Mitochondrial Dysfunction in Apoptosis-Resistant Acute Myeloid Leukemia Cells During a Sterile Inflammatory Response
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Conditions
2.2. Cell Viability Assay
2.3. RNA Sequencing (RNA-Seq)
2.4. Differential Gene Expression Analysis
2.5. Gene Set Enrichment Analysis (GSEA)
2.6. Mitochondrial Metabolism-Related Genes (MMRG) Expression and Functional Analysis
2.7. Construction and Analysis of a Protein–Protein Interaction (PPI) Network
2.8. Transmission Electron Microscopy (TEM)
2.9. Mitochondrial Mass Analysis
2.10. Determination of Mitochondrial Membrane Potential (ΔΨm)
2.11. Determination of Intracellular Reactive Oxygen Species (ROS) Production
2.12. Determination of Lactic Acid (Lactate) Concentration
2.13. Evaluation of ADP/ATP and NAD/NADH Ratios
2.14. Analysis of Mitochondrial Respiration in Permeabilized Cells
2.15. Western Blot Analysis
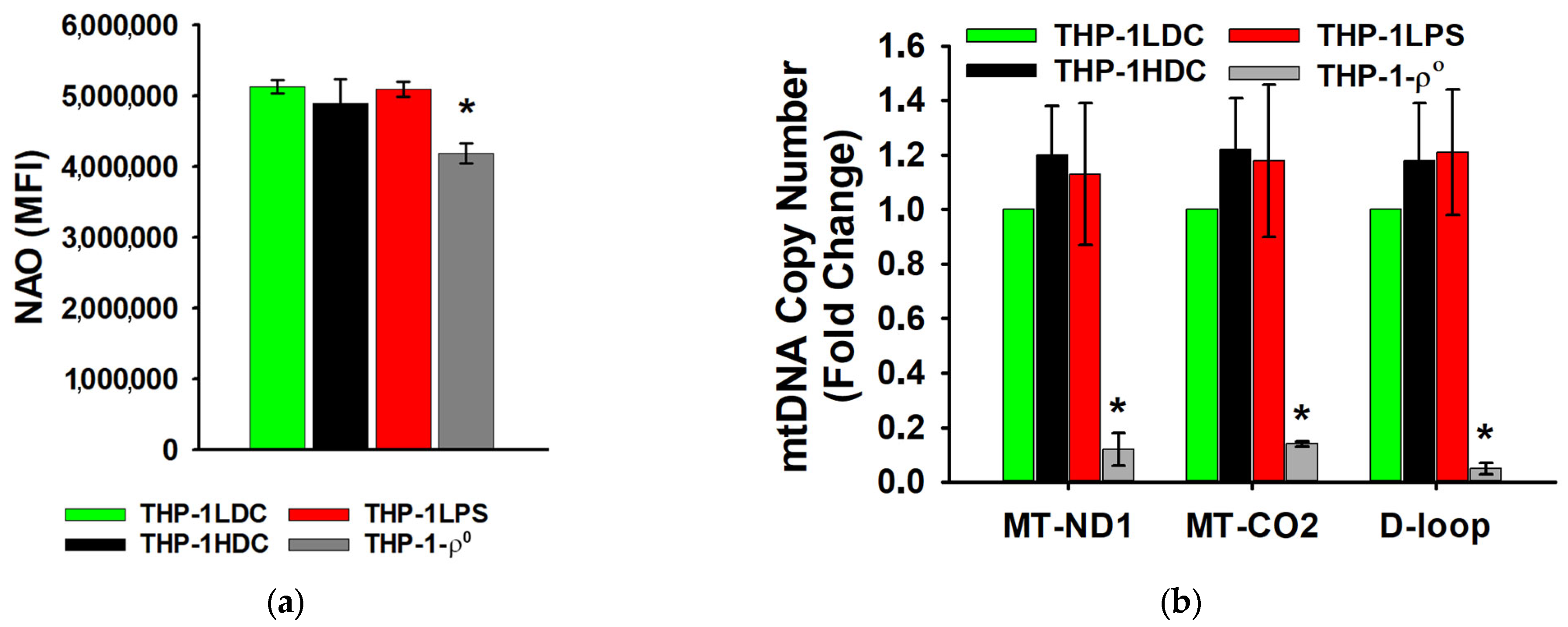
2.16. Real-Time PCR for Mitochondrial DNA Copy Number Quantification
2.17. Statistical Analysis
3. Results
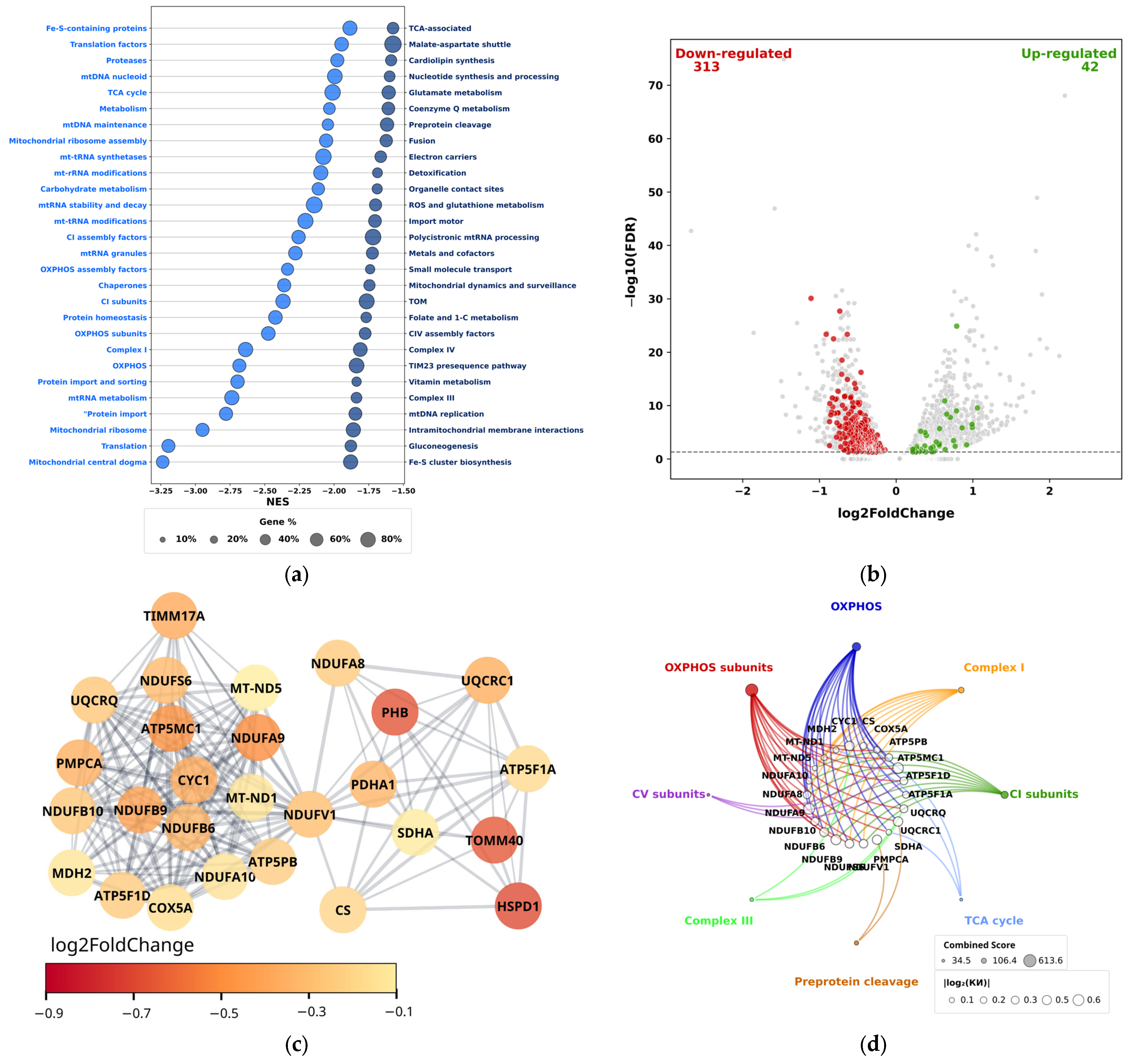
3.1. Transcriptomic Profiling of Mitochondrial Pathways and Metabolism-Related Genes in THP-1 Acute Myeloid Leukemia Cells During Aseptic Pro-Inflammatory Activation
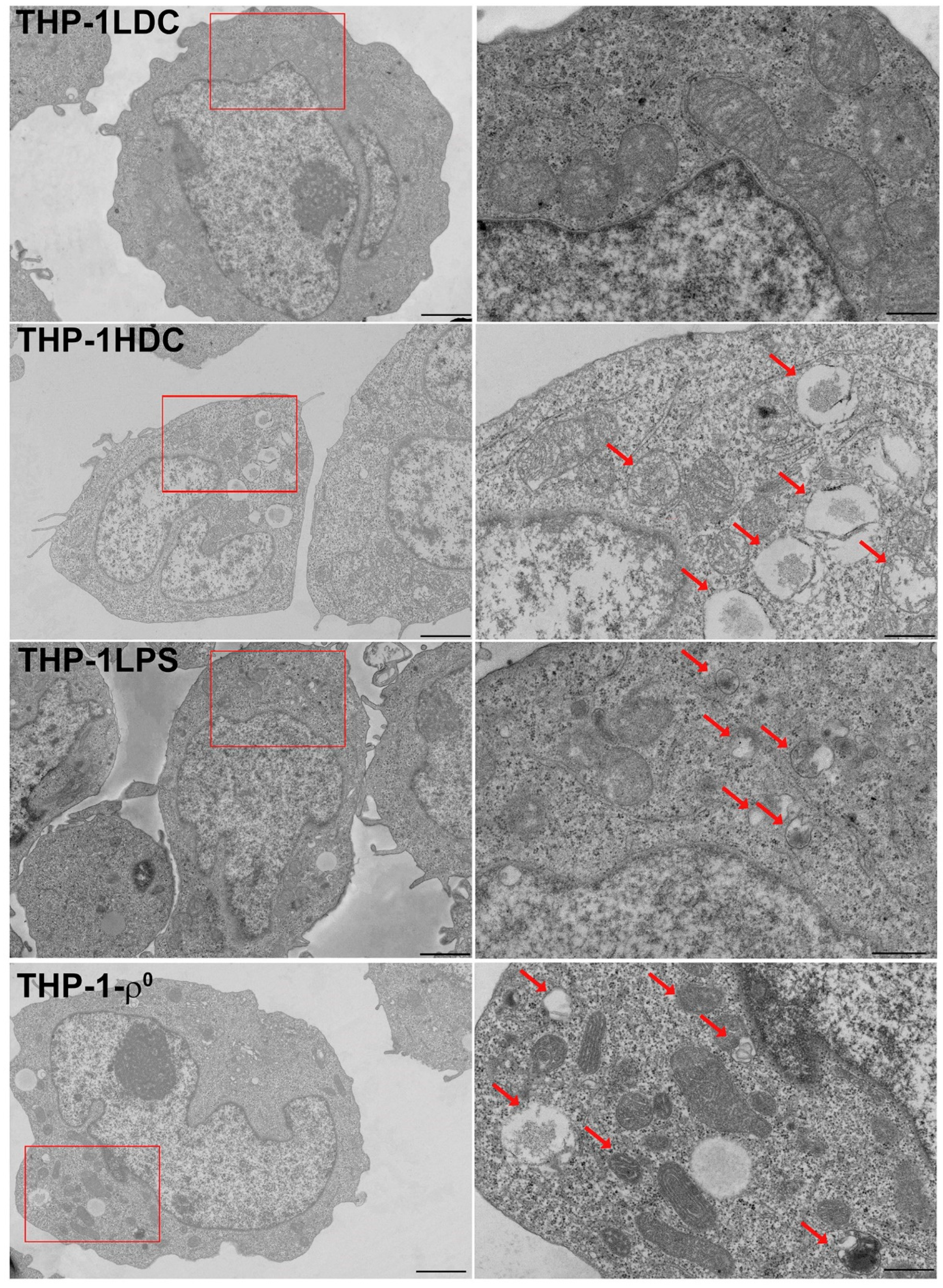
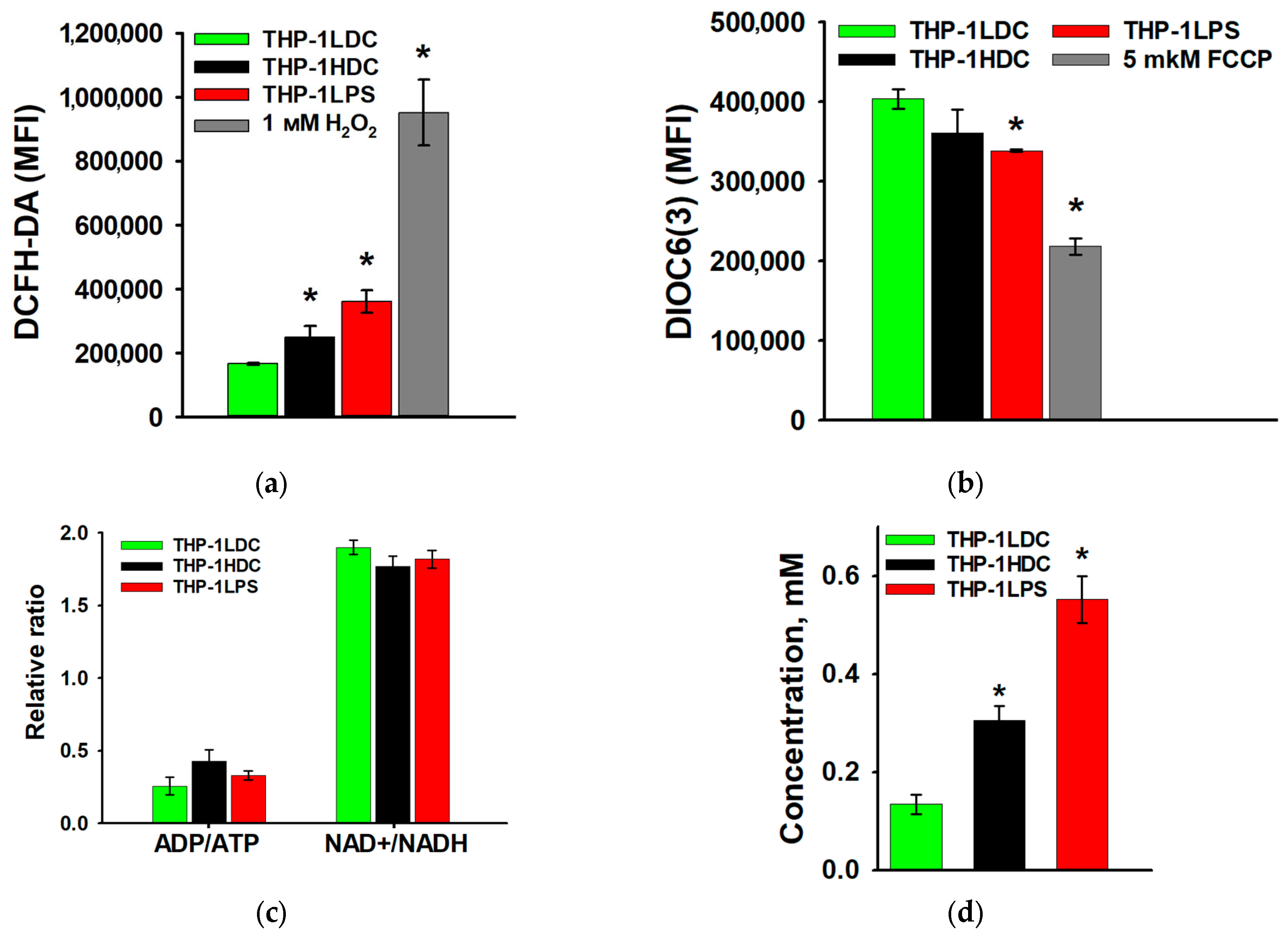
3.2. Analysis of Mitochondrial Content and Ultrastructure in THP-1 Acute Myeloid Leukemia Cells During Aseptic Inflammatory Activation
3.3. Characteristics of Mitochondrial Respiration in THP-1 Acute Myeloid Leukemia Cells During Aseptic Inflammatory Activation
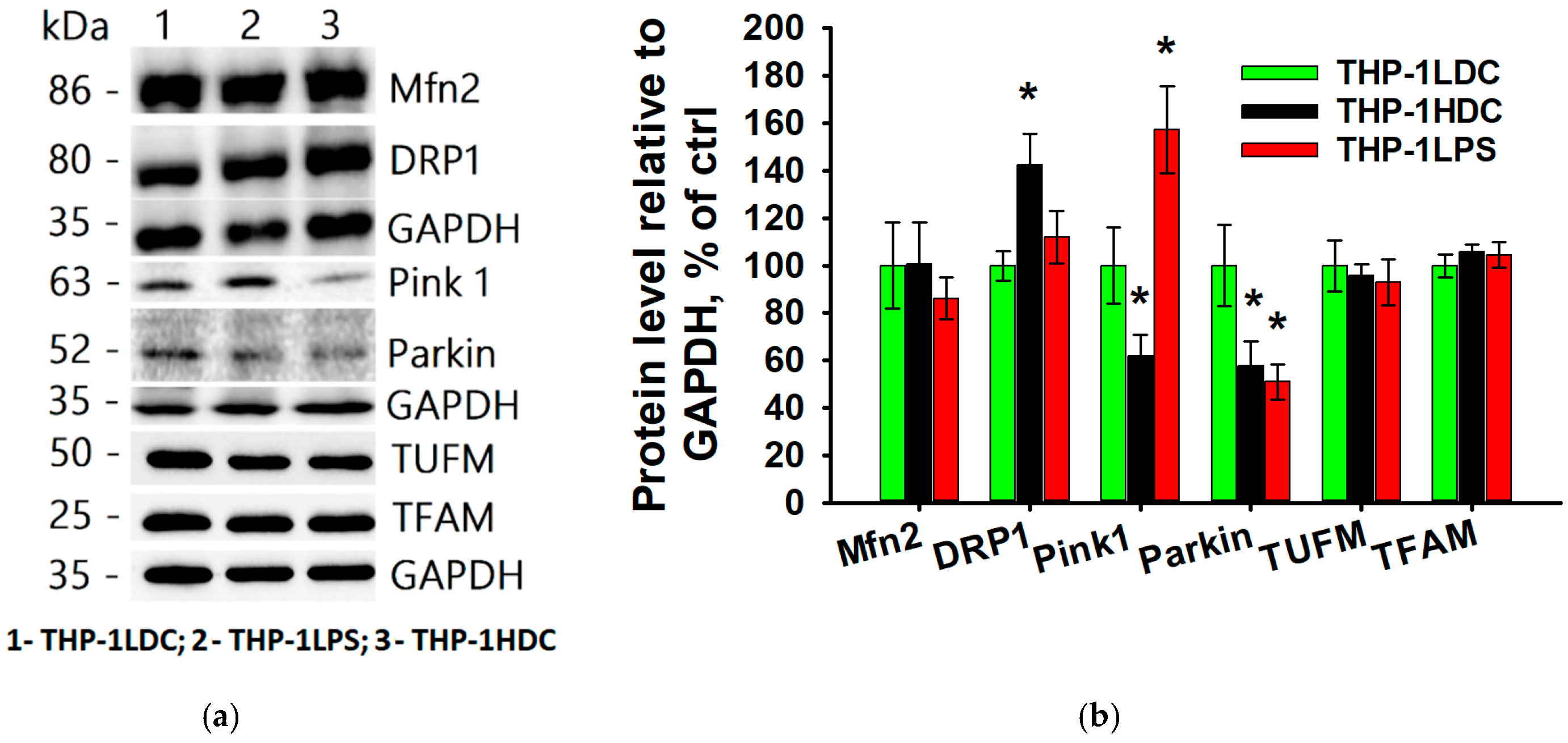
3.4. Key Marker Proteins of Mitochondrial Quality Control System in THP-1 Acute Myeloid Leukemia Cells During Aseptic Inflammatory Activation
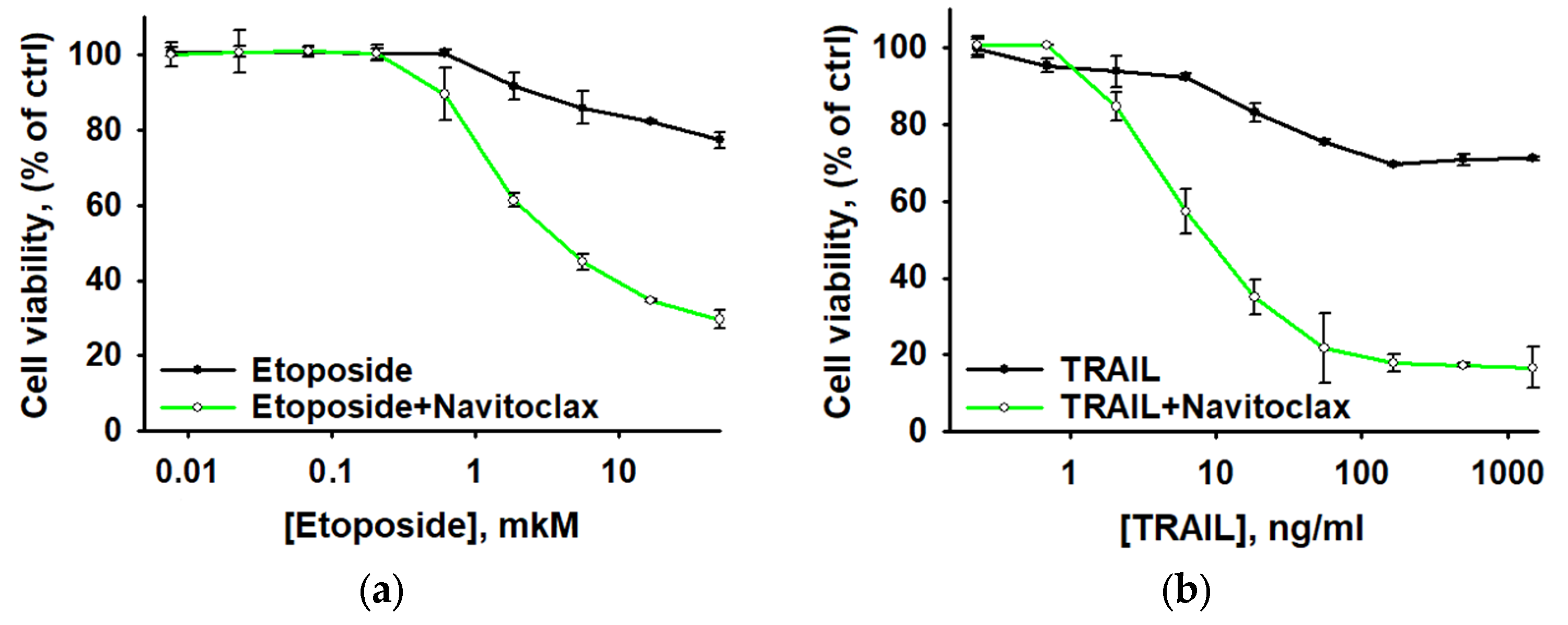
3.5. Navitoclax Sensitizes Aseptically Inflammatory Activated AML Cells to Etoposide and TRAIL-Induced Apoptosis
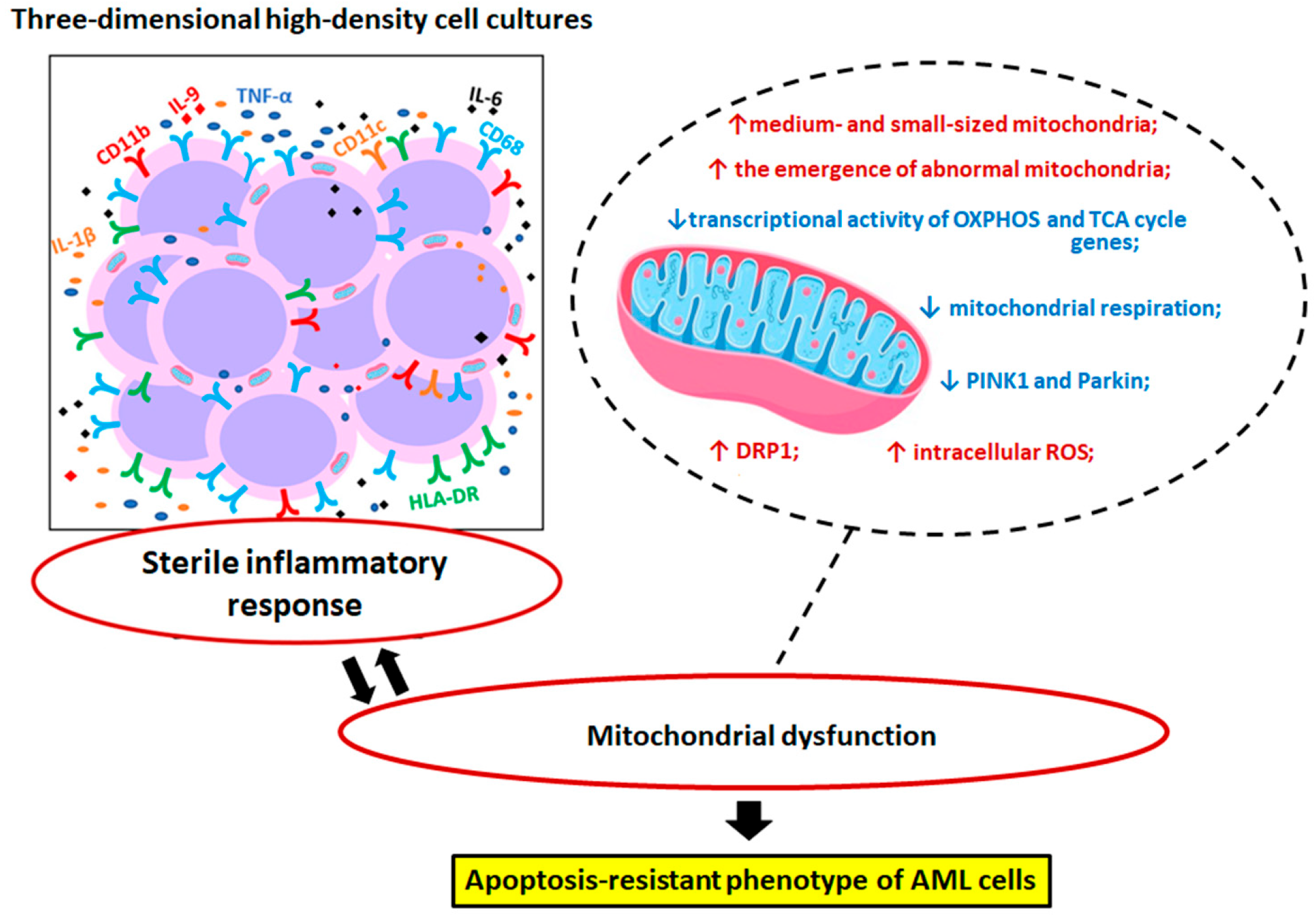
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AML | Acute myeloid leukemia |
| CATR | Carboxyatractyloside |
| DNP | 2,4-dinitrophenol |
| ETC | Electron transport chain |
| HDC | High-density cultures |
| IL | Interleukin |
| LDC | Low-density cultures |
| LPS | Lipopolysaccharide |
| MP | Mitochondrial pathways |
| MMRG | Mitochondrial metabolism-related genes |
| ROS | Reactive oxygen species |
References
- Naji, N.S.; Sathish, M.; Karantanos, T. Inflammation and Related Signaling Pathways in Acute Myeloid Leukemia. Cancers 2024, 16, 3974. [Google Scholar] [CrossRef] [PubMed]
- De Kouchkovsky, I.; Abdul-Hay, M. Acute myeloid leukemia: A comprehensive review and 2016 update. Blood Cancer J. 2016, 6, e441. [Google Scholar] [CrossRef]
- Kantarjian, H.; Borthakur, G.; Daver, N.; DiNardo, C.D.; Issa, G.; Jabbour, E.; Kadia, T.; Sasaki, K.; Short, N.J.; Yilmaz, M.; et al. Current status and research directions in acute myeloid leukemia. Blood Cancer J. 2024, 14, 163. [Google Scholar] [CrossRef]
- Vyas, S.; Zaganjor, E.; Haigis, M.C. Mitochondria and Cancer. Cell 2016, 166, 555–566. [Google Scholar] [CrossRef]
- Mukherjee, S.; Bhatti, G.K.; Chhabra, R.; Reddy, P.H.; Bhatti, J.S. Targeting mitochondria as a potential therapeutic strategy against chemoresistance in cancer. Biomed. Pharmacother. 2023, 160, 114398. [Google Scholar] [CrossRef]
- Hsu, C.C.; Tseng, L.M.; Lee, H.C. Role of mitochondrial dysfunction in cancer progression. Exp. Biol. Med. 2016, 241, 1281–1295. [Google Scholar] [CrossRef]
- Mustafa, M.; Ahmad, R.; Tantry, I.Q.; Ahmad, W.; Siddiqui, S.; Alam, M.; Abbas, K.; Moinuddin; Hassan, M.I.; Habib, S.; et al. Apoptosis: A Comprehensive Overview of Signaling Pathways, Morphological Changes, and Physiological Significance and Therapeutic Implications. Cells 2024, 13, 1838. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Marchi, S.; Guilbaud, E.; Tait, S.W.G.; Yamazaki, T.; Galluzzi, L. Mitochondrial control of inflammation. Nat. Rev. Immunol. 2023, 23, 159–173. [Google Scholar] [CrossRef]
- Vringer, E.; Tait, S.W.G. Mitochondria and cell death-associated inflammation. Cell Death Differ. 2023, 30, 304–312. [Google Scholar] [CrossRef]
