Gut Microbiota and Ferroptosis in Colorectal Cancer: A Comprehensive Review of Mechanisms and Therapeutic Strategies to Overcome Immune Checkpoint Resistance
Abstract
1. Introduction
2. Regulatory Mechanisms of Ferroptosis in Colorectal Cancer and Its Immunological Significance
2.1. Key Regulators of Ferroptotic Pathways in CRC
2.1.1. System Xc−–GPX4 Axis Dysfunction
2.1.2. Post-Translational Regulation of GPX4: The Central Role of Palmitoylation
2.1.3. Iron Metabolism Reprogramming and Iron Overload
2.1.4. Lipid Metabolism Determines Oxidative Susceptibility
2.2. Immunomodulatory Consequences of Ferroptosis in the TME
2.2.1. Immunogenic Cell Death (ICD) and Activation of Antitumor Responses
2.2.2. Bidirectional Regulation of Immune Cell Function
2.2.3. Potential Immunosuppressive Risks Mediated by Ferroptosis
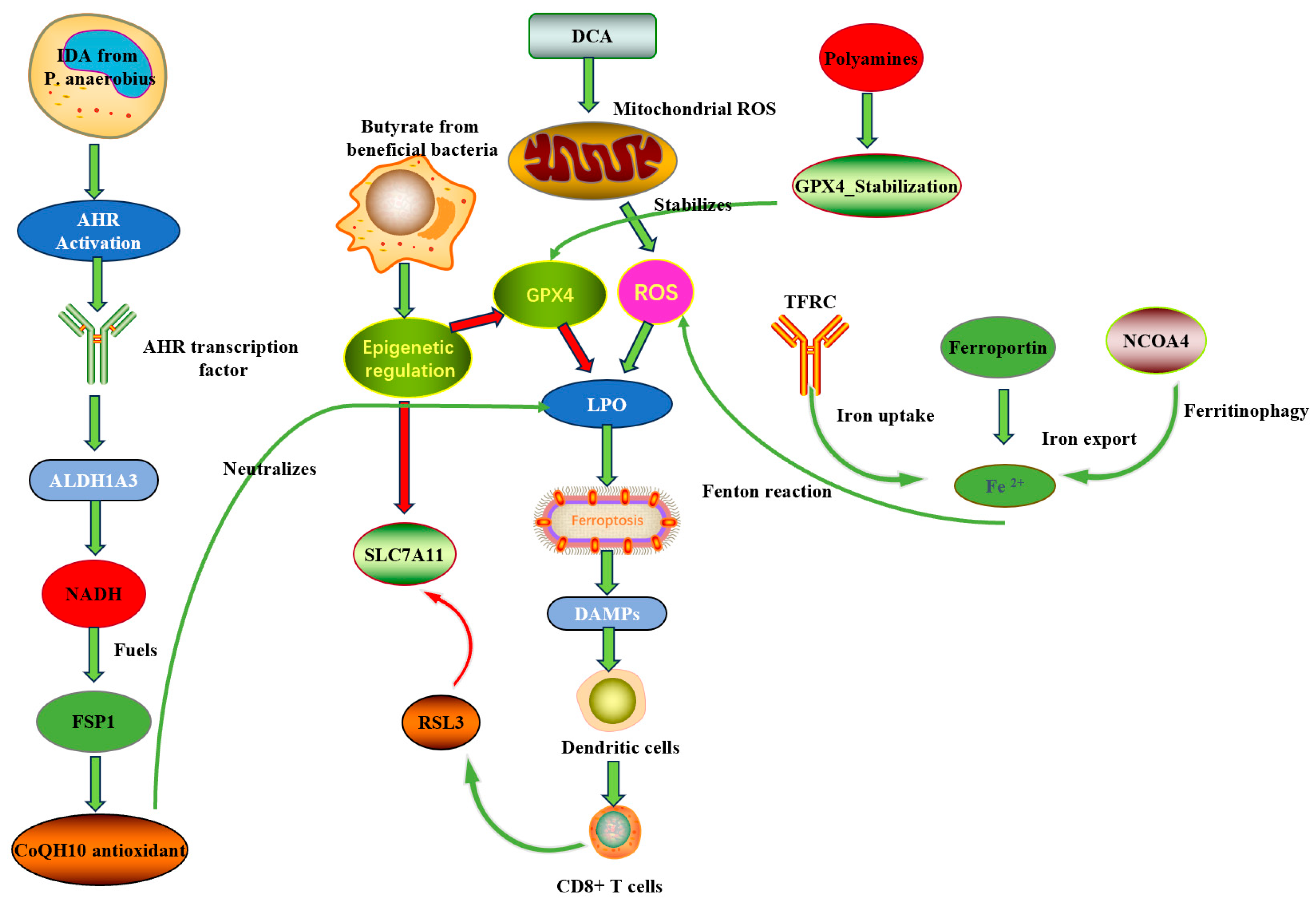
3. Molecular Mechanisms by Which the Gut Microbiota Regulates Ferroptosis and Its Immune Interactions
3.1. Microbial Metabolites Directly Regulate Core Ferroptosis Pathways
3.1.1. Short-Chain Fatty Acids (SCFAs): Epigenetic and Metabolic Regulation
3.1.2. Polyamines and GPX4 Protein Stability
3.1.3. Tryptophan Metabolites: The IDA → AHR → ALDH1A3 → FSP1 Axis
3.1.4. Vitamins and Cofactors: Modulators with Bidirectional Effects
3.2. Microbiota Regulation of Systemic and Local Iron Homeostasis
3.2.1. Nutritional Competition and “Nutritional Immunity”
3.2.2. Regulation of Host Iron Transport Genes
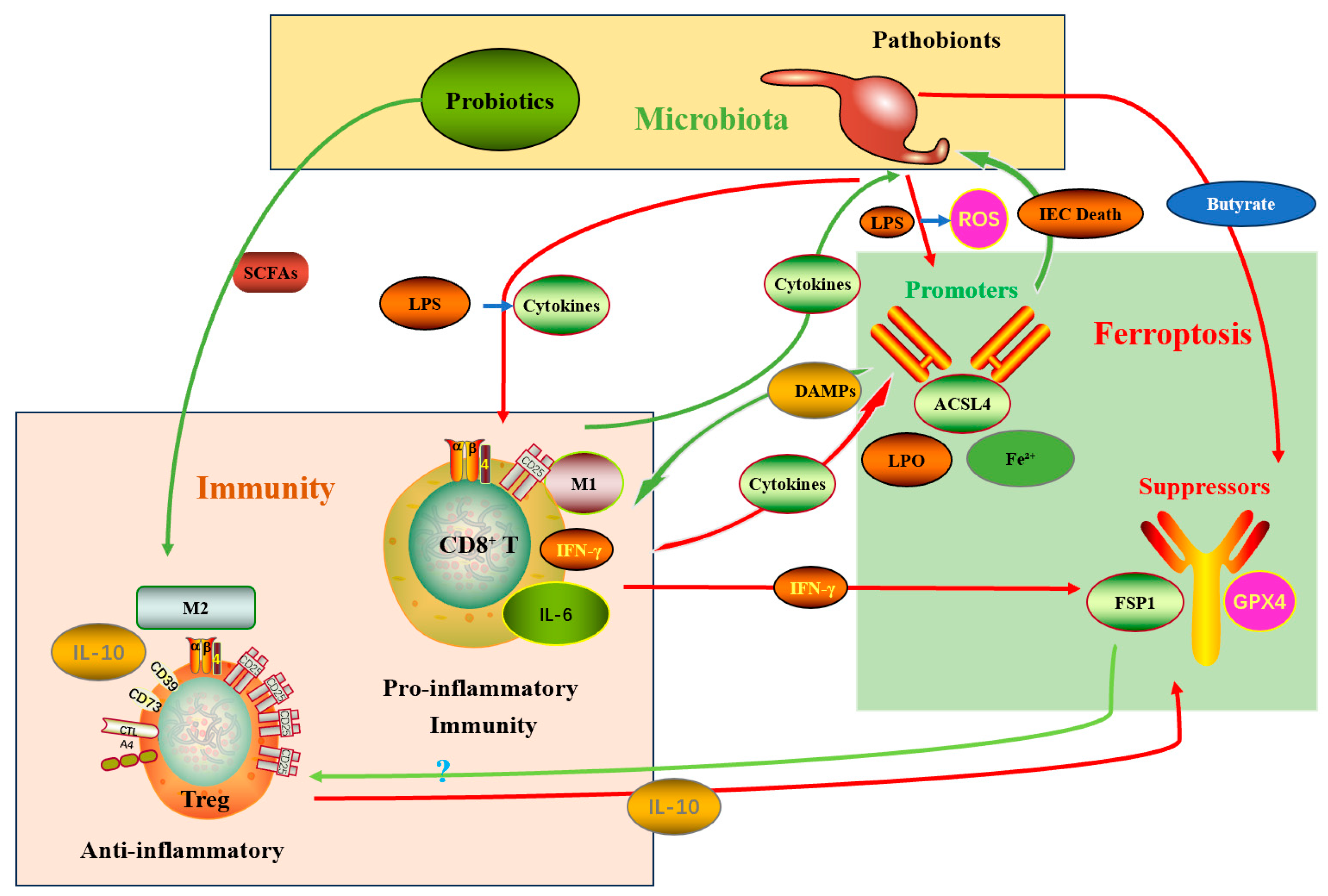
3.3. The “Microbiota–Immune–Ferroptosis” Triangular Interaction Network
3.3.1. Direct Arm (Microbe → Tumor Cell → Ferroptosis)
3.3.2. Indirect Arm (Microbe → Immune System → Tumor Ferroptosis)—The Core Therapeutic Value
3.3.3. Immunological Caveats: The Double-Edged Sword of Ferroptosis
3.3.4. Clinical and Translational Implication of the Triangular Network
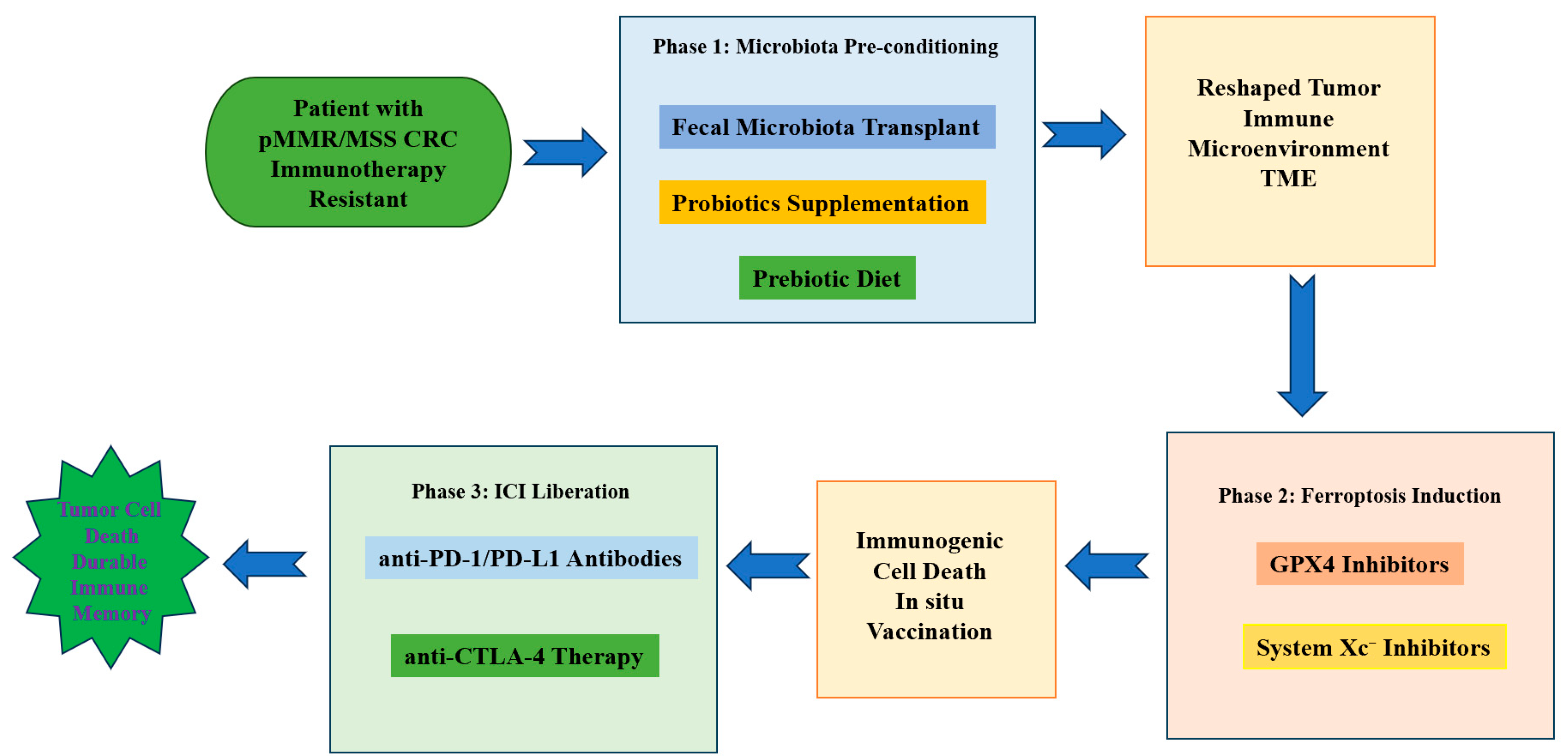
4. Strategies Targeting the “Microbiota–Ferroptosis” Axis to Sensitize CRC Immunotherapy
4.1. Microbiota-Directed Interventions
4.1.1. Probiotics, Prebiotics and Synbiotics
4.1.2. Fecal Microbiota Transplantation (FMT) and Defined Microbial Consortia
4.1.3. Bacteriophage and Precision Antimicrobials
4.1.4. Engineered Commensals and Live Biotherapeutic Products
4.2. Diet and Metabolite-Level Interventions
4.3. Pharmacologic Modulation of Ferroptosis
4.3.1. Direct Ferroptosis Inducers
4.3.2. Iron-Modulating Agents
4.3.3. Combinatorial Small-Molecule Approaches
4.3.4. Role of Ferroptosis Inhibitors in Clinical Oncology
4.4. Combination Strategies with Immune Checkpoint Blockade
4.5. Tumor-Targeted Delivery Platforms
4.6. Biomarker-Driven Patient Selection and Monitoring
4.7. Safety Considerations and Mitigation of Adverse Effects
4.8. Clinical Translation: Trial Design Considerations
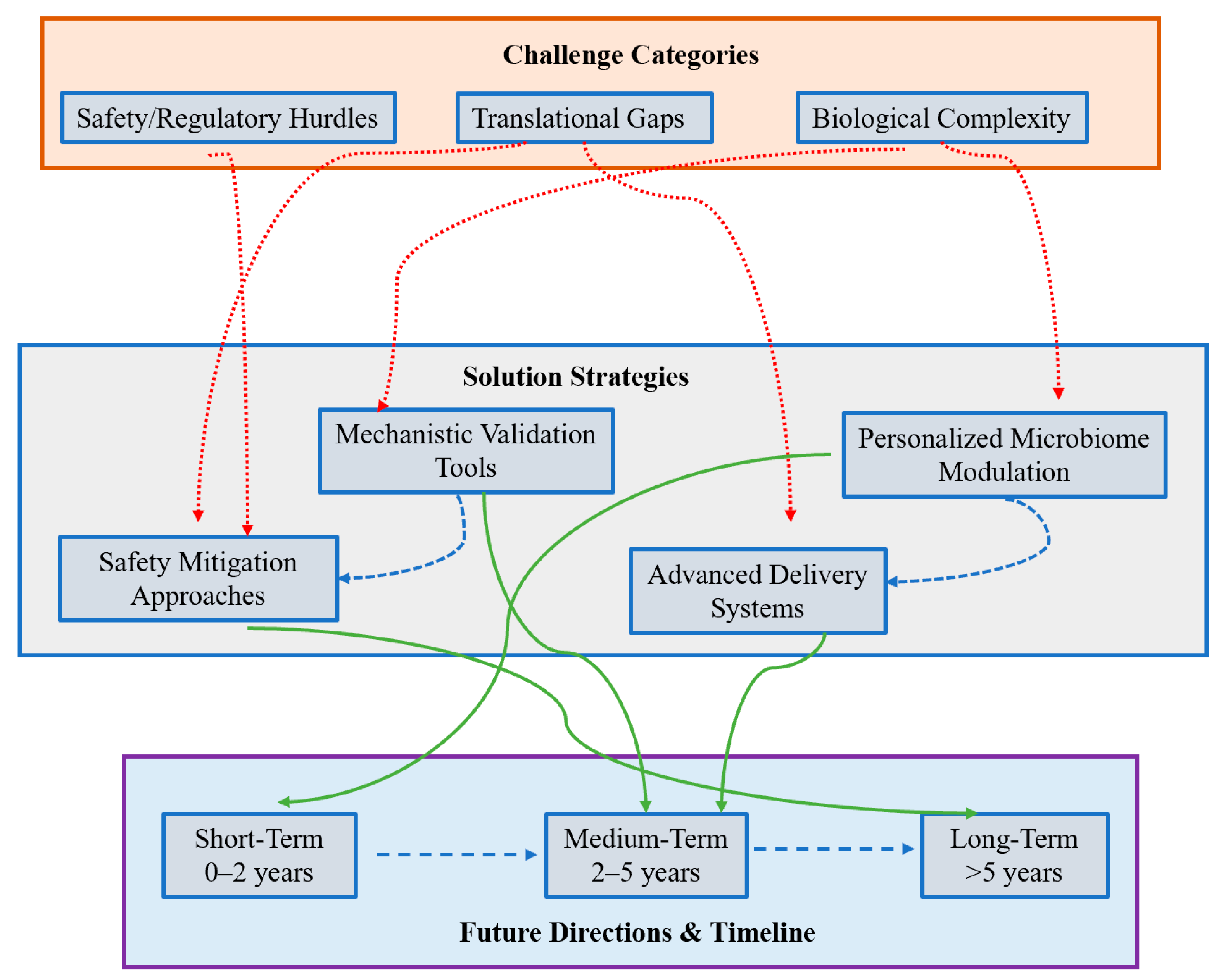
5. Challenges and Future Directions & Conclusions
5.1. Major Challenges
5.1.1. Biological Complexity and Interindividual Variability
5.1.2. Causal Inference Versus Correlation in Human Studies
5.1.3. Differential Sensitivity of Immune and Non-Immune Host Cells
5.1.4. Safety and Infection Risk with Microbiome Manipulation
5.1.5. Adaptive Resistance and Metabolic Compensation
5.1.6. Biomarker Standardization and Assay Limitations
5.1.7. Regulatory, Manufacturing and Ethical Considerations
5.2. Recommended Future Directions
5.2.1. Deep Mechanistic Dissection in Tractable Experimental Systems
5.2.2. Multi-Omics, Spatial and Single-Cell Profiling in Human Cohorts
5.2.3. Rational Design of Combination Regimens and Delivery Modalities
5.2.4. Precision Microbiome Engineering and Targeted Depletion
5.2.5. Biomarker Development and Adaptive Clinical Trial Platforms
5.2.6. Cross-Disciplinary Consortia and Data Sharing
5.3. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, H.; Cheng, Y.; Mao, C.; Liu, S.; Xiao, D.; Huang, J.; Tao, Y. Emerging mechanisms and targeted therapy of ferroptosis in cancer. Mol. Ther. 2021, 29, 2185–2208. [Google Scholar] [CrossRef]
- Liang, Y.; Lan, H.; Li, Q.; Gao, M.; Liu, M.; Xu, Z.; Gao, Y.; Zhang, L.; Li, Y.; Zhao, B. Exploiting metabolic vulnerabilities through synergistic ferroptosis and disulfidptosis for breast cancer therapy. J. Adv. Res. 2025. [Google Scholar] [CrossRef]
- Yang, F.; Xiao, Y.; Ding, J.; Jin, X.; Li, D.-Q.; Shi, J.-X.; Huang, W.; Wang, Y.-P.; Jiang, Y.; Shao, Z. Ferroptosis heterogeneity in triple-negative breast cancer reveals an innovative immunotherapy combination strategy. Cell Metab. 2022, 35, 84–100. [Google Scholar] [CrossRef]
- Li, J.; Liu, J.; Zhou, Z.; Wu, R.; Chen, X.; Yu, C.; Stockwell, B.; Kroemer, G.; Kang, R.; Tang, D. Tumor-specific GPX4 degradation enhances ferroptosis-initiated antitumor immune response in mouse models of pancreatic cancer. Sci. Transl. Med. 2023, 15, eadg3049. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Havel, J.; Chowell, D.; Chan, T. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat. Rev. Cancer 2019, 19, 133–150. [Google Scholar] [CrossRef]
- Taieb, J.; Svrcek, M.; Cohen, R.; Basile, D.; Tougeron, D.; Phelip, J. Deficient mismatch repair/microsatellite unstable colorectal cancer: Diagnosis, prognosis and treatment. Eur. J. Cancer 2022, 175, 136–157. [Google Scholar] [CrossRef]
- Cohen, R.; Colle, R.; Pudlarz, T.; Heran, M.; Duval, A.; Svrcek, M.; André, T. Immune Checkpoint Inhibition in Metastatic Colorectal Cancer Harboring Microsatellite Instability or Mismatch Repair Deficiency. Cancers 2021, 13, 1149. [Google Scholar] [CrossRef]
- Zhou, B.; Liu, J.; Kang, R.; Klionsky, D.; Kroemer, G.; Tang, D. Ferroptosis is a type of autophagy-dependent cell death. Semin. Cancer Biol. 2020, 66, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Ru, Q.; Li, Y.; Chen, L.; Wu, Y.; Min, J.; Wang, F. Iron homeostasis and ferroptosis in human diseases: Mechanisms and therapeutic prospects. Signal Transduct. Target. Ther. 2024, 9, 271. [Google Scholar] [CrossRef]
- Zheng, Y.; Sun, L.; Guo, J.; Ma, J. The crosstalk between ferroptosis and anti-tumor immunity in the tumor microenvironment: Molecular mechanisms and therapeutic controversy. Cancer Commun. 2023, 43, 1071–1096. [Google Scholar] [CrossRef]
