Caveolin-3: A Causative Process of Chicken Muscular Dystrophy
Abstract
1. Introduction
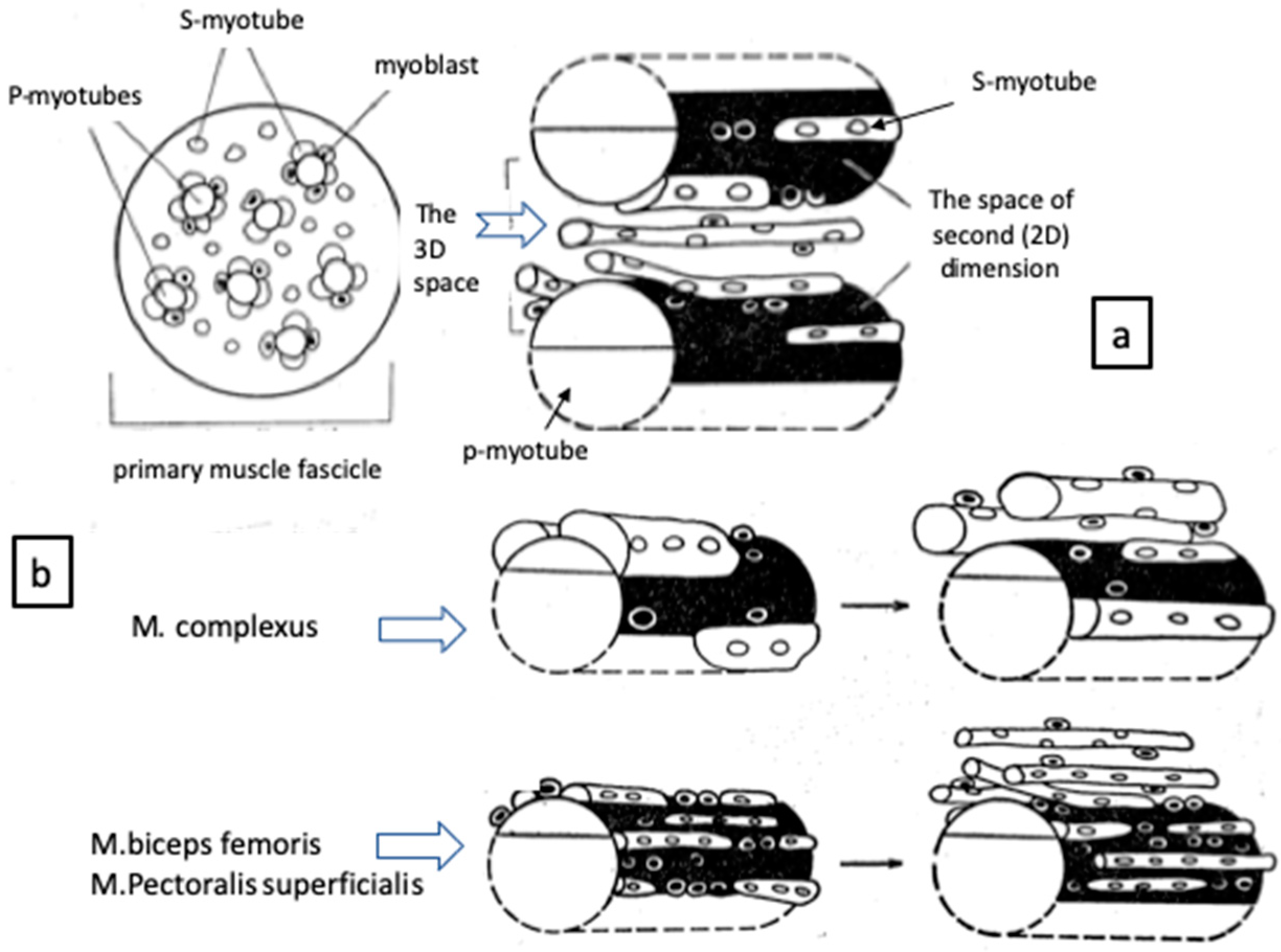
2. Muscle Fiber Formation
3. Are Dystrophic αR Fibers Akin to Embryonic or Denervated?
4. Genetic Linkage Analysis of AM Gene
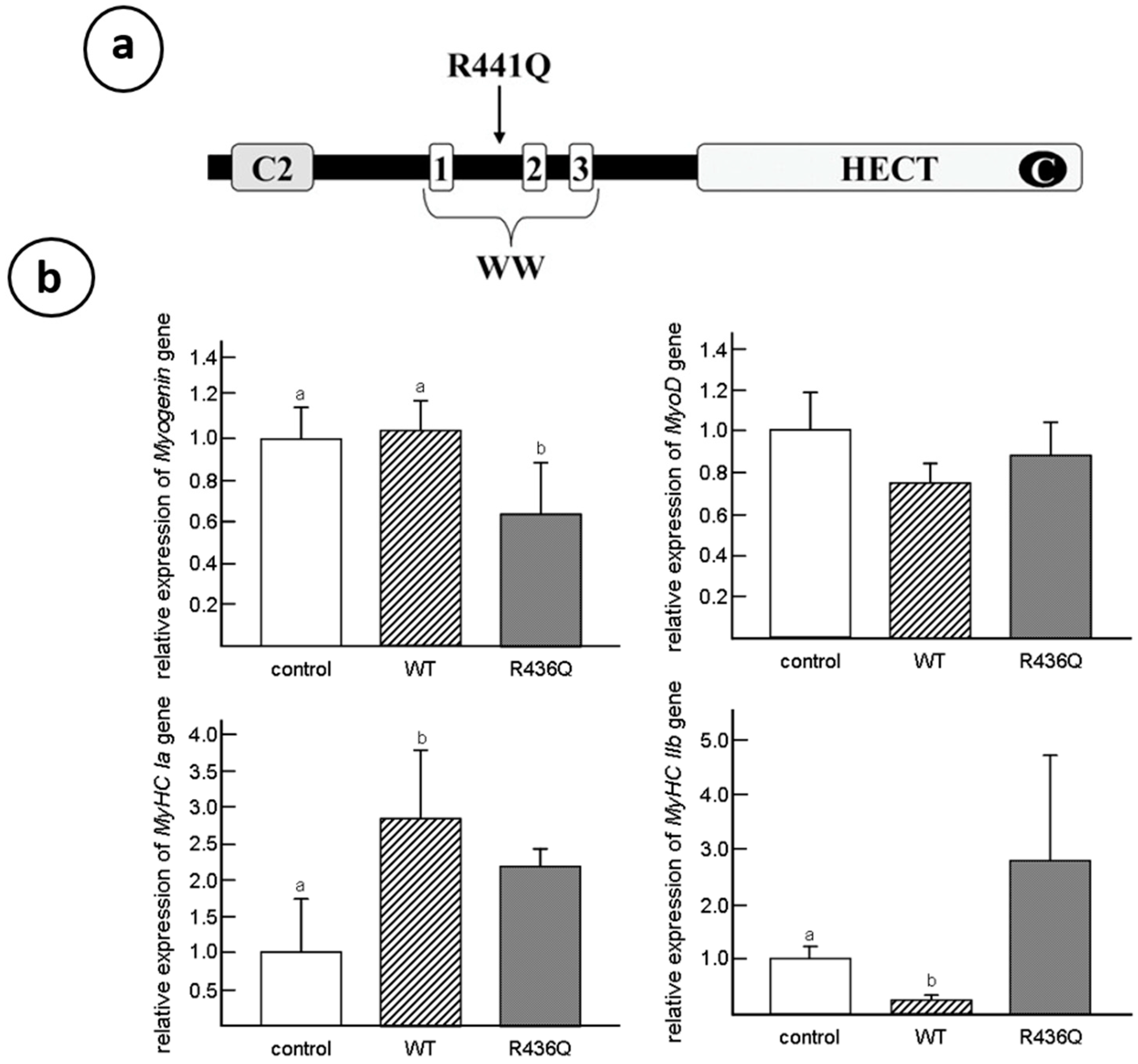
5. WWP1: E3 Ubiquitin Ligases
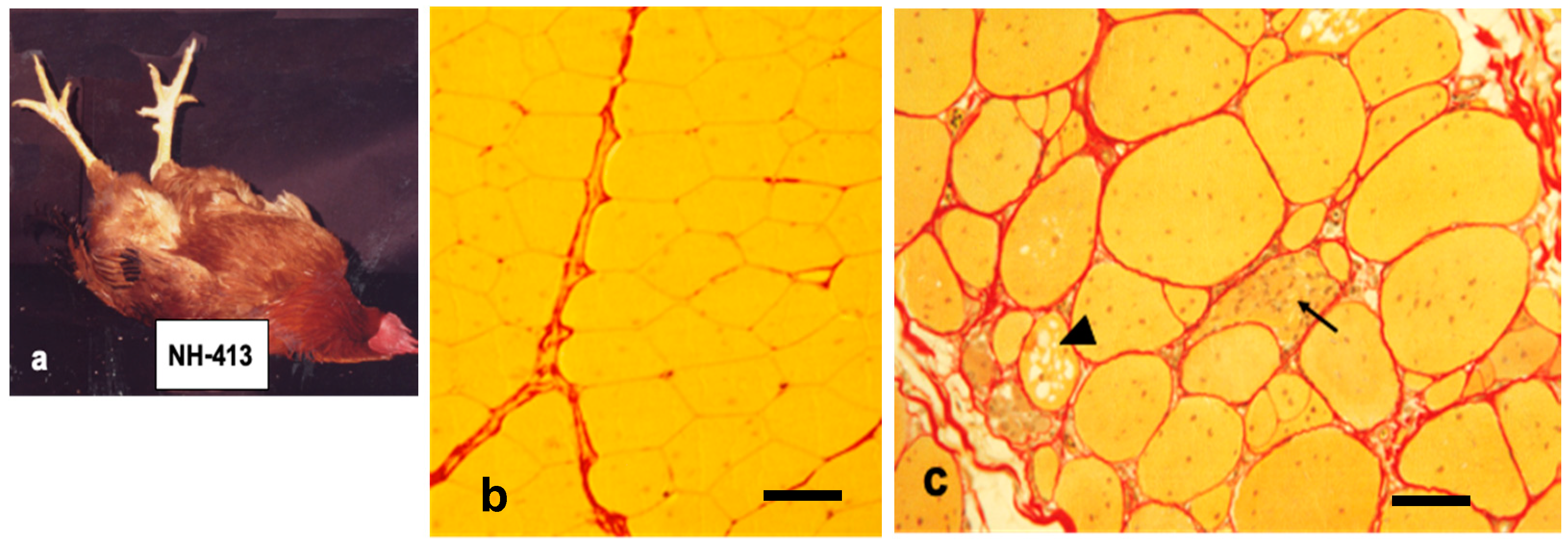
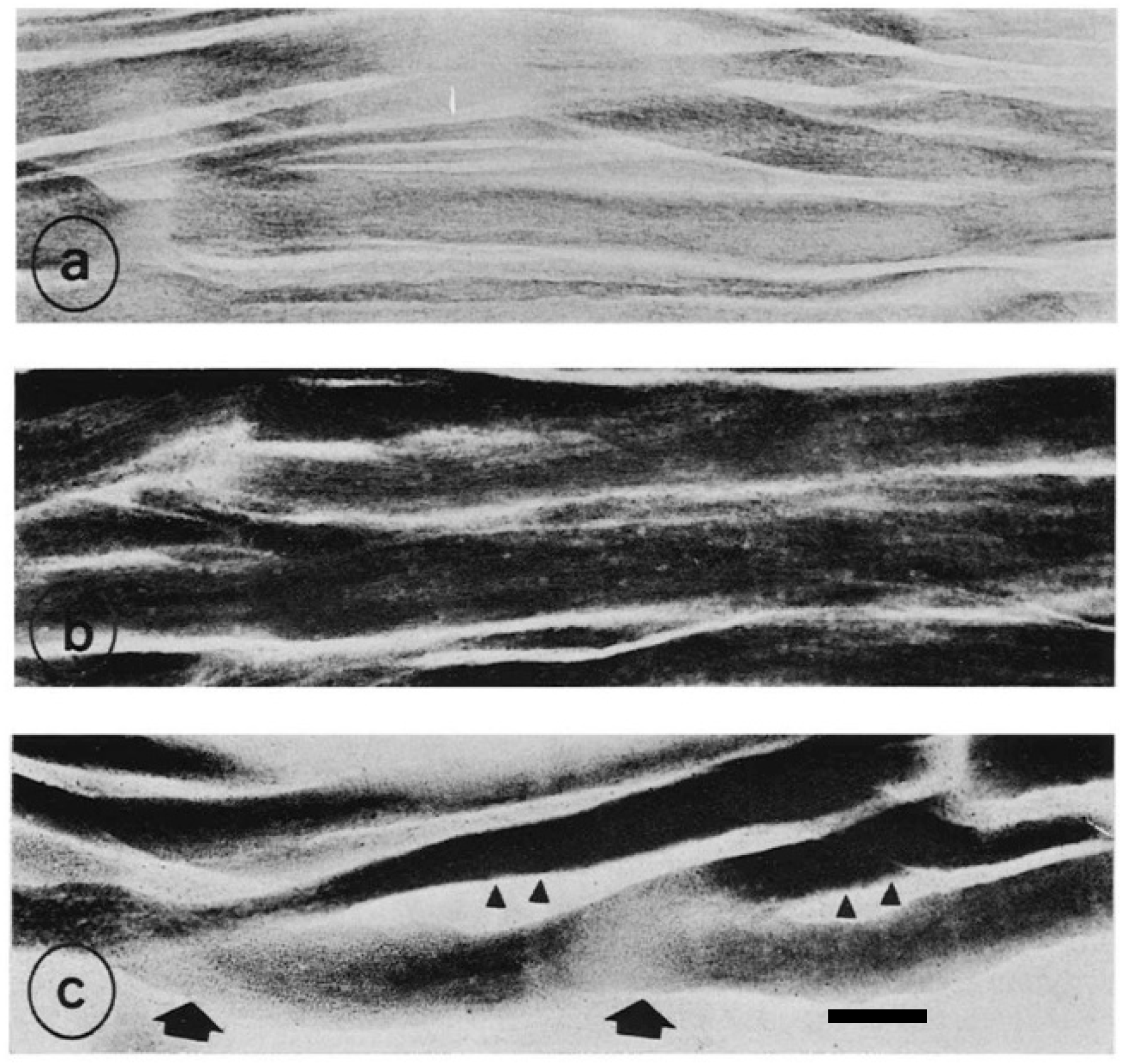
6. Caveolin-3: Another Causative Process of Muscular Dystrophy
Funding
Acknowledgments
Conflicts of Interest
References
- Asmundson, V.S.; Julian, L.M. Inherited muscle abnormality in the domestic fowl. J. Hered. 1956, 47, 248–252. [Google Scholar] [CrossRef]
- Ashmore, C.R.; Doerr, L. Postnatal development of fiber types in normal and dystrophic skeletal muscle of the chick. Exp. Neurol. 1971, 30, 431–446. [Google Scholar] [CrossRef]
- Barnard, E.A.; Lyles, J.M.; Pizzey, J.A. Fibre types in chicken skeletal muscles and their changes in muscular dystrophy. J. Physiol. 1982, 331, 333–354. [Google Scholar] [CrossRef] [PubMed]
- Julian, L.M. Animal model: Hereditary muscular dystrophy of chickens. Am. J. Pathol. 1973, 70, 273–276. [Google Scholar] [PubMed]
- Ashmore, C.R.; Kikuchi, T.; Doerr, L. Some observations on the innervation patterns of different fiber types of chick muscle. Exp. Neurol. 1978, 58, 272–284. [Google Scholar] [CrossRef]
- Ashmore, C.R.; Vigneron, P.; Marger, L.; Doerr, L. Simultaneous cytochemical demonstration of muscle fiber types and acetylcholinesterase in muscle fibers of dystrophic chickens. Exp. Neurol. 1973, 60, 68–82. [Google Scholar] [CrossRef]
- Ashmore, C.R.; Addis, P.B.; Doerr, L.; Stokes, H. Development of muscle fibers in the complexus muscle of normal and dystrophic chicks. J. Histochem. Cytochem. 1973, 21, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Brooke, M.H.; Kaiser, K.K. Muscle Fiber Types: How Many and What Kind? Arch. Neurol. 1970, 23, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, T.; Schmidt, H. Changes in resting and contractile properties of chicken muscles following denervation. Biomed. Res. 1983, 4, 303–314. [Google Scholar] [CrossRef]
- Rafuse, V.F.; Milner, L.D.; Landmesser, L.T. Selective Innervation of Fast and Slow Muscle Regions during Early Chick Neuromuscular Development. J. Neurosci. 1996, 16, 6864–6877. [Google Scholar] [CrossRef] [PubMed]
- Asmundson, V.S.; Kratzer, F.H.; Julian, L.M. Inherited myopathy in the chicken. Ann. N. Y. Acad. Sci. 1966, 138, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Holliday, T.A.; Julian, L.M.; Asmundson, V.S. Muscle growth in selected lines of muscular dystrophic chickens. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 1968, 160, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Randall, W.R.; Wilson, B.W. Properties of muscles from chickens with inherited muscular dystrophy. J. Neurol. Sci. 1980, 46, 145–155. [Google Scholar] [CrossRef]
- Nonaka, I.; Sugita, H. Intracytoplasmic vacuoles in αW fibers of dystrophic chicken muscle—Probable early pathologic event initiates massive fiber necrosis. Acta Neuropathol. 1981, 55, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Ohwada, S.; Kikuchi, T. A microphotometric study on the proliferation pattern of muscle cell nuclei in chickens with hereditary muscle dystrophy. Tohoku J. Agric. Res. 1985, 36, 63–68. [Google Scholar]
