Abstract
Background/Objective: Type 2 diabetes mellitus (T2DM) is characterized by insulin resistance, β-cell dysfunction, and chronic hyperglycemia. Exercise is a cornerstone of non-pharmacological therapy, yet the optimal modalities by which different exercise prescriptions improve metabolic outcomes remain unclear. This review synthesizes evidence on the metabolic effects of aerobic, resistance, high-intensity interval (HIIT), and combined training in individuals with T2DM. Methods: The PubMed, Web of Science, and Scopus databases were searched up to March 30, 2025. A total of 26 articles were included. Articles were selected based on studies conducted on human participants diagnosed with type 2 diabetes mellitus, involving structured exercise interventions, and reporting at least one outcome related to insulin function or glycemic control. Results: This review identified five exercise programs that can improve metabolic outcomes in patients with type 2 diabetes. Evidence levels varied across the 26 studies (n = 20–98), so intensity ranges should be interpreted as indicative rather than prescriptive. Aerobic training was the primary intervention, and evidence from 13 studies (8–48 weeks) showed that moderate-to-vigorous intensity aerobic training (approximately 50–85% of maximum heart rate or 50–75% of VO2max) was generally associated with improvements in β-cell function, insulin sensitivity, and glycated hemoglobin (HbA1c). Strength training (approximately 40–50% to <3RM, 12 weeks) was linked to better glycemic parameters in some studies, though effects on insulin resistance were inconsistent. Most studies indicated that combined aerobic training (60–85% of maximum heart rate) with resistance or other complementary exercise modalities for 8–24 weeks tended to improve HbA1c, fasting glucose, and insulin sensitivity. High-intensity interval training (HIIT, ≥85% of maximum heart rate, 8 weeks) was also associated with enhanced insulin sensitivity, β-cell function, and basal insulin levels. Conclusions: Different exercise modalities improve metabolic health through complementary mechanisms involving enhanced glucose transport, mitochondrial function, anti-inflammatory effects, and increased muscle mass. Tailoring exercise prescriptions based on individual capacity and metabolic targets may optimize outcomes in T2DM management.
1. Introduction
Type 2 diabetes mellitus (T2DM) is a chronic metabolic disorder characterized by impaired insulin secretion, peripheral insulin resistance, and hyperglycemia, which together contribute to microvascular and macrovascular complications, reduced quality of life, and increased mortality [,]. Despite pharmacological advances, long-term glycemic control remains suboptimal in a substantial proportion of patients, highlighting the need for complementary lifestyle interventions targeting the underlying pathophysiological mechanisms of the disease.
Physical exercise has emerged as a cornerstone in the non-pharmacological management of T2DM, with compelling evidence supporting its efficacy in improving glycemic control and mitigating disease progression [,,]. Regular exercise enhances glucose uptake, increases insulin sensitivity, and reduces glycated hemoglobin (HbA1c) levels [,,]. These benefits are underpinned by complex metabolic adaptations in skeletal muscle, liver, and adipose tissue [], including augmented glucose transporter type 4 (GLUT4) translocation, mitochondrial biogenesis, suppression of hepatic gluconeogenesis, and modulation of inflammatory mediators [].
However, the optimal exercise modalities and protocols for improving specific metabolic outcomes in individuals with T2DM remain an area of active investigation. Aerobic training [,,], resistance training [,], high-intensity interval training (HIIT) [,], and various combined regimens exert distinct but sometimes overlapping physiological effects [,], each targeting different nodes of metabolic dysfunction such as insulin resistance and β-cell dysfunction. Furthermore, differences in exercise intensity, duration, and frequency cause variation in their efficacy [,].
This review aims to compare different exercise prescriptions and their underlying metabolic mechanisms—namely aerobic training, resistance training, HIIT, and their combinations—in improving glycemic parameters, insulin sensitivity, pancreatic β-cell function, and HbA1c levels in individuals with T2DM, with a particular focus on AMPK–PGC-1α signaling, GLUT4 translocation, mitochondrial adaptations, and anti-inflammatory pathways. By comparing different exercise modalities, they can be personalized to tackle specific metabolic issues, and a framework for exercise-based therapeutic strategies targeting metabolic dysfunctions in diabetes care can be provided.
2. Materials and Methods
Search Strategy
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) Guideline []. The literature search was conducted across the following databases: PubMed, Web of Science, and Scopus []. Peer-reviewed articles published in English until 30th March 2025 were reviewed. No contact with the studies’ authors was made. The search strategy used in Scopus is displayed in Table 1 as an example, and a similar strategy was used to search the other databases. The detailed search strategies for PubMed and Web of Science are provided in the Supplementary Materials, Tables S1 and S2.

Table 1.
Search strategy for Scopus.
This research was registered in PROSPERO on 21 January 2025 (CRD42025641172) [].
3. Result
3.1. Study Selection
Figure 1 shows a PRISMA flow diagram of the search. The articles were selected for review after thorough screening and exclusion of ineligible articles. The inclusion criteria were as follows: the article was published in English, conducted on human participants, involved a structured exercise intervention, and included individuals diagnosed with type 2 diabetes mellitus.
