RhoA/Rho-Kinase Signaling in Vascular Smooth Muscle and Endothelium: Mechanistic Insights and Translational Implications in Hypertension
Abstract
1. Introduction
2. Mechanistic Pathways of RhoA/ROCK Signaling
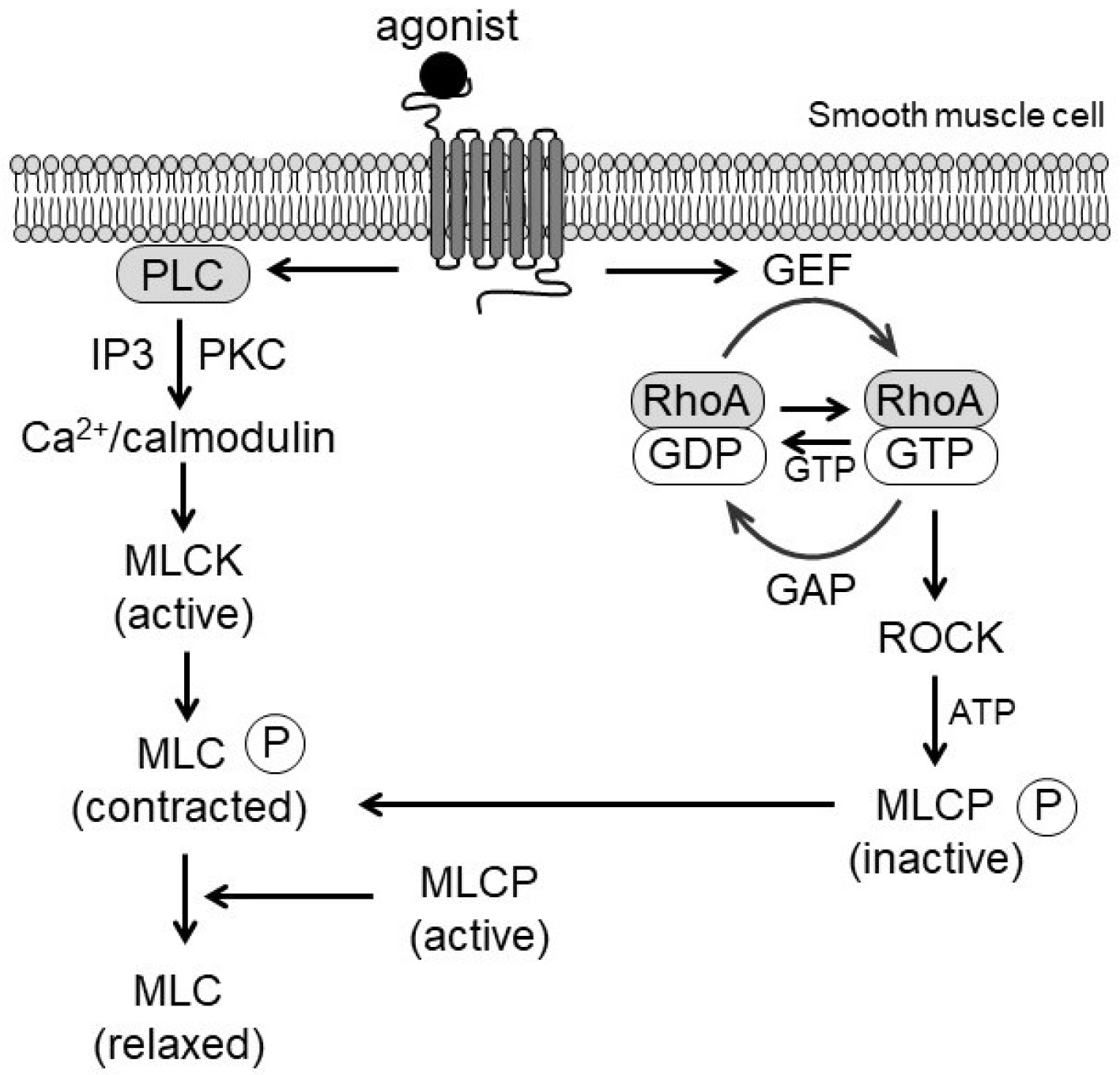
2.1. Signal Initiation and Ca2+ Sensitization
2.2. Myosin Light Chain Phosphorylation and VSMC Contraction
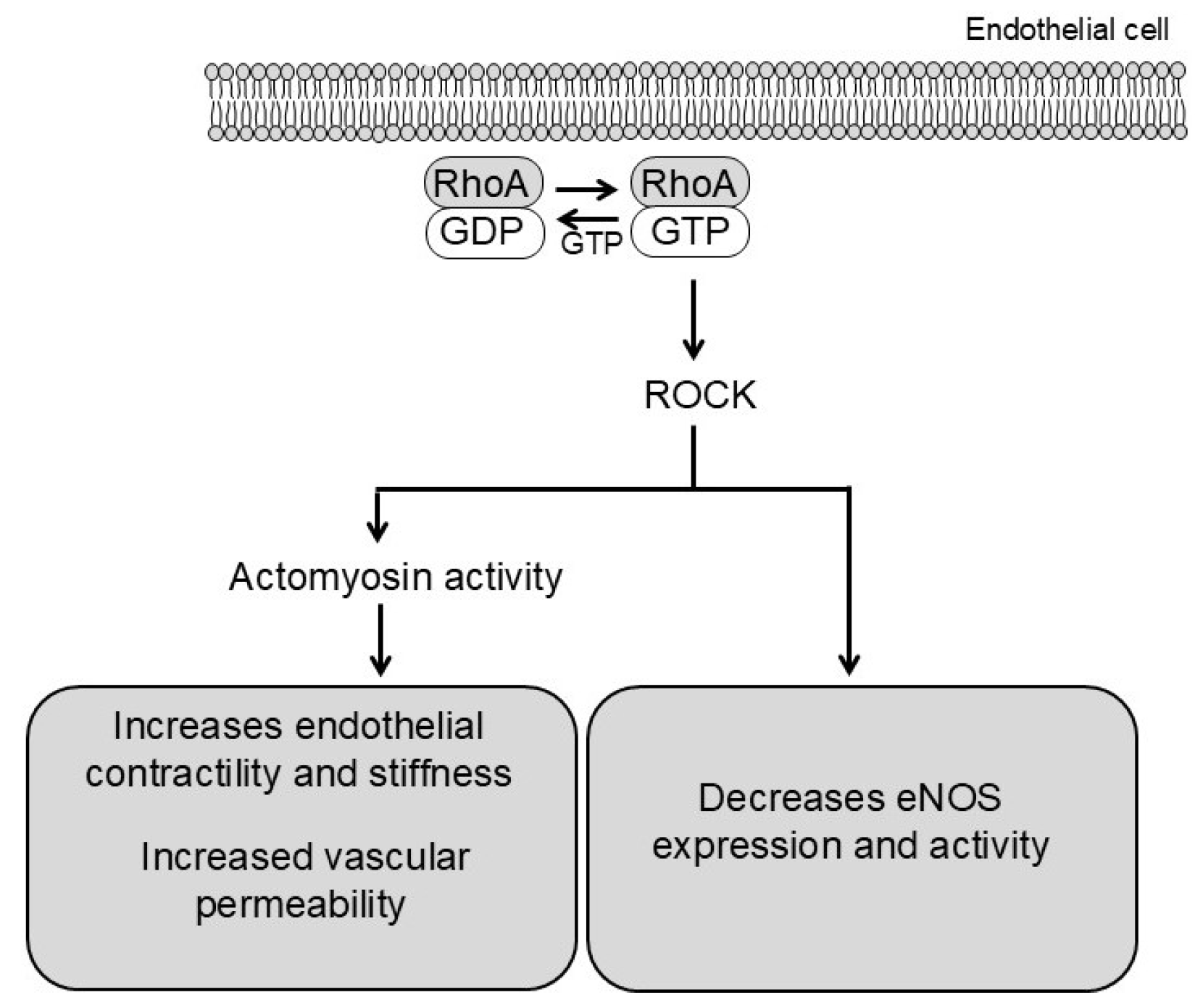
2.3. Endothelial Mechanisms in RhoA/ROCK Signaling
3. Crosstalk with Oxidative Stress and Redox Signaling
3.1. Reactive Oxygen Species (ROS)
3.2. Impact on Ca2+ Handling and Ca2+ Channels
3.3. Ca2+ Microdomains
