Disease and Medication Context Shape Ex Vivo Metabolite Stability: A Pilot Study in Systemic Lupus Erythematosus
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Study Design and Serum Draw
2.3. Automated Metabolite Extraction
2.4. LC-MS Analysis
2.5. Metabolite Annotation
2.6. Statistical Analysis
2.6.1. Quality Control Filtering
2.6.2. Baseline Analyses
2.6.3. Ex Vivo Stability Modeling
2.6.4. Visualization of Ex Vivo Stability Effects
2.6.5. Drug-Related Analyses
2.6.6. Power Analysis
2.7. Visualization and Presentation
3. Results
3.1. Study Design and Experimental Context
3.2. Analytical Pipeline and Feature Filtering
3.3. Baseline Metabolic Differences in SLE vs. HC
3.4. Global Effects of Delayed Centrifugation: PCA Trajectories
3.5. Individual Metabolite Stability Patterns Assessed by Linear Mixed-Effects Modeling
3.6. Effects of Medication on Metabolite Ex Vivo Stability
4. Discussion
4.1. Study Overview and Key Findings
4.2. Methodological Considerations and Analytical Framework
4.3. Baseline Metabolic Context and Sources of Variability
4.4. Multivariate Trends in Ex Vivo Metabolic Drift
4.5. Metabolite-Specific Stability Patterns and Biological Context
4.6. Pharmacological Modulation of Apparent Metabolite Stability
4.7. Implications for Clinical Sampling and Biobank Use
4.8. Implications for SLE and Biomarker Studies
4.9. Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barrett, J.C.; Esko, T.; Fischer, K.; Jostins-Dean, L.; Jousilahti, P.; Julkunen, H.; Jääskeläinen, T.; Kangas, A.; Kerimov, N.; Kerminen, S.; et al. Metabolomic and Genomic Prediction of Common Diseases in 700,217 Participants in Three National Biobanks. Nat. Commun. 2024, 15, 10092. [Google Scholar] [CrossRef]
- Lian, J.; Vardhanabhuti, V. Metabolic Biomarkers Using Nuclear Magnetic Resonance Metabolomics Assay for the Prediction of Aging-Related Disease Risk and Mortality: A Prospective, Longitudinal, Observational, Cohort Study Based on the UK Biobank. GeroScience 2024, 46, 1515–1526. [Google Scholar] [CrossRef]
- Miggiels, P.; Wouters, B.; van Westen, G.J.P.; Dubbelman, A.-C.; Hankemeier, T. Novel Technologies for Metabolomics: More for Less. TrAC Trends Anal. Chem. 2019, 120, 115323. [Google Scholar] [CrossRef]
- Yu, C.T.; Farhat, Z.; Livinski, A.A.; Loftfield, E.; Zanetti, K.A. Characteristics of Cancer Epidemiology Studies That Employ Metabolomics: A Scoping Review. Cancer Epidemiol. Biomark. Prev. 2023, 32, 1130–1145. [Google Scholar] [CrossRef]
- Thachil, A.; Wang, L.; Mandal, R.; Wishart, D.; Blydt-Hansen, T. An Overview of Pre-Analytical Factors Impacting Metabolomics Analyses of Blood Samples. Metabolites 2024, 14, 474. [Google Scholar] [CrossRef] [PubMed]
