Abstract
The treatment landscape for lysosomal storage disorders (LSDs) is rapidly evolving. An increase in the number of preclinical and clinical studies in the last decade has demonstrated that pharmacological chaperones are a feasible alternative to enzyme replacement therapy (ERT) for individuals with LSDs. A systematic search was performed to retrieve and critically assess the evidence from preclinical and clinical applications of pharmacological chaperones in the treatment of LSDs and to elucidate the mechanisms by which they could be effective in clinical practice. Publications were screened according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) reporting guidelines. Fifty-two articles evaluating 12 small molecules for the treatment of seven LSDs are included in this review. Overall, a substantial amount of preclinical and clinical data support the potential of pharmacological chaperones as treatments for Fabry disease, Gaucher disease, and Pompe disease. Most of the available clinical evidence evaluated migalastat for the treatment of Fabry disease. There was a lack of consistency in the terminology used to describe pharmacological chaperones in the literature. Therefore, the new small molecule chaperone (SMC) classification system is proposed to inform a standardized approach for new, emerging small molecule therapies in LSDs.
1. Introduction
Lysosomal storage disorders (LSDs) are a heterogeneous group of rare diseases primarily caused by mutations in genes encoding enzymes responsible for normal lysosomal function [1]. For example, missense mutations in genes encoding lysosomal enzymes may cause them to misfold, leading to endoplasmic reticulum (ER) retention and/or early degradation [2,3]. Partial or complete deficiency of the lysosomal enzyme leads to progressive accumulation of the substrate of the mutant lysosomal enzyme, resulting in additional cell toxicity and death [1]. There are 70 different LSDs, including Gaucher disease, Fabry disease, Pompe disease and Niemann–Pick disease type C (NPC). Gaucher disease is the most common LSD, with a global prevalence of 1.5 cases per 100,000 live births [4]. It occurs due to mutations in the GBA1 gene encoding glucocerebrosidase (GCase), a lysosomal enzyme that converts D-glucosylceramide into ceramide and D-glucose [5]. GBA1 mutations commonly result in GCase misfolding, followed by retention within the ER and premature degradation [5].
Fabry disease is caused by the deficient activity of lysosomal glycosidase due to mutations in the alpha-galactosidase A (α-Gal) gene located on the X-chromosome, which results in the storage of excess cellular glycosphingolipids [6]. In Pompe disease, mutations in the acid-alpha-glucosidase (GAA) gene cause a deficiency of the lysosomal enzyme GAA, leading to progressive, intralysosomal glycogen accumulation in multiple tissues and organs [7]. NPC types 1 and 2 (NPC1 and NPC2) are enzymes involved in cholesterol efflux from late lysosomal and endosomal compartments [8]. NPC disease typically results from missense mutations (70–80% cases) in the NPC1 gene, resulting in misfolding and premature degradation of the NPC1 protein, which leads to the progressive onset of neurological symptoms such as loss of motor function and cognitive impairment [8].
The drug development pipeline for LSDs is rapidly evolving worldwide [9], with several different treatment approaches now used in routine clinical practice, including enzyme replacement therapy (ERT), substrate reduction therapy (SRT), and pharmacological chaperone therapy (PCT) (Table 1). ERT is the most established treatment approach for LSDs resulting from impaired lysosomal enzymes [10]. This approach involves the recurrent administration of exogenous recombinant protein that replaces the specific defective enzyme in order to reduce substrate accumulation [10]. At least 15 ERT products have been developed and approved in Europe for nine different LSDs (Gaucher, Fabry, and Pompe disease, ceroid lipofuscinosis type 2 disease, as well as five mucopolysaccharidoses (MPS I, MPS II, MPS VIA, MPSVI, and MPSVII)) [11]. Although ERT is a highly effective treatment for many individuals with LSDs, it remains a less than favorable therapeutic option for others, e.g., those hypersensitive to the recombinant protein, with mutations in non-lysosomal enzymes, or with LSDs that affect tissues and organs less accessible by intravenous delivery of replacement protein [12,13]). Since ERT can lead to infusion-associated reactions and the formation of neutralizing antidrug antibodies that reduce the efficacy of therapy for some individuals, alternative treatment approaches are warranted [12,13]. Regular ERT infusions can also be inconvenient and have a major impact on a person’s home and work life [14]. Furthermore, some individuals with Pompe disease and who are cross-reactive immunologic material-negative have a poor clinical response to ERT secondary to high sustained antibody titers [15]. Over the past 50 years, there has been an increased focus on the therapeutic role of small molecules, with a remarkable 124 orphan drug designations granted in the US alone for compounds intended to treat 28 LSDs [9]. Within the small molecule development space, SRT and PCT have been shown to provide benefit for the treatment of some LSDs. As the name suggests, rather than directly restoring the activity of the defective enzyme, SRT aims to attenuate the biosynthesis of the accumulated substrate. One SRT drug, N-butyl-deoxynojirimycin (NB-DNJ/miglustat; Zavesca®; Actelion Pharmaceuticals US Inc, San Francisco, CA, USA), is approved in Europe for mild to moderate type 1 Gaucher disease in individuals for whom ERT is unsuitable and for the treatment of progressive neurological manifestations for individuals with NPC disease (approved by the European Medicines Agency (EMA) in 2002 and 2009, respectively) [11]. Miglustat can also act either as a competitive inhibitor of glucosylceramide synthase to decrease the synthesis and accumulation of glucosylceramide in Gaucher disease or as an enzyme stabilizer in Pompe disease [11]. Another SRT, eliglustat (Cerdelga®; Sanofi, Paris, France), was approved by the EMA in 2015 as a first-line treatment for Gaucher disease type 1 [11].
The term “pharmacological chaperone therapy” or “PCT” was first coined in 2000 to describe the category of exogenously administered small molecules that restore folding and trafficking defects of misfolded proteins in LSDs. The EMA approved the first commercially available PCT, migalastat (Galafold®; Amicus Therapeutics Inc., Philadelphia, PA, USA), in 2016, for long-term treatment of adults with Fabry disease who have an amenable mutation (i.e., a mutation that is responsive to treatment) [11]. Migalastat binds and stabilizes endogenous mutant α-Gal enzyme, thereby increasing its activity relative to the specific amenable mutation and improving trafficking to the lysosome, i.e., it reduces ER retention of mutant endogenous α-Gal. Most pharmacological chaperones, such as migalastat, have been reported to bind to the active site of their target enzyme, acting as competitive inhibitors [16]. Second-generation pharmacological chaperones that bind to allosteric sites to stabilize and protect mutant enzymes from degradation without interfering with their activity may also have therapeutic potential [17]. Studies have shown that PCT enables the conformational stabilization of the target protein by promoting more favorable free energy states than the unbound state at neutral pH [18]. Once in the acidic environment of the lysosomes, the pharmacological chaperone dissociates from the mutant enzyme, thus restoring some of its residual catalytic activity [19].
The clinical utility of PCTs to improve the stability of exogenous ERT has also been investigated in Pompe disease, including the use of miglustat in combination with alglucosidase alfa, the first approved recombinant human GAA (rhGAA) for Pompe disease [20], and cipaglucosidase alfa [21], a next-generation rhGAA with cellularly derived bis-phosphorylated N-glycans to improve its cellular uptake through cation-independent mannose-6-phosphate receptors while retaining its capacity to be fully processed into the most active form of the enzyme [21]. In a proof-of-concept study, miglustat increased and prolonged GAA enzyme activity in dried blood spots (DBS) when compared with alglucosidase alfa alone, which the authors concluded is suggestive of a stabilization effect of miglustat on alglucosidase alfa in plasma, as was demonstrated in preclinical studies [20]. Furthermore, building on preclinical data demonstrating that miglustat could bind to, stabilize, and minimize the inactivation of cipaglucosidase alfa [20,22,23,24,25], the safety and effectiveness of cipaglucosidase alfa plus miglustat in adults with Pompe disease was evaluated in the randomized, phase III PROPEL study (NCT03729362) [21]. The two-component miglustat plus cipaglucosidase alfa therapy for Pompe disease was recently approved by the European Commission for treating adults with late-onset Pompe disease (LOPD) and is currently under regulatory review by the US Food and Drug Administration (FDA) [26].