- Wein, T.; Sorek, R. Bacterial origins of human cell-autonomous innate immune mechanisms. Nat. Rev. Immunol. 2022, 22, 629–638. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Barreyro, L.; Chlon, T.M.; Starczynowski, D.T. Chronic immune response dysregulation in MDS pathogenesis. Blood 2018, 132, 1553–1560. [Google Scholar] [CrossRef]
- Wen, Y.; Zhu, Y.; Zhang, C.; Yang, X.; Gao, Y.; Li, M.; Yang, H.; Liu, T.; Tang, H. Chronic inflammation, cancer development and immunotherapy. Front. Pharmacol. 2022, 13, 1040163. [Google Scholar] [CrossRef]
- Brock, S.E.; Rendon, B.E.; Xin, D.; Yaddanapudi, K.; Mitchell, R.A. MIF family members cooperatively inhibit p53 expression and activity. PLoS ONE 2014, 9, e99795. [Google Scholar] [CrossRef]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal Transduct. Target. Ther. 2020, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cao, X. Characteristics and Significance of the Pre-metastatic Niche. Cancer Cell 2016, 30, 668–681. [Google Scholar] [CrossRef]
- Harapas, C.R.; Idiiatullina, E.; Al-Azab, M.; Hrovat-Schaale, K.; Reygaerts, T.; Steiner, A.; Laohamonthonkul, P.; Davidson, S.; Yu, C.H.; Booty, L.; et al. Organellar homeostasis and innate immune sensing. Nat. Rev. Immunol. 2022, 22, 535–549. [Google Scholar] [CrossRef] [PubMed]
- Kobyakova, M.; Lomovskaya, Y.; Senotov, A.; Lomovsky, A.; Minaychev, V.; Fadeeva, I.; Shtatnova, D.; Krasnov, K.; Zvyagina, A.; Odinokova, I.; et al. The Increase in the Drug Resistance of Acute Myeloid Leukemia THP-1 Cells in High-Density Cell Culture Is Associated with Inflammatory-like Activation and Anti-Apoptotic Bcl-2 Proteins. Int. J. Mol. Sci. 2022, 23, 7881. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.W.; Fan, J.M.; Schrey, J.M.; Mitchell, D.V.; Jung, S.K.; Hurwitz, S.N.; Perez, E.B.; Muraro, M.J.; Carroll, M.; Taylor, D.M.; et al. Inflammatory recruitment of healthy hematopoietic stem and progenitor cells in the acute myeloid leukemia niche. Leukemia 2024, 38, 741–750. [Google Scholar] [CrossRef]
- Luciano, M.; Krenn, P.W.; Horejs-Hoeck, J. The cytokine network in acute myeloid leukemia. Front. Immunol. 2022, 13, 1000996. [Google Scholar] [CrossRef]
- Kobyakova, M.I.; Krasnov, K.S.; Krestinina, O.V.; Baburina, Y.L.; Senotov, A.S.; Lomovskaya, Y.V.; Meshcheriakova, E.I.; Lomovsky, A.I.; Zvyagina, A.I.; Pyatina, K.V.; et al. Hypercellular Proinflammatory Microenvironment Inhibits the Etoposide-Induced DNA Damage in Acute Monocytic Leukemia Cells. Biochemistry 2025, 90, 553–567. [Google Scholar] [CrossRef]
- Kobyakova, M.I.; Senotov, A.S.; Krasnov, K.S.; Lomovskaya, Y.V.; Odinokova, I.V.; Kolotova, A.A.; Ermakov, A.M.; Zvyagina, A.I.; Fadeeva, I.S.; Fetisova, E.I.; et al. Pro-Inflammatory Activation Suppresses TRAIL-induced Apoptosis of Acute Myeloid Leukemia Cells. Biochemistry 2024, 89, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yin, S.; Chen, Y.; Wu, Y.; Zheng, W.; Dong, H.; Bai, Y.; Qin, Y.; Li, J.; Feng, S.; et al. LPS induced proinflammatory cytokine expression in human airway epithelial cells and macrophages via NF κB, STAT3 or AP 1 activation. Mol. Med. Rep. 2018, 17, 5484–5491. [Google Scholar] [CrossRef]
- Zhang, R.; Ji, J.; Blaženović, I.; Pi, F.; Wang, T.; Zhang, Y.; Sun, X. Investigation into Cellular Glycolysis for the Mechanism Study of Energy Metabolism Disorder Triggered by Lipopolysaccharide. Toxins 2018, 10, 441. [Google Scholar] [CrossRef]
- Kelly, B.; O’Neill, L.A. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. 2015, 25, 771–784. [Google Scholar] [CrossRef]
- Novotny, M.V.; Xu, W.; Mulya, A.; Janocha, A.J.; Erzurum, S.C. Method for depletion of mitochondria DNA in human bronchial epithelial cells. MethodsX 2023, 12, 102497. [Google Scholar] [CrossRef] [PubMed]
- Khozhukhar, N.; Spadafora, D.; Rodriguez, Y.; Alexeyev, M. Elimination of Mitochondrial DNA from Mammalian Cells. Curr. Protoc. Cell Biol. 2018, 78, 20.11.1–20.11.14. [Google Scholar] [CrossRef]
- Lomovskaya, Y.V.; Kobyakova, M.I.; Senotov, A.S.; Lomovsky, A.I.; Minaychev, V.V.; Fadeeva, I.S.; Shtatnova, D.Y.; Krasnov, K.S.; Zvyagina, A.I.; Akatov, V.S.; et al. Macrophage-like THP-1 Cells Derived from High-Density Cell Culture Are Resistant to TRAIL-Induced Cell Death via Down-Regulation of Death-Receptors DR4 and DR5. Biomolecules 2022, 12, 150. [Google Scholar] [CrossRef] [PubMed]
