Biomimetic Salivary Gland Cancer Spheroid Platform for In Vitro Recapitulation of Three-Dimensional Tumor–Stromal Interactions
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication of Decellularized Matrices
2.2. Assembly of A253 and SGT Spheroids
2.3. Imaging
2.4. Dual-Color Labeling of Co-Culture Spheroids
2.5. Immunofluorescence Staining
2.6. Nucleic Acid and Protein Extraction
2.7. Quantitative Real-Time RT-PCR (qRT-PCR)
2.8. Morphological Characterization of Decellularized Matrices
2.9. Calcein AM/Propidium Iodide (PI) Co-Staining of Spheroids
2.10. Liquid Chromatography-Mass Spectrometry (LC-MS) Analysis of Decellularized Matrices
2.11. Transcriptome Sequencing and Functional Analysis
2.12. Metabolic Activity Using 2-(N-(7-Nitrobenz-2-oxa-1,3-diazol-4-yl) Amino)-2-Deoxyglucose (2-NBDG) Uptake Assay
2.13. Caspase-3/7 Assay for Anticancer Activity
2.14. Pharmacologic Inhibition of FAK and EGFR Signaling
2.15. Statistical Analysis
3. Results and Discussion
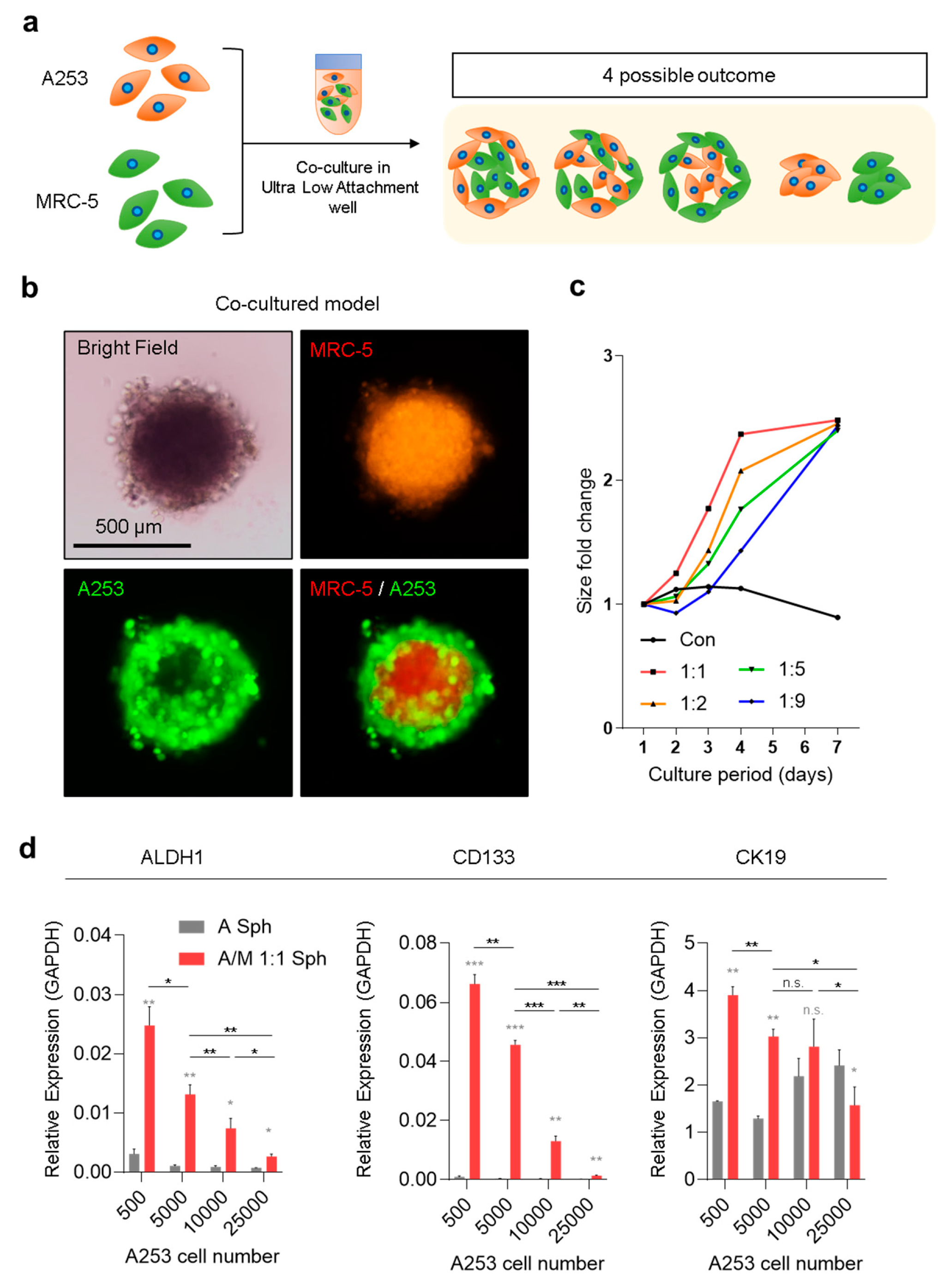
3.1. Spatial Organization of A253/MRC-5 Co-Culture Spheroids and Its Impact on Stemness
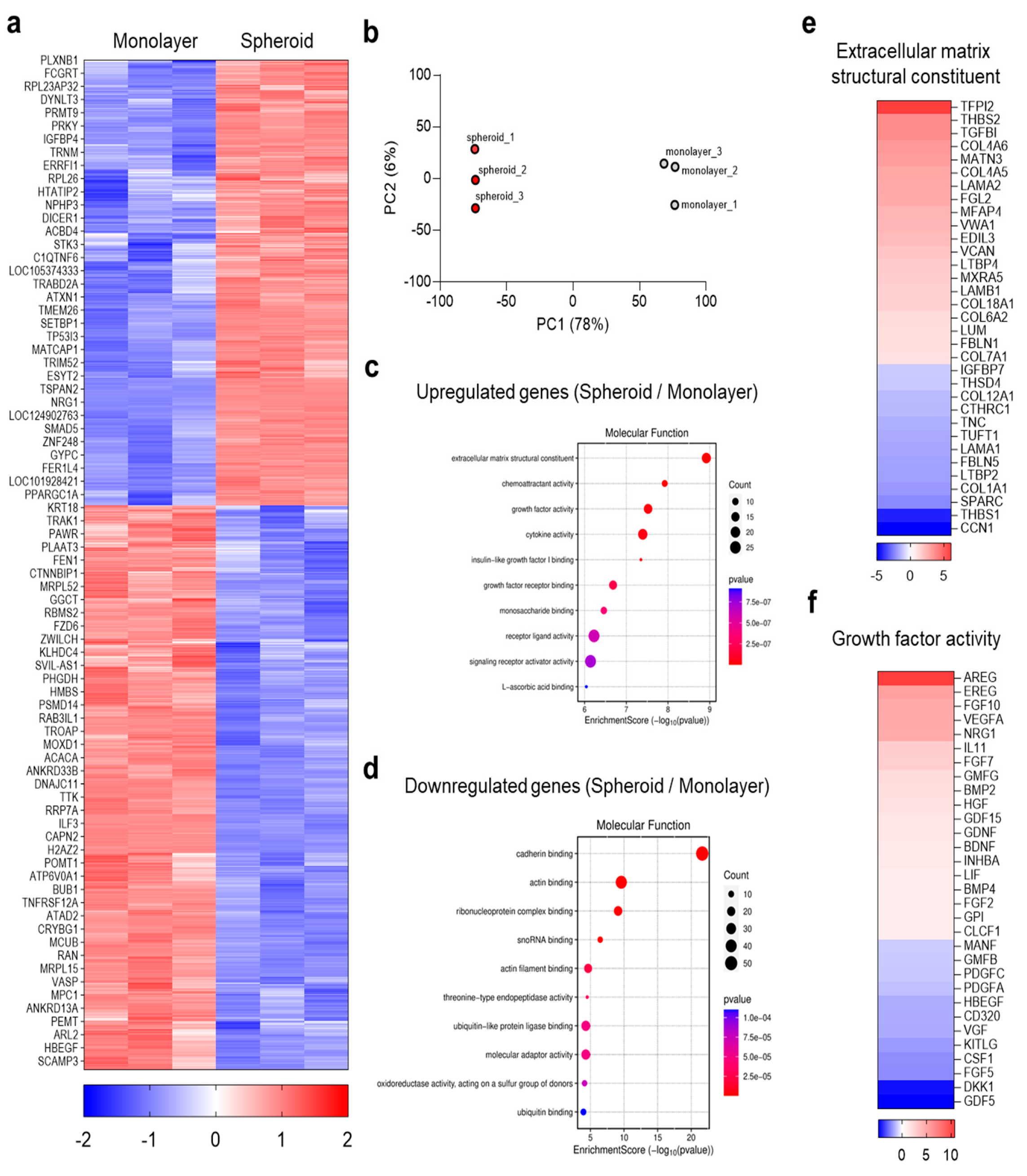
3.2. Global Transcriptomic Analysis of MRC-5 Spheroids
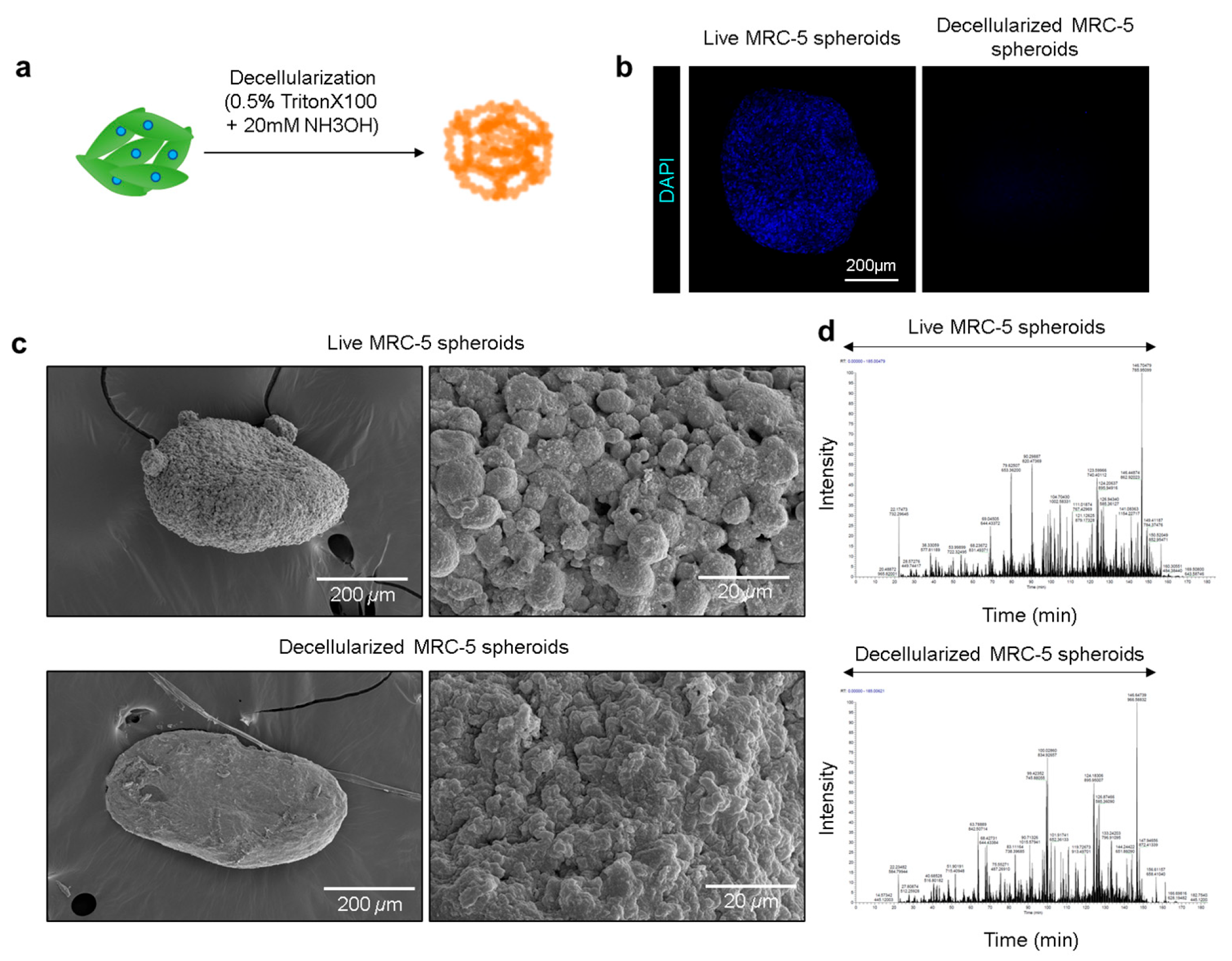
3.3. Generation and Characterization of Decellularized MRC-5 Spheroids
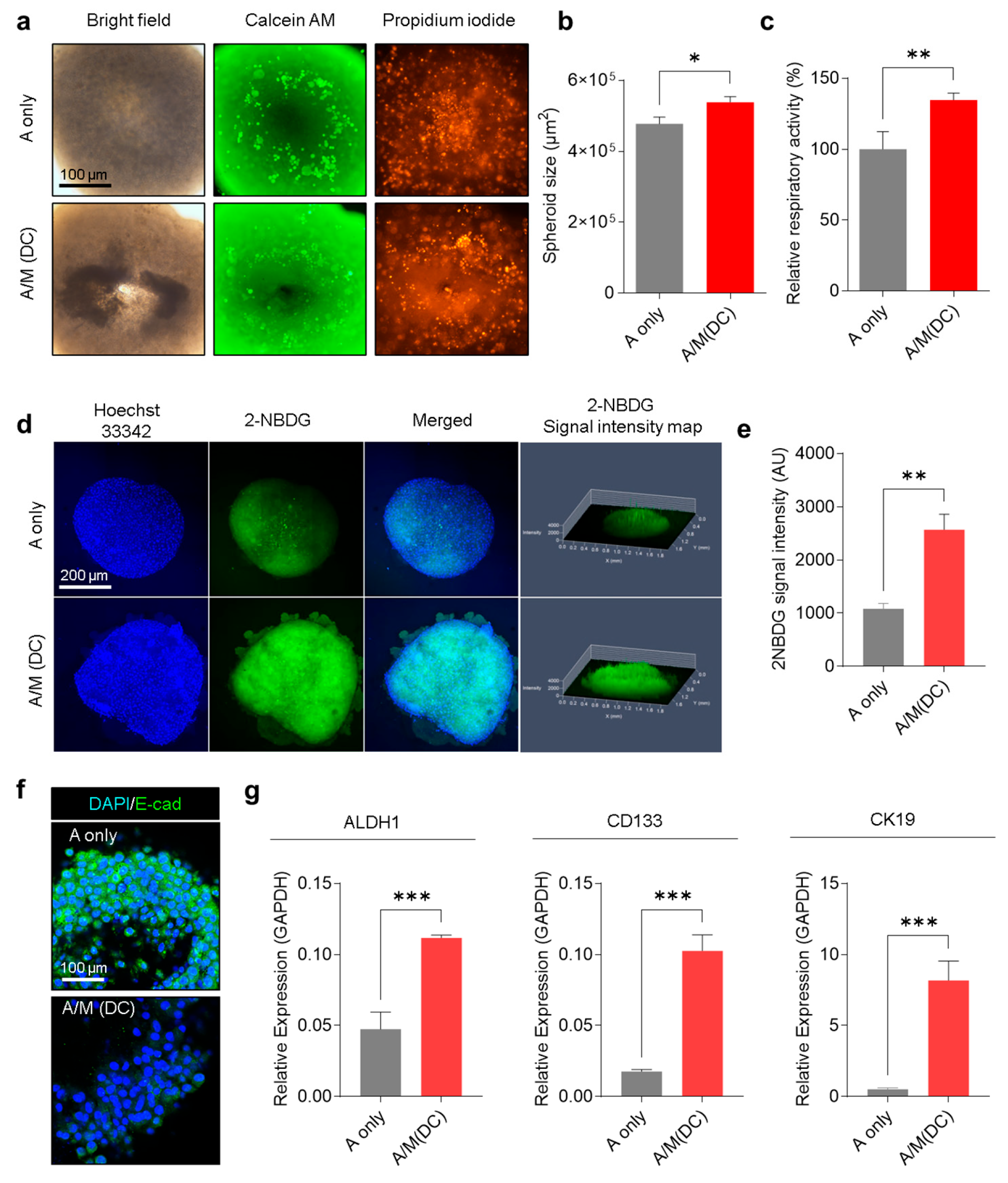
3.4. Physiological Characterization of A253 Cells Cultured with Decellularized MRC-5 Spheroid Scaffolds
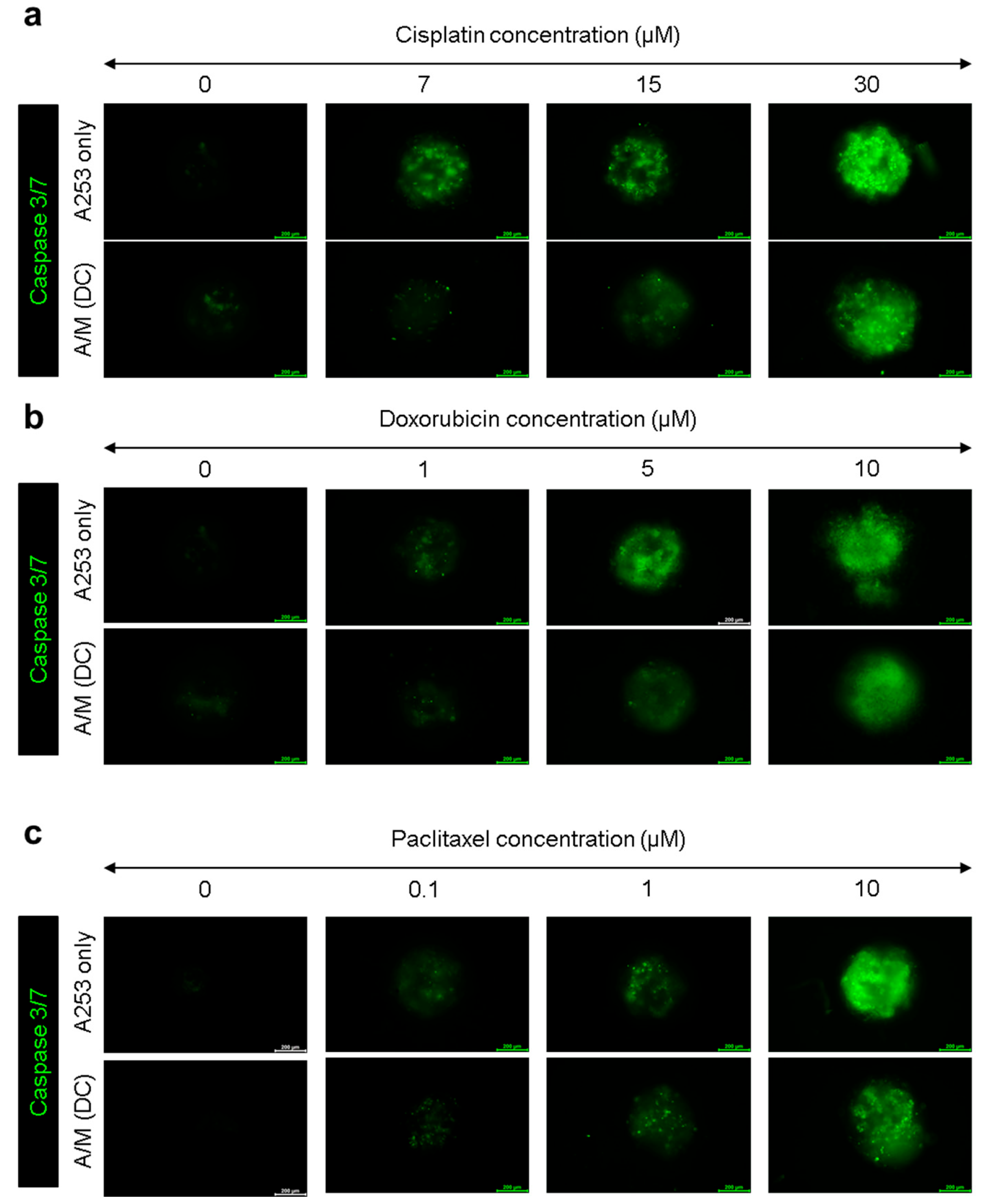
3.5. Evaluation of Chemotherapeutic Resistance in A253 Spheroids Cultured with Decellularized MRC-5 Spheroid Scaffolds
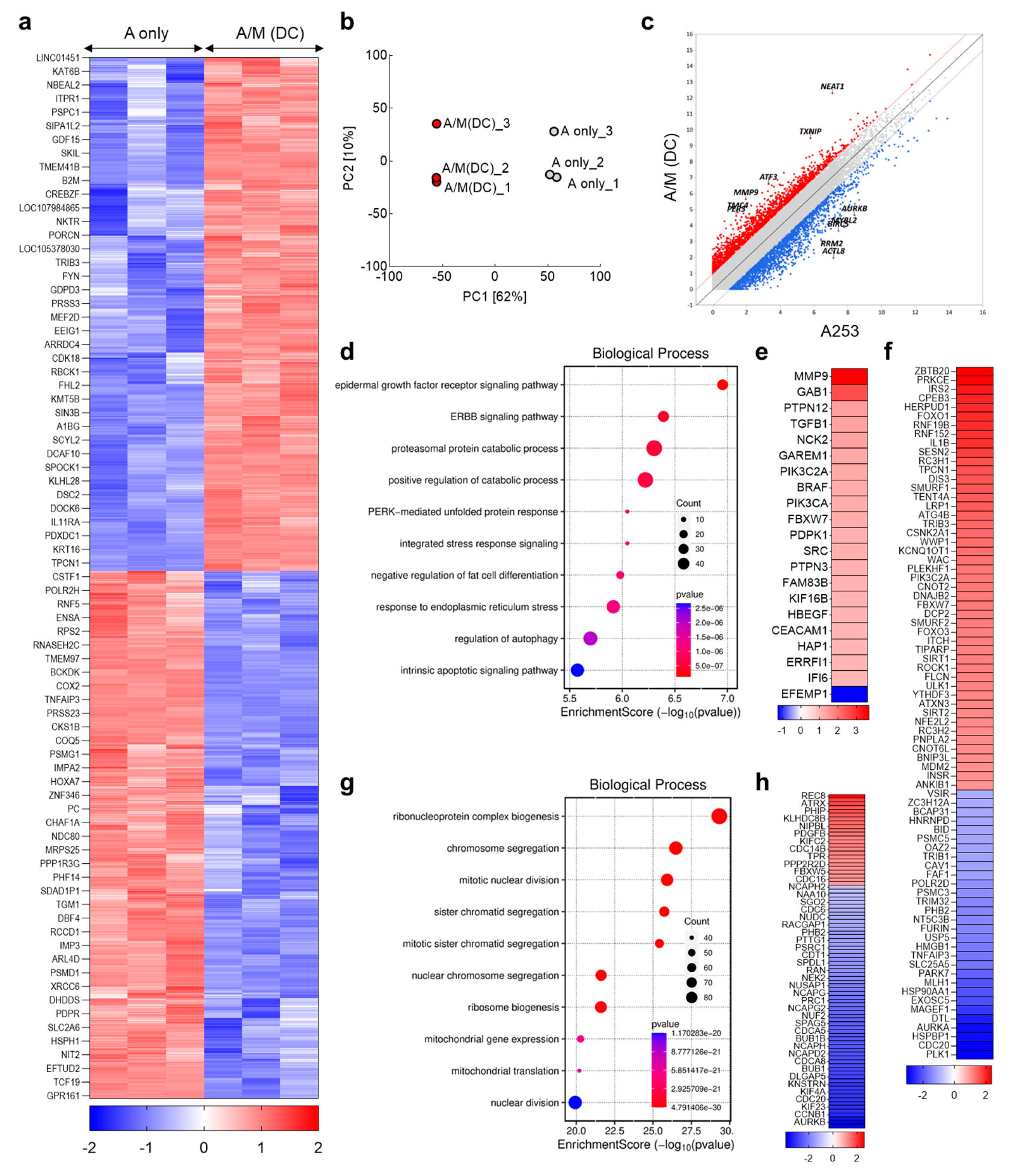
3.6. Transcriptomic Reprogramming of A253 Spheroids Cultured with Decellularized MRC-5 Spheroid Scaffolds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 2D | Two-dimensional |
