Abstract
Cancer remains one of the global leading causes of death and various vaccines have been developed over the years against it, including cell-based, nucleic acid-based, and viral-based cancer vaccines. Although many vaccines have been effective in in vivo and clinical studies and some have been FDA-approved, there are major limitations to overcome: (1) developing one universal vaccine for a specific cancer is difficult, as tumors with different antigens are different for different individuals, (2) the tumor antigens may be similar to the body’s own antigens, and (3) there is the possibility of cancer recurrence. Therefore, developing personalized cancer vaccines with the ability to distinguish between the tumor and the body’s antigens is indispensable. This paper provides a comprehensive review of different types of cancer vaccines and highlights important factors necessary for developing efficient cancer vaccines. Moreover, the application of other technologies in cancer therapy is discussed. Finally, several insights and conclusions are presented, such as the possibility of using cold plasma and cancer stem cells in developing future cancer vaccines, to tackle the major limitations in the cancer vaccine developmental process.
1. Introduction
Vaccines have been used to protect human health against infectious diseases since their first discovery in the late 1700s [1]. The recent success of vaccines against the coronavirus disease is encouraging researchers to extend the underlying concepts to treat cancers [2,3]. Active immunotherapy or vaccination is one of the important aspects of efficient tumor eradication by therapeutic cancer vaccines that can be stimulated and enhanced in two major ways: (1) using nonspecific proinflammatory molecules and adjuvants to improve the antitumor immune response already present in the body or (2) provoking a new immune response against specific tumor antigens in the host [4]. The desired tumor antigens and adjuvants are usually delivered together to stimulate adaptive immune systems, aiming to accomplish the optimal activation of dendritic cells (DCs) and durable responses from effector T cells [5]. Innate immune cells, such as natural killer (NK) cells and phagocytes, also play essential roles in tumor recognition and inhibition [6]. However, the immunosuppressive tumor microenvironment (TME) is one of the key obstacles to tumor-infiltrating immune cells and immunotherapies. The combination of therapeutic cancer vaccines and immune checkpoint inhibitors has become the emerging approach to enhance patients’ response rates and survival [7]. The efficacy of cancer vaccines is still under scrutiny in numerous clinical trials [8]. In this review, we explore the mechanism of the cancer immune cycle in the TME and analyze the effectiveness and limitations of major cancer vaccine platforms. Further, we provide new insights for forthcoming cancer vaccines to be more efficient.
2. Tumor Microenvironment and Cancer Vaccine Mechanisms
The TME contains a plethora of immune cells, such as monocytes, macrophages, natural killer cells (NKs), dendritic cells (DCs), lymphocyte B cells, and lymphocyte T cells (CD4+ and CD8+) that play key role in the antigen-presentation process and cancer immune cycle that can lead to tumor progression; therefore, targeting the TME and its components is considered a major mechanism for effective cancer vaccines [5,9,10,11]. Macrophages, B cells, and DCs in the TME are some examples of the cells called antigen-presenting cells (APCs). These cells promote antigen-specific immune cell interaction and activation (called priming process) by taking up the antigens originating either from vaccine injections through various administration routes (subcutaneous, intradermal, or intramuscular) or from dead cancer cells. The APCs then make the antigens present on the major histocompatibility complex (MHC) class I or II [12,13,14] (in humans, the human leukocyte antigen (HLA) is the MHC system [15]). This is followed by APC migrations from the TME to the lymph nodes to activate the effector T cells (CD4+ or CD8+) [16,17,18]. Lymph nodes are one of the secondary lymphoid organs (SLOs) that provide a three-dimensional structure for immune cells and enhance the interactions between antigen-loaded APCs and effector T cells to activate T cells and produce an effective immune response. Within the lymph nodes, the mature APCs can activate the effector T cells by presenting the MHC-antigen complexes to the effector T cells. This is followed by the infiltration of the activated effector T cells into the TME, where the T cells can recognize the targeted cancer cells and kill them [19,20,21]. Primary (quiescent) B cell follicles in SLOs (called follicular B cells) become activated upon antigen binding to the primary follicles; following activation, primary follicular B cells turn into the secondary follicles, containing a central germinal center (GC) full of B cell blasts, and with antibody maturation, these B cell blasts undergo several phases and processes pertinent to antibody maturation. These steps lead to the differentiation of lymphocytes into effector T (Teff) cells and B memory cells and in this way, their migrations into the TME is facilitated, leading to the eradication of the tumor cells [5,22,23,24,25,26,27,28,29,30]. However, based on studies on the TME, the generation of antitumor defenses occur not only in SLOs, but also directly within SLO-like aggregations called tertiary lymphoid structures (TLS) [31] that develop in the TME through cytokine accumulations, including CXCL13, RANKL, and interleukin (IL)-7. These structures interact with lymphoid tissue-inducer cells (LTi), as well as other cells, specifically DCs, NKs, or CD8+ T cells, leading to the secretion of factors that are essential for high endothelial venule (HEV) formation (which mediate lymphocyte trafficking to lymph nodes), immune cell recruitment, and cell retention. Together, these aforementioned factors recruit and activate the LTi cells [31,32,33,34,35,36,37,38,39,40]. All these stages, from antigen absorption to cancer cell death, are considered as parts of the cancer immune cycle that will be discussed in detail in the following section.
Among the various APCs present in the TME, DCs are the most potent APC compared with B cells and macrophages. These cells mediate the antigen priming-related processes through two general mechanisms: canonical (cross-antigen presentation) and non-canonical (cross-antigen dressing) pathways [41,42]. The canonical pathways are more commonplace and are based on the type of the antigenic proteins (exogenous/endogenous). APCs (like DCs) can drive the canonical antigen presentation mechanism via two major pathways: (1) the cytosolic or proteasome degradation path (specified for endogenous protein presentation), during which the endogenous antigenic proteins, with either a proteosome or a phagosome origin, are cleaved by the cytosolic proteasomes of the DCs to generate peptide fragments, which then become presented by MHC-I molecules and activate the effector T cells against tumor cells, in particular antigen-specific CD8+ cytotoxic T lymphocytes (CTLs) [43,44,45,46], or (2) the vacuolar or endocytosis path (specified for exogenous antigenic protein presentation), during which DCs take up the exogenous antigenic proteins via the endocytosis process to form special vesicular structures called endosomes, which will then be fused with lysosomes, where the lysosomes’ low pH degrades these antigens to peptide fragments, which are then presented on MHC-II molecules, and activate the CD4+ T cells, leading to CTL activation, function, and survival [13,23,41,47,48,49,50,51,52,53,54,55]. Apart from the above-mentioned canonical pathways, several papers have demonstrated the presence of non-canonical/cross-dressing pathways through which the APCs such as DCs do not present the antigen themselves. Instead, the antigen–MHC complexes from the other adjacent DCs or tumor cells (donor cells) get transferred to the APCs, such as DCs (receiver cells), though various mechanisms, including trogocytosis, exosome uptake, and tunneling nanotubes [49,56,57,58,59], and activate the related effector T cells without further antigen processing stages [60,61,62,63,64,65,66]. These processes are shown in Figure 1.
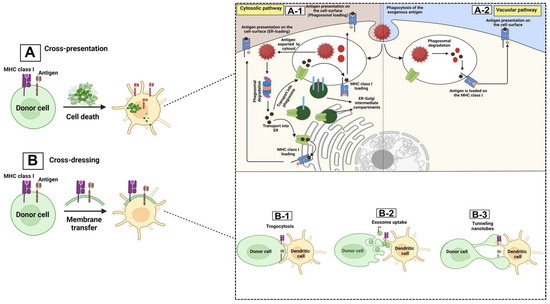
Figure 1.
APC presentation mechanisms. tumor microenvironment and its components: TME contains a wide range of antigen cross-presentation processes that can be mediated by dendritic cells either through the canonical/cross-presentation pathways (A), or through the non-canonical/cross-dressing path (B). The cross-presentation mechanisms are mediated in two ways: cytosolic or proteosome degradation (A-1) and through the vacuolar pathway (A-2). In the cytosolic pathway, antigens that stem from either endosome or phagosome structures move toward the cytosol, forming acidic cytosolic proteosomes that cleave the antigens into shorter peptides. These peptides have two fates: (1) they are transported to the endoplasmic reticulum (ER) for further modifications. The modified antigenic peptides are then loaded on MHC class I molecules and move to the cell surface. (2) the cleaved peptides return to phagosomes/endosomes prior to loading on the MHC I and moving to the cell surface. In the vacuolar pathway, the aforementioned events related to antigen loading on MHC class I occur in the phagosomes or endosomes, which is followed by the moving of the antigen-loaded APCs (DCs) toward the secondary lymphoid organ (SLO) to activate T cells [13,41,49,50,51,52,53]. In the non-canonical/cross-dressing path, the antigen–MHC complex is formed on another cell and is then transformed to the APCs, such as DCs, and the DCs would finally be able to activate the related effector T cell via different pathways: trogocytosis (B-1), exosome uptake (B-2), and tunneling nanotubes (B-3) [59,60,64,65,66,67]. In Trogocytosis, the membrane patch, including the plasma membrane and cytosol from one cell (donor), is transformed to the other cell (trogocytic) [58,59]; exosome uptake by APCs depends on the ability of the exosomes (small membrane-based vesicles formed during the endocytosis process) to transfer particular materials (that can not only be further degraded and reprocessed by APCs for presentation on the MHC molecules, but can also be considered as functional MHC–peptide complexes [59,68]); the tunneling nanotubes are long protrusions derived from cell membranes that not only facilitate the exchange of cell surface molecules and cytoplasmic contents but also can mediate cross-dressing between remote DCs through the transferring of MHC molecules between distant cells [59,69].
3. Cancer Immune Cycle
The cancer immune cycle includes a series of repeated and amplified phases, each of which are mediated by specific cytokines and chemokines which will lead to effective anti-cancer immune response and cancer cell death. Theses stages are as follows: (1) In the first step, the neoantigens that were generated during the tumor formation process are released from the dead tumor cells, which are then carried by the DCs to the adjacent draining lymph node (DLN). (2) The second stage starts with DCs presenting the acquired antigen to T cells through MHC-I and MHC-II molecules to form the MHC-I and MHC-II–antigen complexes, through the cross-presentation pathways discussed earlier. (3) Effector T cells can then recognize the antigen and become activated. (4) Antigen-recognizing tumor-specific T cells present in the DLN, express specific chemokine receptors as well as cell adhesion molecules required for the migration of T cells and their infiltration into the tumor tissue. By virtue of these expressed molecules, T cells leave the DLN and move toward the tumor tissue via the blood stream. (5) This is followed by T cell infiltration into the tumor tissue and (6) the recognition and binding of the MHC-I–antigen complex by virtue of the T cell receptor (TCR), which stimulates the secretion of various cytokines from the DCs that finally activate the T cells. (7) These processes work together to eventually kill the cancer cell [67,70] through various mechanisms, including direct tumor lysis and degranulation [71], antibody-dependent cellular cytotoxicity [72], and/or complement-dependent cytotoxicity [73,74,75]; however, a new pathway for the cancer-killing action of the T cells have been reported recently, which is independent of the antigen presentation by the MHC-I and its recognition by T cells [76]. Considering that additional neoantigens are released upon cancer cell death, causing the immune reaction and continuing the cycle again from the first phase where the neoantigens are upregulated by the cytokines, this mechanism is named cancer immune cycle, the steps of which are shown in Figure 2 in more detail. The cancer immune cycle becomes malfunctioned in cancer patients, as at least one of these steps is defective [67,77]. With this in mind, one of the effective ways to treat cancers would be to develop therapeutic vaccines that can target the cytokines in the cancer immune cycle (Figure 2).
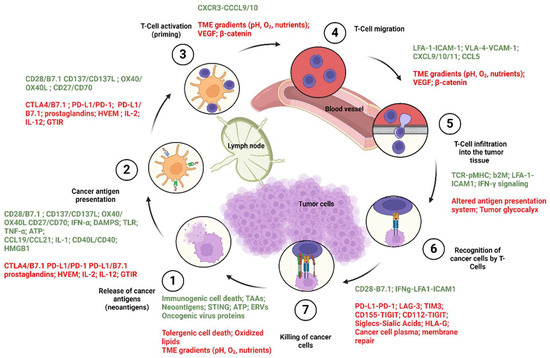
Figure 2.
Caner immune cycle phases and regulation. Cancer immune cycle phases are regulated by a wide spectrum of cytokines and chemokines, some of which stimulate the cancer immune cycle to kill the cancer cells, whereas some cytokines act as inhibitors and down regulate the processes. The stimulatory cytokines work together to mediate the T cell activation. Activated T cells go the TME and induce tumor killing. Each phase is regulated by a wide range of cytokines and other molecular factors, as shown in green for inducers and red as inhibitors.
4. Escaping from the Cancer Immune Cycle
The host immune system constitutes a surveillance part and a protective part; the immune surveillance system constantly inspects the body and boosts antitumor immune responses in order to identify and destroy any existent tumor cells and finally to prevent cancer progression [78,79,80,81]. In general, cancer cells undergo various genetic and epigenetic modifications, leading to the generation of specific antigens; these antigens then stimulate T cells to recognize and kill cancer cells; however, as tumor cells grow, they start to develop mechanisms known as “cancer immunoediting” to escape this host immune surveillance system; thus, the immune system is not able to eliminate these cancer cells [79,82,83]. On the other hand, looking at the protective part of a normal immune system, it consists of specific protein molecules called “immune checkpoints” that are present on the surface of immune cells (including T cells), and their corresponding ligand receptors, which are present on the cancer cells. These immune checkpoints induce inhibitory signals (using the mono-tyrosine-based signaling motifs, in particular, immunoreceptor tyrosine-based inhibitory and switch motifs) that prevent the generation of any strong immune response signals that destroy healthy cells in the body. In this way, immune checkpoints tend to protect normal cells [84,85,86]. PD-1, CTLA-4, LAG3, TIM3, BTLA, and TIGIT are some of the common immune checkpoints that mediate tumor cell recognition by T cells; when the immune checkpoint proteins present on T cells’ surfaces recognize and bind to the tumor cells’ receptors, they send an “off” signal to the T cells and hence prevent the eradication of cancer cells by the immune system. With this in mind, some cancer cells tend to facilitate cancer growth and metastasis by upregulating negative signals via cell surface immune checkpoint molecules and inhibiting T cell activations [87,88], while some other tumor cells may activate immunosuppressive leukocytes (such as eosinophils) to create a TME that is unable to respond to antitumor immune molecules well [89]. Moreover, some tumor-intrinsic genes, including YTHDF1, degrade the MHC-I complex molecules, resulting in immune evasion [90]. According to the mechanisms of action of the cancer vaccines as well as the cancer immunity cycle discussed earlier, some of the key factors for a successful cancer vaccine design are the selection of the appropriate tumor antigen to stimulate effective T cells, the achievement of a sufficient antigen concentration in APCs in such a way as to activate them, as well as the inducement of durable immunogenic responses by activating the effector T cells, i.e., CD4+ and CD8+ [23]. In this respect, the selection of the right antigen along with its delivery method would be of high importance, which will be discussed in detail in the following subsections.
5. Tumor Antigen Classifications
Tumor antigens are any antigenic substances generated in tumor cells that trigger an immunogenic response and serve as biomarkers for tumor recognition that can be used to develop novel therapeutic cancer vaccines. Tumor antigens can appear in phases pertinent to protein synthesis and degradation [82,91]. On the basis of the expression patterns of the HLAs, tumor antigens can be classified into two general groups: tumor-associated antigens (TAAs), in which the antigen is presented by the HLAs that are only expressed on tumor cells (mainly HLA class I), and tumor-specific antigens (TSAs, or neoantigens), in which the antigen is presented by the HLAs that are expressed not only on cancer cells, but also to normal cells [28,92].
Based on the molecular structure and sources of the antigens, TAAs can fall into one of these categories: differentiated (tissue-lineage), oncofetal, cancer–testis, aberrantly glycosylated and expressed, overexpressed, as well as oncoviral antigens. On the other hand, TSAs are classified, according to the frequency of observations, into shared (public) and personalized (private) neoantigens; shared (public) neoantigens arise from alterations that occur specifically in tumors which are observed across other patients/different malignancies, while their personalized (private) counterparts are those originating from tumor-specific alterations that are less likely to occur across other populations/malignancies; thus, personalized neoantigens are patient-specific. To date, a few numbers of public neoantigens have been recognized, whereas the private neoantigens usually originate from non-recurrent driver/passenger mutations and comprise most of the known neoantigens [25,26,54,93,94,95,96]. Furthermore, based on the source from which the antigen is derived, the antigen can be canonical (derived from the protein-coding genes), or non-canonical (derived from non-protein coding genes); in the canonical antigen, the antigen is expressed within the open reading frames (ORFs) of the protein-coding genes [93], such as the overexpression of numerous cancer-related genes [60,65,94], including p53 [97,98,99,100], cancer/testis antigens (CTAs) [100,101], and the human telomerase reverse transcriptase [102,103]. Non-canonical antigens are expressed outside of the ORFs [104], and can stem from alterations of the antigen at various levels, such as genomic, epigenomic, proteomic, transcriptomic, translational, and antigen-processing levels (intronic retention, alternative splicing, codon read-through, and noncanonical/non-AUG translation initiation levels) [93,100,105]. The tumor antigen classifications along with their properties are summarized in Figure 3.
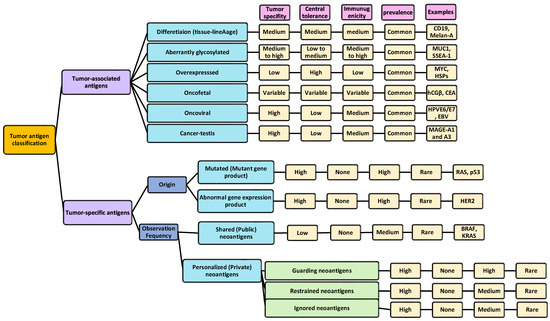
Figure 3.
Tumor antigen classifications. In general, tumor antigens are classified as tumor-specific and tumor-associated antigens, each divided into several categories. Each category is compared in terms of (1) tumor specificity (refers to the degree to which a particular immune response targets and interacts specifically with tumor cells or its antigens while sparing normal cells; hence, a high tumor specificity is desired), (2) central tolerance (mechanisms by which the immune system recognizes the self-antigens from cancer antigens and eliminates cancer cells during their development within the body without mounting immune responses against the self-antigens. Thus, a high central tolerance is desired, as it means that the immune system exhibits a strong level of tolerance towards self-antigens, including those present on normal cells and tissues, and can recognize them better), (3) immunogenicity (indicates the ability of cancer cells to stimulate an immune response from the host immune system. This immune response can involve the activation of immune cells, and the production of antibodies against tumor-specific or tumor-associated antigens; so, high immunogenicity is a desired factor), and (4) prevalence (this shows how common or rare the occurrence of tumor antigens is in patients) [25,26,54,93,94,95,96].
Apart from the type of the antigen, determining an efficacious method for the delivery of the tumor antigen to the APCs would not only make antigen-mediated APC targeting more selective and induce T cell activations, but it would also decrease systemic toxicity. Since different types of antigens have various physical properties, inducing an optimum immune response would depend mostly on the selection of an appropriate delivery system [23]. Some of the major delivery methods are using cells, antigens, peptides, nucleic acids, and viral-based ones, each of which will be discussed in detail in the following sections.
6. Different Cancer Vaccine Platforms
6.1. Peptide-Based Vaccines
In peptide-based cancer vaccines, usually 20–30 amino acids are used to make a wide range of peptides for activating the immune system of patients, enabling them to recognize and kill the tumor cells by enhancing the T cell-mediated immune responses specific to a particular tumor, i.e., CD8+ and CD4+ T cells via the MHC class I and II molecules, respectively [106,107]. These peptides usually belong to one of the TAAs or TSAs (including cancer/testis antigens and neoantigens) that are used for designing personalized vaccines. Peptide-based cancer vaccines can not only activate both B cells and T cell-mediated immune responses, but also induce long-lasting tumor-killing effects [108]; however, to elicit an efficient antitumor T cell response, cancer vaccines usually deliver a mix of tumor antigen peptides including TAAs and TSAs. The identification and discovery of tumor antigen peptides have been discussed in other reviews [54,93,109]. Synthetic long peptides (SLPs) are stronger than short peptides in activating T cell responses because SLPs need to be processed by the APCs and can activate both cytotoxic CD8+ T cell and CD4+ T helper cell responses [110,111]. Due to low immunogenicity, peptide-based vaccines are usually formulated with immune adjuvants. Adjuvants have been licensed by the FDA and EMA for humans, include aluminum salts, MF59, adjuvant systems, and CpG 1018 [112]. Other adjuvants under investigation are polyinosinic-polycytidylic acid stabilized with polylysine and carboxymethylcellulose (poly-ICLC), glucopyranosyl lipid A, Imidazoquinolines, CpG oligodeoxynucleotides, cyclic dinucleotides, etc. [113]. To further increase the immunogenicity of peptide antigens, heteroclitic peptides, which are modified versions of peptides that have been altered (by replacing amino acid residues in the epitope sequence that have similar biochemical properties, overall structure, and function compared to the original amino acid sequences; this is known as conservative amino acid substitution) to enhance their binding affinity to the MHC molecules; in this way, they can induce an enhanced immune response against specific antigens, making heteroclitic peptides a potential tool in vaccine development and immunotherapy for diseases such as cancer [114,115,116,117]. Considering the short half-life and poor stability of the free peptides in the body, tumor antigen peptides are usually incorporated into other delivery systems. Poly lactic-co-glycolic acid (PLGA) nanoparticles and liposomes are two representative delivery systems of antigen peptides and adjuvants because of their proven safety [118,119]. A comparative study by Varypataki, Jiskoot et al. showed that SLP-loaded PLGA nanoparticles and cationic liposomes are more potent for stimulating the T cell responses in vivo than squalene or Montanide-based emulsions [119]. Both vehicles can protect the peptides from degradation and promote dendritic cell uptake and lymph node transport.
In the last decade, liposomal vaccines evaluated in clinical trials include Tecemotide, DepoVax, ISCOMATRIX, Lipo-MERIT, etc.; however, none of them improved the patients’ survival [120,121]. In addition to synthetic nanoparticles, dendritic cell-derived exosomes (DEXs) could play an important role in tumor immunology by transferring MHC/peptide complexes to other immune cells and stimulating T and NK cells directly or indirectly [122,123]. DEXs loaded with antigen peptides have been assessed as cancer vaccines in clinical trials, but failed to generate adequate adaptive immunity in patients with advanced cancer [123]. Nevertheless, DEXs still hold the promise of being a part of combination therapies.