- Wilson, B.W.; Randall, W.R.; Patterson, G.T.; Entrikin, R.K. Major physiologic and histochemical characteristics of inherited dystrophy of the chicken. Ann. N. Y. Acad. Sci. 1979, 317, 224–246. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, T.; Ishiura, S.; Nonaka, I.; Ebashi, S. Genetic heterozygous carriers in hereditary muscular dystrophy of chickens. Tohoku J. Agric. Res. 1981, 32, 14–26. [Google Scholar]
- Kikuchi, T.; Moriya, H.; Matsuzaki, T.; Katoh, M.; Takeda, S. The Development of Laboratory Animal Science for the Study of Human Muscular and Nervous Diseases in Japan. Congenit. Anom. 1987, 27, 447–462. [Google Scholar] [CrossRef]
- Kondo, K.; Kikuchi, T.; Mizutani, M. Breeding of the chick as an animal model for muscular dystrophy. In Proceedings of the International Symposium on Muscular Dystrophy, Tokyo, Japan, 25–27 November 1980; pp. 19–24. [Google Scholar]
- Koenig, M.; Hoffman, E.; Bertelson, C.; Monaco, A.P.; Feener, C.; Kunkel, L. Complete cloning of the duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell 1987, 50, 509–517. [Google Scholar] [CrossRef]
- Matsumoto, H.; Maruse, H.; Inaba, Y.; Yoshizawa, K.; Sasazaki, S.; Fujiwara, A.; Nishibori, M.; Nakamura, A.; Takeda, S.; Ichihara, N.; et al. The ubiquitin ligase gene (WWP1) is responsible for the chicken muscular dystrophy. FEBS Lett. 2008, 582, 2212–2218. [Google Scholar] [CrossRef]
- Kikuchi, T. Studies of development and differentiation of muscle III. Especially on the mode of increase in the number of muscle cells. Tohoku J. Agric. Res. 1971, 22, 1–15. [Google Scholar]
- Kikuchi, T. Ultrastructural evaluation of the myogenic cell fusion in chick embryo using the goniometer stage of electron microscopy. Tohoku J. Agric. Res. 1972, 23, 82–92. [Google Scholar]
- Kikuchi, T.; Nagatani, T.; Tamate, H. Studies on development and differentiation of muscle. VI. Cytokinetic analysis of cell proliferation by using 3H-thymidine autoradiography in various muscle tissues of chick embryo. Tohoku J. Agric. Res. 1974, 25, 22–36. [Google Scholar]
- Draeger, A.; Weeds, A.G.; Fitzsimons, R.B. Primary, secondary and tertiary myotubes in developing skeletal muscle: A new approach to the analysis of human myogenesis. J. Neurol. Sci. 1987, 81, 19–43. [Google Scholar] [CrossRef]
- Harris, A.J.; Duxson, M.J.; Fitzsimons, R.B.; Rieger, F. Myonuclear birthdates distinguish the origins of primary and secondary myotubes in embryonic mammalian skeletal muscles. Development 1989, 107, 771–784. [Google Scholar] [PubMed]
- Fisher, H.I. “Hatching muscle” in the chick. Auk 1958, 75, 391–399. [Google Scholar] [CrossRef]
- Ashmore, C.R.; Robinson, D.W.; Rattray, P.; Doerr, L. Biphasic development of muscle fibers in the fetal lamb. Exp. Neurol. 1972, 37, 241–255. [Google Scholar] [CrossRef]
- Kikuchi, T.; Ashmore, C.R. Developmental aspects of the innervation of skeletal muscle fibers in the chick embryo. Cell Tissue Res. 1976, 171, 233–251. [Google Scholar] [CrossRef]
