Abstract
Background/Objectives: Replacing fish oil with vegetable oil is an important measure for aquaculture to relieve the pressure of fish oil, but it is also easy to cause the growth decline and metabolic disorder of farmed animals, mainly due to the change in dietary fatty acids. This study investigated the regulatory effects of dietary fatty acid composition on glucose metabolism in large yellow croaker (Larimichthys crocea) with an initial weight of 30.51 ± 0.16 g. Methods: Three isonitrogenous (~43% crude protein) and isolipid (~11% crude lipid) diets were formulated as follows: control (CON, DHA/EPA-rich oil as primary lipid), moderate palmitic acid (MPA, 50% of DHA+EPA-rich oil was replaced by glyceryl palmitate), and high palmitic acid (HPA, 100% of DHA+EPA-rich oil was replaced by glyceryl palmitate). Results: After 10 weeks of feeding, the HPA significantly reduced the liver/muscle glycogen contents, increased the liver lipid content, decreased the serum leptin/insulin level, and increased the adiponectin level. The levels of DHA and EPA in liver were decreased significantly. Transcriptionally, HPA upregulated hepatic glucokinase (gk, glycolysis) but down-regulated glycogen synthase (gys) and insulin/irs2 (insulin pathway) while inhibiting muscle ampk and leptin receptor (lepr). Conclusions: This study showed that high dietary PA/(DHA + EPA) impacted glycolipid homeostasis through endocrine and transcriptional regulation, leading to increased crude lipid and decreased glycogen levels, which provides a theoretical basis for scientific aquatic feed fatty acid formulation.
1. Introduction
The growing conflict between global supply and demand of fish oil has made the development of alternative lipid sources a research priority in aquaculture [1]. However, the substitution of fish oil indeed changes the fatty acid profile of feed, which may have profound implications for the metabolic health of farmed species [2]. Numerous studies indicate that the changes in dietary fatty acid composition can significantly influence systemic glucose metabolism [3,4]. In mammalian studies, high dietary saturated fatty acids have been proven to reduce insulin sensitivity in liver [5] and neuron [6]. For example, palmitic acid induces insulin resistance by disrupting hepatic lipid metabolism and insulin secretion, while simultaneously blocking the phosphorylation of key signaling proteins like Akt, the insulin receptor, and IRS1 in HepG2 cells [7]. Therefore, the influence of fatty acid profiles on glucose metabolism warrants close attention when formulating aquafeeds with alternative lipid sources.
The link between dietary fatty acids and glucose metabolism is largely mediated by adipokine regulation [8,9]. High intake of saturated fats raises circulating non-esterified fatty acids (NEFA), which in turn blocks glucose oxidation and drives insulin resistance. Palmitic acid, for instance, is known to trigger hepatic inflammation—a phenomenon verified in species like silver pomfret (Pampus argenteus) [10] and zebrafish (Danio rerio) [11]. Over time, persistent inflammation and high NEFA levels disrupt the insulin signaling cascade. This results in flawed glucose transport and phosphorylation [12], which are more severe in fish with poor glucose tolerance [13]. N-6 polyunsaturated fatty acids (PUFAs) tend to induce inflammation and blunt insulin sensitivity, although these effects are not universal and can be highly dependent on environmental conditions and species-specific factors [14]. N-3 PUFAs do the opposite, acting as anti-inflammatory agents that boost insulin function [15,16]. This protective role is clearly seen in juvenile Nile tilapia (Oreochromis niloticus) [17]. Furthermore, adipokines such as leptin and adiponectin play critical regulatory roles in this process. Leptin targets the hypothalamus to fine-tune the insulin-glucose axis [18], a mechanism highly conserved in teleosts. Specifically, insulin and glucagon trigger the upregulation of leptin A. In turn, Leptin exerts a glucose-dependent biphasic regulation on insulin release: it inhibits insulin a (insa) expression under basal glucose conditions but stimulates insa expression under hyperglycemic conditions [19]. Adiponectin also functions as a metabolic enhancer in skeletal muscle, improving both insulin signaling and glucose utilization [20]. In grass carp, both adiponectin A and B suppress basal glycemia and enhance hepatic glycogen storage [21]. These findings highlight how adipokines serve as a functional bridge connecting fatty acid intake to systemic glucose regulation.
As a cornerstone of the Chinese marine aquaculture industry, the large yellow croaker maintains the highest annual yield among all farmed marine fish [22]. Its widespread appeal stems from a combination of delicate flavor and visually attractive golden pigmentation. The metabolic ability to generate long-chain polyunsaturated fatty acids (LC-PUFA) from LA and ALA is restricted in large yellow croaker, a trait shared by other marine carnivorous fish [23]. Because its physiological health hinges so heavily on the fatty acid composition of its diet, it offers a unique opportunity to study lipid metabolism in depth. However, comprehensive research evaluating how dietary fatty acid composition affects glucolipid metabolism remains scarce, particularly from the perspective of endocrine regulation. To accentuate the contrast between saturated fatty acids (glyceryl palmitate) and n-3 highly unsaturated fatty acids (n-3 HUFAs; EPA/DHA), we formulated diets using glyceryl palmitate alongside oils enriched with EPA and DHA [4]. This research investigates the metabolic interplay between palmitic acid and DHA/EPA using gradient dietary ratios. The purpose of this study is to map their regulatory effects on glucose homeostasis, paving the way for more precise fatty acid formulations in aquaculture.
2. Materials and Methods
2.1. Ethical Statement
Every animal handling and care method was performed under the supervision of Ocean University of China’s Animal Care Committee (OUC-AE-2025-308).
2.2. Diet Preparation
Three experimental diets were formulated to be isonitrogenous and isolipidic, containing approximately 43% crude protein and 11% crude lipid, respectively. Fish meal and soybean meal were utilized as the primary dietary protein sources. The control diet contained DHA+EPA-enriched oil as the main lipid sources. In the experimental diets, 50% and 100% of the DHA+EPA-enriched oil were replaced by glycerin palmitate, named MPA group and HPA group, respectively. The experimental diet details are presented in Table 1. The feed preparation procedure was as follows [4]: feed ingredients were first ground to pass through a 60-mesh sieve. the dry powders were thoroughly mixed step-by-step following the recipe, ensuring consistency via a high-performance mixer (0.5 V Type, Shanghai Tianxiang Jiantai Pharmaceutical Machinery Co., Ltd., China). Simultaneously, the weighed soybean lecithin was fully incorporated into the required oil mixture until clear. The oil containing dissolved lecithin was evenly sprayed onto the mixed powder with manual rubbing to ensure homogeneity. An appropriate amount of water was added, and the mixture was pelleted using a granulator (EL-260, Weihai Friendship Machinery Factory, Shandong, China) to produce pellets with a diameter of 5 mm. The pellets were placed on trays and dried in a blast dryer at 55 °C for 12 h. Upon drying, the finished pellets were stored at −20 °C prior to use. The specific fatty acid profiles of these diets are presented in Table 2.