3.4. Redox Sensitive Proteins
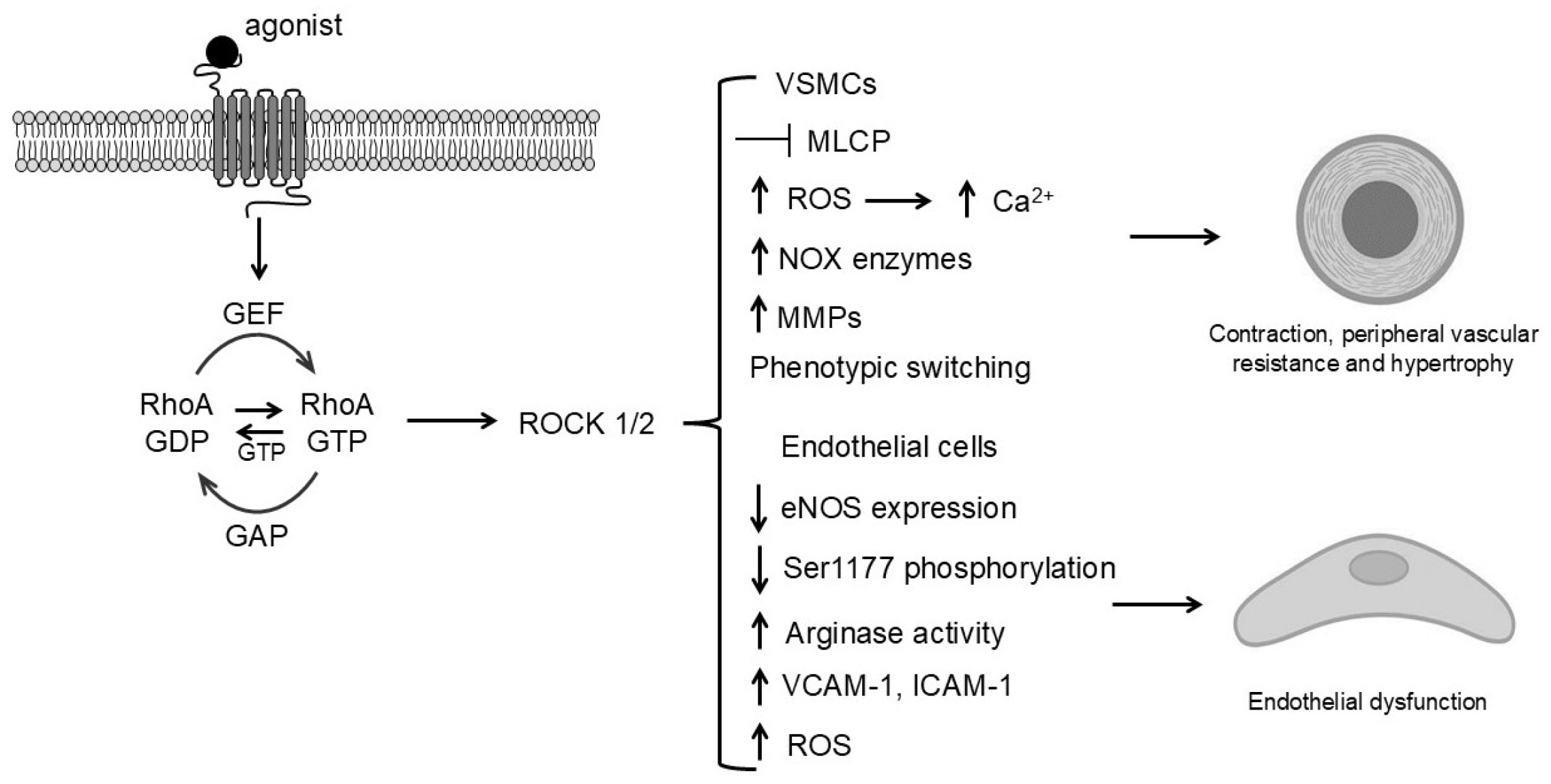
4. RhoA/ROCK in Hypertension and Vascular Diseases
4.1. Increased Contractility and Peripheral Vascular Resistance
4.2. RhoA/ROCK-Induced Endothelial Dysfunction in Hypertension
4.3. RhoA/ROCK-Mediated Suppression of NO Bioavailability and Vascular Remodeling
5. Inflammation and Immune Activation
6. Human Evidence
7. Therapeutic Targeting
7.1. No Pan-ROCK Inhibitors Are in Clinical Use for the Treatment of Hypertension
7.2. Emerging Therapeutic Strategies
8. Future Directions
- Define isoform-specific contributions (ROCK1 vs. ROCK2) in VSMCs vs. ECs.