- Phillips, W.D.; Everett, A.W.; Bennett, M.R. The role of innervation in the establishment of the topographical distribution of primary myotube types during development. J. Neurocytol. 1986, 15, 397–405. [Google Scholar] [CrossRef]
- McLennan, S. The development of the pattern of innervation in chick hindlimb muscles; Evidence for specification of nerve-muscle connection. Dev. Biol. 1983, 97, 229–238. [Google Scholar] [CrossRef]
- Romanul, F.C.; Bannister, R.G. Localized areas of high alkaline phosphatase activity in the terminal arterial tree. J. Cell Biol. 1962, 15, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Ashmore, C.R.; Somes, R.G. Delay of Hereditary Muscular Dystrophy of the Chicken by Oxygen Therapy: Histology. Exp. Biol. Med. 1968, 128, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Ashmore, C.R.; Doerr, L.; Somes, R.G. Microcirculation: Loss of an Enzyme Activity in Chickens with Hereditary Muscular Dystrophy. Science 1968, 160, 319–320. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S.; Nonomura, Y. Presence of the tropomyosin β-chain in dystrophic chicken breast muscle. Biomed. Res. 1980, 1, 176–179. [Google Scholar] [CrossRef]
- Obinata, T.; Saitoh, O.; Takano-Ohmuro, H. Effect of Denervation on the Isoform Transitions of Tropomyosin, Troponin T, and Myosin Isozyme in Chicken Breast Muscle1. J. Biochem. 1984, 95, 585–588. [Google Scholar] [CrossRef]
- Bandman, E. Myosin components of the latissimus dorsi and the pectoralis major muscles in the dystrophic chicken. Muscle Nerve 1984, 7, 312–326. [Google Scholar] [CrossRef]
- Huszar, G.; Vigue, L.; Haines, J. Myosin heavy chain in avian muscular dystrophy corresponds to the neonatal isozyme. J. Biol. Chem. 1985, 260, 9957–9960. [Google Scholar]
- Obinata, T.; Shinbo, K. Slow-type C-protein in dystrophic chicken fast pectoralis muscle. Muscle Nerve 1987, 10, 351–358. [Google Scholar] [CrossRef]
- Kojima, T.; Sano, K.; Hirabayashi, T.; Obinata, T. Characterization of C-Protein Isoforms Expressed in Developing, Denervated, and Dystrophic Chicken Skeletal Muscles by Two-Dimensional Gel Electrophoresis1. J. Biochem. 1990, 107, 470–475. [Google Scholar] [CrossRef]
- Matsuda, R.; Spector, D.; Strohman, R.C. Denervated skeletal muscle displays discoordinate regulation for the synthesis of several myofibrillar proteins. Proc. Natl. Acad. Sci. USA 1984, 81, 1122–1125. [Google Scholar] [CrossRef]
- Le Ray, C.F.; Renaud, D.; Le Douarin, G.H. Change in motor neurone activity modifies the differentiation of a slow muscle in chick embryo. Development 1989, 106, 295–302. [Google Scholar] [PubMed]
- Wilson, B.W.; Montgomery, M.A.; Asmundson, R.V. Cholinesterase Activity and Inherited Muscular Dystrophy of the Chicken. Exp. Biol. Med. 1968, 129, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.W.; Kaplan, M.A.; Merhoff, W.C.; Mori, S.S. Innervation and the regulation of acetylcholinesterase activity during the development of normal and dystrophic chick muscle. J. Exp. Zool. 1970, 174, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.W.; Taylor, R.G.; Fowler, W.M.; Patterson, G.T.; Nieberg, P.A.; Linkhart, S.G.; Linkhart, T.A.; Fry, D. Incidence of acetylcholinesterase in the sarcoplasm of human and chicken muscles. J. Neurol. Sci. 1975, 26, 133–146. [Google Scholar] [CrossRef]