Table 1.
Formulation and chemical proximate composition of the experimental diets (% dry matter).

Table 2.
Fatty acid composition of the experimental diets (% identified fatty acids).
2.3. Experimental Animals and Feeding Management
Juvenile large yellow croaker (Larimichthys crocea) were procured from the Ningde Fufa Fry Hatchery (Ningde, China) and transported to the aquaculture facility of Ningde Fufa Aquatic Products Co., Ltd. (Ningde, China) (temperature: 20.5–25.4 °C, salinity: 23.72–28.72‰). Upon arrival, the fish were acclimatized for two weeks in floating net cages (4.0 m × 4.0 m × 4.0 m). At this stage, fish were fed a control diet to adapt to the artificial compound diet. Following acclimation, 450 healthy fish of uniform size (initial weight: 30.51 ± 0.16 g) were fasted for 24 h and randomly distributed into three treatment groups. Net cages (2 m3, 1.0 m × 1.0 m × 2.0 m) with 50 fish per cage were used to establish each group in triplicate. The aquaculture cycle was 10 weeks. The fish were fed twice daily (05:00 and 17:00) with full food. During the feeding trial, water temperature and salinity fluctuated within the ranges of 20.5–25.4 °C and 23.72–28.72‰, respectively. Dissolved oxygen remained over 6 mg/L. Fish behavior and feeding status were monitored and recorded daily.
2.4. Sample Collection
After the 10-week feeding regimen, all survivors were deprived of feed for 24 h to clear intestinal contents. From each net cage, nine fish were randomly sampled and anesthetized by immersion in MS-222 (Shanghai Reagent Factory, China). Caudal vein blood was collected with syringe immediately upon anesthesia and maintained at 4 °C. Serum was isolated by centrifuging at 836× g (4 °C, 10 min), then aliquoted and snap-frozen in liquid nitrogen. Samples were stored at −80 °C prior to the quantification of various metabolic markers, including glucose, insulin, triglycerides, NEFA, leptin, and adiponectin. Liver and muscle samples were similarly excised, sealed in RNase-free tubes (Biosharp, Hefei, China), and frozen at −80 °C.
2.5. Determination of Glycogen and Lipid Content in the Liver and Muscle
Hepatic and muscular glycogen content was determined by commercial kits (Nanjing Jiancheng Bioengineering Institute, Jiangsu, China). Fresh tissue samples were weighed (≤100 mg) after being cleansed with physiological saline and blotted dry with filter paper. Samples were mixed with three volumes of alkaline solution and hydrolyzed in a boiling water bath for 20 min to yield glycogen hydrolysate. After adding double-distilled water to form the detection solution, color-developing reagent was introduced and mixed thoroughly. The mixture was then boiled for 5 min, followed by cooling under running water, and the absorbance was determined at 620 nm with a microplate reader (SpectraMax i3x, Shanghai, China) with the blank tube zeroed. Glycogen concentration was calculated by comparison with a standard curve.
The quantification of total lipids in liver and muscle tissues was performed using a modified chloroform-methanol method [24]. Prior to analysis, tissues were lyophilized using a freeze-dryer (ALPHA 1–2, Christ, Germany) until constant weight was achieved, and then homogenized. Samples (~100 mg) were treated with 4 mL of chloroform/methanol (2:1, v/v) and agitated for 24 h. Subsequently, another 2 mL of the solvent was introduced, and the supernatant was harvested following centrifugation (3000× g, 15 min). To ensure maximum recovery, the solid residue underwent a second extraction cycle with 2 mL of solvent, and the resulting liquid was added to the first fraction. The pooled extract was treated with 1.2 mL of 1.6% CaCl2 and agitated intensely, after which it was kept undisturbed overnight to allow the phases to stratify. The upper phase was discarded using a pipette and the lower organic phase was evaporated under a stream of nitrogen. The residue was further dried at 75 °C and lipid content was calculated gravimetrically.
2.6. Determination of Fatty Acid Composition in Liver and Muscle
Fatty acid methyl esters (FAMEs) were prepared following the method from Mu et al. [25]. Approximately 100 mg of dry powder was treated with 3 mL of KOH-methanol and heated at 75 °C for 20 min. After the mixture cooled down, 3 mL of HCl-methanol was added, and the sample was heated again at 75 °C for another 20 min. Once at room temperature, the lipids were extracted into 1 mL of n-hexane by vortexing. The mixture was allowed to sit overnight to separate the layers. The top layer was then collected, spun to remove particles, and analyzed by gas chromatography (GC, Santa Clara, CA, USA) to measure the fatty acids.
2.7. Determination of Serum Glucose and Serum Hormones
Serum glucose levels were tested using the glucose oxidase technique using a microplate reader (Multiskan GO, Thermo Fisher Scientific, Waltham, MA, USA). Serum hormone concentrations (adiponectin, insulin and leptin) were quantified by direct enzyme-linked immunosorbent assays (ELISAs) using commercial kits purchased from Cusabio (Wuhan, Hubei, China) according to the methods published before with a microplate reader (Multiskan GO, Thermo Fisher Scientific) [4].
2.8. RNA Extraction, Reverse Transcription, and Quantitative Real-Time PCR
We purified total RNA from the liver and muscle of large yellow croaker using TRIzol reagent (Vazyme, Nanjing, Jiangsu, China). The concentration and purity of the extracts were checked on a NanoDrop 2000 (Thermo Fisher Scientific, Waltham, MA, USA). Subsequently, the PrimeScript RT Kit (Takara, Dalian, China, RR047Q) was employed to generate cDNA from the RNA via a two-step reaction. Quantitative analysis was executed using Vazyme’s SYBR qPCR Master Mix (Nanjing, Jiangsu, China) and specific primers (see Supplementary Table S1). β-actin functioned as the housekeeping gene, selected for its invariant expression under the experimental conditions. Final data were analyzed using the 2(−ΔΔCT) algorithm following amplification efficiency verification [26].