- Kamlage, B.; Neuber, S.; Bethan, B.; González Maldonado, S.; Wagner-Golbs, A.; Peter, E.; Schmitz, O.; Schatz, P. Impact of Prolonged Blood Incubation and Extended Serum Storage at Room Temperature on the Human Serum Metabolome. Metabolites 2018, 8, 6. [Google Scholar] [CrossRef]
- Hernandes, V.V.; Barbas, C.; Dudzik, D. A Review of Blood Sample Handling and Pre-processing for Metabolomics Studies. Electrophoresis 2017, 38, 2232–2241. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, R. From Bedside to Bench—Practical Considerations to Avoid Pre-Analytical Pitfalls and Assess Sample Quality for High-Resolution Metabolomics and Lipidomics Analyses of Body Fluids. Anal. Bioanal. Chem. 2021, 413, 5567–5585. [Google Scholar] [CrossRef] [PubMed]
- Bi, H.; Guo, Z.; Jia, X.; Liu, H.; Ma, L.; Xue, L. The Key Points in the Pre-Analytical Procedures of Blood and Urine Samples in Metabolomics Studies. Metabolomics 2020, 16, 68. [Google Scholar] [CrossRef]
- Bervoets, L.; Louis, E.; Reekmans, G.; Mesotten, L.; Thomeer, M.; Adriaensens, P.; Linsen, L. Influence of Preanalytical Sampling Conditions on the 1H NMR Metabolic Profile of Human Blood Plasma and Introduction of the Standard PREanalytical Code Used in Biobanking. Metabolomics 2015, 11, 1197–1207. [Google Scholar] [CrossRef]
- Lippi, G.; Chance, J.J.; Church, S.; Dazzi, P.; Fontana, R.; Giavarina, D.; Grankvist, K.; Huisman, W.; Kouri, T.; Palicka, V.; et al. Preanalytical Quality Improvement: From Dream to Reality. Clin. Chem. Lab. Med. 2011, 49, 1113–1126. [Google Scholar] [CrossRef]
- Dorochow, E.; Gurke, R.; Rischke, S.; Geisslinger, G.; Hahnefeld, L. Effects of Different Storage Conditions on Lipid Stability in Mice Tissue Homogenates. Metabolites 2023, 13, 504. [Google Scholar] [CrossRef]
- Yin, P.; Lehmann, R.; Xu, G. Effects of Pre-Analytical Processes on Blood Samples Used in Metabolomics Studies. Anal. Bioanal. Chem. 2015, 407, 4879–4892. [Google Scholar] [CrossRef]
- Kirwan, J.A.; Brennan, L.; Broadhurst, D.; Fiehn, O.; Cascante, M.; Dunn, W.B.; Schmidt, M.A.; Velagapudi, V. Preanalytical Processing and Biobanking Procedures of Biological Samples for Metabolomics Research: A White Paper, Community Perspective (for “Precision Medicine and Pharmacometabolomics Task Group”—The Metabolomics Society Initiative). Clin. Chem. 2018, 64, 1158–1182. [Google Scholar] [CrossRef] [PubMed]
- Altmann, H.; Barovic, M.; Straßburger, K.; Tschäpel, M.; Jonas, S.; Poitz, D.M.; Belavgeni, A.; Chavakis, T.; Mirtschink, P.; Funk, A.M. Validating Centralized Biobanking Workflows for NMR Metabolomics Using the PRIMA Panel. Anal. Chem. 2025, 97, 2762–2769. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Mohan, C. Caution in Studying and Interpreting the Lupus Metabolome. Arthritis Res. Ther. 2020, 22, 172. [Google Scholar] [CrossRef] [PubMed]
