Microbial Biotransformation of the Sesquiterpene Carotol: Generation of Hydroxylated Metabolites with Potential Cytotoxic and Target-Specific Binding Activities
Abstract
1. Introduction
2. Materials and Methods
2.1. Substrate Preparation
2.2. Microorganisms
2.3. General Experimental Procedure
2.4. Preparative HPLC
2.5. Screening Procedure
2.6. Preparative-Scale Fermentation
2.7. Single Crystal X-Ray Diffraction Analysis
2.8. Cytotoxicity Evaluation
2.9. Docking Study
2.10. Statistical Analysis
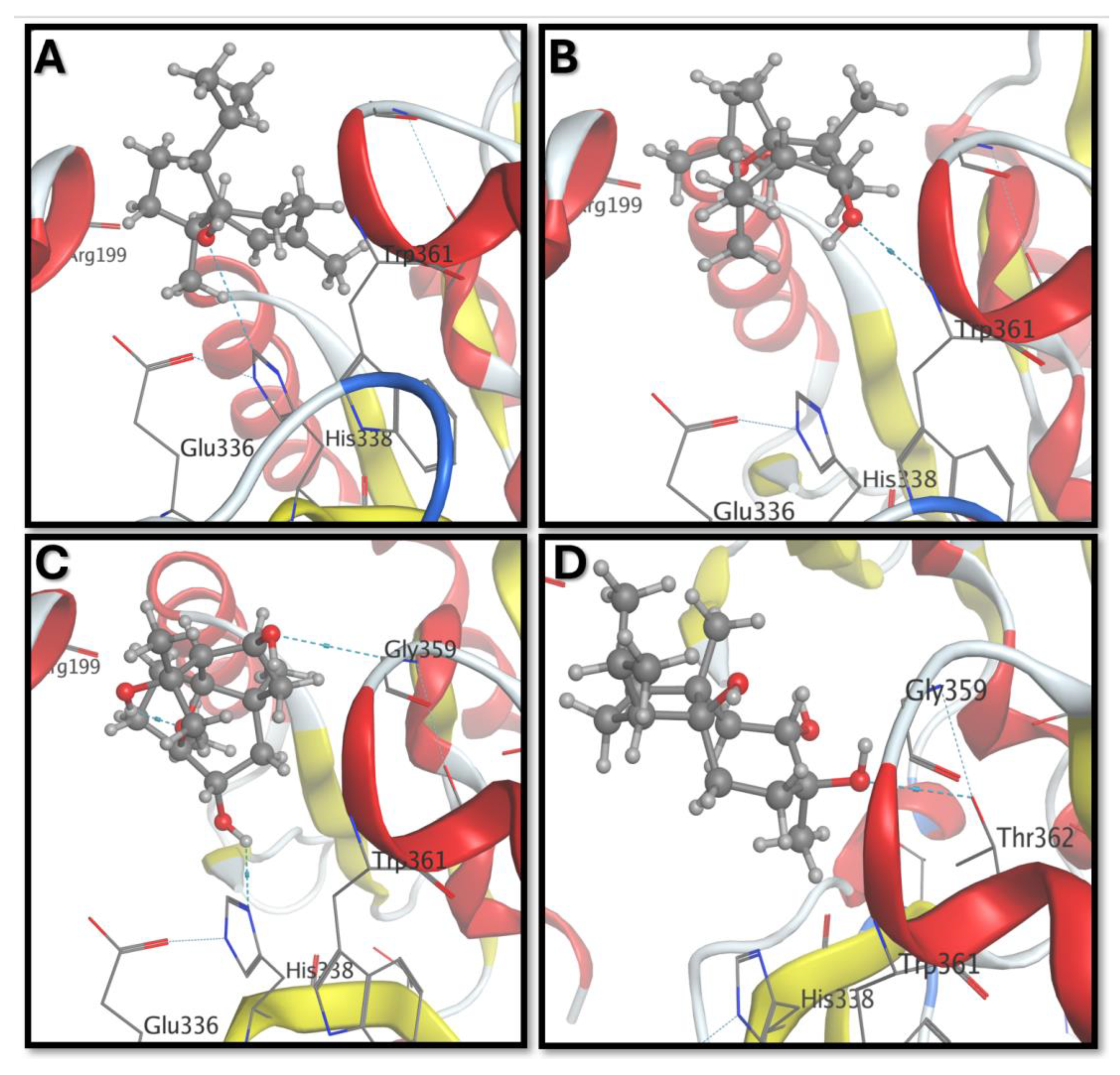
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| API | Active Pharmaceutical Ingredient |
| ATCC | American Type Culture Collection |
| AUMC | Assiut University Mycology Center |
| BChE | Butyrylcholinesterase |
| CM1,2,3 | Carotol metabolites 1, 2, and 3 |
| COX-2 | Cyclooxygenase-2 |
| COSY | Correlation Spectroscopy |
| DEPT | Distortionless Enhancement by Polarization Transfer |
| DMF | N,N-Dimethylformamide |
| EIHRMS | Electron Impact High-Resolution Mass Spectrometry |
| HPLC | High-Performance Liquid Chromatography |
| HSQC | Heteronuclear Single Quantum Coherence |
| HMBC | Heteronuclear Multiple Bond Correlation |
| IC50 | Half Maximal Inhibitory Concentration |
| IR | Infrared Spectroscopy |
| MD | Molecular Dynamic |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide |
| NRRL | Northern Regional Research Laboratories |
| PBS | Phosphate-Buffered Saline |
| PTFE | Polytetrafluoroethylene |
| RMSD | Root Mean Square Deviation |
| RMSF | Root Mean Square Fluctuations |
| TLC | Thin-Layer Chromatography |
| TMS | Tetramethylsilane |
| UV | Ultraviolet |
| XRD | X-Ray Diffraction |
References
- Simon, P.W. Carrot (Daucus carota L.) Breeding. In Advances in Plant Breeding Strategies: Vegetable Crops: Volume 8: Bulbs, Roots and Tubers; Al-Khayri, J.M., Jain, S.M., Johnson, D.V., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 213–238. [Google Scholar]