2.9. Data Statistics and Analysis
All values are expressed as the mean ± standard error of the mean (S.E.M.). Statistical processing was performed using the SPSS 20.0 software package. Group means were compared using a one-way ANOVA combined with Tukey’s multiple range test. Statistical significance was declared when the p-value was lower than 0.05.
3. Results
3.1. Hepatic and Muscular Glycogen and Lipid Levels in Large Yellow Croaker
After the experiment, the survival rate of large yellow croaker in all treatment groups was over 90% (91.33 ± 0.67 vs. 93.33 ± 2.40 vs. 90.00 ± 1.15; %), and there was no significant difference between the groups. Given the no significant effects on growth performance by glyceryl palmitate was found [27], the current study concentrated on the impacts on physiological and biochemical responses, especially on the glucose metabolism. As the proportion of glycerin palmitate in the diet increased, the hepatic glycogen content of large yellow croaker exhibited a decreasing trend. Compared to the CON group, the HPA group showed a significant reduction in hepatic glycogen content (p < 0.05, Figure 1A). Similarly, the HPA group’s muscle glycogen content was substantially lower than the CON group’s (p < 0.05, Figure 1B). Furthermore, the HPA group significantly increased hepatic lipid content (p < 0.05, Figure 1A). However, the MPA and CON groups did not significantly differ from one another (p > 0.05, Figure 1A). Regarding muscular lipid content, the MPA group had the highest level, which was significantly greater than those in the CON and HPA groups (p < 0.05, Figure 1B).
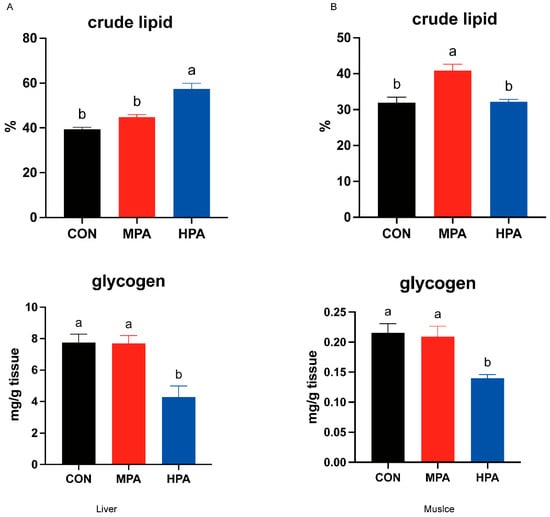
Figure 1.
Effects of glycerin palmitate on hepatic and muscular glycogen and lipid levels in large yellow croaker (mean ± S.E.M., n = 3). (A) the contents of lipid and glycogen in the liver; (B) the contents of lipid and glycogen in the muscle. Following Tukey’s test, the same-letter bars show no discernible changes across treatments (p > 0.05).
3.2. Serum Glucose, Lipid, and Hormone Levels in Large Yellow Croaker
No significant changes were observed in serum glucose or free fatty acid (FFA) levels (p > 0.05, Figure 2A). However, serum triglyceride (TG) levels were found to be significantly higher in the HPA group compared to the CON group (p < 0.05, Figure 2B). Regarding adipokines, the administration of glyceryl palmitate resulted in a significant reduction in leptin (p < 0.05, Figure 2D), whereas adiponectin levels were significantly higher in the HPA group relative to the CON group (p < 0.05, Figure 2F). No significant difference in insulin was found between the MPA and CON groups (p > 0.05), but significantly lower levels were observed in the HPA group (p < 0.05, Figure 2E).
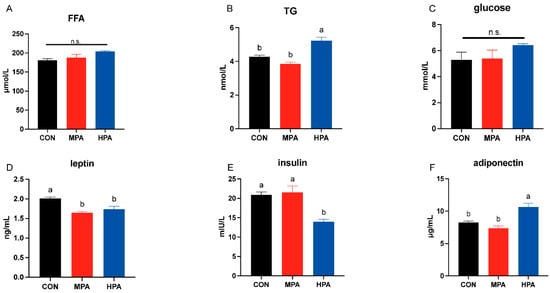
Figure 2.
The serum glucose (C), lipid (A,B), and hormone (D–F) levels in large yellow croaker fed with different diets (mean ± S.E.M., n = 3). FFA: free fatty acid; TG: triglyceride; n.s.: no significance. Following Tukey’s test, the same-letter bars show no discernible changes across treatments (p > 0.05).
3.3. Hepatic Fatty Acid Composition
No significant changes were found in the levels of saturated fatty acids (SFA) or C20:1 in the liver (p > 0.05, Table 3). However, the composition of monounsaturated fatty acids (∑MUFA) was significantly altered. Specifically, a dose-dependent increase was seen in C16:1, C18:1n-9, and total ∑MUFA, and significantly higher levels were recorded in the HPA group compared to the CON and MPA groups. (p < 0.05). Conversely, C18:1n-7 followed an inverse trend, being significantly depleted in the HPA group relative to the CON and MPA treatments. Regarding polyunsaturated fatty acids, total n-3 PUFA levels were negatively correlated with dietary glyceryl palmitate; notably, the HPA group showed significantly reduced ∑n-3 PUFA and C22:6n-3 (DHA) levels compared to the other groups (p < 0.05, Table 3). Additionally, glyceryl palmitate significantly suppressed the content of C20:5n-3 (EPA). The PA/(DHA+EPA) ratio showed an increasing trend with higher dietary glyceryl palmitate, and was significantly elevated in the HPA group relative to the CON and MPA groups (p < 0.05, Table 3). Conversely, the total DHA+EPA content decreased with increasing glyceryl palmitate supplementation and was much lower in the HPA group.

Table 3.
Fatty acid composition in liver (% identified fatty acids).
3.4. Hepatic Gene Expression Profiles in Large Yellow Croaker
3.4.1. Key Enzymes of Glucose Metabolism and Genes Related to Glucose Transport in Liver
Figure 3A illustrates the hepatic transcriptional profiles related to glucose metabolism. Dietary glyceryl palmitate significantly modulated several key genes. Specifically, gk expression was upregulated in both MPA and HPA groups compared to the control (p < 0.05), showing a positive correlation with dietary inclusion. In contrast, gys mRNA levels were significantly suppressed in the treatment groups (p < 0.05). The MPA group specifically exhibited lower pfk expression but higher fbp levels relative to the CON group (p < 0.05). Although pepck appeared to decline with increasing supplementation, no statistically significant differences were found for pepck or g6p (p < 0.05). Similarly, the expression of pyg, g6pd, and glut2 remained stable across all groups. However, g6pd displayed a decreasing trend in response to increasing dietary glyceryl palmitate.