6.2. Recombinant (Pathogen) Vaccines: Viral and Bacterial-Based Vaccines
There are three major classes of recombinant viral/bacterial vaccines: (1) inactivated vaccines (that use killed virus/bacteria that has been cultured in the lab) [124,125], (2) live attenuated vaccines (in which the virus/bacteria is being weakened but not completely killed) [126,127], and (3) subunit vaccines (in which a portion of the virus/bacteria-like protein is used) [126,128,129]. All recombinant vaccines are based on administrating the recombinant genes (such as genes encoding TAAs, cytokines, or costimulatory molecules that are inserted into the viral/bacterial genome) using recombination/selection methods into APCs to stimulate the appropriate antitumor immune responses and by engaging both innate and adaptive immune systems. Viral/bacterial-based vaccines can provide effective and long-lasting immune responses [130,131,132,133,134,135]. These vaccines target the APCs and initiate immune responses through two major mechanisms: (1) the indirect infection of the APCs, which works through cellular damage mediated by viral infection to send danger signals and as well as costimulatory molecules to activate the APCs of bone marrow [130,136,137], and (2) the direct infection of the APCs, which is based on the processing of the antigens in the MHC pathways. The latter mechanism facilitates recombinant viral vaccine modifications for enhancing the antigen presentation [130,138,139]. Some of these modifications are based on expressing the genes encoding the minimal level of MHC class I-restricted peptides [140], inserting endosomal/lysosomal sorting signals into the gene encoding antigen [12,141], as well as using poxviruses to activate T cells [142,143,144], or to be used as a vector to carry specific costimulatory molecules or cytokines [145]. One of the recent effective bacterial-based cancer vaccines was developed by Wu et al.; these researchers used attenuated flagellated bacteria (strain of Salmonella typhimurium) coated with positively charged dendrimer nanoparticles with the ability to bind to negatively charged antigens, and the bacterial had become less immunogenic via gene mutations [146].
6.3. Cell-Based Vaccines: Dendritic Cells (DCs), Stem Cells, and Chimeric Antigen Receptor (CAR) T Cell Therapy
Therapeutic cell-based vaccines are based on the in vitro activation of the APCs (like NK cells or DCs) by the viral peptides, genes, or by using genetically modified tumor cells (killed tumor cells). In this regard, the cell-based vaccines can be classified as tumor cell vaccines and immune cell vaccines [147]. In the tumor cell vaccines, the whole tumor cell is used as the source of the vaccine, which contains whole TAAs, including the CD4+ and Cd8+ T cells’ epitopes. Whole-cell cancer vaccines are currently in clinical trials. Using whole tumor cells as a vaccine that has all the possible antigens in it rather than protein/peptide tumor antigens not only eliminates the need to identify the ideal target antigen; in addition, several tumor antigens can be targeted at once, which would then induce further immune responses to more tumor cells [148,149]. However, there is still a need for a stimulus/stimulatory factor(s) to provoke the antigen absorption process by the APCs to recruit cells from innate and adaptive immune systems. With this in mind, cell-based vaccines have been modified either genetically or via irradiation in such a way as to be able to secrete cytokines without further proliferation in the host [147,150,151]. Most of the recent developed cancer vaccines are based on using whole cells like DCs, which affect the function of the cells in the immune system. The importance of DCs in antigen uptake and presentation processes, as well as T cell activations, which are mediated by a wide spectrum of receptors present on DCs’ surfaces, including those for antigen uptake, antigen presentation, costimulatory molecules, cytokines receptors, receptors for environmental sensors, cytokine production-related receptors, as well as chemokine receptors, have turned DCs into the most commonplace immune cells used in developing immune cell-based cancer vaccines [12,22,39,49,147,152,153,154]. To improve the efficacy of DCs in antigen absorption and T cell activation, researchers have started to use stem cells to develop better cell-based cancer vaccines. The application of stem cells in the realm of cancer vaccines started with embryonic stem cells (ESCs) [155]; considering that ESCs are usually obtained from an unrelated donor, they express a mismatched MHC and minor histocompatibility (miH) antigens (which are peptides derived from normal self-proteins that, in humans, are presented by HLA), and if transplanted in the host, they will cause alloimmune responses [156,157,158]. Although ESCs express a low amount of HLA-I [159,160,161,162,163] and almost no HLA-II [162,163,164,165] and costimulatory molecules [162,164,165], this amount is sufficient to stimulate the cytotoxic T cell-mediated xenorejection of human ESCs [158,166,167]. Following the characterization of human ESC lines, and considering the ability of whole-cell vaccines to deliver multiple oncofetal antigens at once, along with their universal application to all patients regardless of their HLA type [156,168,169], researchers have started to apply these ESCs to whole-cell cancer vaccines to make ECS-based cancer vaccines. Using xenogeneic human ESCs as the plausible cancer vaccine to be tested on mouse and rat models, studies have found that human ESCs resulted in a moderate tumor killing effect, whereas in the case of using allogeneic or autologous ESCs, they observed more potent tumor suppressive effects [169,170]. However, considering that human ESCs were injected into mice, there was the possibility that the aforementioned immune responses were due to the incompatibility of the MHC antigens between the human ESCs and mouse cells rather than the ESC lines [169]; moreover, the tumorigenicity induced by the ESCs hampered their usage as effective cancer vaccines for clinical applications [171,172,173]. These problems led researchers to shift their focus toward using induced pluripotent stem cells (iPSCs), as they share very common features with ESCs in terms of gene expression and epigenetic profiles [174,175,176,177,178,179]. However, iPSCs also have some level of tumorigenicity. Various methods have been reported to overcome their tumorgenicity when developing stem cell-based vaccines: the terminal differentiation or complete elimination of residual iPSCs from culture; interfering with tumor-progression genes to prevent tumor formation from the residual cells; and tumor detection and elimination after its initial formation in the patient’s body [173]. In light of this, most of the recent work with iPSCs have used irradiation to remove the residual iPSCs in the culture and strongly prevent teratoma formation and further iPSC-mediated tumorigenicity [180,181,182,183]. Early studies working on iPSCs transfected to mouse colon cancer demonstrated that although the iPSCs were able to induce cytokines in response to the cancer cells, no tumor rejection was observed, indicating that iPSCs need modifications to be able to induce a strong immune response against tumor cells; for instance, considering that autologous iPSCs have more accurate tumor antigens compared with their xenogeneic counterparts, they can be a better option for developing anti-cancer vaccines than the xenogeneic ones, as they can minimize the alloimmunity; further, to enhance their immune responses against cancers, immunostimulatory adjuvants can be used with them (such as TLR9) [169]. Kooreman et al. used the same strategy to generate an iPSC vaccine against pancreatic ductal adenocarcinoma, in which the autologous iPSCs were irritated, followed by the addition of CPG (a type of TLR 9 adjuvant) to improve the immune response [169]. Another study developed autologous iPSCs from patients with T cell acute lymphoblastic leukemia and were loaded in DCs; this showed efficacy in suppressing acute lymphoblastic leukemia cancer [184]. In a recent study, iPSC-derived exosomes were incubated with DCs (dendritic cells) and their antitumor effects were explored in murine melanoma models; according to their results, the DC+ exosome vaccination significantly inhibited lung metastasis in in vivo models, induced long-term T cell responses, and did not alter the viability of normal cells and mouse viscera [185]. In the same way, another group prepared a nanostructure by combining the iPSCs and DC exosomes that contained the anticancer drug doxorubicin; this improved the in vivo efficacy of chemotherapy drugs as well as the antitumor immunity [186]. Apart from iPSCs, researchers have used inactivated cancer stem cells (CSCs) to develop cancer vaccines [187]. Chimeric antigen receptors (CARs) are recombinant protein receptors that have been engineered in such way as to enable T cells to target a specific antigen in order to generate an antitumor immune response and kill specific tumor cells. The general structure of these receptors are made up of three major domains: (1) an extracellular domain specified for selective binding to a particular tumor antigen, (2) a transmembrane domain, and (3) a intracellular domain; together, these domains facilitate T cell-mediated tumor death by providing T cell signals that are necessary for their activations and for attacking the tumor cells [188,189,190,191]. One of the examples of CAR-T cell therapy is based on using genetically modified autologous T cells expressing CD-19. The therapy reprograms the patient’s own T cells via a transgene that encodes the CAR and is able to recognize and destroy any cells (normal and malignant) that express the CD-19, in a way that, after binding to CD19-expressing cells, the CAR sends a signal that enhances the T cell expansion, activation, and target cell elimination, along with the persistence of the drug. The aforementioned mechanism can be seen in two of the current FDA-approved drugs based on CAR-T cell therapy, i.e., Tisagenlecleucel (used to treat acute lymphoblastic leukemia) and Axicabtagene ciloleucel (used for treating large B cell lymphoma) [192,193]. However, there are some limitations to overcome: there is the possibility of antigen loss, so that patients treated with CAR-T cells may partially express the antigen or may not express it at all [194,195,196]; another problem is the possibility of the expression of the tumor antigen by normal cells [197]. Although the combination of checkpoint inhibitors and CAR-T cell therapy is a new treatment option, this treatment may still be unable to induce efficient T cell infiltration and may lead to cytokine-mediated toxicities that have been reported in several CAR-T cell therapies [198,199,200,201]. This requires looking for a novel method to optimize the CAR-T cell therapy-based cancer vaccines.
6.4. DC Subsets and Their Roles in Priming and Activating T Cells
DCs originate from macrophage–DC progenitors (MDP) in bone marrow and generate common DC progenitors (CDP) that then differentiate into the DCs [154,202], which comprise various types of immune cells that, based on their phenotypes, ontogenetic features, distribution in tissues, as well as transcriptional-related characteristics, are divided into three major groups: classical/conventional DCs (cDCs) (that include cDC1s and cDC2s), plasmacytoid DCs (pDCs), as well as monocyte-derived DCs (moDCs) [203,204]. Each of these DC groups secrete specific types of cytokines that are specialized for priming and activating various classes of effector T cells and regulating particular stages of the cancer immune cycle; thus, in this way, they can affect the result of an immune response in different ways [49,202,203,205]; for example, cDC1s are specialized for antigen priming as well as their cross-presentations to the CD8+ T cells, followed by their recognition via MHC I signaling [202,206,207,208,209,210,211,212]. On the other hand, cDC2s are mostly involved in the cross-presentation of the antigens to CD4+ T cells and their recognition through the MHCII path, promoting Th1, Th2, and Th17 polarization [202,211,212,213,214,215,216]. According to single-cell analysis, a further level of complexity in DCs has been reported via the identification of various types of cDC2 subsets [203,217,218]. pDCs produce type I interferons (IFNs) that are engaged in antiviral and antitumor immune responses [202,212,216,219,220]. Finally, moDCs, which are stimulated by inflammation, become differentiated and recruited to inflammatory parts of the body, such as the TME [216,221,222,223,224]. Prior to encounters with the antigen, DCs are immature and characterized by a high expression of MHC-II inside the cell, low expressions of co-stimulatory molecules and chemokine, and cytokine receptors [225,226,227]. However, these immature DCs uptake the antigen via the cross-presentation process or cross-dressing, and become mature DCs through various pathways, in particular receptor-mediated endocytosis [228,229,230,231]. Due to the presence of various types of receptors on the their surfaces (Figure 4), DC maturations can be stimulated by different factors, ranging from monoclonal antibodies (mAb) to DCs modifications, and the physiological alterations in DCs occur during their maturations, enabling DCs to secrete a wide range of stimulatory cytokines and other chemical molecules to block the inhibitory signals and increase co-stimulatory molecules, cytokine production, and antigen presentation [232,233,234,235,236]. DCs then process and present tumor antigens derived from the vaccinating cells to the effector T cells (CD4 and CD8) via the formation of antigen–MHC complexes on the DCs, and T cells bind to this complex with their T cell receptors (TCRs) [17,234,235,237]. During maturation, DCs undergo physiological alterations, leading to the incremental expression of surface MHC I and MHC II molecules [238,239], co-stimulatory molecules (such as B7-1/CD80, ICAM-1/CD54, LFA-3/CD58, and Tropomodulin1) [240,241,242,243], chemokine receptors [244,245], and cytokine secretions [246,247,248,249], that together regulate the T cell response. Furthermore, DC maturation results in a reduction in the pH of the endocytic vacuoles, leading to proteolysis, the transport of peptide–MHC molecules to the cell surface, and a reduction in the capacity for antigen capture [250,251,252,253,254,255]. Following the upregulated expressions of various stimulatory molecules/receptors, the DCs migrate toward the draining lymph nodes to interact with the T cell and induce the immune response to finally present the tumor antigen derived from the vaccinated cells to the effector T cells (CD4 and CD8) via the formation of antigen–MHC complexes so that T cells can bind to these complexes present on the surface of the DCs with their receptors (TCRs) and become activated; this process leads to tumor killing [17]. If the CD8 T cells are activated efficiently, with addition of other traditional cancer therapy methods, such as monoclonal antibodies, chemotherapy, and radiation therapy, they all can work together synergistically to improve the efficiency of T cell-mediated tumor-killing effects [256,257,258,259,260]. With this perspective, T cell activation regulation is one of the key factors to be considered when developing cancer vaccines. T cell activation is modulated by a wide range of other factors and signals produced by the activated DCs [261], agonist antibodies [262,263], co-stimulatory molecule receptors [264,265], and co-inhibitors (immune checkpoint inhibitors) [84,266]. However, in order to maintain immune homeostasis and self-tolerance, as well as to reduce/prevent inflammation and autoimmunity diseases, it would be necessary to inhibit the effects of stimulatory signals when needed. In light of this, specific molecules, including (1) a heterogeneous Foxp3 expressing a subset of CD4+ T cells known as regulatory T cells (Tregs) that have immunosuppressive properties [267,268,269,270], and (2) other suppressive immune cells, such as myeloid-derived suppressor cells (MDSC) [271,272,273], act and suppress by secreting various inhibitory cytokines and molecules (such as TGF-β, IL-10, and IL-35) [257,274,275,276,277]. Chemotherapy and radiation, if used at immunomodulatory doses, could inhibit the T cell activation [278,279,280,281,282].
6.5. Nucleic Acid-Based Vaccines: DNA and mRNA Vaccines
Nucleic acid vaccines are based on using either DNA or mRNA to deliver genes to the host APCs to encode the tumor antigens and produce antigen proteins so that the expressed tumor antigens induce appropriate immune response to kill/inhibit cancer cells [283].
6.5.1. DNA-Based Cancer Vaccines
The history of using DNA cancer vaccines goes back to 1990 when Wolff et al. studied the effects of the direct injection of naked DNA to murine muscles, which resulted in the expression of their corresponding proteins [284]. And in 1998, the first human trials of a DNA vaccine were reported, which demonstrated the efficiency of DNA vaccines in treating immunodeficiency virus type 1 (HIV) [285]. Cancer DNA vaccines are based on using bacterial plasmids that encode the tumor antigens to activate both innate and adaptive immune responses. In order for the DNA vaccines to be functional, they need to enter to the cell nucleus to be transcribed into mRNA; then, they are transported to the cytoplasm to be translated to the encoded antigens, followed by antigen processing and presentation to CD8+ T (via MHC I) and CD4+ T (via MHC II) cells to activate particular immune responses [286,287,288,289]. The mode of action of DNA vaccines is the activation of adaptive and innate immune systems [290]. Regarding the adaptive immunity activation-based mechanisms, there are three major pathways: (1) the direct insertion of DNA into a somatic cell, such as a muscle cell, followed by translating to antigens, and their direct presentation to the cytotoxic CD8+T cells via the MHC-1 molecules [291]; (2) the releasing of the DNA-encoded antigen in somatic cells through secretion or via apoptotic bodies, followed by phagocytosis and the processing of the released peptides by APCs and their cross-presentations to the CD4+ T cells by the MHC II molecules [291]; and (3) the direct transfection of DNA into the APCs to generate antigens (which would be endogenous antigens). These endogenous antigens are then processed and presented to CD8+ T and CD4+ T cells via MHC I and MHC II molecules, respectively, to induce adaptive cellular immunity (via activation of CD8+ T cells followed by their differentiation to CTLs) as well as humoral immunity (by activating the CD4+ T cells); this direct transfection of DNA into APCs, which mainly takes the form of intradermal delivery, is a momentous pathway for DNA-based cancer vaccines [292]. Turning to the innate immunity activation pathways mediated by DNA vaccines, there are a wide range of factors that regulate the aforementioned pathway, such as CpG (cytosine phosphate guanosine) dinucleotides, which are immunostimulatory motifs within bacterially produced plasmid DNA [293]. These are involved in stimulating innate immunity activation by interacting with one of the key innate immunity stimulators, i.e., Toll-like receptor 9 (TLR9). This is followed by thTLR9 recognizing unmethylated CpG motifs in bacterial DNA, resulting in the triggering of the TLR-mediated signaling pathway of macrophages, dendritic cells, and B cells, which involves activations of the NF-κB, IRAK, and MyD88 signaling pathways to produce proinflammatory cytokines, chemokines, and immunoglobulins [113,294]. Furthermore, DNA itself activates the STING signaling pathway, which is the major pathway controlling the DNA signaling cascades, which occur in cytoplasm independent of TLR. In vivo studies have confirmed that DNA vaccines cannot induce a robust adaptive immune response in the absence of the STING path [113,295]. DNA vaccines offer various advantages, including being highly specific and safe, encoding a wide range of antigens, having low production costs, as well as easy transport and storage; moreover, DNA vaccine have a lower risk of insertional mutation and DNA rarely binds to host chromosomes [296,297,298]. Furthermore optimized DNA vaccines have been efficient in preclinical studies [299,300,301,302]. However, because of their poor immunogenicity, DNA vaccines have gained little progression in clinical trials [303,304]. There are several optimization strategies to tackle the poor immunogenicity problem, including the optimization of plasmid elements (such as the Kozak sequence, intron, and species-specific codons) [305,306], a powerful promoter sequence for an efficient transcription (such as modified viral cytomegalovirus promoters) [306,307,308], using specific adjuvants (such as cytosine–guanine dinucleotide (CPG) motifs, polymers, nanoparticles, liposomes, and small molecule agonists) [305,306,309], and finally, modifying the design of tumor antigens [305,306].
6.5.2. mRNA-Based Vaccines
In vitro transcribed mRNA vaccines are the very early versions of mRNA vaccines developed in 1984 using an in vitro transcribed system containing a plasmid DNA template, RNA polymerases, along with other main components [310]. Although at first mRNA vaccines were not developed for therapeutic purposes, early research, including the first in vitro (using DCs that were pulsed with RNA) and in vivo (in mice) studies pertinent to the mRNA-based cancer vaccine back in the 1990s [311], paved the way for using mRNA vaccines for treating diseases, including cancers. Subsequent research that focused on delivering mRNA into the cells using liposomes further confirmed the therapeutic efficiency of mRNA-based vaccines [312], as mRNA vaccines bring a wide spectrum of benefits, such as tolerability (side effects are controllable and temporary) and the lack of a need for genome integration (because unlike DNA, there is no need for the mRNA to enter the cell nucleus); thus, the risk of insertional mutagenesis is eliminated. There is no need for the usage of any pathogenic/viral agents for developing mRNA-based vaccines; therefore, it is non-infectious. Furthermore, mRNA vaccines are degraded easily (which reduces risk of toxicity), providing humoral and cellular immunity, which are essential for antitumor responses. Additionally, mRNA vaccine production is fast and inexpensive [313,314,315,316]. There are four major types of transcribed mRNA: conventional mRNA, self-amplifying mRNA (samRNA), trans-amplifying RNA (tamRNA), and circular mRNA (circmRNA) [317,318,319,320]. The general structure of these in vitro transcribed conventional mRNAs are similar to natural mRNAs in eukaryotic cells, i.e., they are made up of a 5′ cap, 5′ and 3′ untranslated regions (UTRs), an open reading frame (ORF), and a poly(A) tail [320,321,322,323]. In spite of the advantages of such mRNA vaccines, some drawbacks, including the inherent instability of the mRNA, lack of good manufacturing practices, low protein expression efficacy, high immunogenicity, along with the difficulties related to the in vivo delivery of mRNA into cells, and off-target side effects as a result of repeating the injection dosage to maintain protein expression, have hampered its advancement as an effective therapeutic vaccine [314,324]. With this in mind, in recent decades, considerable efforts have been made to optimize mRNA-based vaccines by improving mRNA stability, reducing its in vitro and in vivo immunogenicity through chemical modifications, product purification, and sequence optimization, such as the 5′ end (autologous) or the 5′ cap (analogues) modifications, ORF modification by codon optimization, guanine plus cytosine (GC) content enrichment, maintaining the length of the 3′ poly(A) tail within 120–150 nucleotides, and adding chemically modified adenosines [325,326,327,328,329,330]. Another way to improve the stability and the protein yield was to develop other types of vaccines which are based on RNA rather than mRNA. For example, self-amplifying RNA (saRNA), trans-amplifying RNA (taRNA), and circular RNA (circRNA) have brought therapeutic benefits in the realm of cancer vaccines [320,331,332,333,334,335,336,337,338,339,340].
However, in order for mRNA vaccines to be used in clinical applications, they should be protected from enzymatic degradation, successfully delivered to the target cells, followed by endocytosis, and escape from endosomes to prevent premature degradation. The physicochemical properties of mRNA complexes should be taken into consideration, as they affect the mRNA uptake mechanisms by the targeted cells [315,341,342]. With this perspective, the efficient delivery of mRNA to the targeted cell/tissue is necessary. To reach this goal, two major approaches have been developed: (1) ex vivo DC transfection via electroporation followed by re-infusion of the transfected cells [343,344,345], and (2) the direct injection of mRNA, with or without a carrier [322,345,346]. In the first approach, mRNA is loaded into the DCs through electroporation (to achieve optimized ex vivo transfection without using any carriers). Upon generating transfection DCs, they would be re-infused to the patient to act as carriers as part of an autologous vaccine and induce immune responses. With the ability to initiate adaptive immune responses as well as anti-body responses (by presenting the intact antigen to B cells), DCs have gained considerable attention to be used as ex vivo and in vivo carriers in the realm of mRNA vaccine delivery [347,348,349,350,351,352].
In the same vein, intradermal and intranodal injections were efficient in providing in vivo immunizations [353,354]. On the other hand, physical methods (using electroporation [355] or a gene gun [356,357,358]) increase the mRNA uptake efficiency by the DCs, but are faced with major limitations that hamper their further development, such as increasing cell death, and confining accessibility to target cells [359]; furthermore, using a gene gun, for instance, demonstrated efficiency only in mouse models but not in human models or larger study scales; electroporation increased the immunogenicity (only in the case of a self-amplifying RNA vaccine) [315,358,360]. Using viral carriers for mRNA delivery has several drawbacks, making them inappropriate carriers; some of these drawbacks include poor in vivo efficacy, the possibility of stimulation of immune responses mediated by the vectors, already-existing immunity against viral vectors, and biosafety issues [133,361,362,363]. These drawbacks led scientists to look for other mRNA carriers, which resulted in taking advantage of nanoparticles, in particular the lipid and polymeric-based nanoparticles, to develop versatile, effective, and safe carriers for mRNA delivery [364,365,366,367,368]. Some of these lipid/polymeric-based methods are based on using protamine (cationic peptide) [369,370,371], cationic lipids [372,373], and polymers, including dendrimers and chitosan [374,375,376], as well as lipid nanoparticles [352,365,377], which are conjugated with other polymers like polyethylene glycol (PEG) to increase the stability. In the case of lipid carriers, cholesterol and other natural lipids present in the membrane have been applied to enhance the efficacy. The lipid-based delivery vector not only improves the efficiency of mRNA delivery and facilitates the selective targeting of organs and/or cells (as it would be possible by adjusting the ratio of various elements in the lipid nanoparticle) [352,364], but also, such lipid-based carriers induce an adjuvant effect, as reported in some of the recent studies showing that lipid nanoparticles induce strong in vivo immune responses, with stronger adjuvant efficacy than AddaVax (a commonplace vaccine adjuvant) [322,378,379,380]; furthermore, lipid nanoparticles can enhance the antitumor efficacy of mRNA cancer vaccines through activating the Toll-like receptor 4 (TLR4) signaling pathway [381,382]. mRNA vaccines can be administered through several routes, such as subcutaneous, intradermal, intranasal, intramuscular, intranodal, intratumorally, and intravenous delivery routes [383]. The ex vivo engineering of autologous DCs with mRNA has been considered as the preferred method for tumor antigen delivery, but most approaches used for developing mRNA vaccines have a tendency to use direct mRNA administration with lipid nanoparticle carriers [343,384]. mRNA-based vaccination is developed to either induce or enhance an effective antitumor immune response. Following the administration and cellular uptake by APCs, mRNA goes to the cytoplasm and undergoes antigen priming and MHC-antigen presentation cascades, leading to APC-mediated antigen presentation via MHC-I and MHC-II and CD8+ and CD4+ T cell activation. Apart from that, CD4+ T cells themselves can induce a humoral immune response through coactivating antigen-specific B cells, and these B cells can serve as APCs to conversely activate CD4+ T cells upon the presentation of antigens to the B cells via MHC class II [385,386,387,388].