- Map upstream RhoGEFs and GAPs activated in hypertensive phenotypes.
- Develop biomarkers (phospho-MYPT1, leukocyte ROCK activity) for patient stratification.
- Explore combination therapies (ROCK inhibition + RAAS blockade or antioxidants).
- Conduct mechanism-based clinical trials assessing chronic ROCK inhibition in hypertension.
9. Conclusions
Author Contributions
Funding
Insitutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADMA | Asymmetric dimethylarginine |
| ADPR | ADP-ribose |
| Ang II | Angiotensin II |
| ATP | Adenosine triphosphate |
| Ca2+ | Calcium ion |
| CaMKII | Ca2+/calmodulin-dependent protein kinase II |
| CPI-17 | PKC-potentiated inhibitory protein for heterotrimeric myosin phosphatase of 17 kDa |
| DAG | Diacylglycerol |
| DOCA | Deoxycorticosterone acetate |
| EC | Endothelial cell |
| eNOS | Endothelial nitric oxide synthase |
| ER | Endoplasmic reticulum |
| ET-1 | Endothelin-1 |
| FMD | Flow-mediated dilation |
| GAP | GTPase activating protein |
| GDP | Guanosine diphosphate |
| GDI | Guanine nucleotide dissociation inhibitor |
| GEF | Guanine nucleotide exchange factor |
| GPCR | G protein–coupled receptor |
| GTP | Guanosine triphosphate |
| H2O2 | Hydrogen peroxide |
| ICAM-1 | Intercellular adhesion molecule 1 |
| IP3 | Inositol 1,4,5-trisphosphate |
| LARG | Leukemia-associated Rho guanine nucleotide exchange factor |
| L-Arg | L-arginine |
| MLC | Myosin light chain |
| MLCK | Myosin light chain kinase |
| MLCP | Myosin light chain phosphatase |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MMP | Matrix metalloproteinase |
| MYPT1 | Myosin phosphatase target subunit 1 |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NFAT | Nuclear factor of activated T cells |
| NO | Nitric oxide |
| NOX | NADPH oxidase |
| NLRP3 | NOD-, LRR- and pyrin domain-containing protein 3 (inflammasome) |
| Nrf2 | Nuclear factor erythroid 2–related factor 2 |
| NUDT9-H | Nudix-type motif 9 homologous domain |
| O2•− | Superoxide anion |
| Orai1 | Calcium release-activated calcium channel protein 1 |
| PAI-1 | Plasminogen activator inhibitor-1 |
| PARP1 | Poly(ADP-ribose) polymerase 1 |
| PDZ-RhoGEF | PDZ domain-containing Rho guanine nucleotide exchange factor |
| PI3K | Phosphatidylinositol 3-kinase |
| PIP2 | Phosphatidylinositol 4,5-bisphosphate |
| PKC | Protein kinase C |
| PKCα | Protein kinase C alpha |
| PLC | Phospholipase C |
| PTK | Protein tyrosine kinase |
| PTP | Protein tyrosine phosphatase |
| PYK2 | Proline-rich tyrosine kinase 2 |
| RAAS | Renin–angiotensin–aldosterone system |
| RBD | Rho-binding domain |
| ROCK | Rho-associated coiled-coil-containing protein kinase (Rho-kinase) |
| ROS | Reactive oxygen species |
| SHR | Spontaneously hypertensive rat |
| STIM1 | Stromal interaction molecule 1 |
| TGF-β1 | Transforming growth factor beta 1 |
| TRPM2 | Transient receptor potential melastatin 2 |
| TRPV4 | Transient receptor potential vanilloid 4 |
| VCAM-1 | Vascular cell adhesion molecule 1 |
| VSMC | Vascular smooth muscle cell |
References
- Durante, A.; Mazzapicchi, A.; Baiardo Redaelli, M. Systemic and Cardiac Microvascular Dysfunction in Hypertension. Int. J. Mol. Sci. 2024, 25, 13294. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Yang, Y. Molecular Mechanisms Underlying Vascular Remodeling in Hypertension. Rev. Cardiovasc. Med. 2024, 25, 72. [Google Scholar] [CrossRef]
- Chitaley, K.; Weber, D.; Webb, R.C. RhoA/Rho-kinase, vascular changes, and hypertension. Curr. Hypertens. Rep. 2001, 3, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Hori, M. Characterization of Contractile Machinery of Vascular Smooth Muscles in Hypertension. Life 2021, 11, 702. [Google Scholar] [CrossRef]
- Zicha, J.; Vaneckova, I. Altered Balance Between Vasoconstrictor and Vasodilator Systems in Experimental Hypertension. Physiol. Res. 2024, 73, 901–928. [Google Scholar] [CrossRef]
- Loirand, G.; Guerin, P.; Pacaud, P. Rho kinases in cardiovascular physiology and pathophysiology. Circ. Res. 2006, 98, 322–334. [Google Scholar] [CrossRef]
- Seccia, T.M.; Rigato, M.; Ravarotto, V.; Calo, L.A. ROCK (RhoA/Rho Kinase) in Cardiovascular-Renal Pathophysiology: A Review of New Advancements. J. Clin. Med. 2020, 9, 1328. [Google Scholar] [CrossRef]
- Sward, K.; Mita, M.; Wilson, D.P.; Deng, J.T.; Susnjar, M.; Walsh, M.P. The role of RhoA and Rho-associated kinase in vascular smooth muscle contraction. Curr. Hypertens. Rep. 2003, 5, 66–72. [Google Scholar] [CrossRef]