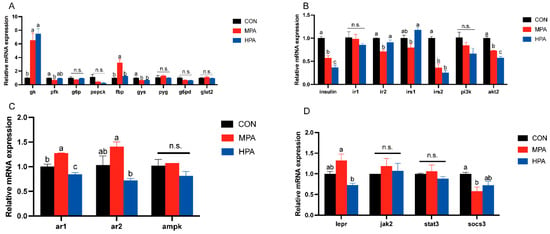
Figure 3.
Hepatic gene expression profiles in large yellow croaker fed with different diets (mean ± S.E.M., n = 3). (A) Key enzymes of glucose metabolism and genes related to glucose transport. (B) Genes associated with insulin pathway. (C) Genes associated with adiponectin pathway. (D) Genes associated with leptin pathway. n.s.: no significance. Following Tukey’s test, the same-letter showed no discernible changes across treatments (p > 0.05), while different-letter showed significant changes across treatments (p < 0.05).
3.4.2. Insulin Pathway-Related Genes in Liver
Figure 3B depicted the hepatic mRNA profiles associated with the insulin signaling cascade. A dose-dependent downregulation was observed for insulin, irs2, and akt2 in response to increasing dietary glyceryl palmitate. Notably, irs2 transcription was significantly inhibited in the MPA group. Conversely, the expression levels of ir1 and pi3k remained stable across all treatments.
3.4.3. Adiponectin and Leptin Pathway-Related Genes in Liver
The expression patterns of genes associated with adiponectin and leptin pathway in the liver are displayed in Figure 3C,D. As the proportion of glyceryl palmitate increased, the expression of ar1, ar2, and lepr exhibited an initial increase followed by a decrease. MPA considerably increased ar1 mRNA expression in comparison to the control group, whereas HPA significantly suppressed it. Even though there were no appreciable variations in the ar2 and lepr mRNA expression between the glyceryl palmitate groups and the control, HPA significantly inhibited the expression of both ar2 and lepr compared to the MPA group. The MPA group significantly inhibited the socs3 mRNA level. In addition, glyceryl palmitate had no significant influence on the mRNA expression levels of jak2 or stat3.
3.5. Muscular Gene Expression Profiles in Large Yellow Croaker
3.5.1. Key Enzymes of Glucose Metabolism and Genes Related to Glucose Transport
Figure 4A illustrates the transcriptional profiles of glucose metabolism and transport genes in muscle tissue. A dose-dependent upregulation was observed for fbp, gys, and pyg, with the HPA group exhibiting significantly higher expression levels compared to the control (p < 0.05). Conversely, dietary glyceryl palmitate significantly suppressed glut1 expression. Specific group effects were also noted: the HPA group showed a marked reduction in pepck, whereas the MPA group displayed significantly lower glut4 levels. Meanwhile, g6pd expression remained stable across all treatments.
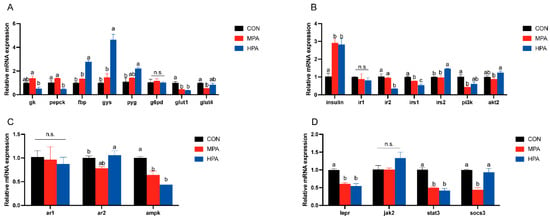
Figure 4.
Muscular gene expression profiles in large yellow croaker fed with different diets (mean ± S.E.M., n = 3). (A) Key enzymes of glucose metabolism and genes related to glucose transport. (B) Genes associated with insulin pathway. (C) Genes associated with adiponectin pathway. (D) Genes associated with leptin pathway. n.s.: no significance. Following Tukey’s test, the same-letter showed no discernible changes across treatments (p > 0.05), while different-letter showed significant changes across treatments (p < 0.05).
3.5.2. Insulin Pathway-Related Genes
Figure 4B illustrates the transcriptional profiles of insulin pathway in muscle tissue. With increasing substitution levels of glyceryl palmitate, the mRNA expression levels of insulin and irs2 exhibited a gradually increasing trend. Specifically, both MPA and HPA significantly up-regulated the expression of insulin, whereas only HPA significantly enhanced the expression of irs2. HPA significantly inhibited the mRNA expression of ir2, and glyceryl palmitate overall significantly suppressed the mRNA expression of irs1. In contrast, the expression of pi3k and akt2 showed an initial decrease followed by an increase in response to rising substitution levels of glyceryl palmitate. However, ir1 expression remained stable across all treatments.
3.5.3. Adiponectin and Leptin Pathway-Related Genes
The expression patterns of genes related to adiponectin and leptin pathway in muscle are shown in Figure 4C,D. With increasing dietary inclusion levels of glyceryl palmitate, the mRNA expression levels of ar2 and socs3 exhibited a biphasic pattern characterized by an initial decrease followed by an increase. Among these, the ar2 mRNA was significantly increased in the HPA group. In contrast, glyceryl palmitate did not exert significant effects on the mRNA expression of ar1 or jak2. Additionally, glyceryl palmitate significantly suppressed the mRNA expression levels of ampk, lepr, and stat3.
