Abstract
Soil salinity is the major limiting factor restricting plant growth and development. Little is known about the comparative and combined effects of gibberellic acid (GA3) seed priming and foliar application on maize under salt stress. The current study determined the impact of different application methods of GA3 on morpho-physiological, biochemical and molecular responses of maize seedlings under three salinity stress treatments (no salinity, moderate salinity-6 dS m−1, and severe salinity-12 dS m−1). The GA3 treatments consisted of control, hydro-priming (HP), water foliar spray (WFS), HP + WFS, seed priming with GA3 (GA3P, 100 mg L−1), foliar spray with GA3 (GA3FS, 100ppm) and GA3P + GA3FS. Salt stress particularly at 12 dS m−1 reduced the length of shoots and roots, fresh and dry weights, chlorophyll, and carotenoid contents, K+ ion accumulation and activities of antioxidant enzymes, while enhanced the oxidative damage and accumulation of the Na+ ion in maize plants. Nevertheless, the application of GA3 improved maize growth, reduced oxidative stress, and increased the antioxidant enzymes activities, antioxidant genes expression, and K+ ion concentration under salt stress. Compared with control, the GA3P + GA3FS recorded the highest increase in roots and shoots length (19–37%), roots fresh and dry weights (31–43%), shoots fresh and dry weights (31–47%), chlorophyll content (21–70%), antioxidant enzymes activities (73.03–150.74%), total soluble protein (13.05%), K+ concentration (13–23%) and antioxidants genes expression levels under different salinity levels. This treatment also reduced the H2O2 content, and Na+ ion concentration. These results indicated that GA3P + GA3FS could be used as an effective tool for improving the maize growth and development, and reducing the oxidative stress in salt-contaminated soils.
1. Introduction
Maize (Zea mays L.) is a staple cereal crop, cultivated throughout the world for its usage as forage, and food grains for human and animal feed. It also provides raw materials to various industries [1]. Maize crop is subjected to various abiotic stresses under field conditions, such as soil salinity, drought, light and temperature, which may lead to a severe decline in its productivity [2]. Among abiotic stresses, soil salinity is one of the major factors limiting crop growth and productivity. It is estimated that about 6% of the total arable land worldwide is affected by soil salinity [3]. Soil salinity not only decreases the seed emergence and germination rates but also severely reduces the growth, development and yield of field crops [4,5]. Also, high salt concentrations in the soil lead to the closure of stomata, and damage photosynthetic machinery and chlorophyll content [6,7,8]. In plants, reactive oxygen species (ROS) are continuously produced as a result of metabolic activities, with more production under environmental stress stimuli [4]. The increase in ROS production can lead to the destruction of lipids, membranes, nucleic acids and proteins, and results in malfunction of cellular machinery [9,10,11]. The overproduction of ROS under salt stress also imposes a disturbance in ion balance [12,13]. In order to reduce the oxidative damage, plants have developed an effective antioxidant defense system comprising of antioxidant enzymes like superoxide dismutase (SOD), peroxidase (POD), ascorbate peroxidase (APX), catalase (CAT) [14].
Gibberellic acid (GA3) application can help to improve plant growth and development under salt stress as it improves the pigment content [15] and reduces the Na+ concentration in shoots and roots [16]. The application of GA3 through seed priming reduces the emergence time by increasing the water absorption and metabolic activities in seeds [17]. When applied at low concentrations, GA3 uplifts the seed dormancy, increases plant growth and overall plant productivity [18]. Gibberellic acid increases the growth of root, shoot and number of leaves by altering the process of cell division and cell elongation [19]. In maize, GA3 application caused a remarkable increase in total chlorophyll contents under salinity [20]. Under salt stress, GA3 application enhanced the dry matter production and plant growth of wheat because of increased photosynthetic activities [21]. In another study, seed priming with GA3 significantly improved the plant height, yield and yield-related traits, Ca2+ and K+ concentrations, and transpiration rates while decreased Na+ concentrations in wheat under salt stress [15]. Although, several studies in recent years have discussed the beneficial role of GA3 application (either as seed priming or foliar spray) for the different field crops including maize [20] wheat [21,22] and rice [23], yet little is known regarding the comparative and combined effects of GA3-seed priming and GA3-foliar spray on morpho-physiological, biochemical and molecular responses of maize seedlings under salinity stress. It was hypothesized that GA3, when applied as combine foliar and seed priming, may effectively alleviate the salinity stress in maize by reducing oxidative stress damage and increasing plant growth and biomass production. The specific objectives of the present study were (a) to investigate the impact of GA3 as a foliar spray and/or seed priming on the growth, physiological parameters, and ionic homeostasis in maize under salinity stress, and (b) to underpin the mechanism of GA3-induced salt stress tolerance in maize.
2. Materials and Methods
2.1. Plant Material and Experimental Site
The seeds of maize cultivar (Hybrid-30T60) were obtained from the Maize Research Institute, Ayub Agricultural Research Institute, Faisalabad, Pakistan. Experimental soil used in pots contained 2.54 g kg−1 soil organic carbon, 45.05 mmol L−1 total soluble salts, 30.89 mmol L−1 sodium (Na+), 0.45 mmol L−1 potassium (K+) and 20.88 mmol L−1 chloride (Cl-) content. The experiment was carried out in the greenhouse at the Old Botanical Garden University of Agriculture Faisalabad, Pakistan (longitude = 73°05′ E, latitude = 31°44′ N and 184.4 m above sea level) during winter season (December 2018–January 2019).
2.2. Experimentation
The pots, having 25 cm depth and 15.25 cm diameter, were filled with 7 kg of well sieved soil. Fertilizers were thoroughly mixed in the soil at the equivalent of 2.50 g N pot−1, 1.4 P2O5 pot−1 and 0.9 g K2O pot−1 (250: 140: 90 kg NPK hectare−1, respectively). Maize seeds (hybrid-30T60) were disinfected and sown on 12 December 2018, and seeds in each pot were equally spaced. The irrigation was applied on daily basis to maintain the field capacity of the soil at 80%. The weeds from each pot were eliminated manually.
2.3. Treatments and Experimental Design
This experiment consisted of two factors: salinity and gibberellic acid (GA3) application. Salt stress was imposed by sodium chloride, and three treatments were as follows: (1) No salinity; (2) moderate salinity (6 dS m−1); (3) and severe salinity (12 dS m−1). A procedure of USDA Laboratory staff (1954) was followed to maintain the required salt level. The salinity was imposed prior to sowing during the filling the pots. There were seven treatments for GA3 application which included a control; hydro-priming (HP); water foliar spray (WFS); HP + WFS; seed priming with GA3 (GA3P, 100 mg L−1); foliar spray with GA3 (GA3FS, 100 ppm); and GA3P + GA3FS. For seed priming, maize seeds were soaked at room temperature in distilled water (for hydro-priming) and in GA3 solution (100 mg L−1), using seed weight to solution volume ratio of 1:5. After 12 h, seeds were thoroughly washed with distilled water thrice and re-dried to their original weight. Treatments of WFS (distilled water) and GA3FS (100 ppm) were imposed at 15 DAS in equal volume (50 mL/pot). In total, there were 21 treatments with three replications, and the treatments were arranged in a completely randomized design (CRD). Ten days after the foliar application of gibberellic acid, the plants were harvested for measuring the observed traits.
2.4. Measurement of Plant Growth Attributes
At 25 DAS, the plants were harvested to measure the root and shoot length, fresh and dry weights, leaf length, and leaf width. A calibrated meter rod was used to measure the seedling length. After measuring the fresh weight, the roots and shoots were dried in the sun for 96 h, and then kept in an electric oven at 70 °C until constant weight, and the dry weight was calculated with an electric balance.