- Ashmore, C.R.; Doerr, L. Oxidative metabolism in skeletal muscle of normal and dystrophic chicks. Biochem. Med. 1970, 4, 246–259. [Google Scholar] [CrossRef]
- Kikuchi, T.; Doerr, L.; Ashmore, C.R. A possible mechanism of phenotypic expression of normal and dystrophic genomes on succinic dehydrogenase activity and fiber size within a single myofiber of muscle transplants. J. Neurol. Sci. 1980, 45, 273–286. [Google Scholar] [CrossRef]
- Kikuchi, T.; Ohwada, S. Effects of denervation on pectoralis muscle of chicken with hereditary muscular dystrophy. Proc. Jpn. Acad. Ser. B 1982, 58, 135–139. [Google Scholar] [CrossRef][Green Version]
- Jirmanová, I.; Zelená, J. Ultrastructural transformation of fast chicken muscle fibres induced by nerve cross-union. Cell Tissue Res. 1973, 146, 103–121. [Google Scholar] [CrossRef]
- Jung, H.W.; Wu, W.Y. The contrasting trophic changes of the anterior and posterior latissimus dorsi of the chick following denervation. Acta Physiol. Sin. 1962, 25, 304–311. [Google Scholar]
- Hoekman, T.B. Isometric contractile properties of the posterior latissimus dorsi muscle in normal and genetically dystrophic chickens. Exp. Neurol. 1976, 53, 729–743. [Google Scholar] [CrossRef]
- Vertel, B.M.; Fischman, D.A. Mitochondrial development during myogenesis. Dev. Biol. 1977, 58, 356–371. [Google Scholar] [CrossRef]
- Linkhart, T.A.; Yee, G.W.; Nieberg, P.S.; Wilson, B.W. Myogenic defect in muscular dystrophy of the chicken. Dev. Biol. 1976, 48, 447–457. [Google Scholar] [CrossRef]
- Lee, E.J.; Yoshizawa, K.; Mannen, H.; Kikuchi, H.; Kikuchi, T.; Mizutani, M.; Tsuji, S. Localization of the muscular dystrophy AM locus using a chicken linkage map constructed with the Kobe University resource family. Anim. Genet. 2002, 33, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Schmid, M.; Nanda, I.; Guttenbach, M.; Steinlein, C.; Hoehn, M.; Schartl, M.; Haaf, T.; Weigend, S.; Fries, R.; Buerstedde, J.-M.; et al. First report on chicken genes and chromosomes 2000. Cytogenet. Genome Res. 2000, 90, 169–218. [Google Scholar] [CrossRef]
- Yoshizawa, K.; Inaba, K.; Mannen, H.; Kikuchi, T.; Mizutani, M.; Tsuji, S. Analyses of beta-1 syntrophin, syndecan 2 and gem GTPase as candidates for chicken muscular dystrophy. Exp. Anim. 2003, 52, 391–396. [Google Scholar] [CrossRef][Green Version]
- Yoshizawa, K.; Inaba, K.; Mannen, H.; Kikuchi, T.; Mizutani, M.; Tsuji, S. Fine mapping of the muscular dystrophy (AM) gene on chicken chromosome 2q. Anim. Genet. 2004, 35, 397–400. [Google Scholar] [CrossRef]
- Matsumoto, H.; Maruse, H.; Yoshizawa, K.; Sasazaki, S.; Fujiwara, A.; Kikuchi, T.; Ichihara, N.; Mukai, F.; Mannen, H. Pinpointing the candidate region for muscular dystrophy in chickens with an abnormal muscle gene. Anim. Sci. J. 2007, 78, 476–483. [Google Scholar] [CrossRef]
- Matsumoto, H.; Sasazaki, S.; Mannen, H. Identification of the gene responsible for chicken muscular dystrophy. Korean J. Poult. Sci. 2011, 38, 1–10. [Google Scholar] [CrossRef]