6.6. Personalized Cancer Vaccines
Personalized cancer vaccines are another type of immunotherapy designed to target a patient’s specific cancer cells based on their unique genetic profiles to stimulate the patient’s immune system to recognize and attack cancer cells more selectively [389]. Based on the different cancer vaccine platforms discussed earlier, several types of personalized cancer vaccines have been developed and used in preclinical and clinical studies, such as personalized cancer vaccines based on peptides [106,390], whole cells [390,391], nucleic acids (DNA and mRNA) [390,392], and neoantigens [390,393]. There are several steps for developing personalized cancer vaccines: (1) a genomic analysis of the patient’s tumor (to identify tumor-specific characteristics, such as mutations, neoantigens, and other related characteristics that can be targeted by the immune system); (2) antigen selection (based on the genomic analysis, tumor-specific antigens are selected for inclusion in the vaccine); and (3) vaccine formulation (vaccines are formulated using different approaches, such as with peptides derived from tumor antigens, DCs loaded with tumor antigens, DNA or RNA encoding tumor antigens, neoantigens [394], or whole tumor cell lysates) [395]. Upon administration of a personalized cancer vaccine, antigens in the vaccine are presented to immune cells by the APCs and stimulate an immune response against the cancer cells bearing the targeted antigens. Cancer vaccines, both conventional and the personalized type, contribute to the growing field of cancer immunotherapy, with personalized vaccines showing promise in improving treatment outcomes for patients with specific tumor profiles, as this type of vaccine offers a highly targeted and individualized approach to cancer immunotherapy, compared to its conventional counterpart that may target common antigens or cancer-associated antigens across broader patient populations. Personalized vaccines are designed to target the patient’s specific cancer cells based on the tumor’s genetic and antigen profile and stimulate the immune system to recognize and attack those specific cancer cells, while conventional cancer vaccines may target common antigens shared by several cancer patients or antigens associated with certain types of cancer but are not personalized to an individual’s tumor. Additionally, the antigens for personalized cancer vaccines are selected based on a genomic analysis of the patient’s tumor and neoantigens, whereas the targeted antigens in the conventional cancer vaccines are more broadly expressed across cancer cells of a particular type or could be associated with cancer but not specific to an individual’s tumor. Considering the production process, the personalized cancer vaccines have a customized production process, (sequencing the patient’s tumor DNA/RNA, identifying specific mutations, synthesizing, or selecting peptides or antigens based on these mutations, and formulating the vaccine); however, the conventional types are produced using standardized antigens or antigen sources that are not personalized to a patient’s tumor). Personalized cancer vaccines activate a targeted immune response against the patient’s specific cancer cells bearing the selected antigens; however, the conventional vaccines induce a generalized immune response against common cancer antigens or antigens associated with specific types of cancer. Personalized cancer vaccines are often used in precision medicine, where treatments are tailored to individual patients based on their unique characteristics. While personalized cancer vaccines offer promising potential in cancer therapy, there are also some challenges that need to be addressed; for instance, they require complex processes (such as genomic sequencing of the tumor, identification of specific antigens), and custom manufacturing of the vaccine for each patient. Considering that these processes are time-consuming, resource-intensive, and costly, these vaccines have limited accessibility among patient populations; this time-consuming problem may lead to other issues and these vaccines are not suitable for patients who require immediate treatment (those with rapidly progressing cancers), as the time required for genomic analysis, antigen selection, and vaccine production may delay treatment initiation [396]. Tumor heterogeneity is another challenge; considering the genetically heterogeneous nature of the tumors, they contain a mix of different cell populations with varying genetic mutations and antigen profiles, and thus, personalized vaccines may not target all relevant antigens present in the tumor, leading to potential escape mechanisms by cancer cells that are not targeted by the vaccine [397]. Furthermore, personalized cancer vaccines may face challenges in overcoming the immune evasion mechanism of the cancer cells, leading to limited efficacy in some cases; other barriers can be attributed to the limited clinical evidence along with other logistical challenges (including storage, transportation, and administration of the personalized cancer vaccines) [393,398,399,400]. Despite these challenges, ongoing research and advancements in cancer immunotherapy continue to improve the development and utilization of personalized cancer vaccines, with the aim of addressing these limitations and enhancing their effectiveness in treating cancer. The most effective approach has been the development of neoantigen-based personal cancer vaccines [394,400,401,402,403,404]. In general, all of the current cancer vaccine platforms have advantages, such as inducing both humoral and adaptive immune systems, long-term stability, flexibility, high immunogenicity, clinical safety, and etc. [106,187,324,364,405,406,407,408,409,410,411,412,413,414,415,416,417,418,419,420,421,422,423,424] along with disadvantages, such as antigen loss, low MHC expression, in appropriate APC uptake and antigen presentation, and etc. [106,201,287,363,383,411,412,416,418,419,422,423] that are summarized in details in Table 1.

Table 1.
Comparing different vaccine platforms.
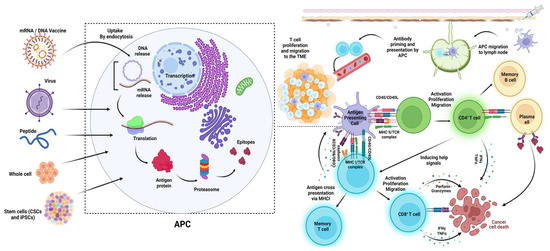
Figure 4.
Cancer vaccine platforms and their mechanisms of action. All of the discussed vaccine platforms tend to be up taken by the antigen-presenting cells to finally induce and enhance the T cell-mediated pathways of killing cancer cells [404].
7. Combining Artificial Intelligence and Cold Plasma Technology as Novel Modality Tools to Develop Cancer Vaccines
Several major companies are currently the leaders of producing therapeutic cancer vaccines, including the following: Immatics, BioNTech, AstraZeneca, Memorial Sloan Kettering Cancer Center, Merck, Massachusetts General Hospital, F. Hoffmann-La Roche, Bristol-Myers Squibb, Regeneron Pharmaceuticals, and Novartis. In spite of numerous patents and the development of various cancer vaccines by the above-mentioned companies, there are several major challenges for the development of efficient and universal cancer vaccines, such as tumor variability in different people, the similarity of the tumor antigens to the body’s own antigens, as well as the possibility of cancer recurrence (caused by an immunosuppressive TME, or tumor heterogenicity) [390,425]. With this in mind, optimizing current cancer vaccines that can cover a wide range of tumor antigens, distinguish tumor antigens from the body’s counterparts, and prevent cancer recurrence is indispensable. As can be seen in Table 1, all of the vaccine platforms have their own particular pros and cons, and this has led scientists to optimize cancer vaccines by combining these different platforms with the aim of reinforcing the advantages and reducing the disadvantages, and to come up with better therapeutic cancer vaccines. Examples of such combination therapies include using lipid-based deliveries (liposomes, lipid nanoparticles, catanionic lipids), various polymers, in particular, PEG, to enhance mRNA stability, infusing polymers with the target cells, tumor specificity, amplifying the tumor antigen response, and reducing possible toxicities [426,427,428,429,430,431,432,433,434,435]. Furthermore, in order to overcome the immunosuppressive TME (which can prevent function and activation of immune cells necessary for destroying cancer cells [436,437]), researchers have combined the usage of mRNA vaccines with immune checkpoint inhibitors [328,438,439]. Tumor heterogenicity refers to the presence of genetically diverse subpopulations with different phenotypic profiles and leads to a diversity of genetic mutations. Tumor homogeneity can be seen between tumors or within the same tumor; tumor homogeneity makes it difficult to detect mutations that occur in subpopulations and has hampered the design of appropriate treatment strategies [440,441]. In terms of tumor heterogenicity, there are spatial (dynamic genome evolution through tumor progression) and temporal (tumor is made up of subclones with different genetic profiles which makes people with the same cancer and tumor subtypes respond differently toward treatments) heterogenicities and both should be overcome [324]. Some of the strategies were based on designing personalized mRNA cancer vaccines based on the variations seen in tumor regions via tissue multipoint sampling; these strategies are able target multiple antigens expressed across various tumor regions at the same time (to target the spatial heterogenicity) and monitor the progression of the disease, which can be followed by modulating treatment plans based on the results of monitoring (to tackle the temporal heterogenicity). However, these strategies and combination therapies make the vaccine design and administration routes more complex, and increase the cost and duration of the treatment time for patients [324]. With the extension of artificial intelligence (AI) applications in various sectors, including medicine, scientists have taken advantage of AI algorithms (such as MHC-binding prediction tools, quantification of mutated transcript expression, and clonality of the mutation, identifying tumor-specific T cell epitopes) to predict the tumor antigens and their properties based on tumor genomic data. Based on the likelihood of eliciting a T cell response, scientists can select some of the specific mutations as vaccine candidates. AI tools may enhance the accuracy of vaccine designs and overcome the challenges associated with the heterogeneity of tumors [442,443,444,445,446].
Using AI algorithms seems to be a promising tool that could help in addressing the major challenges of cancer vaccine development. Another possible technology that could be used along with the AI-related tools is cold plasma-related systems. Cold atmospheric plasma (CAP), also known as nonthermal or cold physical plasma, is a medium consisting of partially ionized gas(es) that provokes the generation of various reactive oxygen and nitrogen species. CAP has been applied in a wide range of industries, including medicine and in particular, in cancer therapy; CAP has shown promising results in destroying cancer cells as well as solid tumors by affecting various related mechanisms at the same time, such as inducing apoptosis, specifically in tumor cells but not in their normal counterparts, reducing cell migration, arresting the cell cycle at the S-phase, damaging the DNA, along with increasing the intracellular concentrations of ROS in the TME, reducing tumor immunosuppression, and improving antigenicity [447,448,449,450,451,452,453]. CAP has gained FDA approval to be used in cancer therapy [454,455,456]. However, this technology has not been used in cancer vaccine development yet. Based on the way that CAP has been applied in cancer therapy, we propose that CAP technology could be applied in cancer vaccine development directly or indirectly. The direct methods can include the direct exposure of cancer cells along with vaccine administrations (for example, simultaneous administration of lipid nanoparticle mRNA cancer vaccines and cold plasma exposure of the cancer cells), and the indirect method can include exposing either the cells in the culture medium (such as patient T cells in case of CAR-T cells, or iPSCs) or the culture medium alone to the CAP first and then growing the cells in the medium. This might help to reduce tumor heterogenicity by preventing genetic mutations with tumor progression, improving the selectivity of the therapy in killing cancer cells without affecting normal cells, and preventing tumor antigen expression by normal cells. Other methods may include trying to develop other new CAR-T cells, rather than CD19 and BCMA, which are able to recognize different tumor antigens, and applying cold plasma. Considering that CAP can enhance the tumor antigenicity (the degree of difference between cancer and normal cells recognized by immune cells) and upregulate immunogenic cell surface molecules such as MHC-I and II, introducing CAP in this field might lead to interesting outcomes. Other methods could include loading the inactivated whole-cell cancer stem cells with lipid nanoparticles containing anti-cancer agents or loading the cancer stem cells with liposomes containing iPSCs, followed by their injection; this could improve the efficiency of DCs and thus the immunogenic response. Moreover, this method may be able to target several factors at the same time; for example, both inactivated cancer stem cells and iPSCs cover a wide range of tumor antigens by themselves, which are poorly immunogenic (do not scape the cancer immune cycle) [185,187,424], and if they are used together in a system, this coverage spectrum might be increased and help to solve tumor heterogenicity problems. Furthermore, this combination system could further enhance the immunogenic response to prevent cancer recurrence. In the end, if the cold plasma is integrated with such a combination system, the combination system could be improved even more, as the CAP itself selectively destroys the tumor cells. At the same time, the liposomes containing iPSCs penetrate the DCs and enhance the variability of tumor antigen presentation, followed by their detection by T cells and the immune response. These are some of the methods that require future investigations, which might open novel and effective approaches to therapeutic cancer vaccine development.
8. Summary and Conclusions
To summarize, we have highlighted the recent research progress of four major vaccine platforms and their limitations. We believe that a comprehensive understanding of the immunosuppressive tumor microenvironment is essential for developing effective cancer vaccines. Besides, particle-based delivery systems have been intensively studied for cancer vaccines in the past few decades, and these hold great promise for improving the immunogenicity of vaccines and facilitating lymph node transport. There is already a consensus that cancer vaccines could achieve a greater therapeutic effect if they were administered in combination with other immunomodulation or standardized therapies. However, sustained endeavors are still needed for identifying tumor-specific neoantigens, effective adjuvants, and optimizing delivery platforms.
Author Contributions
S.S.: organizing the structure, conceptualization, gathering data, writing all sections of the paper, and editing; D.H.L.: help with writing the peptide-based vaccines; S.M.: help with writing the peptide-based vaccines; B.S.: editing, help with writing the introduction, supervising, and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the start-up fund from the College of Pharmacy at the University of Arizona.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Moore, Z.S.; Seward, J.F.; Lane, J.M. Smallpox. Lancet 2006, 367, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Roden, R.B.S.; Stern, P.L. Opportunities and challenges for human papillomavirus vaccination in cancer. Nat. Rev. Cancer 2018, 18, 240–254. [Google Scholar] [CrossRef] [PubMed]
- Pol, S. Hepatitis: HBV vaccine—The first vaccine to prevent cancer. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 190–191. [Google Scholar] [CrossRef] [PubMed]
- Chodon, T.; Koya, R.C.; Odunsi, K. Active Immunotherapy of Cancer. Immunol. Investig. 2015, 44, 817–836. [Google Scholar] [CrossRef] [PubMed]
- Saxena, M.; van der Burg, S.H.; Melief, C.J.M.; Bhardwaj, N. Therapeutic cancer vaccines. Nat. Rev. Cancer 2021, 21, 360–378. [Google Scholar] [CrossRef]
- Lu, C.; Liu, Y.; Ali, N.M.; Zhang, B.; Cui, X. The role of innate immune cells in the tumor microenvironment and research progress in anti-tumor therapy. Front. Immunol. 2023, 13, 1039260. [Google Scholar] [CrossRef]
- Sun, B.; Hyun, H.; Li, L.T.; Wang, A.Z. Harnessing nanomedicine to overcome the immunosuppressive tumor microenvironment. Acta Pharmacol. Sin. 2020, 41, 970–985. [Google Scholar] [CrossRef]
- Kleponis, J.; Skelton, R.; Zheng, L. Fueling the engine and releasing the break: Combinational therapy of cancer vaccines and immune checkpoint inhibitors. Cancer Biol. Med. 2015, 12, 201–208. [Google Scholar] [PubMed]
- Kang, W.; Feng, Z.; Luo, J.; He, Z.; Liu, J.; Wu, J.; Rong, P. Tertiary Lymphoid Structures in Cancer: The Double-Edged Sword Role in Antitumor Immunity and Potential Therapeutic Induction Strategies. Front. Immunol. 2021, 12, 689270. [Google Scholar] [CrossRef]
- Kim, S.K.; Cho, S.W. The Evasion Mechanisms of Cancer Immunity and Drug Intervention in the Tumor Microenvironment. Front. Pharmacol. 2022, 13, 868695. [Google Scholar] [CrossRef]
- Hiam-Galvez, K.J.; Allen, B.M.; Spitzer, M.H. Systemic immunity in cancer. Nat. Rev. Cancer 2021, 21, 345–359. [Google Scholar] [CrossRef] [PubMed]
- Roche, P.A.; Furuta, K. The ins and outs of MHC class II-mediated antigen processing and presentation. Nat. Rev. Immunol. 2015, 15, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Neefjes, J.; Jongsma, M.L.M.; Paul, P.; Bakke, O. Towards a systems understanding of MHC class i and MHC class II antigen presentation. Nat. Rev. Immunol. 2011, 11, 823–836. [Google Scholar] [CrossRef] [PubMed]
- Gaudino, S.J.; Kumar, P. Cross-talk between antigen presenting cells and T cells impacts intestinal homeostasis, bacterial infections, and tumorigenesis. Front. Immunol. 2019, 10, 422031. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Kanai, M.; Choi, W.; Li, X.; Sakaue, S.; Yamamoto, K.; Ogawa, K.; Gutierrez-Arcelus, M.; Gregersen, P.K.; Stuart, P.E.; et al. A high-resolution HLA reference panel capturing global population diversity enables multi-ancestry fine-mapping in HIV host response. Nat. Genet. 2021, 53, 1504–1516. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C.; Velleuer, E. Molecular Immunology: How Science Works; Springer Nature: Berlin/Heidelberg, Germany, 2022; ISBN 9783031040252. [Google Scholar]
- Hilligan, K.L.; Ronchese, F. Antigen presentation by dendritic cells and their instruction of CD4+ T helper cell responses. Cell. Mol. Immunol. 2020, 17, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Eiz-Vesper, B.; Schmetzer, H.M. Antigen-presenting cells: Potential of proven und new players in immune therapies. Transfus. Med. Hemotherapy 2020, 47, 429–431. [Google Scholar] [CrossRef] [PubMed]
- Saxena, V.; Li, L.; Paluskievicz, C.; Kasinath, V.; Bean, A.; Abdi, R.; Jewell, C.M.; Bromberg, J.S. Role of lymph node stroma and microenvironment in T cell tolerance. Immunol. Rev. 2019, 292, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Riven, I.; Feigelson, S.W.; Kartvelishvily, E.; Tohya, K.; Miyasaka, M.; Alon, R.; Haran, G. Three-dimensional localization of T-cell receptors in relation to microvilli using a combination of superresolution microscopies. Proc. Natl. Acad. Sci. USA 2016, 113, E5916–E5924. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wu, J.; Abdi, R.; Jewell, C.M.; Bromberg, J.S. Lymph node fibroblastic reticular cells steer immune responses. Trends Immunol. 2021, 42, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Jou, J.; Harrington, K.J.; Zocca, M.B.; Ehrnrooth, E.; Cohen, E.E.W. The changing landscape of therapeutic cancer vaccines-novel platforms and neoantigen identification. Clin. Cancer Res. 2021, 27, 689–703. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Miao, L.; Sui, J.; Hao, Y.; Huang, G. Nanoparticle cancer vaccines: Design considerations and recent advances. Asian J. Pharm. Sci. 2020, 15, 576–590. [Google Scholar] [CrossRef] [PubMed]
- Jhunjhunwala, S.; Hammer, C.; Delamarre, L. Antigen presentation in cancer: Insights into tumour immunogenicity and immune evasion. Nat. Rev. Cancer 2021, 21, 298–312. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.J.; Svensson-Arvelund, J.; Lubitz, G.S.; Marabelle, A.; Melero, I.; Brown, B.D.; Brody, J.D. Cancer vaccines: The next immunotherapy frontier. Nat. Cancer 2022, 3, 911–926. [Google Scholar] [CrossRef]
- Cuzzubbo, S.; Mangsbo, S.; Nagarajan, D.; Habra, K.; Pockley, A.G.; McArdle, S.E.B. Cancer Vaccines: Adjuvant Potency, Importance of Age, Lifestyle, and Treatments. Front. Immunol. 2021, 11, 615240. [Google Scholar] [CrossRef] [PubMed]
- DeMaria, P.J.; Bilusic, M. Cancer Vaccines. Hematol. Oncol. Clin. N. Am. 2019, 33, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Deniger, D.C.; Pasetto, A.; Robbins, P.F.; Gartner, J.J.; Prickett, T.D.; Paria, B.C.; Malekzadeh, P.; Jia, L.; Yossef, R.; Langhan, M.M.; et al. T-cell responses to TP53 “Hotspot” Mutations and unique neoantigens expressed by human ovarian cancers. Clin. Cancer Res. 2018, 24, 5562–5573. [Google Scholar] [CrossRef]
- Barnett, L.G.; Simkins, H.M.A.; Barnett, B.E.; Korn, L.L.; Johnson, A.L.; Wherry, E.J.; Wu, G.F.; Laufer, T.M. B Cell Antigen Presentation in the Initiation of Follicular Helper T Cell and Germinal Center Differentiation. J. Immunol. 2014, 192, 3607–3617. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Campillo, M.T.; Molina-Hernández, V.; Bautista, M.J.; Pacheco, I.L.; Zafra, R.; Buffoni, L.; Martínez-Moreno, F.J.; Martínez-Moreno, A.; Pérez, J. Characterization of dendritic cells and follicular dendritic cells in the hepatic lymph nodes and liver of sheep experimentally infected with Fasciola hepatica. Vet. Res. 2020, 51, 33. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Silina, K.; van den Broek, M.; Hirahara, K.; Yanagita, M. The roles of tertiary lymphoid structures in chronic diseases. Nat. Rev. Nephrol. 2023, 9, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Warnock, R.A.; Askari, S.; Butcher, E.C.; Von Andrian, U.H. Molecular mechanisms of lymphocyte homing to peripheral lymph nodes. J. Exp. Med. 1998, 187, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Hess, E.; Duheron, V.; Decossas, M.; Lézot, F.; Berdal, A.; Chea, S.; Golub, R.; Bosisio, M.R.; Bridal, S.L.; Choi, Y.; et al. RANKL Induces Organized Lymph Node Growth by Stromal Cell Proliferation. J. Immunol. 2012, 188, 1245–1254. [Google Scholar] [CrossRef] [PubMed]