- Fadeev, R.; Chekanov, A.; Solovieva, M.; Bezborodova, O.; Nemtsova, E.; Dolgikh, N.; Fadeeva, I.; Senotov, A.; Kobyakova, M.; Evstratova, Y.; et al. Improved Anticancer Effect of Recombinant Protein izTRAIL Combined with Sorafenib and Peptide iRGD. Int. J. Mol. Sci. 2019, 20, 525. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Rath, S.; Sharma, R.; Gupta, R.; Ast, T.; Chan, C.; Durham, T.J.; Goodman, R.P.; Grabarek, Z.; Haas, M.E.; Hung, W.H.W.; et al. MitoCarta3.0: An updated mitochondrial proteome now with sub-organelle localization and pathway annotations. Nucleic Acids Res. 2021, 49, D1541–D1547. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Liu, X.; Peltz, G. GSEApy: A comprehensive package for performing gene set enrichment analysis in Python. Bioinformatics 2023, 39, btac757. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Chin, C.H.; Chen, S.H.; Wu, H.H.; Ho, C.W.; Ko, M.T.; Lin, C.Y. cytoHubba: Identifying hub objects and sub-networks from complex interactome. BMC Syst. Biol. 2014, 8, S11. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.-H.; Chou, W.-C.; Wu, C.-H.; Jou, I.-M.; Tu, Y.-K.; Hsieh, P.-L.; Tsai, K.-L. Ginsenoside Rg3 Attenuates TNF-α-Induced Damage in Chondrocytes through Regulating SIRT1-Mediated Anti-Apoptotic and Anti-Inflammatory Mechanisms. Antioxidants 2021, 10, 1972. [Google Scholar] [CrossRef]
- Mishukov, A.; Mndlyan, E.; Berezhnov, A.V.; Kobyakova, M.; Lomovskaya, Y.; Holmuhamedov, E.; Odinokova, I. TR-57 Treatment of SUM159 Cells Induces Mitochondrial Dysfunction without Affecting Membrane Potential. Int. J. Mol. Sci. 2024, 25, 1193. [Google Scholar] [CrossRef]
- Teterina, A.Y.; Smirnov, I.V.; Fadeeva, I.S.; Fadeev, R.S.; Smirnova, P.V.; Minaychev, V.V.; Kobyakova, M.I.; Fedotov, A.Y.; Barinov, S.M.; Komlev, V.S. Octacalcium Phosphate for Bone Tissue Engineering: Synthesis, Modification, and in vitro Biocompatibility Assessment. Int. J. Mol. Sci. 2021, 22, 12747. [Google Scholar] [CrossRef]
- Hedges, L.V. Distribution Theory for Glass’s Estimator of Effect size and Related Estimators. J. Educ. Behav. Stat. 1981, 6, 107–128. [Google Scholar] [CrossRef]
- Chen, Y.; Xian, M.; Ying, W.; Liu, J.; Bing, S.; Wang, X.; Yu, J.; Xu, X.; Xiang, S.; Shao, X.; et al. Succinate dehydrogenase deficiency-driven succinate accumulation induces drug resistance in acute myeloid leukemia via ubiquitin-cullin regulation. Nat. Commun. 2024, 15, 9820. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Ran, Q. Research Progress on NDUFA9 and GBM. Int. J. Biol. Life Sci. 2024, 8, 32–36. [Google Scholar] [CrossRef]
- Li, L.D.; Sun, H.F.; Liu, X.X.; Gao, S.P.; Jiang, H.L.; Hu, X.; Jin, W. Down-Regulation of NDUFB9 Promotes Breast Cancer Cell Proliferation, Metastasis by Mediating Mitochondrial Metabolism. PLoS ONE 2015, 10, e0144441. [Google Scholar] [CrossRef] [PubMed]
- Sotgia, F.; Fiorillo, M.; Lisanti, M.P. Mitochondrial markers predict recurrence, metastasis and tamoxifen-resistance in breast cancer patients: Early detection of treatment failure with companion diagnostics. Oncotarget 2017, 8, 68730–68745. [Google Scholar] [CrossRef]
- Tapia, I.J.; Perico, D.; Wolos, V.J.; Villaverde, M.S.; Abrigo, M.; Di Silvestre, D.; Mauri, P.; De Palma, A.; Fiszman, G.L. Proteomic Characterization of a 3D HER2+ Breast Cancer Model Reveals the Role of Mitochondrial Complex I in Acquired Resistance to Trastuzumab. Int. J. Mol. Sci. 2024, 25, 7397. [Google Scholar] [CrossRef]
- Song, Y.; Zhu, H.; Wei, J.; Yin, S. Prognostic and therapeutic potential of disulfidptosis-related genes in colon adenocarcinoma: A comprehensive multi-omics study. Cancer Cell Int. 2025, 25, 226. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, N.; Saeed, M.E.M.; Sugimoto, Y.; Klauck, S.M.; Greten, H.J.; Efferth, T. Cytotoxicity of nimbolide towards multidrug-resistant tumor cells and hypersensitivity via cellular metabolic modulation. Oncotarget 2018, 9, 35762–35779. [Google Scholar] [CrossRef]
- Cong, H.; Gao, J.; Wang, Q.; Du, M.; Li, H.; Li, Q.; Li, J.; Liang, Y.; Zhao, D.; Yang, H.; et al. Increased Expression of Mitochondrial UQCRC1 in Pancreatic Cancer Impairs Antitumor Immunity of Natural Killer Cells via Elevating Extracellular ATP. Front. Oncol. 2022, 12, 872017. [Google Scholar] [CrossRef]