- Lone, S.; Narayan, S.; Hussain, K.; Malik, M.; Yadav, S.K.; Khan, F.A.; Safa, A.; Ahmad, A.; Masoodi, K.Z. Investigating the antioxidant and anticancer potential of Daucus spp. extracts against human prostate cancer cell line C4-2, and lung cancer cell line A549. J. Ethnopharmacol. 2025, 337, 118855. [Google Scholar] [CrossRef]
- Monica, S.J.; Jemima, D.; Daniel, E.L.; Selvaraju, P.; Preetha, M.A. Rajendran EGMG: Phytochemicals as Anticancer Agents: Investigating Molecular Pathways from Preclinical Research to Clinical Relevance. Curr. Res. Nutr. Food Sci. 2025, 13, 141–164. [Google Scholar] [CrossRef]
- Almuntashiri, S.A.; Alsubaie, F.F.; Alotaybi, M. Plant-Based Diets and Their Role in Preventive Medicine: A Systematic Review of Evidence-Based Insights for Reducing Disease Risk. Cureus 2025, 17, e78629. [Google Scholar] [CrossRef]
- Ahmad, T.; Cawood, M.; Iqbal, Q.; Ariño, A.; Batool, A.; Tariq, R.M.S.; Azam, M.; Akhtar, S. Phytochemicals in Daucus carota and Their Health Benefits—Review Article. Foods 2019, 8, 424. [Google Scholar] [CrossRef] [PubMed]
- Christensen, K.; Kotowska, D.; Olsen, L.; Bhattacharya, S.; Fretté, X.; Færgeman, N.; Kristiansen, K.; Oksbjerg, N.; Grevsen, K.; Christensen, L. Beneficial effects of carrots (Daucus carota) on adipocyte differentiation, glucose uptake, and fat accumulation. Planta Medica 2010, 76, P632. [Google Scholar] [CrossRef]
- Giraud-Robert, A.M. The role of aromatherapy in the treatment of viral hepatitis. Int. J. Aromather. 2005, 15, 183–192. [Google Scholar] [CrossRef]
- Patil, M.V.K.; Kandhare, A.D.; Bhise, S.D. Pharmacological evaluation of ethanolic extract of Daucus carota Linn root formulated cream on wound healing using excision and incision wound model. Asian Pac. J. Trop. Biomed. 2012, 2, S646–S655. [Google Scholar] [CrossRef]
- Shreyasi, S.; Sharma, M.; Sharma, S.; Bishnoi, H. Comparative study with extract of Daucus carota seeds and standard market product for wound healing potency. J. Adv. Sch. Res. Allied Educ. 2025, 22, 236–248. [Google Scholar] [CrossRef]