- Zhi, X.; Chen, C.-S. WWP1: A versatile ubiquitin E3 ligase in signaling and diseases. Cell. Mol. Life Sci. 2011, 69, 1425–1434. [Google Scholar] [CrossRef]
- Matsumoto, H.; Maruse, H.; Sasazaki, S.; Fujiwara, A.; Takeda, S.; Ichihara, N.; Kikuchi, T.; Mukai, F.; Mannen, H. Expression Pattern of WWP1 in Muscular Dystrophic and Normal Chickens. J. Poult. Sci. 2009, 46, 95–99. [Google Scholar] [CrossRef][Green Version]
- Sluimer, J.; Distel, B. Regulating the human HECT E3 ligases. Cell. Mol. Life Sci. 2018, 75, 3121–3141. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Inba, Y.; Sasazaki, S.; Fujiwara, A.; Ichihara, N.; Kikuchi, T.; Mannen, H. Mutated WWP1 Induces an Aberrant Expression of Myosin Heavy Chain Gene in C2C12 Skeletal Muscle Cells. J. Poult. Sci. 2010, 47, 115–119. [Google Scholar] [CrossRef][Green Version]
- Silberstein, L.; Webster, S.G.; Travis, M.; Blau, H.M. Developmental progression of myosin gene expression in cultured muscle cells. Cell 1986, 46, 1075–1081. [Google Scholar] [CrossRef]
- Cho, E.-B.; Yoo, W.; Yoon, S.K.; Yoon, J.-B. β-dystroglycan is regulated by a balance between WWP1-mediated degradation and protection from WWP1 by dystrophin and utrophin. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2018, 1864, 2199–2213. [Google Scholar] [CrossRef] [PubMed]
- Imamura, M.; Nakamura, A.; Mannen, H.; Takeda, S. Characterization of WWP1 protein expression in skeletal muscle of muscular dystrophy chickens. J. Biochem. 2016, 159, 171–179. [Google Scholar] [CrossRef]
- Fambrough, D.M.; Devreotes, P.N. Newly synthesized acetylcholine receptors are located in the Golgi apparatus. J. Cell Biol. 1978, 76, 237–244. [Google Scholar] [CrossRef]
- Mcmahan, U.J. The Agrin Hypothesis. Cold Spring Harb. Symp. Quant. Biol. 1990, 55, 407–418. [Google Scholar] [CrossRef]
- Jacobson, C.; Côté, P.D.; Rossi, S.G.; Rotundo, R.L.; Carbonetto, S. The Dystroglycan Complex Is Necessary for Stabilization of Acetylcholine Receptor Clusters at Neuromuscular Junctions and Formation of the Synaptic Basement Membrane. J. Cell Biol. 2001, 152, 435–450. [Google Scholar] [CrossRef]
- Ohlendieck, K.; Ervasti, J.M.; Snook, J.B.; Campbell, K.P. Dystrophin-glycoprotein complex is highly enriched in isolated skeletal muscle sarcolemma. J. Cell Biol. 1991, 112, 135–148. [Google Scholar] [CrossRef]
- Matsumura, K.; Ervasti, J.M.; Ohlendieck, K.; Kahl, S.D.; Campbell, K.P. Association of dystrophin-related protein with dystrophin-associated proteins in mdx mouse muscle. Nature 1992, 360, 588–591. [Google Scholar] [CrossRef]
- Fambrough, D.M. Control of acetylcholine receptors in skeletal muscle. Physiol. Rev. 1979, 59, 165–227. [Google Scholar] [CrossRef] [PubMed]
- Schuetze, S.M.; Role, L. Developmental Regulation of Nicotinic Acetylcholine Receptors. Annu. Rev. Neurosci. 1987, 10, 403–457. [Google Scholar] [CrossRef] [PubMed]
- Williamson, R.A.; Henry, M.D.; Daniels, K.J.; Hrstka, R.F.; Lee, J.C.; Sunada, Y.; Ibraghimov-Beskrovnaya, O.; Campbell, K.P. Dystroglycan Is Essential for Early Embryonic Development: Disruption of Reichert’s Membrane in Dag1-Null Mice. Hum. Mol. Genet. 1997, 6, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.M.; Smart, E.J. Caveolae structure and function. J. Cell. Mol. Med. 2008, 12, 796–809. [Google Scholar] [CrossRef]