| 2-NBDG | 2-(N-(7-Nitrobenz-2-oxa-1,3-diazol-4-yl) Amino)-2-Deoxyglucose |
| 3D | Three-dimensional |
| ABC | Ammonium bicarbonate |
| ALDH1 | Aldehyde dehydrogenase 1 |
| ANOVA | Analysis of variance |
| ATCC | American type culture collection |
| AURKA | Aurora kinase A |
| CAF | Cancer-associated fibroblasts |
| CCK-8 | Cell counting kit-8 |
| cDNA | Complementary DNA |
| CK19 | Cytokeratin 19 |
| CLSM | Confocal Laser Scanning Microscope |
| CSC | Cancer stem cell |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DEGs | Differentially expressed genes |
| DMEM | Dulbecco’s Modified Eagle Medium |
| ECM | Extracellular matrix |
| EMT | Epithelial–mesenchymal transition |
| FASP | Filter-Aided Sample Preparation |
| FBS | Fetal bovine serum |
| FE-SEM | Field-emission Scanning Electron Microscopy |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| GO | Gene Ontology |
| IAA | Iodoacetic acid |
| IGF | Insulin-like Growth Factor |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| LC-MS | Liquid Chromatography–Mass Spectrometry |
| MRC-5 | Medical Research Council Cell Strain 5 |
| NCE | Normalized Collisional Energy |
| NDS | Normal Donkey Serum |
| NH3OH | Ammonium hydroxide |
| PCA | Principal Component Analysis |
| PI | Propidium iodide |
| qRT-PCR | Quantitative real-time polymerase chain reaction |
| RIPA | Radioimmunoprecipitation assay |
| SD | Standard deviation |
| SGC | Salivary gland carcinoma |
| TCEP | Tris(2-carboxyethyl)phosphine |
| TME | Tumor microenvironment |
| ULA | Ultra-low attachment |
References
- Geiger, J.L.; Ismaila, N.; Beadle, B.; Caudell, J.J.; Chau, N.; Deschler, D.; Glastonbury, C.; Kaufman, M.; Lamarre, E.; Lau, H.Y.; et al. Management of Salivary Gland Malignancy: ASCO Guideline. J. Clin. Oncol. 2021, 39, 1909–1941. [Google Scholar] [CrossRef]