- Rojo-Sánchez, A.; Carmona-Martes, A.; Díaz-Olmos, Y.; Santamaría-Torres, M.; Cala, M.P.; Orozco-Acosta, E.; Aroca-Martínez, G.; Pacheco-Londoño, L.; Navarro-Quiroz, E.; Pacheco-Lugo, L.A. Urinary Metabolomic Profiling of a Cohort of Colombian Patients with Systemic Lupus Erythematosus. Sci. Rep. 2024, 14, 9555. [Google Scholar] [CrossRef]
- Schmitt, F.C.; ten Cate, V.; Fischer, Z.; Hagen, M.; Steigenberger, B.A.; Tenzer, S.; Wild, P.S.; Schmidlin, T. Metabolic Profiling of the EmDia Cohort by a Scalable DIA-LC-MS Workflow. ChemRxiv 2024. [Google Scholar] [CrossRef]
- Pesi, A.; Schmitt, F.; Petridou, T.; Grossmann, L.; Wiegel, M.; Frankenbach, P.; Verdu, E.F.; Bercik, P.; Schmidlin, T.; Schuppan, D. 660: Modulation of behavior via cytokine and neurotransmitter expression in the brain during active celiac disease in the humanized nod DQ8 mouse model. Gastroenterology 2025, 169, S-165. [Google Scholar] [CrossRef]
- Pesi, A.; Lange, S.; Schmitt, F.; Petridou, T.; Grossmann, L.; Wiegel, M.; Frankenbach, P.; Verdu, E.F.; Schmidlin, T.; Steven, S.; et al. Sa1172: Changes in cholesterol synthesis and gender-specific cardiac inflammation during celiac-disease in the nod DQ8 mouse model. Gastroenterology 2025, 169, S-385-S-386. [Google Scholar] [CrossRef]
- Bündgen, G.; Ulges, A.; Pietruschka, J.; Truong-Andrievici, N.; Klein, M.; Romaniuk, K.; Schmitt, F.; Hagen, M.; Seebass, J.G.; Zezlina, L.; et al. Polyamines Regulate Adaptive Antitumor Immunity by Functional Specialization of Regulatory T Cells. Immunity 2025, 58, 2019–2034. [Google Scholar] [CrossRef]
- Takeda, H.; Matsuzawa, Y.; Takeuchi, M.; Takahashi, M.; Nishida, K.; Harayama, T.; Todoroki, Y.; Shimizu, K.; Sakamoto, N.; Oka, T.; et al. MS-DIAL 5 Multimodal Mass Spectrometry Data Mining Unveils Lipidome Complexities. Nat. Commun. 2024, 15, 9903. [Google Scholar] [CrossRef]
- Dührkop, K.; Fleischauer, M.; Ludwig, M.; Aksenov, A.A.; Melnik, A.V.; Meusel, M.; Dorrestein, P.C.; Rousu, J.; Böcker, S. SIRIUS 4: A Rapid Tool for Turning Tandem Mass Spectra into Metabolite Structure Information. Nat. Methods 2019, 16, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Stevens, V.L.; Hoover, E.; Wang, Y.; Zanetti, K.A. Pre-Analytical Factors That Affect Metabolite Stability in Human Urine, Plasma, and Serum: A Review. Metabolites 2019, 9, 156. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Bonelli, P.; Bonfanti, L.; Crevellin, G. The Use of S-Monovette Is Effective to Reduce the Burden of Hemolysis in a Large Urban Emergency Department. Biochem. Medica 2015, 25, 69–72. [Google Scholar] [CrossRef]