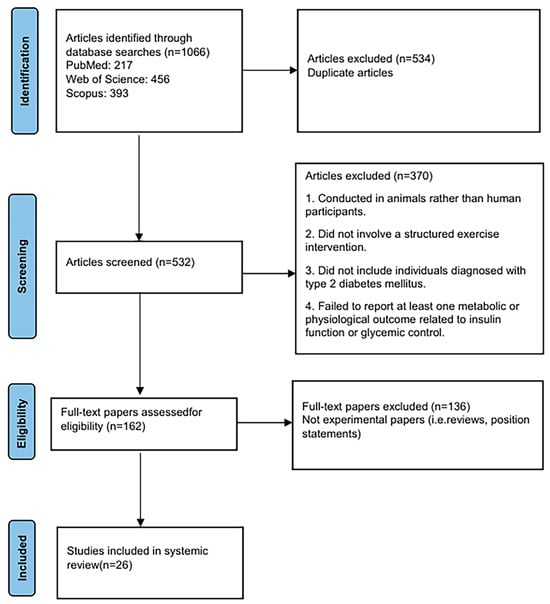
Figure 1.
PRISMA flowchart for study selection.
3.2. Studies Review
A total of 26 studies were included in this review, with participant ages ranging from 39 to 65 years. Baseline glycated hemoglobin (HbA1c) levels across studies ranged from 6.8% to 8.6%, indicating generally poor to moderate glycemic control among participants. The exercise interventions varied in type and intensity, including aerobic training, combined training, resistance training, and high-intensity interval training (HIIT), with intervention durations ranging from 8 to 48 weeks. Aerobic programs were typically performed at moderate-to-vigorous intensity (50–85% HRmax or 50–75% VO2peak), lasting 30–180 min per session, one to six times per week for 8–48 weeks; low-impact exercises such as Tai Chi were also categorized as aerobic training. Combined training protocols integrated aerobic and resistance exercises within the same session or across a weekly schedule. Resistance training, including free-weight and elastic band exercises, was generally performed three times per week for 12 weeks, at moderate-to-high intensity (40–50% to <3RM) with progressive loading (e.g., +0.5 kg per week). HIIT interventions involved repeated short bouts of exercise performed at ≥85% of maximum heart rate, interspersed with active or passive recovery periods. Table 2 presents the detailed characteristics of the included studies, including study design and participant demographics.

Table 2.
Studies reviewed on aerobic exercise.

Table 3.
Studies reviewed on Combined Exercise.

Table 4.
Studies reviewed on Strength Exercise.

Table 5.
Studies reviewed on HIIT Exercise.
3.3. Data Extraction and Quality Assessment
The eligibility criteria were defined according to the PICO framework []. Participants (P): patients diagnosed with type 2 diabetes mellitus (T2DM); Interventions (I): structured exercise training programs; Comparators (C): non-exercise control groups or alternative exercise interventions; and Outcomes (O): indicators of glucose metabolism (e.g., HbA1c, fasting glucose, or insulin sensitivity).
Two reviewers independently conducted the literature screening and data extraction. Titles and abstracts of all retrieved records were screened based on the predefined inclusion and exclusion criteria, and full texts of potentially eligible articles were reviewed in detail to confirm inclusion. Any discrepancies or disagreements between reviewers were resolved through discussion; when consensus could not be reached, a third reviewer adjudicated the decision. A small number of conflicts (n = 3) required adjudication. Inter-rater agreement was assessed qualitatively through discussion rather than statistical testing.
For each included study, data were systematically extracted on (a) participant characteristics, (b) exercise intervention parameters (frequency, intensity, duration, and type), and (c) outcome measures related to glucose metabolism. When relevant information was unclear or missing, the study authors were contacted to obtain additional details (e.g., raw data or clarification of reported outcomes). The methodological quality of the included studies was evaluated using the Cochrane risk-of-bias tool for randomized trials (RoB 2) [].
3.4. Risk of Bias
Two reviewers independently assessed the risk of bias in all included randomized controlled trials using the RoB 2. Any disagreements were verified against the full text and resolved by discussion. Among the included 11 RCTs, 3 were judged as “low risk of bias” overall, while 8 were rated as having “some concerns.” The most common issues were related to deviations from the intended interventions (per-protocol effect) and potential bias in outcome measurement. No study was judged to be at “high risk of bias.”
4. Discussion
This review identifies 5 exercise prescriptions as effective in improving metabolic outcomes in individuals with type 2 diabetes mellitus. Aerobic training alone, when performed at a high intensity (85% of HRmax) three times per week for 8 to 12 weeks, has been shown to enhance pancreatic β-cell function, insulin-related parameters, glycemic control, and HbA1c levels [,]. Resistance training alone, implemented three times weekly for a minimum of 12 weeks at an intensity guided by a Borg 11–13, leads to improvements in glycemic parameters and HbA1c [,]. A combined HIIT (70–90% HRmax or 80–85% VO2peak) and resistance training (55–80% of 1 RM), performed three times per week for at least 8 weeks, has demonstrated efficacy in improving β-cell function, insulin sensitivity, and HbA1c []. Additionally, combined aerobic (70–89% HRmax) and resistance training (60% 1RM), performed three times per week over 12 weeks, effectively improves insulin-related indices, glycemic control, and HbA1c levels [].