- Laurie, S.A.; Licitra, L. Systemic therapy in the palliative management of advanced salivary gland cancers. J. Clin. Oncol. 2006, 24, 2673–2678. [Google Scholar] [CrossRef]
- Rodriguez, C.P.; Wu, Q.V.; Voutsinas, J.; Fromm, J.R.; Jiang, X.; Pillarisetty, V.G.; Lee, S.M.; Santana-Davila, R.; Goulart, B.; Baik, C.S.; et al. A Phase II Trial of Pembrolizumab and Vorinostat in Recurrent Metastatic Head and Neck Squamous Cell Carcinomas and Salivary Gland Cancer. Clin. Cancer Res. 2020, 26, 837–845. [Google Scholar] [CrossRef]
- Onaga, R.; Enokida, T.; Ito, K.; Ueda, Y.; Okano, S.; Fujisawa, T.; Wada, A.; Sato, M.; Tanaka, H.; Takeshita, N.; et al. Combination chemotherapy with taxane and platinum in patients with salivary gland carcinoma: A retrospective study of docetaxel plus cisplatin and paclitaxel plus carboplatin. Front. Oncol. 2023, 13, 1185198. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, K.; Imanishi, Y.; Tada, Y.; Kawakita, D.; Kano, S.; Tsukahara, K.; Shimizu, A.; Ozawa, H.; Okami, K.; Sakai, A.; et al. Clinical Outcomes and Prognostic Factors for Salivary Duct Carcinoma: A Multi-Institutional Analysis of 141 Patients. Ann. Surg. Oncol. 2016, 23, 2038–2045. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 2016, 16, 582–598. [Google Scholar] [CrossRef]
- Hanahan, D.; Coussens, L.M. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell 2012, 21, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Monteran, L.; Erez, N. The Dark Side of Fibroblasts: Cancer-Associated Fibroblasts as Mediators of Immunosuppression in the Tumor Microenvironment. Front. Immunol. 2019, 10, 1835. [Google Scholar] [CrossRef]
- Polak, R.; Zhang, E.T.; Kuo, C.J. Cancer organoids 2.0: Modelling the complexity of the tumour immune microenvironment. Nat. Rev. Cancer 2024, 24, 523–539. [Google Scholar] [CrossRef]