- Luther, S.A.; Ansel, K.M.; Cyster, J.G. Overlapping roles of CXCL13, interleukin 7 receptor α, and CCR7 ligands in lymph node development. J. Exp. Med. 2003, 197, 1191–1198. [Google Scholar] [CrossRef]
- Yoshida, H.; Naito, A.; Inoue, J.I.; Satoh, M.; Santee-Cooper, S.M.; Ware, C.F.; Togawa, A.; Nishikawa, S.; Nishikawa, S.I. Different cytokines induce surface lymphotoxin-αβ on IL-7 receptor-α cells that differentially engender lymph nodes and Peyer’s patches. Immunity 2002, 17, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Kowarik, M.C.; Cepok, S.; Sellner, J.; Grummel, V.; Weber, M.S.; Korn, T.; Berthele, A.; Hemmer, B. CXCL13 is the major determinant for B cell recruitment to the CSF during neuroinflammation. J. Neuroinflammation 2012, 9, 93. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wu, S. Tertiary lymphoid structures are critical for cancer prognosis and therapeutic response. Front. Immunol. 2023, 13, 1063711. [Google Scholar] [CrossRef] [PubMed]
- Meier, D.; Bornmann, C.; Chappaz, S.; Schmutz, S.; Otten, L.A.; Ceredig, R.; Acha-Orbea, H.; Finke, D. Ectopic Lymphoid-Organ Development Occurs through Interleukin 7-Mediated Enhanced Survival of Lymphoid-Tissue-Inducer Cells. Immunity 2007, 26, 643–654. [Google Scholar] [CrossRef]
- Jacquelot, N.; Tellier, J.; Sl, N.; Gt, B. Tertiary lymphoid structures and B lymphocytes in cancer prognosis and response to immunotherapies. Oncoimmunology 2021, 10, 1900508. [Google Scholar] [CrossRef]
- He, M.; He, Q.; Cai, X.; Liu, J.; Deng, H.; Li, F.; Zhong, R.; Lu, Y.; Peng, H.; Wu, X.; et al. Intratumoral tertiary lymphoid structure (TLS) maturation is influenced by draining lymph nodes of lung cancer. J. Immunother. Cancer 2023, 11, e005539. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M. Antigen presentation by MHC-dressed cells. Front. Immunol. 2014, 5, 120340. [Google Scholar] [CrossRef] [PubMed]
- Münz, C. Canonical and Non-Canonical Functions of the Autophagy Machinery in MHC Restricted Antigen Presentation. Front. Immunol. 2022, 13, 868888. [Google Scholar] [CrossRef] [PubMed]
- Rock, K.L.; Gramm, C.; Rothstein, L.; Clark, K.; Stein, R.; Dick, L.; Hwang, D.; Goldberg, A.L. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell 1994, 78, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Sijts, E.J.A.M.; Kloetzel, P.M. The role of the proteasome in the generation of MHC class I ligands and immune responses. Cell. Mol. Life Sci. 2011, 68, 1491–1502. [Google Scholar] [CrossRef] [PubMed]
- Kloetzel, P.M. Antigen processing by the proteasome. Nat. Rev. Mol. Cell Biol. 2001, 2, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Vigneron, N.; Van den Eynde, B.J. Proteasome subtypes and regulators in the processing of antigenic peptides presented by class I molecules of the major histocompatibility complex. Biomolecules 2014, 4, 994–1025. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, N.; Garbi, N.; Reinheckel, T.; Moldenhauer, G.; Hämmerling, G.J.; Momburg, F. A Transporter Associated with Antigen-Processing Independent Vacuolar Pathway for the MHC Class I-Mediated Presentation of Endogenous Transmembrane Proteins. J. Immunol. 2007, 178, 7932–7942. [Google Scholar] [CrossRef]
- Sengupta, D.; Graham, M.; Liu, X.; Cresswell, P. Proteasomal degradation within endocytic organelles mediates antigen cross-presentation. EMBO J. 2019, 38, e99266. [Google Scholar] [CrossRef] [PubMed]
- Joffre, O.P.; Segura, E.; Savina, A.; Amigorena, S. Cross-presentation by dendritic cells. Nat. Rev. Immunol. 2012, 12, 557–569. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, M.; Abualrous, E.T.; Sticht, J.; Álvaro-Benito, M.; Stolzenberg, S.; Noé, F.; Freund, C. Major histocompatibility complex (MHC) class I and MHC class II proteins: Conformational plasticity in antigen presentation. Front. Immunol. 2017, 8, 248429. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.; Hahn, M.; Michel, J.; Sankowski, R.; Kilian, M.; Kehl, N.; Günter, M.; Bunse, T.; Pusch, S.; von Deimling, A.; et al. Dysfunctional dendritic cells limit antigen-specific T cell response in glioma. Neuro Oncol. 2023, 25, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.M.S.; D’Aulerio, R.; Yong, T.; He, M.; Baptista, M.A.P.; Nylén, S.; Westerberg, L.S. Increased cross-presentation by dendritic cells and enhanced anti-tumour therapy using the Arp2/3 inhibitor CK666. Br. J. Cancer 2023, 128, 982–991. [Google Scholar] [CrossRef] [PubMed]
- Connors, J.; Joyner, D.; Mege, N.J.; Cusimano, G.M.; Bell, M.R.; Marcy, J.; Taramangalam, B.; Kim, K.M.; Lin, P.J.C.; Tam, Y.K.; et al. Lipid nanoparticles (LNP) induce activation and maturation of antigen presenting cells in young and aged individuals. Commun. Biol. 2023, 6, 188. [Google Scholar] [CrossRef] [PubMed]
- Haen, S.P.; Löffler, M.W.; Rammensee, H.G.; Brossart, P. Towards new horizons: Characterization, classification and implications of the tumour antigenic repertoire. Nat. Rev. Clin. Oncol. 2020, 17, 595–610. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Leal, Y.; Grubaugh, D.; Nogueira, C.V.; Lopetegui-González, I.; Del Valle, A.; Escalona, F.; Laborde, R.J.; Alvarez, C.; Fernández, L.E.; Starnbach, M.N.; et al. Antigen cross-presentation induced by the pore-forming protein sticholysin II encapsulated into liposomes. Front. Immunol. 2018, 9, 2473. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Usatorre, A.; De Palma, M. Dendritic cell cross-dressing and tumor immunity. EMBO Mol. Med. 2022, 14, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Pishesha, N.; Harmand, T.J.; Ploegh, H.L. A guide to antigen processing and presentation. Nat. Rev. Immunol. 2022, 22, 751–764. [Google Scholar] [CrossRef] [PubMed]
- Schriek, P.; Villadangos, J.A. Trogocytosis and cross-dressing in antigen presentation. Curr. Opin. Immunol. 2023, 83, 102331. [Google Scholar] [CrossRef]
- Campana, S.; De Pasquale, C.; Carrega, P.; Ferlazzo, G.; Bonaccorsi, I. Cross-dressing: An alternative mechanism for antigen presentation. Immunol. Lett. 2015, 168, 349–354. [Google Scholar] [CrossRef]
- Merlotti, A.; Sadacca, B.; Arribas, Y.A.; Ngoma, M.; Burbage, M.; Goudot, C.; Houy, A.; Rocañín-Arjó, A.; Lalanne, A.; Seguin-Givelet, A.; et al. Noncanonical splicing junctions between exons and transposable elements represent a source of immunogenic recurrent neo-antigens in patients with lung cancer. Sci. Immunol. 2023, 8, eabm6359. [Google Scholar] [CrossRef]
- Smyth, L.A.; Harker, N.; Turnbull, W.; El-Doueik, H.; Klavinskis, L.; Kioussis, D.; Lombardi, G.; Lechler, R. The Relative Efficiency of Acquisition of MHC:Peptide Complexes and Cross-Presentation Depends on Dendritic Cell Type. J. Immunol. 2008, 181, 3212–3220. [Google Scholar] [CrossRef]
- Dolan, B.P.; Gibbs, K.D.; Ostrand-Rosenberg, S. Dendritic Cells Cross-Dressed with Peptide MHC Class I Complexes Prime CD8+ T Cells. J. Immunol. 2006, 177, 6018–6024. [Google Scholar] [CrossRef] [PubMed]
- Harshyne, L.A.; Zimmer, M.I.; Watkins, S.C.; Barratt-Boyes, S.M. A Role for Class A Scavenger Receptor in Dendritic Cell Nibbling from Live Cells. J. Immunol. 2003, 170, 2302–2309. [Google Scholar] [CrossRef]
- Wakim, L.M.; Bevan, M.J. Cross-dressed dendritic cells drive memory CD8+ T-cell activation after viral infection. Nature 2011, 471, 629–631. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Rabella, M.; Garcia-Garijo, A.; Palomero, J.; Yuste-Estevanez, A.; Erhard, F.; Farriol-Duran, R.; Martín-Liberal, J.; Ochoa-de-Olza, M.; Matos, I.; Gartner, J.J.; et al. Exploring the Immunogenicity of Noncanonical HLA-I Tumor Ligands Identified through Proteogenomics. Clin. Cancer Res. 2023, 29, 2250–2265. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; He, J.; Liu, J.; Yan, X.; Fan, K. Nanozyme for tumor therapy: Surface modification matters. Exploration 2021, 1, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.S.; Mellman, I. Oncology meets immunology: The cancer-immunity cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, biogenesis and function. Nat. Rev. Immunol. 2002, 2, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Valdebenito, S.; Malik, S.; Luu, R.; Loudig, O.; Mitchell, M.; Okafo, G.; Bhat, K.; Prideaux, B.; Eugenin, E.A. Tunneling nanotubes, TNT, communicate glioblastoma with surrounding non-tumor astrocytes to adapt them to hypoxic and metabolic tumor conditions. Sci. Rep. 2021, 11, 14556. [Google Scholar] [CrossRef] [PubMed]
- Pio, R.; Ajona, D.; Ortiz-Espinosa, S.; Mantovani, A.; Lambris, J.D. Complementing the cancer-immunity cycle. Front. Immunol. 2019, 10, 445513. [Google Scholar] [CrossRef] [PubMed]
- Weigelin, B.; Friedl, P. T cell-mediated additive cytotoxicity—Death by multiple bullets. Trends Cancer 2022, 8, 980–987. [Google Scholar] [CrossRef]
- Coënon, L.; Villalba, M. From CD16a Biology to Antibody-Dependent Cell-Mediated Cytotoxicity Improvement. Front. Immunol. 2022, 13, 913215. [Google Scholar] [CrossRef] [PubMed]
- Macor, P.; Capolla, S.; Tedesco, F. Complement as a biological tool to control tumor growth. Front. Immunol. 2018, 9, 2203. [Google Scholar] [CrossRef] [PubMed]
- Skowyra, A.; Śladowski, D.; Majszyk-Ionescu, J.; Grabska-Liberek, I. The role of the complement system in ocular diseases. Klin. Oczna 2017, 2017, 52–56. [Google Scholar]
- Wang, J.; Zhang, S.; Wang, Y.; Zhu, Y.; Xu, X.; Guo, J. Alternative Complement Pathway Signature Determines Immunosuppression and Resistance to Immunotherapy Plus Tyrosine Kinase Inhibitor Combinations in Renal Cell Carcinoma. Urol. Oncol. Semin. Orig. Investig. 2023, 41, e13–e51. [Google Scholar] [CrossRef] [PubMed]
- Lerner, E.; Woroniecka, K.; Anniballe, V.D.; Wilkinson, D.; Mohan, A.; Cook, S.; Tomaszewski, W.; Cui, X.; Khasraw, M.; Gunn, M.D.; et al. A Novel MHC-Independent Mechanism of Tumor Cell Killing by CD8+ T Cells. bioRxiv 2023. [Google Scholar] [CrossRef]
- Patel, S.A.; Minn, A.J. Combination Cancer Therapy with Immune Checkpoint Blockade: Mechanisms and Strategies. Immunity 2018, 48, 417–433. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.A.; Baer, J.M.; Knolhoff, B.L.; Nywening, T.M.; Panni, R.Z.; Su, X.; Weilbaecher, K.N.; Hawkins, W.G.; Ma, C.; Fields, R.C.; et al. Breast and pancreatic cancer interrupt IRF8-dependent dendritic cell development to overcome immune surveillance. Nat. Commun. 2018, 9, 1250. [Google Scholar] [CrossRef] [PubMed]
- Rothlin, C.V.; Ghosh, S. Lifting the innate immune barriers to antitumor immunity. J. Immunother. Cancer 2020, 8, e000695. [Google Scholar] [CrossRef] [PubMed]
- Chow, M.T.; Möller, A.; Smyth, M.J. Inflammation and immune surveillance in cancer. Semin. Cancer Biol. 2012, 22, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Damhofer, H.; Helin, K. EZH2i unlocks PDAC immune surveillance. Nat. Cancer 2023, 4, 781–783. [Google Scholar] [CrossRef]
- Jongsma, M.L.M.; Neefjes, J.; Spaapen, R.M. Playing hide and seek: Tumor cells in control of MHC class I antigen presentation. Mol. Immunol. 2021, 136, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Takeshima, H.; Ushijima, T. Accumulation of genetic and epigenetic alterations in normal cells and cancer risk. NPJ Precis. Oncol. 2019, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Xu, C. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 2020, 30, 660–669. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, R.; Yang, A.G.; Zheng, G. Diversity of immune checkpoints in cancer immunotherapy. Front. Immunol. 2023, 14, 1121285. [Google Scholar] [CrossRef]
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Goswami, S.; Raychaudhuri, D.; Siddiqui, B.A.; Singh, P.; Nagarajan, A.; Liu, J.; Subudhi, S.K.; Poon, C.; Gant, K.L.; et al. Immune checkpoint therapy—Current perspectives and future directions. Cell 2023, 186, 1652–1669. [Google Scholar] [CrossRef] [PubMed]
- Arner, E.N.; Rathmell, J.C. Metabolic programming and immune suppression in the tumor microenvironment. Cancer Cell 2023, 41, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Blomberg, O.S.; Spagnuolo, L.; Garner, H.; Voorwerk, L.; Isaeva, O.I.; van Dyk, E.; Bakker, N.; Chalabi, M.; Klaver, C.; Duijst, M.; et al. IL-5-producing CD4+ T cells and eosinophils cooperate to enhance response to immune checkpoint blockade in breast cancer. Cancer Cell 2023, 41, 106–123.e10. [Google Scholar] [CrossRef]
- Lin, W.; Chen, L.; Zhang, H.; Qiu, X.; Huang, Q.; Wan, F.; Le, Z.; Geng, S.; Zhang, A.; Qiu, S.; et al. Tumor-intrinsic YTHDF1 drives immune evasion and resistance to immune checkpoint inhibitors via promoting MHC-I degradation. Nat. Commun. 2023, 14, 265. [Google Scholar] [CrossRef]
- Doyle, H.A.; Mamula, M.J. Post-translational protein modifications in antigen recognition and autoimmunity. Trends Immunol. 2001, 22, 443–449. [Google Scholar] [CrossRef]
- Parkhurst, M.R.; Robbins, P.F.; Tran, E.; Prickett, T.D.; Gartner, J.J.; Li, J.; Ivey, G.; Li, Y.F.; El-Gamil, M.; Lalani, A.; et al. Unique neoantigens arise from somatic mutations in patients with gastrointestinal cancers. Cancer Discov. 2019, 9, 1022–1035. [Google Scholar] [CrossRef] [PubMed]
- Chong, C.; Coukos, G.; Bassani-Sternberg, M. Identification of tumor antigens with immunopeptidomics. Nat. Biotechnol. 2022, 40, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Leko, V.; Rosenberg, S.A. Identifying and Targeting Human Tumor Antigens for T Cell-Based Immunotherapy of Solid Tumors. Cancer Cell 2020, 38, 454–472. [Google Scholar] [CrossRef] [PubMed]
- Chandran, S.S.; Ma, J.; Klatt, M.G.; Dündar, F.; Bandlamudi, C.; Razavi, P.; Wen, H.Y.; Weigelt, B.; Zumbo, P.; Fu, S.N.; et al. Immunogenicity and therapeutic targeting of a public neoantigen derived from mutated PIK3CA. Nat. Med. 2022, 28, 946–957. [Google Scholar] [CrossRef] [PubMed]
- Truong, N.H.M.; Vo, N.T.; Vo, P.H.; Nguyen, H.D. Antigen Discovery. In Fish Vaccines Health Management for Sustainable Aquaculture; CRC Press: Boca Raton, FL, USA, 2023; pp. 65–74. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, M.; Zhang, H.; Cao, S.; Li, Y.; Jiang, S.; Song, Y.; Liu, S. Detection of p53 mutation and serum monitoring alert caused by Marek’s disease virus in poultry. BMC Vet. Res. 2020, 16, 303. [Google Scholar] [CrossRef]
- Kaghad, M.; Bonnet, H.; Yang, A.; Creancier, L.; Biscan, J.C.; Valent, A.; Minty, A.; Chalon, P.; Lelias, J.M.; Dumont, X.; et al. Monoallelically expressed gene related to p53 at 1p36, a region frequently deleted in neuroblastoma and other human cancers. Cell 1997, 90, 809–819. [Google Scholar] [CrossRef] [PubMed]
- Bourdon, J.C.; Fernandes, K.; Murray-Zmijewski, F.; Liu, G.; Diot, A.; Xirodimas, D.P.; Saville, M.K.; Lane, D.P. P53 Isoforms Can Regulate P53 Transcriptional Activity. Genes Dev. 2005, 19, 2122–2137. [Google Scholar] [CrossRef] [PubMed]
- Xie, N.; Shen, G.; Gao, W.; Huang, Z.; Huang, C.; Fu, L. Neoantigens: Promising targets for cancer therapy. Signal Transduct. Target. Ther. 2023, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Delgado, A.P.; Brandao, P.; Chapado, M.J.; Hamid, S.; Narayanan, R. Open reading frames associated with cancer in the dark matter of the human genome. Cancer Genom. Proteom. 2014, 11, 201–213. [Google Scholar]
- Meyerson, M.; Counter, C.M.; Eaton, E.N.; Ellisen, L.W.; Steiner, P.; Caddle, S.D.; Ziaugra, L.; Beijersbergen, R.L.; Davidoff, M.J.; Liu, Q.; et al. hEST2, the putative human telomerase catalytic subunit gene, is up-regulated in tumor cells and during immortalization. Cell 1997, 90, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Jie, M.M.; Chang, X.; Zeng, S.; Liu, C.; Liao, G.B.; Wu, Y.R.; Liu, C.H.; Hu, C.J.; Yang, S.M.; Li, X.Z. Diverse regulatory manners of human telomerase reverse transcriptase. Cell Commun. Signal. 2019, 17, 63. [Google Scholar] [CrossRef] [PubMed]
- Chong, C.; Müller, M.; Pak, H.S.; Harnett, D.; Huber, F.; Grun, D.; Leleu, M.; Auger, A.; Arnaud, M.; Stevenson, B.J.; et al. Integrated proteogenomic deep sequencing and analytics accurately identify non-canonical peptides in tumor immunopeptidomes. Nat. Commun. 2020, 11, 1293. [Google Scholar] [CrossRef] [PubMed]
- Laumont, C.M.; Perreault, C. Exploiting non-canonical translation to identify new targets for T cell-based cancer immunotherapy. Cell. Mol. Life Sci. 2018, 75, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Abd-Aziz, N.; Poh, C.L. Development of Peptide-Based Vaccines for Cancer. J. Oncol. 2022, 2022, 9749363. [Google Scholar] [CrossRef] [PubMed]
- Mizukoshi, E.; Nakagawa, H.; Tamai, T.; Kitahara, M.; Fushimi, K.; Nio, K.; Terashima, T.; Iida, N.; Arai, K.; Yamashita, T.; et al. Peptide vaccine-treated, long-term surviving cancer patients harbor self-renewing tumor-specific CD8+ T cells. Nat. Commun. 2022, 13, 3123. [Google Scholar] [CrossRef] [PubMed]
- Buonaguro, L.; Tagliamonte, M. Peptide-based vaccine for cancer therapies. Front. Immunol. 2023, 14, 1210044. [Google Scholar] [CrossRef] [PubMed]
- Alarcon, N.O.; Jaramillo, M.; Mansour, H.M.; Sun, B. Therapeutic Cancer Vaccines—Antigen Discovery and Adjuvant Delivery Platforms. Pharmaceutics 2022, 14, 1448. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, J.; Wang, L.; Liu, B. Personalized neoantigen vaccination with synthetic long peptides: Recent advances and future perspectives. Theranostics 2020, 10, 6011–6023. [Google Scholar] [CrossRef] [PubMed]
- Corradin, G.; Kajava, A.V.; Verdini, A. Long synthetic peptides for the production of vaccines and drugs: A technological platform coming of age. Sci. Transl. Med. 2010, 2, 50rv3. [Google Scholar] [CrossRef] [PubMed]
- Facciolà, A.; Visalli, G.; Laganà, A.; Di Pietro, A. An Overview of Vaccine Adjuvants: Current Evidence and Future Perspectives. Vaccines 2022, 10, 819. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Cai, Y.; Jiang, Y.; He, X.; Wei, Y.; Yu, Y.; Tian, X. Vaccine adjuvants: Mechanisms and platforms. Signal Transduct. Target. Ther. 2023, 8, 283. [Google Scholar] [CrossRef]
- Tagliamonte, M.; Cavalluzzo, B.; Mauriello, A.; Ragone, C.; Buonaguro, F.M.; Tornesello, M.L.; Buonaguro, L. Molecular mimicry and cancer vaccine development. Mol. Cancer 2023, 22, 75. [Google Scholar] [CrossRef]
- Fikes, J.D.; Sette, A. Design of multi-epitope, analogue-based cancer vaccines. Expert Opin. Biol. Ther. 2003, 3, 985–993. [Google Scholar] [CrossRef]
- Mauerer Zirlik, K.; Zahrieh, D.; Neuberg, D.; Gribben, J.G. Cytotoxic T cells generated against heteroclitic peptides kill primary tumor cells independent of the binding affinity of the native tumor antigen peptide. Blood 2006, 108, 3865–3870. [Google Scholar] [CrossRef]
- Cavalluzzo, B.; Ragone, C.; Mauriello, A.; Petrizzo, A.; Manolio, C.; Caporale, A.; Vitagliano, L.; Ruvo, M.; Buonaguro, L.; Tagliamonte, M. Identification and characterization of heteroclitic peptides in TCR-binding positions with improved HLA-binding efficacy. J. Transl. Med. 2021, 19, 89. [Google Scholar] [CrossRef]
- Arbelaez, C.A.; Estrada, J.; Gessner, M.A.; Glaus, C.; Morales, A.B.; Mohn, D.; Phee, H.; Lipford, J.R.; Johnston, J.A. A nanoparticle vaccine that targets neoantigen peptides to lymphoid tissues elicits robust antitumor T cell responses. NPJ Vaccines 2020, 5, 106. [Google Scholar] [CrossRef] [PubMed]
- Varypataki, E.M.; Silva, A.L.; Barnier-Quer, C.; Collin, N.; Ossendorp, F.; Jiskoot, W. Synthetic long peptide-based vaccine formulations for induction of cell mediated immunity: A comparative study of cationic liposomes and PLGA nanoparticles. J. Control. Release 2016, 226, 98–106. [Google Scholar] [CrossRef]
- Gao, S.; Yang, D.; Fang, Y.; Lin, X.; Jin, X.; Wang, Q.; Wang, X.; Ke, L.; Shi, K. Engineering nanoparticles for targeted remodeling of the tumor microenvironment to improve cancer immunotherapy. Theranostics 2019, 9, 126–151. [Google Scholar] [CrossRef] [PubMed]
- Grippin, A.J.; Sayour, E.J.; Mitchell, D.A. Translational nanoparticle engineering for cancer vaccines. Oncoimmunology 2017, 6, e1290036. [Google Scholar] [CrossRef]
- Seo, N.; Akiyoshi, K.; Shiku, H. Exosome-mediated regulation of tumor immunology. Cancer Sci. 2018, 109, 2998–3004. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.M.; André, F.; Amigorena, S.; Soria, J.C.; Eggermont, A.; Kroemer, G.; Zitvogel, L. Dendritic cell-derived exosomes for cancer therapy. J. Clin. Investig. 2016, 126, 1224–1232. [Google Scholar] [CrossRef] [PubMed]
- Sanders, B.; Koldijk, M.; Schuitemaker, H. Inactivated Viral Vaccines. Vaccine Analysis: Strategies, Principles, and Control; Springer Nature: New York, NY, USA, 2015; ISBN 9783662450246. [Google Scholar]
- Fan, Y.; Bai, T.; Tian, Y.; Zhou, B.; Wang, Y.; Yang, L. H2o2-inactivated salmonella typhimurium re88 strain as a new cancer vaccine carrier: Evaluation in a mouse model of cancer. Drug Des. Dev. Ther. 2021, 15, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Lauring, A.S.; Jones, J.O.; Andino, R. Rationalizing the development of live attenuated virus vaccines. Nat. Biotechnol. 2010, 28, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Toussaint, B.; Chauchet, X.; Wang, Y.; Polack, B.; Le Gouëllec, A. Live-attenuated bacteria as a cancer vaccine vector. Expert Rev. Vaccines 2013, 12, 1139–1154. [Google Scholar] [CrossRef] [PubMed]
- Morein, B.; Helenius, A.; Simons, K.; Pettersson, R.; Kääriäinen, L.; Schirrmacher, V. Effective subunit vaccines against an enveloped animal virusories. Nature 1978, 276, 715–718. [Google Scholar] [CrossRef] [PubMed]
- Mayer, R.L.; Impens, F. Immunopeptidomics for next-generation bacterial vaccine development. Trends Microbiol. 2021, 29, 1034–1045. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D. Cancer Vaccines. Nat. Med. 1998, 4, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, S.A.; Lorincz, R.; Boucher, P.; Curiel, D.T. Adenoviral vector vaccine platforms in the SARS-CoV-2 pandemic. NPJ Vaccines 2021, 6, 97. [Google Scholar] [CrossRef] [PubMed]
- de Pinho Favaro, M.T.; Atienza-Garriga, J.; Martínez-Torró, C.; Parladé, E.; Vázquez, E.; Corchero, J.L.; Ferrer-Miralles, N.; Villaverde, A. Recombinant vaccines in 2022: A perspective from the cell factory. Microb. Cell Factories 2022, 21, 203. [Google Scholar] [CrossRef] [PubMed]
- Travieso, T.; Li, J.; Mahesh, S.; Mello, J.D.F.R.E.; Blasi, M. The use of viral vectors in vaccine development. NPJ Vaccines 2022, 7, 75. [Google Scholar] [CrossRef] [PubMed]
- Ragothaman, M.; Yoo, S.Y. Engineered Phage-Based Cancer Vaccines: Current Advances and Future Directions. Vaccines 2023, 11, 919. [Google Scholar] [CrossRef] [PubMed]
- Pranchevicius, M.S.; Vieira, T.R. Production of recombinant immunotherapeutics for anticancer treatment: The role of bioengineering. Bioengineered 2013, 4, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Atkin-Smith, G.K.; Duan, M.; Chen, W.; Poon, I.K.H. The induction and consequences of Influenza A virus-induced cell death. Cell Death Dis. 2018, 9, 1002. [Google Scholar] [CrossRef] [PubMed]
- Imre, G. Since January 2020 Elsevier has created a COVID-19 resource centre with free information in English and Mandarin on the novel coronavirus COVID-19. In The COVID-19 Resource Centre Is Hosted on Elsevier Connect, the Company’s Public News and Information; 2020; Available online: https://cir.nii.ac.jp/crid/1370576118741734285 (accessed on 23 February 2024).