- Tang, R.; Xu, J.; Zhang, B.; Liu, J.; Liang, C.; Hua, J.; Meng, Q.; Yu, X.; Shi, S. Ferroptosis, necroptosis, and pyroptosis in anticancer immunity. J. Hematol. Oncol. 2020, 13, 110. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Hwang, C.-S. Nα-terminal acetylation meets ferroptosis via N-degron pathway. Mol. Cells 2024, 47, 100160. [Google Scholar] [CrossRef]
- Cani, P.; Van Hul, M.; Lefort, C.; Depommier, C.; Rastelli, M.; Everard, A. Microbial regulation of organismal energy homeostasis. Nat. Metab. 2019, 1, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Routy, B.; Chatelier, L.; Derosa, L.; Duong, C.; Alou, M.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.; et al. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef]
- Hakozaki, T.; Richard, C.; Elkrief, A.; Hosomi, Y.; Benlaïfaoui, M.; Mimpen, I.; Terrisse, S.; Derosa, L.; Zitvogel, L.; Routy, B.; et al. The Gut Microbiome Associates with Immune Checkpoint Inhibition Outcomes in Patients with Advanced Non–Small Cell Lung Cancer. Cancer Immunol. Res. 2020, 8, 1243–1250. [Google Scholar] [CrossRef]
- Simpson, R.; Shanahan, E.; Scolyer, R.; Long, G. Towards modulating the gut microbiota to enhance the efficacy of immune-checkpoint inhibitors. Nat. Rev. Clin. Oncol. 2023, 20, 697–715. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Guo, M.; Liu, D.; Xiao, P.; Yang, C.; Huang, H.; Liang, C.; Yang, Y.; Fu, X.; Zhang, Y.; et al. Gut microbial metabolite facilitates colorectal cancer development via ferroptosis inhibition. Nat. Cell Biol. 2024, 26, 124–137. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, N.; Su, X.; Gao, Y.; Yang, R. Gut-Microbiota-Derived Metabolites Maintain Gut and Systemic Immune Homeostasis. Cells 2023, 12, 793. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, X.; Shi, W.; Zhu, W.; Feng, X.; Xin, H.; Zhang, Y.; Cong, B.; Li, Y. The Gut Microbiota Metabolite Butyrate Modulates Acute Stress-Induced Ferroptosis in the Prefrontal Cortex via the Gut–Brain Axis. Int. J. Mol. Sci. 2025, 26, 1698. [Google Scholar] [CrossRef]
- Cheng, X.; Zhao, F.; Ke, B.; Chen, D.; Liu, F. Harnessing Ferroptosis to Overcome Drug Resistance in Colorectal Cancer: Promising Therapeutic Approaches. Cancers 2023, 15, 5209. [Google Scholar] [CrossRef]
- Parker, J.L.; Deme, J.C.; Kolokouris, D.; Kuteyi, G.; Biggin, P.C.; Lea, S.M.; Newstead, S. Molecular basis for redox control by the human cystine/glutamate antiporter system xc−. Nat. Commun. 2021, 12, 7147. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Sun, S.; Johnson, T.; Qi, R.; Zhang, S.; Zhang, J.; Yang, K. The glutathione peroxidase Gpx4 prevents lipid peroxidation and ferroptosis to sustain Treg cell activation and suppression of antitumor immunity. Cell Rep. 2021, 35, 109235. [Google Scholar] [CrossRef]
- Cardoso, B.R.; Hare, D.; Hare, D.; Bush, A.; Roberts, B. Glutathione peroxidase 4: A new player in neurodegeneration? Mol. Psychiatry 2017, 22, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Lin, H.; Huang, Y.; Wang, X.; Lin, H.; Xu, M.; Wu, J.; Wu, Y.; Shen, H.; Zhang, Q.; et al. BAP1-mediated MAFF deubiquitylation regulates tumor growth and is associated with adverse outcomes in colorectal cancer. Eur. J. Cancer 2024, 210, 114278. [Google Scholar] [CrossRef]
- Shakya, A.; McKee, N.W.; Dodson, M.; Chapman, E.; Zhang, D.D. Anti-Ferroptotic Effects of Nrf2: Beyond the Antioxidant Response. Mol. Cells 2023, 46, 165–175. [Google Scholar] [CrossRef]
- Huang, B.; Wang, H.; Liu, S.; Hao, M.; Luo, D.; Zhou, Y.; Huang, Y.; Nian, Y.; Zhang, L.; Chu, B.; et al. Palmitoylation-dependent regulation of GPX4 suppresses ferroptosis. Nat. Commun. 2025, 16, 867. [Google Scholar] [CrossRef]
- Cui, C.; Yang, F.; Li, Q. Post-Translational Modification of GPX4 is a Promising Target for Treating Ferroptosis-Related Diseases. Front. Mol. Biosci. 2022, 9, 901565. [Google Scholar] [CrossRef]
- Vogt, A.; Arsiwala, T.; Mohsen, M.; Vogel, M.; Manolova, V.; Bachmann, M. On Iron Metabolism and Its Regulation. Int. J. Mol. Sci. 2021, 22, 4591. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ma, Q.; Zhang, J.; Li, J.; Wang, Y.E.; Yao, Y.; Ding, Y.; Dai, X.; Luo, X.; Wu, L.; et al. Endogenous Iron(II) Self-Enriched Fenton Nanocatalyst via FTH1 Activity Inhibition and Iron(III) Reduction for Amplified Cancer Ferroptosis Therapy. Mol. Pharm. 2025, 22, 1568–1583. [Google Scholar] [CrossRef]
- Huang, L.; Zhu, J.; Wu, G.; Xiong, W.; Feng, J.; Yan, C.; Yang, J.; Li, Z.; Fan, Q.; Ren, B.; et al. A strategy of “adding fuel to the flames” enables a self-accelerating cycle of ferroptosis-cuproptosis for potent antitumor therapy. Biomaterials 2024, 311, 122701. [Google Scholar] [CrossRef]
- Rodencal, J.; Dixon, S. A tale of two lipids: Lipid unsaturation commands ferroptosis sensitivity. Proteomics 2022, 23, 2100308. [Google Scholar] [CrossRef]
- Ding, K.; Liu, C.; Li, L.; Yang, M.; Jiang, N.; Luo, S.; Sun, L. Acyl-CoA synthase ACSL4: An essential target in ferroptosis and fatty acid metabolism. Chin. Med. J. 2023, 136, 2521–2537. [Google Scholar] [CrossRef] [PubMed]
- Killion, E.; Reeves, A.; Azzouny, M.E.; Yan, Q.; Surujon, D.; Griffin, J.; Bowman, T.; Wang, C.; Matthan, N.; Klett, E.; et al. A role for long-chain acyl-CoA synthetase-4 (ACSL4) in diet-induced phospholipid remodeling and obesity-associated adipocyte dysfunction. Mol. Metab. 2018, 9, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, J.; Kang, R.; Klionsky, D.; Tang, D. Ferroptosis: Machinery and regulation. Autophagy 2020, 17, 2054–2081. [Google Scholar] [CrossRef]
- Dyall, S.; Balas, L.; Bazan, N.; Brenna, J.; Nan, C.; Da Costa Souza, F.; Dalli, J.; Durand, T.; Galano, J.; Lein, P.; et al. Polyunsaturated fatty acids and fatty acid-derived lipid mediators: Recent advances in the understanding of their biosynthesis, structures, and functions. Prog. Lipid Res. 2022, 86, 101165. [Google Scholar] [CrossRef]
- Shi, L.; Liu, Y.; Li, M.; Luo, Z. Emerging roles of ferroptosis in the tumor immune landscape: From danger signals to anti-tumor immunity. FEBS J. 2021, 289, 3655–3665. [Google Scholar] [CrossRef] [PubMed]