- Lazzari, G.; Nicolas, V.; Matsusaki, M.; Akashi, M.; Couvreur, P.; Mura, S. Multicellular spheroid based on a triple co-culture: A novel 3D model to mimic pancreatic tumor complexity. Acta Biomater. 2018, 78, 296–307. [Google Scholar] [CrossRef]
- Ahvaraki, A.; Gheytanchi, E.; Behroodi, E.; Latifi, H.; Vakhshiteh, F.; Bagheri, Z.; Madjd, Z. Advanced co-culture 3D breast cancer model to study cell death and nanodrug sensitivity of tumor spheroids. Biochem. Eng. J. 2024, 209. [Google Scholar] [CrossRef]
- Xu, R.; Zhou, X.; Wang, S.; Trinkle, C. Tumor organoid models in precision medicine and investigating cancer-stromal interactions. Pharmacol. Ther. 2021, 218, 107668. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Sun, L.; Lu, M.; Zhao, Y. Biomimetic gland models with engineered stratagems. Research 2023, 6, 0232. [Google Scholar] [CrossRef]
- Nayak, P.; Bentivoglio, V.; Varani, M.; Signore, A. Three-dimensional in vitro tumor spheroid models for evaluation of anticancer therapy: Recent updates. Cancers 2023, 15, 4846. [Google Scholar] [CrossRef]
- Brancato, V.; Communanza, V.; Imparato, G.; Cora, D.; Urciuolo, F.; Noghero, A.; Bussolino, F.; Netti, P.A. Bioengineered tumoral microtissues recapitulate desmoplastic reaction of pancreatic cancer. Acta Biomater. 2017, 49, 152–166. [Google Scholar] [CrossRef]
- Kuroda, M.; Komatsu, N.; Kosai, A.; Hamakubo, T.; Abe, T. Anti-EGFR antibody immunotoxins improve cytotoxic effects in the salivary gland cancer A253 cell line. J. Oral. Maxillofac. Surg. 2025, 37, 450–454. [Google Scholar] [CrossRef]
- Bea, J.Y.; L, S.W.; Shin, Y.H.; Lee, J.H.; Jahng, J.W.; Park, K. P2X7 receptor and NLRP3 inflammasome activation in head and neck cancer. Oncotarget 2017, 8, 48972. [Google Scholar] [CrossRef]