- Marzouki, H.; Khaldi, A.; Falconieri, D.; Piras, A.; Marongiu, B.; Molicotti, P.; Zanetti, S. Essential oils of Daucus carota subsp. carota of Tunisia obtained by supercritical carbon dioxide extraction. Nat. Prod. Commun. 2010, 5, 1955–1958. [Google Scholar] [CrossRef]
- Kumarasamy, Y.; Nahar, L.; Byres, M.; Delazar, A.; Sarker, S.D. The assessment of biological activities associated with the major constituents of the methanol extract of wild carrot (Daucus carota L.) seeds. J. Herb. Pharmacother. 2005, 5, 61–72. [Google Scholar]
- Sieniawska, E.; Świątek, L.; Rajtar, B.; Kozioł, E.; Polz-Dacewicz, M.; Skalicka-Woźniak, K. Carrot seed essential oil-Source of carotol and cytotoxicity study. Ind. Crops Prod. 2016, 92, 109–115. [Google Scholar] [CrossRef]
- Khan, I.; Abourashed, E. Leung’s Encyclopedia of Common Natural Ingredients Used in Food, Drugs, and Cosmetics, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 147–149. [Google Scholar]
- Mahboubi, M.; Kazempour, N.; Mahboubi, A. The efficacy of essential oils as natural preservatives in vegetable oil. J. Diet. Suppl. 2014, 11, 334–346. [Google Scholar] [CrossRef]
- Ali, A.; Radwan, M.M.; Wanas, A.S.; Khan, I.A. Repellent activity of carrot seed essential oil and its pure compound, carotol, against mosquitoes. J. Am. Mosq. Control Assoc. 2018, 34, 272–280. [Google Scholar] [CrossRef]
- Musnaini, M.; Fransisca, S.; Leslie, W. Effectiveness of cream formulation of carrot seed oil as anti-aging. Int. J. Health Pharm. (IJHP) 2022, 3, 331–340. [Google Scholar] [CrossRef]
- Manjunath, C.; Malpani, A.; Mahurkar, N. In vitro cytotoxicity of cardamom oil and carrot seed oil on mouse fibroblast and urinary bladder cell line. RGUHS J. Pharm. Sci. 2017, 7, 7–12. [Google Scholar]
- Alves-Silva, J.M.; Zuzarte, M.; Gonçalves, M.J.; Cavaleiro, C.; Cruz, M.T.; Cardoso, S.M.; Salgueiro, L. New Claims for Wild Carrot (Daucus carota subsp. carota) Essential Oil. Evid.-Based Complement. Altern. Med. 2016, 2016, 9045196. [Google Scholar] [CrossRef] [PubMed]