- Gong, D.; Chen, M.; Wang, Y.; Shi, J.; Hou, Y. Role of ferroptosis on tumor progression and immunotherapy. Cell Death Discov. 2022, 8, 427. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Tuerxun, H.; Zhao, Y.; Liu, X.; Zhao, Y. Firing up “cold” tumors: Ferroptosis causes immune activation by improving T cell infiltration. Biomed. Pharmacother. Biomed. Pharmacother. 2024, 179, 117298. [Google Scholar] [CrossRef]
- Bell, H.; Stockwell, B.; Zou, W. Ironing out the role of ferroptosis in immunity. Immunity 2024, 57, 941–956. [Google Scholar] [CrossRef]
- Wang, W.; Green, M.; Choi, J.; Gijon, M.; Kennedy, P.; Johnson, J.; Liao, P.; Lang, X.; Kryczek, I.; Sell, A.; et al. CD8+ T cells regulate tumor ferroptosis during cancer immunotherapy. Nature 2019, 569, 270–274. [Google Scholar] [CrossRef]
- Xu, S.; Chaudhary, O.; Rodríguez-Morales, P.; Sun, X.; Chen, D.; Zappasodi, R.; Xu, Z.; Pinto, A.; Williams, A.; Schulze, I.; et al. Uptake of oxidized lipids by the scavenger receptor CD36 promotes lipid peroxidation and dysfunction in CD8+ T cells in tumors. Immunity 2021, 54, 1561–1577. [Google Scholar] [CrossRef]
- Lopes, N.; McIntyre, C.; Martin, S.; Raverdeau, M.; Sumaria, N.; Kohlgruber, A.; Fiala, G.; Agudelo, L.; Dyck, L.; Kane, H.; et al. Distinct metabolic programs established in the thymus control effector functions of γδ T cell subsets in tumor microenvironments. Nat. Immunol. 2021, 22, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Jang, N.; Kim, I.-K.; Jung, D.; Chung, Y.; Kang, Y.P. Regulation of Ferroptosis in Cancer and Immune Cells. Immune Netw. 2025, 25, e6. [Google Scholar] [CrossRef]
- Liang, X.; Luo, M.; Shao, B.; Yang, J.; Tong, A.; Wang, R.-B.; Liu, Y.-T.; Jun, R.; Liu, T.; Yi, T.; et al. Phosphatidylserine released from apoptotic cells in tumor induces M2-like macrophage polarization through the PSR-STAT3-JMJD3 axis. Cancer Commun. 2022, 42, 205–222. [Google Scholar] [CrossRef]
- Dodson, M.; Castro-Portuguez, R.; Zhang, D. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019, 23, 101107. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Zhang, G.; Zhang, X.; Zhao, Q. Nrf2-mediated ferroptosis inhibition: A novel approach for managing inflammatory diseases. Inflammopharmacology 2024, 32, 2961–2986. [Google Scholar] [CrossRef]
- Feng, J.; Read, O.J.; Dinkova-Kostova, A.T. Nrf2 in TIME: The Emerging Role of Nuclear Factor Erythroid 2-Related Factor 2 in the Tumor Immune Microenvironment. Mol. Cells 2023, 46, 142–152. [Google Scholar] [CrossRef]
- Guo, W.; Wu, Z.; Chen, J.; Guo, S.; You, W.; Wang, S.; Ma, J.; Wang, H.; Wang, X.; Wang, H.; et al. Nanoparticle delivery of miR-21-3p sensitizes melanoma to anti-PD-1 immunotherapy by promoting ferroptosis. J. Immunother. Cancer 2022, 10, e004381. [Google Scholar] [CrossRef]
- Tao, Q.; Liu, N.; Wu, J.; Chen, J.; Chen, X.; Peng, C. Mefloquine enhances the efficacy of anti-PD-1 immunotherapy via IFN-γ-STAT1-IRF1-LPCAT3-induced ferroptosis in tumors. J. Immunother. Cancer 2024, 12, e008554. [Google Scholar] [CrossRef] [PubMed]
- Yao, T.; Li, L. The influence of microbiota on ferroptosis in intestinal diseases. Gut Microbes 2023, 15, 2263210. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, V.; Diaconu, C.; Gheorghe, G.; Mihai, M.; Diaconu, C.; Bostan, M.; Bleotu, C. Gut Microbiota and Colorectal Cancer: A Balance Between Risk and Protection. Int. J. Mol. Sci. 2025, 26, 3733. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.; Yu, J. Gut microbiota in colorectal cancer development and therapy. Nat. Rev. Clin. Oncol. 2023, 20, 429–452. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, Y.; Cheng, L.; Cao, X.; Liu, C. Gut microbiota in colorectal cancer: A review of its influence on tumor immune surveillance and therapeutic response. Front. Oncol. 2025, 15, 1557959. [Google Scholar] [CrossRef]
- Sun, S.; Shen, J.; Jiang, J.; Wang, F.; Min, J. Targeting ferroptosis opens new avenues for the development of novel therapeutics. Signal Transduct. Target. Ther. 2023, 8, 372. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, J.; Guo, W.; Li, H.; Lei, L. Periodontitis-level butyrate-induced ferroptosis in periodontal ligament fibroblasts by activation of ferritinophagy. Cell Death Discov. 2020, 6, 119. [Google Scholar] [CrossRef]
- Wang, G.; Qin, S.; Chen, L.; Geng, H.; Zheng, Y.; Xia, C.; Yao, J.; Deng, L. Butyrate dictates ferroptosis sensitivity through FFAR2-mTOR signaling. Cell Death Dis. 2023, 14, 292. [Google Scholar] [CrossRef]
- Ma, X.; Cao, D.; Zhang, Y.; Ding, X.; Hu, Z.; Wang, J. Apatinib combined with paclitaxel suppresses synergistically TNBC progression through enhancing ferroptosis susceptibility regulated SLC7A11/GPX4/ACSL4 axis. Cell. Signal. 2025, 131, 111760. [Google Scholar] [CrossRef]
- He, Y.; Ling, Y.; Zhang, Z.; Mertens, R.; Cao, Q.; Xu, X.; Guo, K.-J.; Shi, Q.; Zhang, X.-L.; Huo, L.; et al. Butyrate reverses ferroptosis resistance in colorectal cancer by inducing c-Fos-dependent xCT suppression. Redox Biol. 2023, 65, 102822. [Google Scholar] [CrossRef]
- Petan, T.; Jovičić, E.J.; Kump, A.; Perne, L.; Koren, Š.; Fedorova, M.; Kuda, O.; Lainšček, D. Abstract 2288 Lipid Droplets as Orchestrators of Lipid Mediator Signaling and Ferroptotic Cell Death. J. Biol. Chem. 2024, 300, 106402. [Google Scholar] [CrossRef]
- Rochette, L.; Dogon, G.; Rigal, E.; Zeller, M.; Cottin, Y.; Vergely, C. Lipid Peroxidation and Iron Metabolism: Two Corner Stones in the Homeostasis Control of Ferroptosis. Int. J. Mol. Sci. 2022, 24, 449. [Google Scholar] [CrossRef]
- Luu, M.; Riester, Z.; Baldrich, A.; Reichardt, N.; Yuille, S.; Busetti, A.; Klein, M.; Wempe, A.; Leister, H.; Raifer, H.; et al. Microbial short-chain fatty acids modulate CD8+ T cell responses and improve adoptive immunotherapy for cancer. Nat. Commun. 2021, 12, 4077. [Google Scholar] [CrossRef]
- Nakkarach, A.; Foo, H.; Song, A.; Mutalib, N.; Nitisinprasert, S.; Withayagiat, U. Anti-cancer and anti-inflammatory effects elicited by short chain fatty acids produced by Escherichia coli isolated from healthy human gut microbiota. Microb. Cell Factories 2021, 20, 36. [Google Scholar] [CrossRef]
- Yi, R.; Wang, H.-D.; Deng, C.; Wang, X.; Yao, L.; Niu, W.; Fei, M.-X.; Zhaba, W. Dihydroartemisinin initiates ferroptosis in glioblastoma through GPX4 inhibition. Biosci. Rep. 2020, 40, BSR20193314. [Google Scholar] [CrossRef]