- Cat, A.; Ucar, K.; Nurlu, N. Could S-Monovette Serum Gel Tubes Be Used for Clinical Chemistry Analysis Instead of Serum Separator Tube II Advance? Clin. Lab. 2022, 68, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gray, A.; Xue, L.; Farb, M.G.; Ayalon, N.; Andersson, C.; Ko, D.; Benjamin, E.J.; Levy, D.; Vasan, R.S.; et al. Metabolomic Profiles, Ideal Cardiovascular Health, and Risk of Heart Failure and Atrial Fibrillation: Insights From the Framingham Heart Study. J. Am. Heart Assoc. 2023, 12, e028022. [Google Scholar] [CrossRef]
- Ho, J.E.; Larson, M.G.; Ghorbani, A.; Cheng, S.; Chen, M.-H.; Keyes, M.; Rhee, E.P.; Clish, C.B.; Vasan, R.S.; Gerszten, R.E.; et al. Metabolomic Profiles of Body Mass Index in the Framingham Heart Study Reveal Distinct Cardiometabolic Phenotypes. PLoS ONE 2016, 11, e0148361. [Google Scholar] [CrossRef]
- Yu, Z.; Kastenmüller, G.; He, Y.; Belcredi, P.; Möller, G.; Prehn, C.; Mendes, J.; Wahl, S.; Roemisch-Margl, W.; Ceglarek, U.; et al. Differences between Human Plasma and Serum Metabolite Profiles. PLoS ONE 2011, 6, e21230. [Google Scholar] [CrossRef]
- Bruns, D.E.; Knowler, W.C. Stabilization of Glucose in Blood Samples: Why It Matters. Clin. Chem. 2009, 55, 850–852. [Google Scholar] [CrossRef]
- Peake, M.J.; Bruns, D.E.; Sacks, D.B.; Horvath, A.R. It’s Time for a Better Blood Collection Tube to Improve the Reliability of Glucose Results. Diabetes Care 2013, 36, e2. [Google Scholar] [CrossRef]
- Chen, D.; Zhao, S.; Li, L.; Li, L. Controlling Pre-Analytical Process in Human Serum/Plasma Metabolomics. TrAC Trends Anal. Chem. 2023, 169, 117364. [Google Scholar] [CrossRef]
- Debik, J.; Isaksen, S.H.; Strømmen, M.; Spraul, M.; Schäfer, H.; Bathen, T.F.; Giskeødegård, G.F. Effect of Delayed Centrifugation on the Levels of NMR-Measured Lipoproteins and Metabolites in Plasma and Serum Samples. Anal. Chem. 2022, 94, 17003–17010. [Google Scholar] [CrossRef]
- Zheng, R.; Brunius, C.; Shi, L.; Zafar, H.; Paulson, L.; Landberg, R.; Naluai, Å.T. Prediction and Evaluation of the Effect of Pre-Centrifugation Sample Management on the Measurable Untargeted LC-MS Plasma Metabolome. Anal. Chim. Acta 2021, 1182, 338968. [Google Scholar] [CrossRef]
- Yoon, N.; Jang, A.-K.; Seo, Y.; Jung, B.H. Metabolomics in Autoimmune Diseases: Focus on Rheumatoid Arthritis, Systemic Lupus Erythematous, and Multiple Sclerosis. Metabolites 2021, 11, 812. [Google Scholar] [CrossRef] [PubMed]
- Guleria, A.; Pratap, A.; Dubey, D.; Rawat, A.; Chaurasia, S.; Sukesh, E.; Phatak, S.; Ajmani, S.; Kumar, U.; Khetrapal, C.L.; et al. NMR Based Serum Metabolomics Reveals a Distinctive Signature in Patients with Lupus Nephritis. Sci. Rep. 2016, 6, 35309. [Google Scholar] [CrossRef]