Protein folding and degradation are naturally regulated by endogenous molecules known as molecular chaperones (e.g., the heat shock protein 70 (HSP70) superfamily), which, together with the ubiquitin–proteasome system and autophagy, are central components of protein quality control [27]. Proteostasis regulators (PRs) have been shown to enhance the expression and functions of endogenous molecular chaperones and regulators of the endoplasmic reticulum quality control system to facilitate protein folding and minimize misfolding in vivo [28]. PRs offer an alternative treatment approach for many human diseases associated with altered protein conformation, including LSDs [28]. Arimoclomol, for example, is an orally available small molecule PR that amplifies the heat shock response and production of heat shock proteins to prevent protein misfolding and activate lysosomal function. The efficacy and safety of arimoclomol to target protein misfolding and improve lysosomal function in individuals with NPC is currently being evaluated in a phase II/III clinical trial (clinicaltrials.gov identifier: NCT02612129) [8].
Due to the rapid expansion of various types of small molecule therapies with different mechanisms of action under investigation for LSDs, we conducted a systematic review to examine the available evidence from the literature for preclinical and clinical applications of pharmacological chaperones in the treatment of LSDs. Based on our findings, we propose a new classification for small molecules in the treatment of LSDs that considers differences in therapeutic approach and mechanism of action.

Table 1.
Different therapeutic approaches for treating LSDs and current descriptions of approved therapies (in at least one geography).
Table 1.
Different therapeutic approaches for treating LSDs and current descriptions of approved therapies (in at least one geography).
| Enzyme Replacement Therapy (ERT) | Substrate Reduction Therapy (SRT) | Pharmacological Chaperone Therapy (PCT) | |
|---|---|---|---|
| Molecular or physiological target | Absent or reduced protein function | Metabolic cascade | Endogenous and/or exogenous protein trafficking/stability |
| Mechanism of action | Substitute or addition of missing or deficient endogenous enzyme with exogenously delivered enzyme | Interferes with the abnormalaccumulation of substrate |
|
| Approved therapy | Agalsidase alfa and beta for Fabry disease; alglucosidase alfa, avalglucosidase alfa and cipaglucosidase * for Pompe disease; and velaglucerase and imiglucerase for Gaucher disease [11] | Eliglustat [29] for Gaucher disease and miglustat [30] for Gaucher disease and NPC disease. |
|
* Cipaglucosidase alfa is approved in Europe with the enzyme stabilizer miglustat as a two-component therapy for Pompe disease. Abbreviations: LSDs, lysosomal storage disorders.
2. Materials and Methods
A systematic review was conducted following the general principles published by the UK NHS Centre for Reviews and Dissemination (CRD) and is reported as per the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) reporting guidelines [42]. PubMed® and Embase® via Ovid® databases were searched using the following strategy: terms for pharmacological chaperones utilizing a combination of free-text terms (i.e., searching in the title/abstract) and subject headings (e.g., MeSH in Medline) and standardized study design filters. Each search was adapted using the appropriate syntax for each database platform (Table S1). All databases were searched from inception to 20 February 2023. No date or language restrictions were applied. The search strategies for both databases are available in the supplementary material (Table S1). All search results were imported into reference management software (Covidence https://www.covidence.org/, accessed on 1 July 2023) and de-duplicated using both EndNote’s de-duplication features and manual checks.
2.1. Study Selection
The inclusion criteria were: (a) research papers and reports where pharmacological chaperones in people with LSDs were the main research topic for the study; (b) only papers written in English or where an English language version was available; and (c) study designs including randomized or non-randomized trials, and economic evaluations. Studies were excluded for the following reasons: abstract only, commentary or non-systematic review articles, letters, and editorials. An initial sample of 10% of abstracts (n = 253) was screened independently by two reviewers to pilot the inclusion criteria and ensure consistency prior to undertaking title and abstract screening (inter-rater agreement was 96.4% and discrepancies were resolved by discussion). Duplicate studies were removed. The remaining studies (n = 2877) were single screened. Where the title or abstract met the criteria (or if this was unclear), the full text was retrieved and screened. Full-text screening was undertaken by two reviewers (the inter-rater agreement was 94.2%).
2.2. Data Extraction Process and Included Studies
Data were extracted by one reviewer and checked by a second using a standardized form that included information on the disease, chaperone, deficient enzyme, mechanism of action, study type, study objective, and summary findings. A total of 52 articles were identified for inclusion (refer to the PRISMA study selection flow diagram; Supplementary Material, Figure S1). Figure 1 summarizes the literature evidence by LSD type and evidence type for the identified chaperones.
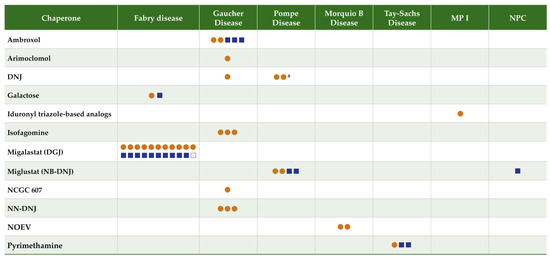
Figure 1.
Literature evidence map for chaperones in lysosomal storage disorders. Orange circles = preclinical studies. Blue squares = clinical studies. Closed squares = one study; open square = 2 articles reporting data from the same study. a One citation reported an analysis including both chaperones N-butyl-deoxynojirimycin (NB-DNJ) and 1-deoxynojirimycin (DNJ), counted twice. Abbreviations: DGJ, 1-deoxygalactonojirimycin/migalastat; DNJ, 1-deoxynojirimycin; NB-DNJ, N-butyl-deoxynojirimycin/miglustat; NN-DNJ, N-Nonyldeoxynojirimycin; NOEV, N-octyl-4-epi-beta-valienamine; NPC, Niemann–Pick Disease Type C; MPSI, Muco-polysaccharidosis 1.
3. Results
3.1. Literature Evidence
Fifty-two articles evaluating 12 chaperones (1-DGJ/migalastat; galactose; isofagomine; arimoclomol; N-Nonyldeoxynojirimycin (NN-DNJ); ambroxol; NCGC607; NB-DNJ/miglustat; 1-DNJ/AT2220/duvoglustat; NOEV; pyrimethamine; iduronyl triazole-based analogs) for the treatment of seven LSDs are included in this review.
3.1.1. Fabry Disease
A total of 25 articles (13 clinical and 12 preclinical studies) were identified evaluating chaperones in Fabry disease, including galactose (two articles; one preclinical and one clinical study) and migalastat (24 articles; 11 preclinical and 12 clinical studies). The split between preclinical and clinical studies was equal. A summary of the included clinical studies for Fabry disease is shown in Table 2 [31,32,33,34,35,36,37,38,39,40,43,44,45]. A summary of the preclinical studies is shown in the Supplementary Material (Table S2) [46,47,48,49,50,51,52,53,54,55,56,57].

Table 2.
Clinical evidence summary: Fabry disease.
3.1.2. Gaucher Disease
A total of 14 articles (three clinical studies) were identified evaluating six chaperones in Gaucher disease: isofagomine (three preclinical articles), arimoclomol (one preclinical article), 1-deoxynojirimycin (DNJ) (one preclinical article), ambroxol (five articles; two preclinical and three clinical studies), NN-DNJ (three preclinical articles), NCGC607 (one preclinical article). Most of the articles were preclinical studies (78.6%). A summary of the included clinical studies for Gaucher disease is shown in Table 3 [58,59,60]. A summary of the preclinical studies is shown in the Supplementary Material (Table S3) [61,62,63,64,65,66,67,68,69,70,71].

Table 3.
Clinical evidence summary: Gaucher disease.