- Eaton, J.; Furst, L.; Ruberto, R.; Moosmayer, D.; Hilpmann, A.; Ryan, M.; Zimmermann, K.; Cai, L.; Niehues, M.; Badock, V.; et al. Selective covalent targeting of GPX4 using masked nitrile-oxide electrophiles. Nat. Chem. Biol. 2020, 16, 497–506. [Google Scholar] [CrossRef]
- Bi, G.; Liang, J.; Bian, Y.; Shan, G.; Huang, Y.; Lu, T.; Zhang, H.; Jin, X.; Chen, Z.; Zhao, M.; et al. Polyamine-mediated ferroptosis amplification acts as a targetable vulnerability in cancer. Nat. Commun. 2024, 15, 2461. [Google Scholar] [CrossRef]
- Wang, J.; Liao, L.; Miao, B.; Yang, B.; Li, B.; Ma, X.; Fitz, A.; Wu, S.; He, J.; Zhang, Q.; et al. Deciphering the role of the MALT1-RC3H1 axis in regulating GPX4 protein stability. Proc. Natl. Acad. Sci. USA 2024, 122, e2419625121. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, Y.-H.; Kong, Y.; Ye, T.; Yu, Q.; Satyanarayanan, S.; Su, K.; Liu, J. Microbiota-derived metabolite Indoles induced aryl hydrocarbon receptor activation and inhibited neuroinflammation in APP/PS1 mice. Brain Behav. Immun. 2022, 106, 76–88. [Google Scholar] [CrossRef]
- Vyhlídalová, B.; Krasulová, K.; Pečinková, P.; Marcalíková, A.; Vrzal, R.; Zemánková, L.; Vančo, J.; Trávníček, Z.; Vondráček, J.; Karasová, M.; et al. Gut Microbial Catabolites of Tryptophan Are Ligands and Agonists of the Aryl Hydrocarbon Receptor: A Detailed Characterization. Int. J. Mol. Sci. 2020, 21, 2614. [Google Scholar] [CrossRef]
- Zhang, R.; Kang, R.; Tang, D. Gut microbiome mediates ferroptosis resistance for colorectal cancer development. Cancer Res. 2024, 84, 796–797. [Google Scholar] [CrossRef]
- Zhou, Y.; Feng, Y.; Zhao, Y.; Wu, Y.; Li, M.; Yang, X.; Wu, X.; Chen, X. Oral 7,8-Dihydroxyflavone Protects Retinal Ganglion Cells by Modulating the Gut-Retina Axis and Inhibiting Ferroptosis via the Indoleacrylic Acid-AhR-ALDH1A3-FSP1 Pathway. CNS Neurosci. Ther. 2025, 31, e70442. [Google Scholar] [CrossRef]
- Wu, Y.; Franzmeier, S.; Liesche-Starnecker, F.; Schlegel, J. Enhanced Sensitivity to ALDH1A3-Dependent Ferroptosis in TMZ-Resistant Glioblastoma Cells. Cells 2023, 12, 2522. [Google Scholar] [CrossRef]
- Ambrożewicz, E.; Muszyńska, M.; Tokajuk, G.; Grynkiewicz, G.; Žarković, N.; Skrzydlewska, E. Beneficial Effects of Vitamins K and D3 on Redox Balance of Human Osteoblasts Cultured with Hydroxyapatite-Based Biomaterials. Cells 2019, 8, 325. [Google Scholar] [CrossRef]
- Mishima, E.; Ito, J.; Wu, Z.; Nakamura, T.; Wahida, A.; Doll, S.; Tonnus, W.; Nepachalovich, P.; Eggenhofer, E.; Aldrovandi, M.; et al. A non-canonical vitamin K cycle is a potent ferroptosis suppressor. Nature 2022, 608, 778–783. [Google Scholar] [CrossRef]
- Nuszkiewicz, J.; Sutkowy, P.; Wróblewski, M.; Pawłowska, M.; Wesołowski, R.; Wróblewska, J.; Woźniak, A. Links between Vitamin K, Ferroptosis and SARS-CoV-2 Infection. Antioxidants 2023, 12, 733. [Google Scholar] [CrossRef]
- Mayneris-Perxachs, J.; Moreno-Navarrete, J.; Fernández-Real, J. The role of iron in host–microbiota crosstalk and its effects on systemic glucose metabolism. Nat. Rev. Endocrinol. 2022, 18, 683–698. [Google Scholar] [CrossRef]
- Golonka, R.; Yeoh, B.; Vijay-Kumar, M. The Iron Tug-of-War between Bacterial Siderophores and Innate Immunity. J. Innate Immun. 2019, 11, 249–262. [Google Scholar] [CrossRef]
- Noordine, M.L.; Seyoum, Y.; Bruneau, A.; Baye, K.; Lefèbvre, T.; Cherbuy, C.; Canonne-Hergaux, F.; Nicolas, G.; Humblot, C.; Thomas, M. The microbiota and the host organism switch between cooperation and competition based on dietary iron levels. Gut Microbes 2024, 16, 2361660. [Google Scholar] [CrossRef]
- González, A.; Gálvez, N.; Martín, J.; Reyes, F.; Pérez-Victoria, I.; Dominguez-Vera, J. Identification of the key excreted molecule by Lactobacillus fermentum related to host iron absorption. Food Chem. 2017, 228, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Das, N.; Schwartz, A.; Barthel, G.; Inohara, N.; Liu, Q.; Sankar, A.; Hill, D.; Ma, X.; Lamberg, O.; Schnizlein, M.; et al. Microbial Metabolite Signaling Is Required for Systemic Iron Homeostasis. Cell Metab. 2019, 31, 115–130. [Google Scholar] [CrossRef]
- Dengler, F.; Rackwitz, R.; Benesch, F.; Pfannkuche, H.; Gäbel, G. Both butyrate incubation and hypoxia upregulate genes involved in the ruminal transport of SCFA and their metabolites. J. Anim. Physiol. Anim. Nutr. 2015, 99, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Pan, S.; Gao, Z.; Qiao, H.; Zhao, Y.; Chuai, X.; Li, J. Roles of hypoxia inducible factors in viral infection: Are they a potential therapeutic target? Virulence 2025, 16, 2546680. [Google Scholar] [CrossRef]
- Nairz, M.; Dichtl, S.; Schroll, A.; Haschka, D.; Tymoszuk, P.; Theurl, I.; Weiss, G. Iron and innate antimicrobial immunity-Depriving the pathogen, defending the host. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. 2018, 48, 118–133. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Li, G.; Liang, Z.; Qi, T.; Deng, K.; Yu, J.; Peng, Y.; Zheng, J.; Song, Y.; Chang, X. Microbiota-assisted iron uptake promotes immune tolerance in the intestine. Nat. Commun. 2023, 14, 2790. [Google Scholar] [CrossRef] [PubMed]
- Hassannia, B.; Vandenabeele, P.; Vanden Berghe, T. Targeting Ferroptosis to Iron Out Cancer. Cancer Cell 2019, 35, 830–849. [Google Scholar] [CrossRef]
- Li, Y.; Li, M.; Liu, L.; Xue, C.; Fei, Y.; Wang, X.; Zhang, Y.; Cai, K.; Zhao, Y.; Luo, Z. Cell-Specific Metabolic Reprogramming of Tumors for Bioactivatable Ferroptosis Therapy. ACS Nano 2022, 16, 3965–3984. [Google Scholar] [CrossRef]
- Wang, X.; Sun, Q.; Liu, T.; Lu, H.; Lin, X.; Wang, W.; Liu, Y.; Huang, Y.; Huang, G.; Sun, H.; et al. Single-cell multi-omics sequencing uncovers region-specific plasticity of glioblastoma for complementary therapeutic targeting. Sci. Adv. 2024, 10, adn4306. [Google Scholar] [CrossRef]
- Liu, R.; Wang, J.; Liu, Y.; Gao, Y.; Yang, R. Regulation of gut microbiota on immune cell ferroptosis: A novel insight for immunotherapy against tumor. Cancer Lett. 2024, 598, 217115. [Google Scholar] [CrossRef]
- Kim, M.B.; Hwangbo, S.; Jang, S.-A.; Jo, Y. Bioengineered Co-culture of organoids to recapitulate host-microbe interactions. Mater. Today Bio 2022, 16, 100345. [Google Scholar] [CrossRef]
- Pei, B.; Peng, S.; Huang, C.; Zhou, F. Bifidobacterium modulation of tumor immunotherapy and its mechanism. Cancer Immunol. Immunother. CII 2024, 73, 94. [Google Scholar] [CrossRef]