- Shrestha, P.; Sumitomo, S.; Lee, C.H.; Nagahara, K.; Kamegai, A.; Yamanaka, Y.; Yakeuchi, H.; Kusakabe, M.; Mori, M. Tenascin: Growth and adhesion modulation—Extracellular matrix degrading function: And in vitro study. Eur. J. Cancer B Oral. Oncol. 1996, 32, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Yoon, H.E.; Kim, D.J.; Kim, S.A.; Ahn, S.G.; Yoon, J.H. Toll-like receptor 5 activation promotes migration and invasion of salivary gland adenocarcinoma. J. Oral. Pathol. Med. 2011, 40, 187–193. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Lee, J.H.; Shin, Y.; Chung, S.; Kuh, H.J. Co-Culture of Tumor Spheroids and Fibroblasts in a Collagen Matrix-Incorporated Microfluidic Chip Mimics Reciprocal Activation in Solid Tumor Microenvironment. PLoS ONE 2016, 11, e0159013. [Google Scholar] [CrossRef]
- Yang, D.; Liu, J.; Qian, H.; Zhuang, Q. Cancer-associated fibroblasts: From basic science to anticancer therapy. Exp. Mol. Med. 2023, 55, 1322–1332. [Google Scholar] [CrossRef]
- Levental, K.R.; Yu, H.; Kass, L.; Lakins, J.N.; Egeblad, M.; Erler, J.T.; Fong, S.F.; Csiszar, K.; Giaccia, A.; Weninger, W.; et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 2009, 139, 891–906. [Google Scholar] [CrossRef]
- Midwood, K.S.; Chiquet, M.; Tucker, R.P.; Orend, G. Tenascin-C at a glance. J. Cell Sci. 2016, 129, 4321–4327. [Google Scholar] [CrossRef]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef]
- Tape, C.J.; Ling, S.; Dimitriadi, M.; McMahon, K.M.; Worboys, J.D.; Leong, H.S.; Norrie, I.C.; Miller, C.J.; Poulogiannis, G.; Lauffenburger, D.A.; et al. Oncogenic KRAS Regulates Tumor Cell Signaling via Stromal Reciprocation. Cell 2016, 165, 910–920. [Google Scholar] [CrossRef]
- Sok, J.C.; Coppelli, F.M.; Thomas, S.M.; Lango, M.N.; Xi, S.; Hunt, J.L.; Freilino, M.L.; Graner, M.W.; Wikstrand, C.J.; Bigner, D.D.; et al. Mutant epidermal growth factor receptor (EGFRvIII) contributes to head and neck cancer growth and resistance to EGFR targeting. Clin. Cancer Res. 2006, 12, 5064–5073. [Google Scholar] [CrossRef] [PubMed]