- Liu, X.; Chen, D.; Liu, G. Overexpression of RhoA promotes the proliferation and migration of cervical cancer cells. Biosci. Biotechnol. Biochem. 2014, 78, 1895–1901. [Google Scholar] [CrossRef]
- Qi, Y.; Liang, X.; Dai, F.; Guan, H.; Sun, J.; Yao, W. RhoA/ROCK Pathway Activation is Regulated by AT1 Receptor and Participates in Smooth Muscle Migration and Dedifferentiation via Promoting Actin Cytoskeleton Polymerization. Int. J. Mol. Sci. 2020, 21, 5398. [Google Scholar] [CrossRef] [PubMed]
- Sawma, T.; Shaito, A.; Najm, N.; Sidani, M.; Orekhov, A.; El-Yazbi, A.F.; Iratni, R.; Eid, A.H. Role of RhoA and Rho-associated kinase in phenotypic switching of vascular smooth muscle cells: Implications for vascular function. Atherosclerosis 2022, 358, 12–28. [Google Scholar] [CrossRef] [PubMed]
- Ishizaki, T.; Maekawa, M.; Fujisawa, K.; Okawa, K.; Iwamatsu, A.; Fujita, A.; Watanabe, N.; Saito, Y.; Kakizuka, A.; Morii, N.; et al. The small GTP-binding protein Rho binds to and activates a 160 kDa Ser/Thr protein kinase homologous to myotonic dystrophy kinase. EMBO J. 1996, 15, 1885–1893. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Amano, M.; Yamamoto, T.; Chihara, K.; Nakafuku, M.; Ito, M.; Nakano, T.; Okawa, K.; Iwamatsu, A.; Kaibuchi, K. Rho-associated kinase, a novel serine/threonine kinase, as a putative target for small GTP binding protein Rho. EMBO J. 1996, 15, 2208–2216. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, O.; Fujisawa, K.; Ishizaki, T.; Saito, Y.; Nakao, K.; Narumiya, S. ROCK-I and ROCK-II, two isoforms of Rho-associated coiled-coil forming protein serine/threonine kinase in mice. FEBS Lett. 1996, 392, 189–193. [Google Scholar] [CrossRef]
- Fukata, Y.; Kaibuchi, K.; Amano, M. Rho-Rho-kinase pathway in smooth muscle contraction and cytoskeletal reorganization of non-muscle cells. Trends Pharmacol. Sci. 2001, 22, 32–39. [Google Scholar] [CrossRef]
- Hartshorne, D.J.; Ito, M.; Erdodi, F. Myosin light chain phosphatase: Subunit composition, interactions and regulation. J. Muscle Res. Cell Motil. 1998, 19, 325–341. [Google Scholar] [CrossRef]
- Kimura, K.; Ito, M.; Amano, M.; Chihara, K.; Fukata, Y.; Nakafuku, M.; Yamamori, B.; Feng, J.; Nakano, T.; Okawa, K.; et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science 1996, 273, 245–248. [Google Scholar] [CrossRef]
- Somlyo, A.P.; Somlyo, A.V. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: Modulated by G proteins, kinases, and myosin phosphatase. Physiol. Rev. 2003, 83, 1325–1358. [Google Scholar] [CrossRef]
- Ying, Z.; Giachini, F.R.; Tostes, R.C.; Webb, R.C. PYK2/PDZ-RhoGEF links Ca2+ signaling to RhoA. Arter. Thromb. Vasc. Biol. 2009, 29, 1657–1663. [Google Scholar] [CrossRef]
- Aida, K.; Mita, M.; Ishii-Nozawa, R. Role of RhoGEFs or RhoGAPs in Pyk2-Mediated RhoA Activation in Depolarization-Induced Contraction of Rat Caudal Arterial Smooth Muscle. Int. J. Mol. Sci. 2025, 26, 8676. [Google Scholar] [CrossRef]
- Martinsen, A.; Yerna, X.; Rath, G.; Gomez, E.L.; Dessy, C.; Morel, N. Different effect of Rho kinase inhibition on calcium signaling in rat isolated large and small arteries. J. Vasc. Res. 2012, 49, 522–533. [Google Scholar] [CrossRef]
- Martinsen, A.; Dessy, C.; Morel, N. Regulation of calcium channels in smooth muscle: New insights into the role of myosin light chain kinase. Channels 2014, 8, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Inaba, H.; Miao, Q.; Nakata, T. Optogenetic control of small GTPases reveals RhoA mediates intracellular calcium signaling. J. Biol. Chem. 2021, 296, 100290. [Google Scholar] [CrossRef]
- Amano, M.; Fukata, Y.; Kaibuchi, K. Regulation and functions of Rho-associated kinase. Exp. Cell Res. 2000, 261, 44–51. [Google Scholar] [CrossRef]
- Ikebe, M.; Hartshorne, D.J. Phosphorylation of smooth muscle myosin at two distinct sites by myosin light chain kinase. J. Biol. Chem. 1985, 260, 10027–10031. [Google Scholar] [CrossRef]
- Koyama, H.; Raines, E.W.; Bornfeldt, K.E.; Roberts, J.M.; Ross, R. Fibrillar collagen inhibits arterial smooth muscle proliferation through regulation of Cdk2 inhibitors. Cell 1996, 87, 1069–1078. [Google Scholar] [CrossRef]