2.5. Determination of Photosynthetic Pigments
The chlorophyll (chlorophyll a (Chla), chlorophyll b (Chlb), Chla + Chlb, and carotenoid contents were determined by the method suggested by Arnon [24] with some modifications. The 0.1 g of fresh and healthy leaves were crushed, and extraction was done with 5 mL of acetone (80%). The extract was transferred to a test tube and left in the dark for 24 h. The absorbance of the supernatant was recorded at wavelengths 663, 645, and 480 nm by using a spectrophotometer. The chlorophyll and carotenoid contents were computed by following these equations and expressed in mg g−1 fresh weight:
where, A663 and A645 are the corresponding wavelengths of light density values, Acar = OD 480 + 0.114 (OD663) − 0.638 (OD645) and Em = 2500.
Chla = [(0.0127 × A663 − 0.00269 × A645) × 100]/0.5
Chlb = [(0.0229 × A645 − 0.00468 × A663) × 100]/0.5
Carotenoids = Acar/Em × 100
2.6. Determination of Hydrogen Peroxide (H2O2)
The H2O2 content was evaluated spectrophotometrically [14]. In a pre-chilled mortar and pestle, about 0.5 g of fresh leaf tissues were ground in 0.1% Trichloroacetic acid (TCA). The extract was then centrifuged at 12,000 rpm for 15 min. The reaction sample composed of 0.5 mL of supernatant, 1 mL of 1M potassium iodide (KI), and 0.5 mL of potassium phosphate buffer was used, and the absorbance was recorded at 390 nm wavelength.
2.7. Determination of Antioxidant Enzymes Activities
Fresh leave tissues (0.25 g) were grounded in 5 mL of potassium phosphate buffer (pH 7.8) with a mortar and pestle and then centrifuged at 15,000 rpm. The activity of catalase (CAT) and peroxidase (POD) enzymes was measured by the method of Chance [25]. For the CAT enzyme, a reaction mixture comprised of 1.9 mL of phosphate buffer (50 mM), 100 µL of extract sample, and 100 µL of H2O2 was prepared, and the absorbance was recorded on a spectrophotometer at a wavelength of 240 nm. For POD activity, a reaction mixture comprised of 750 µL of phosphate buffer (50 mM), 100 µL of H2O2, 10 µL of guaiacol, and 50 µL sample was used, and the absorbance was recorded at 470 nm. The activity of superoxide dismutase was measured spectrophotometrically [26] using the Nitro blue tetrazolium (NBT). The reaction mixture of 250 µL of phosphate buffer (7.8 pH), 100 µL of triton, 50 µL of NBT, 100 µL of L-methionine, 400 µL of distilled water, 50 µL of riboflavin, and 50 µL of the extract was prepared, and the absorbance was recorded at 560 nm.
2.8. Analysis of Antioxidant Genes Expression
The antioxidant gene expression levels (i.e., SOD, POD, and CAT) were evaluated in maize plants by quantitative real-time PCR analysis. Total RNA and cDNA were extracted and synthesized from plant tissues using the RNeasy Plant Mini kit and Reverse Transcription kit (Qiagen, Germany), respectively. Using the protocol of QuantiTect SYBR Green PCR kit (Qiagen, Germany), PCR reactions were performed in triplicates. Amplification procedures were done as follows: 95 °C for 10 min; 40 cycles of 95 °C for 20 s, 60 °C for 30 s, 72 °C for 2 min, 72 °C for 4 min. A melting-curve analysis was assayed to check the amplification specificity. SOD, POD and CAT genes primers [27,28] were used for amplification. Actin2 was assayed as a reference gene [28] and the relative gene expression levels were estimated using the 2−ΔΔCt method.
2.9. Total Soluble Proteins Determination
The total soluble protein was determined by using the method of Bradford [29]. About 0.5 g of fresh leaves were grounded in 10 mL of 50 mM potassium phosphate buffer [K2HPO4 + KH2PO4] in the ice bath. The extract was then centrifuged at 10,000 rpm for 15 min at 4 °C. The absorbance mixture containing 100 mL of H2PO4, 5 μL of extract sample, 95 μL of NaCl, and 1 mL of Bradford dye was prepared, and the absorbance was recorded at 595 nm with a spectrophotometer.
2.10. Estimation of Phenolic Contents
In this study, the total phenolic contents were determined according to Ainsworth and Gillespie [30]. About 0.5 g leaves were extracted in 5 mL of 80% acetone, and after filtration, the volume was increased to 10 mL by using acetone. The reaction mixture comprised 20 μL of sample solution, 1.58 mL of water, and 100 μL of Folin-Ciocalteu reagents. The reaction sample was then mixed with 300 μL of sodium carbonate and kept at 40 °C for 30 min. The absorbance of the reaction sample was recorded at 760 nm using a spectrophotometer and the phenolic content was computed using the standard curve stated by Ainsworth and Gillespie [30].
2.11. Mineral Ions Determination (Na+, K+ and Ca2+) in Plant Tissues
In this study, the Flame photometer (flame photometer 410) was used to determine the mineral ions (Na+, K+, and Ca2+) in plant tissues [31]. Digest about 0.1 g dried sample in 2 mL H2SO4 in the digestion flask, and then placed the sample at room temperature for overnight. The samples were then heated at 150 °C by using the hot plate. The samples were grinded in 2 mL of H2O2 and use distilled water to increase the volume to 50 mL. Then, sodium (Na+), potassium (K+) and calcium (Ca2+) ions were determined by using a Flame photometer (Flame photometer 410).
2.12. Statistical Analysis
The growth, physiological and biochemical observations were proceeded to Microsoft Excel for the calculation of mean values and standard deviation. The ANOVA was used to statistically analyze the data, and multiple comparisons were performed using the least significant difference test at p-value of less than 5% (p < 0.05) to compare the treatment means [32] using Statistix 8.1. SigmaPlot was used for graphical presentation.
3. Results
3.1. Fresh and Dry Weight of Roots and Shoots
Plant growth significantly differed with gibberellic acid (GA3) application under salinity stress (Figure 1; Table S1). Salt stress significantly reduced the fresh and dry weight of roots and shoots as compared to no salinity treatment control (Figure 1). As compared to the control, moderate and severe salinity treatments reduced the shoot fresh weight by 34.27% and 102.59%, the shoot dry weight by 6.48% and 82.07%, the root fresh weight by 6.76% and 68% and root dry biomass by 12.84% and 39.93%, respectively. The application of GA3 significantly increased the fresh and dry weight of roots and shoots than control (Figure 1). Under severe salinity, GA3 priming with foliar spray (GA3P + GA3FS) increased the shoot fresh and dry weight, root fresh weight and dry weight by 138.71%, 232.89%, 145.08% and 67.19%.
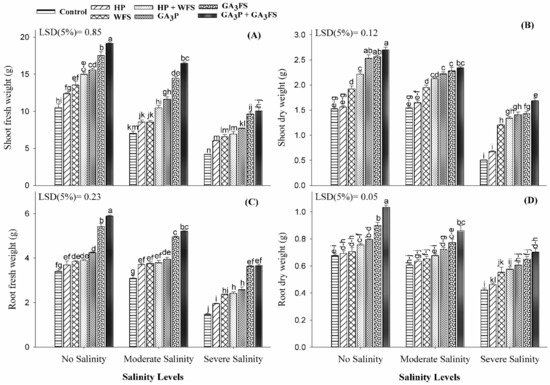
Figure 1.