4.1. Effects of Aerobic Exercise on Improving Metabolic Outcomes
The metabolic benefits of aerobic exercise in individuals with type 2 diabetes mellitus are mediated through multiple interrelated mechanisms. At the core of these effects is the enhancement of skeletal muscle insulin sensitivity [,,], largely attributed to increased translocation of GLUT4 to the cell membrane, which facilitates greater glucose uptake and utilization []. This effect is known to last for up to 6 h after exercise, which leaves the muscle sensitized to insulin for up to 48 h []. Aerobic exercise also promotes mitochondrial biogenesis and oxidative enzyme activity in muscle tissue, thereby improving glucose oxidation efficiency and reducing reliance on anaerobic metabolism []. Concurrently, aerobic training suppresses hepatic gluconeogenesis by decreasing intrahepatic lipid accumulation and inflammatory cytokine expression [], ultimately leading to reduced fasting and postprandial blood glucose levels [,]. These changes alleviate chronic hyperglycemia, thus lowering the availability of glucose substrates for non-enzymatic glycation of hemoglobin, reflected in decreased HbA1c levels [,,,,,,,,]. According to the American College of Physicians (2018), maintaining HbA1c between 7% and 8% is considered clinically meaningful for most patients with type 2 diabetes, suggesting that the exercise-induced improvements observed in these studies are not only statistically significant but also clinically relevant []. Moreover, aerobic exercise modulates adipose tissue function by reducing visceral fat mass and circulating free fatty acids, which diminishes lipotoxicity and restores insulin signaling pathways []. The anti-inflammatory effects of regular aerobic activity, marked by reduced levels of C-reactive protein (CRP), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α), further support β-cell preservation and improve insulin secretory dynamics []. It is worth noting that low-intensity aerobic modalities, such as Tai Chi, have also been examined in individuals with type 2 diabetes. For instance, a 16-week, twice-weekly Tai Chi program (HR during exercise: 83.3 ± 13.7) produced no significant changes in HbA1c or insulin resistance []. This finding suggests that exercise intensity and frequency are key determinants of metabolic adaptation and that insufficient training stimulus may limit measurable improvements. Collectively, these adaptive responses form the physiological basis by which aerobic exercise exerts its multifaceted effects on β-cell function, insulin sensitivity, glycemic control, and long-term glycemic markers such as HbA1c. The proposed mechanism involves several key tissues (Figure 2).
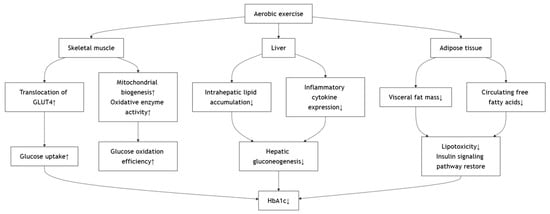
Figure 2.
Effects of Aerobic Exercise on Improving Metabolic Outcomes.
4.2. Effects of Resistance Training on Improving Metabolic Outcomes
Compared to aerobic exercise, resistance training improves glycemic parameters and HbA1c through distinct but complementary metabolic pathways [,]. While aerobic training primarily enhances glucose uptake via increased oxidative capacity and mitochondrial adaptations, resistance exercise promotes muscle hypertrophy and increases lean body mass, thereby expanding the overall reservoir for glucose disposal []. The increased muscle mass augments basal metabolic rate and facilitates sustained glucose clearance from circulation, which is particularly relevant for reducing fasting and postprandial glycemia over time [,]. Resistance training also reduces visceral adiposity and attenuates chronic low-grade inflammation by lowering circulating pro-inflammatory cytokines (e.g., IL-6, TNF-α), thereby mitigating their inhibitory effects on insulin signaling []. Although the improvement in oxidative metabolism is less pronounced than in aerobic training, resistance exercise exerts substantial effects on glucose regulation through structural and hormonal adaptations. Nonetheless, Kwon et al. reported no improvement in insulin resistance after 12 weeks of elastic band training, indicating that low-to-moderate-intensity resistance exercise may be insufficient to induce measurable metabolic adaptations in certain diabetic populations. This finding suggests that exercise intensity and loading play a critical role in optimizing metabolic outcomes []. These mechanisms collectively account for the observed reductions in blood glucose and HbA1c following regular moderate-intensity resistance training, as guided by a Borg rating of 11–13 over at least 12 weeks []. Like aerobic training, these improvements in HbA1c are considered clinically meaningful within the target range recommended by current diabetes management guidelines. When compared to aerobic modalities, resistance training provides a muscle-centric strategy for glycemic control, and its incorporation into a combined regimen may yield additive or synergistic metabolic benefits in patients with type 2 diabetes [,,]. The proposed mechanism involves several key tissues (Figure 3).
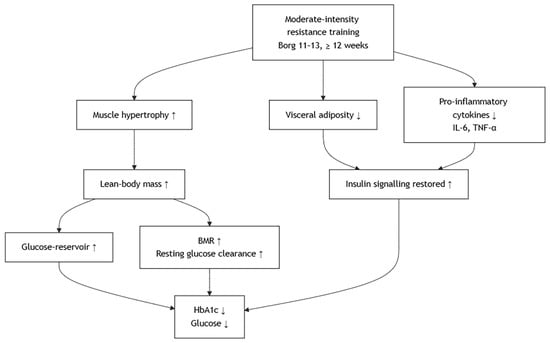
Figure 3.
Effects of Resistance Training on Improving Metabolic Outcomes.
4.3. Effects of HIIT on Improving Metabolic Outcomes
HIIT, characterized by repeated short bouts of vigorous activity interspersed with recovery periods [], exerts unique metabolic effects that significantly improve insulin-related parameters in individuals with type 2 diabetes mellitus [,,]. One of the primary mechanisms underlying these benefits is the rapid activation of the AMP-activated protein kinase (AMPK)–peroxisome proliferator-activated receptor gamma coactivator-1α (PGC-1α) signaling pathway, which enhances mitochondrial biogenesis and oxidative phosphorylation capacity in skeletal muscle []. This was achieved, albeit with a lower magnitude of AMPK activation compared to high-volume continuous endurance exercise, which leads to less protein degradation []. Furthermore, the high metabolic stress induced by HIIT upregulates GLUT4 translocation to the muscle cell membrane, improving insulin sensitivity in both fasted and fed states []. HIIT has also been shown to reduce intramyocellular lipid accumulation and circulating free fatty acids, thereby alleviating lipotoxicity and restoring insulin receptor signaling []. In addition, HIIT reduces systemic inflammation by decreasing pro-inflammatory cytokines such as Tumor Necrosis Factor-alpha (TNF-α), which is known to impair insulin action [,]. The proposed mechanism involves several key tissues (Figure 4).