- Jasicka-Misiak, I.; Lipok, J.; Nowakowska, E.M.; Wieczorek, P.P.; Młynarz, P.; Kafarski, P. Antifungal activity of the carrot seed oil and its major sesquiterpene compounds. Z. Fur Naturforschung C J. Biosci. 2004, 59, 791–796. [Google Scholar] [CrossRef]
- Radulovic, N.; Ðordevic, N.; Stojanovic-Radic, Z. Volatiles of Balkan Endemic Daucus guttatus ssp: Zahariadii and cultivated and wild-growing D. carota- a comparison study. Food Chem. 2011, 125, 35–43. [Google Scholar] [CrossRef]
- Ozcan, M.M.; Chalchat, J.C. Chemical composition of carrot seeds (Daucus carota L.) cultivated in Turkey: Characterization of the seed oil and essential oil. Grasas Y Aceites 2007, 58, 359–365. [Google Scholar] [CrossRef]
- Chahal, K.; Kaur, P.; Kataria, D.; Kaur, R. Carotol: A sesquiterpenoid isolated from carrot seed oil. Asian J. Chem. 2016, 28, 1004–1006. [Google Scholar] [CrossRef]
- Dehghani, L.; Fattahi, M.; Ashrafi-Saeidlou, S. Morpho-phytochemical screening biological assessments of aerial parts of Iranian populations of wild carrot (Daucus carota, L. subsp. carota). Sci. Rep. 2025, 15, 12619. [Google Scholar] [CrossRef]
- Sharma, M.K.; Chahal, K.K.; Kaur, R.; Singh, R.; Kataria, D. Antifungal potential and structure activity relationship of carrot seed constituents. J. Food Biochem. 2019, 43, e12971. [Google Scholar] [CrossRef]
- Orabi, K.Y. Microbial Models of Mammalian Metabolism. Sampangines; Elsevier: New York, NY, USA, 2000. [Google Scholar]
- Loughlin, W.A. Biotransformations in Organic Synthesis. Bioresour. Technol. 2000, 74, 49–62. [Google Scholar] [CrossRef]
- Aminudin, N.; Ridzuan, M.; Susanti, D.; Abidin, Z.; Abidin, Z. Biotransformation of sesquiterpenoids: A recent insight. J. Asian Nat. Prod. Res. 2021, 24, 103–145. [Google Scholar] [CrossRef]