Shoot fresh weight (A), shoot dry weight (B), root fresh weight (C) and root dry weight (D) of maize as affected by different treatments of gibberellic acid under no salinity (control), moderate salinity (6 dS m−1), and severe salinity (12 dS m−1). Values are means ± SD (n = 3). Hydropriming (HP); water foliar spray (WFS); hydropriming and water foliar spray (HP + WFS); seed priming with GA3 (GA3P); foliar spray with GA3 (GA3FS); seed priming and foliar spray of GA3 (GA3P + GA3FS). Bars with the same letters do not differ significantly at p ≤ 0.05.
3.2. Seedling Length and Leaves Growth
When plants were exposed to salinity stress, shoot and root length, maximum leaf length and width were reduced significantly, but the application of GA3 alleviated the damage caused by salinity stress (Figure 2). Plants treated with GA3 had a higher shoot and root length, and maximum leaf length and width compared to the control; the maximum values were reported for GA3P + GA3FS treatment. Under severe salinity, GA3P + GA3FS application increased the shoot and root length, maximum leaf length and width by 96.86%, 251.95%, 74.65% and 59.63%, respectively.
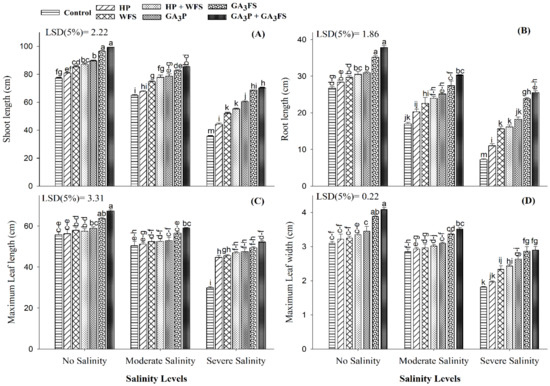
Figure 2.
Shoot length (A), root length (B), maximum leaf length (C) and maximum leaf width (D) of maize as affected by different treatments of gibberellic acid under no salinity (control), moderate salinity (6 dS m−1), and severe salinity (12 dS m−1). Values are means ± SD (n = 3). Hydropriming (HP); water foliar spray (WFS); hydropriming and water foliar spray (HP + WFS); seed priming with GA3 (GA3P); foliar spray with GA3 (GA3FS); seed priming and foliar spray of GA3 (GA3P + GA3FS). Bars with the same letters do not differ significantly at p ≤ 0.05.
3.3. Chlorophyll and Carotenoid Content
Salinity stress significantly reduced the Chla, Chlb and total chlorophyll content in maize leaves (Figure 3A–C; Table S1). Under severe salinity, Chla, Chlb and total chlorophyll were decreased by 17.06%, 29.66% and 20.10% respectively compared with no salinity treatment. The application of GA3 significantly increased the chlorophyll content compared with the corresponding treatments without GA3 application; the maximum increase in chlorophyll content was recorded by GA3P + GA3FS. Under severe salinity, GA3P + GA3FS increased the Chla, Chlb and total chlorophyll by 58.73%, 139.75% and 77.84%, respectively compared to control plants. Overall, the positive influence of GA3 treatments for chlorophyll content was in the order of GA3P + GA3FS > GA3FS > GA3P > HP + WFS > WFS > HP. Interestingly, foliar spray of water also significantly improved the chlorophyll content as compared with hydropriming and control plants, even under non-stressed conditions.
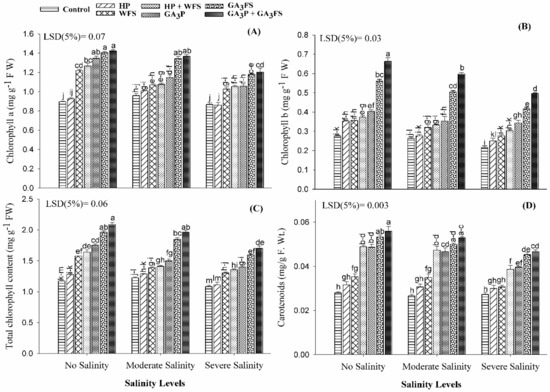
Figure 3.
Chlorophyll a (A), chlorophyll b (B), total chlorophyll (C) and carotenoid content (D) of maize as affected by different treatments of gibberellic acid under no salinity, moderate salinity (6 dS m−1), and severe salinity (12 dS m−1). Values are means ± SD (n = 3). Hydropriming (HP); water foliar spray (WFS); hydropriming and water foliar spray (HP + WFS); seed priming with GA3 (GA3P); foliar spray with GA3 (GA3FS); seed priming and foliar spray of GA3 (GA3P + GA3FS). Bars with the same letters do not differ significantly at p ≤ 0.05.
Salt stress decreased the carotenoid content than the control (Figure 3D). Under severe salinity, carotenoid content was 16.90% lower than the non-stressed plants. The GA3 application significantly increased the carotenoid content than respective treatments without GA3 application. Under severe salinity, GA3FS and GA3P + GA3FS increased the carotenoid content by 65.86% and 70.73% respectively compared with the control.
3.4. Hydrogen Peroxide (H2O2) Concentration
Salinity stress enhanced the H2O2 concentration in maize leaves (Figure 4). The highest concentration was observed in severe salinity without GA3 application which was 12% higher than non-stressed plants. The GA3 application significantly (p < 0.05) reduced the H2O2 content than respective hydro-treatments and control. Under severe salinity, the maximum reduction was reported with GA3P + GA3FS treatment, which decreased the H2O2 content by 85.84% than control.
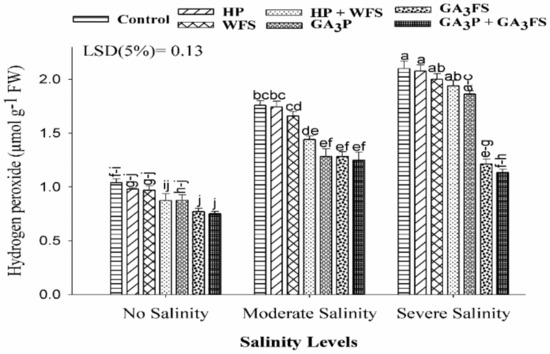
Figure 4.
Hydrogen peroxide (H2O2) content in maize leaves as affected by different treatments of gibberellic acid under no salinity, moderate salinity (6 dS m−1), and severe salinity (12 dS m−1). Values are means ± SD (n = 3). Hydropriming (HP); water foliar spray (WFS); hydropriming and water foliar spray (HP + WFS); seed priming with GA3 (GA3P); foliar spray with GA3 (GA3FS); seed priming and foliar spray of GA3 (GA3P + GA3FS). Bars with the same letters do not differ significantly at p ≤ 0.05.
3.5. Antioxidant Activities
The application of GA3 and salt stress significantly affected the activities of SOD, POD, and CAT in maize leaves (Figure 5; Table S1).
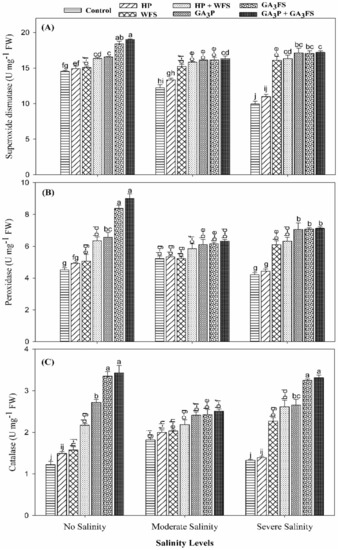
Figure 5.