4. Discussion
Due to the limited supply of fish oil resources, the substitution of fish oil with vegetable oils has become an inevitable trend [28,29]. Under these conditions, a risk of nutritional stress is posed to aquatic animals by changes in fatty acid profiles, and the negative effects of saturated fatty acids are particularly noted [30]. The proportion of fatty acids in feed did not affect the survival and growth of fish [31], this was also confirmed in this study. A complex connection between lipid and glucose metabolism is observed in living organisms [32,33]. It has been indicated that glucose balance in farmed animals can be disrupted by changes in dietary fats [34,35]. Since a limited ability to process carbohydrates is naturally possessed by fish [36], it is not yet clear if the reduced growth seen after switching to vegetable oils is linked to glucose metabolism issues. Therefore, glyceryl palmitate was selected as a representative saturated fat in this study, and the effect of the dietary saturated/polyunsaturated fatty acid ratio on glucose metabolism in large yellow croaker (Larimichthys crocea) was investigated. Hepatic and muscle glycogen levels were significantly reduced when DHA+EPA-rich oil was fully replaced by glyceryl palmitate. This finding is supported by observations in large yellow croaker, where muscle glycogen was decreased by the substitution of soybean oil [37]. The reduction in glycogen levels likely results from a dual mechanism of impaired synthesis and enhanced mobilization. First, the replacement of n-3 HUFAs depressed the PI3K/AKT pathway and downregulates glycogenesis [37]. Second, metabolic stress triggers glycogen depletion. As shown in carnivorous fish, hepatic glycogen is preferentially mobilized as the primary energy substrate when lipid utilization is suboptimal [38]. Additionally, dietary glyceryl palmitate significantly increased the crude lipid content in both muscle and liver tissues. Similar results have been reported in tilapia (Oreochromis niloticus) [39], grass carp (Ctenopharyngodon idella) [40], and blunt snout bream (Megalobrama amblycephala) [41]. The specific mechanism may be related to the overactivation of de novo fat synthesis by palmitic acid. The liver is recognized as the central organ for processing fats, and its fatty acid profile is known to be easily changed by the diet [42,43]. In this study, the total amount of saturated fatty acids (SFA) in the liver was not significantly altered by the addition of glyceryl palmitate, but the level of monounsaturated fatty acids (MUFA) was markedly increased. Since the harmful effects of high saturated fat levels are known to be reduced by MUFA [44,45], the observed rise in MUFA might be interpreted as an internal defense response triggered to keep liver cells healthy and metabolism balanced. Also, a drop in these polyunsaturated fats in the liver was directly caused by the lower amount of EPA and DHA provided in the feed. Because DHA and EPA cannot be easily produced by the large yellow croaker [46], it is held that adequate amounts must be supplied in the diet to maintain both muscle quality and overall health.
The regulation of glucose and fat metabolism is heavily relied upon by adipokines, which are proteins released by fat tissue [47]. Key roles in metabolism, inflammation, insulin response, and appetite control are played by these proteins [48]. In this experiment, serum leptin levels were significantly lowered by glyceryl palmitate. Given that leptin has been shown to promote fatty acid oxidation in aquatic animals [49], the observed lipid increase in these tissues may be attributed to an attenuated capacity for leptin-mediated lipid catabolism [50]. Serum adiponectin levels were significantly raised by glyceryl palmitate. This finding is supported by mammalian studies, where it has been noted that circulating adiponectin is markedly increased by diets high in saturated fats [51], which reflected the conservation of adiponectin regulation between fish and mammals. An essential role in the regulation of fat and sugar metabolism is played by adiponectin, a cytokine secreted by adipose tissue. It has been shown that lipid oxidation is promoted and fat formation is blocked by this protein [52]. Thus, the rise in adiponectin triggered by glyceryl palmitate might be viewed as a feedback response to abnormal fat storage. Insulin is regarded as a key hormone for blood sugar control [53]. In this study, serum insulin levels were reduced by glyceryl palmitate, and the ability of the large yellow croaker to regulate blood glucose was consequently impaired. Ultimately, it is suggested that the disturbance of glucose and lipid metabolism caused by high saturated fatty acids is partially explained by these hormonal changes.
Focusing on the signaling pathways of insulin, leptin, and adiponectin, this work uncovered how large yellow croaker responds to glyceryl palmitate at the transcriptional level. The findings provide insight into the underlying network linking hormonal signals to glucose homeostasis. In hepatic tissue, the expression of glucokinase (GK), the initial rate-limiting enzyme of the glycolytic pathway [54], was significantly activated by glyceryl palmitate treatment. At the same time, the transcriptional level of glycogen synthase (GYS), the key rate-limiting enzyme for glycogen synthesis, was significantly reduced [55]. The decrease in liver glycogen observed in this study was matched by the gene expression pattern. It is indicated that glycolysis is promoted and glycogen storage is inhibited by glyceryl palmitate, so the glucose balance in the liver is affected. Regarding the insulin signaling pathway, a significant drop in the expression of the insulin gene and its downstream molecule, insulin receptor substrate 2 (IRS2), was caused by the treatment. This result is supported by reports in aquatic animals where insulin resistance is induced by saturated fatty acids [56]. It is suggested that insulin signal transmission might be disrupted by the activation of ceramide synthesis. The role of protein kinase B subtype 2 (AKT2) in improving insulin sensitivity is becoming better understood in fish. It is known that metabolic problems are treated by drugs like fenofibrate via AKT2 [57], so the value of this pathway is highlighted. In muscle tissue, insulin gene expression was raised by glyceryl palmitate. This change could be seen as a defense attempt to keep blood sugar balanced. However, the expression of the insulin receptor (IR2) and IRS1 was suppressed. It is indicated that the signaling path was blocked even with more insulin made, and the effective work of insulin was prevented. In the present study, we observed that glyceryl palmitate exerted a tissue-specific regulatory effect on the expression of genes related to the adiponectin and leptin signaling pathways. Specifically, it had no effect on ampk expression in the liver, whereas it significantly downregulated ampk expression in the muscle. This differential regulation is likely attributed to the distinct metabolic buffering capacities of the liver and muscle against palmitic acid. As the primary site for lipid oxidation, skeletal muscle has a limited capacity for lipid efflux, which renders it more susceptible to the conversion of excess palmitic acid into cytotoxic C16:0-ceramide under conditions of lipid overload [58]. In contrast, the liver can effectively clear excess lipids through its robust very-low-density lipoprotein (VLDL) secretion machinery, thereby alleviating the intracellular accumulation of lipotoxic substances. A similar tissue-specific regulatory pattern was also observed in the modulation of stat3 by glyceryl palmitate. The decreased expression of the leptin receptor (LEPR) corresponded with reduced circulating leptin levels. Since fatty acid oxidation is promoted by leptin, increased fat storage might be explained by a weakening of the leptin signaling pathway. Overall, metabolic balance in the liver and muscle of large yellow croaker is affected by glyceryl palmitate. This is driven by the fact that the expression of key glucose metabolism enzymes, insulin signaling components, and energy sensors (AMPK and the leptin system) is regulated by the diet. Consequently, lipid deposition and growth performance are potentially modulated by these factors.