- Kramer, N.; Walzl, A.; Unger, C.; Rosner, M.; Krupitza, G.; Hengstschlager, M.; Dolznig, H. In vitro cell migration and invasion assays. Mutat. Res. 2013, 752, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, S.; Bhola, N.E.; Grandis, J.R. HGF/Met Signaling in Head and Neck Cancer: Impact on the Tumor Microenvironment. Clin. Cancer Res. 2016, 22, 4005–4013. [Google Scholar] [CrossRef]
- Yu, H.; Pardoll, D.; Jove, R. STATs in cancer inflammation and immunity: A leading role for STAT3. Nat. Rev. Cancer 2009, 9, 798–809. [Google Scholar] [CrossRef]
- Clay, M.R.; Tabor, M.; Owen, J.H.; Carey, T.E.; Bradford, C.R.; Wolf, G.T.; Wicha, M.S.; Prince, M.E. Single-marker identification of head and neck squamous cell carcinoma cancer stem cells with aldehyde dehydrogenase. Head Neck 2010, 32, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; He, X.; Wei, W.; Wang, J.; Zhang, T.; Shen, X. Tenascin-C induces resistance to apoptosis in pancreatic cancer cell through activation of ERK/NF-kappaB pathway. Apoptosis 2015, 20, 843–857. [Google Scholar] [CrossRef] [PubMed]
- Kesh, K.; Gupta, V.K.; Durden, B.; Garrido, V.; Mateo-Victoriano, B.; Lavania, S.P.; Banerjee, S. Therapy Resistance, Cancer Stem Cells and ECM in Cancer: The Matrix Reloaded. Cancers 2020, 12, 3067. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.; Price, Z.K.; Lokman, N.A.; Wang, W.; Goonetilleke, L.; Kadife, E.; Oehler, M.K.; Ricciardelli, C.; Kannourakis, G.; Ahmed, N. Platinum-resistance in epithelial ovarian cancer: An interplay of epithelial-mesenchymal transition interlinked with reprogrammed metabolism. J. Transl. Med. 2022, 20, 556. [Google Scholar] [CrossRef]
- Marona, P.; Gorka, J.; Kotlinowski, J.; Majka, M.; Jura, J.; Miekus, K. C-Met as a Key Factor Responsible for Sustaining Undifferentiated Phenotype and Therapy Resistance in Renal Carcinomas. Cells 2019, 8, 272. [Google Scholar] [CrossRef]