- Luo, Z.; Zeng, W.; Tang, W.; Long, T.; Zhang, J.; Xie, X.; Kuang, Y.; Chen, M.; Su, J.; Chen, X. CD147 interacts with NDUFS6 in regulating mitochondrial complex I activity and the mitochondrial apoptotic pathway in human malignant melanoma cells. Curr. Mol. Med. 2014, 14, 1252–1264. [Google Scholar] [CrossRef]
- Hirpara, J.; Eu, J.Q.; Tan, J.K.M.; Wong, A.L.; Clement, M.V.; Kong, L.R.; Ohi, N.; Tsunoda, T.; Qu, J.; Goh, B.C.; et al. Metabolic reprogramming of oncogene-addicted cancer cells to OXPHOS as a mechanism of drug resistance. Redox Biol. 2019, 25, 101076. [Google Scholar] [CrossRef]
- Kopecka, J.; Gazzano, E.; Castella, B.; Salaroglio, I.C.; Mungo, E.; Massaia, M.; Riganti, C. Mitochondrial metabolism: Inducer or therapeutic target in tumor immune-resistance? Semin. Cell Dev. Biol. 2020, 98, 80–89. [Google Scholar] [CrossRef]
- Liu, W.; Sun, Y.; Ge, W.; Zhang, F.; Gan, L.; Zhu, Y.; Guo, T.; Liu, K. DIA-Based Proteomics Identifies IDH2 as a Targetable Regulator of Acquired Drug Resistance in Chronic Myeloid Leukemia. Mol. Cell. Proteom. 2022, 21, 100187. [Google Scholar] [CrossRef]
- Zhang, H.; Shao, Y.; Chen, W.; Chen, X. Identifying Mitochondrial-Related Genes NDUFA10 and NDUFV2 as Prognostic Markers for Prostate Cancer through Biclustering. BioMed Res. Int. 2021, 2021, 5512624. [Google Scholar] [CrossRef] [PubMed]
- Shao, F.; Lyu, X.; Miao, K.; Xie, L.; Wang, H.; Xiao, H.; Li, J.; Chen, Q.; Ding, R.; Chen, P.; et al. Enhanced Protein Damage Clearance Induces Broad Drug Resistance in Multitype of Cancers Revealed by an Evolution Drug-Resistant Model and Genome-Wide siRNA Screening. Adv. Sci. 2020, 7, 2001914. [Google Scholar] [CrossRef]
- Welch, D.R.; Foster, C.; Rigoutsos, I. Roles of mitochondrial genetics in cancer metastasis. Trends Cancer 2022, 8, 1002–1018. [Google Scholar] [CrossRef]
- Kabelikova, P.; Ivovic, D.; Sumbalova, Z.; Karhanek, M.; Tatayova, L.; Skopkova, M.; Cagalinec, M.; Bruderova, V.; Roska, J.; Jurkovicova, D. Mitochondrial genome variability and metabolic alterations reveal new biomarkers of resistance in testicular germ cell tumors. Cancer Drug Resist. 2024, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Mu, C.; Zheng, R.; Zhang, Z.; Zhang, Q.; Liang, T. The Cancer Antioxidant Regulation System in Therapeutic Resistance. Antioxidants 2024, 13, 778. [Google Scholar] [CrossRef]
- Parma, B.; Wurdak, H.; Ceppi, P. Harnessing mitochondrial metabolism and drug resistance in non-small cell lung cancer and beyond by blocking heat-shock proteins. Drug Resist. Updates 2022, 65, 100888. [Google Scholar] [CrossRef]
- Yun, C.W.; Kim, H.J.; Lim, J.H.; Lee, S.H. Heat Shock Proteins: Agents of Cancer Development and Therapeutic Targets in Anti-Cancer Therapy. Cells 2019, 9, 60. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Guo, X.; Liu, X.M.; Liu, L.; Weng, Q.F.; Dong, S.J.; Knowlton, A.A.; Yuan, W.J.; Lin, L. Extracellular HSP60 induces inflammation through activating and up-regulating TLRs in cardiomyocytes. Cardiovasc. Res. 2013, 98, 391–401. [Google Scholar] [CrossRef]
- Cabibi, D.; Conway de Macario, E.; Ingrao, S.; Porcasi, R.; Zucco, F.; Macario, A.J.L.; Cappello, F.; Rappa, F. CD1A-positive cells and HSP60 (HSPD1) levels in keratoacanthoma and squamous cell carcinoma. Cell Stress Chaperones 2016, 21, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Samoylova, N.; Cummings, M. TOMM40, a risk gene for Alzheimer’s disease, is upregulated during proinflammatory response. Alzheimers Dement. 2021, 17, e058711. [Google Scholar] [CrossRef]
- Gao, X.; Liu, J.; Jia, B.; Guo, J. USP30 Aggravating the Malignant Progression of Breast Cancer and Its Resistance to Tamoxifen by Inhibiting the Ubiquitination of TOMM40. J. Biochem. Mol. Toxicol. 2025, 39, e70258. [Google Scholar] [CrossRef]
- Rout, S.K.; Priya, V.; Setia, A.; Mehata, A.K.; Mohan, S.; Albratty, M.; Najmi, A.; Meraya, A.M.; Makeen, H.A.; Tambuwala, M.M.; et al. Mitochondrial targeting theranostic nanomedicine and molecular biomarkers for efficient cancer diagnosis and therapy. Biomed. Pharmacother. 2022, 153, 113451. [Google Scholar] [CrossRef]
- Cevatemre, B.; Ulukaya, E.; Dere, E.; Dilege, S.; Acilan, C. Pyruvate Dehydrogenase Contributes to Drug Resistance of Lung Cancer Cells Through Epithelial Mesenchymal Transition. Front. Cell Dev. Biol. 2022, 9, 738916. [Google Scholar] [CrossRef]