- Yoo, J.S.; Sasaki, M.; Cho, S.X.; Kasuga, Y.; Zhu, B.; Ouda, R.; Orba, Y.; de Figueiredo, P.; Sawa, H.; Kobayashi, K.S. SARS-CoV-2 inhibits induction of the MHC class I pathway by targeting the STAT1-IRF1-NLRC5 axis. Nat. Commun. 2021, 12, 6602. [Google Scholar] [CrossRef] [PubMed]
- Pishesha, N.; Harmand, T.J.; Rothlauf, P.W.; Praest, P.; Alexander, R.K.; Van den Doel, R.; Liebeskind, M.J.; Vakaki, M.A.; McCaul, N.; Wijne, C.; et al. A class II MHC-targeted vaccine elicits immunity against SARS-CoV-2 and its variants. Proc. Natl. Acad. Sci. USA 2021, 118, e2116147118. [Google Scholar] [CrossRef] [PubMed]
- Minev, B.R.; McFarland, B.J.; Spiess, P.J.; Rosenberg, S.A.; Restifo, N.P. Insertion Signal Sequence Fused to Minimal Peptides Elicits Specific CD8+ T-Cell Responses and Prolongs Survival of Thymoma-bearing Mice. Cancer Res. 1994, 54, 4155–4161. [Google Scholar] [PubMed]
- Olson, E.; Ceccarelli, T.; Raghavan, M. Endo-lysosomal assembly variations among human leukocyte antigen class I (HLA class I) allotypes. Elife 2023, 12, e79144. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhong, Q.; Tian, T.; Dubin, K.; Athale, S.K.; Kupper, T.S. Epidermal injury and infection during poxvirus immunization is crucial for the generation of highly protective T cell-mediated immunity. Nat. Med. 2010, 16, 224–227. [Google Scholar] [CrossRef]
- Chiuppesi, F.; Salazar, A.; Contreras, H.; Nguyen, V.H.; Martinez, J. Development of a Synthetic Poxvirus-Based SARS-CoV-2 Vaccine. bioRxiv 2020. [Google Scholar] [CrossRef]
- Esposito, I.; Cicconi, P.; D’Alise, A.M.; Brown, A.; Esposito, M.; Swadling, L.; Holst, P.J.; Bassi, M.R.; Stornaiuolo, M.; Mori, F.; et al. MHC Class II Invariant Chain-Adjuvanted Viral Vectored Vaccines Enhances T Cell Responses in Humans. Sci. Transl. Med. 2020, 12, eaaz7715. [Google Scholar] [CrossRef]
- Fiander, A.; Nimako, M.; Man, S.; Wilkinson, G.W.G.; Boursnell, M.E.G.; Rutherford, E.; Hickling, J.K.; Inglis, S.C.; Evans, A.S.; Adams, M.; et al. A recombinant vaccinia virus encoding human papillomavirus types 16 and 18, E6 and E7 proteins as immunotherapy for cervical cancer. Lancet 1996, 347, 1523–1527. [Google Scholar]
- Wang, W.; Xu, H.; Ye, Q.; Tao, F.; Wheeldon, I.; Yuan, A.; Hu, Y.; Wu, J. Systemic immune responses to irradiated tumours via the transport of antigens to the tumour periphery by injected flagellate bacteria. Nat. Biomed. Eng. 2022, 6, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Keenan, B.P.; Jaffee, E.M. Whole cell vaccines—Past progress and future strategies. Semin. Oncol. 2012, 39, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Bencherif, S.A.; Sands, R.W.; Ali, O.A.; Li, W.A.; Lewin, S.A.; Braschler, T.M.; Shih, T.Y.; Verbeke, C.S.; Bhatta, D.; Dranoff, G.; et al. Injectable cryogel-based whole-cell cancer vaccines. Nat. Commun. 2015, 6, 7556. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.L.L.; Benencia, F.; Coukos, G. Whole tumor antigen vaccines. Semin. Immunol. 2010, 22, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Warfel, J.M.; Zimmerman, L.I.; Merkel, T.J. Acellular pertussis vaccines protect against disease butfail to prevent infection and transmission ina nonhuman primate model. Proc. Natl. Acad. Sci. USA 2014, 111, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Alghounaim, M.; Alsaffar, Z.; Alfraij, A.; Bin-Hasan, S.; Hussain, E. Whole-Cell and Acellular Pertussis Vaccine: Reflections on Efficacy. Med. Princ. Pract. 2022, 31, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Eisenbarth, S.C. Dendritic cell subsets in T cell programming: Location dictates function. Alzheimer’s Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef] [PubMed]
- Stoitzner, P.; Romani, N.; Rademacher, C.; Probst, H.C.; Mahnke, K. Antigen targeting to dendritic cells: Still a place in future immunotherapy? Eur. J. Immunol. 2022, 52, 1909–1924. [Google Scholar] [CrossRef]
- Liu, K. Dendritic Cells. Encycl. Cell Biol. 2016, 3, 741–749. [Google Scholar] [CrossRef]
- Dong, W.; Du, J.; Shen, H.; Gao, D.; Li, Z.; Wang, G.; Mu, X.; Liu, Q. Administration of embryonic stem cells generates effective antitumor immunity in mice with minor and heavy tumor load. Cancer Immunol. Immunother. 2010, 59, 1697–1705. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, X.; Telli, M.L.; Wu, J.C. Induced pluripotent stem cell-based cancer vaccines. Front. Immunol. 2019, 10, 460435. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.J.; Bolton, E.M.; Pocock, S.; Sharples, L.D.; Pedersen, R.A.; Bradley, J.A. Banking on human embryonic stem cells: Estimating the number of donor cell lines needed for HLA matching. Lancet 2005, 366, 2019–2025. [Google Scholar] [CrossRef] [PubMed]
- Robertson, N.J.; Brook, F.A.; Gardner, R.L.; Cobbold, S.P.; Waldmann, H.; Fairchild, P.J. Embryonic stem cell-derived tissues are immunogenic but their inherent immune privilege promotes the induction of tolerance. Proc. Natl. Acad. Sci. USA 2007, 104, 20920–20925. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.F.; Yu, C.Y.; Chen, M.J.; Chou, S.H.; Chiang, M.S.; Chou, W.H.; Ko, B.S.; Huang, H.P.; Kuo, H.C.; Ho, H.N. Characteristic expression of major histocompatibility complex and immune privilege genes in human pluripotent stem cells and their derivatives. Cell Transplant. 2015, 24, 845–864. [Google Scholar] [CrossRef] [PubMed]
- Drukker, M.; Katz, G.; Urbach, A.; Schuldiner, M.; Markel, G.; Itskovitz-Eldor, J.; Reubinoff, B.; Mandelboim, O.; Benvenisty, N. Characterization of the expression of MHC proteins in human embryonic stem cells. Proc. Natl. Acad. Sci. USA 2002, 99, 9864–9869. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, J.; Gong, L.; Yu, D.; An, C.; Bunpetch, V.; Dai, J.; Huang, H.; Zou, X.; Ouyang, H.; et al. The Plasticity of Mesenchymal Stem Cells in Regulating Surface HLA-I. iScience 2019, 15, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Mehler, V.J.; Burns, C.J.; Stauss, H.; Francis, R.J.; Moore, M.L. Human iPSC-Derived Neural Crest Stem Cells Exhibit Low Immunogenicity. Mol. Ther.-Methods Clin. Dev. 2020, 16, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Álvarez, B.; Rodriguez, R.M.; Calvanese, V.; Blanco-Gelaz, M.A.; Suhr, S.T.; Ortega, F.; Otero, J.; Cibelli, J.B.; Moore, H.; Fraga, M.F.; et al. Epigenetic mechanisms regulate MHC and antigen processing molecules in human embryonic and induced pluripotent stem cells. PLoS ONE 2010, 5, e10192. [Google Scholar] [CrossRef]
- Chen, H.; Li, Y.; Lin, X.; Cui, D.; Cui, C.; Li, H.; Xiao, L. Functional disruption of human leukocyte antigen II in human embryonic stem cell. Biol. Res. 2015, 48, 59. [Google Scholar] [CrossRef] [PubMed]
- Drukker, M.; Katchman, H.; Katz, G.; Even-Tov Friedman, S.; Shezen, E.; Hornstein, E.; Mandelboim, O.; Reisner, Y.; Benvenisty, N. Human Embryonic Stem Cells and Their Differentiated Derivatives Are Less Susceptible to Immune Rejection Than Adult Cells. Stem Cells 2006, 24, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Paskin, N. Toward unique identifiers. Proc. IEEE 1999, 87, 1208–1227. [Google Scholar] [CrossRef]
- Bifari, F. Immunological properties of embryonic and adult stem cells. World J. Stem Cells 2010, 2, 50. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.L.L.; Coukos, G.; Kandalaft, L.E. Whole tumor antigen vaccines: Where are we? Vaccines 2015, 3, 344–372. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zeng, H.; Xu, R.H.; Liu, B.; Li, Z. Vaccination with human pluripotent stem cells generates a broad spectrum of immunological and clinical responses against colon cancer. Stem Cells 2009, 27, 3103–3111. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Chen, X.H.; Chang, X.H.; Ye, X.; Li, Y.; Cui, H. Human embryonic stem cells—A potential vaccine for ovarian cancer. Asian Pac. J. Cancer Prev. 2012, 13, 4295–4300. [Google Scholar] [CrossRef] [PubMed]
- Gordeeva, O.; Khaydukov, S. Tumorigenic and Differentiation Potentials of Embryonic Stem Cells Depend on TGF β Family Signaling: Lessons from Teratocarcinoma Cells Stimulated to Differentiate with Retinoic Acid. Stem Cells Int. 2017, 2017, 7284872. [Google Scholar] [CrossRef] [PubMed]
- Lezmi, E.; Benvenisty, N. The Tumorigenic Potential of Human Pluripotent Stem Cells. Stem Cells Transl. Med. 2022, 11, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Ben-David, U.; Benvenisty, N. The tumorigenicity of human embryonic and induced pluripotent stem cells. Nat. Rev. Cancer 2011, 11, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Bock, C.; Kiskinis, E.; Verstappen, G.; Gu, H.; Boulting, G.; Smith, Z.D.; Ziller, M.; Croft, G.F.; Amoroso, M.W.; Oakley, D.H.; et al. Reference maps of human es and ips cell variation enable high-throughput characterization of pluripotent cell lines. Cell 2011, 144, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Zhang, Y. Embryonic stem cell and induced pluripotent stem cell: An epigenetic perspective. Cell Res. 2013, 23, 49–69. [Google Scholar] [CrossRef] [PubMed]
- Efrat, S. Epigenetic Memory: Lessons From iPS Cells Derived From Human β Cells. Front. Endocrinol. 2021, 11, 614234. [Google Scholar] [CrossRef] [PubMed]
- Nishino, K.; Takasawa, K.; Okamura, K.; Arai, Y.; Sekiya, A.; Akutsu, H.; Umezawa, A. Identification of an epigenetic signature in human induced pluripotent stem cells using a linear machine learning model. Hum. Cell 2021, 34, 99–110. [Google Scholar] [CrossRef]
- de Boni, L.; Gasparoni, G.; Haubenreich, C.; Tierling, S.; Schmitt, I.; Peitz, M.; Koch, P.; Walter, J.; Wüllner, U.; Brüstle, O. DNA methylation alterations in iPSC- and hESC-derived neurons: Potential implications for neurological disease modeling. Clin. Epigenetics 2018, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Poetsch, M.S.; Strano, A.; Guan, K. Human Induced Pluripotent Stem Cells: From Cell Origin, Genomic Stability, and Epigenetic Memory to Translational Medicine. Stem Cells 2022, 40, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Inui, S.; Minami, K.; Ito, E.; Imaizumi, H.; Mori, S.; Koizumi, M.; Fukushima, S.; Miyagawa, S.; Sawa, Y.; Matsuura, N. Irradiation strongly reduces tumorigenesis of human induced pluripotent stem cells. J. Radiat. Res. 2017, 58, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Ito, E.; Minami, K.; Harada, A.; Mochizuki-Oda, N.; Sawa, Y.; Miyagawa, S. Elimination of residual undifferentiated induced pluripotent stem cells (iPSCs) using irradiation for safe clinical applications of iPSC-derived cardiomyocytes. Biochem. Biophys. Res. Commun. 2021, 574, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Mohammadpour, H.; Pourfathollah, A.A.; Nikougoftar Zarif, M.; Shahbazfar, A.A. Irradiation enhances susceptibility of tumor cells to the antitumor effects of TNF-α activated adipose derived mesenchymal stem cells in breast cancer model. Sci. Rep. 2016, 6, 28433. [Google Scholar] [CrossRef] [PubMed]
- Kooreman, N.G.; Kim, Y.; de Almeida, P.E.; Termglinchan, V.; Diecke, S.; Shao, N.Y.; Wei, T.T.; Yi, H.; Dey, D.; Nelakanti, R.; et al. Autologous iPSC-Based Vaccines Elicit Anti-tumor Responses In Vivo. Cell Stem Cell 2018, 22, 501–513.e7. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, X.; Liu, L.; Zhou, M.; Zhou, G.; Liu, T. Development of the T-ALLiPSC-based therapeutic cancer vaccines for T-cell acute lymphoblastic leukemia. Med. Oncol. 2022, 39, 200. [Google Scholar] [CrossRef]
- Wang, R.; Zhu, T.; Hou, B.; Huang, X. An iPSC-derived exosome-pulsed dendritic cell vaccine boosts antitumor immunity in melanoma. Mol. Ther. 2023, 31, 2376–2390. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tian, L.; Zhao, T.; Zhang, J. A nanotherapeutic system for gastric cancer suppression by synergistic chemotherapy and immunotherapy based on iPSCs and DCs exosomes. Cancer Immunol. Immunother. 2023, 72, 1673–1683. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, F.; Razmi, M.; Tajik, F.; Zöller, M.; Dehghan Manshadi, M.; Mahdavinezhad, F.; Tiyuri, A.; Ghods, R.; Madjd, Z. Efficacy of Whole Cancer Stem Cell-Based Vaccines: A Systematic Review of Preclinical and Clinical Studies. Stem Cells 2023, 41, 207–232. [Google Scholar] [CrossRef] [PubMed]
- Hwang, M.S.; Miller, M.S.; Thirawatananond, P.; Douglass, J.; Wright, K.M.; Hsiue, E.H.C.; Mog, B.J.; Aytenfisu, T.Y.; Murphy, M.B.; Aitana Azurmendi, P.; et al. Structural engineering of chimeric antigen receptors targeting HLA-restricted neoantigens. Nat. Commun. 2021, 12, 5271. [Google Scholar] [CrossRef] [PubMed]
- Guedan, S.; Calderon, H.; Posey, A.D.; Maus, M.V. Engineering and Design of Chimeric Antigen Receptors. Mol. Ther.-Methods Clin. Dev. 2019, 12, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro-Vornhagen, A.; Böll, B.; Schellongowski, P.; Valade, S.; Metaxa, V.; Azoulay, E.; von Bergwelt-Baildon, M. Critical care management of chimeric antigen receptor T-cell therapy recipients. CA Cancer J. Clin. 2022, 72, 78–93. [Google Scholar] [CrossRef] [PubMed]
- Syed, F.; El Fakih, R.; Alahmari, A.D.; Osman Ali, A.S.; Aljurf, M. Chimeric Antigen Receptor Structure and Manufacturing of Clinical Grade CAR Engineered Cells using Different Bioreactors. Hematol. Oncol. Stem Cell Ther. 2022, 15, 137–152. [Google Scholar] [CrossRef]
- Porter, D.L.; Levine, B.L.; Kalos, M.; Bagg, A.; June, C.H. Chimeric Antigen Receptor–Modified T Cells in Chronic Lymphoid Leukemia. N. Engl. J. Med. 2011, 365, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, C.A.; Chavez, J.C.; Sehgal, A.R.; William, B.M.; Munoz, J.; Salles, G.; Munshi, P.N.; Casulo, C.; Maloney, D.G.; de Vos, S.; et al. Axicabtagene ciloleucel in relapsed or refractory indolent non-Hodgkin lymphoma (ZUMA-5): A single-arm, multicentre, phase 2 trial. Lancet Oncol. 2022, 23, 91–103. [Google Scholar] [CrossRef]
- Mishra, A.; Maiti, R.; Mohan, P.; Gupta, P. Antigen loss following CAR-T cell therapy: Mechanisms, implications, and potential solutions. Eur. J. Haematol. 2023, 112, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Sterner, R.C.; Sterner, R.M. CAR-T cell therapy: Current limitations and potential strategies. Blood Cancer J. 2021, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- Rasche, L.; Vago, L.; Mutis, T. Tumour Escape from CAR-T Cells. In The EBMT/EHA CAR-T Cell Handbook; Springer: Cham, Germany, 2022; pp. 15–22. [Google Scholar] [CrossRef]
- Ma, L.; Hostetler, A.; Morgan, D.M.; Maiorino, L.; Sulkaj, I.; Whittaker, C.A.; Neeser, A.; Pires, I.S.; Yousefpour, P.; Gregory, J.; et al. Vaccine-boosted CAR T crosstalk with host immunity to reject tumors with antigen heterogeneity. Cell 2023, 186, 3148–3165.e20. [Google Scholar] [CrossRef] [PubMed]
- Brentjens, R.J.; Davila, M.L.; Riviere, I.; Park, J.; Wang, X.; Cowell, L.G.; Bartido, S.; Stefanski, J.; Taylor, C.; Olszewska, M.; et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci. Transl. Med. 2013, 5, 177ra38. [Google Scholar] [CrossRef] [PubMed]
- Morris, E.C.; Neelapu, S.S.; Giavridis, T.; Sadelain, M. Cytokine release syndrome and associated neurotoxicity in cancer immunotherapy. Nat. Rev. Immunol. 2022, 22, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Porter, D.L.; Hwang, W.T.; Frey, N.V.; Lacey, S.F.; Shaw, P.A.; Loren, A.W.; Bagg, A.; Marcucci, K.T.; Shen, A.; Gonzalez, V.; et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci. Transl. Med. 2015, 7, 303ra139. [Google Scholar] [CrossRef] [PubMed]
- Neelapu, S.S.; Tummala, S.; Kebriaei, P.; Wierda, W.; Gutierrez, C.; Locke, F.L.; Komanduri, K.V.; Lin, Y.; Jain, N.; Daver, N.; et al. Chimeric antigen receptor T-cell therapy-assessment and management of toxicities. Nat. Rev. Clin. Oncol. 2018, 15, 47–62. [Google Scholar] [CrossRef]
- Murphy, T.L.; Murphy, K.M. Dendritic cells in cancer immunology. Cell. Mol. Immunol. 2022, 19, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, A.; Salvi, V.; Soriani, A.; Laffranchi, M.; Sozio, F.; Bosisio, D.; Sozzani, S. Dendritic cell subsets in cancer immunity and tumor antigen sensing. Cell. Mol. Immunol. 2023, 20, 432–447. [Google Scholar] [CrossRef]
- Katakam, A.K.; Brightbill, H.; Franci, C.; Kung, C.; Nunez, V.; Jones, C.; Peng, I.; Jeet, S.; Wu, L.C.; Mellman, I.; et al. Dendritic cells require NIK for CD40-dependent cross-priming of CD8+ T cells. Proc. Natl. Acad. Sci. USA 2015, 112, 14664–14669. [Google Scholar] [CrossRef] [PubMed]
- Manh, T.P.V.; Alexandre, Y.; Baranek, T.; Crozat, K.; Dalod, M. Plasmacytoid, conventional, and monocyte-derived dendritic cells undergo a profound and convergent genetic reprogramming during their maturation. Eur. J. Immunol. 2013, 43, 1706–1715. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Jiang, A. Dendritic Cells and CD8 T Cell Immunity in Tumor Microenvironment. Front. Immunol. 2018, 9, 3059. [Google Scholar] [CrossRef] [PubMed]
- Pulendran, B.; Smith, J.L.; Caspary, G.; Brasel, K.; Pettit, D.; Maraskovsky, E.; Maliszewski, C.R. Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc. Natl. Acad. Sci. USA 1999, 96, 1036–1041. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.; Theisen, D. The role of cDC1s in vivo: CD8 T cell priming through cross-presentation. F1000Research 2017, 6, 98. [Google Scholar] [CrossRef]
- Ferris, S.T.; Durai, V.; Wu, R.; Theisen, D.J.; Ward, J.P.; Bern, M.D.; Iv, J.T.D.; Bagadia, P.; Liu, T.; Carlos, G.; et al. cDC1 prime and are licensed by CD4 T cells to induce anti-tumour immunity. Nature 2021, 584, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Segura, E. Cross-dressed cDC1s instruct T cells in allorecognition. Immunol. Cell Biol. 2020, 98, 520–523. [Google Scholar] [CrossRef]
- Bosteels, C.; Neyt, K.; Vanheerswynghels, M.; van Helden, M.J.; Sichien, D.; Debeuf, N.; De Prijck, S.; Bosteels, V.; Vandamme, N.; Martens, L.; et al. Inflammatory Type 2 cDCs Acquire Features of cDC1s and Macrophages to Orchestrate Immunity to Respiratory Virus Infection. Immunity 2020, 52, 1039–1056.e9. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Ikegawa, M.; Kawai, T. Antigen Presentation in the Lung. Front. Immunol. 2022, 13, 860915. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.W.; Do Kim, K.; Lee, H.K. The role of dendritic cells in tumor microenvironments and their uses as therapeutic targets. BMB Rep. 2021, 54, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Komori, S.; Kotani, T.; Murata, Y.; Matozaki, T. The Role of Type-2 Conventional Dendritic Cells in the Regulation of Tumor Immunity. Cancers 2022, 14, 1976. [Google Scholar] [CrossRef] [PubMed]
- Ugur, M.; Mueller, S.N. T cell and dendritic cell interactions in lymphoid organs: More than just being in the right place at the right time. Immunol. Rev. 2019, 289, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, X.; Cheng, Y.; Cao, X. Dendritic cell migration in inflammation and immunity. Cell. Mol. Immunol. 2021, 18, 2461–2471. [Google Scholar] [CrossRef] [PubMed]