Superoxide dismutase (A), peroxidase (B) and catalase (C) activity in maize leaves as affected by different treatments of gibberellic acid under no salinity (control), moderate salinity (6 dS m−1), and severe salinity (12 dS m−1). Values are means ± SD (n = 3). Hydropriming (HP); water foliar spray (WFS); hydropriming and water foliar spray (HP + WFS); seed priming with GA3 (GA3P); foliar spray with GA3 (GA3FS); seed priming and foliar spray of GA3 (GA3P + GA3FS). Bars with the same letters do not differ significantly at p ≤ 0.05.
Salinity stress caused a marked reduction in the activities of SOD, POD and CAT compared with the unstressed treatment. Nonetheless, GA3 application significantly (p < 0.05) increased the activities of antioxidant enzymes under salinity as well as the unstressed conditions. At moderate salinity, GA3 application increased the activities of SOD, POD and CAT in the range of 12.23–38.12% relative to the control plants. Under severe salinity, the maximum increase in SOD, POD and CAT enzyme activities was reported for GA3P + GA3FS, which were 73.03%, 69.52% and 150.74% than respective control, however, these values were statistically similar with GA3FS treatments.
3.6. Expression Analysis of Antioxidant Genes
The application of GA3 and salt stress significantly affected the expression of antioxidant genes (SOD, POD, and CAT) in maize leaves (Figure 6).
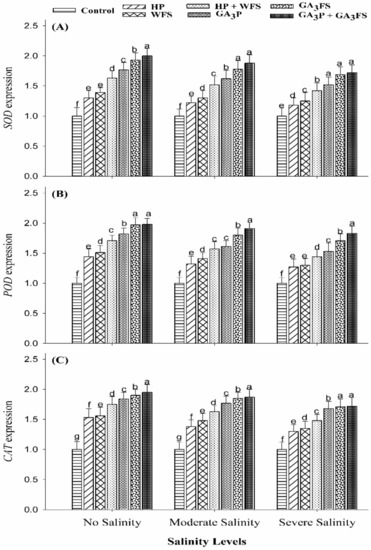
Figure 6.
Expression level of SOD gene (A), POD gene (B) and CAT gene (C) in maize leaves as affected by different treatments of gibberellic acid under no salinity (control), moderate salinity (6 dS m−1), and severe salinity (12 dS m−1). Values are means ± SD (n = 3). Hydropriming (HP); water foliar spray (WFS); hydropriming and water foliar spray (HP + WFS); seed priming with GA3 (GA3P); foliar spray with GA3 (GA3FS); seed priming and foliar spray of GA3 (GA3P + GA3FS). Bars with the same letters do not differ significantly at p ≤ 0.05.
Severe salinity stress caused a marked decrease in the expression levels of antioxidant genes (SOD, POD, and CAT) compared with unstressed plants (Figure 6). Nonetheless, GA3 application significantly (p < 0.05) enhanced the expression levels of antioxidant genes particularly under moderate and severe salinity. Under severe salinity, the maximum increase in the expression levels of antioxidant genes (SOD, POD, and CAT) was recorded for GA3P + GA3FS (Figure 6).
3.7. Total Soluble Protein and Total Phenolics
Salinity stress significantly affected the total soluble protein contents in maize leaves as compared with unstressed plants (Figure 7A). The total soluble protein was increased significantly under GA3 application, the maximum values were recorded for GA3P + GA3FS indicating that the application of GA3 as seed priming and foliar spray effectively enhance the soluble protein under salinity stress. Salinity stress significantly reduced the total phenolic content in maize leaves, and the maximum reduction was found under severe salinity (Figure 7B). Under severe salinity, GA3P + GA3FS increased the phenolic content by 70.72% compared with the respective control. Averaged across different stress treatment, the increase in phenolics contents was in the order of GA3P + GA3FS > GA3FS > GA3P > HP + WFS > WFS > HP. Interestingly, foliar water spray also significantly increased the total phenolic content as compared to control plants, even under non-stressed conditions.
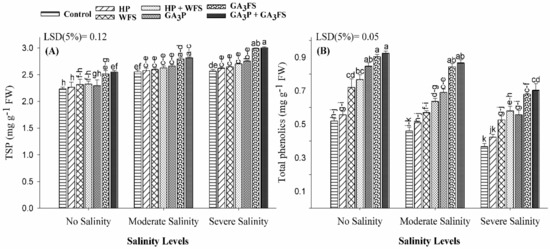
Figure 7.
Total soluble protein-TSP (A) and total phenolic content (B) of maize as affected by different treatments of gibberellic acid under no salinity (control), moderate salinity (6 dS m−1), and severe salinity (12 dS m−1). Values are means ± SD (n = 3). Hydropriming (HP); water foliar spray (WFS); hydropriming and water foliar spray (HP + WFS); seed priming with GA3 (GA3P); foliar spray with GA3 (GA3FS); seed priming and foliar spray of GA3 (GA3P + GA3FS). Bars with the same letters do not differ significantly at p ≤ 0.05.
3.8. Mineral Ions (Na+, K+ and Ca2+) Concentrations
Salinity stress and GA3 application exhibited a significant effect on Na+ and K+ ion concentrations in different parts of maize plants (Figure 8; Table S1). Salt stress significantly increased the Na+ ion concentration in the roots and leaves as compared to unstressed plants. Severe salinity caused the highest increase in Na+ ions in roots and leaves, which was about 30.22% and 47.43%, higher than unstressed treatment, respectively. The application of GA3 significantly reduced the Na+ ion concentrations in roots and leaves than the control. Under severe salinity, GA3P, GA3FS and GA3P + GA3FS lowered the Na+ ion concentration in roots by 16.50%, 37.93% and 41.17%, respectively compared to the control. Similarly, GA3 significantly reduced the Na+ ion concentration in leaves, with maximum reduction under GA3P + GA3FS, which was 31.56% lower than control under severe salinity stress. Salinity stress reduced the accumulation of K+ ion in different plant parts; the lowest concentration of K+ ion in roots and shoots was found under severe salinity. The application of GA3 significantly increased the concentration of K+ ions in shoots and roots, with the maximum increase in GA3P + GA3FS compared with control. Under severe salinity stress, GA3P + GA3FS increased the concentration of K+ ions in roots and shoots by 54.38% and 20.13%, respectively than the respective control.
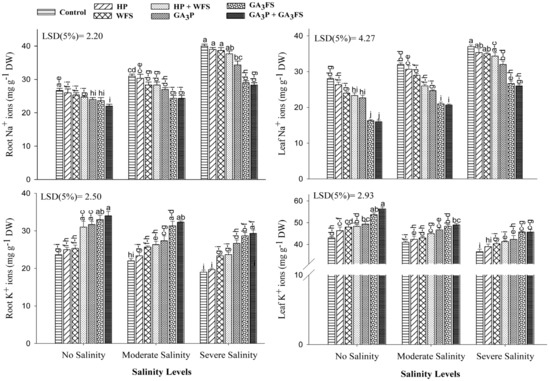
Figure 8.
Sodium (Na+) and potassium (K+) ion concentration in different maize parts as affected by different treatments of gibberellic acid under no salinity (control), moderate salinity (6 dS m−1), and severe salinity (12 dS m−1). Values are means ± SD (n = 3). Hydropriming (HP); water foliar spray (WFS); hydropriming and water foliar spray (HP + WFS); seed priming with GA3 (GA3P); foliar spray with GA3 (GA3FS); seed priming and foliar spray of GA3 (GA3P + GA3FS). Bars with the same letters do not differ significantly at p ≤ 0.05.