- Campbell, C.; Kandalgaonkar, M.; Golonka, R.; Yeoh, B.; Vijay-Kumar, M.; Saha, P. Crosstalk between Gut Microbiota and Host Immunity: Impact on Inflammation and Immunotherapy. Biomedicines 2023, 11, 294. [Google Scholar] [CrossRef]
- Cheng, X.; Wang, Y.; Liu, L.-Y.; Lv, C.; Liu, C.-L.; Xu, J.-T. SLC7A11, a Potential Therapeutic Target Through Induced Ferroptosis in Colon Adenocarcinoma. Front. Mol. Biosci. 2022, 9, 889688. [Google Scholar] [CrossRef] [PubMed]
- Zitvogel, L.; Kroemer, G. Interferon-γ induces cancer cell ferroptosis. Cell Res. 2019, 29, 692–693. [Google Scholar] [CrossRef] [PubMed]
- Almonte, A.; Thomas, S.; Zitvogel, L. Microbiota-centered interventions to boost immune checkpoint blockade therapies. J. Exp. Med. 2025, 222, e20250378. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Lichterman, J.; Coughlin, L.; Poulides, N.; Li, W.; Del Valle, P.; Palmer, S.; Gan, S.; Kim, J.; Zhan, X.; et al. Immune checkpoint blockade induces gut microbiota translocation that augments extraintestinal anti-tumor immunity. Sci. Immunol. 2022, 8, eabo2003. [Google Scholar] [CrossRef]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.; Aquino-Michaels, K.; Earley, Z.; Benyamin, F.; Lei, Y.M.; Jabri, B.; Alegre, M.; et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti–PD-L1 efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef]
- Preet, R.; Islam, M.A.; Shim, J.; Rajendran, G.; Mitra, A.; Vishwakarma, V.; Kutz, C.; Choudhury, S.; Pathak, H.; Dai, Q.; et al. Gut commensal Bifidobacterium-derived extracellular vesicles modulate the therapeutic effects of anti-PD-1 in lung cancer. Nat. Commun. 2025, 16, 3500. [Google Scholar] [CrossRef]
- Myers, K.; Amend, S.; Pienta, K. Targeting Tyro3, Axl and MerTK (TAM receptors): Implications for macrophages in the tumor microenvironment. Mol. Cancer 2019, 18, 94. [Google Scholar] [CrossRef]
- Liu, F.; Qiu, H.; Xue, M.; Zhang, S.; Zhang, X.; Xu, J.; Chen, J.; Yang, Y.; Xie, J. MSC-secreted TGF-β regulates lipopolysaccharide-stimulated macrophage M2-like polarization via the Akt/FoxO1 pathway. Stem Cell Res. Ther. 2019, 10, 345. [Google Scholar] [CrossRef]
- Kuang, F.; Liu, J.; Tang, D.; Kang, R. Oxidative Damage and Antioxidant Defense in Ferroptosis. Front. Cell Dev. Biol. 2020, 8, 586578. [Google Scholar] [CrossRef]
- Liao, P.; Wang, W.; Wang, W.; Kryczek, I.; Li, X.; Bian, Y.; Sell, A.; Wei, S.; Grove, S.; Johnson, J.; et al. CD8+ T cells and fatty acids orchestrate tumor ferroptosis and immunity via ACSL4. Cancer Cell 2022, 40, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Jian, J.; Wei, J. Ferroptosis: A New Pathway in the Interaction between Gut Microbiota and Multiple Sclerosis. Front. Biosci. 2025, 30, 26265. [Google Scholar] [CrossRef]
- Zhuge, A.; Li, S.; Han, S.; Yuan, Y.; Shen, J.; Wu, W.-R.; Wang, K.; Xia, J.; Wang, Q.; Gu, Y.; et al. Akkermansia muciniphila-derived acetate activates the hepatic AMPK/SIRT1/PGC-1α axis to alleviate ferroptosis in metabolic-associated fatty liver disease. Acta Pharm. Sin. B 2024, 15, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Yu, S.; Qin, S.; Liu, Q.; Xu, H.; Zhao, W.; Chu, Q.; Wu, K. Gut microbiome modulates efficacy of immune checkpoint inhibitors. J. Hematol. Oncol. 2018, 11, 47. [Google Scholar] [CrossRef]
- Guan, Z.-W.; Jin, X.; Guan, Z.; Liu, S.; Tao, K.; Luo, L. The gut microbiota metabolite capsiate regulate SLC2A1 expression by targeting HIF-1α to inhibit knee osteoarthritis-induced ferroptosis. Aging Cell 2023, 22, e13807. [Google Scholar] [CrossRef]
- Si, W.; Liang, H.; Bugno, J.; Xu, Q.; Ding, X.-C.; Yang, K.; Fu, Y.; Weichselbaum, R.; Zhao, X.; Wang, L. Lactobacillus rhamnosus GG induces cGAS/STING- dependent type I interferon and improves response to immune checkpoint blockade. Gut 2021, 71, 521–533. [Google Scholar] [CrossRef]
- Park, J.; Hsueh, P.; Li, Z.; Ho, P.-C. Microenvironment-driven metabolic adaptations guiding CD8+ T cell anti-tumor immunity. Immunity 2023, 56, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Mills, S.; Yang, B.; Smith, G.; Stanton, C.; Ross, R. Efficacy of Bifidobacterium longum alone or in multi-strain probiotic formulations during early life and beyond. Gut Microbes 2023, 15, 2186098. [Google Scholar] [CrossRef]
- Sharma, S.; Bhatia, R.; Devi, K.; Rawat, A.; Singh, S.; Bhadada, S.; Bishnoi, M.; Sharma, S.; Kondepudi, K. A synbiotic combination of Bifidobacterium longum Bif10 and Bifidobacterium breve Bif11, isomaltooligosaccharides and finger millet arabinoxylan prevents dextran sodium sulphate induced ulcerative colitis in mice. Int. J. Biol. Macromol. 2023, 231, 123326. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Q.; Ye, F.; Yang, C.; Jiang, H. Gut microbiota-derived short-chain fatty acids promote prostate cancer progression via inducing cancer cell autophagy and M2 macrophage polarization. Neoplasia 2023, 43, 100928. [Google Scholar] [CrossRef]
- Sadik, A.; Patterson, L.S.; Öztürk, S.; Mohapatra, S.; Panitz, V.; Secker, P.; Pfänder, P.; Loth, S.; Salem, H.; Prentzell, M.; et al. IL4I1 Is a Metabolic Immune Checkpoint that Activates the AHR and Promotes Tumor Progression. Cell 2020, 182, 1252–1270. [Google Scholar] [CrossRef]
- Vecchio, E.; Caiazza, C.; Mimmi, S.; Avagliano, A.; Iaccino, E.; Brusco, T.; Nisticò, N.; Maisano, D.; Aloisio, A.; Quinto, I.; et al. Metabolites Profiling of Melanoma Interstitial Fluids Reveals Uridine Diphosphate as Potent Immune Modulator Capable of Limiting Tumor Growth. Front. Cell Dev. Biol. 2021, 9, 730726. [Google Scholar] [CrossRef] [PubMed]
- Jobin, K.; Seetharama, D.; Rüttger, L.; Fenton, C.; Kharybina, E.; Wirsching, A.; Huang, A.; Knöpper, K.; Kaisho, T.; Busch, D.; et al. A distinct priming phase regulates CD8 T cell immunity by orchestrating paracrine IL-2 signals. Science 2025, 388, adq1405. [Google Scholar] [CrossRef]
- Hatfull, G.; Dedrick, R.; Schooley, R. Phage Therapy for Antibiotic-Resistant Bacterial Infections. Annu. Rev. Med. 2021, 73, 197–211. [Google Scholar] [CrossRef]
- Darby, E.; Trampari, E.; Siasat, P.; Gaya, M.S.; Alav, I.; Webber, M.; Blair, J. Molecular mechanisms of antibiotic resistance revisited. Nat. Rev. Microbiol. 2022, 21, 280–295. [Google Scholar] [CrossRef]