- Dutertre, C.A.; Becht, E.; Irac, S.E.; Khalilnezhad, A.; Narang, V.; Khalilnezhad, S.; Ng, P.Y.; van den Hoogen, L.L.; Leong, J.Y.; Lee, B.; et al. Single-Cell Analysis of Human Mononuclear Phagocytes Reveals Subset-Defining Markers and Identifies Circulating Inflammatory Dendritic Cells. Immunity 2019, 51, 573–589.e8. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.C.; Gudjonson, H.; Pritykin, Y.; Deep, D.; Lavallée, V.P.; Mendoza, A.; Fromme, R.; Mazutis, L.; Ariyan, C.; Leslie, C.; et al. Transcriptional Basis of Mouse and Human Dendritic Cell Heterogeneity. Cell 2019, 179, 846–863.e24. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Peng, P.; Loschko, J.; Feng, L.; Pham, P.; Cui, W.; Lee, K.P.; Krug, A.B.; Jiang, A. Plasmacytoid dendritic cells cross-prime naive CD8 T cells by transferring antigen to conventional dendritic cells through exosomes. Proc. Natl. Acad. Sci. USA 2020, 117, 23730–23741. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Kasahara, S.; Jhingran, A.; Tosini, N.L.; Zhai, B.; Aufiero, M.A.; Mills, K.A.M.; Gjonbalaj, M.; Espinosa, V.; Rivera, A.; et al. During Aspergillus Infection, Monocyte-Derived DCs, Neutrophils, and Plasmacytoid DCs Enhance Innate Immune Defense through CXCR3-Dependent Crosstalk. Cell Host Microbe 2020, 28, 104–116.e4. [Google Scholar] [CrossRef] [PubMed]
- Marzaioli, V.; Canavan, M.; Floudas, A.; Wade, S.C.; Low, C.; Veale, D.J.; Fearon, U. Monocyte-Derived Dendritic Cell Differentiation in Inflammatory Arthritis Is Regulated by the JAK/STAT Axis via NADPH Oxidase Regulation. Front. Immunol. 2020, 11, 1406. [Google Scholar] [CrossRef] [PubMed]
- Boulet, S.; Daudelin, J.F.; Odagiu, L.; Pelletier, A.N.; Yun, T.J.; Lesage, S.; Cheong, C.; Labrecque, N. The orphan nuclear receptor NR4A3 controls the differentiation of monocyte-derived dendritic cells following microbial stimulation. Proc. Natl. Acad. Sci. USA 2019, 116, 15150–15159. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.L.; Dalod, M. The Quest for Faithful In Vitro Models of Human Dendritic Cells Types. Mol. Immunol. 2020, 123, 40–59. [Google Scholar] [CrossRef] [PubMed]
- Sutti, S.; Bruzzì, S.; Heymann, F.; Liepelt, A.; Krenkel, O.; Toscani, A.; Ramavath, N.N.; Cotella, D.; Albano, E.; Tacke, F. CX3CR1 Mediates the Development of Monocyte-Derived Dendritic Cells during Hepatic Inflammation. Cells 2019, 8, 1099. [Google Scholar] [CrossRef] [PubMed]
- Leblanc-Hotte, A.; Audiger, C.; Chabot-Roy, G.; Lombard-Vadnais, F.; Delisle, J.S.; Peter, Y.A.; Lesage, S. Immature and mature bone marrow-derived dendritic cells exhibit distinct intracellular mechanical properties. Sci. Rep. 2023, 13, 1967. [Google Scholar] [CrossRef] [PubMed]
- Wilson, N.S.; El-Sukkari, D.; Villadangos, J.A. Dendritic cells constitutively present self antigens in their immature state in vivo and regulate antigen presentation by controlling the rates of MHC class II synthesis and endocytosis. Blood 2004, 103, 2187–2195. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Kim, J. Properties of immature and mature dendritic cells: Phenotype, morphology, phagocytosis, and migration. RSC Adv. 2019, 9, 11230–11238. [Google Scholar] [CrossRef] [PubMed]
- Garrett, W.S.; Chen, L.M.; Kroschewski, R.; Ebersold, M.; Turley, S.; Trombetta, S.; Galán, J.E.; Mellman, I. Developmental control of endocytosis in dendritic cells by Cdc42. Cell 2000, 102, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Villadangos, J.A.; Schnorrer, P. Intrinsic and cooperative antigen-presenting functions of dendritic-cell subsets in vivo. Nat. Rev. Immunol. 2007, 7, 543–555. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiang, Y.; Xin, V.W.; Wang, X.W.; Peng, X.C.; Liu, X.Q.; Wang, D.; Li, N.; Cheng, J.T.; Lyv, Y.N.; et al. Dendritic cell biology and its role in tumor immunotherapy. J. Hematol. Oncol. 2020, 13, 107. [Google Scholar] [CrossRef] [PubMed]
- Platt, C.D.; Ma, J.K.; Chalouni, C.; Ebersold, M.; Bou-Reslan, H.; Carano, R.A.D.; Mellman, I.; Delamarre, L. Mature dendritic cells use endocytic receptors to capture and present antigens. Proc. Natl. Acad. Sci. USA 2010, 107, 4287–4292. [Google Scholar] [CrossRef] [PubMed]
- Hunter, T.B.; Alsarraj, M.; Gladue, R.P.; Bedian, V.; Antonia, S.J. An agonist antibody specific for CD40 induces dendritic cell maturation and promotes autologous anti-tumour T-cell responses in an in vitro mixed autologous tumour cell/lymph node cell model. Scand. J. Immunol. 2007, 65, 479–486. [Google Scholar] [CrossRef] [PubMed]
- DiLillo, D.J.; Ravetch, J.V. Differential Fc-receptor engagement drives an anti-tumor vaccinal effect. Cell 2015, 161, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.V.; Nino-Castro, A.C.; Schultze, J.L. Regulatory dendritic cells: There is more than just immune activation. Front. Immunol. 2012, 3, 274. [Google Scholar] [CrossRef] [PubMed]
- Melero, I.; Hervas-Stubbs, S.; Glennie, M.; Pardoll, D.M.; Chen, L. Immunostimulatory monoclonal antibodies for cancer therapy. Nat. Rev. Cancer 2007, 7, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Holtkamp, S.; Kreiter, S.; Selmi, A.; Simon, P.; Koslowski, M.; Huber, C.; Türeci, Ö.; Sahin, U. Modification of antigen-encoding RNA increases stability, translational efficacy, and T-cell stimulatory capacity of dendritic cells. Blood 2006, 108, 4009–4017. [Google Scholar] [CrossRef] [PubMed]
- Alloatti, A.; Kotsias, F.; Magalhaes, J.G.; Amigorena, S. Dendritic cell maturation and cross-presentation: Timing matters! Immunol. Rev. 2016, 272, 97–108. [Google Scholar] [CrossRef] [PubMed]
- van der Wel, N.N.; Sugita, M.; Fluitsma, D.M.; Cao, X.; Schreibelt, G.; Brenner, M.B.; Peters, P.J. CD1 and Major Histocompatibility Complex II Molecules Follow a Different Course during Dendritic Cell Maturation. Mol. Biol. Cell 2003, 14, 3378–3388. [Google Scholar] [CrossRef] [PubMed]
- Villadangos, J.A.; Schnorrer, P.; Wilson, N.S. Control of MHC class II antigen presentation in dendritic cells: A balance between creative and destructive forces. Immunol. Rev. 2005, 207, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Hubo, M.; Trinschek, B.; Kryczanowsky, F.; Tuettenberg, A.; Steinbrink, K.; Jonuleit, H. Costimulatory molecules on immunogenic versus tolerogenic human dendritic cells. Front. Immunol. 2013, 4, 82. [Google Scholar] [CrossRef] [PubMed]
- Carenza, C.; Calcaterra, F.; Oriolo, F.; Di Vito, C.; Ubezio, M.; Della Porta, M.G.; Mavilio, D.; Della Bella, S. Costimulatory molecules and immune checkpoints are differentially expressed on different subsets of dendritic cells. Front. Immunol. 2019, 10, 1325. [Google Scholar] [CrossRef] [PubMed]
- Hodge, J.W.; Rad, A.N.; Grosenbach, D.W.; Sabzevari, H.; Gómez Yafal, A.; Gritz, L.; Schlom, J. Enhanced activation of T cells by dendritic cells engineered to hyperexpress a triad of costimulatory molecules. J. Natl. Cancer Inst. 2000, 92, 1228–1239. [Google Scholar] [CrossRef]
- Liu, X.; Xia, X.; Wang, X.; Zhou, J.; Sung, L.A.; Long, J.; Geng, X.; Zeng, Z.; Yao, W. Tropomodulin1 Expression Increases Upon Maturation in Dendritic Cells and Promotes Their Maturation and Immune Functions. Front. Immunol. 2021, 11, 587441. [Google Scholar] [CrossRef] [PubMed]
- Tiberio, L.; Del Prete, A.; Schioppa, T.; Sozio, F.; Bosisio, D.; Sozzani, S. Chemokine and chemotactic signals in dendritic cell migration review-article. Cell. Mol. Immunol. 2018, 15, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Oppenheim, J.J.; Yang, D.; Biragyn, A.; Howard, O.M.Z.; Plotz, P. Chemokine receptors on dendritic cells promote autoimmune reactions. Arthritis Res. 2002, 4 (Suppl. S3), 183–188. [Google Scholar] [CrossRef] [PubMed]
- Banchereau, J.; Pascual, V.; Palucka, A.K. Autoimmunity through cytokine-induced dendritic cell activation. Immunity 2004, 20, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Ebner, S.; Ratzinger, G.; Krösbacher, B.; Schmuth, M.; Weiss, A.; Reider, D.; Kroczek, R.A.; Herold, M.; Heufler, C.; Fritsch, P.; et al. Production of IL-12 by Human Monocyte-Derived Dendritic Cells Is Optimal When the Stimulus Is Given at the Onset of Maturation, and Is Further Enhanced by IL-4. J. Immunol. 2001, 166, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.B.; Guerra, J.; Firek, A.; Langridge, W.H.R. Extracellular vimentin modulates human dendritic cell activation. Mol. Immunol. 2018, 104, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.-R.; Muckersie, E.; Robertson, M.; Xu, H.; Liversidge, J.; Forrester, J.V. Secretion of interleukin-10 or interleukin-12 by LPS-activated dendritic cells is critically dependent on time of stimulus relative to initiation of purified DC culture. J. Leukoc. Biol. 2002, 72, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Erra Díaz, F.; Ochoa, V.; Merlotti, A.; Dantas, E.; Mazzitelli, I.; Gonzalez Polo, V.; Sabatté, J.; Amigorena, S.; Segura, E.; Geffner, J. Extracellular Acidosis and mTOR Inhibition Drive the Differentiation of Human Monocyte-Derived Dendritic Cells. Cell Rep. 2020, 31, 107613. [Google Scholar] [CrossRef] [PubMed]
- Boudier, A.; Aubert-Pouëssel, A.; Louis-Plence, P.; Gérardin, C.; Jorgensen, C.; Devoisselle, J.M.; Bégu, S. The control of dendritic cell maturation by pH-sensitive polyion complex micelles. Biomaterials 2009, 30, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Hanč, P.; Schulz, O.; Fischbach, H.; Martin, S.R.; Kjær, S.; Reis e Sousa, C. A pH- and ionic strength-dependent conformational change in the neck region regulates DNGR-1 function in dendritic cells. EMBO J. 2016, 35, 2484–2497. [Google Scholar] [CrossRef]
- Lee, H.; Park, H.; Yu, H.S.; Na, K.; Oh, K.T.; Lee, E.S. Dendritic cell-targeted ph-responsive extracellular vesicles for anticancer vaccination. Pharmaceutics 2019, 11, 54. [Google Scholar] [CrossRef] [PubMed]
- Savina, A.; Jancic, C.; Hugues, S.; Guermonprez, P.; Vargas, P.; Moura, I.C.; Lennon-Duménil, A.M.; Seabra, M.C.; Raposo, G.; Amigorena, S. NOX2 Controls Phagosomal pH to Regulate Antigen Processing during Crosspresentation by Dendritic Cells. Cell 2006, 126, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Mantegazza, A.R.; Savina, A.; Vermeulen, M.; Pérez, L.; Geffner, J.; Hermine, O.; Rosenzweig, S.D.; Faure, F.; Amigorena, S. NADPH oxidase controls phagosomal pH and antigen cross-presentation in human dendritic cells. Blood 2008, 112, 4712–4722. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.; Mahmud, A.R.; Siddiquee, M.F.-R.-; Shahriar, A.; Biswas, P.; Shimul, M.E.K.; Ahmed, S.Z.; Ema, T.I.; Rahman, N.; Khan, M.A.; et al. Role of T cells in cancer immunotherapy: Opportunities and challenges. Cancer Pathog. Ther. 2023, 1, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Park, J.J.; Peng, L.; Yang, Q.; Chow, R.D.; Dong, M.B.; Lam, S.Z.; Guo, J.; Tang, E.; Zhang, Y.; et al. A genome-scale gain-of-function CRISPR screen in CD8 T cells identifies proline metabolism as a means to enhance CAR-T therapy. Cell Metab. 2022, 34, 595–614.e14. [Google Scholar] [CrossRef] [PubMed]
- Ribas, A.; Haining, W.N.; Schumacher, T.N.M. When Cancer Cells Become the Enablers of an Antitumor Immune Response. Cancer Discov. 2022, 12, 2244–2248. [Google Scholar] [CrossRef] [PubMed]
- Al Moussawy, M.; Abdelsamed, H.A. Non-cytotoxic functions of CD8 T cells: “repentance of a serial killer”. Front. Immunol. 2022, 13, 1001129. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; Zhou, L.; Wang, B.; Sun, X.; Zhang, Q.; Chen, H.; Wan, N.; Ye, H.; Wu, X.; Sun, D.; et al. Targeting cathepsin B by cycloastragenol enhances antitumor immunity of CD8 T cells via inhibiting MHC-I degradation. J. Immunother. Cancer 2022, 10, e004874. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.; Wang, Q.; Korner, H.; Zhang, L.; Wei, W. Molecular mechanisms of T cells activation by dendritic cells in autoimmune diseases. Front. Pharmacol. 2018, 9, 642. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Vesely, M.D. Stimulating T Cells against Cancer with Agonist Immunostimulatory Monoclonal Antibodies. Int. Rev. Cell Mol. Biol. 2019, 342, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Shi, Y.; Haymaker, C.L.; Naing, A.; Ciliberto, G.; Hajjar, J. T-cell agonists in cancer immunotherapy. J. Immunother. Cancer 2020, 8, e000966. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Dallas, B. Flies Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat. Rev. Immunol. 2013, 13, 227–242. [Google Scholar] [CrossRef] [PubMed]
- Goronzy, J.J.; Weyand, C.M. T-cell co-stimulatory pathways in autoimmunity. Arthritis Res. Ther. 2008, 10, S3. [Google Scholar] [CrossRef] [PubMed]
- Goleva, E.; Lyubchenko, T.; Kraehenbuehl, L.; Lacouture, M.E.; Leung, D.Y.M.; Kern, J.A. Our current understanding of checkpoint inhibitor therapy in cancer immunotherapy. Ann. Allergy Asthma Immunol. 2021, 126, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Mccallion, O.; Bilici, M.; Hester, J.; Issa, F. Regulatory T-cell therapy approaches. Clin. Exp. Immunol. 2023, 211, 96–107. [Google Scholar] [CrossRef]
- Dieckmann, D.; Plottner, H.; Berchtold, S.; Berger, T.; Schuler, G. Ex vivo isolation and characterization of CD4+CD25+ T cells with regulatory properties from human blood. J. Exp. Med. 2001, 193, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Jiang, P.; Wei, S.; Xu, X.; Wang, J. Regulatory T cells in tumor microenvironment: New mechanisms, potential therapeutic strategies and future prospects. Mol. Cancer 2020, 19, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Rocamora-Reverte, L.; Melzer, F.L.; Würzner, R.; Weinberger, B. The Complex Role of Regulatory T Cells in Immunity and Aging. Front. Immunol. 2021, 11, 116. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Cheng, X.; Zhang, L.; Lu, X.; Chaudhary, S.; Teng, R.; Frederickson, C.; Champion, M.M.; Zhao, R.; Cheng, L.; et al. Myeloid-derived suppressor cells inhibit T cell activation through nitrating LCK in mouse cancers. Proc. Natl. Acad. Sci. USA 2018, 115, 10094–10099. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Li, K.; Ni, Y.; Liang, X.; Zhao, X. Myeloid-Derived Suppressor Cells: Implications in the Resistance of Malignant Tumors to T Cell-Based Immunotherapy. Front. Cell Dev. Biol. 2021, 9, 707198. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, C.; Liu, T.; Dai, X.; Bazhin, A.V. Myeloid-Derived Suppressor Cells in Tumors: From Mechanisms to Antigen Specificity and Microenvironmental Regulation. Front. Immunol. 2020, 11, 1371. [Google Scholar] [CrossRef] [PubMed]
- Dario, A.A.; Vignali, L.W.C.; Creg, J.W. How regulatory T cells work. Nat. Rev. Immunol. 2008, 8, 523–532. [Google Scholar] [CrossRef]
- Collison, L.W.; Workman, C.J.; Kuo, T.T.; Boyd, K.; Wang, Y.; Vignali, K.M.; Cross, R.; Sehy, D.; Blumberg, R.S.; Vignali, D.A.A. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature 2007, 450, 566–569. [Google Scholar] [CrossRef]
- Yan, S.; Kotschenreuther, K.; Deng, S.; Kofler, D.M. Regulatory T cells in rheumatoid arthritis: Functions, development, regulation, and therapeutic potential. Cell. Mol. Life Sci. 2022, 79, 533. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Li, S.; Gao, X.; Zhou, J.; Zhu, X.; Wang, D.; Cai, Y.; Li, F.; Yang, Q.; Gu, X.; et al. Interleukin-6-mediated CCR9+ interleukin-17-producing regulatory T cells polarization increases the severity of necrotizing enterocolitis. EBioMedicine 2019, 44, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Gough, M.J.; Crittenden, M.R. The paradox of radiation and T cells in tumors. Neoplasia 2022, 31, 100808. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liu, Y.; Cui, B. Effect of radiotherapy on T cell and PD-1 / PD-L1 blocking therapy in tumor microenvironment. Hum. Vaccines Immunother. 2021, 17, 1555–1567. [Google Scholar] [CrossRef]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef] [PubMed]
- Bernal-Estévez, D.; Sánchez, R.; Tejada, R.E.; Parra-López, C. Chemotherapy and radiation therapy elicits tumor specific T cell responses in a breast cancer patient. BMC Cancer 2016, 16, 104257. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, I.J.; Bernal-Estévez, D.A.; Llano-León, M.; Bonilla, C.E.; Parra-López, C.A. Neoadjuvant chemotherapy modulates exhaustion of T cells in breast cancer patients. PLoS ONE 2023, 18, e0280851. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Shi, Q.; Huang, X.; Koo, S.; Kong, N.; Tao, W. mRNA-based cancer therapeutics. Nat. Rev. Cancer 2023, 23, 526–543. [Google Scholar] [CrossRef] [PubMed]
- Wolff, J.A.; Malone, R.W.; Williams, P.; Chong, W.; Acsadi, G.; Jani, A.; Felgner, P.L. Direct gene transfer into mouse muscle in vivo. Science 1990, 247, 1465–1468. [Google Scholar] [CrossRef] [PubMed]
- MacGregor, R.R.; Boyer, J.D.; Ugen, K.E.; Lacy, K.E.; Gluckman, S.J.; Bagarazzi, M.L.; Chattergoon, M.A.; Baine, Y.; Higgins, T.J.; Ciccarelli, R.B.; et al. First human trial of a DNA-based vaccine for treatment of human immunodeficiency virus type 1 infection: Safety and host response. J. Infect. Dis. 1998, 178, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z. DNA Vaccine. Adv. Genet. 2005, 54, 257–289. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.; Vandermeulen, G.; Préat, V. Cancer DNA vaccines: Current preclinical and clinical developments and future perspectives. J. Exp. Clin. Cancer Res. 2019, 38, 146. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, F.K.; Ottensmeier, C.H.; Johnson, P.; Zhu, D.; Buchan, S.L.; McCann, K.J.; Roddick, J.S.; King, A.T.; McNicholl, F.; Savelyeva, N.; et al. DNA vaccines to attack cancer. Proc. Natl. Acad. Sci. USA 2004, 101, 14646–14652. [Google Scholar] [CrossRef] [PubMed]
- Jorritsma, S.H.T.; Gowans, E.J.; Grubor-Bauk, B.; Wijesundara, D.K. Delivery methods to increase cellular uptake and immunogenicity of DNA vaccines. Vaccine 2016, 34, 5488–5494. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Luo, Y.; Lo, J.F.; Kaplan, C.D.; Mizutani, M.; Mizutani, N.; Lee, J.D.; Primus, F.J.; Becker, J.C.; Xiang, R.; et al. DNA-based vaccines activate innate and adaptive antitumor immunity by engaging the NKG2D receptor. Proc. Natl. Acad. Sci. USA 2005, 102, 10846–10851. [Google Scholar] [CrossRef]
- Rezaei, T.; Davoudian, E.; Khalili, S.; Amini, M.; Hejazi, M.; de la Guardia, M.; Mokhtarzadeh, A. Strategies in DNA vaccine for melanoma cancer. Pigment Cell Melanoma Res. 2021, 34, 869–891. [Google Scholar] [CrossRef] [PubMed]
- Sudowe, S.; Dominitzki, S.; Montermann, E.; Bros, M.; Grabbe, S.; Reske-Kunz, A.B. Uptake and presentation of exogenous antigen and presentation of endogenously produced antigen by skin dendritic cells represent equivalent pathways for the priming of cellular immune responses following biolistic DNA immunization. Immunology 2009, 128, e193–e205. [Google Scholar] [CrossRef] [PubMed]
- Krieg, A.M. CpG motifs: The active ingredient in bacterial extracts? Nat. Med. 2003, 9, 831–835. [Google Scholar] [CrossRef] [PubMed]
- Bode, C.; Zhao, G.; Steinhagen, F.; Kinjo, T.; Klinman, D.M. CpG DNA as a vaccine adjuvant (author manuscript). Expert Rev. Vaccines 2011, 10, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Ma, Z.; Barber, G.N. STING regulates intracellular DNA-mediated, type i interferon-dependent innate immunity. Nature 2009, 461, 788–792. [Google Scholar] [CrossRef] [PubMed]
- Kutzler, M.A.; Weiner, D.B. DNA vaccines: Ready for prime time? Nat. Rev. Genet. 2008, 9, 776–788. [Google Scholar] [CrossRef] [PubMed]