5. Conclusions
This study confirmed that a high dietary ratio of glyceryl palmitate (PA) to DHA+EPA disrupted glucolipid homeostasis in large yellow croaker. The imbalance induced by replacing fish oil with glyceryl palmitate did not merely alter tissue fatty acid profiles; it actively triggered metabolic disorders characterized by hepatic lipid accumulation and glycogen depletion. Mechanistically, we identified that this disruption was mediated through an endocrine axis involving suppressed insulin and leptin signaling, alongside elevated adiponectin, which subsequently upregulated glycolysis while inhibiting glycogen synthesis and insulin sensitivity at the transcriptional level. From an aquaculture industry perspective, these findings highlight a critical physiological limit in replacing fish oil with saturated fatty acid sources. To prevent metabolic disorders and fatty liver conditions, feed formulations must prioritize an optimal PA/(DHA+EPA) ratio rather than focusing solely on lipid energy provision. This study provides a theoretical basis for precision nutrition strategies to maintain the metabolic health and flesh quality of farmed marine fish.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/metabo16010072/s1, Table S1: Primer table for Q-RT-PCR.
Author Contributions
Q.W.: Methodology, Data curation, Writing—original draft; H.G.: Resources, Software, Writing—review and editing; Z.G.: Validation, Writing—review and editing; H.M.: Resources, Methodology, Writing—review and editing; H.C.: Resources, Investigation; K.M.: Project administration, Supervision; W.Z.: Funding acquisition, Conceptualization, Supervision, Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Science and Technology Research and Development Projects of China National Fisheries Corp. (CNFCCA2025002) and The APC was funded by (CNFCCA2025002).
Institutional Review Board Statement
The animal study protocol was approved by Ocean University of China’s Animal Care Committee (Protocol code OUC-AE-2025-308 and Approval date: 1 January 2022).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Acknowledgments
We thank Jiahuan Liu, Mingzhu Pan, Fangli Xie, and Dong Liu for their guidance and help in sample selection and experimental techniques.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hodar, A.; Vasava, R.; Mahavadiya, D.; Joshi, N. Fish meal and fish oil replacement for aqua feed formulation by using alternative sources: A review. J. Exp. Zool. India 2020, 23, 13–21. [Google Scholar]
- Alhazzaa, R.; Nichols, P.D.; Carter, C.G. Sustainable alternatives to dietary fish oil in tropical fish aquaculture. Rev. Aquacult. 2019, 11, 1195–1218. [Google Scholar] [CrossRef]
- Castro, C.; Corraze, G.; Panserat, S.; Oliva-Teles, A. Effects of fish oil replacement by a vegetable oil blend on digestibility, postprandial serum metabolite profile, lipid and glucose metabolism of E uropean sea bass (Dicentrarchus labrax) juveniles. Aquac. Nutr. 2015, 21, 592–603. [Google Scholar]
- Wang, Q.; Mu, H.; Shen, H.; Gu, Z.; Liu, D.; Yang, M.; Zhang, Y.; Xu, W.; Zhang, W.; Mai, K. Comparative analysis of glucose metabolism responses of large yellow croaker Larimichthys crocea fed diet with fish oil and palm oil. Fish Physiol. Biochem. 2019, 45, 1603–1614. [Google Scholar] [CrossRef]
- Garcia-Martinez, I.; Alen, R.; Pereira, L.; Povo-Retana, A.; Astudillo, A.M.; Hitos, A.B.; Gomez-Hurtado, I.; Lopez-Collazo, E.; Boscá, L.; Francés, R. Saturated fatty acid-enriched small extracellular vesicles mediate a crosstalk inducing liver inflammation and hepatocyte insulin resistance. JHEP Rep. 2023, 5, 100756. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Alegría, K.; Bastián-Eugenio, C.E.; Vaca, L.; Arias, C. Palmitic acid induces insulin resistance by a mechanism associated with energy metabolism and calcium entry in neuronal cells. FASEB J. 2021, 35, e21712. [Google Scholar] [CrossRef]
- Malik, S.; Inamdar, S.; Acharya, J.; Goel, P.; Ghaskadbi, S. Characterization of palmitic acid toxicity induced insulin resistance in HepG2 cells. Toxicol. Vitr. 2024, 97, 105802. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, S.; Li, Z. Adipokines in glucose and lipid metabolism. Adipocyte 2023, 12, 2202976. [Google Scholar] [CrossRef] [PubMed]
- Morigny, P.; Boucher, J.; Arner, P.; Langin, D. Lipid and glucose metabolism in white adipocytes: Pathways, dysfunction and therapeutics. Nat. Rev. Endocrinol. 2021, 17, 276–295. [Google Scholar] [CrossRef] [PubMed]
- Jia, K.; Shi, P.; Zhang, L.; Yan, X.; Xu, J.; Liao, K. HSF1 Mediates Palmitic Acid-Disrupted Lipid Metabolism and Inflammatory Response by Maintaining Endoplasmic Reticulum Homeostasis in Fish. J. Agric. Food Chem. 2025, 73, 5236–5247. [Google Scholar] [CrossRef]
- Shi, P.; Liao, K.; Xu, J.; Wang, Y.; Xu, S.; Yan, X. Eicosapentaenoic acid mitigates palmitic acid-induced heat shock response, inflammation and repair processes in fish intestine. Fish Shellfish Immunol. 2022, 124, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Miklankova, D.; Markova, I.; Hüttl, M.; Stankova, B.; Malinska, H. The different insulin-sensitising and anti-inflammatory effects of palmitoleic acid and oleic acid in a prediabetes model. J. Diabetes Res. 2022, 2022, 4587907. [Google Scholar] [CrossRef]
- Polakof, S.; Panserat, S.; Soengas, J.L.; Moon, T.W. Glucose metabolism in fish: A review. J. Comp. Physiol. B 2012, 182, 1015–1045. [Google Scholar] [CrossRef]
- Poli, A.; Agostoni, C.; Visioli, F. Dietary fatty acids and inflammation: Focus on the n-6 series. Int. J. Mol. Sci. 2023, 24, 4567. [Google Scholar] [CrossRef]
