Abstract
The exponentially correlated Hylleraas–configuration interaction method (E-Hy-CI) is a generalization of the Hylleraas–configuration interaction method (Hy-CI) in which the single of an Hy-CI wave function is generalized to a form of the generic type . This work continues the exploration, begun in the first two papers in this series (on the helium atom and on ground and excited states of Li II), of whether wave functions containing both linear and exponential factors converge more rapidly than either one alone. In the present study, we examined not only 1 states but 1s2p states for the He I, Li II, Be III, C V and O VII members of the He isoelectronic sequence as well. All energies except He I are better than previous results. The wave functions obtained were used to calculate oscillator strengths, including upper and lower bounds, for the He-sequence lowest (resonance) transition. Interpolation techniques were used to make a graphical study of the oscillator strength behavior along the isoelectronic sequence. Comparisons were made with previous experimental and theoretical results. The results of this study are oscillator strengths for the 1 1s2 He isoelectronic sequence with rigorous non-relativistic quantum mechanical upper and lower bounds of (0.001–0.003)% and probable precision ≤ 0.0000003, and were obtained by extending the previously developed E-Hy-CI formalism to include the calculation of transition moments (oscillator strengths).
1. Introduction
The Hylleraas–configuration interaction (Hy-CI) technique (developed by Sims and Hagstrom (SH) [1] and also independently by Woźnicki [2]) is a hybrid approach that attempts to combine the high precision of explicitly correlated traditional Hylleraas (Hy) [3] methods with the analytical tractability of strictly orbital-based configuration interaction (CI) methods [4]. The use of configurations wherever possible leads to less difficult integrals than in a purely Hy1 approach whose progress beyond the three electron atomic level has been hindered by the intractability of integrals involving nonseparable products of factors of the form . If one restricts the wave function to at most a single, linear interelectronic distance factor in each configuration state function (CSF), then the most difficult integrals are already dealt with at the four-electron level and the calculation retains the precision of Hy techniques, but is greatly simplified by the use of CI basis sets. However, for ≥ 4, there exists an ultimately slowly convergent “” double cusp that is built into Hy-CI for four or more electrons. Consequently, methods that speed up the convergence of an factor can facilitate the extension of Hy-CI to systems with > 4.
In this paper, we continue to explore2 a generalization of the Hy-CI method proposed by Wang, Mei, Kurokawa, Nakashima and Nakatsuji [8], the exponentially correlated Hylleraas–configuration interaction (E-Hy-CI) method, in which the single of an Hy-CI wave function is generalized to a form of the generic type . Pairing an exponential factor with linear was first suggested for helium by Hirschfelder who, in 1960 [9], suggested that this type of correlation factor has the right behavior in the vicinity of the cusp, and also has the right behavior as goes to infinity. Whether functions having both linear and exponential factors might be more rapidly convergent than either one alone was not demonstrated until our recent studies of the ground S state of the helium atom [6] and not only the ground 1 state of the Li ion (Li II), but the 2 through 6 excited states as well [7].3 The present contribution continues this work by comparing the convergence of the E-Hy-CI wave function expansion to that of the Hy-CI wave function without exponential factors for states of non- symmetry, specifically the 1s2 (2 ) state of some members of the He isoelectronic sequence.
The computed 1s2p state wave functions were used with the previously obtained wave functions (and some new ones for Li II, Be III, C V and O VII) to calculate He-sequence oscillator strengths, including upper and lower bounds, for the lowest transition.
The calculations reported in this work were obtained by solving the non-relativistic Schrödinger equation. Chung et al. [15] discussed the relative importance of correlation effects versus relativistic effects and stated that, for Z ≤ 27, correlation effects dominate relativistic effects. Consequently, one should start out with the best possible solutions to the non-relativistic Schrödinger equation and treat relativistic effects as a perturbation. Since this work considers first-row atoms for which Z ≤ 10, solving the non-relativistic Schrödinger equation is meaningful.
2. Variational Calculations
For electrons, the total non-relativistic, stationary-point-nucleus energy is defined as the exact solution (eigenvalue) of the time-independent, non-relativistic Schrödinger equation
where the Hamiltonian is defined as (in hartree atomic units)
Here, = , being a one-electron operator (electron i) consisting of a kinetic energy part and a nuclear attraction part . denotes the number of electrons and Z the corresponding nuclear charge.
The E-Hy-CI wave function for electron atomic states is
where
denotes the th antisymmetrized orbital and spin angular momentum projected explicitly correlated configuration state function (CSF). is the symmetry adaptation operator that performs the projection and is a product of an idempotent projection operator that makes the state antisymmetric, , and , , the idempotent orbital and spin angular momentum projection operators of Löwdin type [16] for a state of total quantum numbers (Russell–Saunders () coupling is assumed). Each term K contains at most one explicitly correlated factor that, in addition to the Hy-CI case with , can also have . In practice, it is sufficient to take equal to 0 or 1, with , the Hy-CI case and , the CI case.4 is a primitive spin product function for term K and represents the sth basis orbital in the th term.
The basis orbitals are un-normalized Slater-type orbitals (STOs) that are defined as
where is a normalized spherical harmonic in the Condon and Shortley phase convention [19]. With this choice of phase convention, the spherical harmonics satisfy
The coefficients in Equation (3) were found in this work by solving the generalized eigenvalue problem
where the matrix elements are given by and with the Hamiltonian H given by Equation (2) and being some starting approximation for the eigenvalue E of interest.
The details of how the authors solve this real symmetric-definite eigenvalue problem are discussed in [20], including how to obtain a quadruple precision version of our computationally fast Fortran 90+ portable parallel package suitable for large (80,000 × 80,000 or greater) dense matrices. All results reported in this article were obtained using real*32 extended precision (quad-double-with exponent or QDE, 256-bit, ≈64 digits) floating point arithmetic, and the message-passing interface (MPI) standard [21] was used to parallelize the code.
Method of Calculation
For two electrons, following Hy-CI [22], spin can be eliminated, and the E-Hy-CI wave function becomes [6,7]
where the terms are specifically of the form
is the operator that permutes electrons 1 and 2; the plus sign is for singlet levels and the minus sign is for triplet levels. Equation (2) becomes
where .
In the generalized eigenvalue problem , matrix element is
where denotes the th primitive (unprojected) function as given in Equation (11) above. is similar, with replaced by the unit operator 1.
The projection reduction of these matrix elements is the same projection reduction used in Hy-CI calculations on lithium [23] and on beryllium [24] and is discussed in Appendix A of those references. The helium case is simpler so just the result is given here, which is that is given by
where is the correlation factor and is a product of un-normalized STOs (Equation (5)). In Equation (14), one can apply either before or after applying B = 1 + . The projection is applied only on the orbital products since commutes with [1].
In generalizing Hy-CI to E-Hy-CI for the two-electron He-sequence case, one merely has to take the correlation factor to be = in the formulas above since has the same symmetry properties as . Thus, becomes
A detailed discussion of the most general exponentially correlated integrals that have to be evaluated when using this wave function can be found in Section 3 of our recent study [6] of the ground state of helium.
3. Oscillator Strengths
The basic theory of electric dipole radiation in atomic systems was given by Condon and Shortley [19]; their terminology is followed closely herein. Assuming Russell–Saunders () coupling and the non-relativistic many-electron Hamiltonian (Equation (2)), the energy states of an atom are characterized by the set of quantum numbers (here, denotes the electronic configuration5). For an electric dipole transition connecting the terms (multiplets) and , Condon and Shortley introduced the quantity ;), called the absolute multiplet oscillator strength (line strength). It is defined in terms of the electric dipole moment operator
by
The f-value or multiplet oscillator strength is related to S by
The familiar electric dipole selection rules = 0, = 0, = and = 0, hold for the Hamiltonian that we used. Moreover, given and , ,(=S), as a consequence of the Wigner–Eckart theorem, every nonvanishing matrix element of the operator can be expressed in terms of a single parameter multiplied by expressions involving only and . The parameter is independent of the quantum numbers and , the summation indices occurring in Equation (17). Consequently, the expressions for the multiplet oscillator strength may be written as products of a parameter and a single algebraic function of L and S resulting from the summation. Finally, notice that only one nonvanishing matrix element needs to be evaluated to determine a , so, instead of computing all the matrix elements occurring in the summations in Equation (17), a single matrix element suffices to find the multiplet oscillator strength for a given .
For the 1 1s2 transition, the term consists of the one state with , . The 1s2 term consists of three states corresponding to , and . We choose to express (→) in terms of the matrix element corresponding to the state with , and . Then, Equation (17) for becomes (un-normalized wave functions), from Goldberg [25],
and we only need to construct = ) and = ) and evaluate Equation (19) to compute the line strength S and then the non-relativistic oscillator strength f.6
To evaluate Equation (19), we use the method developed by one of the authors (see Section 2.B in [29]) which leads to the following expression for S:
where , the electronic dipole transition moment, is defined by
in terms of un-normalized STOs (Equation (5)). To make the notation clearer, is just the STO , i.e., it is a STO whose orbital exponent equals 0.
To evaluate Equation (20), we use the fact that the wave functions employed to evaluate Equation (20) are given by Equations (3) and (4) and are linear combinations of antisymmetrized projected products of orbitals and at most one correlation factor of the form .
Thus,
where
In Equation (23), is a linear combination of orbitals and at most one correlation factor, so Equation (23) can be evaluated by expanding the product of and the appropriate STO of in terms of STOs by the formulas given in a previous paper [30]. Then, the integrals required to evaluate Equation (20) are similar in type to those required to calculate overlap matrix elements using these wave functions and can be evaluated as described in the next section.