In this study, a non-significant difference (p > 0.05) for Ca2+ ion in maize shoot was observed among salt stress and GA3 treatments (Figure 9A; Table S1). However, salinity and GA3 treatments significantly influenced the Ca2+ ions in maize roots. Salinity stress significantly decreased the accumulation of Ca2+ ions in maize roots compared to the unstressed treatment, and the maximum reduction was recorded under severe salinity (Figure 9B). The GA3-treated plants had higher concentrations of Ca2+ ion in roots as compared with non-GA treated plants. Under moderate and severe salinity, GA3P + GA3FS increased the concentration of Ca2+ ions in maize roots by 59.25% and 28.57% than their respective control (Figure 9B).
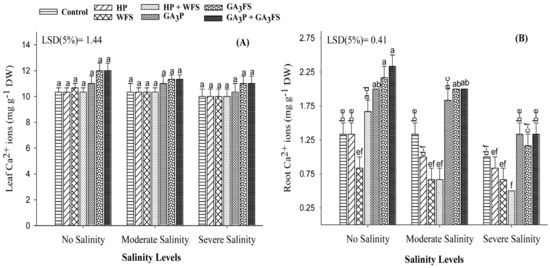
Figure 9.
Calcium (Ca2+) ions in maize shoots (A) and roots (B) as affected by different treatments of gibberellic acid under no salinity (control), moderate salinity (6 dS m−1), and severe salinity (12 dS m−1). Values are means ± SD (n = 3). Hydropriming (HP); water foliar spray (WFS); hydropriming and water foliar spray (HP + WFS); seed priming with GA3 (GA3P); foliar spray with GA3 (GA3FS); seed priming and foliar spray of GA3 (GA3P + GA3FS). Bars with the same letters do not differ significantly at p ≤ 0.05.
4. Discussion
Abiotic stresses restrict plant growth and development [33,34]. The present study demonstrated that salinity stress, particularly at 12 dS m−1, significantly hampered the plant growth and biomass accumulation compared the unstressed control (Figure 1 and Figure 2). Consistently, several previous studies [35,36] have also documented that salinity is highly detrimental to plant growth and biomass production. Salt stress in maize reduced the root and shoot length, dry and fresh biomass and leaves growth compared to the control [36]. The decrease in plant biomass might be due to the higher Na+ ion concentration in roots and outside the plant cell [35,37]. In the soil, higher salt concentration limits the absorption of water and nutrients by plant roots. Moreover, the higher accumulation of Na+ ions in roots can cause osmotic stress, reduce water potential, and disturb the nutrient balance in plants. The higher concentrations of Na+ inside and outside the plant cell have a negative impact on K+ influx in the cell, the latter being an essential element required for plant growth [35,37]. In the present study, application of GA3 as priming and/or foliar spray significantly improved the growth of maize crop under salinity stress (Figure 1 and Figure 2). The beneficial effect of GA3-seed priming or GA3-foliar spray has previously been reported by several researchers in different field crops [17,19,20,21,22,38]. The exogenous supply of GA3 may enhance its endogenous accumulation, which may facilitate the better growth of plants [15]. The GA3 is considered as an important hormone for cell elongation, therefore, the better growth and seedling length of GA3-treated maize in the present study (Figure 1 and Figure 2) might be due to the higher cell and stem elongation [20,39]. The application of GA3 can also increase the plant growth because of improved carbohydrate metabolism [40]. In the past, GA3 application-induced growth stimulation of wheat under zinc oxide nanoparticle stress was attributed to improved nutritional status [22].
Chlorophyll is the main pigment of plant photosynthesis and plays an important role in different physiological processes of plants [41]. In this study, salinity stress significantly reduced leaf chlorophyll content (shown as Chla, Chlb and total chlorophyll) as compared to untreated plants (Figure 3). Various previous reports have also confirmed that salt stress can reduce the activity of photosynthetic pigments [42,43]. The decrease in chlorophyll content can be attributed to the formation of proteolytic enzymes at high salt concentrations [20]; and these enzymes are responsible for the degradation of chlorophyll [44], and reduction of photosynthesis under salt conditions [45]. The reduction in maize biomass under salt stress (Figure 1 and Figure 2) may also be due to the decrease in chlorophyll contents (Figure 3) and photosynthesis rate [46]. The application of GA3 increased the chlorophyll content of maize leaves exposed to salt stress, with a maximum increase in case of the GA3P + GA3FS treatment (Figure 3). The higher accumulation of chlorophyll content in GA3-treated maize seedlings under salinity stress might be linked to lower Na+ accumulation (Figure 8), lower oxidative damage and an improved antioxidant defense system (Figure 4, Figure 5 and Figure 6). These findings are consistent with the results of previous studies [47,48,49], which reported that the application of GA3 increased leaf chlorophyll content. Foliar applied GA3 significantly improved the chlorophyll content in maize under salt stress [20]. In our work, seed priming and foliar application of GA3 decreased Na+ contents in maize tissue, suggesting that translocation of Na+ from roots to shoots might be inhibited by GA3 treatments (Figure 8). Less accumulation of Na+ in maize tissue might show less harmful effects on leaf functions, which may affect the changes of photosynthetic pigments in the present study (Figure 3). Additionally, in our work, the positive influence of GA3 treatments was more pronounced for chlorophyll b (Figure 3). However, the mechanism behind this requires further exploration.
Oxidative stress has an adverse effect on plant cell functions [50]. Under oxidative stress, excessive production of ROS leads to damage to plant tissue [51]. More production of ROS under salt stress has been reported before [52,53,54]. Consistent with published studies, higher concentrations of H2O2 under salt stress were observed in maize seedlings (Figure 4). However, plants have evolved many defense mechanisms to reduce ROS-induced damage by modulating the activities of the antioxidant enzymes as well as the expression of antioxidant genes. It is well documented that CAT enzyme converts H2O2 into H2O and O2, and POD enzyme may also play an important role in the catalysis of H2O2. In addition, increased SOD enzyme activity has been reported to enhance the ability of seedlings to scavenging O2−radicals under stress conditions [55]. However, under stress conditions, plants cannot sufficiently eliminate ROS, which can lead to oxidative stress [56]. In this study, the application of GA3 significantly reduced the oxidative stress under salt stress, because of the less production of ROS and higher activities of antioxidant enzymes (Figure 4, Figure 5 and Figure 6). Our results also showed that at moderate and severe salinity, GA3 application significantly restored antioxidant enzyme activities, as compared to untreated plants. Under severe salinity, the application of GA3 increased the activities of antioxidant enzymes and their expression levels, and similar findings have been reported in previously published data [57,58]. Furthermore, GA3 application improved the carotenoid (Figure 3), and total phenolic contents (Figure 7), which also protects plants from oxidative damage. In the present study, SOD activity in control plants decreased significantly with increasing the salinity levels. Similar results have previously been reported by Al-Hassan et al. [59] under salt stress.
The restriction in the concentration of Na+ ions and maintenance or high level of Ca2+ and K+ ions are pivotal for plant survival under salt stress [60,61]. Under salinity, higher Ca2+ concentration helps plants to maintain their growth [62]. Our results showed that Na+ concentration decreased in maize roots and leaves under GA3 application. Also, GA3 application caused a significant increase in Ca2+ and K+ ions in different maize parts under salinity stress (Figure 8 and Figure 9). Various studies have shown that the application of GA3 significantly reduced the concentration of Na+ ions and increased the concentrations of Ca2+ and K+ ions under salt stress [21,63,64,65]. Interestingly, water spray decreased the Ca2+ concentration in maize roots as compared to untreated plants. Perhaps water causes a dilution effect or the priming process itself caused the different responses.