- Liu, W.; Zheng, Q.; Zhu, M.; Liu, X.; Liu, J.; Lu, Y.; Cheng, J.; Chen, Y. Regulatory effects of n-3 PUFAs on pancreatic β-cells and insulin-sensitive tissues. Curr. Drug Metab. 2021, 22, 1017–1034. [Google Scholar] [CrossRef]
- Arnemo, M.; Kavaliauskis, A.; Andresen, A.M.S.; Bou, M.; Berge, G.M.; Ruyter, B.; Gjøen, T. Effects of dietary n-3 fatty acids on Toll-like receptor activation in primary leucocytes from Atlantic salmon (Salmo salar). Fish Physiol. Biochem. 2017, 43, 1065–1080. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Sun, B.; Guan, W.; Bi, Y.; Li, P.; Ma, J.; Chen, F.; Pan, Q.; Xie, Q. N-3 essential fatty acids in Nile tilapia, Oreochromis niloticus: Effects of linolenic acid on non-specific immunity and anti-inflammatory responses in juvenile fish. Aquaculture 2016, 450, 250–257. [Google Scholar] [CrossRef]
- Russo, B.; Menduni, M.; Borboni, P.; Picconi, F.; Frontoni, S. Autonomic nervous system in obesity and insulin-resistance—The complex interplay between leptin and central nervous system. Int. J. Mol. Sci. 2021, 22, 5187. [Google Scholar] [CrossRef]
- Deck, C.A.; Mankiewicz, J.L.; Borski, R.J. Evidence for a leptin–insulin axis in a fish, the tilapia (Oreochromis mossambicus). J. Endocrinol. 2022, 253, 13–25. [Google Scholar] [CrossRef]
- Balakrishnan, R.; Thurmond, D.C. Mechanisms by which skeletal muscle myokines ameliorate insulin resistance. Int. J. Mol. Sci. 2022, 23, 4636. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Zhao, W.; Yan, X.; Yang, G.; Yang, L.; Lu, R.; Pi, D.; Nie, G. Effects of adiponectin on glucose metabolism in the hepatopancreas of grass carp (Ctenopharyngodon idella). Aquac. Nutr. 2022, 2022, 5699931. [Google Scholar] [CrossRef]
- Yuan, J.; Zhuang, X.; Wu, L.; Lin, H.; Li, Y.; Wu, L.; Yao, J.; Liu, J.; Ding, S. Assessing the population genetic structure of yellow croaker in China: Insights into the ecological and genetic consequences of artificial breeding on natural populations. Aquaculture 2024, 590, 741026. [Google Scholar] [CrossRef]
- Parzanini, C.; Colombo, S.M.; Kainz, M.J.; Wacker, A.; Parrish, C.C.; Arts, M.T. Discrimination between freshwater and marine fish using fatty acids: Ecological implications and future perspectives. Environ. Rev. 2020, 28, 546–559. [Google Scholar] [CrossRef]
- Bennett, P.M.; Weber, L.P.; Janz, D.M. Comparison of chloroform–methanol-extracted and solvent-free triglyceride determinations in four fish species. J. Aquat. Anim. Health 2007, 19, 179–185. [Google Scholar] [PubMed]
- Mu, H.; Shen, H.; Liu, J.; Xie, F.; Zhang, W.; Mai, K. High level of dietary soybean oil depresses the growth and anti-oxidative capacity and induces inflammatory response in large yellow croaker Larimichthys crocea. Fish Shellfish Immunol. 2018, 77, 465–473. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Wang, Q. Effects of Dietary Palmitic Acid and DHA/EPA on Glucose Metabolism in Large Yellow Croaker (Larimichthys crocea). Master’s Thesis, Ocean University of China, Qingdao, China, 2019. [Google Scholar]
- Álvarez, A.; Fontanillas, R.; Hernández-Contreras, A.; Hernández, M. Partial replacement of fish oil with vegetal oils in commercial diets: The effect on the quality of gilthead seabream (Sparus aurata). Anim. Feed Sci. Technol. 2020, 265, 114504. [Google Scholar] [CrossRef]
- Qian, Y.F.; Wang, J.X.; Qiao, F.; Luo, Y.; Chen, L.Q.; Zhang, M.L.; Du, Z.Y. Modelling the impact of replacing fish oil with plant oils: A meta-analysis to match the optimal plant oil for major cultured fish. Rev. Aquacult. 2024, 16, 1395–1422. [Google Scholar] [CrossRef]
- Marques, V.H.; Moreira, R.G.; Branco, G.S.; Honji, R.M.; Rombenso, A.N.; Viana, M.T.; de Mello, P.H.; Mata-Sotres, J.A.; Araujo, B.C. Different saturated and monounsaturated fatty acids levels in fish oil-free diets to cobia (Rachycentron canadum) juveniles: Effects in growth performance and lipid metabolism. Aquaculture 2021, 541, 736843. [Google Scholar] [CrossRef]
- Zhang, F.; Li, L.; Li, P.; Meng, X.; Cui, X.; Ma, Q.; Wei, Y.; Liang, M.; Xu, H. Fish oil replacement by beef tallow in juvenile turbot diets: Effects on growth performance, body composition and volatile flavor compounds in the muscle. Aquaculture 2023, 564, 739070. [Google Scholar] [CrossRef]
- Ramatchandirin, B.; Pearah, A.; He, L. Regulation of liver glucose and lipid metabolism by transcriptional factors and coactivators. Life 2023, 13, 515. [Google Scholar] [CrossRef]
- Yan, X.; Qin, C.; Deng, D.; Yang, G.; Feng, J.; Lu, R.; Wang, G.; Nie, G. Regulation of glucose and lipid metabolism by insulin and glucagon in vivo and in vitro in common carp Cyprinus carpio L. Aquac. Rep. 2020, 18, 100427. [Google Scholar] [CrossRef]
- Egalini, F.; Guardamagna, O.; Gaggero, G.; Varaldo, E.; Giannone, B.; Beccuti, G.; Benso, A.; Broglio, F. The effects of omega 3 and omega 6 fatty acids on glucose metabolism: An updated review. Nutrients 2023, 15, 2672. [Google Scholar] [CrossRef]
- Menoyo, D.; Diez, A.; Lopez-Bote, C.J.; Casado, S.; Obach, A.; Bautista, J.M. Dietary fat type affects lipid metabolism in Atlantic salmon (Salmo salar L.) and differentially regulates glucose transporter GLUT4 expression in muscle. Aquaculture 2006, 261, 294–304. [Google Scholar] [CrossRef]
- Li, X.; Han, T.; Zheng, S.; Wu, G. Hepatic glucose metabolism and its disorders in fish. In Recent Advances in Animal Nutrition and Metabolism; Springer: Berlin/Heidelberg, Germany, 2021; pp. 207–236. [Google Scholar]