- Pan, S.S.; Wang, F.; Hui, Y.P.; Chen, K.Y.; Zhou, L.; Gao, W.L.; Wu, H.K.; Zhang, D.S.; Yang, S.Y.; Hu, X.Y.; et al. Insulin reduces pyroptosis-induced inflammation by PDHA1 dephosphorylation-mediated NLRP3 activation during myocardial ischemia-reperfusion injury. Perfusion 2023, 38, 1277–1287. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Jiang, A.; Zeng, H.; Peng, X.; Song, L. Comprehensive analyses of PDHA1 that serves as a predictive biomarker for immunotherapy response in cancer. Front. Pharmacol. 2022, 13, 947372. [Google Scholar] [CrossRef]
- Lo, Y.W.; Lin, S.T.; Chang, S.J.; Chan, C.H.; Lyu, K.W.; Chang, J.F.; May, E.W.; Lin, D.Y.; Chou, H.C.; Chan, H.L. Mitochondrial proteomics with siRNA knockdown to reveal ACAT1 and MDH2 in the development of doxorubicin-resistant uterine cancer. J. Cell. Mol. Med. 2015, 19, 744–759. [Google Scholar] [CrossRef]
- Liu, W.; Li, J.; Xu, S.; Wang, Y.; Li, J.; Wang, S.; Fu, L.; Jiang, M.; Bai, G. Phillyrin and its metabolites exert antipyretic effects by targeting the NAD+ binding domain of GAPDH, MDH2 and IDH2. Phytomedicine 2024, 134, 155955. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, C.; El-Kholy, M.; Parekh, F.; Robson, M.; Lamb, K.; Allen, C.; Sillibourne, J.; Cordoba, S.; Thomas, S.; Pule, M. Novel Fas-TNFR chimeras that prevent Fas ligand-mediated kill and signal synergistically to enhance CAR T cell efficacy. Mol. Ther. Nucleic Acids 2023, 32, 603–621. [Google Scholar] [CrossRef]
- Chen, L.; Liu, T.; Zhou, J.; Wang, Y.; Wang, X.; Di, W.; Zhang, S. Citrate synthase expression affects tumor phenotype and drug resistance in human ovarian carcinoma. PLoS ONE 2014, 9, e115708. [Google Scholar] [CrossRef]
- Goldsmith, J.A.; Lai, R.E.; Garten, R.S.; Chen, Q.; Lesnefsky, E.J.; Perera, R.A.; Gorgey, A.S. Visceral Adiposity, Inflammation, and Testosterone Predict Skeletal Muscle Mitochondrial Mass and Activity in Chronic Spinal Cord Injury. Front. Physiol. 2022, 13, 809845. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Fu, D.; Liang, W.; Fan, L.; Chen, K.; Shan, L.; Hu, S.; Ma, X.; Zhou, K.; Cheng, B. CYC1 silencing sensitizes osteosarcoma cells to TRAIL-induced apoptosis. Cell. Physiol. Biochem. 2014, 34, 2070–2080. [Google Scholar] [CrossRef] [PubMed]
- Dai, R.; Lei, X.; Liu, X.; Bian, C. UQCRB regulates mitochondrial energy metabolism by promoting COX5A in atherosclerotic endothelial dysfunction. Naunyn-Schmiedebergs Arch. Pharmacol. 2025, 398, 16181–16193. [Google Scholar] [CrossRef]
- Balaji, S.; Rao, A.; Saraswathi, K.K.; Sethu Nagarajan, R.; Santhi, R.; Kim, U.; Muthukkaruppan, V.; Vanniarajan, A. Focused cancer pathway analysis revealed unique therapeutic targets in retinoblastoma. Med. Oncol. 2024, 41, 168. [Google Scholar] [CrossRef]
- García-Aranda, M.; Pérez-Ruiz, E.; Redondo, M. Bcl-2 Inhibition to Overcome Resistance to Chemo- and Immunotherapy. Int. J. Mol. Sci. 2018, 19, 3950. [Google Scholar] [CrossRef]
- Cousin, F.J.; Jouan-Lanhouet, S.; Théret, N.; Brenner, C.; Jouan, E.; Le Moigne-Muller, G.; Dimanche-Boitrel, M.T.; Jan, G. The probiotic Propionibacterium freudenreichii as a new adjuvant for TRAIL-based therapy in colorectal cancer. Oncotarget 2016, 7, 7161–7178. [Google Scholar] [CrossRef]
- Matsushima-Miyagi, T.; Hatano, K.; Nomura, M.; Li-Wen, L.; Nishikawa, T.; Saga, K.; Shimbo, T.; Kaneda, Y. TRAIL and Noxa are selectively upregulated in prostate cancer cells downstream of the RIG-I/MAVS signaling pathway by nonreplicating Sendai virus particles. Clin. Cancer Res. 2012, 18, 6271–6283. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, S.; Bhattacharya, A.; Adhikary, S.; Singh, V.; Gadad, S.S.; Roy, S.; Das, C. The paradigm of drug resistance in cancer: An epigenetic perspective. Biosci. Rep. 2022, 42, BSR20211812. [Google Scholar] [CrossRef]
- Ke, H.; Chen, Z.; Chen, L.; Zhang, H.; Wang, Y.; Song, T.; Bi, A.; Li, Q.; Sheng, H.; Jia, Y.; et al. FK506-binding proteins: Emerging target and therapeutic opportunity in multiple tumors. Int. J. Biol. Macromol. 2025, 307, 141914. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Wang, D.; Weng, J.; Zhang, S.; Zhang, Y. BNIP3 decreases the LPS-induced inflammation and apoptosis of chondrocytes by promoting the development of autophagy. J. Orthop. Surg. Res. 2020, 15, 284. [Google Scholar] [CrossRef]