- Bengtsson, A.A.; Trygg, J.; Wuttge, D.M.; Sturfelt, G.; Theander, E.; Donten, M.; Moritz, T.; Sennbro, C.-J.; Torell, F.; Lood, C.; et al. Metabolic Profiling of Systemic Lupus Erythematosus and Comparison with Primary Sjögren’s Syndrome and Systemic Sclerosis. PLoS ONE 2016, 11, e0159384. [Google Scholar] [CrossRef]
- Hwang, J.L.; Weiss, R.E. Steroid-induced Diabetes: A Clinical and Molecular Approach to Understanding and Treatment. Diabetes Metab. Res. Rev. 2014, 30, 96–102. [Google Scholar] [CrossRef]
- Etchegaray-Morales, I.; Mendoza-Pinto, C.; Munguía-Realpozo, P.; Solis-Poblano, J.C.; Méndez-Martínez, S.; Ayón-Aguilar, J.; Abud-Mendoza, C.; García-Carrasco, M.; Cervera, R. Risk of Diabetes Mellitus in Systemic Lupus Erythematosus: Systematic Review and Meta-Analysis. Rheumatology 2024, 63, 2047–2055. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, H.; Yin, L.; Mao, Y.; Zhou, W. Immunometabolism in the Pathogenesis of Systemic Lupus Erythematosus. J. Transl. Autoimmun. 2020, 3, 100046. [Google Scholar] [CrossRef] [PubMed]
- Terrell, M.; Morel, L. The Intersection of Cellular and Systemic Metabolism: Metabolic Syndrome in Systemic Lupus Erythematosus. Endocrinology 2022, 163, bqac067. [Google Scholar] [CrossRef]
- Jiang, M.-Y.; Hwang, J.-C.; Feng, I.-J. Impact of Diabetes Mellitus on the Risk of End-Stage Renal Disease in Patients with Systemic Lupus Erythematosus. Sci. Rep. 2018, 8, 6008. [Google Scholar] [CrossRef]
- Miyake, C.N.H.; Gualano, B.; Dantas, W.S.; Pereira, R.T.; Neves, W.; Zambelli, V.O.; Shinjo, S.K.; Pereira, R.M.; Silva, E.R.; Sá-Pinto, A.L.; et al. Increased Insulin Resistance and Glucagon Levels in Mild/Inactive Systemic Lupus Erythematosus Patients Despite Normal Glucose Tolerance. Arthritis Care Res. 2018, 70, 114–124. [Google Scholar] [CrossRef]
- Dawood, A.; Fayez, D.; Essa, E.; El-zorkany, K.; El-Najjar, M.; Gazareen, S. Study of Insulin Resistance in Patients with Systemic Lupus Erythematosus and Rheumatoid Arthritis. Menoufia Med. J. 2014, 27, 215. [Google Scholar] [CrossRef]
- Javaid, S.; Farooq, T.; Rehman, Z.; Afzal, A.; Ashraf, W.; Rasool, M.F.; Alqahtani, F.; Alsanea, S.; Alasmari, F.; Alanazi, M.M.; et al. Dynamics of Choline-Containing Phospholipids in Traumatic Brain Injury and Associated Comorbidities. Int. J. Mol. Sci. 2021, 22, 11313. [Google Scholar] [CrossRef]
- Zhou, B.; Xia, Y.; She, J. Dysregulated Serum Lipid Profile and Its Correlation to Disease Activity in Young Female Adults Diagnosed with Systemic Lupus Erythematosus: A Cross-Sectional Study. Lipids Health Dis. 2020, 19, 40. [Google Scholar] [CrossRef]