- Yao, L.; Romero, M.J.; Toque, H.A.; Yang, G.; Caldwell, R.B.; Caldwell, R.W. The role of RhoA/Rho kinase pathway in endothelial dysfunction. J. Cardiovasc. Dis. Res. 2010, 1, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Kinzenbaw, D.A.; Langmack, L.; Faraci, F.M. Angiotensin II-induced endothelial dysfunction: Impact of sex, genetic background, and rho kinase. Physiol. Rep. 2022, 10, e15336. [Google Scholar] [CrossRef]
- de Almeida, A.; de Oliveira, J.; da Silva Pontes, L.V.; de Souza Junior, J.F.; Goncalves, T.A.F.; Dantas, S.H.; de Almeida Feitosa, M.S.; Silva, A.O.; de Medeiros, I.A. ROS: Basic Concepts, Sources, Cellular Signaling, and its Implications in Aging Pathways. Oxid. Med. Cell. Longev. 2022, 2022, 1225578. [Google Scholar] [CrossRef] [PubMed]
- Jitca, G.; Osz, B.E.; Tero-Vescan, A.; Miklos, A.P.; Rusz, C.M.; Batrinu, M.G.; Vari, C.E. Positive Aspects of Oxidative Stress at Different Levels of the Human Body: A Review. Antioxidants 2022, 11, 572. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Afzal, S.; Abdul Manap, A.S.; Attiq, A.; Albokhadaim, I.; Kandeel, M.; Alhojaily, S.M. From imbalance to impairment: The central role of reactive oxygen species in oxidative stress-induced disorders and therapeutic exploration. Front. Pharmacol. 2023, 14, 1269581. [Google Scholar] [CrossRef]
- Checa, J.; Aran, J.M. Reactive Oxygen Species: Drivers of Physiological and Pathological Processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef]
- Amberg, G.C.; Earley, S.; Glapa, S.A. Local regulation of arterial L-type calcium channels by reactive oxygen species. Circ. Res. 2010, 107, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- AlAhmad, M.; Shitaw, E.E.; Sivaprasadarao, A. A TRPM2-Driven Signalling Cycle Orchestrates Abnormal Inter-Organelle Crosstalk in Cardiovascular and Metabolic Diseases. Biomolecules 2025, 15, 1193. [Google Scholar] [CrossRef] [PubMed]
- Giachini, F.R.; Chiao, C.W.; Carneiro, F.S.; Lima, V.V.; Carneiro, Z.N.; Dorrance, A.M.; Tostes, R.C.; Webb, R.C. Increased activation of stromal interaction molecule-1/Orai-1 in aorta from hypertensive rats: A novel insight into vascular dysfunction. Hypertension 2009, 53, 409–416. [Google Scholar] [CrossRef]
- Parekh, A.B. Ca2+ microdomains near plasma membrane Ca2+ channels: Impact on cell function. J. Physiol. 2008, 586, 3043–3054. [Google Scholar] [CrossRef] [PubMed]
- Billaud, M.; Lohman, A.W.; Johnstone, S.R.; Biwer, L.A.; Mutchler, S.; Isakson, B.E. Regulation of cellular communication by signaling microdomains in the blood vessel wall. Pharmacol. Rev. 2014, 66, 513–569. [Google Scholar] [CrossRef]
- Suzuki, Y. Ca2+ microdomains in vascular smooth muscle cells: Roles in vascular tone regulation and hypertension. J. Pharmacol. Sci. 2025, 158, 59–67. [Google Scholar] [CrossRef]
- El Atab, O.; Ghantous, C.M.; El-Zein, N.; Farhat, R.; Agouni, A.; Korashy, H.M.; Djouhri, L.; Kamareddine, L.; Zibara, K.; Zeidan, A. Involvement of caveolae in hyperglycemia-induced changes in adiponectin and leptin expressions in vascular smooth muscle cells. Eur. J. Pharmacol. 2022, 919, 174701. [Google Scholar] [CrossRef]
- Webb, R.C. Smooth muscle contraction and relaxation. Adv. Physiol. Educ. 2003, 27, 201–206. [Google Scholar] [CrossRef]
- Sumoza-Toledo, A.; Penner, R. TRPM2: A multifunctional ion channel for calcium signalling. J. Physiol. 2011, 589, 1515–1525. [Google Scholar] [CrossRef] [PubMed]
- Welsh, C.L.; Madan, L.K. Protein Tyrosine Phosphatase regulation by Reactive Oxygen Species. Adv. Cancer Res. 2024, 162, 45–74. [Google Scholar] [CrossRef] [PubMed]
- Trebak, M.; Ginnan, R.; Singer, H.A.; Jourd’heuil, D. Interplay Between calcium and reactive oxygen/nitrogen species: An essential paradigm for vascular smooth muscle signaling. Antioxid. Redox Signal. 2010, 12, 657–674. [Google Scholar] [CrossRef]
- Roberts-Craig, F.T.; Worthington, L.P.; O’Hara, S.P.; Erickson, J.R.; Heather, A.K.; Ashley, Z. CaMKII Splice Variants in Vascular Smooth Muscle Cells: The Next Step or Redundancy? Int. J. Mol. Sci. 2022, 23, 7916. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Li, W.; Jin, R.; Yu, S.; Xie, D.; Zheng, X.; Zhong, W.; Cheng, X.; Hu, S.; Li, M.; et al. PI3Kγ (Phosphoinositide 3-Kinase γ) Regulates Vascular Smooth Muscle Cell Phenotypic Modulation and Neointimal Formation Through CREB (Cyclic AMP-Response Element Binding Protein)/YAP (Yes-Associated Protein) Signaling. Arter. Thromb. Vasc. Biol. 2019, 39, e91–e105. [Google Scholar] [CrossRef]