- Faurez, F.; Dory, D.; Le Moigne, V.; Gravier, R.; Jestin, A. Biosafety of DNA vaccines: New generation of DNA vectors and current knowledge on the fate of plasmids after injection. Vaccine 2010, 28, 3888–3895. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Troilo, P.J.; Wang, X.; Griffiths, T.G.; Pacchione, S.J.; Barnum, A.B.; Harper, L.B.; Pauley, C.J.; Niu, Z.; Denisova, L.; et al. Detection of integration of plasmid DNA into host genomic DNA following intramuscular injection and electroporation. Gene Ther. 2004, 11, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.; Vanvarenberg, K.; Préat, V.; Vandermeulen, G. Codon-Optimized P1A-Encoding DNA Vaccine: Toward a Therapeutic Vaccination against P815 Mastocytoma. Nucleic Acids-Mol. Ther. 2017, 8, 404–415. [Google Scholar] [CrossRef] [PubMed]
- Stachyra, A.; Redkiewicz, P.; Kosson, P.; Protasiuk, A.; Góra-Sochacka, A.; Kudla, G.; Sirko, A. Codon optimization of antigen coding sequences improves the immune potential of DNA vaccines against avian influenza virus H5N1 in mice and chickens. Virol. J. 2016, 13, 143. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Ferrall, L.; Gaillard, S.; Wang, C.; Chi, W.Y.; Huang, C.H.; Roden, R.B.S.; Wu, T.C.; Chang, Y.N.; Hung, C.F. Development of dna vaccine targeting e6 and e7 proteins of human papillomavirus 16 (Hpv16) and hpv18 for immunotherapy in combination with recombinant vaccinia boost and pd-1 antibody. mBio 2021, 12, 10–1128. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, L.; Lin, A.; Xu, C.; Li, Z.; Liu, K.; Liu, B.; Ma, X.; Zhao, F.; Jiang, H.; et al. Algorithm for Optimized mRNA Design Improves Stability and Immunogenicity. Nature 2023, 621, 396–403. [Google Scholar] [CrossRef]
- Ferraro, B.; Morrow, M.P.; Hutnick, N.A.; Shin, T.H.; Lucke, C.E.; Weiner, D.B. Clinical applications of DNA vaccines: Current progress. Clin. Infect. Dis. 2011, 53, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Jeang, J.; Yang, A.; Wu, T.C.; Hung, C.F. DNA vaccine for cancer immunotherapy. Hum. Vaccines Immunother. 2014, 10, 3153–3164. [Google Scholar] [CrossRef] [PubMed]
- Target, A.M.; Reference, B.F. Molecular mechanisms for enhanced DNA vaccine immunogenicity. Expert Rev. Vaccines 2016, 15, 313–329. [Google Scholar] [CrossRef]
- Saade, F.; Petrovsky, N. Technologies for enhanced efficacy of DNA vaccines. Expert Rev. Vaccines 2012, 11, 189–209. [Google Scholar] [CrossRef] [PubMed]
- Ledgerwood, J.E.; Pierson, T.C.; Hubka, S.A.; Desai, N.; Rucker, S.; Gordon, I.J.; Enama, M.E.; Nelson, S.; Nason, M.; Gu, W.; et al. A west nile virus DNA vaccine utilizing a modified promoter induces neutralizing antibody in younger and older healthy adults in a phase I clinical trial. J. Infect. Dis. 2011, 203, 1396–1404. [Google Scholar] [CrossRef] [PubMed]
- Garrod, T.J.; Grubor-Bauk, B.; Gargett, T.; Li, Y.; Miller, D.S.; Yu, W.; Major, L.; Burrell, C.J.; Wesselingh, S.; Suhrbier, A.; et al. DNA vaccines encoding membrane-bound or secreted forms of heat shock protein 70 exhibit improved potency. Eur. J. Immunol. 2014, 44, 1992–2002. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Song, W.; Xu, Q.; Liu, Y.; Liu, H.; Guo, R.; Chiou, C.J.; Gao, K.; Jin, B.; Chen, C.; et al. Adjuvant DNA vaccine pNMM promotes enhanced specific immunity and anti-tumor effects. Hum. Vaccines Immunother. 2023, 19, 2202127. [Google Scholar] [CrossRef] [PubMed]
- Krieg, P.A.; Melton, D.A. Functional messenger RNAs are produced by SP6 in vitro transcription of cloned cDNAs. Nucleic Acids Res. 1984, 12, 7057–7070. [Google Scholar] [CrossRef] [PubMed]
- Boczkowski, B.D.; Nair, S.K.; Snyder, D.; Gilboa, E. Dendritic Cells Pulsed with RNA Are Potent Antigen—Presenting Cells In Vitro and In Vivo. J. Exp. Med. 1996, 184, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Martinon, F.; Krishnan, S.; Lenzen, G.; Magné, R.; Gomard, E.; Guillet, J.-G.; Lévy, J.-P.; Meulien, P. Induction of virus-specific cytotoxic T lymphocytes in vivo by liposome-entrapped mRNA. Eur. J. Immunol. 1993, 23, 1719–1722. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, M.; Peng, X.; Yang, Y.; Chen, Q.; Liu, J.; Tan, J.; Lou, C.; Liao, Z. mRNA vaccine in cancer therapy: Current advance and future outlook. Clin. Transl. Med. 2023, 13, e1384. [Google Scholar] [CrossRef]
- Duan, L.; Wang, Q.; Zhang, C.; Yang, D.; Zhang, X. Potentialities and Challenges of mRNA Vaccine in Cancer Immunotherapy. Front. Immunol. 2022, 13, 923647. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef]
- Greener, M. mRNA anti-cancer vaccine research gains momentum. Prescriber 2023, 34, 5–8. [Google Scholar] [CrossRef]
- Bloom, K.; van den Berg, F.; Arbuthnot, P. Self-amplifying RNA vaccines for infectious diseases. Gene Ther. 2021, 28, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Jiang, L.; Liao, S.; Wu, F.; Yang, G.; Hou, L.; Liu, L. Vaccines’ New Era—RNA Vaccine. Viruses 2023, 15, 1760. [Google Scholar] [CrossRef] [PubMed]
- Szabó, G.T.; Mahiny, A.J.; Vlatkovic, I. COVID-19 mRNA vaccines: Platforms and current developments. Mol. Ther. 2022, 30, 1850–1868. [Google Scholar] [CrossRef] [PubMed]
- Perenkov, A.D.; Sergeeva, A.D.; Vedunova, M.V. In Vitro Transcribed RNA-Based Platform Vaccines: Past, Present, and Future. Vaccines 2023, 11, 1600. [Google Scholar] [CrossRef] [PubMed]
- McNamara, M.A.; Nair, S.K.; Holl, E.K. RNA-Based Vaccines in Cancer Immunotherapy. J. Immunol. Res. 2015, 2015, 794528. [Google Scholar] [CrossRef] [PubMed]
- Tockary, T.A.; Abbasi, S.; Matsui-Masai, M.; Hayashi, A.; Yoshinaga, N.; Boonstra, E.; Wang, Z.; Fukushima, S.; Kataoka, K.; Uchida, S. Comb-structured mRNA vaccine tethered with short double-stranded RNA adjuvants maximizes cellular immunity for cancer treatment. Proc. Natl. Acad. Sci. USA 2023, 120, 2017. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, A.; Aljabbari, A.; Lokras, A.; Foged, C.; Thakur, A. Opportunities and challenges in the delivery of mrna-based vaccines. Pharmaceutics 2020, 12, 102. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Pei, J.; Xu, S.; Liu, J.; Yu, J. Recent advances in mRNA cancer vaccines: Meeting challenges and embracing opportunities. Front. Immunol. 2023, 14, 1246682. [Google Scholar] [CrossRef]
- Kim, S.C.; Sekhon, S.S.; Shin, W.R.; Ahn, G.; Cho, B.K.; Ahn, J.Y.; Kim, Y.H. Modifications of mRNA vaccine structural elements for improving mRNA stability and translation efficiency. Mol. Cell. Toxicol. 2022, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Wang, X. RNA modification in mRNA cancer vaccines. Clin. Exp. Med. 2023, 23, 1917–1931. [Google Scholar] [CrossRef] [PubMed]
- Shabalina, S.A.; Ogurtsov, A.Y.; Spiridonov, N.A. A periodic pattern of mRNA secondary structure created by the genetic code. Nucleic Acids Res. 2006, 34, 2428–2437. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, L.; Xu, Z.; Miao, L.; Huang, L. mRNA Vaccine with Antigen-Specific Checkpoint Blockade Induces an Enhanced Immune Response against Established Melanoma. Mol. Ther. 2018, 26, 420–434. [Google Scholar] [CrossRef] [PubMed]
- Hassett, K.J.; Benenato, K.E.; Jacquinet, E.; Lee, A.; Woods, A.; Yuzhakov, O.; Himansu, S.; Deterling, J.; Geilich, B.M.; Ketova, T.; et al. Optimization of Lipid Nanoparticles for Intramuscular Administration of mRNA Vaccines. Mol. Ther.-Nucleic Acids 2019, 15, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bernard, M.C.; Bazin, E.; Petiot, N.; Lemdani, K.; Commandeur, S.; Verdelet, C.; Margot, S.; Perkov, V.; Ripoll, M.; Garinot, M.; et al. The impact of nucleoside base modification in mRNA vaccine is influenced by the chemistry of its lipid nanoparticle delivery system. Mol. Ther.-Nucleic Acids 2023, 32, 794–806. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Y.; Hu, Q. Advances in saRNA Vaccine Research against Emerging/Re-Emerging Viruses. Vaccines 2023, 11, 1142. [Google Scholar] [CrossRef] [PubMed]
- Deviatkin, A.A.; Simonov, R.A.; Trutneva, K.A.; Maznina, A.A.; Soroka, A.B.; Kogan, A.A.; Feoktistova, S.G.; Khavina, E.M.; Mityaeva, O.N.; Volchkov, P.Y. Cap-Independent Circular mRNA Translation Efficiency. Vaccines 2023, 11, 238. [Google Scholar] [CrossRef] [PubMed]
- Brito, L.A.; Kommareddy, S.; Maione, D.; Uematsu, Y.; Giovani, C.; Berlanda Scorza, F.; Otten, G.R.; Yu, D.; Mandl, C.W.; Mason, P.W.; et al. Self-Amplifying mRNA Vaccines; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; Volume 89. [Google Scholar]
- Schmidt, C.; Schnierle, B.S. Self-Amplifying RNA Vaccine Candidates: Alternative Platforms for mRNA Vaccine Development. Pathogens 2023, 12, 138. [Google Scholar] [CrossRef] [PubMed]
- Voigt, E.A.; Gerhardt, A.; Hanson, D.; Jennewein, M.F.; Battisti, P.; Reed, S.; Singh, J.; Mohamath, R.; Bakken, J.; Beaver, S.; et al. A self-amplifying RNA vaccine against COVID-19 with long-term room-temperature stability. NPJ Vaccines 2022, 7, 136. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.; Haefner, E.; Gerbeth, J.; Beissert, T.; Sahin, U.; Perkovic, M.; Schnierle, B.S. A taRNA vaccine candidate induces a specific immune response that protects mice against Chikungunya virus infections. Mol. Ther.-Nucleic Acids 2022, 28, 743–754. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, H.; Lu, S.S.; Jones, K.A.; Govind, A.P.; Jeyifous, O.; Simmons, C.Q.; Tabatabaei, N.; Green, W.N.; Holder, J.L.; et al. RNA-based translation activators for targeted gene upregulation. Nat. Commun. 2023, 14, 6827. [Google Scholar] [CrossRef] [PubMed]
- Niu, D.; Wu, Y.; Lian, J. Circular RNA vaccine in disease prevention and treatment. Signal Transduct. Target. Ther. 2023, 8, 341. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Y.; Zhou, S.; Dain, L.; Mei, L.; Zhu, G. Circular RNA: An emerging frontier in RNA therapeutic targets, RNA therapeutics, and mRNA vaccines. J. Control. Release 2022, 348, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Wang, S.K.; Belk, J.A.; Amaya, L.; Li, Z.; Cardenas, A.; Abe, B.T.; Chen, C.K.; Wender, P.A.; Chang, H.Y. Engineering circular RNA for enhanced protein production. Nat. Biotechnol. 2023, 41, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, C.; Fotin-Mleczek, M.; Roth, G.; Becker, C.; Dam, T.C.; Verdurmen, W.P.R.; Brock, R.; Probst, J.; Schlake, T. Protein expression from exogenous mRNA: Uptake by receptor-mediated endocytosis and trafficking via the lysosomal pathway. RNA Biol. 2011, 8, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Del Toro Runzer, C.; Anand, S.; Mota, C.; Moroni, L.; Plank, C.; van Griensven, M.; Balmayor, E.R. Cellular uptake of modified mRNA occurs via caveolae-mediated endocytosis, yielding high protein expression in slow-dividing cells. Mol. Ther.-Nucleic Acids 2023, 32, 960–979. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.Z.; Zhao, X.; Song, X.R. Ex vivo pulsed dendritic cell vaccination against cancer. Acta Pharmacol. Sin. 2020, 41, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Weissman, D.; Ni, H.; Scales, D.; Dude, A.; Capodici, J.; McGibney, K.; Abdool, A.; Isaacs, S.N.; Cannon, G.; Karikó, K. HIV Gag mRNA Transfection of Dendritic Cells (DC) Delivers Encoded Antigen to MHC Class I and II Molecules, Causes DC Maturation, and Induces a Potent Human In Vitro Primary Immune Response. J. Immunol. 2000, 165, 4710–4717. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhu, Y.; He, J.; Sun, X. Path towards mRNA delivery for cancer immunotherapy from bench to bedside. Theranostics 2024, 14, 96–115. [Google Scholar] [CrossRef]
- Tateshita, N.; Miura, N.; Tanaka, H.; Masuda, T.; Ohtsuki, S.; Tange, K.; Nakai, Y.; Yoshioka, H.; Akita, H. Development of a lipoplex-type mRNA carrier composed of an ionizable lipid with a vitamin E scaffold and the KALA peptide for use as an ex vivo dendritic cell-based cancer vaccine. J. Control. Release 2019, 310, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Borch, T.H.; Engell-Noerregaard, L.; Zeeberg Iversen, T.; Ellebaek, E.; Met, Ö.; Hansen, M.; Andersen, M.H.; thor Straten, P.; Svane, I.M. mRNA-transfected dendritic cell vaccine in combination with metronomic cyclophosphamide as treatment for patients with advanced malignant melanoma. Oncoimmunology 2016, 5, e1207842. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.A.; Nair, S.K. RNA-transfected dendritic cells in cancer immunotherapy. J. Clin. Investig. 2000, 106, 1065–1069. [Google Scholar] [CrossRef] [PubMed]
- Dörrie, J.; Schaft, N.; Schuler, G.; Schuler-Thurner, B. Therapeutic cancer vaccination with ex vivo rna-transfected dendritic cells—An update. Pharmaceutics 2020, 12, 92. [Google Scholar] [CrossRef] [PubMed]
- Perez, C.R.; De Palma, M. Engineering dendritic cell vaccines to improve cancer immunotherapy. Nat. Commun. 2019, 10, 5408. [Google Scholar] [CrossRef] [PubMed]
- Benteyn, D.; Heirman, C.; Bonehill, A.; Thielemans, K.; Breckpot, K. mRNA-based dendritic cell vaccines. Expert Rev. Vaccines 2014, 14, 161–176. [Google Scholar] [CrossRef] [PubMed]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles from Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef] [PubMed]
- Jewell, C.M.; Bustamante López, S.C.; Irvine, D.J. In situ engineering of the lymph node microenvironment via intranodal injection of adjuvant-releasing polymer particles. Proc. Natl. Acad. Sci. USA 2011, 108, 15745–15750. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.; Gao, W.; Alber, S.; Trichel, A.; Murphey-Corb, M.; Watkins, S.C.; Gambotto, A.; Barratt-Boyes, S.M. Adenovirus-Transduced Dendritic Cells Injected into Skin or Lymph Node Prime Potent Simian Immunodeficiency Virus-Specific T Cell Immunity in Monkeys. J. Immunol. 2003, 171, 6875–6882. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.; Sayegh, N.; Graciotti, M.; Kandalaft, L.E. Electroporation as a method of choice to generate genetically modified dendritic cell cancer vaccines. Curr. Opin. Biotechnol. 2020, 65, 142–155. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Nishikawa, M.; Kobayashi, N.; Takakura, Y. Gene silencing in primary and metastatic tumors by small interfering RNA delivery in mice: Quantitative analysis using melanoma cells expressing firefly and sea pansy luciferases. J. Control. Release 2005, 105, 332–343. [Google Scholar] [CrossRef]
- Steitz, J.; Britten, C.M.; Wölfel, T.; Tüting, T. Effective induction of anti-melanoma immunity following genetic vaccination with synthetic mRNA coding for the fusion protein EGFP.TRP2. Cancer Immunol. Immunother. 2006, 55, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Mandl, C.W.; Aberle, J.H.; Aberle, S.W.; Holzmann, H.; Allison, S.L.; Heinz, F.X. In vitro-synthesized infectious RNA as an attenuated live vaccine in a flavivirus model. Nat. Med. 1998, 4, 1438–1440. [Google Scholar] [CrossRef]
- Bolhassani, A.; Khavari, A.; Oraf, Z. Electroporation—Advantages and Drawbacks for Delivery of Drug, Gene and Vaccine. In Application of Nanotechnology in Drug Delivery; InTech: Rijeka, Croatia, 2014; pp. 369–397. [Google Scholar] [CrossRef]
- Choi, Y.S.; Lee, M.Y.; David, A.E.; Park, Y.S. Nanoparticles for gene delivery: Therapeutic and toxic effects. Mol. Cell. Toxicol. 2014, 10, 1–8. [Google Scholar] [CrossRef]
- Ramamoorth, M.; Narvekar, A. Non viral vectors in gene therapy—An overview. J. Clin. Diagn. Res. 2015, 9, GE01–GE06. [Google Scholar] [CrossRef] [PubMed]
- Riecken, K.; Głów, D.; Fehse, B. How to package and SEND mRNA: A novel “humanized” vector system based on endogenous retroviruses. Signal Transduct. Target. Ther. 2021, 6, 384. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liang, B.; Wang, W.; Li, L.; Feng, N.; Zhao, Y.; Wang, T.; Yan, F.; Yang, S.; Xia, X. Viral vectored vaccines: Design, development, preventive and therapeutic applications in human diseases. Signal Transduct. Target. Ther. 2023, 8, 149. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Lim, J.; Wang, C.P.J.; Han, J.H.; Shin, H.E.; Kim, S.N.; Jeong, D.; Lee, S.H.; Chun, B.H.; Park, C.G.; et al. Lipid nanoparticle-based mRNA delivery systems for cancer immunotherapy. Nano Converg. 2023, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Strelkova Petersen, D.M.; Chaudhary, N.; Arral, M.L.; Weiss, R.M.; Whitehead, K.A. The mixing method used to formulate lipid nanoparticles affects mRNA delivery efficacy and organ tropism. Eur. J. Pharm. Biopharm. 2023, 192, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Lin, Y.; Wei, T.; Cheng, Q. Lipid Nanoparticle (LNP) Enables mRNA Delivery for Cancer Therapy. Adv. Mater. 2023, 35, e2303261. [Google Scholar] [CrossRef]
- Chander, N.; Basha, G.; Yan Cheng, M.H.; Witzigmann, D.; Cullis, P.R. Lipid nanoparticle mRNA systems containing high levels of sphingomyelin engender higher protein expression in hepatic and extra-hepatic tissues. Mol. Ther.-Methods Clin. Dev. 2023, 30, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Kordalivand, N.; Tondini, E.; Lau, C.Y.J.; Vermonden, T.; Mastrobattista, E.; Hennink, W.E.; Ossendorp, F.; van Nostrum, C.F. Cationic synthetic long peptides-loaded nanogels: An efficient therapeutic vaccine formulation for induction of T-cell responses. J. Control. Release 2019, 315, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Haug, B.E.; Camilio, K.A.; Eliassen, L.T.; Stensen, W.; Svendsen, J.S.; Berg, K.; Mortensen, B.; Serin, G.; Mirjolet, J.F.; Bichat, F.; et al. Discovery of a 9-mer Cationic Peptide (LTX-315) as a Potential First in Class Oncolytic Peptide. J. Med. Chem. 2016, 59, 2918–2927. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, A.; Rajendrakumar, S.K.; Cherukula, K.; Park, M.S.; Padmanaban, S.; Vasukuty, A.; Mohanty, A.; Lee, J.Y.; Bae, W.K.; Park, I.K. A sugar modified amphiphilic cationic nano-adjuvant ceased tumor immune suppression and rejuvenated peptide vaccine induced antitumor immunity in cervical cancer. Biomater. Sci. 2023, 11, 1853–1866. [Google Scholar] [CrossRef] [PubMed]
- Heuts, J.; Jiskoot, W.; Ossendorp, F.; Maaden, K. Van Der Cationic Nanoparticle-Based Cancer Vaccines. Pharmaceutics 2021, 13, 596. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.N.; Li, M.; Luo, Y.L.; Chen, Q.; Wang, L.; Zhang, H.B.; Shen, S.; Gu, Z.; Wang, J. Cationic lipid-assisted nanoparticles for delivery of mRNA cancer vaccine. Biomater. Sci. 2018, 6, 3009–3018. [Google Scholar] [CrossRef] [PubMed]
- Le, T.M.D.; Yoon, A.R.; Thambi, T.; Yun, C.O. Polymeric Systems for Cancer Immunotherapy: A Review. Front. Immunol. 2022, 13, 826876. [Google Scholar] [CrossRef] [PubMed]
- Ben-Akiva, E.; Karlsson, J.; Hemmati, S.; Yu, H.; Tzeng, S.Y.; Pardoll, D.M.; Green, J.J. Biodegradable lipophilic polymeric mRNA nanoparticles for ligand-free targeting of splenic dendritic cells for cancer vaccination. Proc. Natl. Acad. Sci. USA 2023, 120, e2301606120. [Google Scholar] [CrossRef] [PubMed]
- Duinkerken, S.; Horrevorts, S.K.; Kalay, H.; Ambrosini, M.; Rutte, L.; de Gruijl, T.D.; Garcia-Vallejo, J.J.; van Kooyk, Y. Glyco-dendrimers as intradermal anti-tumor vaccine targeting multiple skin DC subsets. Theranostics 2019, 9, 5797–5809. [Google Scholar] [CrossRef]