- Lee, J.D.; Jung, H.; Min, S.H. Identification of proteins suppressing the functions of oncogenic phosphatase of regenerating liver 1 and 3. Exp. Ther. Med. 2016, 12, 2974–2982. [Google Scholar] [CrossRef][Green Version]
- Xu, H.; Zheng, S.; Zhang, Q.; Xu, Y.; Zhang, H.; Hu, T.; Zhang, X.; E, J.; Li, X.; Wang, R.; et al. CUL1-neddylation contributes to K29-linked ubiquitination on p27 for autophagic degradation in sorafenib-resistant liver cancer. Cell Biol. Toxicol. 2025, 41, 61. [Google Scholar] [CrossRef]
- Hernandez, E.D.; Lee, S.J.; Kim, J.Y.; Duran, A.; Linares, J.F.; Yajima, T.; Müller, T.D.; Tschöp, M.H.; Smith, S.R.; Diaz-Meco, M.T.; et al. A macrophage NBR1-MEKK3 complex triggers JNK-mediated adipose tissue inflammation in obesity. Cell Metab. 2014, 20, 499–511. [Google Scholar] [CrossRef]
- Lu, C.; Yang, B.; Liu, Y.; Liu, W.; Yan, J.; Guo, C.; Song, T.; Wang, X. Carvacrol Regulates the Expression of SLC25A6 by Inhibiting VDAC1 to Improve Mitochondrial Function and Reduce LPS-Induced Inflammatory Injury in HMEC-1 Cells. ACS Omega 2025, 10, 8512–8522. [Google Scholar] [CrossRef]
- Soto-Heredero, G.; Gómez de Las Heras, M.M.; Gabandé-Rodríguez, E.; Oller, J.; Mittelbrunn, M. Glycolysis—A key player in the inflammatory response. FEBS J. 2020, 287, 3350–3369. [Google Scholar] [CrossRef] [PubMed]
- Everts, B.; Amiel, E.; van der Windt, G.J.; Freitas, T.C.; Chott, R.; Yarasheski, K.E.; Pearce, E.L.; Pearce, E.J. Commitment to glycolysis sustains survival of NO-producing inflammatory dendritic cells. Blood 2012, 120, 1422–1431. [Google Scholar] [CrossRef]
- Samavati, L.; Lee, I.; Mathes, I.; Lottspeich, F.; Hüttemann, M. Tumor necrosis factor alpha inhibits oxidative phosphorylation through tyrosine phosphorylation at subunit I of cytochrome c oxidase. J. Biol. Chem. 2008, 283, 21134–21144. [Google Scholar] [CrossRef]
- Fujieda, Y.; Manno, A.; Hayashi, Y.; Rhodes, N.; Guo, L.; Arita, M.; Bamba, T.; Fukusaki, E. Inflammation and resolution are associated with upregulation of fatty acid β-oxidation in Zymosan-induced peritonitis. PLoS ONE 2013, 8, e66270. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.H.; Chae, C.W.; Chang, H.S.; Choi, G.E.; Lee, H.J.; Han, H.J. Silencing SIRT5 induces the senescence of UCB-MSCs exposed to TNF-α by reduction of fatty acid β-oxidation and anti-oxidation. Free Radic. Biol. Med. 2022, 192, 1–12. [Google Scholar] [CrossRef]
- Miettinen, T.P.; Björklund, M. Mitochondrial Function and Cell Size: An Allometric Relationship. Trends Cell Biol. 2017, 27, 393–402. [Google Scholar] [CrossRef]
- Wang, H.; Sun, X.; Lu, Q.; Zemskov, E.A.; Yegambaram, M.; Wu, X.; Wang, T.; Tang, H.; Black, S.M. The mitochondrial redistribution of eNOS is involved in lipopolysaccharide induced inflammasome activation during acute lung injury. Redox Biol. 2021, 41, 101878. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Cui, R.; Geng, X.; Li, J.; Zhou, Y.; He, L.; Cao, C.; Zhang, C.; Chen, Z.; Ying, S. LPS-induced mitochondrial DNA synthesis and release facilitate RAD50-dependent acute lung injury. Signal Transduct. Target. Ther. 2021, 6, 103. [Google Scholar] [CrossRef]
- Kobyakova, M.I.; Evstratova, Y.V.; Senotov, A.S.; Lomovski, A.I.; Minaicev, V.V.; Zvjagina, A.I.; Solovea, M.E.; Fadeva, I.S.; Akatov, V.S.; Fadev, R.S. Appearance of signs of differentiation and pro-inflammatory phenotype in acute myeloid leukemia cells THP-1 with an increase in their trail resistance in cell aggregates in vitro. Biol. Membr. 2021, 15, 97–105. [Google Scholar] [CrossRef]
- Mercy, L.; Pauw, A.D.; Payen, L.; Tejerina, S.; Houbion, A.; Demazy, C.; Raes, M.; Renard, P.; Arnould, T. Mitochondrial biogenesis in mtDNA-depleted cells involves a Ca2+-dependent pathway and a reduced mitochondrial protein import. FEBS J. 2005, 272, 5031–5055. [Google Scholar] [CrossRef]
- Kukat, A.; Kukat, C.; Brocher, J.; Schäfer, I.; Krohne, G.; Trounce, I.A.; Villani, G.; Seibel, P. Generation of rho0 cells utilizing a mitochondrially targeted restriction endonuclease and comparative analyses. Nucleic Acids Res. 2008, 36, e44. [Google Scholar] [CrossRef]
- Schubert, S.; Heller, S.; Löffler, B.; Schäfer, I.; Seibel, M.; Villani, G.; Seibel, P. Generation of Rho Zero Cells: Visualization and Quantification of the mtDNA Depletion Process. Int. J. Mol. Sci. 2015, 16, 9850–9865. [Google Scholar] [CrossRef]