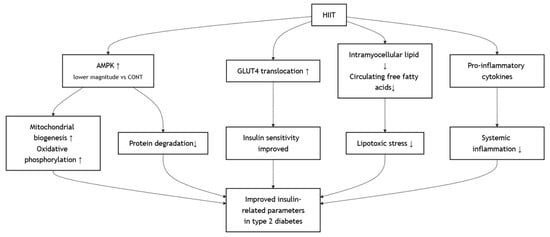
Figure 4.
Effects of HIIT on Improving Metabolic Outcomes.
4.4. Effects of Combined HIIT and Resistance Training
A combined protocol of HIIT and resistance training effectively improved insulin sensitivity and HbA1c levels in individuals with type 2 diabetes mellitus [,]. Compared to high-volume, low-intensity aerobic exercise, resistance training with HIIT still maintains the ability to synthesize mitochondrial protein while attenuating AMPK-induced protein degradation and maintaining mTOR-induced protein synthesis []. Considering that a diabetic individual does need to maintain their cardiovascular fitness and muscle mass for not only diabetic management but also health and fitness maintenance in general, they should choose the most beneficial method that has the least disadvantage, and it seems that the HIIT–resistance training combination can achieve that. However, only 2 studies have been conducted on diabetic patients, and more evidence should be examined before a stand can be established. In addition, HIIT may not be beginner- or sedentary-friendly, and progressive overloading of aerobic intensity should be prescribed. The proposed mechanism involves several key tissues (Figure 5).
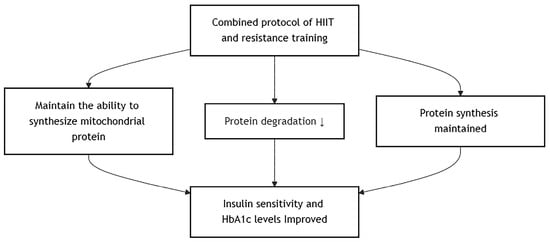
Figure 5.
Effects of Combined HIIT and Resistance Training.
4.5. Effects of Combined Aerobic and Resistance Training
Combined aerobic and resistance training elicits complementary physiological adaptations that contribute to significant improvements in insulin sensitivity, glycemic control, and HbA1c levels in individuals with type 2 diabetes mellitus [,,,]. Aerobic training primarily enhances skeletal muscle oxidative capacity and mitochondrial efficiency through activation of the AMPK–PGC-1α pathway [], leading to increased GLUT4 expression and translocation, thereby facilitating insulin-independent glucose uptake []. Concurrently, resistance training increases skeletal muscle mass and improves glucose transporter density and insulin receptor function, expanding the capacity for insulin-mediated glucose disposal []. This dual-modality approach also effectively reduces visceral adiposity and circulating levels of free fatty acids and pro-inflammatory cytokines (e.g., TNF-α, IL-6), mitigating lipotoxicity and chronic low-grade inflammation []—two major contributors to insulin resistance and β-cell dysfunction []. Additionally, resistance training improves muscle glycogen synthesis capacity [], while aerobic exercise attenuates hepatic gluconeogenesis and enhances peripheral glucose oxidation []. These synergistic effects result in improved fasting and postprandial glucose regulation, reduced glucose variability, and a lower glycation burden on hemoglobin over time [,,,]. However, the direct comparison between continuous aerobic exercise with HIIT in conjunction with resistance training is lacking, and thus no direct comparison can be made. It is theorized that continuous aerobic exercise, especially that with low intensity and longer duration, may diminish muscle mass []. Studies comparing HIIT and continuous aerobic exercise alongside resistance training should examine not only the typical diabetic outcome measures but also muscle mass and cardiovascular fitness to fully elucidate its holistic benefits to establish a stronger stand on which training method is optimal. The proposed mechanism involves several key tissues (Figure 6).
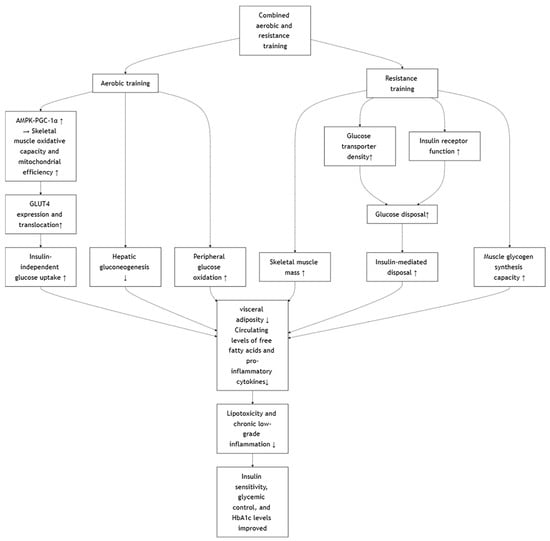
Figure 6.
Effects of Combined Aerobic and Resistance Training.