- Ponnapalli, M.G.; Sura, M.B.; Sudhakar, R.; Govindarajalu, G.; Sijwali, P.S. Biotransformation of artemisinin to 14-hydroxydeoxyartemisinin: C-14 hydroxylation by Aspergillus flavus. J. Agric. Food Chem. 2018, 66, 10490–10495. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Wu, Y.; Xu, F.; Bai, Y.; Guan, Y.; Williamson, J.S.; Liu, B. A novel dihydroxylated derivative of artemisinin from microbial transformation. Fitoterapia 2017, 120, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.F.; Abdel Bar, F.M.; Sallam, A.; Galala, A.A. New neuroprotective sesquiterpene lactate esters from carotol biotransformation. S. Afr. J. Bot. 2023, 153, 163–171. [Google Scholar] [CrossRef]
- Kataria, D.; Chahal, K.; Kumar, A. Chemical transformations of carotol isolated from carrot seed oil. Asian J. Chem. 2016, 28, 1790–1792. [Google Scholar] [CrossRef]
- Kula, J.; Bonikowski, R.; Staniszewska, M.; Krakowiak, A.; Wieczorek, M.; Majzner, R.; Bujacz, G. Transformation of Carotol into the Hydroindane-Derived Musk Odorant. Eur. J. Org. Chem. 2002, 2002, 1826–1829. [Google Scholar] [CrossRef]
- Hashidoko, Y.; Tahara, S.; Mizutani, J. Conversion of (+)-Carotol into (−)-Carota-1,4-dienaldehyde. Biosci. Biotechnol. Biochem. 1992, 56, 1892–1893. [Google Scholar] [CrossRef]
- Smith, R.V.; Rosazza, J.P. Microbial models of mammalian metabolism. J. Pharm. Sci. 1975, 64, 1737–1759. [Google Scholar] [CrossRef] [PubMed]
- Aslantürk, Ö.S. In Vitro Cytotoxicity Cell Viability Assays: Principles Advantages Disadvantages. In Genotoxicity—AP Redictable Risk to Our Actual World; Larramendy, M.L., Soloneski, S., Eds.; IntechOpen: London, UK, 2017. [Google Scholar]
- Pisani, P.; Berrino, F.; Macaluso, M.; Pastorino, U.; Crosignani, P.; Baldasseroni, A. Carrots, Green Vegetables and Lung Cancer: A Case-Control Study. Int. J. Epidemiol. 1986, 15, 463–468. [Google Scholar] [CrossRef]
- Chen, H.; Shao, F.; Zhang, F.; Miao, Q. Association between dietary carrot intake and breast cancer: A meta-analysis. Medicine 2018, 97, e12164. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Rui, L.; Kangcheng, S.; Jing-Xiang, W.; Xiao-Peng, G.; Zheng, L.; Xiaoyin, G.; Hailin, P.; Lei, C. Structure of human phagocyte NADPH oxidase in the resting state. eLife 2022, 11, e837432022. [Google Scholar] [CrossRef]