3.1.3. Pompe Disease
A total of six articles (two clinical studies) were identified evaluating chaperones in Pompe disease: NB-DNJ/miglustat (two preclinical articles), DNJ (two preclinical articles; note one article included both NB-DNJ and 1-DNJ), NB-DNJ/miglustat plus ERT (one clinical article), and miglustat plus cipaglucosidase alfa combination (one clinical article). The majority of the articles were preclinical studies. A summary of the included clinical studies for Pompe disease is shown in Table 4 [20,21]. A summary of the preclinical studies is shown in the Supplementary Material (Table S4) [22,72,73,74].

Table 4.
Clinical evidence summary: Pompe disease.
3.1.4. GM1 Gangliosidosis—Morquio B Disease
A total of two articles (no clinical studies) were identified evaluating chaperones in Morquio B disease: N-octyl-4-epi-beta-valienamine (NOEV). A summary of the preclinical studies is shown in the Supplementary Material (Table S5) [75,76].
3.1.5. GM2 Gangliosidosis—Tay–Sachs Disease
A total of three articles (one preclinical study and two clinical studies) were identified evaluating chaperones in Tay–Sachs disease: pyrimethamine (three articles). A summary of the included clinical studies for Tay–Sachs disease is shown in Table 5 [77,78]. A summary of the preclinical studies is shown in the Supplementary Material (Table S6) [79].

Table 5.
Clinical evidence summary: GM2 gangliosidosis—Tay–Sachs disease.
3.1.6. Mucopolysaccharidosis I
One preclinical article was identified evaluating pharmacological chaperones in mucopolysaccharidosis I disease (iduronyl triazole-based analogs). A summary of the preclinical study is shown in the Supplementary Material (Table S7) [80].
3.1.7. NPC
One clinical article was identified evaluating pharmacological chaperones in NPC: miglustat. A summary of the included clinical study for NPC disease is shown in Table 6 [81].

Table 6.
Clinical evidence summary: NPC disease.
3.2. Mechanism of Action and Rationale for Reclassification of Small Molecule Chaperones
The mode of action (MoA) for individual small molecule therapies analyzed in this paper varies and the exact MoA remains to be fully elucidated for many. In Fabry disease, migalastat reversibly binds to the active site of amenable mutations of endogenous agalactosidase and stabilizes the mutant enzyme to facilitate trafficking to the lysosome [16]. Migalastat is described in the literature as having multiple modes of action, including molecular binding and rescue of protein folding, stabilization and ‘’active’’ trafficking, i.e., it ‘‘accompanies’’ the deficient endogenous α-Gal protein as it is transported from the ER through the trans-Golgi network to the lysosomes [16]. In preclinical studies, the expectorant ambroxol showed pH-dependent affinity for the lysosomal hydrolase GCase with decreasing inhibition at lysosomal pH. The exact mode of action of ambroxol in Gaucher disease is unclear [82]. More recently, Pantoom et al. (2022) showed that ambroxol has little in vitro ability to increase the specific activity of GCase [83,84]. Further studies are required to elucidate whether ambroxol plays a regulatory role in the proteostasis network [83,84].
Some small molecules may exert a different MoA in different LSDs. Miglustat acts as a competitive, reversible glucosylceramide synthase inhibitor and has been approved as an SRT in Gaucher disease and NPC [11]. However, in Pompe disease, miglustat, in combination with ERT, has demonstrated potential as a stabilizer of exogenously administered recombinant GAA [20]. Our literature review also identified differences in how chaperones are described and classified. For example, Parenti et al. (2015) classify pharmacological chaperones as small molecule ligands that selectively bind, stabilize, and traffic unstable proteins [16]. However, Frustaci et al. (2001) evaluated the role of exogenously administered galactose infusion therapy for the treatment of an individual with Fabry disease, describing galactose as a ‘‘chemical chaperone’’ able to bind reversibly, stabilize, and assist in the trafficking of endogenous mutant α-Gal from the ER to the lysosome [43]. Notably, the terms chemical or molecular chaperone are typically misapplied in the literature, e.g., these terms have been used to describe endogenous protein chaperones such as HSP70 rather than an exogenously administered pharmacological chaperone that binds to deficient endogenous α-Gal. Abian et al. (2011) used the term ‘’chemical chaperones’’ for small molecules unable to leave the ER [85]. In contrast, Okumiya et al. (2007) described miglustat as a ‘’chemical chaperone’’ that promotes export from the ER to the lysosomes and stabilizes the activity of endogenous mutant GAA species in individuals with Pompe disease [72].
Differences in the MoA of small molecule chaperones identified in the literature and the terminology used to describe them vary significantly and are often contradictory. Moreover, the current term ‘’pharmacological chaperone’’ is inadequate to fully differentiate between the emerging small molecule therapies for LSDs. Therefore, we propose a reclassification to standardize terminology based on the MoA of the small molecule therapy in treating LSDs (Table 7 and Figure 2). The SMC classification system is a simple tool to better describe and categorize existing and future small molecule pharmacological chaperones approved to treat LSDs by type of therapy (i.e., monotherapy or combination), type of action (i.e., chaperone or stabilizer), target enzyme (i.e., exogenous ERT or endogenous mutant), and site of action/trafficking (i.e., intracellular or circulation). The differences between the two proposed SMC groups are depicted in Figure 2. Notably, the SMC classification currently includes two distinct groups and may need to be further revisited as novel MoAs are validated.

Table 7.
The small molecule chaperone (SMC) classification system—a new classification system to describe small molecule chaperones based on their action.
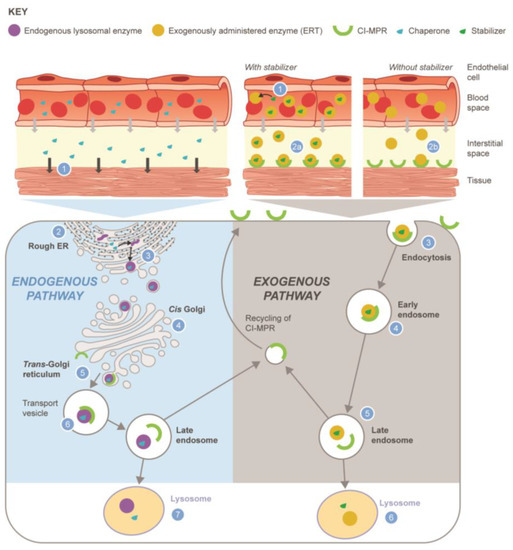
Figure 2.
Reclassification of small molecule chaperones (SMC) into two distinct pathways: monotherapy chaperone (left panel) or combined/co-administered stabilizer (right panel). Chaperone/endogenous lysosomal enzyme (left): (1) Chaperone leaves circulation by paracellular diffusion, enters the cell, and distributes to the ER within target tissues; (2) synthesis of unstable endogenous mutant enzyme protein on the rough ER; (3) chaperone binds and stabilizes endogenous enzyme by facilitating the correct folding required to progress from the ER to the Golgi; (4) enzyme–chaperone complex transported through Golgi; (5) enzyme–chaperone complex phosphorylated and binds to CI-MPR; (6) enzyme–chaperone complex transported through endosomes to lysosome; (7) chaperone dissociates from endogenous enzyme due to low pH and high substrate concentration in the lysosome, resulting in endogenous enzyme being available within lysosome. Stabilizer/exogenously administered lysosomal enzyme (right): (1) Stabilizer binds to exogenously administered enzyme in the blood; (2a) enzyme–stabilizer complex binds to CI-MPR; (2b) without the stabilizer, less active enzyme is available to bind to the CI-MPR; (3) enzyme–stabilizer complex undergoes endocytosis into the muscle cell after binding CI-MPR; (4) enzyme–stabilizer complex transported through endosomes; (5) enzyme–stabilizer complex transported to lysosome; (6) stabilizer dissociates from exogenous enzyme in the lysosome. Abbreviations: CI-MPR, cation-independent mannose 6-phosphate receptor; ER, endoplasmic reticulum; ERT, enzyme replacement therapy.