5. Conclusions
This review highlights the efficacy of various exercise modalities in improving metabolic health among individuals with type 2 diabetes mellitus. Aerobic, resistance, HIIT, and combined training protocols each exert distinct yet complementary effects on glycemic control, insulin sensitivity, and pancreatic β-cell function. These benefits are mediated through multiple mechanisms, including enhanced GLUT4 translocation, activation of the AMPK–PGC-1α signaling pathway, increased mitochondrial biogenesis, and reductions in inflammation and visceral adiposity.
Notably, combined exercise programs—particularly those integrating aerobic or HIIT components with resistance training—appear to produce superior outcomes by simultaneously engaging multiple metabolic pathways. Tailoring exercise prescriptions to individual capabilities, preferences, and glycemic profiles, therefore, represents a powerful, non-pharmacological approach to optimizing diabetes management.
Several key variables, including diet, medication use, baseline fitness status, and adherence to exercise programs, were insufficiently reported in many included studies. This limitation may have affected the interpretation of results and constrained the ability to determine the independent effects of exercise. In addition, the age and physical limitations of participants may influence exercise tolerance and safety, particularly for high-intensity protocols such as HIIT. Accordingly, exercise intensity should be individualized, especially for older adults or those with mobility restrictions.
The existing evidence base is further limited by variability in exercise protocols and the lack of long-term follow-up in many studies, which hinders direct comparison and assessment of the durability of observed benefits. These findings should therefore be interpreted with caution, as they are derived from heterogeneous studies with differing designs, sample sizes, and reporting standards. Additional data directly comparing continuous aerobic and HIIT protocols on glycemic and fitness outcomes are needed to clarify the optimal exercise strategies for individuals with diabetes. Furthermore, the long-term sustainability and potential risks associated with different exercise modalities—such as musculoskeletal injury or overtraining—remain insufficiently explored. Future high-quality randomized controlled trials with standardized reporting of exercise intensity and mechanistic outcomes are warranted to validate and refine these insights.
6. Implications for Clinical Practice and Public Health
From a clinical standpoint, the integration of individualized exercise prescriptions into standard diabetes care has the potential to substantially improve metabolic control and reduce pharmacological burden. Given the heterogeneity in patient response, exercise prescriptions should be tailored to account for baseline fitness, metabolic profiles, and comorbid conditions. Furthermore, the complementary mechanisms elicited by combined training regimens support their prioritization in clinical guidelines.
At the population level, these findings advocate for the expansion of community-based and preventive exercise programs targeting individuals with or at risk for type 2 diabetes. Examples may include community walking groups, workplace-based HIIT sessions, or structured exercise counseling integrated into primary healthcare. Embedding exercise strategies into public health frameworks may contribute to delaying disease onset, mitigating progression, and reducing the long-term burden of diabetes-related complications. As such, exercise should be regarded not only as a lifestyle recommendation but also as a clinically and biologically validated component of comprehensive diabetes management.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/metabo15110739/s1, Table S1: Search strategy for web of science.; Table S2: Search strategy for PubMed.
Author Contributions
Y.L., P.L. and Y.W. wrote the first draft of the manuscript and conducted the systematic search. Y.L. gained funding for the publication. Y.W., R.W. and P.L. extracted the data from each study. Y.L. conceptualized the review idea. Y.L. and Y.W. interpreted and reviewed the data and discussion. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Natural Science Foundation of Changchun Normal University (Grant number: 2021004) awarded to Y.L. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of type 2 diabetes mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Ferrannini, E.; Groop, L.; Henry, R.R.; Herman, W.H.; Holst, J.J.; Hu, F.B.; Kahn, C.R.; Raz, I.; Shulman, G.I. Type 2 diabetes mellitus. NRDP 2015, 1, 15019. [Google Scholar] [CrossRef]
- de Lade, C.G.; Marins, J.C.B.; Lima, L.M.; de Carvalho, C.J.; Teixeira, R.B.; Albuquerque, M.R.; Reis, J.S.; Amorim, P.R.d.S. Effects of different exercise programs and minimal detectable changes in hemoglobin A1c in patients with type 2 diabetes. Diabetol. Metab. Syndr. 2016, 8, 13. [Google Scholar] [CrossRef]