- Larsson, D.; Flach, C.-F. Antibiotic resistance in the environment. Nat. Rev. Microbiol. 2021, 20, 257–269. [Google Scholar] [CrossRef]
- Balcha, F.B.; Neja, S.A. CRISPR-Cas9 mediated phage therapy as an alternative to antibiotics. Anim. Dis. 2023, 3, 4. [Google Scholar] [CrossRef]
- Manohar, P.; Royam, M.M.; Loh, B.; Bozdoğan, B.; Nachimuthu, R.; Leptihn, S. Synergistic Effects of Phage-Antibiotic Combinations against Citrobacter amalonaticus. ACS Infect. Dis. 2022, 8, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Canale, F.; Basso, C.; Antonini, G.; Perotti, M.; Li, N.; Sokolovska, A.; Neumann, J.; James, M.; Geiger, S.; Jin, W.-J.; et al. Metabolic modulation of tumours with engineered bacteria for immunotherapy. Nature 2021, 598, 662–666. [Google Scholar] [CrossRef]
- Fan, J.X.; Niu, M.T.; Qin, Y.; Sun, Y.X.; Zhang, X. Progress of Engineered Bacteria for Tumor Therapy. Adv. Drug Deliv. Rev. 2022, 185, 114296. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Castro, S.; Coker, C.; Hinchliffe, T.; Arpaia, N.; Danino, T. Programmable bacteria induce durable tumor regression and systemic antitumor immunity. Nat. Med. 2019, 25, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Y.; Wang, Y.; Xie, H.; Gong, R.; Wu, X.; Chen, J.; Sun, C.; Gu, Y. Anti-tumor activity of an αPD-L1-PE38 immunotoxin delivered by engineered Nissle 1917. Int. J. Biol. Macromol. 2025, 295, 139537. [Google Scholar] [CrossRef]
- Holmes, Z.; Villa, M.; Durand, H.; Jiang, S.; Dallow, E.; Petrone, B.; Silverman, J.; Lin, P.; David, L. Microbiota responses to different prebiotics are conserved within individuals and associated with habitual fiber intake. Microbiome 2021, 10, 114. [Google Scholar] [CrossRef]
- Lu, Z.; Zhang, C.; Zhang, J.; Su, W.; Wang, G.; Wang, Z. The Kynurenine Pathway and Indole Pathway in Tryptophan Metabolism Influence Tumor Progression. Cancer Med. 2025, 14, e70703. [Google Scholar] [CrossRef]
- Liu, D.; Liang, C.-H.; Huang, B.; Zhuang, X.; Cui, W.; Yang, L.; Yang, Y.; Zhang, Y.; Fu, X.; Zhang, X.; et al. Tryptophan Metabolism Acts as a New Anti-Ferroptotic Pathway to Mediate Tumor Growth. Adv. Sci. 2023, 10, 2204006. [Google Scholar] [CrossRef]
- Murphy, S.; Rahmy, S.; Gan, D.; Liu, G.; Zhu, Y.; Manyak, M.; Duong, L.; He, J.-Y.; Schofield, J.; Schafer, Z.; et al. Ketogenic diet alters the epigenetic and immune landscape of prostate cancer to overcome resistance to immune checkpoint blockade therapy. Cancer Res. 2024, 84, 1597–1612. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Kusumi, R.; Hamashima, S.; Kobayashi, S.; Sasaki, S.; Komiyama, Y.; Izumikawa, T.; Conrad, M.; Bannai, S.; Sato, H. The ferroptosis inducer erastin irreversibly inhibits system x(c)- and synergizes with cisplatin to increase cisplatin’s cytotoxicity in cancer cells. Sci. Rep. 2018, 8, 968. [Google Scholar] [CrossRef]
- Eaton, J.K.; Furst, L.; Cai, L.L.; Viswanathan, V.S.; Schreiber, S.L. Structure–activity relationships of GPX4 inhibitor warheads family of glutathione peroxidases that enables reduction of structurally-diverse lipid. Bioorganic Med. Chem. Lett. 2021, 30, 127538. [Google Scholar]
- Cheu, J.; Lee, D.; Li, Q.; Goh, C.; Bao, M.H.-R.; Yuen, V.; Zhang, M.S.; Yang, C.; Chan, C.Y.K.; Tse, A.P.W.; et al. Ferroptosis Suppressor Protein 1 Inhibition Promotes Tumor Ferroptosis and Anti-tumor Immune Responses in Liver Cancer. Cell. Mol. Gastroenterol. Hepatol. 2023, 16, 133–159. [Google Scholar] [CrossRef]
- Li, K.; Lin, C.; Li, M.; Xu, K.; He, Y.; Mao, Y.; Lu, L.; Geng, W.; Li, X.; Luo, Z.; et al. Multienzyme-like Reactivity Cooperatively Impairs Glutathione Peroxidase 4 and Ferroptosis Suppressor Protein 1 Pathways in Triple-Negative Breast Cancer for Sensitized Ferroptosis Therapy. ACS Nano 2022, 16, 2381–2398. [Google Scholar] [CrossRef] [PubMed]
- Phipps, O.; Al-Hassi, H.; Quraishi, M.; Dickson, E.; Segal, J.; Steed, H.; Kumar, A.; Acheson, A.; Beggs, A.; Brookes, M. Oral and Intravenous Iron Therapy Differentially Alter the On- and Off-Tumor Microbiota in Anemic Colorectal Cancer Patients. Cancers 2021, 13, 1341. [Google Scholar] [CrossRef]
- Cuisiniere, T.; Hajjar, R.; Oliero, M.; Calvé, A.; Fragoso, G.; Rendos, H.; Gerkins, C.; Taleb, N.; Gagnon-Konamna, M.; Dagbert, F.; et al. Initial gut microbiota composition is a determining factor in the promotion of colorectal cancer by oral iron supplementation: Evidence from a murine model. Microbiome 2025, 13, 100. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Fang, W.; Guo, Y.; Hu, P.; Shi, J. Nebulized Therapy of Early Orthotopic Lung Cancer by Iron-Based Nanoparticles: Macrophage-Regulated Ferroptosis of Cancer Stem Cells. J. Am. Chem. Soc. 2023, 145, 24153–24165. [Google Scholar] [CrossRef]
- Vadhan-Raj, S.; Abonour, R.; Goldman, J.; Smith, D.; Slapak, C.; Ilaria, R.; Tiu, R.; Wang, X.; Callies, S.; Cox, J.; et al. A first-in-human phase 1 study of a hepcidin monoclonal antibody, LY2787106, in cancer-associated anemia. J. Hematol. Oncol. 2017, 10, 73. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Chen, X.; Yu, C.; Xu, G.; Nie, X.; Cheng, Y.; Luan, Y.; Song, Q. Radiotherapy-Mediated Redox Homeostasis-Controllable Nanomedicine for Enhanced Ferroptosis Sensitivity in Tumor Therapy. Acta Biomater. 2023, 159, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Bersuker, K.; Hendricks, J.; Li, Z.; Magtanong, L.; Ford, B.; Tang, P.; Roberts, M.; Tong, B.; Maimone, T.; Zoncu, R.; et al. The CoQ oxidoreductase FSP1 acts in parallel to GPX4 to inhibit ferroptosis. Nature 2019, 575, 688–692. [Google Scholar] [CrossRef]
- Yu, X.; Ruan, Y.; Huang, X.; Dou, L.; Lan, M.; Cui, J.; Chen, B.; Gong, H.; Wang, Q.; Yan, M.; et al. Dexrazoxane ameliorates doxorubicin-induced cardiotoxicity by inhibiting both apoptosis and necroptosis in cardiomyocytes. Biochem. Biophys. Res. Commun. 2019, 523, 140–146. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, Y.; Qi, Z.; Li, X.; Zhao, Y. Ferroptosis: CD8+T cells’ blade to destroy tumor cells or poison for self-destruction. Cell Death Discov. 2025, 11, 128. [Google Scholar] [CrossRef]
- Liu, J.; Zhan, J.; Zhang, Y.; Huang, L.; Yang, J.; Feng, J.; Ding, L.; Shen, Z.; Chen, X. Ultrathin Clay Nanoparticles-Mediated Mutual Reinforcement of Ferroptosis and Cancer Immunotherapy. Adv. Mater. 2023, 36, 2309562. [Google Scholar] [CrossRef]