- Szymczyk, J.; Sluzalska, K.D.; Materla, I.; Opalinski, L.; Otlewski, J.; Zakrzewska, M. FGF/FGFR-Dependent Molecular Mechanisms Underlying Anti-Cancer Drug Resistance. Cancers 2021, 13, 5796. [Google Scholar] [CrossRef]
- Zhu, Y.; Hu, H.; Yuan, Z.; Zhang, Q.; Xiong, H.; Hu, Z.; Wu, H.; Huang, R.; Wang, G.; Tang, Q. LncRNA NEAT1 remodels chromatin to promote the 5-FU resistance by maintaining colorectal cancer stemness. Cell Death Dis. 2020, 11, 962. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Dewille, J.W.; Hai, T. A potential dichotomous role of ATF3, an adaptive-response gene, in cancer development. Oncogene 2008, 27, 2118–2127. [Google Scholar] [CrossRef]
- Kim, S.; Ge, J.; Kim, D.; Lee, J.J.; Choi, Y.J.; Chen, W.; Bowman, J.W.; Foo, S.S.; Chang, L.C.; Liang, Q.M.; et al. TXNIP-mediated crosstalk between oxidative stress and glucose metabolism. PLoS ONE 2024, 19, e0292655. [Google Scholar] [CrossRef]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix Metalloproteinases: Regulators of the Tumor Microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef]
- Chen, L.F.; Cohen, E.E.W.; Grandis, J.R. New Strategies in Head and Neck Cancer: Understanding Resistance to Epidermal Growth Factor Receptor Inhibitors. Clin. Canc Res. 2010, 16, 2489–2495. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.L.; Wang, M.Z.; Chen, X.T.; Chen, S.L.; Liu, L.; Zhu, J.B.; Wang, J.H.; Yang, X.R.; Cai, X.S. Expression of Cytokeratin 19 in Adipose-Derived Stem Cells Is Induced by Epidermal Growth Factor. Med. Sci. Monit. 2018, 24, 4254–4261. [Google Scholar] [CrossRef] [PubMed]
- Urra, H.; Dufey, E.; Avril, T.; Chevet, E.; Hetz, C. Endoplasmic Reticulum Stress and the Hallmarks of Cancer. Trends Cancer 2016, 2, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Pakos-Zebrucka, K.; Koryga, I.; Mnich, K.; Ljujic, M.; Samali, A.; Gorman, A.M. The integrated stress response. EMBO Rep. 2016, 17, 1374–1395. [Google Scholar] [CrossRef]