4. Discussion
This review presents the available literature evidence in relation to the therapeutic role of small molecule chaperones in LSDs. The clinical evidence suggests that small molecule chaperones can be effective and well tolerated. Most of the clinical evidence identified evaluated migalastat for the treatment of Fabry disease. This was expected since the first studies using pharmacological chaperones were conducted for Fabry disease, and migalastat was the first pharmacological chaperone approved for treating individuals with Fabry disease and amenable mutations [11]. Our literature review highlighted a lack of consistency in terminology in articles that describe pharmacological chaperones, which could be attributable to evolution in their development and varying and often unclear MoAs. Therefore, we propose a new classification system to inform a standardized approach, which we have referred to as the SMC classification system.
Preclinical evidence suggested pharmacological chaperones could ‘‘theoretically’’ enhance enzyme activity for all identified LSDs. However, while some mutations for LSDs could respond to small molecule chaperone therapy, amenability will likely continue to be a limiting factor. The systematic literature review identified a wealth of active preclinical research in Fabry disease (n = 12 studies) and Gaucher disease (n = 11 studies). The concept for chaperone-stabilized recombinant enzyme therapy has been explored in preclinical models of Pompe disease. Coadministration of miglustat with cipaglucosidase alfa has been shown to improve rhGAA activity compared to administration of cipaglucosidase alfa alone in GAA knockout mice [22,23]. Miglustat was also shown to increase the circulation half-life of cipaglucosidase alfa in mouse, rat, and monkey models of Pompe disease [25]. When miglustat was administered with cipaglucosidase alfa, the two-component treatment reversed or significantly improved all aspects of the Pompe disease pathogenesis in non-symptomatic GAA-deficient mice with fully developed muscle pathology [25]. These studies suggest that cipaglucosidase alfa plus miglustat can reverse not only the primary defect of Pompe disease, lysosomal glycogen accumulation, but also secondary events resulting from lysosomal dysfunction in the muscle of GAA knockout mice [20,22,23,24,25]. Fewer preclinical studies were identified for rarer LSDs (Pompe disease, n = 4; Morquio B disease, n = 2; Tay–Sachs disease, n = 1; mucopolysaccharidosis I, n = 1; and NPC, n = 0).
The literature identified that chaperone treatment was effective in individuals with Fabry disease with a wide range of genotypes, phenotypes, and disease severity, including those with amenable mutations with multiple organ system involvement at baseline [36,39]. In Fabry disease, chaperone therapy reduced clinical deterioration (i.e., renal, cardiac and neurologic function, pain symptoms, and health status were unchanged), suggesting those on chaperone therapy maintained disease stability [31]. The long-term benefit of chaperone treatment has been demonstrated through maintenance of renal function over ≤8.6 years, irrespective of treatment status, sex, and phenotype in individuals with Fabry disease and amenable mutations [40].
In Pompe disease, the use of miglustat as a stabilizer of rhGAA has been reported, first for alglucosidase alfa [20] and subsequently for cipaglucosidase alfa [21]. The evaluation of miglustat in combination with cipaglucosidase alfa is particularly relevant since this two-component therapy has now received regulatory approval for the treatment of adults with LOPD disease by the EMA and is currently under investigation by the US FDA [26]. Miglustat has been reported to minimize inactivation of cipaglucosidase alfa in the circulation, making more targeted enzyme available to target tissue. A phase III clinical study demonstrated meaningful improvements in musculoskeletal and respiratory endpoints for individuals switching from alglucosidase alfa to cipaglucosidase alfa plus miglustat [21].
Overall, although the clinical studies identified herein show chaperones and/or stabilizers are promising therapeutic tools in LSDs, their success and clinical application are currently limited for most LSDs, except for Fabry disease, and potentially in the future for Pompe disease.
The strengths of this literature review are that the searches were systematic, and two authors reviewed each article. This enhances and adds robustness to the non-systematic approach previously reported in the review published by Ligouri et al. (2020) [86] because systematic reviews typically use more comprehensive search strategies that reduce biases [87]. However, the full text of a few articles could not be accessed, and studies not written in the English language that may have also been relevant to the findings in our literature review were excluded. Additionally, in LSDs, the mechanism of action of some small molecule entities can vary by disease type, e.g., miglustat acts as a substrate reduction therapy in Gaucher disease and as an enzyme stabilizer in Pompe disease. Furthermore, given the specified research question, a quality assessment of the included studies was not conducted. An additional strength is that our results update previous reviews and move the pharmacological chaperone discussion forward by proposing a new classification for emerging small molecule therapies in LSDs.
5. Conclusions
A substantial amount of preclinical data support the potential of small molecule chaperones/stabilizers as treatments for Fabry disease, Gaucher disease, and Pompe disease. However, additional preclinical studies are required for other LSDs, including Morquio B disease, Tay–Sachs disease, mucopolysaccharidosis I, and NPC, to elucidate the mechanisms by which small molecule chaperones/stabilizers could be effective in clinical practice. Most clinical studies identified in this review were with migalastat for Fabry disease, which demonstrated a beneficial effect with respect to disease outcomes.
With approval of the first oral small molecule chaperone migalastat (Galafold®) in Fabry disease, using small molecules to treat other lysosomal storage disorders is a rational approach to explore. Accompanying exogenously administered ERTs with a chaperone/stabilizer, as in the case of cipaglucosidase alfa with miglustat, is another approach that has potential clinical validity in treating LSD individuals. Our study identified an unmet need to understand better in the future the biological pathways that lead to extreme variability in the phenotypes of LSD. Furthermore, our proposed reclassification of small molecule chaperone therapies based on their MoAs will help standardize the terminology of these molecules in clinical development, which will subsequently help researchers, clinicians, and drug developers to focus on and rationally apply these promising approaches in the treatment of people with LSDs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biom13081227/s1. Figure S1: PRISMA figure; Table S1: Literature search strategy and study selection process; Tables S2–S7: Preclinical evidence summary: Fabry disease; Gaucher disease; Pompe disease; GM1 gangliosidosis—Morquio B disease; GM2 gangliosidosis—Tay–Sachs disease; Mucopolysaccharidosis I.
Author Contributions
Substantial contributions to the conception or design of work, I.K., S.S. (Simon Shohet) and L.C.; methodology, I.K., S.S. (Simon Shohet) and L.C.; software, L.C.; validation, I.K. and S.S. (Simon Shohet); acquisition, analysis, and interpretation of data for work, L.C. and K.J.B.; writing—original draft preparation, K.J.B.; interpretation of the data and critical review of the content and conclusions, I.K., S.S. (Simon Shohet), J.C., S.S. (Sheela Sitaraman), B.V.-R., J.M.W., B.F., T.W., S.T., L.C. and K.J.B.; project administration, K.J.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Amicus Therapeutics, Ltd.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the Supplementary Materials described above.
Acknowledgments
With thanks to the team at Prescript Communications Ltd. for research and project management support, and to the team at Comradis (an AMICULUM® agency) for their support with visualization and image creation, funded by Amicus Therapeutics, Inc.
Conflicts of Interest
Ian Keyzor, Simon Shohet, Jeff Castelli, Sheela Sitaraman, Biliana Veleva-Rotse, Jill M. Weimer, Brian Fox, Tobias Willer and Steve Tuske are employees of and hold stock in Amicus Therapeutics. Louise Crathorne and Klara J. Belzar were involved in this project on behalf of Prescript Communications Ltd. There are no other conflict of interest.