- Griffin, M.; Hang, H. Microbial mechanisms to improve immune checkpoint blockade responsiveness. Neoplasia 2022, 31, 100818. [Google Scholar] [CrossRef]
- Renga, G.; Nunzi, E.; Pariano, M.; Puccetti, M.; Bellet, M.; Pieraccini, G.; D’Onofrio, F.; Santarelli, I.; Stincardini, C.; Aversa, F.; et al. Optimizing therapeutic outcomes of immune checkpoint blockade by a microbial tryptophan metabolite. J. Immunother. Cancer 2022, 10, e003725. [Google Scholar] [CrossRef]
- Ma, X.; Xiao, L.; Liu, L.; Ye, L.; Su, P.; Bi, E.; Wang, Q.; Yang, M.; Qian, J.; Yi, Q. CD36-mediated ferroptosis dampens intratumoral CD8+ T cell effector function and impairs their antitumor ability. Cell Metab. 2021, 33, 1001–1012. [Google Scholar] [CrossRef] [PubMed]
- Leone, R.; Powell, J. Metabolism of immune cells in cancer. Nat. Rev. Cancer 2020, 20, 516–531. [Google Scholar] [CrossRef] [PubMed]
- Kao, K.-C.; Vilbois, S.; Tsai, C.-H.; Ho, P.-C. Metabolic communication in the tumour–immune microenvironment. Nat. Cell Biol. 2022, 24, 1574–1583. [Google Scholar] [CrossRef]
- Lyamzaev, K.; Panteleeva, A.; Simonyan, R.; Avetisyan, A.; Chernyak, B. Mitochondrial Lipid Peroxidation Is Responsible for Ferroptosis. Cells 2023, 12, 611. [Google Scholar] [CrossRef] [PubMed]
- Kalkavan, H.; Chen, M.; Crawford, J.; Quarato, G.; Fitzgerald, P.; Tait, S.; Goding, C.; Green, D. Sublethal cytochrome c release generates drug-tolerant persister cells. Cell 2022, 185, 3356–3374. [Google Scholar] [CrossRef]
- Liang, H.; Wu, X.; Zhao, G.; Feng, K.; Ni, K.; Sun, X. Renal Clearable Ultrasmall Single-Crystal Fe Nanoparticles for Highly Selective and Effective Ferroptosis Therapy and Immunotherapy. J. Am. Chem. Soc. 2021, 143, 15812–15823. [Google Scholar] [CrossRef]
- Liu, Y.; Quan, X.; Li, J.; Huo, J.; Li, X.; Zhao, Z.; Li, S.; Wan, J.; Li, J.; Liu, S.; et al. Liposomes embedded with PEGylated iron oxide nanoparticles enable ferroptosis and combination therapy in cancer. Natl. Sci. Rev. 2022, 10, nwac167. [Google Scholar] [CrossRef]
- Sun, L.; Liu, H.; Ye, Y.; Lei, Y.; Islam, R.; Tan, S.; Tong, R.; Miao, Y.; Cai, L. Smart nanoparticles for cancer therapy. Signal Transduct. Target. Ther. 2023, 8, 418. [Google Scholar] [CrossRef]
- Fu, J.; Liu, X.; Cui, Z.; Zheng, Y.; Jiang, H.; Zhang, Y.; Li, Z.; Liang, Y.; Zhu, S.; Chu, P.; et al. Probiotic-based nanoparticles for targeted microbiota modulation and immune restoration in bacterial pneumonia. Natl. Sci. Rev. 2022, 10, nwac221. [Google Scholar] [CrossRef]
- Wu, L.; Han, Y.; Zheng, Z.; Peng, G.; Liu, P.; Yue, S.; Zhu, S.; Chen, J.; Lv, H.; Shao, L.; et al. Altered Gut Microbial Metabolites in Amnestic Mild Cognitive Impairment and Alzheimer’s Disease: Signals in Host–Microbe Interplay. Nutrients 2021, 13, 228. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Cui, H.; Qin, L.; Liu, R.; Fang, F.; Wang, Z. Soybean Lecithin–Gallic Acid Complex Sensitizes Lung Cancer Cells to Radiation Through Ferroptosis Regulated by Nrf2/SLC7A11/GPX4 Pathway. Nutrients 2025, 17, 1262. [Google Scholar] [CrossRef] [PubMed]
- Olley, M.; Ward, N. Abstract 5429: NRF2 activation sensitizes the Fe-S cluster synthesis pathway to iron restriction. Cancer Res. 2025, 85, 5429. [Google Scholar] [CrossRef]
- Golesworthy, B.; Wang, Y.; Tanti, A.; Pacis, A.; Romero, J.; Cuggia, A.; Domecq, C.; Bourdel, G.; Denroche, R.; Jang, G.; et al. Intra-Tumoral CD8+ T-Cell Infiltration and PD-L1 Positivity in Homologous Recombination Deficient Pancreatic Ductal Adenocarcinoma. Front. Oncol. 2022, 12, 860767. [Google Scholar] [CrossRef]
- Criscuolo, A.; Nepachalovich, P.; Rio, D.F.G.-D.; Lange, M.; Ni, Z.; Baroni, M.; Cruciani, G.; Goracci, L.; Blüher, M.; Fedorova, M. Analytical and computational workflow for in-depth analysis of oxidized complex lipids in blood plasma. Nat. Commun. 2022, 13, 6547. [Google Scholar] [CrossRef]
- Seidl, K.; Briamonte, C.; Higgins, M.; Hoffman, C.; Holtzlaw, S.; McHugh, M.; Meyer, A.; Rangan, K.; Schmus, C.; Strachan, M.; et al. NURS-21. Exploration of correlative studies in early phase trials for pediatric and young adult Central Nervous System (CNS) tumors and the potential translation to clinical care. Neuro-Oncology 2024, 26, iv192. [Google Scholar] [CrossRef]
- Silva, A.C.; Piccinno, G.; Suissa, D.; Bourgin, M.; Schreibelt, G.; Durand, S.; Birebent, R.; Fidelle, M.; Sow, C.; Aprahamian, F.; et al. Influence of microbiota-associated metabolic reprogramming on clinical outcome in patients with melanoma from the randomized adjuvant dendritic cell-based MIND-DC trial. Nat. Commun. 2024, 15, 1633. [Google Scholar] [CrossRef]
- Nakamura, T.; Hipp, C.; Mourão, A.S.D.; Borggräfe, J.; Aldrovandi, M.; Henkelmann, B.; Wanninger, J.; Mishima, E.; Lytton, E.; Emler, D.; et al. Phase separation of FSP1 promotes ferroptosis. Nature 2023, 619, 371–377. [Google Scholar] [CrossRef]
- Chia, S.; Seow, J.J.W.; Da Silva, R.P.; Suphavilai, C.; Shirgaonkar, N.; Murata-Hori, M.; Zhang, X.; Yong, E.Y.; Pan, J.; Thangavelu, M.; et al. CAN-Scan: A multi-omic phenotype-driven precision oncology platform identifies prognostic biomarkers of therapy response for colorectal cancer. Cell Rep. Med. 2025, 6, 102053. [Google Scholar] [CrossRef]
- Conlon, M.; Poltorack, C.; Forcina, G.; Armenta, D.; Mallais, M.; Perez, M.; Wells, A.; Kahanu, A.; Magtanong, L.; Watts, J.; et al. A Compendium of Kinetic Modulatory Profiles Identifies Ferroptosis Regulators. Nat. Chem. Biol. 2021, 17, 665–674. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Wang, Y.; Chen, Y.; Zhang, W.; Pan, X.; Su, C.; Li, Z.; Wang, L.; Gu, J. IgG4-mediated M2 macrophage polarization in tertiary lymphoid structures of esophageal cancer: Implications for immunosuppression. Front. Immunol. 2025, 15, 1497783. [Google Scholar] [CrossRef]