Transition Moment Integrals
In Equation (23), the exponentially correlated transition moment integrals that have to be evaluated are of the form
where the operator = for transition moment integrals. The key to evaluating these integrals is to recognize that we can convert these integrals into overlap integrals ( = 1) by recognizing that and are STOs and can be expanded in terms of STO-like functions
in a manner similar to the way “charge distributions”
are expanded. When is given by
the formula becomes
where and the exponents are . satisfies the triangular inequality , with (g integral, i.e., the summation goes in steps of 2), and with the further restriction , . The are standard Condon and Shortley coefficients (the so-called s) [19,30,31,32]. Using Equation (28) transforms the transition moment integrals into the form of Equation (19) of the first paper in this series [6] with = 1, which can be evaluated by the methods of Section 3.1 of that paper.
4. Upper and Lower Bounds to Oscillator Strengths
The f-value or multiplet oscillator strength is notoriously sensitive to the wave functions employed in computing S via Equation (19) (S is then used in Equation (18) to compute f) [33,34] because significant contributions to the integral may arise from regions that do not significantly affect the energies of either state. To remedy this situation, Weinhold showed how to calculate rigorous upper and lower bounds to f-values (dipole oscillator strengths) from non-relativistic quantum mechanical principles [35,36]. While bounds were computed for a few atomic and molecular oscillator strengths [29,37,38,39], the rigorous bounds did not reflect the true precision of the computed oscillator strengths. For example, one of the co-authors calculated oscillator strengths, including rigorous (non-relativistic, infinite nuclear mass) quantum mechanical upper and lower bounds, for the lowest → transition of Be I, C III and O V [29] of (7–10)% and probable accuracy of ≤2%. The same co-author computed oscillator strengths, with rigorous upper and lower bounds, for the 16 lines arising from allowed transitions among the four lowest and four lowest states of Li I [38] of 3.5% in the best case () (the error in the best case was later shown to actually be approximately 0.3% [26]).
To obtain good bounds, the calculations have to be of higher precision, as was the case in the Anderson and Weinhold calculations of dipole oscillator strengths for He and Li in 1974 [37] in which a third of the calculated f-values were guaranteed to be 1% or better; in the best case (Li 2 ), the bounds tightened to approximately 0.05%, around two orders of magnitude better than the corresponding experimental results. Perhaps because of the need for high precision, the most recent bounds calculation took place in 1976 (except for one in 1986 for the special case of the hydrogen atom [40] and one in 1988 for the special case of Hartree–Fock wave functions [41]). However, computational capacity has continued to evolve so that more and more high-precision wave functions are becoming available, and, in the case of the two-electron helium atom, the E-Hy-CI wave functions are precise enough energetically to suggest using them to compute oscillator strengths with upper and lower bounds to provide rigorous estimates of the uncertainty in the computed f-values.
Method of Calculation
Weinhold’s procedure [35] for calculating rigorous upper and lower bounds to dipole strengths actually computes bounds to the transition moment, from which, as we have seen, f-values (oscillator strengths) can be computed. In terms of approximate wave functions and for states a and b, respectively, the approximate transition moment is
The following additional quantities are required for the calculation of error bounds (see Section 2.C in [29]): the overlap integrals of the approximate wave functions with the true wave functions ,
the corresponding overlap errors , ,
and an uncertainty that is defined by
Upper and lower bounds can then be calculated from the inequalities
where the subscript ± signs mean the upper (+) or lower (-) bounds to the corresponding quantities.
With ’s defined by Equation (23), we have that
which relates upper and lower bounds to transition moments and upper and lower bounds to oscillator strengths.
5. Wave Functions
The basis set orbitals in Hy-CI and E-Hy-CI atomic calculations are STOs; see Equation (5). For the nonlinear orbital exponent parameters for 1 (1 ) states, the use of the “doubled” (orbital exponent) basis set E-Hy-CI introduced in paper I and continued in paper II was also continued here. In this treatment, the first set has an orbital with an orbital exponent that makes it essentially a valence shell orbital, whereas, for the second set, the orbital has a large exponent that brings it in closer to the nucleus. This approach is due to Drake [42,43], who pointed out the need for “doubling” basis sets so there is a natural partition of the basis set into two distinct distance scales: one appropriate for the complex correlated motion near the nucleus, and the other appropriate further out. The results are very good for the ground state of helium [6] and Li ground and excited states [7] of symmetry; that approach was continued here in the treatment of other members of the helium isoelectronic sequence.
In contrast to the closed-shell 1 states, the states are open-shell 1s2p states with the two electrons having different symmetries. The two different orbital exponent basis sets for are chosen to be representative of the two different electrons; there is no natural partition of the basis set into two distinct distance scales. In this case, pairing an exponential factor with linear may have an enhanced role in providing the right behavior in the vicinitiy of the cusp and also the right behavior as goes to infinity. How well this approach works for non- states was studied by carrying out calculations on the lowest helium state (2 ) and then extending the He 2 state calculation to other members of the He 2 isoelectronic sequence.
It is worth noting that the calculations are formally no different from the calculations since the idempotent orbital and spin angular momentum projection operators of the Löwdin type in Equation (4) will project out linear combinations of the basis set orbitals with the correct orbital and spin angular momentum.
5.1. Comparison with Orthogonal Hy-CI
Table 1 presents a comparison of the convergence of an E-Hy-CI wave function expansion for He with that of the Hy-CI wave function without exponential factors. Included also for comparison with these non-orthogonal wave function results are the orthogonal Hy-CI (O-Hy-CI) results of Zhang et al. [17]. The groundwork for O-Hy-CI was laid out in the 1960s by Szasz in his “General Theory of Correlated Wave Functions” [44], but his calculations were orthogonal Hy [45,46] (the orbitals were s-only STOs and not p-, d-, … orbitals, which are the hallmark of CI). The first genuine O-Hy-CI was carried out by Plute in 1984 [47] with calculations on four He states, the He being the one relevant to this research. Using an s-, p-, d- and f- STO basis, Plute was able to achieve an energy of −2.1238 4260 hartree with 90 terms in the expansion, better at the time than all but the perimetric calculation of Schiff et al. [48] and the Hy calculation of Anderson and Weinhold [37]. To the authors’ knowledge, this remained the only O-Hy-CI calculation until the O-Hy-CI calculation of Zhang et al. [17], which is significant, as will be seen, in that the O-Hy-CI results, as do the E-Hy-CI results, speed up convergence over non-orthogonal Hy-CI wave functions and hence might help to overcome the ultimately slow convergence of “the double cusp”, analogous to the cusp problem for CI, that arises for ≥ four-electron systems.
5.2. Comparison of E-Hy-CI and Hy-CI
To clarify the E-Hy-CI and Hy-CI approach of the authors to orbital basis sets (and their optimization) for states, consider Table 1. In contrast to states, each line in the table specifies an inner set for the first electron and its associated l quantum number (=), and an outer set with its associated and quantum number for the second electron. In the table, only the minimum information needed to specify the basis set is listed, namely an exponent , the l quantum number for orbitals with that exponent, , the number of orbitals with that exponent (the in Equation (5) run from to ), and , the exponent of the exponentially correlated factor if this is an E-Hy-CI calculation. In all of the tables, N is used to refer to the number of terms in the wave function (see Equation (3)).
Table 1 displays the steps leading to the final 7744-term 2 state wave function used in this work. Starting with a fixed = 1.085 for = {0,1}, was varied until the energy went through a minimum and there were enough data points to fit a smooth curve and yield an optimized = 2.272. Next, was fixed at 2.272 and was optimized in a similar fashion, yielding the optimized values of = {2.272,1.291} for the 480 term wave function used in the optimization. and were then extended to 19 for an = {0,1} 722-term Hy-CI wave function of ≈0.015 millihartree precision. The effect of the exponential on the precision of the calculation is clearly evident when adding to each term an exponentially correlated factor whose exponent is chosen to be the one (0.5) that satisfies the Kato cusp condition [49], yielding a 1444-term E-Hy-CI wave function of ≈15 hartree precision. The speedup of O-Hy-CI over non-orthogonal Hy-CI wave functions is readily apparent at this point as the 514-term O-Hy-CI calculation of Zhang et al. [17] improves the 1444-term Hy-CI to greater than nanohartee precision (but the E-Hy-CI precision is better). This strategy was continued to the = {1,2} block, yielding optimized values of = {1.955,2.25} for the 1444-term Hy-CI wave function and the 2888-term E-Hy-CI wave function, which is of ≈ picohartree precision. Thanks to the very high-precision exponentially correlated Hylleraas (E-Hy) calculation of Aznabaev et al. [50], it is clear that the = 3 (s-, p-, d- and f- STO) Hy-CI precision for this state is 10 decimal places and the E-Hy-CI precision is >13 decimal places, which is better than all previous calculations except for the Hy calculation of Drake [43] and the calculation by Aznabaev et al. The results from continuing the comparison through = 7 are tabulated in Table 1.


Table 1.
Comparison of theoretical Hy-CI, E-Hy-CI and O-Hy-CI energies for the He 2 state using two different orbital exponents () for electrons 1 and 2. Calculations utilized in obtaining the optimal , are included. For each , + 1. N is the number of terms in the expansion.
Table 1.