- Xuan, J.; Deng, C.; Lu, H.; He, Y.; Zhang, J.; Zeng, X.; Sun, Y.; Chen, S.; Liu, Y. Serum Lipid Profile in Systemic Lupus Erythematosus. Front. Immunol. 2025, 15, 1503434. [Google Scholar] [CrossRef]
- Toms, T.E.; Panoulas, V.F.; Kitas, G.D. Dyslipidaemia in Rheumatological Autoimmune Diseases. Open Cardiovasc. Med. J. 2011, 5, 64. [Google Scholar] [CrossRef]
- Maia, C.; Fung, C.W.; Sanchez-Lopez, E. Choline in Immunity: A Key Regulator of Immune Cell Activation and Function. Front. Immunol. 2025, 16, 1617077. [Google Scholar] [CrossRef]
- Cas, M.D.; Roda, G.; Li, F.; Secundo, F. Functional Lipids in Autoimmune Inflammatory Diseases. Int. J. Mol. Sci. 2020, 21, 3074. [Google Scholar] [CrossRef]
- Wang, J.-C.; Gray, N.E.; Kuo, T.; Harris, C.A. Regulation of Triglyceride Metabolism by Glucocorticoid Receptor. Cell Biosci. 2012, 2, 19. [Google Scholar] [CrossRef]
- Macfarlane, D.P.; Forbes, S.; Walker, B.R. Glucocorticoids and Fatty Acid Metabolism in Humans: Fuelling Fat Redistribution in the Metabolic Syndrome. J. Endocrinol. 2008, 197, 189–204. [Google Scholar] [CrossRef]
- Peng-Cheng, L.; Meng-Na, L.; Jian-Bin, L.; Shu-Jiao, Y.; Wu, R. Advancements on the Impact of Hydroxychloroquine in Systemic Lupus Erythematosus. Heliyon 2024, 10, e30393. [Google Scholar] [CrossRef]
- Lyu, J.; Gu, Z.; Zhang, Y.; Vu, H.S.; Lechauve, C.; Cai, F.; Cao, H.; Keith, J.; Brancaleoni, V.; Granata, F.; et al. A Glutamine Metabolic Switch Supports Erythropoiesis. Science 2024, 386, eadh9215. [Google Scholar] [CrossRef]
- Curthoys, N.P.; Watford, M. Regulation of Glutaminase Activity and Glutamine Metabolism. Annu. Rev. Nutr. 1995, 15, 133–159. [Google Scholar] [CrossRef]
- King, G.F.; Kuchel, P.W. Assimilation of α-Glutamyl-Peptides by Human Erythrocytes. A Possible Means of Glutamate Supply for Glutathione Synthesis. Biochem. J. 1985, 227, 833–842. [Google Scholar] [CrossRef]
- Cruzat, V.; Macedo Rogero, M.; Noel Keane, K.; Curi, R.; Newsholme, P. Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation. Nutrients 2018, 10, 1564. [Google Scholar] [CrossRef]
- Thorn, B.; Dunstan, R.H.; Macdonald, M.M.; Borges, N.; Roberts, T.K. Evidence That Human and Equine Erythrocytes Could Have Significant Roles in the Transport and Delivery of Amino Acids to Organs and Tissues. Amino Acids 2020, 52, 711–724. [Google Scholar] [CrossRef]
- Seifert, F.; Schulz, K.; Koch, B.; Manhart, S.; Demuth, H.-U.; Schilling, S. Glutaminyl Cyclases Display Significant Catalytic Proficiency for Glutamyl Substrates. Biochemistry 2009, 48, 11831–11833. [Google Scholar] [CrossRef]
- Chelius, D.; Jing, K.; Lueras, A.; Rehder, D.S.; Dillon, T.M.; Vizel, A.; Rajan, R.S.; Li, T.; Treuheit, M.J.; Bondarenko, P.V. Formation of Pyroglutamic Acid from N-Terminal Glutamic Acid in Immunoglobulin Gamma Antibodies. Anal. Chem. 2006, 78, 2370–2376. [Google Scholar] [CrossRef]