- Charitos, I.; Scacco, S.; Cotoia, A.; Castellaneta, F.; Castellana, G.; Pasqualotto, F.; Venneri, M.; Ferrulli, A.; Aliani, M.; Santacroce, L.; et al. Intestinal Microbiota Dysbiosis Role and Bacterial Translocation as a Factor for Septic Risk. Int. J. Mol. Sci. 2025, 26, 2028. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Li, J.; Zhang, J.; Feng, W.; Lu, J.-Y.; Ma, X.; Ding, W.; Ouyang, S.; Lu, J.-J.; Yue, P.; et al. Metabolic reprogramming driven by IGF2BP3 promotes acquired resistance to EGFR inhibitors in non-small cell lung cancer. Cancer Res. 2023, 83, 2187–2207. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Hua, S.; Lin, X.; Lu, F.; Zhang, W.; Zhou, L.; Cui, J.; Wang, R.; Xia, J.-Y.; Xu, F.; et al. Hybrid Biomimetic Membrane Coated Particles-Mediated Bacterial Ferroptosis for Acute MRSA Pneumonia. ACS Nano 2023, 17, 11692–11712. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Qin, B.-D.; Jiao, X.; Liu, K.; Wang, Z.; Zang, Y. New clinical trial design in precision medicine: Discovery, development and direction. Signal Transduct. Target. Ther. 2024, 9, 57. [Google Scholar] [CrossRef]
- Zhu, X.; Lu, H.; Li, W.; Niu, S.; Xue, J.; Sun, H.; Zhang, J.; Zhang, Z. Ferroptosis Induces gut microbiota and metabolic dysbiosis in Collagen-Induced arthritis mice via PAD4 enzyme. Gene 2024, 936, 149106. [Google Scholar] [CrossRef]
- Dixon, S.; Olzmann, J. The cell biology of ferroptosis. Nat. Rev. Mol. Cell Biol. 2024, 25, 424–442. [Google Scholar] [CrossRef]
- Pope, L.; Dixon, S. Regulation of ferroptosis by lipid metabolism. Trends Cell Biol. 2023, 33, 1077–1087. [Google Scholar] [CrossRef]
- Kim, R.; Hashimoto, A.; Markosyan, N.; Tyurin, V.; Tyurina, Y.; Kar, G.; Fu, S.; Sehgal, M.; Garcia-Gerique, L.; Kossenkov, A.; et al. Ferroptosis of tumour neutrophils causes immune suppression in cancer. Nature 2022, 612, 338–346. [Google Scholar] [CrossRef]
- Chen, P.; Black, A.; Sobel, A.; Zhao, Y.; Mukherjee, P.; Molparia, B.; Moore, N.; Muench, G.A.; Wu, J.; Chen, W.; et al. Directed remodeling of the mouse gut microbiome inhibits the development of atherosclerosis. Nat. Biotechnol. 2020, 38, 1288–1297. [Google Scholar] [CrossRef]
- Chen, R.; Wen, L.; Guo, F.; He, J.; Wong, K.H.; Chen, M. Glutathione-scavenging natural-derived ferroptotic nano-amplifiers strengthen tumor therapy through aggravating iron overload and lipid peroxidation. J. Control. Release 2025, 379, 866–878. [Google Scholar] [CrossRef] [PubMed]
- Barberio, D. Navigating regulatory and analytical challenges in live biotherapeutic product development and manufacturing. Front. Microbiomes 2024, 3, 1441290. [Google Scholar] [CrossRef]
- Kaistha, S.; Devi, P.; Sharma, N.; Sagar, S. Navigating Regulatory Frameworks and Compliances for Bacteriophages as Therapeutic Agents. Curr. Pharm. Biotechnol. 2025, 27, 334–346. [Google Scholar] [CrossRef]
- Li, Y.; Ran, Q.; Duan, Q.; Jin, J.; Wang, Y.; Yu, L.; Wang, C.; Zhu, Z.; Chen, X.; Weng, L.; et al. 7-Dehydrocholesterol dictates ferroptosis sensitivity. Nature 2024, 626, 411–418. [Google Scholar] [CrossRef]
- Gunasekara, C.; Scott, C.; Laritsky, E.; Baker, M.; MacKay, H.; Duryea, J.; Kessler, N.; Kessler, N.; Hellenthal, G.; Wood, A.; et al. A genomic atlas of systemic interindividual epigenetic variation in humans. Genome Biol. 2019, 20, 105. [Google Scholar] [CrossRef]
- Thomas, A.; Fidelle, M.; Routy, B.; Kroemer, G.; Wargo, J.; Segata, N.; Zitvogel, L. Gut OncoMicrobiome Signatures (GOMS) as next-generation biomarkers for cancer immunotherapy. Nat. Rev. Clin. Oncol. 2023, 20, 583–603. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xie, L. AI-driven multi-omics integration for multi-scale predictive modeling of genotype-environment-phenotype relationships. Comput. Struct. Biotechnol. J. 2024, 27, 265–277. [Google Scholar] [CrossRef]
- Cheng, J.; Yu, Q.; Li, J.; Xu, Z.; Li, J.; Guan, L.; Xiao, J.; Duan, X. Intrinsic tumor-targeted murine Ferritin nanocage co-delivers GPX4 and FSP1 inhibitors for synergistic ferroptosis-immunotherapy. Nano Today 2024, 58, 102411. [Google Scholar] [CrossRef]
- Jain, M.; Prasanthi, S.; Bommala, N.D.; Goudanavar, P.; Naveen, N. Harnessing the Human Microbiome for Innovative Drug Delivery Systems: Exploring Pharmacomicrobiomics and Targeted Therapies. Curr. Pharm. Des. 2025, 31, 2959–2971. [Google Scholar] [CrossRef]
- Selle, K.; Fletcher, J.; Tuson, H.; Schmitt, D.; McMillan, L.; Vridhambal, G.; Rivera, A.; Montgomery, S.; Fortier, L.; Barrangou, R.; et al. In Vivo Targeting of Clostridioides difficile Using Phage-Delivered CRISPR-Cas3 Antimicrobials. mBio 2020, 11, 12. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; He, C.; Zhang, Q.; Bayakmetov, S.; Wang, X. Modularized Design and Construction of Tunable Microbial Consortia with Flexible Topologies. ACS Synth. Biol. 2024, 13, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Teng, Z.; Zhu, J.; Tang, R. An improved biomarker-guided adaptive patient enrichment design for oncology trials. J. Biopharm. Stat. 2025. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Turjeman, S.; Rozera, T.; Elinav, E.; Ianiro, G.; Koren, O. From big data and experimental models to clinical trials: Iterative strategies in microbiome research. Cell 2025, 188, 1178–1197. [Google Scholar] [CrossRef]
- Chianumba, E.C.; Ikhalea, N.; Mustapha, A.Y.; Forkuo, A.Y. Developing a Framework for Using AI in Personalized Medicine to Optimize Treatment Plans. J. Front. Multidiscip. Res. 2022, 3, 57–71. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, Y.; Zhao, F.; Cheng, X. Gut Microbiota and Ferroptosis in Colorectal Cancer: A Comprehensive Review of Mechanisms and Therapeutic Strategies to Overcome Immune Checkpoint Resistance. Biomolecules 2025, 15, 1546. https://doi.org/10.3390/biom15111546
Cai Y, Zhao F, Cheng X. Gut Microbiota and Ferroptosis in Colorectal Cancer: A Comprehensive Review of Mechanisms and Therapeutic Strategies to Overcome Immune Checkpoint Resistance. Biomolecules. 2025; 15(11):1546. https://doi.org/10.3390/biom15111546
Chicago/Turabian StyleCai, Yingchang, Feng Zhao, and Xiaofei Cheng. 2025. "Gut Microbiota and Ferroptosis in Colorectal Cancer: A Comprehensive Review of Mechanisms and Therapeutic Strategies to Overcome Immune Checkpoint Resistance" Biomolecules 15, no. 11: 1546. https://doi.org/10.3390/biom15111546
APA StyleCai, Y., Zhao, F., & Cheng, X. (2025). Gut Microbiota and Ferroptosis in Colorectal Cancer: A Comprehensive Review of Mechanisms and Therapeutic Strategies to Overcome Immune Checkpoint Resistance. Biomolecules, 15(11), 1546. https://doi.org/10.3390/biom15111546