Plants have various defense responses against abiotic stress, including salinity [66], which leads to the production of several secondary metabolites, such as phenolics [67]. In this study, a significant increase in phenolic compounds was reported in GA3-treated plants exposed to salt stress (Figure 7). Similar findings were reported in S. miltiorrhiza [68] and Artemisia absinthium (L.) [69]. Moreover, the role of phenolics in scavenging of ROS in different plant species has also been documented [69,70,71] indicating that higher accumulation of phenolics could be pivotal for plant tolerance against oxidative stress. In this experiment, the application of GA3 also significantly increased the total soluble protein under salt stress. These results are consistent with previous reports [72,73], which demonstrated that the application of GA3 increased the protein content in maize. The supply of GA3 plays an essential role in protein biosynthesis because it can increase the uptake of N from the soil [72]. Accumulation of organic solutes, including soluble protein and phenolics could contribute to osmotic adjustment and stabilization of membranes in plants [58]. It is well-known that organic solutes can enhance plant tolerance against different stresses, including salinity, through maintaining pressure potential and membrane integrity, protection of proteins, and scavenging of free radicals [74,75].
5. Conclusions
In summary, salinity stress significantly inhibited the growth and development of maize crop, however, the application of GA3 can effectively ameliorate the damage caused by salt stress. Gibberellic acid amendment enhanced the maize growth, chlorophyll content, total soluble protein and K+ ion concentration, while reduced the oxidative stress and Na+ ion accumulation under salinity. The GA3P + GA3FS was the most effective treatment for enhancing the growth and development of maize under salt stress. Better stress tolerance and greater growth of maize in this treatment was associated with higher antioxidative defense, maintenance of photosynthetic pigments, higher osmolyte accumulation and better ionic homeostasis in salt-affected soil.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/biom11071005/s1, Supplement Table S1: Two-way ANOVA analysis of salt stress, gibberellic acid (GA3) application, and their interaction for growth, physiological traits, antioxidant enzymes and ionic contents of maize.
Author Contributions
Conceptualization S.H. (Saddam Hussain), M.A. and M.A.E.-E.; investigation, K.S., S.H. (Sadam Hussain), and S.Z.; methodology, K.S., S.H. (Sadam Hussain), M.S. and M.A.E.-E.; data analysis: S.H. (Saddam Hussain) and M.A.E.-E.; visualization and resources, E.A.W., A.R., C.H., M.A.E.-E. and K.Y.K.; writing—original draft, K.S., S.H. (Sadam Hussain), M.A. and M.A.E.-E.; writing—review and editing, M.A.E-E., S.H. (Saddam Hussain), E.A.W., M.S., S.Z., A.R., K.Y.K and C.H. Moreover, S.H. (Saddam Hussain) and M.A.E.-E. performed the molecular gene expression analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data supporting the findings of this study are included in this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jiang, C.; Zu, C.; Lu, D.; Zheng, Q.; Shen, J.; Wang, H.; Li, D. Effect of exogenous selenium supply on photosynthesis, Na+ accumulation and antioxidative capacity of maize (Zea mays L.) under salinity stress. Sci. Rep. 2017, 7, 42039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, P. Growth and antioxidant responses in mustard (Brassica juncea L.) plants subjected to combined effect of gibberellic acid and salinity. Arch. Agron. Soil Sci. 2010, 56, 575–588. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [Green Version]
- Gill, S.S.; Anjum, N.A.; Hasanuzzaman, M.; Gill, R.; Trivedi, D.K.; Ahmad, I.; Pereira, E.; Tuteja, N. Glutathione and glutathione reductase: A boon in disguise for plant abiotic stress defense operations. Plant Physiol. Biochem. 2013, 70, 204–212. [Google Scholar] [CrossRef]
- Saade, S.; Maurer, A.; Shahid, M.; Oakey, H.; Schmöckel, S.M.; Negrão, S.; Pillen, K.; Tester, M. Yield-related salinity tolerance traits identified in a nested association mapping (NAM) population of wild barley. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anuradha, S.; Rao, S.S.R. Effect of brassinosteroids on salinity stress induced inhibition of seed germination and seedling growth of rice (Oryza sativa L.). Plant Growth Regul. 2001, 33, 151–153. [Google Scholar] [CrossRef]
- Tuteja, N.; Sahoo, R.K.; Garg, B.; Tuteja, R. O s SUV 3 dual helicase functions in salinity stress tolerance by maintaining photosynthesis and antioxidant machinery in rice (Oryza sativa L. cv. IR 64). Plant J. 2013, 76, 115–127. [Google Scholar]
- Deinlein, U.; Stephan, A.B.; Horie, T.; Luo, W.; Xu, G.; Schroeder, J.I. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014, 19, 371–379. [Google Scholar] [CrossRef] [Green Version]
- Pérez-López, U.; Robredo, A.; Lacuesta, M.; Sgherri, C.; Muñoz-Rueda, A.; Navari-Izzo, F.; Mena-Petite, A. The oxidative stress caused by salinity in two barley cultivars is mitigated by elevated CO2. Physiol. Plant. 2009, 135, 29–42. [Google Scholar] [CrossRef]
- Suzuki, N.; Koussevitzky, S.; Mittler, R.; Miller, G. ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ. 2012, 35, 259–270. [Google Scholar] [CrossRef]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef]
- Chaparzadeh, N.; D’Amico, M.L.; Khavari-Nejad, R.-A.; Izzo, R.; Navari-Izzo, F. Antioxidative responses of Calendula officinalis under salinity conditions. Plant Physiol. Biochem. 2004, 42, 695–701. [Google Scholar] [CrossRef]
- Chawla, S.; Jain, S.; Jain, V. Salinity induced oxidative stress and antioxidant system in salt-tolerant and salt-sensitive cultivars of rice (Oryza sativa L.). J. Plant Biochem. Biotechnol. 2013, 22, 27–34. [Google Scholar] [CrossRef]
- Parveen, A.; Liu, W.; Hussain, S.; Asghar, J.; Perveen, S.; Xiong, Y. Silicon Priming Regulates Morpho-Physiological Growth and Oxidative Metabolism in Maize under Drought Stress. Plants 2019, 8, 431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aldesuquy, H.; Ibrahim, A. Interactive effect of seawater and growth bioregulators on water relations, abscisic acid concentration and yield of wheat plants. J. Agron. Crop Sci. 2001, 187, 185–193. [Google Scholar] [CrossRef]
- Iqbal, M.; Ashraf, M. Gibberellic acid mediated induction of salt tolerance in wheat plants: Growth, ionic partitioning, photosynthesis, yield and hormonal homeostasis. Environ. Exp. Bot. 2013, 86, 76–85. [Google Scholar] [CrossRef]