- Song, K.S.; Scherer, P.E.; Tang, Z.; Okamoto, T.; Li, S.; Chafel, M.; Chu, C.; Kohtz, D.S.; Lisanti, M.P. Expression of Caveolin-3 in Skeletal, Cardiac, and Smooth Muscle Cells. Caveolin-3 Is a Component of the Sarcolemma and Cofractionates with Dystrophin and dystrophin -associated glycoproteins. J. Biol. Chem. 1996, 271, 15160–15165. [Google Scholar] [CrossRef]
- Hagiwara, Y.; Nishina, Y.; Yorifuji, H.; Kikuchi, T. Immunolocalization of caveolin-1 and caveolin-3 in monkey skeletal, cardiac and uterine smooth muscles. Cell Struct. Funct. 2002, 27, 375–382. [Google Scholar] [CrossRef]
- Hezel, M.; De Groat, W.C.; Galbiati, F. Caveolin-3 Promotes Nicotinic Acetylcholine Receptor Clustering and Regulates Neuromuscular Junction Activity. Mol. Biol. Cell 2010, 21, 302–310. [Google Scholar] [CrossRef]
- Parton, R.G.; Way, M.; Zorzi, N.; Stang, E. Caveolin-3 Associates with Developing T-tubules during Muscle Differentiation. J. Cell Biol. 1997, 136, 137–154. [Google Scholar] [CrossRef]
- Ralston, E.; Ploug, T. Caveolin-3 Is Associated with the T-Tubules of Mature Skeletal Muscle Fibers. Exp. Cell Res. 1999, 246, 510–515. [Google Scholar] [CrossRef]
- Galbiati, F.; Engelman, J.A.; Volonte, D.; Zhang, X.L.; Minetti, C.; Li, M.; Hou, H.; Kneitz, B.; Edelmann, W.; Lisanti, M.P. Caveolin-3 Null Mice Show a Loss of Caveolae, Changes in the Microdomain Distribution of the Dystrophin-Glycoprotein Complex, and T-tubule Abnormalities. J. Biol. Chem. 2001, 276, 21425–21433. [Google Scholar] [CrossRef]
- Hagiwara, Y.; Sasaoka, T.; Araishi, K.; Imamura, M.; Yorifuji, H.; Nonaka, I.; Ozawa, E.; Kikuchi, T. Caveolin-3 deficiency causes muscle degeneration in mice. Hum. Mol. Genet. 2000, 9, 3047–3054. [Google Scholar] [CrossRef]
- Galbiati, F.; Volonté, D.; Minetti, C.; Bregman, D.B.; Lisanti, M.P. Limb-girdle Muscular Dystrophy (LGMD-1C) Mutants of Caveolin-3 Undergo Ubiquitination and Proteasomal Degradation. J. Biol. Chem. 2000, 275, 37702–37711. [Google Scholar] [CrossRef] [PubMed]
- Galbiati, F.; Volonté, D.; Chu, J.B.; Li, M.; Fine, S.W.; Fu, M.; Bermudez, J.; Pedemonte, M.; Weidenheim, K.M.; Pestell, R.G.; et al. Transgenic overexpression of caveolin-3 in skeletal muscle fibers induces a Duchenne-like muscular dystrophy phenotype. Proc. Natl. Acad. Sci. USA 2000, 97, 9689–9694. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Sasazaki, S.; Fujiwara, A.; Ichihara, N.; Kikuchi, T.; Mannen, H. Accumulation of caveolin-3 protein is limited in damaged muscle in chicken muscular dystrophy. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2010, 157, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Weinstock, I.; Behrendt, J.; Jones, K. Pyruvate kinase and creatine phosphokinase during development of the chicken with muscular dystrophy. Life Sci. 1977, 21, 1199–1205. [Google Scholar] [CrossRef]
- Echarri, A.; Del Pozo, M.A. Caveolae-mechanosensitive membrane invaginations linked to actin filaments. J. Cell Sci. 2015, 128, 2747–2758. [Google Scholar] [CrossRef]