- Deng, J.; Chalhoub, N.E.; Sherwin, C.M.; Li, C.; Brunner, H.I. Glucocorticoids Pharmacology and Their Application in the Treatment of Childhood-Onset Systemic Lupus Erythematosus. Semin. Arthritis Rheum. 2019, 49, 251–259. [Google Scholar] [CrossRef]
- Ellero-Simatos, S.; Szymańska, E.; Rullmann, T.; Dokter, W.H.; Ramaker, R.; Berger, R.; van Iersel, T.M.; Smilde, A.K.; Hankemeier, T.; Alkema, W. Assessing the Metabolic Effects of Prednisolone in Healthy Volunteers Using Urine Metabolic Profiling. Genome Med. 2012, 4, 94. [Google Scholar] [CrossRef]
- Burt, M.G.; Gibney, J.; Ho, K.K.Y. Protein Metabolism in Glucocorticoid Excess: Study in Cushing’s Syndrome and the Effect of Treatment. Am. J. Physiol.-Endocrinol. Metab. 2007, 292, E1426–E1432. [Google Scholar] [CrossRef]
- Kumar, M.A.; Baba, S.K.; Khan, I.R.; Khan, M.S.; Husain, F.M.; Ahmad, S.; Haris, M.; Singh, M.; Akil, A.S.A.-S.; Macha, M.A.; et al. Glutamine Metabolism: Molecular Regulation, Biological Functions, and Diseases. MedComm 2025, 6, e70120. [Google Scholar] [CrossRef]
- Muñoz-Urbano, M.; Quintero-González, D.C.; Vasquez, G. T Cell Metabolism and Possible Therapeutic Targets in Systemic Lupus Erythematosus: A Narrative Review. Immunopharmacol. Immunotoxicol. 2022, 44, 457–470. [Google Scholar] [CrossRef]
- Perl, A. Oxidative Stress in the Pathology and Treatment of Systemic Lupus Erythematosus. Nat. Rev. Rheumatol. 2013, 9, 674–686. [Google Scholar] [CrossRef]
- Shah, D.; Sah, S.; Nath, S.K. Interaction between Glutathione and Apoptosis in Systemic Lupus Erythematosus. Autoimmun. Rev. 2013, 12, 741–751. [Google Scholar] [CrossRef]
- Wang, K.; Li, X.; Tang, Y.; Zhao, L. Exploring Key Genes of Glutathione Metabolism in Systemic Lupus Erythematosus Based on Mendelian Randomisation, Single-Cell RNA Sequencing and Multiple Machine Learning Approaches. IET Syst. Biol. 2025, 19, e70021. [Google Scholar] [CrossRef]
- Van Der Werf, P.; Stephani, R.A.; Meister, A. Accumulation of 5-Oxoproline in Mouse Tissues After Inhibition of 5-Oxoprolinase and Administration of Amino Acids: Evidence for Function of the γ-Glutamyl Cycle. Proc. Natl. Acad. Sci. USA 1974, 71, 1026–1029. [Google Scholar] [CrossRef]
- Ruiz-Irastorza, G.; Bertsias, G. Treating Systemic Lupus Erythematosus in the 21st Century: New Drugs and New Perspectives on Old Drugs. Rheumatology 2020, 59, v69–v81. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, X.; Yin, X.; Wang, H.; Fu, C.; Wang, H.; Li, K.; Li, Y.; Zhang, X.; Liang, H.; et al. Metabolomic Profiling Reveals Serum L-Pyroglutamic Acid as a Potential Diagnostic Biomarker for Systemic Lupus Erythematosus. Rheumatology 2021, 60, 598–606. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Davis, T.A.; Kim, S.W.; Li, P.; Marc Rhoads, J.; Carey Satterfield, M.; Smith, S.B.; Spencer, T.E.; Yin, Y. Arginine Metabolism and Nutrition in Growth, Health and Disease. Amino Acids 2009, 37, 153–168. [Google Scholar] [CrossRef]