- Lin, J.; Duan, J.; Wang, Q.; Xu, S.; Zhou, S.; Yao, K. Mitochondrial Dynamics and Mitophagy in Cardiometabolic Disease. Front. Cardiovasc. Med. 2022, 9, 917135. [Google Scholar] [CrossRef]
- Morton, H.; Kshirsagar, S.; Orlov, E.; Bunquin, L.E.; Sawant, N.; Boleng, L.; George, M.; Basu, T.; Ramasubramanian, B.; Pradeepkiran, J.A.; et al. Defective mitophagy and synaptic degeneration in Alzheimer’s disease: Focus on aging, mitochondria and synapse. Free Radic. Biol. Med. 2021, 172, 652–667. [Google Scholar] [CrossRef]
- Kim, H.K.; Kang, Y.G.; Jeong, S.H.; Park, N.; Marquez, J.; Ko, K.S.; Rhee, B.D.; Shin, J.W.; Han, J. Cyclic stretch increases mitochondrial biogenesis in a cardiac cell line. Biochem. Biophys. Res. Commun. 2018, 505, 768–774. [Google Scholar] [CrossRef]
- Chen, H.; Detmer, S.A.; Ewald, A.J.; Griffin, E.E.; Fraser, S.E.; Chan, D.C. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J. Cell Biol. 2003, 160, 189–200. [Google Scholar] [CrossRef]
- Narendra, D.P.; Youle, R.J. The role of PINK1-Parkin in mitochondrial quality control. Nat. Cell Biol. 2024, 26, 1639–1651. [Google Scholar] [CrossRef]
- Smirnova, E.; Griparic, L.; Shurland, D.L.; van der Bliek, A.M. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol. Biol. Cell 2001, 12, 2245–2256. [Google Scholar] [CrossRef]
- Qian, S.; Wei, Z.; Yang, W.; Huang, J.; Yang, Y.; Wang, J. The role of BCL-2 family proteins in regulating apoptosis and cancer therapy. Front. Oncol. 2022, 12, 985363. [Google Scholar] [CrossRef]
- Yuan, S.; Zhou, Y.; Li, Y.; Chen, Z.; Xiao, W.; Jiang, D.; Zhang, P.; Zhang, Y.; Bai, F.; Deng, J.; et al. Mitoxantrone-liposome Sensitizes FLT3-ITD Acute Myeloid Leukemia to Gilteritinib Treatment. J. Cancer 2025, 16, 1905–1917. [Google Scholar] [CrossRef]
- Takahashi, S. Downstream molecular pathways of FLT3 in the pathogenesis of acute myeloid leukemia: Biology and therapeutic implications. J. Hematol. Oncol. 2011, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- King, Z.; Desai, S.R.; Frank, D.A.; Shastri, A. STAT signaling in the pathogenesis and therapy of acute myeloid leukemia and myelodysplastic syndromes. Neoplasia 2025, 61, 101137. [Google Scholar] [CrossRef] [PubMed]
- Banella, C.; Catalano, G.; Calvani, M.; Candi, E.; Noguera, N.I.; Travaglini, S. Metabolic Signature of FLT3-Mutated AML: Clinical and Therapeutic Implications. J. Pers. Med. 2025, 15, 431. [Google Scholar] [CrossRef] [PubMed]
- Alshamleh, I.; Kurrle, N.; Makowka, P.; Bhayadia, R.; Kumar, R.; Süsser, S.; Seibert, M.; Ludig, D.; Wolf, S.; Koschade, S.E.; et al. PDP1 is a key metabolic gatekeeper and modulator of drug resistance in FLT3-ITD-positive acute myeloid leukemia. Leukemia 2023, 37, 2367–2382. [Google Scholar] [CrossRef]
- Lagunas-Rangel, F.A. DNA damage accumulation and repair defects in FLT3-ITD acute myeloid leukemia: Implications for clonal evolution and disease progression. Hematol. Oncol. 2023, 41, 26–38. [Google Scholar] [CrossRef]
- Kannan, S.; Irwin, M.E.; Herbrich, S.M.; Cheng, T.; Patterson, L.L.; Aitken, M.J.L.; Bhalla, K.; You, M.J.; Konopleva, M.; Zweidler-McKay, P.A.; et al. Targeting the NRF2/HO-1 Antioxidant Pathway in FLT3-ITD-Positive AML Enhances Therapy Efficacy. Antioxidants 2022, 11, 717. [Google Scholar] [CrossRef]
- Lin, L.; Wei, J.; Xue, J.; Fan, G.; Zhu, W.; Zhu, Y.; Wu, R. Drp1 Promotes Macrophage M1 Polarization and Inflammatory Response in Autoimmune Myocarditis by Driving Mitochondrial Fission. J. Cardiovasc. Transl. Res. 2025, 18, 237–246. [Google Scholar] [CrossRef]
- Chegodaev, Y.S.; Nikiforov, N.; Zhuravlev, A.; Postnov, A.Y.; Orekhov, A.N. Inflammatory stimulation of macrophages inhibits mitophagy. Atherosclerosis 2024, 395, 117666. [Google Scholar] [CrossRef]
- Haileselassie, B.; Joshi, A.U.; Minhas, P.S.; Mukherjee, R.; Andreasson, K.I.; Mochly-Rosen, D. Mitochondrial dysfunction mediated through dynamin-related protein 1 (Drp1) propagates impairment in blood brain barrier in septic encephalopathy. J. Neuroinflamm. 2020, 17, 36. [Google Scholar] [CrossRef]
- Alula, K.M.; Delgado-Deida, Y.; Callahan, R.; Till, A.; Underwood, L.; Thompson, W.E.; Souza, R.F.; Dassopoulos, T.; Onyiah, J.; Venuprasad, K.; et al. Inner mitochondrial membrane protein Prohibitin 1 mediates Nix-induced, Parkin-independent mitophagy. Sci. Rep. 2023, 13, 18. [Google Scholar] [CrossRef] [PubMed]