- Costello, B.R.; Shafiq, S.A. Freeze-fracture study of muscle plasmalemma in normal and dystrophic chickens. Muscle Nerve 1979, 2, 191–201. [Google Scholar] [CrossRef]
- McLean, B.; Mazen-Lynch, L.; Shotton, D.; McLean, G.A. Quantitative freeze?fracture studies of membrane changes in chicken muscular dystrophy. Muscle Nerve 1986, 9, 501–514. [Google Scholar] [CrossRef]
- Sotgia, F.; Lee, J.K.; Das, K.; Bedford, M.; Petrucci, C.; Macioce, P.; Sargiacomo, M.; Bricarelli, F.D.; Minetti, C.; Sudol, M.; et al. Caveolin-3 directry interact with the C-terminal tail of β-dystroglycan. J. Biol. Chem. 2000, 275, 38048–38058. [Google Scholar] [CrossRef]
- Allen, E.R.; Murphy, B.J. Early detection of inherited muscular dystrophy in chickens. Cell Tissue Res. 1979, 197, 165–167. [Google Scholar] [CrossRef]
- Cohn, R.D.; Campbell, K.P. Molecular basis of muscular dystrophies. Muscle Nerve 2000, 23, 1456–1471. [Google Scholar] [CrossRef]
- Dulhunty, A.F.; Franzini-Armstrong, C. The relative contributions of the folds and caveolae to the surface membrane of frog skeletal muscle fibres at different sarcomere lengths. J. Physiol. 1975, 250, 513–539. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Schmid-Schönbein, G.W. Biomechanics of skeletal muscle capillaries: Hemodynamic resistance, endothelial distensibility, and pseudopod formation. Ann. Biomed. Eng. 1995, 23, 226–246. [Google Scholar] [CrossRef] [PubMed]
- Sinha, B.; Köster, D.V.; Ruez, R.; Gonnord, P.; Bastiani, M.; Abankwa, D.; Stan, R.V.; Butler-Browne, G.; Védie, B.; Johannes, L.; et al. Cells Respond to Mechanical Stress by Rapid Disassembly of Caveolae. Cell 2011, 144, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Feit, H.; Kawai, M.; Schulman, M.I. Stiffness and contractile properties of avian normal and dystrophic muscle bundles as measured by sinusoidal length perturbations. Muscle Nerve 1985, 8, 503–510. [Google Scholar] [CrossRef]
- Feit, H.; Kawai, M.; Mostafapour, A.S. The role of collagen crosslinking in the increased stiffness of avian dystrophic muscle. Muscle Nerve 1989, 12, 486–492. [Google Scholar] [CrossRef]
- Fujii, K.; Murota, K.; Tanzer, M.L. Abnormal collagen synthesis in skeletal muscle of dystrophic chicken. Biochem. Biophys. Res. Commun. 1983, 111, 933–938. [Google Scholar] [CrossRef]
- Sweeny, P.R. Ultrastructure of the developing myotendinous junction of genetic dystrophic chickens. Muscle Nerve 1983, 6, 207–217. [Google Scholar] [CrossRef]
- Holly, R.G.; Barnett, J.G.; Ashmore, C.R.; Taylor, R.G.; Molé, P.A. Stretch-induced growth in chicken wing muscles: A new model of stretch hypertrophy. Am. J. Physiol. Physiol. 1980, 238, C62–C71. [Google Scholar] [CrossRef]
- Barnett, J.G.; Holly, R.G.; Ashmore, C.R. Stretch-induced growth in chicken wing muscles: Biochemical and morphological characterization. Am. J. Physiol. Physiol. 1980, 239, C39–C46. [Google Scholar] [CrossRef]
- Loughna, P.T.; Izumo, S.; Goldspink, G.; Nadal-Ginard, B. Disuse and passive stretch cause rapid alterations in expression of developmental and adult contractile protein genes in skeletal muscle. Development 1990, 109, 217–223. [Google Scholar] [PubMed]