- Ghodrat, V.; Rousta, M.J. Effect of priming with Gibberellic acid (GA3) on germination and growth of corn (Zea mays L.) under saline conditions. Int. J. Agric. Crop Sci. 2012, 4, 882–885. [Google Scholar]
- Binenbaum, J.; Weinstain, R.; Shani, E. Gibberellin localization and transport in plants. Trends Plant Sci. 2018, 23, 410–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel-Hamid, A. Physiological and molecular markers for salt tolerance in four barley cultivars. Eur. Sci. J. 2014, 10, 252–272. [Google Scholar]
- Tuna, A.L.; Kaya, C.; Dikilitas, M.; Higgs, D. The combined effects of gibberellic acid and salinity on some antioxidant enzyme activities, plant growth parameters and nutritional status in maize plants. Environ. Exp. Bot. 2008, 62, 1–9. [Google Scholar] [CrossRef]
- Ashraf, M.; Karim, F.; Rasul, E. Interactive effects of gibberellic acid (GA3) and salt stress on growth, ion accumulation and photosynthetic capacity of two spring wheat (Triticum aestivum L.) cultivars differing in salt tolerance. Plant Growth Regul. 2002, 36, 49–59. [Google Scholar] [CrossRef]
- Iftikhar, A.; Ali, S.; Yasmeen, T.; Arif, M.S.; Zubair, M.; Rizwan, M.; Alhaithloul, H.A.S.; Alayafi, A.A.; Soliman, M.H. Effect of gibberellic acid on growth, photosynthesis and antioxidant defense system of wheat under zinc oxide nanoparticle stress. Environ. Pollut. 2019, 254, 113109. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, S.; Sudhakar, P.; Elankavi, S.; Suseendran, K.; Jawahar, S. Effect of gibberellic acid (GA3) on growth and yield of rice (Oryza sativa L.). Plant Arch. 2019, 19, 1369–1372. [Google Scholar]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chance, B.; Maehly, A. Assay of catalases and peroxidases. Methods Enzymol. 1955, 2, 764–775. [Google Scholar]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Vwioko, E.; Adinkwu, O.; El-Esawi, M.A. Comparative Physiological, Biochemical and Genetic Responses to Prolonged Waterlogging Stress in Okra and Maize Given Exogenous Ethylene Priming. Front. Physiol. 2017, 8, 632. [Google Scholar] [CrossRef]
- Huang, H.; Lv, L.; Wang, D.; Guo, B.; Lv, J.; Luo, L.; Wen, B.; Kang, Y. Biochemical and molecular responses of maize (Zea mays L.) to 1,2-dibromo-4-(1,2 dibromoethyl) cyclohexane (TBECH) diastereomers: Oxidative stress, DNA damage, antioxidant enzyme gene expression and diversity of root exudates. Sci. Total Environ. 2021, 753, 141872. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Yin, L.; Wang, S.; Tanaka, K.; Fujihara, S.; Itai, A.; Den, X.; Zhang, S. Silicon-mediated changes in polyamines participate in silicon-induced salt tolerance in Sorghum bicolor L. Plant Cell Environ. 2016, 39, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Steel, R.; Torrie, J. Principles and Procedures of Statistics: A Biometrical Approach, 2nd ed.; McGraw-Hill Book Company: New York, NY, USA, 1980. [Google Scholar]
- Alhaithloul, H.A.; Soliman, M.H.; Ameta, K.L.; El-Esawi, M.A.; Elkelish, A. Changes in Ecophysiology, Osmolytes, and Secondary Metabolites of the Medicinal Plants of Mentha piperita and Catharanthus roseus Subjected to Drought and Heat Stress. Biomolecules 2020, 10, 43. [Google Scholar] [CrossRef] [Green Version]
- El-Esawi, M.A.; Alayafi, A.A. Overexpression of StDREB2 Transcription Factor Enhances Drought Stress Tolerance in Cotton (Gossypium barbadense L.). Genes 2019, 10, 142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Singh, S.; Gaurav, A.K.; Srivastava, S.; Verma, J.P. Plant Growth-Promoting Bacteria: Biological Tools for the Mitigation of Salinity Stress in Plants. Front. Microbiol. 2020, 11, 1216. [Google Scholar] [CrossRef] [PubMed]
- Hoque, M.M.I.; Jun, Z.; Guoying, W. Evaluation of salinity tolerance in maize (Zea mays L.) genotypes at seedling stage. J. Biosci. Biotechnol. 2015, 4, 39–49. [Google Scholar]
- Singh, R.P.; Jha, P.; Jha, P.N. The plant-growth-promoting bacterium Klebsiella sp. SBP-8 confers induced systemic tolerance in wheat (Triticum aestivum) under salt stress. J. Plant Physiol. 2015, 184, 57–67. [Google Scholar] [CrossRef]
- Naeem, M.; Muhammad, S. Effect of seed priming on growth of barley (Hordeum vulgare) by using brackish water in salt affected soils. Pak. J. Bot. 2006, 38, 613. [Google Scholar]
- Kaur, S.; Gupta, A.K.; Kaur, N. Gibberellin A3 reverses the effect of salt stress in chickpea (Cicer arietinum L.) seedlings by enhancing amylase activity and mobilization of starch in cotyledons. Plant Growth Regul. 1998, 26, 85–90. [Google Scholar] [CrossRef]
- Salimi, K.; Afshari, R.T.; Hosseini, M.; Struik, P. Effects of gibberellic acid and carbon disulphide on sprouting of potato minitubers. Sci. Hortic. 2010, 124, 14–18. [Google Scholar] [CrossRef]
- Gu, X.; Li, Y.; Du, Y. Compensative impact of winter oilseed rape (Brassica napus L.) affected by water stress at re-greening stage under different nitrogen rates. Chin. J. Eco Agric. 2016, 24, 572–581. [Google Scholar]
- El-Esawi, M.A.; Al-Ghamdi, A.A.; Ali, H.M.; Alayafi, A.A.; Witczak, J.; Ahmad, M. Analysis of Genetic Variation and Enhancement of Salt Tolerance in French Pea (Pisum Sativum L.). Int. J. Mol. Sci. 2018, 19, 2433. [Google Scholar] [CrossRef] [Green Version]
- Qu, C.; Liu, C.; Gong, X.; Li, C.; Hong, M.; Wang, L.; Hong, F. Impairment of maize seedling photosynthesis caused by a combination of potassium deficiency and salt stress. Environ. Exp. Bot. 2012, 75, 134–141. [Google Scholar] [CrossRef]
- Sabater, B.; Rodrguez, M.T. Control of chlorophyll degradation in detached leaves of barley and oat through effect of kinetin on chlorophyllase levels. Physiol. Plant. 1978, 43, 274–276. [Google Scholar] [CrossRef]
- Yasseen, B. An Analysis of the Effects of Salinity on Leaf Growth in Mexican Wheats. Ph.D. Thesis, University of Leeds, Leeds, UK, 1983. [Google Scholar]
- Xia, S.; Wang, X.; Su, G.; Shi, G. Effects of drought on cadmium accumulation in peanuts grown in a contaminated calcareous soil. Environ. Sci. Pollut. Res. 2015, 22, 18707–18717. [Google Scholar] [CrossRef]
- Bose, S.K.; Yadav, R.K.; Mishra, S.; Sangwan, R.S.; Singh, A.; Mishra, B.; Srivastava, A.; Sangwan, N.S. Effect of gibberellic acid and calliterpenone on plant growth attributes, trichomes, essential oil biosynthesis and pathway gene expression in differential manner in Mentha arvensis L. Plant Physiol. Biochem. 2013, 66, 150–158. [Google Scholar] [CrossRef]
- Shah, S. Effects of salt stress on mustard as affected by gibberellic acid application. Gen. Appl. Plant Physiol. 2007, 33, 97–106. [Google Scholar]
