.; et al. Maternal Dietary Imbalance between Omega-6 and Omega-3 Fatty Acids Triggers the Offspring’s Overeating in Mice. Commun. Biol. 2020, 3, 473. [Google Scholar] [CrossRef]
- Yavin, E.; Menkes, J.H. Effect of Temperature on Fatty Acid Metabolism in Dissociated Cell Cultures of Developing Brain. Pediatr. Res. 1974, 8, 263–269. [Google Scholar] [CrossRef]
- Bosch-Bouju, C.; Layé, S. Dietary Omega-6/Omega-3 and Endocannabinoids: Implications for Brain Health and Diseases. In Cannabinoids Health Disease; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef]
- Borasio, F.; De Cosmi, V.; D’Oria, V.; Scaglioni, S.; Syren, M.L.E.; Turolo, S.; Agostoni, C.; Coniglio, M.; Molteni, M.; Antonietti, A.; et al. Associations between Dietary Intake, Blood Levels of Omega-3 and Omega-6 Fatty Acids and Reading Abilities in Children. Biomolecules 2023, 13, 368. [Google Scholar] [CrossRef]
- Zock, P.L.; Mensink, R.P.; Harryvan, J.; De Vries, J.H.M.; Katan, M.B. Fatty Acids in Serum Cholesteryl Esters as Quantitative Biomarkers of Dietary Intake in Humans. Am. J. Epidemiol. 1997, 145, 1114–1122. [Google Scholar] [CrossRef]
- Corrigan, F.M.; Horrobin, D.F.; Skinner, E.R.; Besson, J.A.O.; Cooper, M.B. Abnormal Content of N−6 and N−3 Long-Chain Unsaturated Fatty Acids in the Phosphoglycerides and Cholesterol Esters of Parahippocampal Cortex from Alzheimer’s Disease Patients and Its Relationship to Acetyl CoA Content. Int. J. Biochem. Cell Biol. 1998, 30, 197–207. [Google Scholar] [CrossRef]
- Wood, P.L.; Medicherla, S.; Sheikh, N.; Terry, B.; Phillipps, A.; Kaye, J.A.; Quinn, J.F.; Woltjer, R.L. Targeted Lipidomics of Fontal Cortex and Plasma Diacylglycerols (DAG) in Mild Cognitive Impairment (MCI) and Alzheimer’s Disease: Validation of DAG Accumulation Early in the Pathophysiology of Alzheimer’s Disease. J. Alzheimers’s Dis. 2015, 48, 537–546. [Google Scholar] [CrossRef]
- Strosznajder, J. Incorporation of Linoleic Acid into Membrane Glycerophospholipids from Rat Brain Submitted to Ischemia and Hypoxia. Neurochem. Res. 1980, 5, 1265–1277. [Google Scholar] [CrossRef]
- Almena, M.; Mérida, I. Shaping up the Membrane: Diacylglycerol Coordinates Spatial Orientation of Signaling. Trends Biochem. Sci. 2011, 36, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Park, C.C.; Hennessey, T.; Ahmed, Z. Manipulation of Plasma Membrane Fatty Acid Composition of Fetal Rat Brain Cells Grown in a Serum-Free Denned Medium. J. Neurochem. 1990, 55, 1537–1545. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The Omega-6/Omega-3 Fatty Acid Ratio: Health Implications. Ol. Corps Gras Lipides 2010, 17, 267–275. [Google Scholar] [CrossRef]
- Kang, J.X.; Wan, J.B.; He, C. Concise Review: Regulation of Stem Cell Proliferation and Differentiation by Essential Fatty Acids and Their Metabolites. Stem Cells 2014, 32, 1092–1098. [Google Scholar] [CrossRef]
- Connor, W.E.; Neuringer, M.; Lin, D.S. Dietary Effects on Brain Fatty Acid Composition: The Reversibility of n-3 Fatty Acid Deficiency and Turnover of Docosahexaenoic Acid in the Brain, Erythrocytes, and Plasma of Rhesus Monkeys. J. Lipid Res. 1990, 31, 237–247. [Google Scholar] [CrossRef]
- Kim, H.Y.; Akbar, M.; Kim, Y.S. Phosphatidylserine-Dependent Neuroprotective Signaling Promoted by Docosahexaenoic Acid. Prostaglandins Leukot. Essent. Fat. Acids 2010, 82, 165–172. [Google Scholar] [CrossRef]
- Hamilton, J.; Greiner, R.; Salem, N.J.; Kim, H.Y. N-3 Fatty Acid Deficiency Decreases Phosphatidylserine Accumulation Selectively in Neuronal Tissues. Lipids 2000, 35, 863–869. [Google Scholar] [CrossRef]