- Byon, C.H.; Heath, J.M.; Chen, Y. Redox signaling in cardiovascular pathophysiology: A focus on hydrogen peroxide and vascular smooth muscle cells. Redox Biol. 2016, 9, 244–253. [Google Scholar] [CrossRef]
- Erickson, J.R.; Joiner, M.L.; Guan, X.; Kutschke, W.; Yang, J.; Oddis, C.V.; Bartlett, R.K.; Lowe, J.S.; O’Donnell, S.E.; Aykin-Burns, N.; et al. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell 2008, 133, 462–474. [Google Scholar] [CrossRef]
- Luczak, E.D.; Anderson, M.E. CaMKII oxidative activation and the pathogenesis of cardiac disease. J. Mol. Cell. Cardiol. 2014, 73, 112–116. [Google Scholar] [CrossRef]
- Ashino, T.; Yamamoto, M.; Yoshida, T.; Numazawa, S. Redox-sensitive transcription factor Nrf2 regulates vascular smooth muscle cell migration and neointimal hyperplasia. Arter. Thromb. Vasc. Biol. 2013, 33, 760–768. [Google Scholar] [CrossRef]
- Ginnan, R.; Guikema, B.J.; Halligan, K.E.; Singer, H.A.; Jourd’heuil, D. Regulation of smooth muscle by inducible nitric oxide synthase and NADPH oxidase in vascular proliferative diseases. Free Radic. Biol. Med. 2008, 44, 1232–1245. [Google Scholar] [CrossRef]
- Meng, T.C.; Fukada, T.; Tonks, N.K. Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol. Cell 2002, 9, 387–399. [Google Scholar] [CrossRef]
- Touyz, R.M.; Alves-Lopes, R.; Rios, F.J.; Camargo, L.L.; Anagnostopoulou, A.; Arner, A.; Montezano, A.C. Vascular smooth muscle contraction in hypertension. Cardiovasc. Res. 2018, 114, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Denniss, S.G.; Jeffery, A.J.; Rush, J.W. RhoA-Rho kinase signaling mediates endothelium- and endoperoxide-dependent contractile activities characteristic of hypertensive vascular dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H1391–H1405. [Google Scholar] [CrossRef]
- Wirth, A. Rho kinase and hypertension. Biochim. Biophys. Acta 2010, 1802, 1276–1284. [Google Scholar] [CrossRef]
- Seko, T.; Ito, M.; Kureishi, Y.; Okamoto, R.; Moriki, N.; Onishi, K.; Isaka, N.; Hartshorne, D.J.; Nakano, T. Activation of RhoA and inhibition of myosin phosphatase as important components in hypertension in vascular smooth muscle. Circ. Res. 2003, 92, 411–418. [Google Scholar] [CrossRef]
- Uehata, M.; Ishizaki, T.; Satoh, H.; Ono, T.; Kawahara, T.; Morishita, T.; Tamakawa, H.; Yamagami, K.; Inui, J.; Maekawa, M.; et al. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature 1997, 389, 990–994. [Google Scholar] [CrossRef]
- Li, C.; Ma, L.; Wang, Q.; Shao, X.; Guo, L.; Chen, J.; Wang, W.; Yu, J. Rho kinase inhibition ameliorates vascular remodeling and blood pressure elevations in a rat model of apatinib-induced hypertension. J. Hypertens. 2022, 40, 675–684. [Google Scholar] [CrossRef]
- Johnson, A.C.; Wu, W.; Attipoe, E.M.; Sasser, J.M.; Taylor, E.B.; Showmaker, K.C.; Kyle, P.B.; Lindsey, M.L.; Garrett, M.R. Loss of Arhgef11 in the Dahl Salt-Sensitive Rat Protects Against Hypertension-Induced Renal Injury. Hypertension 2020, 75, 1012–1024. [Google Scholar] [CrossRef] [PubMed]
- Toth, A.; Balogh, E.; Jeney, V. Regulation of Vascular Calcification by Reactive Oxygen Species. Antioxidants 2020, 9, 963. [Google Scholar] [CrossRef] [PubMed]
- Ming, X.F.; Barandier, C.; Viswambharan, H.; Kwak, B.R.; Mach, F.; Mazzolai, L.; Hayoz, D.; Ruffieux, J.; Rusconi, S.; Montani, J.P.; et al. Thrombin stimulates human endothelial arginase enzymatic activity via RhoA/ROCK pathway: Implications for atherosclerotic endothelial dysfunction. Circulation 2004, 110, 3708–3714. [Google Scholar] [CrossRef]
- Takemoto, M.; Sun, J.; Hiroki, J.; Shimokawa, H.; Liao, J.K. Rho-kinase mediates hypoxia-induced downregulation of endothelial nitric oxide synthase. Circulation 2002, 106, 57–62. [Google Scholar] [CrossRef]
- Cao, Y.; Fang, Y.; Mu, J.; Liu, X. High salt medium activates RhoA/ROCK and downregulates eNOS expression via the upregulation of ADMA. Mol. Med. Rep. 2016, 14, 606–612. [Google Scholar] [CrossRef][Green Version]
- Gavard, J.; Gutkind, J.S. Protein kinase C-related kinase and ROCK are required for thrombin-induced endothelial cell permeability downstream from Gα12/13 and Gα11/q. J. Biol. Chem. 2008, 283, 29888–29896. [Google Scholar] [CrossRef] [PubMed]
- Gimbrone, M.A., Jr.; Garcia-Cardena, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef]
- Renna, N.F.; de Las Heras, N.; Miatello, R.M. Pathophysiology of vascular remodeling in hypertension. Int. J. Hypertens. 2013, 2013, 808353. [Google Scholar] [CrossRef]