- Granot-Matok, Y.; Ezra, A.; Ramishetti, S.; Sharma, P.; Naidu, G.S.; Benhar, I.; Peer, D. Lipid nanoparticles-loaded with toxin mRNA represents a new strategy for the treatment of solid tumors. Theranostics 2023, 13, 3497–3508. [Google Scholar] [CrossRef] [PubMed]
- Alameh, M.G.; Tombácz, I.; Bettini, E.; Lederer, K.; Ndeupen, S.; Sittplangkoon, C.; Gaudette, B.T.; Soliman, O.Y.; Wilmore, J.R.; Pine, M.; et al. Lipid nanoparticles enhance the efficacy of mRNA and protein subunit vaccines by inducing robust T follicular helper cell and humoral responses. Immunity 2021, 54, 287–2892. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Jeong, M.; Park, J.; Jung, H.; Lee, H. Immunogenicity of lipid nanoparticles and its impact on the efficacy of mRNA vaccines and therapeutics. Exp. Mol. Med. 2023, 55, 2085–2096. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Wang, Z.G.; Liu, S.L. Lipid Nanoparticles for mRNA Delivery to Enhance Cancer Immunotherapy. Molecules 2022, 27, 5607. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; You, X.; Wang, X.; Cui, L.; Wang, Z.; Xu, F.; Li, M.; Yang, Z.; Liu, J.; Huang, P.; et al. Delivery of mRNA vaccine with a lipid-like material potentiates antitumor efficacy through toll-like receptor 4 signaling. Proc. Natl. Acad. Sci. USA 2021, 118, e2005191118. [Google Scholar] [CrossRef] [PubMed]
- Jackson Hoffman, B.A.; Pumford, E.A.; Enueme, A.I.; Fetah, K.L.; Friedl, O.M.; Kasko, A.M. Engineered macromolecular Toll-like receptor agents and assemblies. Trends Biotechnol. 2023, 41, 1139–1154. [Google Scholar] [CrossRef] [PubMed]
- Mohamad Razif, M.I.; Nizar, N.; Zainal Abidin, N.H.; Muhammad Ali, S.N.; Wan Zarimi, W.N.N.; Khotib, J.; Susanti, D.; Mohd Jailani, M.T.; Taher, M. Emergence of mRNA vaccines in the management of cancer. Expert Rev. Vaccines 2023, 22, 629–642. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, K.; Antognini, D.; Combes, A.; Paden, M.; Zakhary, B.; Ogino, M.; Maclaren, G.; Brodie, D. Clinical advances and ongoing trials of mRNA vaccines for cancer treatment. Lancet Oncol. 2022, 23, 450–458. [Google Scholar]
- Wang, Y.; Zhang, R.; Tang, L.; Yang, L. Nonviral Delivery Systems of mRNA Vaccines for Cancer Gene Therapy. Pharmaceutics 2022, 14, 512. [Google Scholar] [CrossRef] [PubMed]
- Ni, L. Advances in mRNA-Based Cancer Vaccines. Vaccines 2023, 11, 1599. [Google Scholar] [CrossRef] [PubMed]
- Rojas, L.A.; Sethna, Z.; Soares, K.C.; Olcese, C.; Pang, N.; Patterson, E.; Lihm, J.; Ceglia, N.; Guasp, P.; Chu, A.; et al. Personalized RNA neoantigen vaccines stimulate T cells in pancreatic cancer. Nature 2023, 618, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Lindgren, G.; Lin, A.; Thompson, E.A.; Ols, S.; Röhss, J.; John, S.; Hassett, K.; Yuzhakov, O.; Bahl, K.; et al. Efficient Targeting and Activation of Antigen-Presenting Cells In Vivo after Modified mRNA Vaccine Administration in Rhesus Macaques. Mol. Ther. 2017, 25, 2635–2647. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Goedegebuure, P.; Mardis, E.R.; Ellis, M.J.C.; Zhang, X.; Herndon, J.M.; Fleming, T.P.; Carreno, B.M.; Hansen, T.H.; Gillanders, W.E. Cancer genome sequencing and its implications for personalized cancer vaccines. Cancers 2011, 3, 4191–4211. [Google Scholar] [CrossRef]
- Fritah, H.; Rovelli, R.; Chiang, C.L.L.; Kandalaft, L.E. The current clinical landscape of personalized cancer vaccines. Cancer Treat. Rev. 2022, 106, 102383. [Google Scholar] [CrossRef] [PubMed]
- Hawlina, S.; Zorec, R.; Chowdhury, H.H. Potential of Personalized Dendritic Cell-Based Immunohybridoma Vaccines to Treat Prostate Cancer. Life 2023, 13, 1498. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Lin, E.Z.; Anekoji, M.; Ichim, T.E.; Hu, J.; Marincola, F.M.; Jones, L.D.; Kesari, S.; Ashili, S. Advancing personalized medicine in brain cancer: Exploring the role of mRNA vaccines. J. Transl. Med. 2023, 21, 830. [Google Scholar] [CrossRef] [PubMed]
- Shemesh, C.S.; Hsu, J.C.; Hosseini, I.; Shen, B.Q.; Rotte, A.; Twomey, P.; Girish, S.; Wu, B. Personalized Cancer Vaccines: Clinical Landscape, Challenges, and Opportunities. Mol. Ther. 2021, 29, 555–570. [Google Scholar] [CrossRef] [PubMed]
- Perrinjaquet, M.; Richard Schlegel, C. Personalized neoantigen cancer vaccines: An analysis of the clinical and commercial potential of ongoing development programs. Drug Discov. Today 2023, 28, 103773. [Google Scholar] [CrossRef] [PubMed]
- Richard, G.; Princiotta, M.F.; Bridon, D.; Martin, W.D.; Steinberg, G.D.; De Groot, A.S. Neoantigen-based personalized cancer vaccines: The emergence of precision cancer immunotherapy. Expert Rev. Vaccines 2022, 21, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Ott, P.A.; Hu, Z.; Keskin, D.B.; Shukla, S.A.; Sun, J.; Bozym, D.J.; Zhang, W.; Luoma, A.; Giobbie-Hurder, A.; Peter, L.; et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 2017, 547, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Dagogo-Jack, I.; Shaw, A.T. Tumour heterogeneity and resistance to cancer therapies. Nat. Rev. Clin. Oncol. 2018, 15, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Türeci, Ö.; Löwer, M.; Schrörs, B.; Lang, M.; Tadmor, A.; Sahin, U. Challenges towards the realization of individualized cancer vaccines. Nat. Biomed. Eng. 2018, 2, 566–569. [Google Scholar] [CrossRef] [PubMed]
- Katsikis, P.D.; Ishii, K.J.; Schliehe, C. Challenges in developing personalized neoantigen cancer vaccines. Nat. Rev. Immunol. 2024, 24, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Richard, G.; Ruggiero, N.; Steinberg, G.D.; Martin, W.D.; De Groot, A.S. Neoadjuvant personalized cancer vaccines: The final frontier? Expert Rev. Vaccines 2024, 23, 205–212. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, X.; Wang, S.Z.; Xu, Y.; Jia, H.R.; Zhu, Y.X.; Wu, S.Y.; Zhang, X.; Feng, H.H.; Gao, G.; et al. Turning tumor cells into microvesicles as personalized cancer vaccines for cancer prevention and treatment. Nano Today 2024, 55, 102219. [Google Scholar] [CrossRef]
- Blass, E.; Ott, P.A. Advances in the development of personalized neoantigen-based therapeutic cancer vaccines. Nat. Rev. Clin. Oncol. 2021, 18, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Amaya-Ramirez, D.; Martinez-Enriquez, L.C.; Parra-López, C. Usefulness of Docking and Molecular Dynamics in Selecting Tumor Neoantigens to Design Personalized Cancer Vaccines: A Proof of Concept. Vaccines 2023, 11, 1174. [Google Scholar] [CrossRef] [PubMed]
- Pollard, A.J.; Bijker, E.M. A guide to vaccinology: From basic principles to new developments. Nat. Rev. Immunol. 2021, 21, 83–100. [Google Scholar] [CrossRef] [PubMed]
- Adotévi, O.; Vernerey, D.; Jacoulet, P.; Meurisse, A.; Laheurte, C.; Almotlak, H.; Jacquin, M.; Kaulek, V.; Boullerot, L.; Malfroy, M.; et al. Safety, Immunogenicity, and 1-Year Efficacy of Universal Cancer Peptide-Based Vaccine in Patients with Refractory Advanced Non-Small-Cell Lung Cancer: A Phase Ib/Phase IIa De-Escalation Study. J. Clin. Oncol. 2023, 41, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Kaumaya, P.T.P.; Guo, L.; Overholser, J.; Penichet, M.L.; Bekaii-Saab, T. Immunogenicity and antitumor efficacy of a novel human PD-1 B-cell vaccine (PD1-Vaxx) and combination immunotherapy with dual trastuzumab/pertuzumab-like HER-2 B-cell epitope vaccines (B-Vaxx) in a syngeneic mouse model. Oncoimmunology 2020, 9, 1818437. [Google Scholar] [CrossRef] [PubMed]
- Berry, J.S.; Vreeland, T.J.; Hale, D.F.; Jackson, D.O.; Trappey, A.F. Evaluation of attenuated tumor antigens and the implications for peptide-based cancer vaccine development. J. Cancer 2017, 8, 1255–1262. [Google Scholar] [CrossRef]
- Purcell, A.W.; McCluskey, J.; Rossjohn, J. More than one reason to rethink the use of peptides in vaccine design. Nat. Rev. Drug Discov. 2007, 6, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Wada, H.; Goto, R.; Osada, T.; Yamamura, K.; Fukaya, S.; Shimizu, A.; Okubo, M.; Minamiguchi, K.; Ikizawa, K.; et al. TAS0314, a novel multi-epitope long peptide vaccine, showed synergistic antitumor immunity with PD-1/PD-L1 blockade in HLA-A*2402 mice. Sci. Rep. 2020, 10, 17284. [Google Scholar] [CrossRef] [PubMed]
- Jordan, K.R.; McMahan, R.H.; Kemmler, C.B.; Kappler, J.W.; Slansky, J.E. Peptide vaccines prevent tumor growth by activating T cells that respond to native tumor antigens. Proc. Natl. Acad. Sci. USA 2010, 107, 4652–4657. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Admistration (FDA). 2021. Available online: www.fda.gov (accessed on 1 March 2024).
- Stephens, A.J.; Burgess-Brown, N.A.; Jiang, S. Beyond Just Peptide Antigens: The Complex World of Peptide-Based Cancer Vaccines. Front. Immunol. 2021, 12, 696791. [Google Scholar] [CrossRef]
- bin Umair, M.; Akusa, F.N.; Kashif, H.; Seerat-e-Fatima, F.B.; Azhar, M.; Munir, I.; Ahmed, M.; Khalil, W.; Sharyar, H.; Rafique, S.; et al. Viruses as tools in gene therapy, vaccine development, and cancer treatment. Arch. Virol. 2022, 167, 1387–1404. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, J.; Kim, E.S. Oncolytic Viruses and Cancer Immunotherapy. Curr. Oncol. Rep. 2023, 25, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Feola, S.; Chiaro, J.; Cerullo, V. Integrating immunopeptidome analysis for the design and development of cancer vaccines. Semin. Immunol. 2023, 67, 101750. [Google Scholar] [CrossRef] [PubMed]
- Hollingsworth, R.E.; Jansen, K. Turning the corner on therapeutic cancer vaccines. NPJ Vaccines 2019, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Raina, N.; Pal, A.K.; Rani, R.; Sharma, A.; Gupta, M. Functional Nanomaterials and Nanocomposite in Cancer Vaccines; Elsevier Inc.: Amsterdam, The Netherlands, 2022; ISBN 9780128236864. [Google Scholar]
- Pandya, A.; Shah, Y.; Kothari, N.; Postwala, H.; Shah, A.; Parekh, P.; Chorawala, M.R. The Future of Cancer Immunotherapy: DNA Vaccines Leading the Way; Springer: Berlin/Heidelberg, Germany, 2023; Volume 40, ISBN 0123456789. [Google Scholar]
- Das, S.S.; Moitra, I.; Singh, S.K.; Verma, P.R.P.; Swain, S. DNA Vaccines for Cancer Treatment: Challenges and Promises; Elsevier Inc.: Amsterdam, The Netherlands, 2022; ISBN 9780128236864. [Google Scholar]
- Rinaldi, M.; Signori, E.; Rosati, P.; Cannelli, G.; Parrella, P.; Iannace, E.; Monego, G.; Ciafrè, S.A.; Farace, M.G.; Iurescia, S.; et al. Feasibilty of in utero DNA vaccination following naked gene transfer into pig fetal muscle: Transgene expression, immunity and safety. Vaccine 2006, 24, 4586–4591. [Google Scholar] [CrossRef] [PubMed]
- Le, T.; Sun, C.; Chang, J.; Zhang, G.; Yin, X. mRNA Vaccine Development for Emerging Animal and Zoonotic Diseases. Viruses 2022, 14, 401. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Tang, T.; Chen, Y.; Huang, X.; Liang, T. mRNA vaccines in disease prevention and treatment. Signal Transduct. Target. Ther. 2023, 8, 365. [Google Scholar] [CrossRef] [PubMed]
- Chehelgerdi, M.; Chehelgerdi, M. The Use of RNA-Based Treatments in the Field of Cancer Immunotherapy; BioMed Central: London, UK, 2023; Volume 22, ISBN 1294302301807. [Google Scholar]
- Wang, L.; Pegram, M.D.; Wu, J.C. Induced pluripotent stem cells as a novel cancer vaccine. Expert Opin. Biol. Ther. 2019, 19, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.; Zhang, M.; Yang, J.; Zhu, Z.; Cao, W.; Dong, C. Therapeutic cancer vaccines: Advancements, challenges, and prospects. Signal Transduct. Target. Ther. 2023, 8, 450. [Google Scholar] [CrossRef] [PubMed]
- Tahaghoghi-Hajghorbani, S.; Yazdani, M.; Nikpoor, A.R.; Hatamipour, M.; Ajami, A.; Jaafari, M.R.; Badiee, A.; Rafiei, A. Targeting the tumor microenvironment by liposomal Epacadostat in combination with liposomal gp100 vaccine. Sci. Rep. 2023, 13, 5802. [Google Scholar] [CrossRef] [PubMed]
- Twilhaar, M.K.N.; Czentner, L.; van Nostrum, C.F.; Storm, G.; Den Haan, J.M.M. Mimicking pathogens to augment the potency of liposomal cancer vaccines. Pharmaceutics 2021, 13, 954. [Google Scholar] [CrossRef] [PubMed]
- Alhamhoom, Y.; Kakinani, G.; Rahamathulla, M.; Ali, M.; Osmani, R.; Hani, U.; Yoonus Thajudeen, K.; Kiran Raj, G.; Gowda, D.V. Recent advances in the liposomal nanovesicles based immunotherapy in the treatment of cancer: A review. Saudi Pharm. J. 2023, 31, 279–294. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Shi, H.; Fu, P.; Shi, P.; Yang, J. Liposomal Dendritic Cell Vaccine in Breast Cancer Immunotherapy. ACS Omega 2021, 6, 3991–3998. [Google Scholar] [CrossRef] [PubMed]
- Rommasi, F.; Esfandiari, N. Liposomal Nanomedicine: Applications for Drug Delivery in Cancer Therapy. Nanoscale Res. Lett. 2021, 16, 95. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Suita, Y.; Miriyala, S.; Dean, J.; Tapinos, N.; Shen, J. Advances in lipid-based nanoparticles for cancer chemoimmunotherapy. Pharmaceutics 2021, 13, 520. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.; Duan, S.; Ye, F.; Hou, X.; Li, X.; Zhao, J.; Yu, X.; Hu, Z.; Tang, Z.; Mo, F.; et al. The enhanced antitumor-specific immune response with mannose- and CpG-ODN-coated liposomes delivering TRP2 peptide. Theranostics 2018, 8, 1723–1739. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fang, H.; Zhang, T.; Wang, Y.; Qi, T.; Li, B.; Jiao, H. Lipid-mRNA nanoparticles landscape for cancer therapy. Front. Bioeng. Biotechnol. 2022, 10, 1053197. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Shi, K.; Jia, Y.P.; Hao, Y.; Peng, J.R.; Qian, Z.Y. Advanced biomaterials for cancer immunotherapy. Acta Pharmacol. Sin. 2020, 41, 911–927. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.M.; Gattis, B.; Choi, M.R.; Zhang, B.; Gianneschi, N. Use of proteomimetic polymers for delivery of tumor antigens and adjuvants through formation of stable electrostatic complexes with small molecule STING agonists. J. Clin. Oncol. 2023, 41, 2583. [Google Scholar] [CrossRef]
- Verma, N.K.; Wong, B.H.S.; Poh, Z.S.; Udayakumar, A.; Verma, R.; Goh, R.K.J.; Duggan, S.P.; Shelat, V.G.; Chandy, K.G.; Grigoropoulos, N.F. Obstacles for T-lymphocytes in the tumour microenvironment: Therapeutic challenges, advances and opportunities beyond immune checkpoint. eBioMedicine 2022, 83, 104216. [Google Scholar] [CrossRef] [PubMed]
- Bilotta, M.T.; Antignani, A.; Fitzgerald, D.J. Managing the TME to improve the efficacy of cancer therapy. Front. Immunol. 2022, 13, 954992. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Chen, J.; Zhou, H.; Zeng, X.; Ruan, Z.; Pu, Z.; Jiang, X.; Matsui, A.; Zhu, L.; Amoozgar, Z.; et al. Combining p53 mRNA nanotherapy with immune checkpoint blockade reprograms the immune microenvironment for effective cancer therapy. Nat. Commun. 2022, 13, 758. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, T. Personalized anti-cancer vaccine combining mRNA and immunotherapy tested in melanoma trial. Nat. Med. 2023, 29, 2379–2380. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.; Pusztai, L.; Swanton, C. Cancer heterogeneity: Implications for targeted therapeutics. Br. J. Cancer 2013, 108, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Bedard, P.L.; Hansen, A.R.; Ratain, M.J.; Siu, L.L. Tumour heterogeneity in the clinic. Nature 2013, 501, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S. Vaccine Design: Methods and Protocols, Volume 3. Resources for Vaccine Development; Springer: New York, NY, USA, 2022; Volume 3, ISBN 9781071618912. [Google Scholar]
- Schmidt, J.; Guillaume, P.; Dojcinovic, D.; Karbach, J.; Coukos, G.; Luescher, I. In silico and cell-based analyses reveal strong divergence between prediction and observation of T-cell-recognized tumor antigen T-cell epitopes. J. Biol. Chem. 2017, 292, 11840–11849. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Su, G.H.; Ma, D.; Xiao, Y.; Shao, Z.M.; Jiang, Y.Z. Technological advances in cancer immunity: From immunogenomics to single-cell analysis and artificial intelligence. Signal Transduct. Target. Ther. 2021, 6, 312. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wang, X.; Zeng, S.; Ren, X.; Yan, Y.; Gong, Z. Applying artificial intelligence for cancer immunotherapy. Acta Pharm. Sin. B 2021, 11, 3393–3405. [Google Scholar] [CrossRef] [PubMed]
- Boehm, K.M.; Bhinder, B.; Raja, V.J.; Dephoure, N.; Elemento, O. Predicting peptide presentation by major histocompatibility complex class I using one million peptides. BMC Bioinform. 2019, 20, 7. [Google Scholar] [CrossRef] [PubMed]
- Woo, K.; Padalhin, A. Reactive oxygen species overload: A review of plasma therapy and photobiomodulation for cancer treatment. Med. Lasers 2023, 12, 18–28. [Google Scholar] [CrossRef]
- Živanić, M.; Espona-Noguera, A.; Lin, A.; Canal, C. Current State of Cold Atmospheric Plasma and Cancer-Immunity Cycle: Therapeutic Relevance and Overcoming Clinical Limitations Using Hydrogels. Adv. Sci. 2023, 10, e2205803. [Google Scholar] [CrossRef] [PubMed]
- Labay, C.; Roldán, M.; Tampieri, F.; Stancampiano, A.; Bocanegra, P.E.; Ginebra, M.P.; Canal, C. Enhanced generation of reactive species by cold plasma in gelatin solutions for selective cancer cell death. ACS Appl. Mater. Interfaces 2020, 12, 47256–47269. [Google Scholar] [CrossRef] [PubMed]
- Murillo, D.; Huergo, C.; Gallego, B.; Rodríguez, R.; Tornín, J. Exploring the Use of Cold Atmospheric Plasma to Overcome Drug Resistance in Cancer. Biomedicines 2023, 11, 208. [Google Scholar] [CrossRef] [PubMed]
- Hirst, A.M.; Simms, M.S.; Mann, V.M.; Maitland, N.J.; O’connell, D.; Frame, F.M. Low-temperature plasma treatment induces DNA damage leading to necrotic cell death in primary prostate epithelial cells. Br. J. Cancer 2015, 112, 1536–1545. [Google Scholar] [CrossRef] [PubMed]
- Gaur, N.; Kurita, H.; Oh, J.S.; Miyachika, S.; Ito, M.; Mizuno, A.; Cowin, A.J.; Allinson, S.; Short, R.D.; Szili, E.J. On cold atmospheric-pressure plasma jet induced DNA damage in cells. J. Phys. D Appl. Phys. 2021, 54, 035203. [Google Scholar] [CrossRef]
- Braný, D.; Dvorská, D.; Strnádel, J.; Matáková, T.; Halašová, E.; Škovierová, H. Effect of cold atmospheric plasma on epigenetic changes, dna damage and possibilities for its use in synergistic cancer therapy. Int. J. Mol. Sci. 2021, 22, 12252. [Google Scholar] [CrossRef] [PubMed]
- Kumar Dubey, S.; Dabholkar, N.; Narayan Pal, U.; Singhvi, G.; Kumar Sharma, N.; Puri, A.; Kesharwani, P. Emerging innovations in cold plasma therapy against cancer: A paradigm shift. Drug Discov. Today 2022, 27, 2425–2439. [Google Scholar] [CrossRef] [PubMed]
- Ruidiaz, M.E.; Cortes-Mateos, M.J.; Sandoval, S.; Martin, D.T.; Wang-Rodriguez, J.; Hasteh, F.; Wallace, A.; Vose, J.G.; Kummel, A.C.; Blair, S.L. Quantitative comparison of surgical margin histology following excision with traditional electrosurgery and a low-thermal-injury dissection device. J. Surg. Oncol. 2011, 104, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Pomicter, A.D.; Li, F.; Bhatt, S.; Chen, C.; Li, W.; Qi, M.; Huang, C.; Deininger, M.W.; Kong, M.G.; et al. Trident cold atmospheric plasma blocks three cancer survival pathways to overcome therapy resistance. Proc. Natl. Acad. Sci. USA 2021, 118, e2107220118. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).