References
- Jackson, M.P.; Hewitt, E.W. Cellular proteostasis: Degradation of misfolded proteins by lysosomes. Essays Biochem. 2016, 60, 173–180. [Google Scholar] [CrossRef]
- Miller, J.J.; Kanack, A.J.; Dahms, N.M. Progress in the understanding and treatment of Fabry disease. Biochim. Biophys. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2020, 1864, 129437. [Google Scholar] [CrossRef] [PubMed]
- Consolato, F.; De Fusco, M.; Schaeffer, C.; Pieruzzi, F.; Scolari, F.; Gallieni, M.; Lanzani, C.; Feriozzi, S.; Rampoldi, L. α-Gal A missense variants associated with Fabry disease can lead to ER stress and induction of the unfolded protein response. Mol. Genet. Metab. Rep. 2022, 33, 100926. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, F.; Zhang, J.; Lu, C.; Kong, W. Global epidemiology of Gaucher disease: An updated systematic review and meta-analysis. J. Pediatr. Hematol. Oncol. 2022, 45, 181. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Sud, N.; Hettinghouse, A.; Liu, C.-J. Molecular regulations and therapeutic targets of Gaucher disease. Cytokine Growth Factor. Rev. 2018, 41, 65–74. [Google Scholar] [CrossRef]
- Motabar, O.; Sidransky, E.; Goldin, E.; Zheng, W. Fabry disease—Current treatment and new drug development. Curr. Chem. Genom. 2010, 4, 50–56. [Google Scholar] [CrossRef]
- Meena, N.K.; Raben, N. Pompe disease: New developments in an old lysosomal storage disorder. Biomolecules 2020, 10, 1339. [Google Scholar] [CrossRef]
- Mengel, E.; Patterson, M.C.; Da Riol, R.M.; Del Toro, M.; Deodato, F.; Gautschi, M.; Grunewald, S.; Grønborg, S.; Harmatz, P.; Héron, B.; et al. Efficacy and safety of arimoclomol in Niemann-Pick disease type C: Results from a double-blind, randomised, placebo-controlled, multinational phase 2/3 trial of a novel treatment. J. Inherit. Metab. Dis. 2021, 44, 1463–1480. [Google Scholar] [CrossRef]
- Garbade, S.F.; Zielonka, M.; Mechler, K.; Kölker, S.; Hoffmann, G.F.; Staufner, C.; Mengel, E.; Ries, M. FDA orphan drug designations for lysosomal storage disorders—A cross-sectional analysis. PLoS ONE 2020, 15, e0230898. [Google Scholar] [CrossRef]
- Desnick, R.J.; Schuchman, E.H. Enzyme replacement therapy for lysosomal diseases: Lessons from 20 years of experience and remaining challenges. Ann. Rev. Genom. Hum. Genet. 2012, 13, 307–335. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). Available online: https://www.ema.europa.eu/en (accessed on 30 May 2023).
- Lenders, M.; Brand, E. Fabry disease: The Current Treatment Landscape. Drugs 2021, 81, 635–645. [Google Scholar] [CrossRef]
- McEachern, K.A.; Fung, J.; Komarnitsky, S.; Siegel, C.S.; Chuang, W.-L.; Hutto, E.; Shayman, J.A.; Grabowski, G.A.; Aerts, J.M.; Cheng, S.H.; et al. A specific and potent inhibitor of glucosylceramide synthase for substrate inhibition therapy of Gaucher disease. Mol. Genet. Metab. 2007, 91, 259–267. [Google Scholar] [CrossRef]
- Concolino, D.; Deodato, F.; Parini, R. Enzyme replacement therapy: Efficacy and limitations. Italian J. Pediatr. 2018, 44, 120. [Google Scholar] [CrossRef]
- Banugaria, S.G.; Prater, S.N.; Ng, Y.-K.; Kobori, J.A.; Finkel, R.S.; Ladda, R.L.; Chen, Y.-T.; Rosenberg, A.S.; Kishnani, P.S. The impact of antibodies on clinical outcomes in diseases treated with therapeutic protein: Lessons learned from infantile Pompe disease. Genet. Med. 2011, 13, 729–736. [Google Scholar] [CrossRef]
- Parenti, G.; Andria, G.; Valenzano, K.J. Pharmacological chaperone therapy: Preclinical development, clinical translation, and prospects for the treatment of lysosomal storage disorders. Mol. Ther. 2015, 23, 1138–1148. [Google Scholar] [CrossRef] [PubMed]
- Porto, C.; Ferrara, M.C.; Meli, M.; Acampora, E.; Avolio, V.; Rosa, M.; Cobucci-Ponzano, B.; Colombo, G.; Moracci, M.; Andria, G.; et al. Pharmacological enhancement of α-glucosidase by the allosteric chaperone N-acetylcysteine. Mol. Ther. 2012, 20, 2201–2211. [Google Scholar] [CrossRef] [PubMed]
- Valenzano, K.J.; Khanna, R.; Powe, A.C., Jr.; Boyd, R.; Lee, G.; Flanagan, J.J.; Benjamin, E.R. Identification and characterization of pharmacological chaperones to correct enzyme deficiencies in lysosomal storage disorders. Assay Drug Dev. Technol. 2011, 9, 213–235. [Google Scholar] [CrossRef]
- Wang, Y.J.; Di, X.J.; Mu, T.W. Using pharmacological chaperones to restore proteostasis. Pharmacol. Res. 2014, 83, 3–9. [Google Scholar] [CrossRef]
- Parenti, G.; Fecarotta, S.; la Marca, G.; Rossi, B.; Ascione, S.; Donati, M.A.; Morandi, L.O.; Ravaglia, S.; Pichiecchio, A.; Ombrone, D.; et al. A chaperone enhances blood α-glucosidase activity in Pompe disease patients treated with enzyme replacement therapy. Mol. Ther. 2014, 22, 2004–2012. [Google Scholar] [CrossRef] [PubMed]
- Schoser, B.; Roberts, M.; Byrne, B.J.; Sitaraman, S.; Jiang, H.; Laforêt, P.; Toscano, A.; Castelli, J.; Díaz-Manera, J.; Goldman, M.; et al. Safety and efficacy of cipaglucosidase alfa plus miglustat versus alglucosidase alfa plus placebo in late-onset Pompe disease (PROPEL): An international, randomised, double-blind, parallel-group, phase 3 trial. Lancet Neurol. 2021, 20, 1027–1037. [Google Scholar]
- Porto, C.; Cardone, M.; Fontana, F.; Rossi, B.; Tuzzi, M.R.; Tarallo, A.; Barone, M.V.; Andria, G.; Parenti, G. The pharmacological chaperone N-butyldeoxynojirimycin enhances enzyme replacement therapy in Pompe disease fibroblasts. Mol. Ther. 2009, 17, 964–971. [Google Scholar] [CrossRef]
- Selvan, N.; Venkateswaran, S.; Feng, J.; Madrid, M.; Mehta, N.; Graziano, M. Enhancing delivery of acid alpha-glucosidae (GAA) to skeletal muscle in Pompe disease (PD): Key challenges and attributes of AT-GAA. In Proceedings of the Poster presented at the virtual Muscular Dystrophy Association (MDA) Clinical & Scientific Conference, Dallas, TX, USA, 15–18 March 2021. Poster No. 11. [Google Scholar]