Comparison of theoretical Hy-CI, E-Hy-CI and O-Hy-CI energies for the He 2 state using two different orbital exponents () for electrons 1 and 2. Calculations utilized in obtaining the optimal , are included. For each , + 1. N is the number of terms in the expansion.
| Technique | Author | N | Energy (Hartree) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hy-CI 1 | This work | 0 | 16 | 2.28 | 1 | 15 | 1.085 | 480 | −2.1238 2778 5160 1660 5130 | |
| Hy-CI | This work | 0 | 16 | 2.272 | 1 | 15 | 1.085 | 480 | −2.1238 2778 5160 4328 8615 | |
| Hy-CI | This work | 0 | 16 | 2.270 | 1 | 15 | 1.085 | 480 | −2.1238 2778 5160 4106 3517 | |
| Hy-CI | This work | 0 | 16 | 2.272 | 1 | 15 | 1.300 | 480 | −2.1238 2780 3592 4727 1690 | |
| Hy-CI | This work | 0 | 16 | 2.272 | 1 | 15 | 1.291 | 480 | −2.1238 2780 3657 1217 9787 | |
| Hy-CI | This work | 0 | 16 | 2.272 | 1 | 15 | 1.290 | 480 | −2.1238 2780 3655 3377 1118 | |
| Hy-CI | This work | 0 | 16 | 2.272 | 1 | 15 | 1.291 | 480 | −2.1238 2780 3657 1217 9787 | |
| E-Hy-CI | This work | 0 | 16 | 2.272 | 1 | 15 | 1.291 | 0.5 | 960 | −2.1238 4308 3927 0924 8309 |
| Hy-CI | This work | 0 | 19 | 2.272 | 1 | 19 | 1.291 | 722 | −2.1238 2782 6872 0251 1341 | |
| E-Hy-CI | This work | 0 | 19 | 2.272 | 1 | 19 | 1.291 | 0.5 | 1444 | −2.1238 4308 5062 4897 8422 |
| Hy-CI | This work | 1 | 15 | 2.000 | 2 | 14 | 0.75 | 900 | −2.1238 4303 4769 4360 5998 | |
| Hy-CI | This work | 1 | 15 | 1.955 | 2 | 14 | 0.75 | 900 | −2.1238 4303 4770 5696 3685 | |
| Hy-CI | This work | 1 | 15 | 1.900 | 2 | 14 | 0.75 | 900 | −2.1238 4303 4768 6097 2667 | |
| Hy-CI | This work | 1 | 15 | 1.955 | 2 | 14 | 2.30 | 900 | −2.1238 4307 3318 1068 7228 | |
| Hy-CI | This work | 1 | 15 | 1.955 | 2 | 14 | 2.25 | 900 | −2.1238 4307 3463 3468 4622 | |
| Hy-CI | This work | 1 | 15 | 1.955 | 2 | 14 | 2.20 | 900 | −2.1238 4307 2960 1221 3901 | |
| Hy-CI | This work | 1 | 15 | 1.955 | 2 | 14 | 2.25 | 900 | −2.1238 4307 3463 3468 4622 | |
| E-Hy-CI | This work | 1 | 15 | 1.955 | 2 | 14 | 2.25 | 0.5 | 1800 | −2.1238 4308 6441 2634 4197 |
| Hy-CI | This work | 1 | 19 | 1.955 | 2 | 19 | 2.25 | 1444 | −2.1238 4307 5774 3704 6662 | |
| O-Hy-CI | Zhang et al. [17] | 6 2 | 1.169 3 | 4 4 | 0.28419 3 | 514 | −2.1238 4308 6457 05 | |||
| E-Hy-CI | This work | 1 | 19 | 1.955 | 2 | 19 | 2.25 | 0.5 | 2888 | −2.1238 4308 6497 9644 7703 |
| Hy-CI | This work | 2 | 19 | 2.40 | 3 | 18 | 2.30 | 2128 | −2.1238 4308 6479 8938 8807 | |
| E-Hy-CI | This work | 2 | 19 | 2.40 | 3 | 18 | 2.30 | 0.5 | 4256 | −2.1238 4308 6498 0912 9534 |
| Hy-CI | This work | 3 | 18 | 2.40 | 4 | 15 | 2.30 | 2668 | −2.1238 4308 6491 8108 5041 | |
| E-Hy-CI | This work | 3 | 18 | 2.40 | 4 | 15 | 2.30 | 0.5 | 5336 | −2.1238 4308 6498 0989 3227 |
| Hy-CI | This work | 4 | 15 | 2.40 | 5 | 14 | 2.30 | 3088 | −2.1238 4308 6491 9211 6091 | |
| E-Hy-CI | This work | 4 | 15 | 2.40 | 5 | 14 | 2.30 | 0.5 | 6176 | −2.1238 4308 6498 1004 0079 |
| Hy-CI | This work | 5 | 14 | 2.40 | 6 | 14 | 2.30 | 3480 | −2.1238 4308 6491 9773 5726 | |
| E-Hy-CI | This work | 5 | 14 | 2.40 | 6 | 14 | 2.30 | 0.5 | 6960 | −2.1238 4308 6498 1008 1958 |
| Hy-CI | This work | 6 | 14 | 2.40 | 7 | 14 | 2.30 | 3872 | −2.1238 4308 6492 0145 5741 | |
| E-Hy-CI | This work | 6 | 14 | 2.40 | 7 | 14 | 2.30 | 0.5 | 7744 | −2.1238 4308 6498 1009 6317 |
| O-Hy-CI | Plute (1984) [47] | 90 | −2.1238 4260 | |||||||
| Hy | AW 5 (1974) [37] | 2.00 | 0.88 | 137 | −2.1238 4303 14 | |||||
| Hy | SLPR 6 (1965) [48] | 560 | −2.1238 4308 5800 | |||||||
| O-Hy-CI | Zhang et al. [17] | 6 2 | 1.169 3 | 4 4 | 0.28419 3 | 514 | −2.1238 4308 6457 05 | |||
| Hy | Drake (1996) [43] | 804 | −2.1238 4308 6498 091 | |||||||
| E-Hy-CI | This work | 6 | 14 | 2.40 | 7 | 14 | 2.30 | 0.5 | 7744 | −2.1238 4308 6498 1009 6317 |
| Reference | Aznabaev et al. [50] | 22,000 | −2.1238 4308 6498 1013 5924 | |||||||
| (E-Hy) 7 | (2018) | … 7333 1423 74 |
Note: All energies in this work are variational; all calculations were carried out in quadruple-double-with exponent (QDE) precision. 1 For a detailed discussion of both E-Hy-CI and Hy-CI, including how they compare with the other methods in this table and elsewhere in this research, see the authors’ review article [14]. 2 6 here represents = 7, 6, 5, 4, 3, 2 and 1, respectively, for . 3 Jiao [51]. 4 4 here represents = 5, 4, 3, 2 and 1, respectively, for . 5 Anderson and Weinhold. 6 Schiff, Lifson, Pekeris and Rabinowitz. 7 This line continues the line above, giving the trailing digits from Table II of Aznabaev et al. [50].
The formula of Pekeris and co-workers [48],
where the , and values are the 6176, 6960 and 7744-term results in Table 1, is used to extrapolate to an estimated exact non-relativistic energy of this state.
These Table 1 results show dramatic improvements:
- Hy-CI -wave 4 decimal place precision becomes 8 decimal place E-Hy-CI -wave precision (> Hy-CI -wave precision),
- Hy-CI -wave 7 decimal place precision becomes 11 decimal place E-Hy-CI -wave precision (> Hy-CI l = 7 precision),
- already at the E-Hy-CI -wave expansion, the result is better than the Hy-CI l = 7 result.
It is clear from the Table 1 results that the 14 decimal place l = 7 Hy-CI result can be improved on with just an l = 3 E-Hy-CI basis; this demonstrates that the E-Hy-CI speed-up convergence over non-orthogonal Hy-CI wave functions for states continues for non- states as well (specifically, for states).
6. Isoelectronic Sequences
The 8568-term wave function for the 1 state of He I of estimated 20 decimal digit precision and the 7744-term wave function for the 1s2p state of estimated 16 decimal digit precision were used to calculate wave functions and energies for other members of the He isoelectronic sequence, namely Li II, Be III, C V and O VII. For these results, no exponent minimization was performed, just a simple nuclear charge-based scaling of the wave functions of Table 4 of Sims et al. [6] and Table 1, respectively. For example, the results for Be III were obtained by scaling the orbital exponents by a factor of 4.0/2.0 and, for C V, the orbital exponents were scaled by a factor of 6.0/2.0. Our results are tabulated in Table 2 and compared with the best previous calculations for these states. Since the 7744-term result is better than the best previous calculation for Li II through O VIII, the formula of Pekeris and co-workers [48] given in Equation (35) was again used to extrapolate to an estimated exact non-relativistic energy for these states; the , and 6176, 6960 and 7744-term results are included in Table 2. The complete E-Hy-CI wave function expansions for all calculations used in this study are enumerated in Appendix A Table A1 () and Table A2 ().

Table 2.
Correlated wave function He isoelectronic sequence 1 and 2 non-relativistic energies. N is the number of terms in the expansion.
7. f-Values and Bounds
As discussed in Section 3 and Section 4, theoretical upper and lower bounds to f-values (multiplet oscillator strengths7) can be computed from non-relativistic quantum mechanical principles. These bounds do not, however, reflect the true precision of the calculation and are usually too crude to provide useful error estimates, even with fairly high-precision electronic wave functions. This has led to efforts by Roginsky and co-workers ([40,41] to tighten the error bounds. While these efforts have led to improved results for the special cases of the hydrogen atom [40] and alkali atoms [41], it is not clear to what extent they will be effective in the more general case. However, in the case of the two-electron helium atom, the E-Hy-CI wave functions are precise enough energetically to suggest using them to compute oscillator strengths with upper and lower bounds to provide rigorous estimates of the uncertainty in the computed f-values. Our computed f-values8 (multiplet oscillator strengths), along with rigorous non-relativistic quantum mechanical upper and lower bounds for the 1 1s2p resonance transition for some members of the He isoelectronic sequence, are presented in Table 3.