- Xu, S.; Touyz, R.M. Reactive oxygen species and vascular remodelling in hypertension: Still alive. Can. J. Cardiol. 2006, 22, 947–951. [Google Scholar] [CrossRef]
- Staiculescu, M.C.; Foote, C.; Meininger, G.A.; Martinez-Lemus, L.A. The role of reactive oxygen species in microvascular remodeling. Int. J. Mol. Sci. 2014, 15, 23792–23835. [Google Scholar] [CrossRef]
- Chen, Z.; Guo, L.; Hadas, J.; Gutowski, S.; Sprang, S.R.; Sternweis, P.C. Activation of p115-RhoGEF requires direct association of Gα13 and the Dbl homology domain. J. Biol. Chem. 2012, 287, 25490–25500. [Google Scholar] [CrossRef] [PubMed]
- Eckenstaler, R.; Hauke, M.; Benndorf, R.A. A current overview of RhoA, RhoB, and RhoC functions in vascular biology and pathology. Biochem. Pharmacol. 2022, 206, 115321. [Google Scholar] [CrossRef] [PubMed]
- Rivera, P.; Ocaranza, M.P.; Lavandero, S.; Jalil, J.E. Rho kinase activation and gene expression related to vascular remodeling in normotensive rats with high angiotensin I converting enzyme levels. Hypertension 2007, 50, 792–798. [Google Scholar] [CrossRef]
- Leguina-Ruzzi, A.; Pereira, J.; Pereira-Flores, K.; Valderas, J.P.; Mezzano, D.; Velarde, V.; Saez, C.G. Increased RhoA/Rho-Kinase Activity and Markers of Endothelial Dysfunction in Young Adult Subjects with Metabolic Syndrome. Metab. Syndr. Relat. Disord. 2015, 13, 373–380. [Google Scholar] [CrossRef]
- Dai, Y.; Luo, W.; Chang, J. Rho kinase signaling and cardiac physiology. Curr. Opin. Physiol. 2018, 1, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, R.; Li, Y.; Noma, K.; Hiroi, Y.; Liu, P.Y.; Taniguchi, M.; Ito, M.; Liao, J.K. FHL2 prevents cardiac hypertrophy in mice with cardiac-specific deletion of ROCK2. FASEB J. 2013, 27, 1439–1449. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Boiti, A.; Vallone, D.; Foulkes, N.S. Reactive Oxygen Species Signaling and Oxidative Stress: Transcriptional Regulation and Evolution. Antioxidants 2024, 13, 312. [Google Scholar] [CrossRef]
- Tran, N.; Mills, E.L. Redox regulation of macrophages. Redox Biol. 2024, 72, 103123. [Google Scholar] [CrossRef]
- Rossol, M.; Pierer, M.; Raulien, N.; Quandt, D.; Meusch, U.; Rothe, K.; Schubert, K.; Schoneberg, T.; Schaefer, M.; Krugel, U.; et al. Extracellular Ca2+ is a danger signal activating the NLRP3 inflammasome through G protein-coupled calcium sensing receptors. Nat. Commun. 2012, 3, 1329. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.M.; Ling, Y.H.; Huuskes, B.M.; Ferens, D.M.; Saini, N.; Chan, C.T.; Diep, H.; Kett, M.M.; Samuel, C.S.; Kemp-Harper, B.K.; et al. Pharmacological inhibition of the NLRP3 inflammasome reduces blood pressure, renal damage, and dysfunction in salt-sensitive hypertension. Cardiovasc. Res. 2019, 115, 776–787. [Google Scholar] [CrossRef]
- Zheng, Y.; Xu, L.; Dong, N.; Li, F. NLRP3 inflammasome: The rising star in cardiovascular diseases. Front. Cardiovasc. Med. 2022, 9, 927061. [Google Scholar] [CrossRef]
- Bai, B.; Yang, Y.; Wang, Q.; Li, M.; Tian, C.; Liu, Y.; Aung, L.H.H.; Li, P.F.; Yu, T.; Chu, X.M. NLRP3 inflammasome in endothelial dysfunction. Cell Death Dis. 2020, 11, 776. [Google Scholar] [CrossRef]
- Burger, F.; Baptista, D.; Roth, A.; da Silva, R.F.; Montecucco, F.; Mach, F.; Brandt, K.J.; Miteva, K. NLRP3 Inflammasome Activation Controls Vascular Smooth Muscle Cells Phenotypic Switch in Atherosclerosis. Int. J. Mol. Sci. 2021, 23, 340. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhu, Q.; Yu, X.; Liang, X.; Guan, H.; Zhao, H.; Yao, W. RhoGDI3 at the trans-Golgi network participates in NLRP3 inflammasome activation, VSMC phenotypic modulation, and neointima formation. Atherosclerosis 2023, 387, 117391. [Google Scholar] [CrossRef]
- Nunes, K.P.; Webb, R.C. New insights into RhoA/Rho-kinase signaling: A key regulator of vascular contraction. Small GTPases 2021, 12, 458–469. [Google Scholar] [CrossRef]
- Batty, M.; Bennett, M.R.; Yu, E. The Role of Oxidative Stress in Atherosclerosis. Cells 2022, 11, 3843. [Google Scholar] [CrossRef]
- Calo, L.A.; Davis, P.A.; Pagnin, E.; Dal Maso, L.; Maiolino, G.; Seccia, T.M.; Pessina, A.C.; Rossi, G.P. Increased level of p63RhoGEF and RhoA/Rho kinase activity in hypertensive patients. J. Hypertens. 2014, 32, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Nohria, A.; Grunert, M.E.; Rikitake, Y.; Noma, K.; Prsic, A.; Ganz, P.; Liao, J.K.; Creager, M.A. Rho kinase inhibition improves endothelial function in human subjects with coronary artery disease. Circ. Res. 2006, 99, 1426–1432. [Google Scholar] [CrossRef]