- Solomon, T.P.J.; Haus, J.M.; Kelly, K.R.; Rocco, M.; Kashyap, S.R.; Kirwan, J.P. Improved Pancreatic β-Cell Function in Type 2 Diabetic Patients After Lifestyle-Induced Weight Loss Is Related to Glucose-Dependent Insulinotropic Polypeptide. Diabetes Care 2010, 33, 1561–1566. [Google Scholar] [CrossRef] [PubMed]
- Sanz, C.; Gautier, J.-F.; Hanaire, H. Physical Exercise for the Prevention and Treatment of Type 2 Diabetes. Diabetes Metab. 2010, 36, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.H.; Stidsen, J.V.; de Almeida, M.E.; Wentorf, E.K.; Jensen, K.; Ørtenblad, N.; Højlund, K. High-Intensity Interval Training Combining Rowing and Cycling Improves but Does Not Restore Beta-Cell Function in Type 2 Diabetes. Endocr. Connect. 2024, 13, 5. [Google Scholar]
- Johansen, M.Y.; Karstoft, K.; MacDonald, C.S.; Hansen, K.B.; Ellingsgaard, H.; Hartmann, B.; Wewer Albrechtsen, N.J.; Vaag, A.A.; Holst, J.J.; Pedersen, B.K. Effects of an Intensive Lifestyle Intervention on the Underlying Mechanisms of Improved Glycaemic Control in Individuals with Type 2 Diabetes: A Secondary Analysis of a Randomised Clinical Trial. Diabetologia 2020, 63, 2410–2422. [Google Scholar] [CrossRef]
- Thyfault, J.P.; Bergouignan, A. Exercise and Metabolic Health: Beyond Skeletal Muscle. Diabetologia 2020, 63, 1464–1474. [Google Scholar] [CrossRef]
- Roden, M.; Shulman, G.I. The Integrative Biology of Type 2 Diabetes. Nature 2019, 576, 51–60. [Google Scholar] [CrossRef]
- Burns, N.; Finucane, F.M.; Hatunic, M.; Gilman, M.; Murphy, M.; Gasparro, D.; Mari, A.; Gastaldelli, A.; Nolan, J.J. Early-Onset Type 2 Diabetes in Obese White Subjects Is Characterised by a Marked Defect in Beta Cell Insulin Secretion, Severe Insulin Resistance and a Lack of Response to Aerobic Exercise Training. Diabetologia 2007, 50, 1500–1508. [Google Scholar] [CrossRef]
- Zhang, W.; Guo, Q.; Shen, S. Effects of Various Exercise Intensities with the Same Energy Expenditure on Glucose Homeostasis and Insulin Sensitivity in Type 2 Diabetes Mellitus. Chin. J. Rehabil. Med. 2014, 29, 228–233. [Google Scholar]
- Meex, R.C.R.; Schrauwen-Hinderling, V.B.; Moonen-Kornips, E.; Schaart, G.; Mensink, M.; Phielix, E.; van de Weijer, T.; Sels, J.P.; Schrauwen, P.; Hesselink, M.K.C. Restoration of muscle mitochondrial function and metabolic flexibility in type 2 diabetes by exercise training is paralleled by increased myocellular fat storage and improved insulin sensitivity. Diabetes 2010, 59, 572–579. [Google Scholar] [CrossRef]
- Solomon, T.P.J.; Malin, S.K.; Karstoft, K.; Kashyap, S.R.; Haus, J.M.; Kirwan, J.P. Pancreatic β-cell function is a stronger predictor of changes in glycemic control after an aerobic exercise intervention than insulin sensitivity. J. Clin. Endocrinol. Metab. 2013, 98, 4176–4186. [Google Scholar] [CrossRef] [PubMed]
- Enteshary, M.; Esfarjani, F.; Reisi, J. Comparison of the effects of two different intensities of combined training on irisin, betatrophin, and insulin levels in women with type 2 diabetes. Asian J. Sports Med. 2019, 10, e85937. [Google Scholar] [CrossRef]
- Balducci, S.; Sacchetti, M.; Haxhi, J.; Orlando, G.; D’Errico, V.; Fallucca, S.; Menini, S.; Pugliese, G. Physical exercise as therapy for type 2 diabetes mellitus. Diabetes Metab. Res. Rev. 2014, 30 (Suppl. 1), 13–23. [Google Scholar] [CrossRef] [PubMed]
- Grace, A.; Chan, E.; Giallauria, F.; Graham, P.L.; Smart, N.A. Clinical outcomes and glycaemic responses to different aerobic exercise training intensities in type II diabetes: A systematic review and meta-analysis. Cardiovasc. Diabetol. 2017, 16, 37. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Healthcare Interventions: Explanation and Elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef]
- Bramer, W.M.; de Jonge, G.B.; Rethlefsen, M.L.; Mast, F.; Kleijnen, J. A systematic approach to searching: An efficient and complete method to develop literature searches. J. Med. Libr. Assoc. 2018, 106, 531–537. [Google Scholar] [CrossRef]
- Booth, A.; Clarke, M.; Dooley, G.; Ghersi, D.; Moher, D.; Petticrew, M.; Stewart, L. The nuts and bolts of PROSPERO: An international prospective register of systematic reviews. Syst. Rev. 2012, 1, 2. [Google Scholar] [CrossRef]
- Michishita, R.; Shono, N.; Kasahara, T.; Tsuruta, T. Effects of low intensity exercise therapy on early phase insulin secretion in overweight subjects with impaired glucose tolerance and type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2008, 82, 291–297. [Google Scholar] [CrossRef]
- Tsang, T.; Orr, R.; Lam, P.; Comino, E.; Fiatarone Singh, M. Effects of Tai Chi on Glucose Homeostasis and Insulin Sensitivity in Older Adults with Type 2 Diabetes: A Randomised Double-Blind Sham-Exercise-Controlled Trial. Age Ageing 2008, 37, 64–71. [Google Scholar] [CrossRef]