- Zang, Y.-X.; Chun, I.-J.; Zhang, L.-L.; Hong, S.-B.; Zheng, W.-W.; Xu, K. Effect of gibberellic acid application on plant growth attributes, return bloom, and fruit quality of rabbiteye blueberry. Sci. Hortic. 2016, 200, 13–18. [Google Scholar] [CrossRef]
- Soliman, M.; Alhaithloul, H.A.; Hakeem, K.R.; Alharbi, B.M.; El-Esawi, M.; Elkelish, A. Exogenous nitric oxide mitigates nickel-induced oxidative damage in eggplant by upregulating antioxidants, osmolyte metabolism, and glyoxalase systems. Plants 2019, 8, 562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaskulak, M.; Rorat, A.; Grobelak, A.; Kacprzak, M. Antioxidative enzymes and expression of rbcL gene as tools to monitor heavy metal-related stress in plants. J. Environ. Manag. 2018, 218, 71–78. [Google Scholar] [CrossRef]
- El-Esawi, M.A.; Alaraidh, I.A.; Alsahli, A.A.; Alzahrani, S.M.; Ali, H.M.; Alayafi, A.A.; Ahmad, M. Serratia liquefaciens KM4 Improves Salt Stress Tolerance in Maize by Regulating Redox Potential, Ion Homeostasis, Leaf Gas Exchange and Stress-Related Gene Expression. Int. J. Mol. Sci. 2018, 19, 3310. [Google Scholar] [CrossRef] [Green Version]
- El-Esawi, M.A.; Alaraidh, I.A.; Alsahli, A.A.; Alamri, S.A.; Ali, H.M.; Alayafi, A.A. Bacillus firmus (SW5) augments salt tolerance in soybean (Glycine max L.) by modulating root system architecture, antioxidant defense systems and stress-responsive genes expression. Plant Physiol. Biochem. 2018, 132, 375–384. [Google Scholar] [CrossRef] [PubMed]
- El-Esawi, M.A.; Al-Ghamdi, A.A.; Ali, H.M.; Alayafi, A.A. Azospirillum lipoferum FK1 confers improved salt tolerance in chickpea (Cicer arietinum L.) by modulating osmolytes, antioxidant machinery and stress-related genes expression. Environ. Exp. Bot. 2019, 159, 55–65. [Google Scholar] [CrossRef]
- Agarwal, S.; Pandey, V. Antioxidant enzyme responses to NaCl stress in Cassia angustifolia. Biol. Plant. 2004, 48, 555–560. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Qayyum, M.F.; Ibrahim, M.; Zia-ur-Rehman, M.; Abbas, T.; Ok, Y.S. Mechanisms of biochar-mediated alleviation of toxicity of trace elements in plants: A critical review. Environ. Sci. Pollut. Res. 2016, 23, 2230–2248. [Google Scholar] [CrossRef]
- Khalid, A.; Aftab, F. Effect of exogenous application of IAA and GA 3 on growth, protein content, and antioxidant enzymes of Solanum tuberosum L. grown in vitro under salt stress. Vitr. Cell. Dev. Biol. Plant 2020, 56, 377–389. [Google Scholar] [CrossRef]
- Saeidi-Sar, S.; Abbaspour, H.; Afshari, H.; Yaghoobi, S.R. Effects of ascorbic acid and gibberellin A3 on alleviation of salt stress in common bean (Phaseolus vulgaris L.) seedlings. Acta Physiol. Plant. 2013, 35, 667–677. [Google Scholar] [CrossRef]
- Al Hassan, M.; Chaura, J.; Donat-Torres, M.P.; Boscaiu, M.; Vicente, O. Antioxidant responses under salinity and drought in three closely related wild monocots with different ecological optima. AoB Plants 2017, 9, plx009. [Google Scholar] [CrossRef] [PubMed]
- Tester, M.; Davenport, R. Na+ tolerance and Na+ transport in higher plants. Ann. Bot. 2003, 91, 503–527. [Google Scholar] [CrossRef] [PubMed]
- Gupta, B.; Huang, B. Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization. Int. J. Genom. 2014, 2014, 701596. [Google Scholar] [CrossRef]
- Tuna, A.L.; Kaya, C.; Ashraf, M.; Altunlu, H.; Yokas, I.; Yagmur, B. The effects of calcium sulphate on growth, membrane stability and nutrient uptake of tomato plants grown under salt stress. Environ. Exp. Bot. 2007, 59, 173–178. [Google Scholar] [CrossRef]
- Shomeili, M.; Nabipour, M.; Meskarbashee, M.; Memari, H.R. Effects of gibberellic acid on sugarcane plants exposed to salinity under a hydroponic system. Afr. J. Plant Sci. 2011, 5, 609–616. [Google Scholar]
- Samad, R.; Karmoker, J. Effects of gibberellic acid and Kn on seed germination and accumulation of Na+ and K+ in the seedlings of triticale-I under salinity stress. Bangladesh J. Bot. 2012, 41, 123–129. [Google Scholar] [CrossRef] [Green Version]
- Riboldi, L.B.; da Cruz Araújo, S.H.; Murcia, J.A.G.; de Freitas, S.T.; de Camargo e Castro, P.R. Abscisic acid and 24-epibrassinolide regulate blossom-end rot (BER) development in tomato fruit under Ca2+ deficiency. Aust. J. Crop Sci. 2018, 12, 1440–1446. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, H.; Song, C.; Zhu, J.-K.; Shabala, S. Mechanisms of plant responses and adaptation to soil salinity. Innovation 2020, 1, 100017. [Google Scholar] [CrossRef]
- Ali, M.B.; Singh, N.; Shohael, A.M.; Hahn, E.J.; Paek, K.-Y. Phenolics metabolism and lignin synthesis in root suspension cultures of Panax ginseng in response to copper stress. Plant Sci. 2006, 171, 147–154. [Google Scholar] [CrossRef]
- Liang, Z.; Ma, Y.; Xu, T.; Cui, B.; Liu, Y.; Guo, Z.; Yang, D. Effects of abscisic acid, gibberellin, ethylene and their interactions on production of phenolic acids in Salvia miltiorrhiza Bunge hairy roots. PLoS ONE 2013, 8, e72806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, M.; Abbasi, B.H.; Ali, G.S. Elicitation of antioxidant secondary metabolites with jasmonates and gibberellic acid in cell suspension cultures of Artemisia absinthium L. Plant Cell Tissue Organ Cult. 2015, 120, 1099–1106. [Google Scholar] [CrossRef]
- Amid, A.; Johan, N.N.; Jamal, P.; Zain, W.N.W.M. Observation of antioxidant activity of leaves, callus and suspension culture of Justicia gendarusa. Afr. J. Biotechnol. 2011, 10, 18653–18656. [Google Scholar]
- El-Esawi, M.; Glascoe, A.; Engle, D.; Ritz, T.; Link, J.; Ahmad, M. Cellular metabolites modulate in vivo signaling of Arabidopsis cryptochrome-1. Plant Signal. Behav. 2015, 10, e1063758. [Google Scholar] [CrossRef] [Green Version]
- Hamdia, M.; El-Komy, H. Effect of salinity, gibberellic acid and Azospirillum inoculation on growth and nitrogen uptake of Zea mays. Biol. Plant. 1997, 40, 109–120. [Google Scholar] [CrossRef]
- Mohammed, A. Physiological aspects of mungbean plant (Vigna radiata L. Wilczek) in response to salt stress and gibberellic acid treatment. Res. J. Agr. Biol. Sci. 2007, 3, 200–213. [Google Scholar]
- Ashraf, M.; Athar, H.R.; Harris, P.J.C.; Kwon, T.R. Some prospective strategies for improving crop salt tolerance. Adv. Agron. 2008, 97, 45–110. [Google Scholar]
- Habib, N.; Ashraf, M.; Ali, Q.; Perveen, R. Response of salt stressed okra (Abelmoschus esculentus Moench) plants to foliar-applied glycine betaine and glycine betaine containing sugarbeet extract. S. Afr. J. Bot. 2012, 83, 151–158. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).