Froese Fischer [26] presented some general variational methods that rely on precision indicators and discussed precision in a general way. She pointed out that, for light atoms (using C III as an example), the relativistic correction is important for a precise energy but the line strength (and hence the oscillator strength) is close to the non-relativistic one. Helium is the simplest multi-electron atom and there exists a recent high-precision multi-configuration Dirac–Hartree–Fock (MCDHF) calculation (using the GRASP2K [54,55,56] program9) of the transition [28]. Two lines in the data for He I in the table (the LLWQ lines [28]) are from this study and are included here to ascertain the magnitude of the relativistic effects for the He I transition, which turn out to be ≈0.00003 (≈0.01%) and indeed show relativistic effects to be small. Note that all of the high-precision studies (all correlated wave function calculations) are in agreement that the oscillator strength is 0.27616. Our results are in agreement and extend the precision of the non-relativistic (NR) oscillator strength by two orders of magnitude, based on the rigorous quantum mechanical upper and lower bounds. We note here that these results show that the Anderson and Weinhold [37] result is better than their bounds suggest, which is probably true for our results as well. Specifically, we know from the 3040 × 2888 result that the 1088 × 960 and 1520 × 1444-term results are in error in the seventh decimal digit, but the bounds suggest that the results are only good to three decimal places. We recommend the NR oscillator strength to be 0.2761 647(28) for He I.
For Li II, our results are in agreement with the previous high-precision correlated wave function calculations for this ion, those of Schiff, Pekeris and Accad [58], Anderson and Weinhold [37] and Cann and Thakkar [53]. These results for Li II are similar to the He I results in that they show that the Anderson and Weinhold [37] result for Li II is better than their bounds suggest, which again is probably true for our results as well. We again note that, from the 3040 × 2888 result, it is clear that the 1088 × 960 and 1520 × 1444-term results are in error in the seventh decimal digit, but the bounds suggest that the results are only good to three decimal places. For Li II, we recommend the NR oscillator strength to be 0.4566 298(18) and note the excellent agreement with Cann and Thakkar [53]. Comparing our recommended NR oscillator strength with the Wiese and Fuhr [59] reference value, which includes relativistic effects, puts the magnitude of the relativistic effects for the Li II transition as ≈0.00005 (again ≈0.01%) and shows relativistic effects to be small for this member of the isoelectronic sequence (similar to He I). For Be III, C V and O VII (chosen to be representative of the first row of the He-isoelectronic sequence), our results again agree with the previous high-precision correlated wave function calculations for these ions. We recommend the NR oscillator strengths to be 0.5515 538(21), 0.6470 674(22) and 0.6944 496 (22).


Table 3.
Non-relativistic multiplet oscillator strengths (f-values) with rigorous upper and lower bounds for the 1 1s2 transition for some members of the helium isoelectronic sequence. Oscillator strengths computed by other authors are included for comparison.
Table 3.
Non-relativistic multiplet oscillator strengths (f-values) with rigorous upper and lower bounds for the 1 1s2 transition for some members of the helium isoelectronic sequence. Oscillator strengths computed by other authors are included for comparison.
| State | Technique | Author | N () | E () (Hartree) | N () | E () (Hartree) | f-Value |
|---|---|---|---|---|---|---|---|
| He | E-Hy-CI | This work | 1088 | −2.9037 2435 4189 0888 5088 58 | 960 | −2.1238 4308 3927 0924 8309 | 0.2761 6466 27 ±0.0012 |
| He | E-Hy-CI | This work | 1520 | −2.9037 2435 4275 7390 6714 30 | 1444 | −2.1238 4308 5062 4897 8422 | 0.2761 6463 24 ±0.0012 |
| He | E-Hy-CI | This work | 3040 | −2.9037 2437 7034 1043 9282 03 | 2888 | −2.1238 4308 6497 9644 7703 | 0.2761 6470 03 ±0.0000 028 |
| He | E-Hy-CI | Recommended | 0.2761 647(28) | ||||
| He | Hy | SPA 1 (1971) [58] | 1078 | −2.9037 2437 48 | 364 | −2.1238 4308 26 | 0.2761 6 |
| He | Hy-CI | AW 2 (1974) [37] | 135 | −2.9037 2436 62 | 137 | −2.1238 4303 14 | 0.2761 ±0.0014 |
| He | Hy | KH 3 (1984) [60] | 138 | −2.9037 2437 51 | 140 | −2.1238 4308 | 0.2761 6 |
| He | E-Hy | CT 4 (1992) [53] | 100 | −2.9037 2437 36 | 100 | −2.1238 4308 02 | 0.2761 7 |
| He | Hy | DM 5 (2007) [61] | 0.2761 6 | ||||
| He | MCHF 6 | LLWQ 7 (2021) [28] | 0.2762 9 | ||||
| He | MCDHF 8 | LLWQ 9 (2021) [28] | 0.2763 2 | ||||
| He | Reference 9 | WF 10 (2009) [59] | 0.2762 5 | ||||
| Li II | E-Hy-CI | This work | 1088 | −7.2799 1332 7713 3474 7954 38 | 960 | −4.9933 5106 5519 3789 3549 | 0.4566 2972 51 ±0.0016 |
| Li II | E-Hy-CI | This work | 1520 | −7.2799 1332 8098 0780 5105 69 | 1444 | −4.9933 5106 5763 9277 0706 | 0.4566 2972 83 ±0.0016 |
| Li II | E-Hy-CI | This work | 3040 | −7.2799 1341 2669 2298 8163 74 | 2888 | −4.9933 5107 7779 9718 5893 | 0.4566 2984 06 ±0.0000 018 |
| Li II | E-Hy-CI | Recommended | 0.4566 298(18) | ||||
| Li II | Hy | SPA (1971) [58] | 1078 | −7.2799 1341 03 | 364 | −4.9933 5107 46 | 0.4566 |
| Li II | Hy-CI | AW (1974) [37] | 135 | −7.2799 1339 60 | 137 | −4.9933 5102 30 | 0.4566 ±0.0010 |
| Li II | E-Hy | CT (1992) [53] | 100 | −7.2799 1340 96 | 100 | −4.9933 5107 21 | 0.4566 27 |
| Li II | Reference | WF (2009) [59] | 0.4566 8 | ||||
| Be III | E-Hy-CI | This work | 1088 | −13.6555 6607 5022 1216 4675 55 | 960 | −9.1107 7158 4011 3245 7902 | 0.5515 5367 16 ±0.0019 |
| Be III | E-Hy-CI | This work | 1520 | −13.6555 6607 5806 8585 0501 71 | 1444 | −9.1107 7158 4761 1835 3319 | 0.5515 5367 29 ±0.0019 |
| Be III | E-Hy-CI | This work | 3040 | −13.6555 6623 8423 4190 7983 70 | 2888 | −9.1107 7162 2916 2965 8769 | 0.5515 5380 86 ±0.0000 021 |
| Be III | E-Hy-CI | Recommended | 0.5515 538(21) | ||||
| Be III | Hy | SPA (1971) [58] | 1078 | −13.6555 6623 60 | 364 | −9.1107 7161 94 | 0.5515 5 |
| Be III | E-Hy | CT (1992) [53] | 100 | −13.6555 6623 40 | 100 | −9.1107 7161 42 | 0.5515 55 |
| C V | E-Hy-CI | This work | 1088 | −32.4062 4629 6959 6060 3495 12 | 960 | −21.0933 3219 8896 1611 3442 | 0.6470 6730 80 ±0.0018 |
| C V | E-Hy-CI | This work | 1520 | −32.4062 4629 8488 0267 6781 26 | 1444 | −21.0933 3220 0942 1122 7462 | 0.6470 6730 91 ±0.0016 |
| C V | E-Hy-CI | This work | 3040 | −32.4062 4660 1898 1683 2715 76 | 2888 | −21.0933 3231 3387 9577 8140 | 0.6470 6746 83 ±0.0000 022 |
| C V | E-Hy-CI | Recommended | 0.6470 674(22) | ||||
| C V | Hy | SPA (1971) [58] | 1078 | −32.4062 4659 96 | 364 | −21.0933 3230 94 | 0.6470 7 |
| C V | E-Hy | CT (1992) [53] | 100 | −32.4062 4659 80 | 100 | −21.0933 3230 09 | 0.6470 67 |
| O VII | E-Hy-CI | This work | 1088 | −59.1565 9471 2148 2530 7571 52 | 960 | −38.0747 3504 8048 8747 6964 | 0.6944 4942 27 ±0.0014 |
| O VII | E-Hy-CI | This work | 1520 | −59.1565 9471 4241 7214 8946 79 | 1444 | −38.0747 3505 0653 6634 6644 | 0.6944 4942 33 ±0.0014 |
| O VII | E-Hy-CI | This work | 3040 | −59.1565 9512 2757 3982 3860 09 | 2888 | −38.0747 3523 5874 9991 6695 | 0.6944 4959 41±0.0000 022 |
| O VII | E-Hy-CI | Recommended | 0.6944 496 (22) | ||||
| O VII | Hy | SPA (1971) [58] | 1078 | −59.1565 9512 02 | 364 | −38.0747 3523 21 | 0.6944 5 |
| O VII | E-Hy | CT (1992) [53] | 100 | −59.1565 9511 90 | 100 | −38.0747 3522 16 | 0.6944 49 |
Note: All energies in this work are variational except for the recommended energy; all calculations were performed in quadruple-double-with exponent (QDE) precision. 1 Schiff, Pekeris and Accad. 2 Anderson andWeinhold. 3 Kono and Hattori. 4 Cann and Thakkar. 5 Drake and Morton. 6 Multi-configuration Hartree–Fock [62]. 7 Liu, Li, Wang and Qu [28]. 8 Multi-configuration Dirac–Hartree–Fock [636465]. 9 Relativistic effects included. 10 Wiese and Fuhr.