- Kirwan, J.P.; Solomon, T.P.; Wojta, D.M.; Staten, M.A.; Holloszy, J.O. Effects of 7 days of exercise training on insulin sensitivity and responsiveness in type 2 diabetes mellitus. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E151–E156. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, W.; Guo, Q.; Liu, X.; Zhang, Q.; Dong, R.; Dou, H.; Shi, J.; Wang, J.; Yu, D. Duration of exercise as a key determinant of improvement in insulin sensitivity in type 2 diabetes patients. Tohoku J. Exp. Med. 2012, 227, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Duvivier, B.; Schaper, N.C.; Hesselink, M.K.C.; van Kan, L.; Stienen, N.; Winkens, B.; Koster, A.; Savelberg, H. Breaking sitting with light activities vs. structured exercise: A randomised crossover study demonstrating benefits for glycaemic control and insulin sensitivity in type 2 diabetes. Diabetologia 2017, 60, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Ho, C.W.; Chen, L.C.; Chang, C.C.; Wang, Y.W.; Chiou, C.P.; Chiang, S.L. Effects of a 12-week exercise training on insulin sensitivity, quality of life, and depression status in patients with type 2 diabetes. J. Med. Sci. 2017, 37, 227–236. [Google Scholar]
- Rahbar, S.; Naimi, S.S.; Soltani, A.R.; Rahimi, A.; Akbarzadeh Baghban, A.; Rashedi, V.; Tavakkoli, H.M. Improvement in biochemical parameters in patients with type 2 diabetes after twenty-four sessions of aerobic exercise: A randomized controlled trial. Iran. Red Crescent Med. J. 2017, 19, e55094. [Google Scholar] [CrossRef]
- Shakil-Ur-Rehman, S.; Karimi, H.; Gillani, S.A. Effects of supervised structured aerobic exercise training program on fasting blood glucose level, plasma insulin level, glycemic control, and insulin resistance in type 2 diabetes mellitus. Pak. J. Med. Sci. 2017, 33, 576–580. [Google Scholar] [CrossRef]
- Nuhu, J.M.; Maharaj, S.S. Influence of a mini-trampoline rebound exercise program on insulin resistance, lipid profile and central obesity in individuals with type 2 diabetes. J. Sports Med. Phys. Fitness 2018, 58, 503–509. [Google Scholar] [CrossRef]
- Zhang, H.; Simpson, L.K.; Carbone, N.P.; Hirshman, M.F.; Nigro, P.; Vamvini, M.; Goodyear, L.J.; Middelbeek, R.J. Moderate-intensity endurance training improves late phase β-cell function in adults with type 2 diabetes. iScience 2023, 26, 107123. [Google Scholar] [CrossRef]
- Bruce, C.R.; Kriketos, A.D.; Cooney, G.J.; Hawley, J.A. Disassociation of muscle triglyceride content and insulin sensitivity after exercise training in patients with type 2 diabetes. Diabetologia 2004, 47, 23–30. [Google Scholar] [CrossRef]
- Tokmakidis, S.P.; Zois, C.E.; Volaklis, K.A.; Kotsa, K.; Touvra, A.M. The effects of a combined strength and aerobic exercise program on glucose control and insulin action in women with type 2 diabetes. Eur. J. Appl. Physiol. 2004, 92, 437–442. [Google Scholar] [CrossRef]
- Glans, F.; Eriksson, K.F.; Segerström, Å.; Thorsson, O.; Wollmer, P.; Groop, L. Evaluation of the effects of exercise on insulin sensitivity in Arabian and Swedish women with type 2 diabetes. Diabetes Res. Clin. Pract. 2009, 85, 69–74. [Google Scholar] [CrossRef]
- Mir, E.; Moazzami, M.; Bijeh, N.; Dokht, E.H.; Rahimi, N. Changes in SFRP5, WNT5A, HbA1c, BMI, PBF, and insulin resistance in men with type 2 diabetes after 12 weeks of combined exercise (HIIT and resistance). Int. J. Diabetes Dev. Ctries. 2020, 40, 248–254. [Google Scholar] [CrossRef]
- Mancilla, R.; Brouwers, B.; Schrauwen-Hinderling, V.B.; Hesselink, M.K.; Hoeks, J.; Schrauwen, P. Exercise training elicits superior metabolic effects when performed in the afternoon compared to morning in metabolically compromised humans. Physiol. Rep. 2021, 8, e14669. [Google Scholar] [CrossRef] [PubMed]
- Amaravadi, S.K.; Maiya, G.A.; K, V.; Shastry, B.A. Effectiveness of structured exercise program on insulin resistance and quality of life in type 2 diabetes mellitus—A randomized controlled trial. PLoS ONE 2024, 19, e0302831. [Google Scholar] [CrossRef] [PubMed]
- Misra, A.; Alappan, N.K.; Vikram, N.K.; Goel, K.; Gupta, N.; Mittal, K.; Bhatt, S.; Luthra, K. Effect of supervised progressive resistance-exercise training protocol on insulin sensitivity, glycemia, lipids, and body composition in Asian Indians with type 2 diabetes. Diabetes Care 2008, 31, 1282–1287. [Google Scholar] [CrossRef]
- Kwon, H.R.; Han, K.A.; Ku, Y.H.; Ahn, H.J.; Koo, B.K.; Kim, H.C.; Min, K.W. The effects of resistance training on muscle and body fat mass and muscle strength in type 2 diabetic women. Korean Diabetes J. 2010, 34, 101–110. [Google Scholar] [CrossRef]
- Schardt, C.; Adams, M.B.; Owens, T.; Keitz, S.; Fontelo, P. Utilization of the PICO Framework to Improve Searching PubMed for Clinical Questions. BMC Med. Inform. Decis. Mak. 2007, 7, 16. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Zanuso, S.; Sacchetti, M.; Sundberg, C.J.; Orlando, G.; Benvenuti, P.; Balducci, S. Exercise in type 2 diabetes: Genetic, metabolic and neuromuscular adaptations. A review of the evidence. Br. J. Sports Med. 2017, 51, 1533–1538. [Google Scholar] [CrossRef]