- Xu, S.; Lun, Y.; Frascella, M.; Garcia, A.; Soska, R.; Nair, A.; Ponery, A.S.; Schilling, A.; Feng, J.; Tuske, S.; et al. Improved efficacy of a next-generation ERT in murine Pompe disease. JCI Insight 2019, 4, e125358. [Google Scholar] [CrossRef]
- Meena, N.K.; Ralston, E.; Raben, N.; Puertollano, R. Enzyme Replacement Therapy Can Reverse Pathogenic Cascade in Pompe Disease. Mol. Ther. Methods Clin. Dev. 2020, 18, 199–214. [Google Scholar] [CrossRef]
- Blair, H.A. Cipaglucosidase alfa: First approval. Drugs 2023, 83, 739–745. [Google Scholar] [CrossRef]
- Tedesco, B.; Ferrari, V.; Cozzi, M.; Chierichetti, M.; Casarotto, E.; Pramaggiore, P.; Mina, F.; Galbiati, M.; Rusmini, P.; Crippa, V.; et al. The role of small heat shock proteins in protein misfolding associated motoneuron diseases. Int. J. Mol. Sci. 2022, 23, 11759. [Google Scholar] [CrossRef] [PubMed]
- Calamini, B.; Morimoto, R.I. Protein homeostasis as a therapeutic target for diseases of protein conformation. Curr. Top. Med. Chem. 2012, 12, 2623–2640. [Google Scholar] [CrossRef] [PubMed]
- Mistry, P.K.; Lukina, E.; Ben Turkia, H.; Shankar, S.P.; Feldman, H.B.; Ghosn, M.; Mehta, A.; Packman, S.; Lau, H.; Petakov, M.; et al. Clinical outcomes after 4.5 years of eliglustat therapy for Gaucher disease type 1: Phase 3 ENGAGE trial final results. Am. J. Hematol. 2021, 96, 1156–1165. [Google Scholar] [CrossRef] [PubMed]
- Schiffmann, R.; FitzGibbon, E.J.; Harris, C.; DeVile, C.; Davies, E.H.; Abel, L.; Van Schaik, I.N.; Benko, W.S.; Timmons, M.; Ries, M.; et al. Randomized, controlled trial of miglustat in Gaucher’s disease type 3. Ann. Neurol. 2008, 64, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Riccio, E.; Zanfardino, M.; Ferreri, L.; Santoro, C.; Cocozza, S.; Capuano, I.; Imbriaco, M.; Feriozzi, S.; Pisani, A.; AFFIINITY Group. Switch from enzyme replacement therapy to oral chaperone migalastat for treating fabry disease: Real-life data. Eur. J. Hum. Genet. 2020, 28, 1662–1668. [Google Scholar] [CrossRef]
- Müntze, J.; Gensler, D.; Maniuc, O.; Liu, D.; Cairns, T.; Oder, D.; Hu, K.; Lorenz, K.; Frantz, S.; Wanner, C.; et al. Oral Chaperone Therapy Migalastat for Treating Fabry Disease: Enzymatic Response and Serum Biomarker Changes After 1 Year. Clin. Pharmacol. Ther. 2019, 105, 1224–1233. [Google Scholar] [CrossRef]
- Lenders, M.; Nordbeck, P.; Kurschat, C.; Karabul, N.; Kaufeld, J.; Hennermann, J.B.; Patten, M.; Cybulla, M.; Müntze, J.; Üçeyler, N.; et al. Treatment of fabry’s disease with migalastat: Outcome from a prospective observational multicenter study (FAMOUS). Clin. Pharmacol. Ther. 2020, 108, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Lenders, M.; Nordbeck, P.; Kurschat, C.; Eveslage, M.; Karabul, N.; Kaufeld, J.; Hennermann, J.B.; Patten, M.; Cybulla, M.; Müntze, J.; et al. Treatment of fabry disease with migalastat-outcome from a prospective 24 months observational multicenter study (FAMOUS). Eur. Heart J.-Cardiovasc. Pharmacother. 2021, 8, 272–281. [Google Scholar] [CrossRef]
- Lamari, F.; Mauhin, W.; Koraichi, F.; Khrouf, W.; Bordet, C.; London, J.; Lidove, O.; Charron, P. Strong increase of leukocyte apha-galactosidase A activity in two male patients with Fabry disease following oral chaperone therapy. Mol. Genet. Genom. Med. 2019, 7, e894. [Google Scholar] [CrossRef]
- Hughes, D.A.; Nicholls, K.; Shankar, S.P.; Sunder-Plassmann, G.; Koeller, D.; Nedd, K.; Vockley, G.; Hamazaki, T.; Lachmann, R.; Ohashi, T.; et al. Oral pharmacological chaperone migalastat compared with enzyme replacement therapy in Fabry disease: 18-month results from the randomised phase III ATTRACT study. J Med. Genet. 2017, 54, 288–296. [Google Scholar] [CrossRef]
- Giugliani, R.; Waldek, S.; Germain, D.; Nicholls, K.; Bichet, D.; Simosky, J.; Bragat, A.; Castelli, J.; Benjamin, E.; Boudes, P. A Phase 2 study of migalastat hydrochloride in females with Fabry disease: Selection of population, safety and pharmacodynamic effects. Mol. Genet. Metab. 2013, 109, 86–92. [Google Scholar] [CrossRef]
- Germain, D.P.; Nicholls, K.; Giugliani, R.; Bichet, D.G.; Hughes, D.A.; Barisoni, L.M.; Colvin, R.B.; Jennette, J.C.; Skuban, N.; Castelli, J.P.; et al. Efficacy of the pharmacologic chaperone migalastat in a subset of male patients with the classic phenotype of Fabry disease and migalastat-amenable variants: Data from the phase 3 randomized, multicenter, double-blind clinical trial and extension study. Genet. Med. 2019, 21, 1987–1997. [Google Scholar] [CrossRef]
- Germain, D.P.; Hughes, D.A.; Nicholls, K.; Bichet, D.G.; Giugliani, R.; Wilcox, W.R.; Feliciani, C.; Shankar, S.P.; Ezgu, F.; Amartino, H.; et al. Treatment of Fabry’s Disease with the Pharmacologic Chaperone Migalastat. N. Engl. J. Med. 2016, 375, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Bichet, D.G.; Torra, R.; Wallace, E.; Hughes, D.; Giugliani, R.; Skuban, N.; Krusinska, E.; Feldt-Rasmussen, U.; Schiffmann, R.; Nicholls, K. Long-term follow-up of renal function in patients treated with migalastat for Fabry disease. Mol. Genet. Metab. Rep. 2021, 28, 100786. [Google Scholar] [CrossRef]
- Feldt-Rasmussen, U.; Hughes, D.; Sunder-Plassmann, G.; Shankar, S.; Nedd, K.; Olivotto, I.; Ortiz, D.; Ohashi, T.; Hamazaki, T.; Skuban, N.; et al. Long-term efficacy and safety of migalastat treatment in Fabry disease: 30-month results from the open-label extension of the randomized, phase 3 ATTRACT study. Mol. Genet. Metab. 2020, 131, 219–228. [Google Scholar] [CrossRef]
- PRISMA. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). Available online: http://www.prisma-statement.org/ (accessed on 30 June 2023).