- Orabi, K.Y.; El-Feraly, F.S.; Al-Sulmy, W.A.; Al-Yahya, M.A. Biotransformation of Vulgarin. Mini Rev. Med. Chem. 2013, 13, 777–782. [Google Scholar] [CrossRef]
- ElGamal, R.A.; Galala, A.A.; Abdel-Kader, M.S.; Badria, F.A.; Soliman, A.F. Microbial Transformation of the Sesquiterpene Lactone, Vulgarin, by Aspergillus niger. Molecules 2023, 28, 3729. [Google Scholar] [CrossRef]
- Mishra, S.K.; Bae, Y.S.; Lee, Y.M.; Kim, J.S.; Oh, S.H.; Kim, H.M. Sesquiterpene Alcohol Cedrol Chemosensitizes Human Cancer Cells and Suppresses Cell Proliferation by Destabilizing Plasma Membrane Lipid Rafts. Front. Cell Dev. Biol. 2020, 8, 571676. [Google Scholar] [CrossRef]
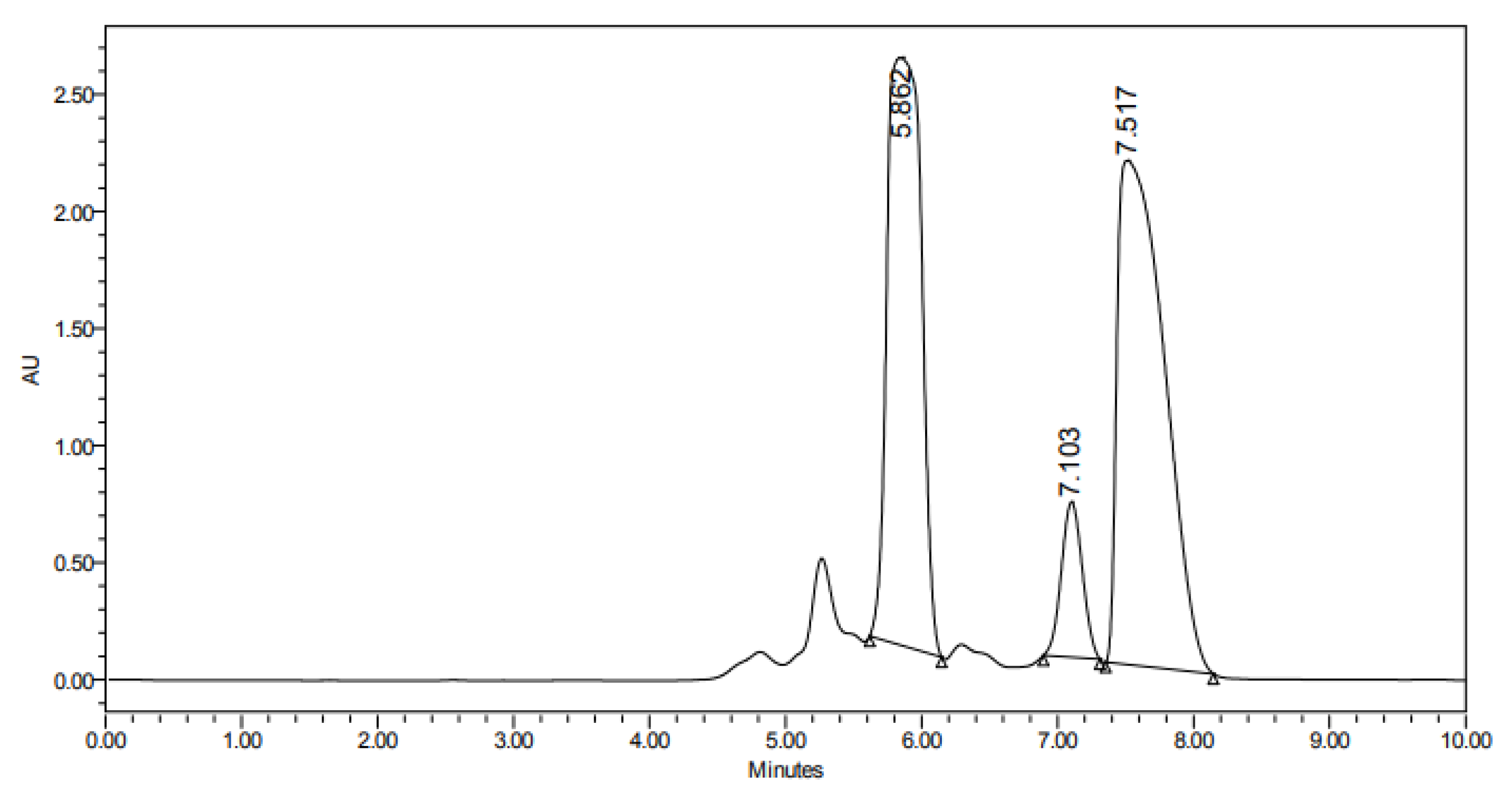
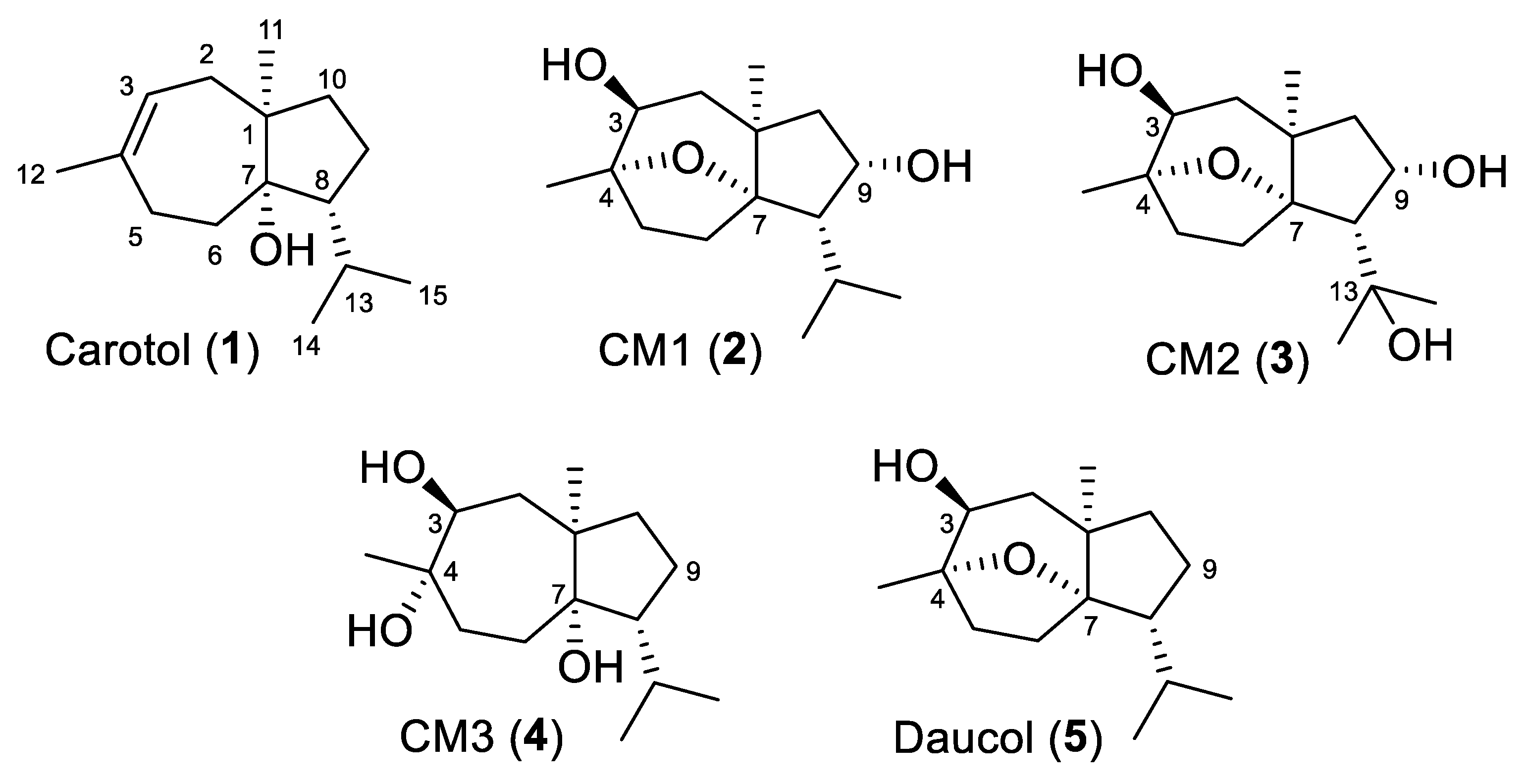
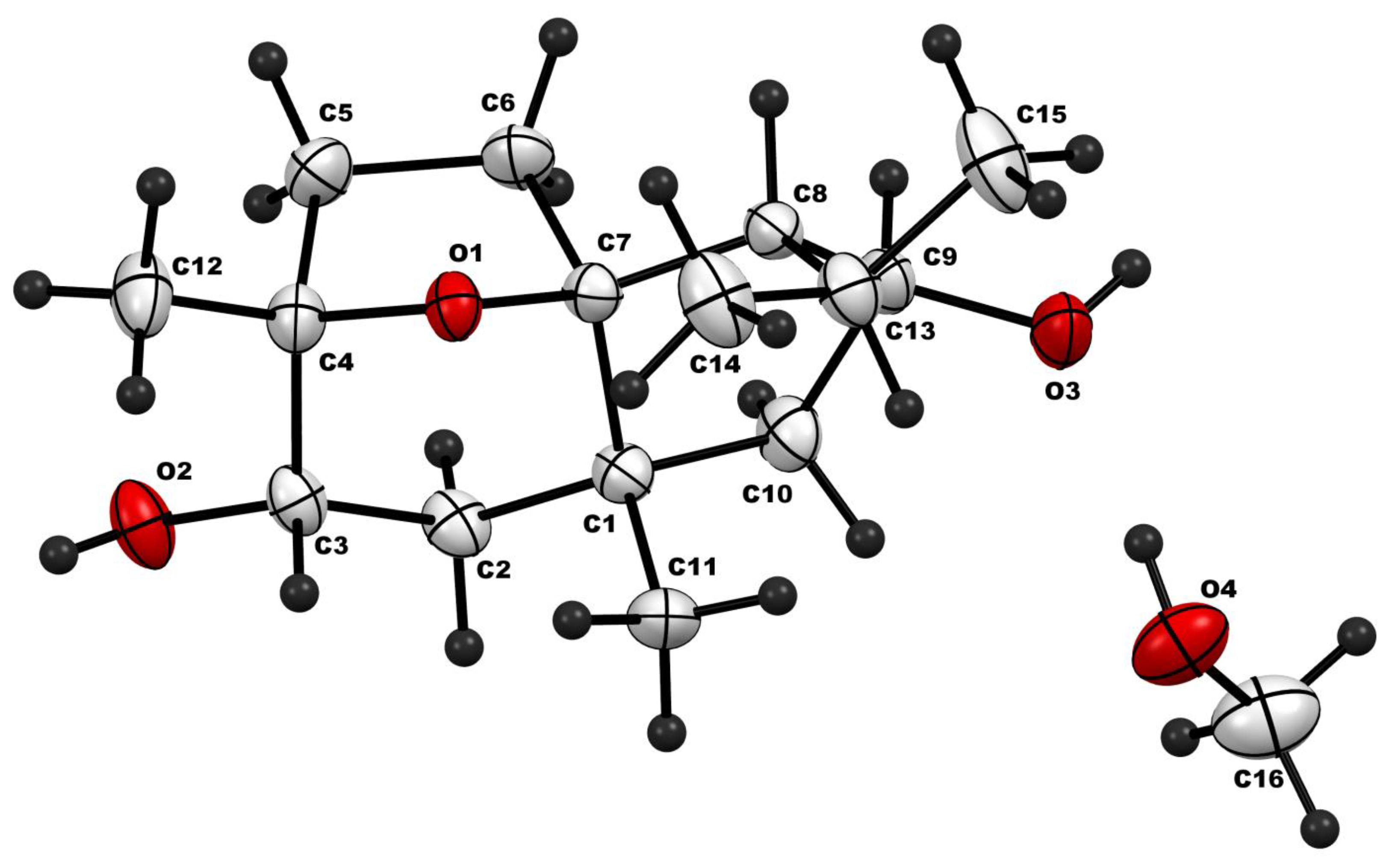
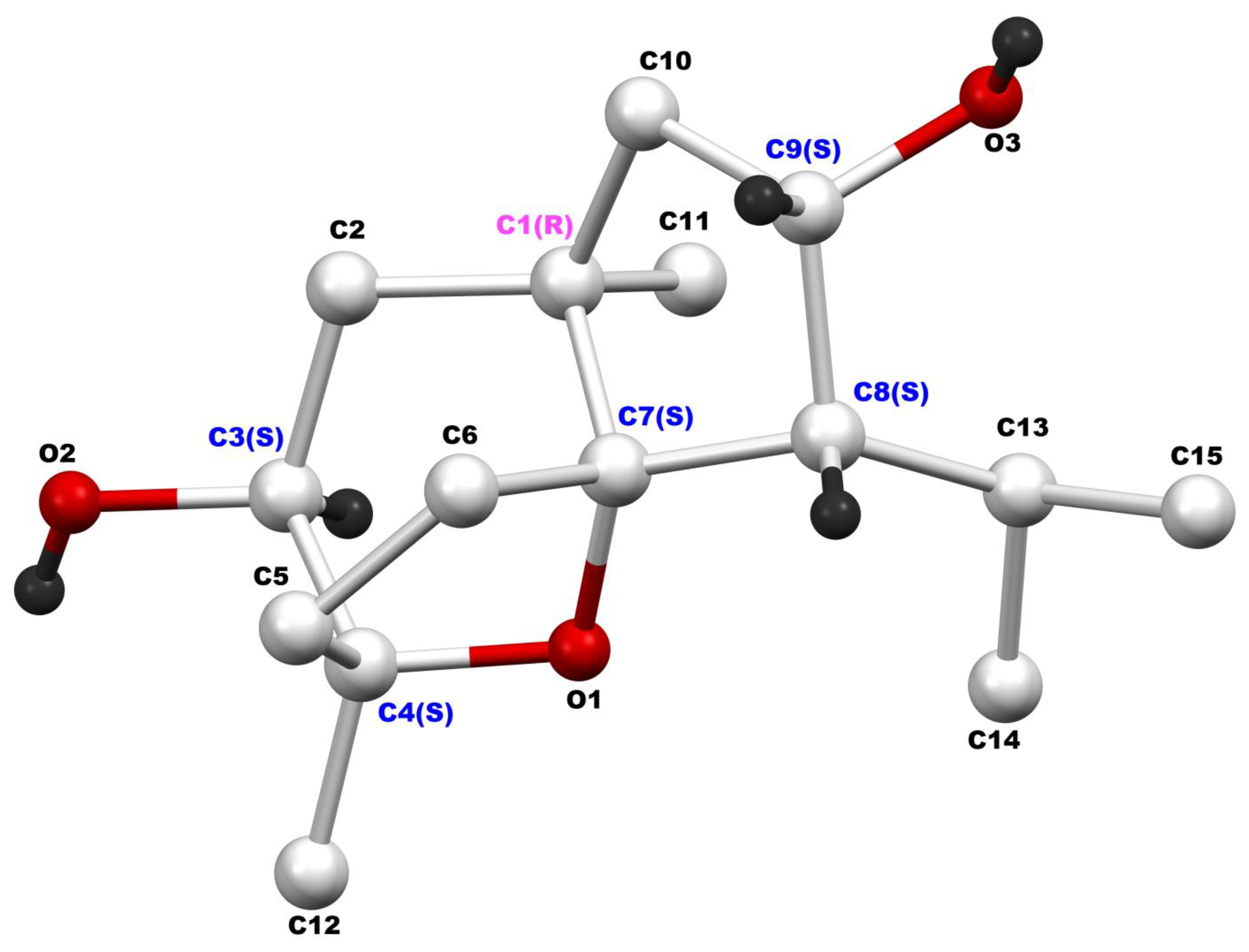

| ẟH, Multiplicity (J in Hz) | ||||
|---|---|---|---|---|
| Carotol | CM1 | CM2 | CM3 | |
| 2 | 1.73, dd (12.1, 8.4) 2.29, d (16.2) | 1.22, dd (12.0, 12.0) 1.75, dd (12.9, 6.0) | 1.79, dd (12.2, 5.6) 2.27, m | 1.39, m 1.55, dd (12.0, 10.2) |
| 3 | 5.35, d | 3.76, m | 3.85, m | 3.62, d (10.2) |
| 5 | 2.10, dd (5.4, 5.4) - | 1.34, dd (5.4, 1.2) 1.85, m | 1.35, m 1.92, m | 1.46, m 1.88, m |
| 6 | 1.65, m 1.97, m | 1.54, ddd (13.0, 13.0, 4.8) 2.14, m | 1.25, m 1.78, m | 1.68, m 1.74, m |
| 8 | 1.82, m | 1.85, m | 2.45, d (8.0) | 1.47, m |
| 9 | 1.52, m 1.67, m | 4.47, ddd (7.2, 7.2, 0.6) | 4.56, m | 1.45, m 1.63, m |
| 10 | 1.32, m 1.58, m | 1.45, dd (13.2, 0.6) 1.80, ddd (13.8, 7.2, 0.6) | 1.60, m 1.65, m | 1.47, m 1.93, m |
| 11 | 0.96, s | 1.36, s | 1.38, s | 1.01, s |
| 12 | 1.68, s | 1.30, s | 1.37, s | 1.24, s |
| 13 | 1.83, m | 2.10, m | - | 1.64, m |
| 14 | 1.02, d (6.6) | 1.04, d (6.0) | 1.47, s | 0.98, d (6.6) |
| 15 | 0.97, d (6.0) | 1.17, d (6.6) | 1.48, s | 0.90, d (6.6) |
| ẟc, Multiplicity a | ||||
|---|---|---|---|---|
| Carotol | CM1 | CM2 | CM3 | |
| 1 | 49.3, s | 44.8, s | 45.5, s | 47.5, s |