The use of interpolation techniques based on Rayleigh–Schrödinger perturbation theory to study systematic trends of f-values along an isoelectronic sequence is due to Wiese and Weiss [66]. The fundamental idea is to scale all distances by the nuclear charge Z and then apply conventional perturbation theory, treating the interelectronic repulsion as the perturbation. The dependence of the f-value on Z for an isoelectronic sequence is a power of Z expansion given by
where is just the f-value computed in a purely hydrogenic approximation (since there are no zeroth-order degeneracies). Hence, the infinite Z limit is for the He-isoelectronic sequence given by = 0.8324 [67] and is the natural parameter against which to plot f-values in a graphical display of the isoelectronic sequence. The entire sequence is then compressed in the region between Z = 0 and the value of the neutral atom. Since the value at Z is known, the results for Li II, Be III, C V and O VII can be extrapolated to Z = 0 in the line presented in Figure 1. The table (Table 3) and graph (Figure 1) give our recommended f-values for the entire non-relativistic He isoelectronic sequence.
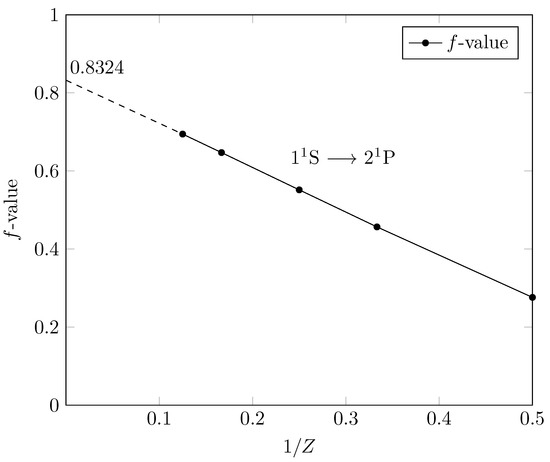
Figure 1.
f-values for the helium isoelectronic sequence versus 1/Z.
This research was focused on (1) determining high-precision wave functions and (2) using these wave functions to compute properties along with obtaining theoretical bounds to the property of interest; in this case, the oscillator strength. We were not concerned with obtaining bounds for the energy states because the variational method guarantees that the solution obtained is an upper bound to the exact energy of the state, and the error in the calculation (which provides a lower bound) can be estimated from the convergence of the eigenvalue with respect to our basis sets. It would be nice to be able to compute lower bounds theoretically, which has been carried out in the past (see, for example, the calculations on Li of Lüchow and Kleindienst [68] and King [69]), but the bounds were not very precise and the procedure involved computing matrix elements of in addition to just H. Fortunately, progress is being made in this regard [70] using the Pollak–Martinazzo (PM) [71] lower bound theory. Ronto et al. [72] recently used the explicitly correlated Gaussian (ECG) method to calculate upper and lower bounds to sub-parts-per-million precision for ground and excited states of the He, Li and Be atoms, i.e., lower bounds were computed to the same level of accuracy as the upper bounds.
8. Conclusions
The renewed interest in these methods is coming from the incredible precision of experiments these days, which is the product of sophisticated and refined theoretical methods. This necessitates the use of correlated wave functions to perform as well or better theoretically. Of these methods, Hy-CI and E-Hy-CI are unique in that they were designed (by allowing at most a single factor per term or, in the case of Hy-CI, a single factor per term in the wave function) in a way that leads to solvable integrals for > 4 electrons and hence does not limit the methods to systems with ≤ 4 while retaining the precision of the Hy methods. However, as mentioned in the introduction, there is ultimately a slow convergence built into Hy-CI that is driving our interest in ascertaining whether the presence of an exponential correlation factor in E-Hy-CI will lead to a convergence acceleration in Hy-CI.
In Paper I of this series, we demonstrated that Hy-CI and E-Hy-CI are capable of greater than 20-digit precision for the ground 1 state of helium and that E-Hy-CI does indeed accelerate convergence. Excited states are more diffuse, so, in the second paper, we demonstrated that E-Hy-CI accelerates convergence for excited states of symmetry by examining excited states of the Li ion. In this study, states of non- symmetry were treated by carrying out calculations on the lowest helium state (2 ), demonstrating convergence acceleration for non-S states as well, and then extending the analysis to other members of the He 2 isoelectronic sequence. The very dramatic convergence improvements of s-, p-, d- and f-wave E-Hy-CI expansions compared to the Hy-CI l-wave expansions are important because they suggest that the E-Hy-CI method may be the key to extending Hy-CI to systems with .
Our interest in the states was also to utilize these very precise wave functions to compute properties other than the energy; in this case, to compute transition rates in the form of oscillator strengths for various members of the the lowest (resonance) transition. In addition, the uncertainty in atomic data is of interest, which fueled an examination of what the upper and lower bounds of these properties are, both from the consideration of the convergence of our calculated values and from rigorous quantum mechanical upper and lower bounds.
Our present study produced not just high-precision 1 and 1s2p energies for the He I, Li II, Be III, C V and O VII members of the He isoelectronic sequence. Wave functions were generated to enable the calculation of oscillator strengths, including upper and lower bounds, for the lowest transition. Table 2 summarizes our results along with the best previous correlated wave function method calculations employing Slater-type orbitals. Interpolation techniques were used to produce a graph of the oscillator strengths along the isoelectronic sequence. While it has been demonstrated again that the precision of the oscillator strengths is better than the bounds predict, recommended values are presented for the 1 1s2 He isoelectronic sequence with rigorous non-relativistic quantum mechanical upper and lower bounds of (0.001–0.003)% and probable precision ≤ 0.0000003.
Author Contributions
All authors contributed to developing the methodology. J.S.S. wrote the software and performed the calculations. All authors contributed in some way to the writing, review and editing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
One of us (J.S.S.) would like to thank John R. Rumble for suggesting that we revisit upper and lower bounds, Milo Rumble Becker for his encouraging “We aren’t quitters, Poppy”, William L. George for message passing interface (MPI) support, Carl Spangler, Mark C. Williams Sr. and Collin X. Guan for parallel systems support, and Christie Sims, Chris Schanzle and Judith Devaney Terrill for supporting this endeavor. The authors would like to thank Li Guang Jiao for correspondence concerning his group’s orthogonal Hy-CI calculations and dedicate this work to the memory of our great teacher and friend Stanley A. Hagstrom, the inspiration behind Hy-CI.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
This appendix contains the complete E-Hy-CI wave function expansions for all calculations used in this study. Table A1 contains the expansions and Table A2 contains the expansions.
Each line in the Table A1 specifies the {outer,outer} and {inner,inner} sets for each l quantum number. In the table, only the minimum information needed to specify the basis set is listed, namely an exponent , the l quantum number for orbitals with that exponent, , the number of orbitals with that exponent (the n in Equation (5) run from to ), and , the exponent of the exponentially correlated factor for these E-Hy-CI calculations. There is an outer and inner basis set for these “doubled” basis sets. In all of the tables, N is used to refer to the number of terms in the wave function (see Equation (3)).
In contrast to the states, each line in Table A2 specifies an inner set for the first electron and its associated l quantum number (= ), and an outer set with its associated and quantum number for the second electron, but is otherwise the same.

Table A1.
E-Hy-CI wave function He 1 S isoelectronic sequence non-relativistic energies (in hartree atomic units using a doubled basis set of orbitals for each l-wave). N is the number of terms in the expansion.
Table A1.
E-Hy-CI wave function He 1 S isoelectronic sequence non-relativistic energies (in hartree atomic units using a doubled basis set of orbitals for each l-wave). N is the number of terms in the expansion.