- Kim, H.Y.; Huang, B.X.; Spector, A.A. Phosphatidylserine in the Brain: Metabolism and Function. Prog. Lipid Res. 2014, 56, 1–18. [Google Scholar] [CrossRef]
- Dyall, S.C. Long-Chain Omega-3 Fatty Acids and the Brain: A Review of the Independent and Shared Effects of EPA, DPA and DHA. Front. Aging Neurosci. 2015, 7, 52. [Google Scholar] [CrossRef]
- DeMar, J.C.; Lee, H.J.; Ma, K.; Chang, L.; Bell, J.M.; Rapoport, S.I.; Bazinet, R.P. Brain Elongation of Linoleic Acid Is a Negligible Source of the Arachidonate in Brain Phospholipids of Adult Rats. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2006, 1761, 1050–1059. [Google Scholar] [CrossRef]
- Gimenez da Silva-Santi, L.; Masetto Antunes, M.; Mori, M.A.; Biesdorf de Almeida-Souza, C.; Vergílio Visentainer, J.; Carbonera, F.; Rabello Crisma, A.; Nunes Masi, L.; Massao Hirabara, S.; Curi, R.; et al. Brain Fatty Acid Composition and Inflammation in Mice Fed with High-Carbohydrate Diet or High-Fat Diet. Nutrients 2018, 10, 1277. [Google Scholar] [CrossRef]
- Taha, A.Y.; Cheon, Y.; Ma, K.; Rapoport, S.I.; Rao, J.S. Altered Fatty Acid Concentrations in Prefrontal Cortex of Schizophrenic Patients. J. Psychiatr. Res. 2013, 47, 636–643. [Google Scholar] [CrossRef]
- Taha, A.Y.; Basselin, M.; Ramadan, E.; Modi, H.R.; Rapoport, S.I.; Cheon, Y. Altered Lipid Concentrations of Liver, Heart and Plasma but Not Brain in HIV-1 Transgenic Rats. Prostaglandins Leukot. Essent. Fat. Acids 2012, 87, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Gozlan-Devillierre, N.; Baumann, N.; Bourre, J.M. Incorporation of Stearic Acid Into Brain Lipids in the Developing Brain: Blood-Brain Relationships during Development. Developmental Neuroscience. Dev. Neurosci. 1978, 1, 153–158. [Google Scholar] [CrossRef]
- Gozlan-Devillierre, N.; Baumann, N.A.; Bourre, J.M. Mouse Brain Uptake and Metabolism of Stearic Acid. Biochimie 1976, 58, 1129–1133. [Google Scholar] [CrossRef] [PubMed]
- Morand, O.; Baumann, N.; Bourre, J.M. In Vivo Incorporation of Exogenous [1-14C]Stearic Acid into Neurons and Astrocytes. Neurosci. Lett. 1979, 13, 177–181. [Google Scholar] [CrossRef]
- Hussain, G.; Wang, J.; Rasul, A.; Anwar, H.; Imran, A.; Qasim, M.; Zafar, S.; Kamran, S.K.S.; Razzaq, A.; Aziz, N.; et al. Role of Cholesterol and Sphingolipids in Brain Development and Neurological Diseases. Lipids Health Dis. 2019, 18, 26. [Google Scholar] [CrossRef]
- Camdzic, M.; Aga, D.S.; Atilla-Gokcumen, G.E. Cellular Lipidome Changes during Retinoic Acid (RA)-Induced Differentiation in SH-SY5Y Cells: A Comprehensive In Vitro Model for Assessing Neurotoxicity of Contaminants. Environ. Health 2023, 1, 110–120. [Google Scholar] [CrossRef]
- Martínez, M.; Mougan, I. Fatty Acid Composition of Human Brain Phospholipids during Normal Development. J. Neurochem. 1998, 71, 2528–2533. [Google Scholar] [CrossRef]
- Knobloch, M.; Pilz, G.-A.; Ghesquière, B.; Kovacs, W.J.; Wegleiter, T.; Moore, D.L.; Hruzova, M.; Zamboni, N.; Carmeliet, P.; Jessberger, S. A Fatty Acid Oxidation-Dependent Metabolic Shift Regulates Adult Neural Stem Cell Activity. Cell Rep. 2017, 20, 2144–2155. [Google Scholar] [CrossRef]
- Wallis, T.P.; Venkatesh, B.G.; Narayana, V.K.; Kvaskoff, D.; Ho, A.; Sullivan, R.K.; Windels, F.; Sah, P.; Meunier, F.A. Saturated Free Fatty Acids and Association with Memory Formation. Nat. Commun. 2021, 12, 3443. [Google Scholar] [CrossRef]
- Schönfeld, P.; Wojtczak, L. Short- and Medium-Chain Fatty Acids in Energy Metabolism: The Cellular Perspective. J. Lipid Res. 2016, 57, 943–954. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).