- Frustaci, A.; Chimenti, C.; Ricci, R.; Natale, L.; Russo, M.A.; Pieroni, M.; Eng, C.M.; Desnick, R.J. Improvement in cardiac function in the cardiac variant of Fabry’s disease with galactose-infusion therapy. N. Engl. J. Med. 2001, 345, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Müntze, J.; Lau, K.; Cybulla, M.; Brand, E.; Cairns, T.; Lorenz, L.; Üçeyler, N.; Sommer, C.; Wanner, C.; Nordbeck, P. Patient reported quality of life and medication adherence in Fabry disease patients treated with migalastat: A prospective, multicenter study. Mol. Genet. Metab. 2023, 138, 106981. [Google Scholar] [CrossRef] [PubMed]
- Camporeale, A.; Bandera, F.; Pieroni, M.; Pieruzzi, F.; Spada, M.; Bersano, A.; Econimo, L.; Lanzillo, C.; Rubino, M.; Mignani, R.; et al. Effect of Migalastat on cArdiac Involvement in FabRry Disease: MAIORA study. J. Med. Genet. 2023, 42, ehab724.1744. [Google Scholar] [CrossRef]
- Okumiya, T.; Ishii, S.; Takenaka, T.; Kase, R.; Kamei, S.; Sakuraba, H.; Suzuki, Y. Galactose stabilizes various missense mutants of alpha-galactosidase in Fabry disease. Biochem. Biophys. Res. Commun. 1995, 214, 1219–1224. [Google Scholar] [CrossRef]
- Andreotti, G.; Citro, V.; De Crescenzo, A.; Orlando, P.; Cammisa, M.; Correra, A.; Cubellis, M.V. Therapy of Fabry disease with pharmacological chaperones: From in silico predictions to in vitro tests. Orphanet J. Rare Dis. 2011, 6, 66. [Google Scholar] [CrossRef]
- Asano, N.; Ishii, S.; Kizu, H.; Ikeda, K.; Yasuda, K.; Kato, A.; Martin, O.R.; Fan, J.-Q. In vitro inhibition and intracellular enhancement of lysosomal alpha-galactosidase A activity in Fabry lymphoblasts by 1-deoxygalactonojirimycin and its derivatives. Eur. J. Biochem. 2000, 267, 4179–4186. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.R.; Flanagan, J.J.; Schilling, A.; Chang, H.H.; Agarwal, L.; Katz, E.; Wu, X.; Pine, C.; Wustman, B.; Desnick, R.J.; et al. The pharmacological chaperone 1-deoxygalactonojirimycin increases alpha-galactosidase A levels in Fabry patient cell lines. J. Inherit. Metab. Dis. 2009, 32, 424–440. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.-Q.; Ishii, S.; Asano, N.; Suzuki, Y. Accelerated transport and maturation of lysosomal alpha-galactosidase A in Fabry lymphoblasts by an enzyme inhibitor. Nat. Med. 1999, 5, 112–115. [Google Scholar] [CrossRef]
- Lukas, J.; Giese, A.K.; Markoff, A.; Grittner, U.; Kolodny, E.; Mascher, H.; Lackner, K.J.; Meyer, W.; Wree, P.; Saviouk, V.; et al. Functional characterisation of alpha-galactosidase a mutations as a basis for a new classification system in fabry disease. PLoS Genet. 2013, 9, e1003632. [Google Scholar] [CrossRef]
- Lukas, J.; Cimmaruta, C.; Liguori, L.; Pantoom, S.; Iwanov, K.; Petters, J.; Hund, C.; Bunschkowski, M.; Hermann, A.; Cubellis, M.V.; et al. Assessment of gene variant amenability for pharmacological chaperone therapy with 1-deoxygalactonojirimycin in Fabry disease. Int. J. Mol. Sci. 2020, 21, 956. [Google Scholar] [CrossRef]
- Seemann, S.; Ernst, M.; Cimmaruta, C.; Struckmann, S.; Cozma, C.; Koczan, D.; Knospe, A.-M.; Haake, L.R.; Citro, V.; Bräuer, A.U.; et al. Proteostasis regulators modulate proteasomal activity and gene expression to attenuate multiple phenotypes in Fabry disease. Biochem. J. 2020, 477, 359–380. [Google Scholar] [CrossRef]
- Sugawara, K.; Ohno, K.; Saito, S.; Sakuraba, H. Structural characterization of mutant alpha-galactosidases causing Fabry disease. J. Hum. Genet. 2008, 53, 812–824. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Katz, E.; Della Valle, M.C.; Mascioli, K.; Flanagan, J.J.; Castelli, J.P.; Schiffmann, R.; Boudes, P.; Lockhart, D.J.; Valenzano, K.J.; et al. A pharmacogenetic approach to identify mutant forms of α-galactosidase A that respond to a pharmacological chaperone for Fabry disease. Hum. Mutat. 2011, 32, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Mena-Barragán, T.; Higaki, K.; Johnson, J.L.; Drury, J.E.; Lieberman, R.L.; Nakasone, N.; Ninomiya, H.; Tsukimura, T.; Sakuraba, H.; et al. Molecular basis of 1-deoxygalactonojirimycin arylthiourea binding to human α-galactosidase a: Pharmacological chaperoning efficacy on Fabry disease mutants. ACS Chem. Biol. 2014, 9, 1460–1469. [Google Scholar] [CrossRef]
- Porto, C.; Pisani, A.; Rosa, M.; Acampora, E.; Avolio, V.; Tuzzi, M.R.; Visciano, B.; Gagliardo, C.; Materazzi, S.; la Marca, G.; et al. Synergy between the pharmacological chaperone 1-deoxygalactonojirimycin and the human recombinant alpha-galactosidase A in cultured fibroblasts from patients with Fabry disease. J. Inherit. Metab. Dis. 2012, 35, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Narita, A.; Shirai, K.; Itamura, S.; Matsuda, A.; Ishihara, A.; Matsushita, K.; Fukuda, C.; Kubota, N.; Takayama, R.; Shigematsu, H.; et al. Ambroxol chaperone therapy for neuronopathic Gaucher disease: A pilot study. Ann. Clin. Transl. Neurol. 2016, 3, 200–215. [Google Scholar] [CrossRef] [PubMed]
- Zimran, A.; Altarescu, G.; Elstein, D. Pilot study using ambroxol as a pharmacological chaperone in type 1 Gaucher disease. Blood Cells Mol. Dis. 2013, 50, 134–137. [Google Scholar] [CrossRef]
- Aries, C.; Lohmöller, B.; Tiede, S.; Täuber, K.; Hartmann, G.; Rudolph, C.; Muschol, N. Promising Effect of High Dose Ambroxol Treatment on Neurocognition and Motor Development in a Patient With Neuropathic Gaucher Disease 2. Front. Neurol. 2022, 13, 907317. [Google Scholar] [CrossRef]
- Dasgupta, N.; Xu, Y.-H.; Li, R.; Peng, Y.; Pandey, M.K.; Tinch, S.L.; Liou, B.; Inskeep, V.; Zhang, W.; Setchell, K.D.; et al. Neuronopathic Gaucher disease: Dysregulated mRNAs and miRNAs in brain pathogenesis and effects of pharmacologic chaperone treatment in a mouse model. Hum. Mol. Genet. 2015, 24, 7031–7048. [Google Scholar] [CrossRef]
- Khanna, R.; Benjamin, E.R.; Pellegrino, L.; Schilling, A.; Rigat, B.A.; Soska, R.; Nafar, H.; Ranes, B.E.; Feng, J.; Lun, Y.; et al. The pharmacological chaperone isofagomine increases the activity of the Gaucher disease L444P mutant form of beta-glucosidase. FEBS J. 2010, 277, 1618–1638. [Google Scholar] [CrossRef]
- Sun, Y.; Liou, B.; Xu, Y.-H.; Quinn, B.; Zhang, W.; Hamler, R.; Setchell, K.D.R.; Grabowski, G.A. Ex vivo and in vivo effects of isofagomine on acid β-glucosidase variants and substrate levels in Gaucher disease. J. Biol. Chem. 2012, 287, 4275–4287. [Google Scholar] [CrossRef]
- Mena-Barragán, T.; García-Moreno, M.I.; Sevšek, A.; Okazaki, T.; Nanba, E.; Higaki, K.; Martin, N.I.; Pieters, R.J.; Fernández, J.M.G.; Mellet, C.O. Probing the inhibitor versus chaperone properties of sp2-Iminosugars towards human β-glucocerebrosidase: A picomolar chaperone for Gaucher disease. Molecules 2018, 23, 927. [Google Scholar] [CrossRef] [PubMed]