| State | N | Energy (Hartree) | ||||||
|---|---|---|---|---|---|---|---|---|
| He I 1 | 0 | 16 | 2.20 | 16 | 25.0 | 0.5 | 1088 | −2.9037 2435 4189 0888 5088 58 |
| He I 1 | 0 | 19 | 2.20 | 19 | 25.0 | 0.5 | 1520 | −2.9037 2435 4275 7390 6714 30 |
| He I 1 | 1 | 19 | 3.05 | 19 | 40.5 | 0.5 | 3040 | −2.9037 2437 7034 1043 9282 03 |
| He I 1 | 2 | 19 | 3.50 | 19 | 40.5 | 0.5 | 4560 | −2.9037 2437 7034 1195 9808 72 |
| He I 1 | 3 | 18 | 3.90 | 18 | 40.5 | 0.5 | 5928 | −2.9037 2437 7034 1195 9830 82 |
| He I 1 | 4 | 15 | 4.50 | 15 | 40.5 | 0.5 | 6888 | −2.9037 2437 7034 1195 9830 89 |
| He I 1 | 5 | 14 | 5.20 | 15 | 40.5 | 0.5 | 7728 | −2.9037 2437 7034 1195 9830 92 |
| He I 1 | 6 | 14 | 6.00 | 15 | 40.5 | 0.5 | 8568 | −2.9037 2437 7034 1195 9830 94 |
| Li II 1 | 0 | 16 | 3.30 | 16 | 37.5 | 0.5 | 1088 | −7.2799 1332 7713 3474 7954 38 |
| Li II 1 | 0 | 19 | 3.30 | 19 | 37.5 | 0.5 | 1520 | −7.2799 1332 8098 0780 5105 69 |
| Li II 1 | 1 | 19 | 4.575 | 19 | 60.75 | 0.5 | 3040 | −7.2799 1341 2669 2298 8163 74 |
| Li II 1 | 2 | 19 | 5.25 | 19 | 60.75 | 0.5 | 4560 | −7.2799 1341 2669 3059 6348 60 |
| Li II 1 | 3 | 18 | 5.85 | 18 | 60.75 | 0.5 | 5928 | −7.2799 1341 2669 3059 6491 28 |
| Li II 1 | 4 | 15 | 6.75 | 15 | 60.75 | 0.5 | 6888 | −7.2799 1341 2669 3059 6491 59 |
| Li II 1 | 5 | 14 | 7.80 | 15 | 60.75 | 0.5 | 7728 | −7.2799 1341 2669 3059 6491 62 |
| Li II 1 | 6 | 14 | 9.00 | 15 | 60.75 | 0.5 | 8568 | −7.2799 1341 2669 3059 6491 66 |
| Be III 1 | 0 | 16 | 4.40 | 16 | 50.0 | 0.5 | 1088 | −13.6555 6607 5022 1216 4675 55 |
| Be III 1 | 0 | 19 | 4.40 | 19 | 50.0 | 0.5 | 1520 | −13.6555 6607 5806 8585 0501 71 |
| Be III 1 | 1 | 19 | 6.10 | 19 | 81.0 | 0.5 | 3040 | −13.6555 6623 8423 4190 7983 71 |
| Be III 1 | 2 | 19 | 7.00 | 19 | 81.0 | 0.5 | 4560 | −13.6555 6623 8423 5866 9800 72 |
| Be III 1 | 3 | 18 | 7.80 | 18 | 81.0 | 0.5 | 5928 | −13.6555 6623 8423 5867 0206 92 |
| Be III 1 | 4 | 15 | 9.00 | 15 | 81.0 | 0.5 | 6888 | −13.6555 6623 8423 5867 0207 73 |
| Be III 1 | 5 | 14 | 10.40 | 15 | 81.0 | 0.5 | 7728 | −13.6555 6623 8423 5867 0207 76 |
| Be III 1 | 6 | 14 | 12.00 | 15 | 81.0 | 0.5 | 8568 | −13.6555 6623 8423 5867 0207 77 |
| C V 1 | 0 | 16 | 6.60 | 16 | 75.0 | 0.5 | 1088 | −32.4062 4629 6959 6060 3495 12 |
| C V 1 | 0 | 19 | 6.60 | 19 | 75.0 | 0.5 | 1520 | −32.4062 4629 8488 0267 6781 26 |
| C V 1 | 1 | 19 | 9.15 | 19 | 121.5 | 0.5 | 3040 | −32.4062 4660 1898 1683 2715 76 |
| C V 1 | 2 | 19 | 10.50 | 19 | 121.5 | 0.5 | 4560 | −32.4062 4660 1898 5302 9749 39 |
| C V 1 | 3 | 18 | 11.70 | 18 | 121.5 | 0.5 | 5928 | −32.4062 4660 1898 5303 1052 76 |
| C V 1 | 4 | 15 | 13.50 | 15 | 121.5 | 0.5 | 6888 | −32.4062 4660 1898 5303 1055 18 |
| C V 1 | 5 | 14 | 15.60 | 15 | 121.5 | 0.5 | 7728 | −32.4062 4660 1898 5303 1055 21 |
| C V 1 | 6 | 14 | 18.00 | 15 | 121.5 | 0.5 | 8568 | −32.4062 4660 1898 5303 1055 17 |
| O VII 1 | 0 | 16 | 8.80 | 16 | 100.0 | 0.5 | 1088 | −59.1565 9471 2148 2530 7571 52 |
| O VII 1 | 0 | 19 | 8.80 | 19 | 100.0 | 0.5 | 1520 | −59.1565 9471 4241 7214 8946 79 |
| O VII 1 | 1 | 19 | 12.20 | 19 | 162.0 | 0.5 | 3040 | −59.1565 9512 2757 3982 3860 09 |
| O VII 1 | 2 | 19 | 14.00 | 19 | 162.0 | 0.5 | 4560 | −59.1565 9512 2757 9255 3478 69 |
| O VII 1 | 3 | 18 | 15.60 | 18 | 162.0 | 0.5 | 5928 | −59.1565 9512 2757 9255 5849 99 |
| O VII 1 | 4 | 15 | 18.00 | 15 | 162.0 | 0.5 | 6888 | −59.1565 9512 2757 9255 5854 37 |
| O VII 1 | 5 | 14 | 20.80 | 15 | 162.0 | 0.5 | 7728 | −59.1565 9512 2757 9255 5854 39 |
| O VII 1 | 6 | 14 | 24.00 | 15 | 162.0 | 0.5 | 8568 | −59.1565 9512 2757 9255 5854 43 |
Note: All energies are variational; all calculations were performed in quadruple-double-with exponent (QDE) precision.

Table A2.
E-Hy-CI energies (in hartrees) for the He 2 isoelectronic sequence using two separate basis sets of orbitals () for the inner and outer electrons. For each , + 1. N is the number of terms in the expansion.
Table A2.
E-Hy-CI energies (in hartrees) for the He 2 isoelectronic sequence using two separate basis sets of orbitals () for the inner and outer electrons. For each , + 1. N is the number of terms in the expansion.
| State | N | Energy (hartree) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| He I 2 | 0 | 16 | 2.272 | 1 | 15 | 1.291 | 0.5 | 960 | −2.1238 4308 3927 0924 8309 |
| He I 2 | 0 | 19 | 2.272 | 1 | 19 | 1.291 | 0.5 | 1444 | −2.1238 4308 5062 4897 8422 |
| He I 2 | 1 | 19 | 1.955 | 2 | 19 | 2.25 | 0.5 | 2888 | −2.1238 4308 6497 9644 7703 |
| He I 2 | 2 | 19 | 2.40 | 3 | 18 | 2.30 | 0.5 | 4256 | −2.1238 4308 6498 0912 9534 |
| He I 2 | 3 | 18 | 2.40 | 4 | 15 | 2.30 | 0.5 | 5336 | −2.1238 4308 6498 0989 3227 |
| He I 2 | 4 | 15 | 2.40 | 5 | 14 | 2.30 | 0.5 | 6176 | −2.1238 4308 6498 1004 0079 |
| He I 2 | 5 | 14 | 2.40 | 6 | 14 | 2.30 | 0.5 | 6960 | −2.1238 4308 6498 1008 1958 |
| He I 2 | 6 | 14 | 2.40 | 7 | 14 | 2.30 | 0.5 | 7744 | −2.1238 4308 6498 1009 6317 |
| Li II 2 | 0 | 16 | 3.408 | 1 | 15 | 1.9365 | 0.5 | 960 | −4.9933 5106 5519 3789 3549 |
| Li II 2 | 0 | 19 | 3.408 | 1 | 19 | 1.9365 | 0.5 | 1444 | −4.9933 5106 5763 9277 0706 |
| Li II 2 | 1 | 19 | 2.9325 | 2 | 19 | 3.375 | 0.5 | 2888 | −4.9933 5107 7779 9718 5893 |
| Li II 2 | 2 | 19 | 3.60 | 3 | 18 | 3.45 | 0.5 | 4256 | −4.9933 5107 7779 9724 9272 |
| Li II 2 | 3 | 18 | 3.60 | 4 | 15 | 3.45 | 0.5 | 5336 | −4.9933 5107 7780 0140 3745 |
| Li II 2 | 4 | 15 | 3.60 | 5 | 14 | 3.45 | 0.5 | 6176 | −4.9933 5107 7780 0153 5568 |
| Li II 2 | 5 | 14 | 3.60 | 6 | 14 | 3.45 | 0.5 | 6960 | −4.9933 5107 7780 0155 3584 |
| Li II 2 | 6 | 14 | 3.60 | 7 | 14 | 3.45 | 0.5 | 7744 | −4.9933 5107 7780 0159 3316 |
| Be III 2 | 0 | 16 | 4.544 | 1 | 15 | 2.582 | 0.5 | 960 | −9.1107 7158 4011 3245 7902 |
| Be III 2 | 0 | 19 | 4.544 | 1 | 19 | 2.582 | 0.5 | 1444 | −9.1107 7158 4761 1835 3319 |
| Be III 2 | 1 | 19 | 3.910 | 2 | 19 | 4.500 | 0.5 | 2888 | −9.1107 7162 2916 2965 8769 |
| Be III 2 | 2 | 19 | 4.910 | 3 | 18 | 4.600 | 0.5 | 4256 | −9.1107 7162 2916 2983 6771 |
| Be III 2 | 3 | 18 | 4.800 | 4 | 15 | 4.600 | 0.5 | 5336 | −9.1107 7162 2916 4364 1540 |
| Be III 2 | 4 | 15 | 4.800 | 5 | 14 | 4.600 | 0.5 | 6176 | −9.1107 7162 2916 4383 9458 |
| Be III 2 | 5 | 14 | 4.800 | 6 | 14 | 4.600 | 0.5 | 6960 | −9.1107 7162 2916 4397 2592 |
| Be III 2 | 6 | 14 | 4.800 | 7 | 14 | 4.600 | 0.5 | 7744 | −9.1107 7162 2916 4406 7410 |
| C V 2 | 0 | 16 | 6.816 | 1 | 15 | 3.873 | 0.5 | 960 | −21.0933 3219 8896 1611 3442 |
| C V 2 | 0 | 19 | 6.816 | 1 | 19 | 3.873 | 0.5 | 1444 | −21.0933 3220 0942 1122 7462 |
| C V 2 | 1 | 19 | 5.865 | 2 | 19 | 6.750 | 0.5 | 2888 | −21.0933 3231 3387 9577 8140 |
| C V 2 | 2 | 19 | 7.200 | 3 | 18 | 6.900 | 0.5 | 4256 | −21.0933 3231 3387 9617 4846 |
| C V 2 | 3 | 18 | 7.200 | 4 | 15 | 6.900 | 0.5 | 5336 | −21.0933 3231 3388 3922 5630 |
| C V 2 | 4 | 15 | 7.200 | 5 | 14 | 6.900 | 0.5 | 6176 | −21.0933 3231 3388 3968 4013 |
| C V 2 | 5 | 14 | 7.200 | 6 | 14 | 6.900 | 0.5 | 6960 | −21.0933 3231 3388 3993 9969 |
| C V 2 | 6 | 14 | 7.200 | 7 | 14 | 6.900 | 0.5 | 7744 | −21.0933 3231 3388 4009 9911 |
| O VII 2 | 0 | 16 | 10.224 | 1 | 15 | 5.8095 | 0.5 | 960 | −38.0747 3504 8048 8747 6964 |
| O VII 2 | 0 | 19 | 10.224 | 1 | 19 | 5.8095 | 0.5 | 1444 | −38.0747 3505 0653 6634 6644 |
| O VII 2 | 1 | 19 | 8.7975 | 2 | 19 | 10.125 | 0.5 | 2888 | −38.0747 3523 5874 9991 6695 |
| O VII 2 | 2 | 19 | 10.800 | 3 | 18 | 9.200 | 0.5 | 4256 | −38.0747 3523 5875 0015 0005 |
| O VII 2 | 3 | 18 | 10.800 | 4 | 15 | 10.350 | 0.5 | 5336 | −38.0747 3523 5875 4462 8699 |
| O VII 2 | 4 | 15 | 10.800 | 5 | 14 | 10.350 | 0.5 | 6176 | −38.0747 3523 5875 4922 2755 |
| O VII 2 | 5 | 14 | 10.800 | 6 | 14 | 10.350 | 0.5 | 6960 | −38.0747 3523 5875 5174 8235 |
| O VII 2 | 6 | 14 | 10.800 | 7 | 14 | 10.350 | 0.5 | 7744 | −38.0747 3523 5875 5321 5035 |
Note: All energies are variational; all calculations were performed in quadruple-double-with exponent (QDE) precision.