| 2 | 38.8, t | 40.8, t | 43.4, t | 38.7, t |
| 3 | 122.3, d | 71.5, d | 71.1, d | 75.4, d |
| 4 | 138.8, s | 85.6, s | 87.4, s | 75.5, s |
| 5 | 29.6, t | 29.2, t | 29.1, t | 35.5, t |
| 6 | 34.6, t | 41.7, t | 40.8, t | 41.3, t |
| 7 | 84.8, s | 92.6, s | 91.9, s | 83.6, s |
| 8 | 52.7, d | 58.8, d | 60.1, d | 56.6, d |
| 9 | 24.6, t | 73.3, d | 74.3, d | 26.5, t |
| 10 | 39.6, t | 45.4, t | 43.7, t | 34.1, t |
| 11 | 21.7, q | 23.1, q | 23.0, q | 25.9, q |
| 12 | 25.2, q | 24.1, q | 24.3, q | 24.4, q |
| 13 | 27.8, d | 29.4, d | 74.1, s | 28.9, d |
| 14 | 24.3, q | 26.0, q | 31.1, q | 23.5, q |
| 15 | 21.6, q | 22.7, q | 30.2, q | 21.8, q |
| Parameter | Value |
|---|---|
| Chemical formula | C16H30O4 |
| Mr | 286.40 |
| Crystal system, space group | Orthorhombic, P212121 |
| Temperature (K) | 150 |
| a, b, c (Å) | 6.4762 (3), 15.7204 (9), 15.9679 (9) |
| α, β, γ (°) | 90, 90, 90 |
| V (Å3) | 1625.67 (15) |
| Z | 4 |
| Radiation type | Cu Kα |
| µ (mm−1) | 0.66 |
| Crystal size (mm) | 0.21 × 0.19 × 0.15 |
| Diffractometer | Bruker APEX-II CCD |
| Absorption correction | Multi-scan: SADABS2016/2—Bruker AXS area detector scaling and absorption correction |
| Tmin, Tmax | 0.86, 0.91 |
| No. of measured, independent & observed [I > 2σ(I)] reflections | 10,554, 2864, 2713 |
| Rint | 0.037 |
| (sin θ/λ)max (Å−1) | 0.596 |
| R[F2 > 2σ(F2)], wR(F2), S | 0.037, 0.099, 1.08 |
| No. of reflections | 2864 |
| No. of parameters | 189 |
| H-atom treatment | Constrained |
| Δρmax, Δρmin (e Å−3) | 0.13−0.20 |
| Cell Line a | Carotol | CM1 | CM2 | CM3 | Cis-Platin | Doxorubicin |
|---|---|---|---|---|---|---|
| HepG-2 | 52.34 ± 4.23 b SI = 3.36 | 220.74 ± 12.26 a SI = 2.11 | 154.45 ± 9.39 a SI = 1.60 | 195.53 ± 10.57 a SI = 1.62 | 11.96 ± 1.23 c SI = 13.12 | 1.27 ± 0.20 d SI = 34.62 |
| HCT-116 | 25.68 ± 0.53 b SI = 6.85 | 339.38 ± 15.56 a SI = 1.37 | 180.64 ± 9.95 a SI = 1.37 | 226.38 ± 11.04 a SI = 1.40 | 17.96 ± 1.76 b SI = 8.74 | 2.99 ± 0.22 c SI = 14.66 |