- Hidaka, T.; Hata, T.; Soga, J.; Fujii, Y.; Idei, N.; Fujimura, N.; Kihara, Y.; Noma, K.; Liao, J.K.; Higashi, Y. Increased leukocyte rho kinase (ROCK) activity and endothelial dysfunction in cigarette smokers. Hypertens. Res. 2010, 33, 354–359. [Google Scholar] [CrossRef]
- Hahmann, C.; Schroeter, T. Rho-kinase inhibitors as therapeutics: From pan inhibition to isoform selectivity. Cell. Mol. Life Sci. 2010, 67, 171–177. [Google Scholar] [CrossRef]
- Cheng, Z.; Erhardt, S.; Wang, J. Rho/ROCK Pathway as a Therapeutic Target in Multiple Diseases. Int. J. Drug Discov. Pharmacol. 2025, 4, 100018. [Google Scholar] [CrossRef]
- Liu, L.C.; Chen, Y.H.; Lu, D.W. The Application of Rho Kinase Inhibitors in the Management of Glaucoma. Int. J. Mol. Sci. 2024, 25, 5576. [Google Scholar] [CrossRef]
- Fehrenbach, D.J.; Nguyen, B.; Alexander, M.R.; Madhur, M.S. Modulating T Cell Phenotype and Function to Treat Hypertension. Kidney360 2023, 4, e534–e543. [Google Scholar] [CrossRef]
- Althaf Umar, K.P.; Anagha, R.A.; Sreeja, C.N.; Kanthlal, S.K. Nanoparticle-based approaches for vascular inflammation in managing hypertension: Advancing molecular mechanisms and treatment strategies. Drug Deliv. Transl. Res. 2025, 1–31. [Google Scholar] [CrossRef]
- Loirand, G.; Pacaud, P. Involvement of Rho GTPases and their regulators in the pathogenesis of hypertension. Small GTPases 2014, 5, e983866. [Google Scholar] [CrossRef]
- Wang, J.; Yuan, Z.; Yu, N.; Jiao, Q.; Zhou, H.; Liao, W.; Shan, J.; Ruan, S.; Zhao, Y.; Mo, Y.; et al. Inactivation of RhoA for Hypertension Treatment Through the TRPV4-RhoA-RhoGDI1 Axis. Circulation 2025, 152, 519–536. [Google Scholar] [CrossRef]
- Dong, M.; Yan, B.P.; Liao, J.K.; Lam, Y.Y.; Yip, G.W.; Yu, C.M. Rho-kinase inhibition: A novel therapeutic target for the treatment of cardiovascular diseases. Drug Discov. Today 2010, 15, 622–629. [Google Scholar] [CrossRef]
- Hartmann, S.; Ridley, A.J.; Lutz, S. The Function of Rho-Associated Kinases ROCK1 and ROCK2 in the Pathogenesis of Cardiovascular Disease. Front. Pharmacol. 2015, 6, 276. [Google Scholar] [CrossRef]
- Fayed, H.S.; Bakleh, M.Z.; Ashraf, J.V.; Howarth, A.; Ebner, D.; Al Haj Zen, A. Selective ROCK Inhibitor Enhances Blood Flow Recovery After Hindlimb Ischemia. Int. J. Mol. Sci. 2023, 24, 14410. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Hall, A. Guanine nucleotide exchange factors for Rho GTPases: Turning on the switch. Genes Dev. 2002, 16, 1587–1609. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.I.; Lin, Y.C.; Tsai, T.H.; Lin, H.S.; Liou, C.W.; Chang, W.N.; Lu, C.H.; Yuen, C.M.; Yip, H.K. The prognostic values of leukocyte Rho kinase activity in acute ischemic stroke. Biomed. Res. Int. 2014, 2014, 214587. [Google Scholar] [CrossRef]
- Liu, P.Y.; Chen, J.H.; Lin, L.J.; Liao, J.K. Increased Rho kinase activity in a Taiwanese population with metabolic syndrome. J. Am. Coll. Cardiol. 2007, 49, 1619–1624. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.M.; Nagrale, P. Rho Kinase Inhibitors as a Neuroprotective Pharmacological Intervention for the Treatment of Glaucoma. Cureus 2022, 14, e28445. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Randar, S.; Silva-Velasco, D.L.; Priviero, F.; Webb, R.C. RhoA/Rho-Kinase Signaling in Vascular Smooth Muscle and Endothelium: Mechanistic Insights and Translational Implications in Hypertension. Biomolecules 2025, 15, 1607. https://doi.org/10.3390/biom15111607
Randar S, Silva-Velasco DL, Priviero F, Webb RC. RhoA/Rho-Kinase Signaling in Vascular Smooth Muscle and Endothelium: Mechanistic Insights and Translational Implications in Hypertension. Biomolecules. 2025; 15(11):1607. https://doi.org/10.3390/biom15111607
Chicago/Turabian StyleRandar, Stephanie, Diana L. Silva-Velasco, Fernanda Priviero, and R. Clinton Webb. 2025. "RhoA/Rho-Kinase Signaling in Vascular Smooth Muscle and Endothelium: Mechanistic Insights and Translational Implications in Hypertension" Biomolecules 15, no. 11: 1607. https://doi.org/10.3390/biom15111607
APA StyleRandar, S., Silva-Velasco, D. L., Priviero, F., & Webb, R. C. (2025). RhoA/Rho-Kinase Signaling in Vascular Smooth Muscle and Endothelium: Mechanistic Insights and Translational Implications in Hypertension. Biomolecules, 15(11), 1607. https://doi.org/10.3390/biom15111607