Notes
| 1 | Some authors refer to this as fully correlated [5]. |
| 2 | For our previous studies, papers I and II in this series, see [6,7]. |
| 3 | For papers discussing the integrals arising in E-Hy-CI, see [8,10,11,12,13]; for a review of both Hy-CI and E-Hy-CI, see [14]. |
| 4 | In the orthogonal Hy-CI of Zhang et al. [17] (see also Jiao et al. [18]) discussed later, can be 2 or 3. |
| 5 | Actually, the configuration designation is rigorous only for Russell–Saunders () coupling in a central field. We retain the terminology here to conform to standard spectroscopic notation with the understanding that, by 1 and 1s2p , we mean the lowest terms of those respective symmetries (the He 1 ground state and the 2 lowest state of symmetry, respectively). |
| 6 | In light atoms, the relativistic correction is important for an accurate transition energy but has a small effect on the line strength [26]. For a detailed treatment of line strengths (proportional to transition rates), see Morton et al. [27], where the corrections are shown to be less than 0.1% for helium, and Liu et al. [28], where they are shown to be ≈0.01% for helium. |
| 7 | In this paper, we use f-value and oscillator strength interchangably. |
| 8 | Computed using truncations of our final non-relativistic and wave functions. |
| 9 | For an introduction to relativistic theory as implemented in GRASP, see [57]. |
References
- Sims, J.S.; Hagstrom, S. Combined Configuration-Interaction—Hylleraas-Type Wave-Function Study of the Ground State of the Beryllium Atom. Phys. Rev. A 1971, 4, 908–916. [Google Scholar] [CrossRef]
- Woźnicki, W. On the method of constructing the variational wave functions for many-electron atoms. In Theory of Electronic Shells in Atoms and Molecules; Jucys, A., Ed.; Mintis: Vilnius, Lithuania, 1971; pp. 103–106. [Google Scholar]
- Hylleraas, E.A. Neue berechnung der energie des heliums im grundzustande, sowie des tiefsten terms von ortho-helium. Z. Phys. 1929, 54, 347–366. [Google Scholar] [CrossRef]
- Bunge, C.F. Electronic Wave Functions for Atoms. II. Some Aspects of the Convergence of the Configuration Interaction Expansion for the Ground States of the He Isoelectronic Series. Theor. Chem. Acta 1970, 16, 124–144. [Google Scholar]
- Jentschura, U.D.; Adkins, G.S. Quantum Electrodynamics: Atoms, Lasers and Gravity; World Scientific Publishing Company: Singapore, 2023. [Google Scholar]
- Sims, J.S.; Padhy, B.; Ruiz, M.B. Exponentially correlated Hylleraas-configuration interaction nonrelativistic energy of the 1S ground state of the helium atom. Int. J. Quantum Chem. 2020, 121, e26470. [Google Scholar]
- Sims, J.S.; Padhy, B.; Ruiz, M.B. Exponentially correlated Hylleraas-configuration interaction studies of atomic systems. II. Non-relativistic energies of the 1 1S through 6 1S states of the Li+ ion. Int. J. Quantum Chem. 2022, 122, e26823. [Google Scholar] [CrossRef]
- Wang, C.; Mei, P.; Kurokawa, Y.; Nakashima, H.; Nakatsuji, H. Analytical evaluations of exponentially correlated unlinked one-center, three- and four-electron integrals. Phys. Rev. A 2012, 85, 042512. [Google Scholar] [CrossRef]
- Hirschfelder, J.O. Removal of electron-electron poles from many-electron Hamiltonians. J. Chem. Phys. 1963, 39, 3145–3146. [Google Scholar] [CrossRef]
- Padhy, B. Kinetic energy matrix elements for a two-electron atom with extended Hylleraas-CI wave function. Orissa J. Phys. 2018, 25, 9–21. [Google Scholar]
- Padhy, B. Analytic evaluation of two-electron atomic integrals involving extended Hylleraas-CI functions with STO basis. Orissa J. Phys. 2018, 25, 99–112. [Google Scholar]
- Harris, F.E. Exponentially correlated wave functions for four-body systems. Adv. Quantum Chem. 2016, 73, 81–102. [Google Scholar]
- Harris, F.E. Matrix elements for explicitly-correlated atomic wave functions. In Concepts, Methods and Applications of Quantum Systems in Chemistry and Physics. Progress in Theoretical Chemistry and Physics; Wang, Y., Thachuk, M., Krems, R., Maruani, J., Eds.; Springer: New York, NY, USA, 2018; Volume 31. [Google Scholar]
- Ruiz, M.B.; Sims, J.S.; Padhy, B. High-precision Hy-CI and E-Hy-CI studies of atomic and molecular properties. Adv. Quantum Chem. 2021, 83, 171–208. [Google Scholar]
- Chung, H.-K.; Braams, B.J.; Bartschat, K.; Csaszar, A.G.; Drake, G.W.F.; Kirchner, T.; Kokoouline, V.; Tennyson, J. Uncertainty Estimates for Theoretical Atomic and Molecular Data. J. Phys. D Appl. Phys. 2016, 49, 363002. [Google Scholar] [CrossRef]
- Löwdin, P.O. Angular momentum wavefunctions constructed by projector operators. Rev. Mod. Phys. 1964, 36, 966–976. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Gao, Y.C.; Jiao, L.G.; Liu, F.; Ho, Y.K. Linear dependence in Hylleraas-configuration interaction calculations of He atom. Int. J. Quantum Chem. 2019, 120, e26136. [Google Scholar] [CrossRef]
- Jiao, L.G.; Zan, L.R.; Zhu, L.; Zhang, Y.Z.; Ho, Y.K. High-precision calculation of the geometric quantities of two-electron atoms based on the Hylleraas-configuration-interaction basis. Phys. Rev. A 2019, 100, 022509. [Google Scholar] [CrossRef]
- Condon, E.U.; Shortley, G.H. The Theory of Atomic Spectra; Cambridge University Press: Cambridge, UK, 1963. [Google Scholar]
- Sims, J.S.; Ruiz, M.B. Parallel generalized real symmetric-definite eigenvalue problem. J. Res. Natl. Inst. Stand. Technol. 2020, 125, 125032. [Google Scholar] [CrossRef]
- Message Passing Interface Forum. MPI: A Message-Passing Interface Standard. Int. J. Supercomput. Appl. High Perform. Comput. 1994, 8, 159–416. [Google Scholar]
- Sims, J.S.; Hagstrom, S.A. High-precision Hy-CI variational calculations for the ground state of neutral helium and helium-like ions. Int. J. Quantum Chem. 2002, 90, 1600–1609. [Google Scholar] [CrossRef]
- Sims, J.S.; Hagstrom, S.A. Hylleraas-configuration interaction study of the 2 2S ground state of neutral lithium and the first five excited 2S states. Phys. Rev. A 2009, 80, 052507. [Google Scholar] [CrossRef]
- Sims, J.S.; Hagstrom, S.A. Hylleraas-configuration interaction study of the 1S ground state of neutral beryllium. Phys. Rev. A 2011, 83, 032518. [Google Scholar] [CrossRef]
- Goldberg, L. Relative multiplet strengths in L-S coupling. Astrophys. J. 1935, 82, 1–25. [Google Scholar] [CrossRef]
- Fischer, C.F. Evaluating the accuracy of theoretical transition data. J. Phys. B At. Mol. Opt. Phys. 2009, T134, 014019. [Google Scholar] [CrossRef]
- Morton, D.C.; Moffatt, P.; Drake, G.W.F. Relativistic corrections to the He I transition rates. Can. J. Phys. 2011, 89, 129–134. [Google Scholar] [CrossRef]
- Liu, Q.; Li, J.; Wang, J.; Qu, Y. Effect of electron correlation and Breit interaction on energies, oscillator strengths, and transition rates for low-lying states of helium. Chin. Phys. Lett. 2021, 38, 113101. [Google Scholar] [CrossRef]
- Sims, J.S.; Whitten, R.C. Upper and lower bounds to atomic and molecular properties. I. Be-sequence oscillator strengths (Dipole-length formulation) for the 1s2 2s2 1S → 1s2 2s2p 1P transition. Phys. Rev. A 1973, 8, 2220–2230. [Google Scholar] [CrossRef]
- Sims, J.S.; Hagstrom, S.A. One center rij integrals over Slater-type orbitals. J. Chem. Phys. 1971, 55, 4699–4710. [Google Scholar] [CrossRef]