- Fog, C.K.; Zago, P.; Malini, E.; Solanko, L.M.; Peruzzo, P.; Bornaes, C.; Magnoni, R.; Mehmedbasic, A.; Petersen, N.H.; Bembi, B.; et al. The heat shock protein amplifier arimoclomol improves refolding, maturation and lysosomal activity of glucocerebrosidase. EBioMedicine 2018, 38, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Sawkar, A.R.; Cheng, W.-C.; Beutler, E.; Wong, C.-H.; Balch, W.E.; Kelly, J.W. Chemical chaperones increase the cellular activity of N370S beta-glucosidase: A therapeutic strategy for Gaucher disease. Proc. Natl. Acad. Sci. USA 2002, 99, 15428–15433. [Google Scholar] [CrossRef] [PubMed]
- Sawkar, A.R.; Schmitz, M.; Zimmer, K.-P.; Reczek, D.; Edmunds, T.; Balch, W.E.; Kelly, J.W. Chemical chaperones and permissive temperatures alter localization of Gaucher disease associated glucocerebrosidase variants. ACS Chem. Biol. 2006, 1, 235–251. [Google Scholar] [CrossRef]
- Sawkar, A.R.; Adamski-Werner, S.L.; Cheng, W.-C.; Wong, C.-H.; Beutler, E.; Zimmer, K.-P.; Kelly, J.W. Gaucher disease-associated glucocerebrosidases show mutation-dependent chemical chaperoning profiles. Chem. Biol. 2005, 12, 1235–1244. [Google Scholar] [CrossRef]
- Bendikov-Bar, I.; Maor, G.; Filocamo, M.; Horowitz, M. Ambroxol as a pharmacological chaperone for mutant glucocerebrosidase. Blood Cells Mol. Dis. 2013, 50, 141–145. [Google Scholar] [CrossRef]
- Maegawa, G.H.B.; Tropak, M.B.; Buttner, J.D.; Rigat, B.A.; Fuller, M.; Pandit, D.; Tang, L.; Kornhaber, G.J.; Hamuro, Y.; Clarke, J.T.R.; et al. Identification and characterization of ambroxol as an enzyme enhancement agent for Gaucher disease. J. Biol. Chem. 2009, 284, 23502–23516. [Google Scholar] [CrossRef]
- Aflaki, E.; Borger, D.K.; Moaven, N.; Stubblefield, B.K.; Rogers, S.A.; Patnaik, S.; Schoenen, F.J.; Westbroek, W.; Zheng, W.; Sullivan, P.; et al. A new glucocerebrosidase chaperone reduces α-synuclein and glycolipid levels in iPSC-derived dopaminergic neurons from patients with Gaucher disease and Parkinsonism. J. Neurosci. 2016, 36, 7441–7452. [Google Scholar] [CrossRef]
- Okumiya, T.; Kroos, M.A.; Van Vliet, L.; Takeuchi, H.; Van der Ploeg, A.T.; Reuser, A.J. Chemical chaperones improve transport and enhance stability of mutant alpha-glucosidases in glycogen storage disease type II. Mol. Genet. Metab. 2007, 90, 49–57. [Google Scholar] [CrossRef]
- Flanagan, J.J.; Rossi, B.; Tang, K.; Wu, X.; Mascioli, K.; Donaudy, F.; Tuzzi, M.R.; Fontana, F.; Cubellis, M.V.; Porto, C.; et al. The pharmacological chaperone 1-deoxynojirimycin increases the activity and lysosomal trafficking of multiple mutant forms of acid alpha-glucosidase. Hum. Mutat. 2009, 30, 1683–1692. [Google Scholar] [CrossRef]
- Parenti, G.; Zuppaldi, A.; Pittis, M.G.; Tuzzi, M.R.; Annunziata, I.; Meroni, G.; Porto, C.; Donaudy, F.; Rossi, B.; Rossi, M.; et al. Pharmacological enhancement of mutated alpha-glucosidase activity in fibroblasts from patients with Pompe disease. Mol. Ther. 2007, 15, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, J.; Suzuki, O.; Oshima, A.; Yamamoto, Y.; Noguchi, A.; Takimoto, K.; Itoh, M.; Matsuzaki, Y.; Yasuda, Y.; Ogawa, S.; et al. Chemical chaperone therapy for brain pathology in G(M1)-gangliosidosis. Proc. Natl. Acad. Sci. 2003, 100, 15912–15917. [Google Scholar] [CrossRef]
- Suzuki, Y.; Ichinomiya, S.; Kurosawa, M.; Ohkubo, M.; Watanabe, H.; Iwasaki, H.; Matsuda, J.; Noguchi, Y.; Takimoto, K.; Itoh, M.; et al. Chemical chaperone therapy: Clinical effect in murine G(M1)-gangliosidosis. Ann. Neurol. 2007, 62, 671–675. [Google Scholar] [CrossRef]
- Clarke, J.T.; Mahuran, D.J.; Sathe, S.; Kolodny, E.H.; Rigat, B.A.; Raiman, J.A.; Tropak, M.B. An open-label Phase I/II clinical trial of pyrimethamine for the treatment of patients affected with chronic GM2 gangliosidosis (Tay-Sachs or Sandhoff variants). Mol. Genet. Metab. 2011, 102, 6–12. [Google Scholar] [CrossRef]
- Osher, E.; Fattal-Valevski, A.; Sagie, L.; Urshanski, N.; Sagiv, N.; Peleg, L.; Lerman-Sagie, T.; Zimran, A.; Elstein, D.; Navon, R.; et al. Effect of cyclic, low dose pyrimethamine treatment in patients with Late Onset Tay Sachs: An open label, extended pilot study. Orphanet J. Rare Dis. 2015, 10, 45. [Google Scholar] [CrossRef]
- Maegawa, G.H.B.; Tropak, M.; Buttner, J.; Stockley, T.; Kok, F.; Clarke, J.T.R.; Mahuran, D.J. Pyrimethamine as a potential pharmacological chaperone for late-onset forms of GM2 gangliosidosis. J. Biol. Chem. 2007, 282, 9150–9161. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.-C.; Lin, C.-K.; Li, H.-Y.; Chang, Y.-C.; Lu, S.-J.; Chen, Y.-S.; Chang, S.-Y. A combinatorial approach towards the synthesis of non-hydrolysable triazole-iduronic acid hybrid inhibitors of human α-l-iduronidase: Discovery of enzyme stabilizers for the potential treatment of MPSI. Chem. Commun. 2018, 54, 2647–2650. [Google Scholar] [CrossRef] [PubMed]
- Patterson, M.C.; Vecchio, D.; Prady, H.; Abel, L.; Wraith, J.E. Miglustat for treatment of Niemann-Pick C disease: A randomised controlled study. Lancet Neurol. 2007, 6, 765–772. [Google Scholar] [CrossRef]
- Lukas, J.; Pockrandt, A.-M.; Seemann, S.; Sharif, M.; Runge, F.; Pohlers, S.; Zheng, C.; Gläser, A.; Beller, M.; Rolfs, A.; et al. Enzyme enhancers for the treatment of Fabry and Pompe disease. Mol. Ther. 2015, 23, 456–464. [Google Scholar] [CrossRef]
- Pantoom, S.; Hules, L.; Schöll, C.; Petrosyan, A.; Monticelli, M.; Pospech, J.; Cubellis, M.V.; Hermann, A.; Lukas, J. Mechanistic insight into the mode of action of acid β-glucosidase enhancer ambroxol. Int. J. Mol. Sci. 2022, 23, 3536. [Google Scholar] [CrossRef]
- Ciana, G.; Dardis, A.; Pavan, E.; Da Riol, R.M.; Biasizzo, J.; Ferino, D.; Zanatta, M.; Boni, A.; Antonini, L.; Crichiutti, G.; et al. In vitro and in vivo effects of ambroxol chaperone therapy in two Italian patients affected by neuronopathic Gaucher disease and epilepsy. Mol. Genet. Metab. Rep. 2020, 25, 100678. [Google Scholar] [CrossRef]
- Abian, O.; Alfonso, P.; Velazquez-Campoy, A.; Giraldo, P.; Pocovi, M.; Sancho, J. Therapeutic strategies for Gaucher disease: Miglustat (NB-DNJ) as a pharmacological chaperone for glucocerebrosidase and the different thermostability of velaglucerasealfa and imiglucerase. Mol. Pharm. 2011, 8, 2390–2397. [Google Scholar] [CrossRef] [PubMed]
- Liguori, L.; Monticelli, M.; Allocca, M.; Mele, B.H.; Lukas, J.; Cubellis, M.V.; Andreotti, G. Pharmacological chaperones: A therapeutic approach for diseases caused by destabilizing missense mutations. Int. J. Mol. Sci. 2020, 21, 489. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.J.; Mulrow, C.D.; Haynes, R.B. Systematic reviews: Synthesis of best evidence for clinical decisions. Ann. Intern. Med. 1997, 126, 376–380. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).