- Levy, J.M.M.; Towers, C.G.; Thorburn, A. Targeting autophagy in cancer. Nat. Rev. Cancer 2017, 17, 528–542. [Google Scholar] [CrossRef]
- Inoue, A.; Outani, H.; Imura, Y.; Nakai, S.; Takami, H.; Kotani, Y.; Mae, H.; Okada, S. AURKA/PLK1/CDC25C Axis as a Novel Therapeutic Target in INI1-Deficient Epithelioid Sarcoma. Cancer Sci. 2025, 116, 976–989. [Google Scholar] [CrossRef]
- Recasens, A.; Munoz, L. Targeting Cancer Cell Dormancy. Trends Pharmacol. Sci. 2019, 40, 128–141. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Kwon, S.; Park, S.; Namkoong, E.; Kim, J.; Sim, H.-Y.; Sharker, S.M.; Lee, S.-w. Biomimetic Salivary Gland Cancer Spheroid Platform for In Vitro Recapitulation of Three-Dimensional Tumor–Stromal Interactions. Biomolecules 2025, 15, 1634. https://doi.org/10.3390/biom15121634
Wang L, Kwon S, Park S, Namkoong E, Kim J, Sim H-Y, Sharker SM, Lee S-w. Biomimetic Salivary Gland Cancer Spheroid Platform for In Vitro Recapitulation of Three-Dimensional Tumor–Stromal Interactions. Biomolecules. 2025; 15(12):1634. https://doi.org/10.3390/biom15121634
Chicago/Turabian StyleWang, Lele, Seokjun Kwon, Sujin Park, Eun Namkoong, Junchul Kim, Hye-Young Sim, Shazid Md. Sharker, and Sang-woo Lee. 2025. "Biomimetic Salivary Gland Cancer Spheroid Platform for In Vitro Recapitulation of Three-Dimensional Tumor–Stromal Interactions" Biomolecules 15, no. 12: 1634. https://doi.org/10.3390/biom15121634
APA StyleWang, L., Kwon, S., Park, S., Namkoong, E., Kim, J., Sim, H.-Y., Sharker, S. M., & Lee, S.-w. (2025). Biomimetic Salivary Gland Cancer Spheroid Platform for In Vitro Recapitulation of Three-Dimensional Tumor–Stromal Interactions. Biomolecules, 15(12), 1634. https://doi.org/10.3390/biom15121634