- Sims, J.S.; Hagstrom, S.A. Mathematical and computational science issues in high precision Hylleraas-configuration interaction variational calculations: I. Three-electron integrals. J. Phys. B At. Mol. Opt. Phys. 2004, 37, 1519–1540. [Google Scholar] [CrossRef]
- Sims, J.S.; Hagstrom, S.A. Mathematical and computational science issues in high precision Hylleraas-configuration interaction variational calculations: III. Four-electron integrals. J. Phys. B At. Mol. Opt. Phys. 2015, 48, 175003. [Google Scholar] [CrossRef]
- Chandrasekhar, S. Some remarks on the negative hydrogen ion and its absorption coefficient. Astrophys. J. 1944, 100, 176. [Google Scholar] [CrossRef]
- Chandrasekhar, S. Theoretical molecular transition probabilities. I. The V-N transition in H2. J. Chem. Phys. 1961, 34, 1224–1231. [Google Scholar]
- Weinhold, F. Calculation of upper and lower bounds to oscillator strengths. J. Chem. Phys. 1971, 54, 1874–1881. [Google Scholar] [CrossRef]
- Weinhold, F. Upper and lower bounds to quantum-mechanical properties. Adv. Quant. Chem. 1972, 6, 299–331. [Google Scholar]
- Anderson, M.T.; Weinhold, F. Dipole oscillator strengths, with rigorous limits of error, for He and Li+. Phys. Rev. A 1974, 9, 118–128. [Google Scholar] [CrossRef]
- Sims, J.S.; Hagstrom, S.A.; Rumble, J.R., Jr. Upper and lower bounds to atomic and molecular properties. III. Lithium oscillator strengths for various doublet S→doublet P transitions. Phys. Rev. A 1976, 13, 242–250. [Google Scholar] [CrossRef]
- Rumble, J.R., Jr.; Sims, J.S.; Purdy, D.R. Upper and lower bounds for the oscillator strength of the X → C transition of the hydrogen molecule. J. Phys. B: At. Mol. Opt. Phys. 1977, 10, 2553–2559. [Google Scholar] [CrossRef]
- Roginsky, D.V.I.; Laughin, C.; Cohen, M. Improved lower and upper bounds for dipole transition moments. J. Phys. B At. Mol. Phys. 1986, 19, 1115–1123. [Google Scholar] [CrossRef]
- Roginsky, D.V.I.; Weiss, A.W. Calculations of tighter error bounds for theoretical atomic-oscillator strengths. Phys. Rev. A 1988, 38, 1760–1766. [Google Scholar] [CrossRef] [PubMed]
- Drake, G.W.F. High precision theory of atomic helium. Phys. Scr. 1999, T83, 83–92. [Google Scholar] [CrossRef]
- Drake, G.W.F. High precision calculations for helium. In Atomic, Molecular, and Optical Physics Handbook; Drake, G.W.F., Ed.; API Press: New York, NY, USA, 1996; pp. 154–171. [Google Scholar]
- Szasz, L. Atomic many-body problem. I. General theory of correlated wave functions. Phys. Rev. 1962, 126, 169–181. [Google Scholar] [CrossRef]
- Szasz, L. Pair correlations in the Be atom. Phys. Lett. 1963, 126, 263–264. [Google Scholar] [CrossRef]
- Szasz, L.; Byrne, J. Atomic many-body problem. III. The calculation of Hylleraas-type correlated wave functions for the beryllium atom. Phys. Rev. 1967, 158, 34–48. [Google Scholar] [CrossRef]
- Plute, E.J., Jr. Orthogonal Combined Configuration Interaction—Hylleraas Study of Two Electron Atoms. Ph.D. Thesis, Indiana University, Bloomington, IN, USA, 1984. [Google Scholar]
- Schiff, B.; Lifson, H.; Pekeris, C.L.; Rabinowitz, P. 2 1,3P, 3 1,3P, and 4 1,3P states of He and the 2 1P State of Li+. Phys. Rev. 1965, 140, 1104–1121. [Google Scholar] [CrossRef]
- Kato, T. On the eigenfunctions of many-particle systems in quantum mechanics. Commun. Pure Appl. Math. 1957, 10, 151–177. [Google Scholar] [CrossRef]
- Aznabaev, D.T.; Bekbaev, A.K.; Korobov, V.I. Nonrelativistic energy levels of helium atoms. Phys. Rev. A 2018, 98, 012510. [Google Scholar] [CrossRef]
- Jiao, L.G. (Jilin University, Changchun, China). Personal communication, 2021.
- Nakashima, H.; Nakatsuji, H. Solving the Schrödinger equation for helium atom and its isoelectronic ions with the free iterative complement interaction method. J. Chem. Phys. 2008, 128, 154107. [Google Scholar] [CrossRef]
- Cann, N.M.; Thakkar, A.J. Oscillator strengths for S→P and P→D transitions in heliumlike ions. Phys. Rev. A 1992, 46, 5397–5404. [Google Scholar] [CrossRef]
- Jönsson, P.; He, X.; Fischer, C.F.; Grant, I.P. The GRASP2K atomic structure package. Comput. Phys. Commun. 2007, 177, 597–622. [Google Scholar] [CrossRef]
- Fischer, C.F.; Gaigalas, G.; Jönsson, P.; Bierón, J. Grasp2018—A Fortran 95 version of the general relativistic atomic structure package. Comput. Phys. Commun. 2019, 237, 184–187. [Google Scholar] [CrossRef]
- Jönsson, P.; Gaigalas, G.; Bierón, J.; Fischer, C.F.; Grant, I.P. New version: GRASP2K relativistic atomic structure package. Comput. Phys. Commun. 2013, 184, 2197–2203. [Google Scholar] [CrossRef]
- Jönsson, P.; Godefroid, M.; Gaigalas, G.; Ekman, J.; Grumer, J.; Li, W.; Li, J.; Brage, T.; Grant, I.P.; Bierón, J.; et al. An Introduction to Relativistic Theory as Implemented in GRASP. Atoms 2023, 11, 7. [Google Scholar] [CrossRef]
- Schiff, B.; Pekeris, C.L.; Accad, Y. f values for transitions between the low-lying S and P states of the helium isoelectronic sequence up to Z = 10. Phys. Rev. A 1971, 4, 885–893. [Google Scholar] [CrossRef]
- Wiese, W.L.; Fuhr, J.R. Accurate atomic transition probabilities for hydrogen through lithium. J. Phys. Chem. Ref. Data 2009, 38, 565–719. [Google Scholar] [CrossRef]
- Kono, A.; Hattori, S. Accurate oscillator strengths for neutral helium. Phys. Rev. A 1984, 29, 2981–2988. [Google Scholar] [CrossRef]
- Drake, G.W.F.; Morton, D.C. A Multiplet Table for Neutral Helium (4He I) with Transition Rates. Astrophys. J. Supp. Ser. 2007, 170, 251–260. [Google Scholar] [CrossRef]
- Fischer, C.F. A multi-configuration Hartree-Fock program. Comput. Phys. Commun. 1970, 1, 151–166. [Google Scholar] [CrossRef]
- Fischer, C.F.; Brage, T.; Jönsson, P. Computational atomic structure: An MCHF approach. In Concepts, Methods and Applications of Quantum Systems in Chemistry and Physics. Progress in Theoretical Chemistry and Physics; Routledge: New York, NY, USA, 1997. [Google Scholar]
- Fischer, C.F. The MCHF atomic-structure package. Comput. Phys. Commun. 2000, 128, 635–636. [Google Scholar] [CrossRef]
- Fischer, C.F. The MCHF atomic-structure package. Comput. Phys. Commun. 2013, 184, 2197–2203. [Google Scholar]
- Wiese, W.L.; Weiss, A.W. Regularities in atomic oscillator strengths. Phys. Rev. 1968, 175, 50–65. [Google Scholar] [CrossRef]
- Weiss, A.W. Oscillator Strengths for the Helium Isoelectronic Sequence. J. Res. Natl. Inst. Stand. Technol. 1967, 71A, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Lüchow, A.; Kleindienst, H. Accurate upper and lower bounds to the 2S states of the lithium atom. Int. J. Quantum Chem. 1994, 51, 211–224. [Google Scholar] [CrossRef]
- King, F.W. Lower bound for the nonrelativistic ground state energy of the lithium atom. J. Chem. Phys. 1995, 102, 8053–8058. [Google Scholar] [CrossRef]
- Ireland, R.T.; Jeszenszki, P.; Mâtyus, E.; Martinazzo, R.; Ronto, M.; Pollak, E. Lower Bounds for Nonrelativistic Atomic Energies. ACS Phys. Chem. Au 2022, 2, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Pollak, E.; Martinazzo, R. Lower Bounds for Coulombic Systems. ACS Phys. Chem. Au 2021, 17, 1535–1547. [Google Scholar] [CrossRef]
- Ronto, M.; Jeszenszki, P.; Mâtyus, E.; Pollak, E. Lower bounds on par with upper bounds for few-electron atomic energies. Phys. Rev. A 2023, 107, 012204. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).