- Pattullo, M.C.; Cotter, M.A.; Cameron, N.E.; Barry, J.A. Effects of lengthened immobilization on functional and histochemical properties of rabbit tibialis anterior muscle. Exp. Physiol. 1992, 77, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Ashmore, C.R. Stretch-induced growth in chicken wing muscles: Effects on hereditary muscular dystrophy. Am. J. Physiol. Physiol. 1982, 242, C178–C183. [Google Scholar] [CrossRef]
- Lee, J.O.; Lee, S.K.; Kim, N.; Kim, J.H.; You, G.Y.; Moon, J.W.; Jie, S.; Kim, S.J.; Lee, Y.W.; Kang, H.J.; et al. E3 ubiquitin ligase, WWP1, Interacts with AMPKα2 and down-regulates its expression in skeletal muscle C2C12 cells. J. Biol. Chem. 2013, 288, 4673–4680. [Google Scholar] [CrossRef]
- Winder, W.W.; Holmes, B.F.; Rubink, D.S.; Jensen, E.B.; Chen, M.; Holloszy, J.O. Activation of AMP-activated protein kinase increases mitochondrial enzymes in skeletal muscle. J. Appl. Physiol. 2000, 88, 2219–2226. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, R.; Ren, J.M.; Cadman, K.S.; Moore, I.K.; Perret, P.; Pypaert, M.; Young, L.H.; Semenkovich, C.F.; Shulman, G.I. Chronic activation of AMP kinase results in NRF-1 activation and mitochondrial biogenesis. Am. J. Physiol. Metab. 2001, 281, E1340–E1346. [Google Scholar] [CrossRef] [PubMed]
- Saito, F.; Blank, M.; Schröder, J.; Manya, H.; Shimizu, T.; Campbell, K.P.; Endo, T.; Mizutani, M.; Kröger, S.; Matsumura, K. Aberrant glycosylation of α-dystroglycan causes defective binding of laminin in the muscle of chicken muscular dystrophy. FEBS Lett. 2005, 579, 2359–2363. [Google Scholar] [CrossRef]


| Muscle Fiber Types | Twitch Fibers | Tonic Fibers | |||
|---|---|---|---|---|---|
| Ashmore and Doerr (1971) [2] | αW | αR | βR | α’ | β’ |
| Brooke and Kaiser (1970) [8] | ||B | ||A | | | |||A | |||B |
| Histochemical criteria | |||||
| ATPase (pH 10) | ● | ● | ○ | ● | ● |
| ATPase (pH 4.1) | ○ | ○ | ● | ◎ | ● |
| SDH or NADH-TR | ○ | ◎ | ● | ◎ | ● |
| Phosphorylase | ● | ◎ | ○ | ◎ | ◎ |
| Innervation pattern | Focal | Focal | Multiple | Multiple | Multiple |
| Enzyme | Age (Days) | Normal b (mU/mL) | Dystrophy b (mU/mL) | Heterozygote b (mU/mL) |
|---|---|---|---|---|
| PK | 37 | 401 ± 111(8) | 12,430 ± 6,269(4) c | 630 ± 209(5) |
| 70–86 | 405 ± 73(4) | 10,773 ± 6,800(7) c | 1,213 ± 552(10) d | |
| 475 | 229 ± 33(7) | 8,940 ± 4,032(5) c | 516 ± 138(7) d | |
| CPK | 37 | 141 ± 55(8) | 1,071 ± 812(4) c | 180 ± 31(5) |
| 70–86 | 164 ± 41(4) | 1,146 ± 599(7) c | 183 ± (10) | |
| 475 | 30 ± 9(7) | 986 ± 586(5) c | 46 ± 10(7) e |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kikuchi, T. Caveolin-3: A Causative Process of Chicken Muscular Dystrophy. Biomolecules 2020, 10, 1206. https://doi.org/10.3390/biom10091206
Kikuchi T. Caveolin-3: A Causative Process of Chicken Muscular Dystrophy. Biomolecules. 2020; 10(9):1206. https://doi.org/10.3390/biom10091206
Chicago/Turabian StyleKikuchi, Tateki. 2020. "Caveolin-3: A Causative Process of Chicken Muscular Dystrophy" Biomolecules 10, no. 9: 1206. https://doi.org/10.3390/biom10091206
APA StyleKikuchi, T. (2020). Caveolin-3: A Causative Process of Chicken Muscular Dystrophy. Biomolecules, 10(9), 1206. https://doi.org/10.3390/biom10091206