| MCF-7 | 68.38 ± 6.04 b SI = 2.57 | 400.75 ± 20.28 a SI = 1.16 | 212.85 ± 13.72 a SI = 1.16 | 242.14 ± 15.68 a SI = 1.31 | 19.03 ± 2.10 c SI = 8.25 | 3.07 ± 0.18 d SI = 14.31 |
| A-549 | 28.65 ± 2.75 b SI = 6.14 | 225.38 ± 13.40 a SI = 2.07 | 138.21 ± 9.20 a SI = 1.79 | 205.24 ± 10.61 a SI = 1.54 | 24.53 ± 2.26 b SI = 6.40 | 3.31 ± 0.26 c SI = 12.71 |
| MRC-5 | 175.61 ± 10.93 ab | 466.17 ± 20.32 a | 247.62 ± 11.24 ab | 316.36 ± 15.17 a | 156.46 ± 6.48 ab | 43.96 ± 3.24 b |
| Compound | Free Energy of Binding (Kcal/mol) | RMSD (Å) | Interacted Residues |
|---|---|---|---|
| Carotol | −5.65 | 1.35 | His338, Trp361 |
| CM1 | −5.11 | 1.91 | Trp361 |
| CM2 | −5.41 | 1.50 | Gly359, His338 |
| CM3 | −5.16 | 1.85 | Thr362 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sary, H.G.; Khedr, M.A.; Radwan, M.M.; Vinodh, M.; Orabi, K.Y. Microbial Biotransformation of the Sesquiterpene Carotol: Generation of Hydroxylated Metabolites with Potential Cytotoxic and Target-Specific Binding Activities. Biomolecules 2025, 15, 1651. https://doi.org/10.3390/biom15121651
Sary HG, Khedr MA, Radwan MM, Vinodh M, Orabi KY. Microbial Biotransformation of the Sesquiterpene Carotol: Generation of Hydroxylated Metabolites with Potential Cytotoxic and Target-Specific Binding Activities. Biomolecules. 2025; 15(12):1651. https://doi.org/10.3390/biom15121651
Chicago/Turabian StyleSary, Hanan G., Mohammed A. Khedr, Mohamed M. Radwan, Mickey Vinodh, and Khaled Y. Orabi. 2025. "Microbial Biotransformation of the Sesquiterpene Carotol: Generation of Hydroxylated Metabolites with Potential Cytotoxic and Target-Specific Binding Activities" Biomolecules 15, no. 12: 1651. https://doi.org/10.3390/biom15121651
APA StyleSary, H. G., Khedr, M. A., Radwan, M. M., Vinodh, M., & Orabi, K. Y. (2025). Microbial Biotransformation of the Sesquiterpene Carotol: Generation of Hydroxylated Metabolites with Potential Cytotoxic and Target-Specific Binding Activities. Biomolecules, 15(12), 1651. https://doi.org/10.3390/biom15121651