- Gu, Z.; Mu, H.; Shen, H.; Deng, K.; Liu, D.; Yang, M.; Zhang, Y.; Zhang, W.; Mai, K. High level of dietary soybean oil affects the glucose and lipid metabolism in large yellow croaker Larimichthys crocea through the insulin-mediated PI3K/AKT signaling pathway. Comp. Biochem. Phys. B 2019, 231, 34–41. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, X.; Han, Z.; Gong, Y.; Huang, X.; Chen, N.; Li, S. The preferential utilization of hepatic glycogen as energy substrates in largemouth bass (Micropterus salmoides) under short-term starvation. Fish Physiol. Biochem. 2024, 50, 785–796. [Google Scholar] [PubMed]
- Ayisi, C.L.; Zhao, J.; Rupia, E.J. Growth performance, feed utilization, body and fatty acid composition of Nile tilapia (Oreochromis niloticus) fed diets containing elevated levels of palm oil. Aquac. Fish. 2017, 2, 67–77. [Google Scholar] [CrossRef]
- Yang, S.-S.; Yu, C.-B.; Luo, Z.; Luo, W.-L.; Zhang, J.; Xu, J.-X.; Xu, W.-N. Berberine attenuates sodium palmitate-induced lipid accumulation, oxidative stress and apoptosis in grass carp (Ctenopharyngodon idella) hepatocyte in vitro. Fish Shellfish Immunol. 2019, 88, 518–527. [Google Scholar]
- Yu, C.; Zhang, M.; Liu, J.; Zhang, J.; Xu, J.; Xu, W. Effects of sodium acetate on lipid metabolism, antioxidant capability and cell apoptosis of blunt snout bream (Megalobrama amblycephala) hepatocytes treated by sodium palmitate. Aquac. Res. 2022, 53, 1098–1109. [Google Scholar] [CrossRef]
- Mzengereza, K.; Ishikawa, M.; Koshio, S.; Yokoyama, S.; Yukun, Z.; Shadrack, R.S.; Seo, S.; Duy Khoa, T.N.; Moss, A.; Dossou, S. Effect of substituting fish oil with camelina oil on growth performance, fatty acid profile, digestibility, liver histology, and antioxidative status of red seabream (Pagrus major). Animals 2021, 11, 1990. [Google Scholar] [CrossRef]
- Gou, N.; Ji, H.; Zhong, M.; Chang, Z.; Deng, W. Effects of dietary fish oil replacements with three vegetable oils on growth, fatty acid composition, antioxidant capacity, serum parameters and expression of lipid metabolism related genes in juvenile Onychostoma macrolepis. Aquac. Nutr. 2021, 27, 163–175. [Google Scholar] [CrossRef]
- Howe, A.-M.; Burke, S.; O’Reilly, M.E.; McGillicuddy, F.C.; Costello, D.A. Palmitic acid and oleic acid differently modulate TLR2-mediated inflammatory responses in microglia and macrophages. Mol. Neurobiol. 2022, 59, 2348–2362. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiang, Z.; Xu, Y.; Cheung, L.S.; Wang, X.; Wang, M.; Wong, H.H.W.; Zhu, Z.; Zhang, W.; Gao, Y. Oleic acid restores the impaired antitumor immunity of γδ-T cells induced by palmitic acid. Signal Transduct. Target. Ther. 2025, 10, 209. [Google Scholar] [CrossRef]
- Mu, H.; Wei, C.; Zhang, Y.; Zhou, H.; Pan, Y.; Chen, J.; Zhang, W.; Mai, K. Impacts of replacement of dietary fish oil by vegetable oils on growth performance, anti-oxidative capacity, and inflammatory response in large yellow croaker Larimichthys crocea. Fish Physiol. Biochem. 2020, 46, 231–245. [Google Scholar] [CrossRef]
- Shi, J.; Fan, J.; Su, Q.; Yang, Z. Cytokines and abnormal glucose and lipid metabolism. Front. Endocrinol. 2019, 10, 703. [Google Scholar] [CrossRef]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef]
- Song, Y.-F.; Tan, X.-Y.; Pan, Y.-X.; Zhang, L.-H.; Chen, Q.-L. Fatty acid β-oxidation is essential in leptin-mediated oocytes maturation of yellow catfish Pelteobagrus fulvidraco. Int. J. Mol. Sci. 2018, 19, 1457. [Google Scholar] [CrossRef] [PubMed]
- Picó, C.; Palou, M.; Pomar, C.A.; Rodríguez, A.M.; Palou, A. Leptin as a key regulator of the adipose organ. Rev. Endocr. Metab. Disord. 2022, 23, 13–30. [Google Scholar] [CrossRef] [PubMed]
- You, M.; Considine, R.V.; Leone, T.C.; Kelly, D.P.; Crabb, D.W. Role of adiponectin in the protective action of dietary saturated fat against alcoholic fatty liver in mice. Hepatology 2005, 42, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.; Xu, X.; Turchini, G.M.; Mai, K.; Ai, Q. Adiponectin’s roles in lipid and glucose metabolism modulation in fish: Mechanisms and perspectives. Rev. Aquacult. 2021, 13, 2305–2321. [Google Scholar] [CrossRef]
- Aronoff, S.L.; Berkowitz, K.; Shreiner, B.; Want, L. Glucose metabolism and regulation: Beyond insulin and glucagon. Diabetes Spectr. 2004, 17, 183–190. [Google Scholar] [CrossRef]
- Gupta, R.; Gupta, N. Glycolysis and gluconeogenesis. In Fundamentals of Bacterial Physiology and Metabolism; Springer: Berlin/Heidelberg, Germany, 2021; pp. 267–287. [Google Scholar]
- Tang, B.; Frasinyuk, M.S.; Chikwana, V.M.; Mahalingan, K.K.; Morgan, C.A.; Segvich, D.M.; Bondarenko, S.P.; Mrug, G.P.; Wyrebek, P.; Watt, D.S. Discovery and development of small-molecule inhibitors of glycogen synthase. J. Med. Chem. 2020, 63, 3538–3551. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Chen, Q.; Xiang, X.; Dai, W.; Fang, W.; Cui, K.; Li, B.; Liu, Q.; Liu, Y.; Shen, Y. Tip60-mediated Rheb acetylation links palmitic acid with mTORC1 activation and insulin resistance. J. Cell Biol. 2024, 223, e202309090. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zhang, X.; Ma, F.; Sun, W.; Wang, W.; Yu, J.; Shi, Y.; Cai, L.; Xu, Z. The role of Akt2 in the protective effect of fenofibrate against diabetic nephropathy. Int. J. Biol. Sci. 2020, 16, 553. [Google Scholar] [CrossRef]
- Blachnio-Zabielska, A.U.; Chacinska, M.; Vendelbo, M.H.; Zabielski, P. The crucial role of C18-Cer in fat-induced skeletal muscle insulin resistance. Cell. Physiol. Biochem. 2016, 40, 1207–1220. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.