- Widdrington, J.D.; Gomez-Duran, A.; Pyle, A.; Ruchaud-Sparagano, M.H.; Scott, J.; Baudouin, S.V.; Rostron, A.J.; Lovat, P.E.; Chinnery, P.F.; Simpson, A.J. Exposure of Monocytic Cells to Lipopolysaccharide Induces Coordinated Endotoxin Tolerance, Mitochondrial Biogenesis, Mitophagy, and Antioxidant Defenses. Front. Immunol. 2018, 9, 2217. [Google Scholar] [CrossRef] [PubMed]
- Agha-Mir-Salim, D.; Bhayadia, R.; Heckl, D.; Klusmann, J.H. Evaluation of Navitoclax (ABT-263) as a promising Therapy for Pediatric Acute Myeloid Leukemia. Klin. Pädiatr. 2024, 236, 200. [Google Scholar] [CrossRef]
- Gao, K.; Liu, Y.; Sun, C.; Wang, Y.; Bao, H.; Liu, G.; Ou, J.; Sun, P. TNF-α induces mitochondrial dysfunction to drive NLRP3/Caspase-1/GSDMD-mediated pyroptosis in MCF-7 cells. Sci. Rep. 2024, 14, 25880. [Google Scholar] [CrossRef]
- Willemsen, J.; Neuhoff, M.T.; Hoyler, T.; Noir, E.; Tessier, C.; Sarret, S.; Thorsen, T.N.; Littlewood-Evans, A.; Zhang, J.; Hasan, M.; et al. TNF leads to mtDNA release and cGAS/STING-dependent interferon responses that support inflammatory arthritis. Cell Rep. 2021, 37, 109977. [Google Scholar] [CrossRef]
- Xian, H.; Watari, K.; Sanchez-Lopez, E.; Offenberger, J.; Onyuru, J.; Sampath, H.; Ying, W.; Hoffman, H.M.; Shadel, G.S.; Karin, M. Oxidized DNA fragments exit mitochondria via mPTP- and VDAC-dependent channels to activate NLRP3 inflammasome and interferon signaling. Immunity 2022, 55, 1370–1385.e8. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.S.; Chung, J.H. Molecular mechanisms of mitochondrial DNA release and activation of the cGAS-STING pathway. Exp. Mol. Med. 2023, 55, 510–519. [Google Scholar] [CrossRef]
- Janssen, M.; Müller-Tidow, C. Inflammation conjoins differentiation and resistance in AML. Blood 2025, 145, 2405–2407. [Google Scholar] [CrossRef]

| Primer Name | Sequence (5′→3′) |
|---|---|
| MT-ND-1 | F: CACCCAAGAACAGGGTTTGT R: TGGCCATGGGTATGTTGTTAA |
| MT-CO2 | F: AATCGAGTAGTACTCCCGATTG R: TTCTAGGACGATGGGCATGAAA |
| D-loop | F: CTATCACCCTATTAACCACTCA R: TTCGCCTGTAATATTGAACGTA |
| 18S | F: CTACCACATCCAAGGAAGCA R: TTTTTCGTCACTACCTCCCCG |
| % of Normal Mitochondria per Cell | % of Abnormal Mitochondria per Cell | |||||
|---|---|---|---|---|---|---|
| Small | Medium | Large | Small | Medium | Large | |
| THP-1LDC | 13.5 ± 8.4 | 30.2 ± 5.5 | 56.3 ± 13 | - | - | - |
| THP-1HDC | 7.0 ± 4.6 | 45.8 ± 14.6 | 13.5 ± 6.2 | 18.6 ± 7.8 | - | 8.5 ± 5.9 |
| THP-1LPS | 14.9 ± 5.2 | 45.1 ± 3.8 | 24.2 ± 3.2 | 7.0 ± 1.7 | 5.6 ± 3.7 | 3.1 ± 1.8 |
| THP-1-ρ0 | 19.6 ± 7.8 | 29.2 ± 8.9 | 13.4 ± 2.9 | 9.1 ± 2.3 | 11.4 ± 5.4 | 17.2 ± 10.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meshcheriakova, E.I.; Krasnov, K.S.; Odinokova, I.V.; Lomovsky, A.I.; Krestinina, O.V.; Baburina, Y.L.; Mikheeva, I.B.; Mikhailova, G.Z.; Senotov, A.S.; Nekhochina, P.S.; et al. Mitochondrial Dysfunction in Apoptosis-Resistant Acute Myeloid Leukemia Cells During a Sterile Inflammatory Response. Biomolecules 2025, 15, 1635. https://doi.org/10.3390/biom15121635
Meshcheriakova EI, Krasnov KS, Odinokova IV, Lomovsky AI, Krestinina OV, Baburina YL, Mikheeva IB, Mikhailova GZ, Senotov AS, Nekhochina PS, et al. Mitochondrial Dysfunction in Apoptosis-Resistant Acute Myeloid Leukemia Cells During a Sterile Inflammatory Response. Biomolecules. 2025; 15(12):1635. https://doi.org/10.3390/biom15121635
Chicago/Turabian StyleMeshcheriakova, Elena I., Kirill S. Krasnov, Irina V. Odinokova, Aleksey I. Lomovsky, Olga V. Krestinina, Yuliya L. Baburina, Irina B. Mikheeva, Gulnara Z. Mikhailova, Anatoly S. Senotov, Polina S. Nekhochina, and et al. 2025. "Mitochondrial Dysfunction in Apoptosis-Resistant Acute Myeloid Leukemia Cells During a Sterile Inflammatory Response" Biomolecules 15, no. 12: 1635. https://doi.org/10.3390/biom15121635
APA StyleMeshcheriakova, E. I., Krasnov, K. S., Odinokova, I. V., Lomovsky, A. I., Krestinina, O. V., Baburina, Y. L., Mikheeva, I. B., Mikhailova, G. Z., Senotov, A. S., Nekhochina, P. S., Lomovskaya, Y. V., Minaychev, V. V., Fadeeva, I. S., Kobyakova, M. I., & Fadeev, R. S. (2025). Mitochondrial Dysfunction in Apoptosis-Resistant Acute Myeloid Leukemia Cells During a Sterile Inflammatory Response. Biomolecules, 15(12), 1635. https://doi.org/10.3390/biom15121635