- Carruthers, A. Facilitated diffusion of glucose. Physiol. Rev. 1990, 70, 1135–1176. [Google Scholar] [CrossRef]
- Syeda, U.A.; Battillo, D.; Visaria, A.; Malin, S.K. The importance of exercise for glycemic control in type 2 diabetes. Am. J. Med. Open 2023, 9, 100031. [Google Scholar] [CrossRef] [PubMed]
- Irrcher, I.; Adhihetty, P.J.; Joseph, A.-M.; Ljubicic, V.; Hood, D.A. Regulation of mitochondrial biogenesis in muscle by endurance exercise. Sports Med. 2003, 33, 783–793. [Google Scholar] [CrossRef]
- Kazeminasab, F.; Baharlooie, M.; Rezazadeh, H.; Soltani, N.; Rosenkranz, S.K. The effects of aerobic exercise on liver function, insulin resistance, and lipid profiles in prediabetic and type 2 diabetic mice. Physiol. Behav. 2023, 271, 114340. [Google Scholar] [CrossRef] [PubMed]
- Mika, A.; Macaluso, F.; Barone, R.; Di Felice, V.; Sledzinski, T. Effect of exercise on fatty acid metabolism and adipokine secretion in adipose tissue. Front. Physiol. 2019, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Teixeira-Lemos, E.; Nunes, S.; Teixeira, F.; Reis, F. Regular physical exercise training assists in preventing type 2 diabetes development: Focus on its antioxidant and anti-inflammatory properties. Cardiovasc. Diabetol. 2011, 10, 12. [Google Scholar] [CrossRef]
- Lee, J.; Kim, D.; Kim, C. Resistance training for glycemic control, muscular strength, and lean body mass in old type 2 diabetic patients: A meta-analysis. Diabetes Ther. 2017, 8, 459–473. [Google Scholar] [CrossRef]
- Zierler, K. Whole body glucose metabolism. Am. J. Physiol. Endocrinol. Metab. 1999, 276, E409–E426. [Google Scholar] [CrossRef]
- Laursen, P.B.; Jenkins, D.G. The scientific basis for high-intensity interval training. Sports Med. 2002, 32, 53–73. [Google Scholar] [CrossRef]
- Towler, M.C.; Hardie, D.G. AMP-activated protein kinase in metabolic control and insulin signaling. Circ. Res. 2007, 100, 328–341. [Google Scholar] [CrossRef]
- Methenitis, S. A brief review on concurrent training: From laboratory to the field. Sports 2018, 6, 127. [Google Scholar] [CrossRef]
- Hansen, P.A.; Nolte, L.A.; Chen, M.M.; Holloszy, J.O. Increased GLUT-4 translocation mediates enhanced insulin sensitivity of muscle glucose transport after exercise. J. Appl. Physiol. 1998, 85, 1218–1222. [Google Scholar] [CrossRef] [PubMed]
- Min, C.; Lei, Z.; Siyu, C.; Yaqian, Q.; Jingquan, S. Acute and chronic effects of high-intensity interval training (HIIT) on postexercise intramuscular lipid metabolism in rats. Physiol. Res. 2021, 70, 735. [Google Scholar] [CrossRef] [PubMed]
- Krogh-Madsen, R.; Plomgaard, P.; Møller, K.; Mittendorfer, B.; Pedersen, B.K. Influence of TNF-α and IL-6 infusions on insulin sensitivity and expression of IL-18 in humans. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E108–E114. [Google Scholar] [CrossRef]
- Leiva-Valderrama, J.M.; Montes-de-Oca-Garcia, A.; Opazo-Diaz, E.; Ponce-Gonzalez, J.G.; Molina-Torres, G.; Velázquez-Díaz, D.; Galán-Mercant, A. Effects of high-intensity interval training on inflammatory biomarkers in patients with type 2 diabetes: A systematic review. Int. J. Environ. Res. Public Health 2021, 18, 12644. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.-H.; Rhyu, H.-S.; Kim, J.Y. The effects of combined aerobic and resistance training on inflammatory markers in obese men. J. Exerc. Rehabil. 2018, 14, 660–666. [Google Scholar] [CrossRef]
- Drake, J.C.; Wilson, R.J.; Yan, Z. Molecular mechanisms for mitochondrial adaptation to exercise training in skeletal muscle. FASEB J. 2016, 30, 13–22. [Google Scholar] [CrossRef]
- Ferrari, F.; Bock, P.M.; Motta, M.T.; Helal, L. Biochemical and molecular mechanisms of glucose uptake stimulated by physical exercise in insulin resistance state: Role of inflammation. Arq. Bras. Cardiol. 2019, 113, 1139–1148. [Google Scholar] [CrossRef]
- Cartee, G.D. Mechanisms for greater insulin-stimulated glucose uptake in normal and insulin-resistant skeletal muscle after acute exercise. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E949–E959. [Google Scholar] [CrossRef]
- Römer, A.; Linn, T.; Petry, S.F. Lipotoxic impairment of mitochondrial function in β-cells: A review. Antioxidants 2021, 10, 293. [Google Scholar] [CrossRef]
- Greiwe, J.S.; Hickner, R.C.; Hansen, P.A.; Racette, S.B.; Chen, M.M.; Holloszy, J.O. Effects of endurance exercise training on muscle glycogen accumulation in humans. J. Appl. Physiol. 1999, 87, 222–226. [Google Scholar] [CrossRef]
- Gregory, J.M.; Muldowney, J.A.; Engelhardt, B.G.; Tyree, R.; Marks-Shulman, P.; Silver, H.J.; Donahue, E.P.; Edgerton, D.S.; Winnick, J.J. Aerobic exercise training improves hepatic and muscle insulin sensitivity, but reduces splanchnic glucose uptake in obese humans with type 2 diabetes. Nutrients 2019, 9, 25. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).