- Halaby, M.J.; McGaha, T.L. Amino Acid Transport and Metabolism in Myeloid Function. Front. Immunol. 2021, 12, 695238. [Google Scholar] [CrossRef]
- Kuo, T.; Harris, C.A.; Wang, J.-C. Metabolic Functions of Glucocorticoid Receptor in Skeletal Muscle. Mol. Cell. Endocrinol. 2013, 380, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.; McQueen, A.; Chen, T.-C.; Wang, J.-C. Regulation of Glucose Homeostasis by Glucocorticoids. Adv. Exp. Med. Biol. 2015, 872, 99–126. [Google Scholar] [CrossRef] [PubMed]
- Allison, A. Mechanisms of Action of Mycophenolate Mofetil. Lupus 2005, 14, 2–8. [Google Scholar] [CrossRef]

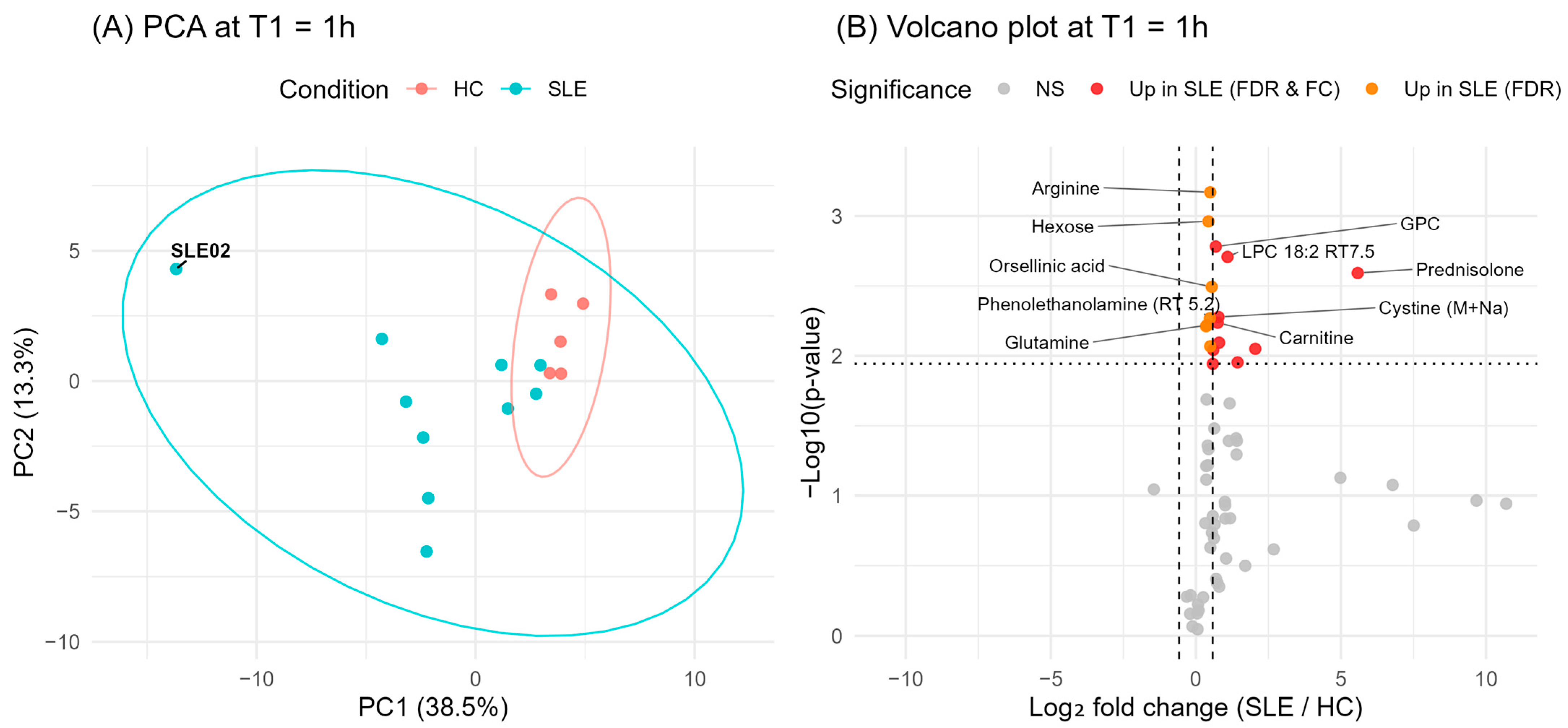
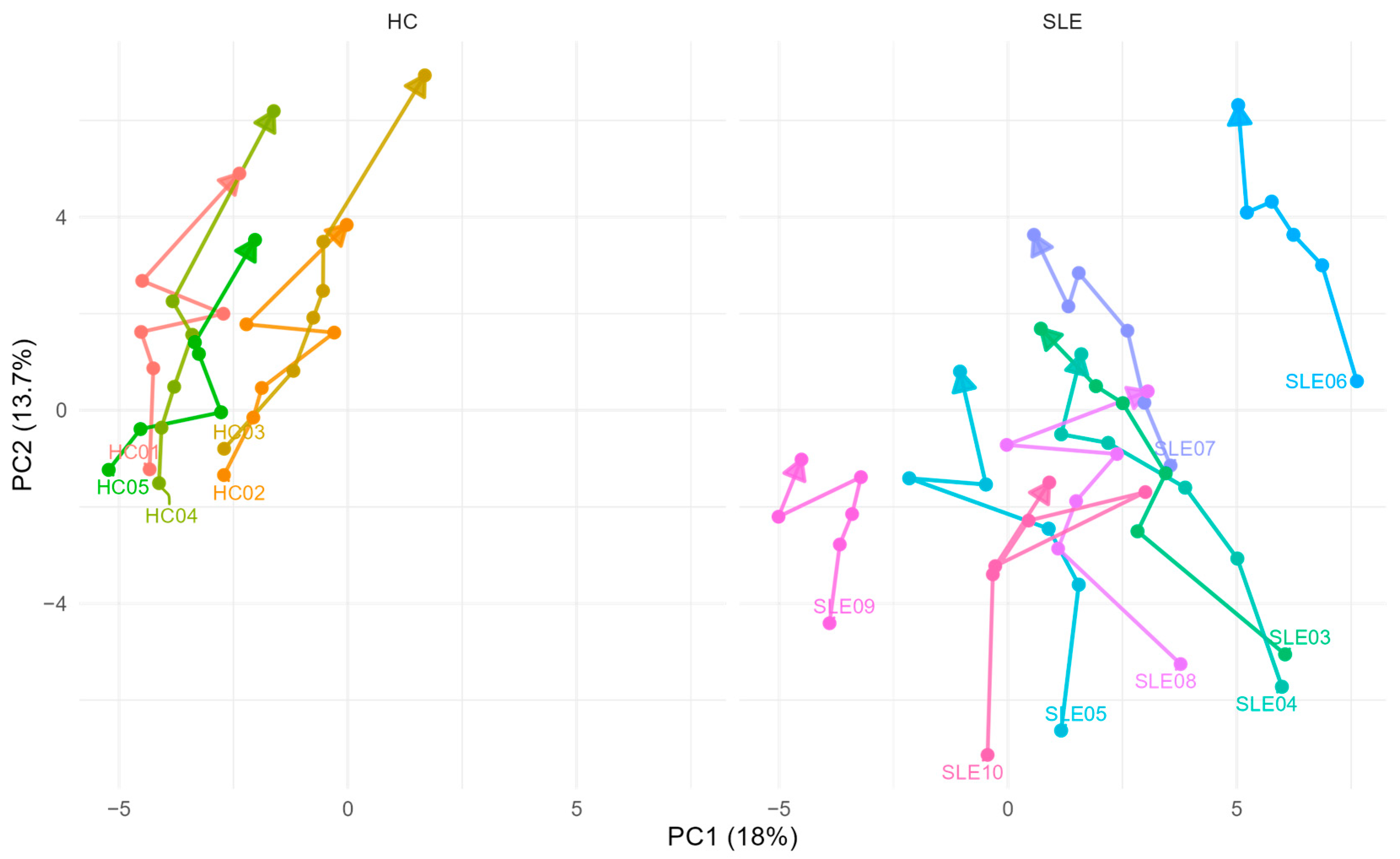
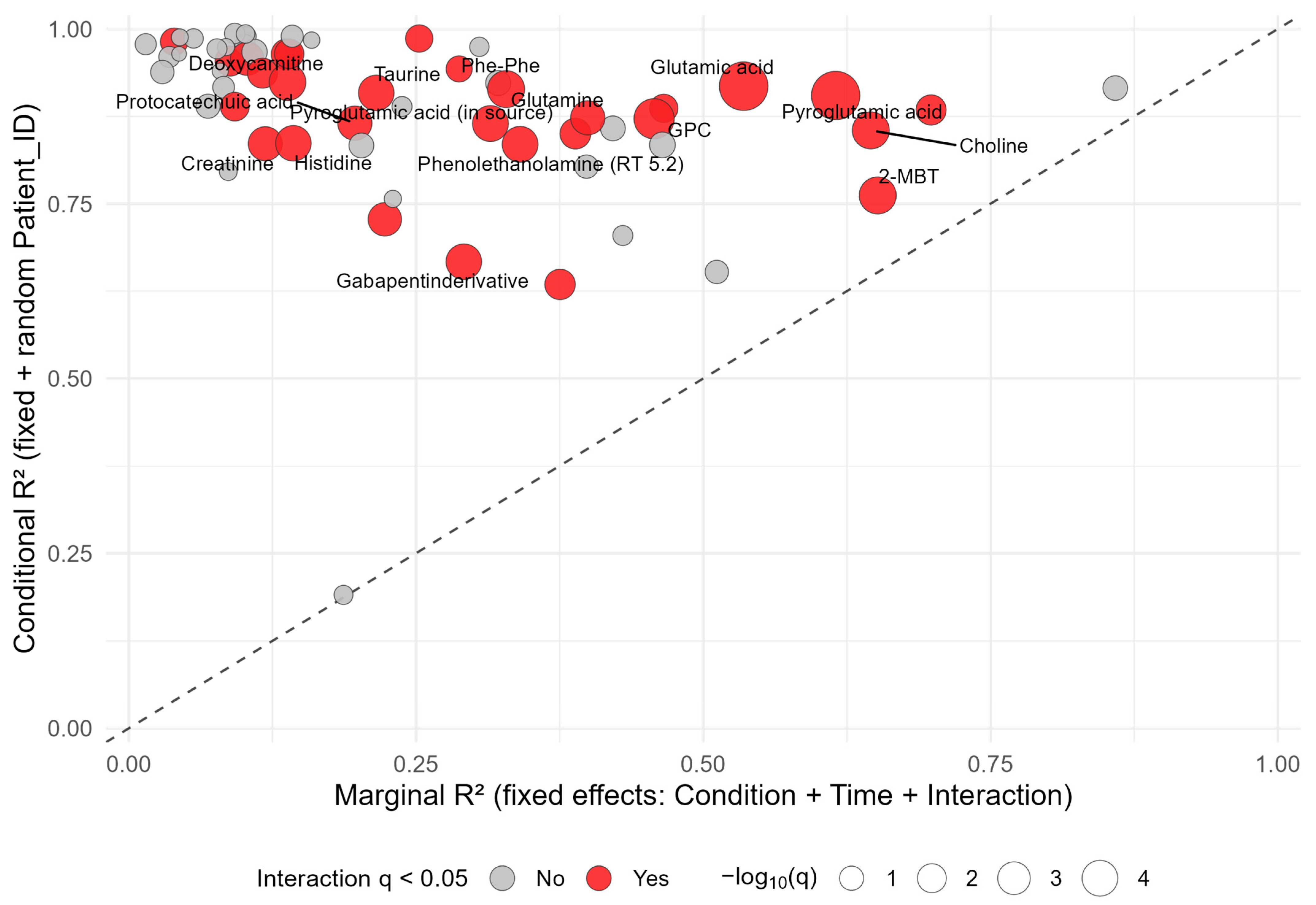
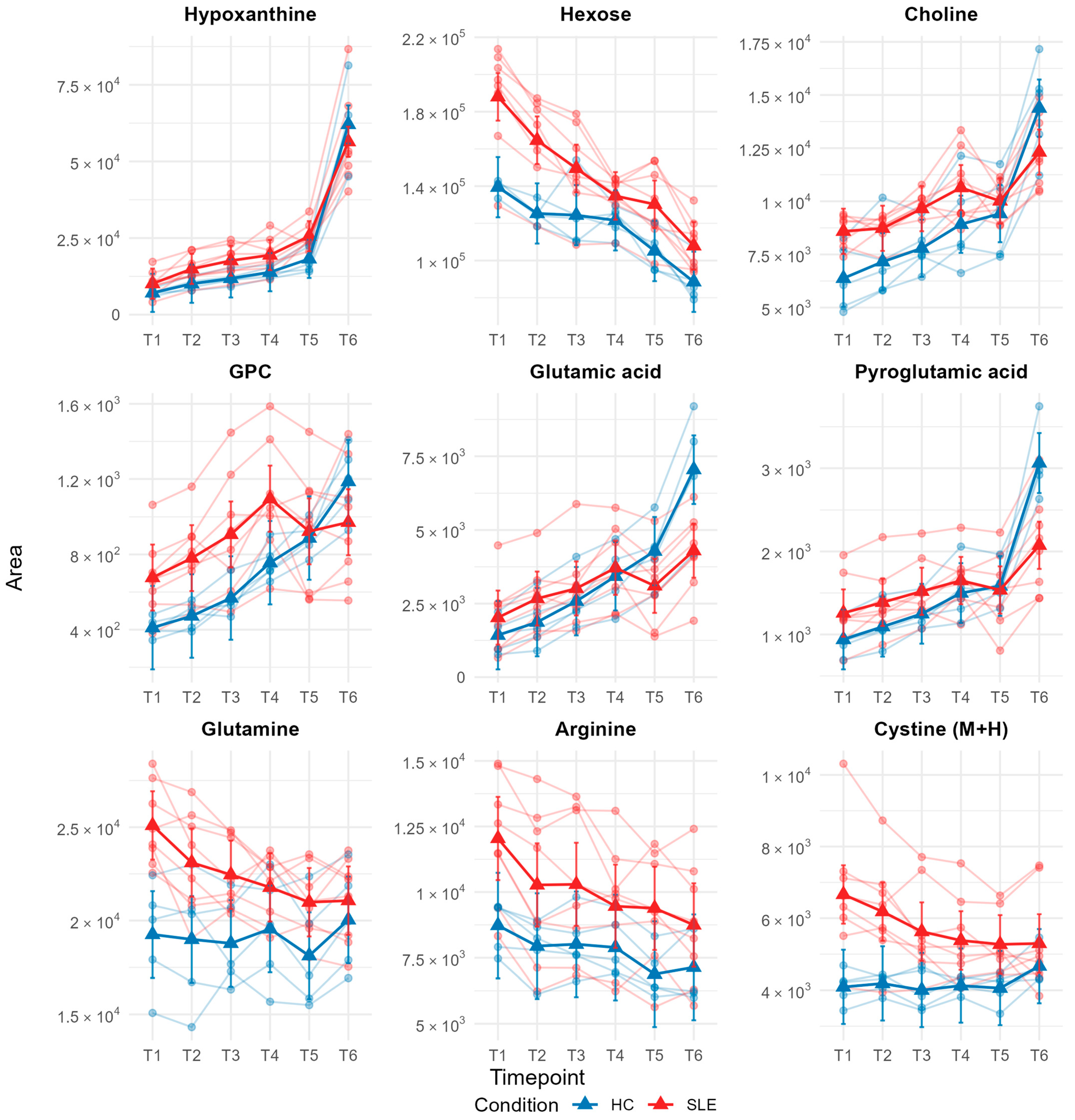

| Characteristics | SLE (n = 10 1) | HC (n = 5 1) | p-Value 2 |
|---|---|---|---|
| Age—yr | 48 ± 16 | 33 ± 5 | 0.043 |
| Female sex | 9/10 (90%) | 3/5 (60%) | 0.2 |
| Immunosuppressive medication | |||
| Antimalarial agent | 7/10 (70%) | ||
| Glucocorticoids | 5/10 (50%) | ||
| Mycophenolate | 3/10 (30%) | ||
| Belimumab | 5/10 (50%) | ||
| Azathioprine | 2/10 (20%) | ||
| Tacrolimus | 1/10 (10%) | ||
| Disease activity | |||
| SLEDAI-2k | 2.60 ± 2.12 | ||
| PGA Score | 0.80 ± 0.63 | ||
| LLDAS | 6/10 (60%) | ||
| DORIS | 2/10 (20%) | ||
| Serology | |||
| Anti-ds-DNA-Antibodies—IU/mL | 31 ± 41 | ||
| Hypocomplementemia C3c | 3/10 (30%) | ||
| Hypocomplementemia C4 | 6/10 (60%) | ||
| Antiphospholipid antibodies | 4/10 (40%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmitt, F.; Nguyen, S.; Claßen, P.C.; Meineck, M.; Hagen, M.; Weinmann-Menke, J.; Schmidlin, T. Disease and Medication Context Shape Ex Vivo Metabolite Stability: A Pilot Study in Systemic Lupus Erythematosus. Metabolites 2025, 15, 738. https://doi.org/10.3390/metabo15110738
Schmitt F, Nguyen S, Claßen PC, Meineck M, Hagen M, Weinmann-Menke J, Schmidlin T. Disease and Medication Context Shape Ex Vivo Metabolite Stability: A Pilot Study in Systemic Lupus Erythematosus. Metabolites. 2025; 15(11):738. https://doi.org/10.3390/metabo15110738
Chicago/Turabian StyleSchmitt, Fabian, Susanne Nguyen, Paul Christoph Claßen, Myriam Meineck, Mathias Hagen, Julia Weinmann-Menke, and Thierry Schmidlin. 2025. "Disease and Medication Context Shape Ex Vivo Metabolite Stability: A Pilot Study in Systemic Lupus Erythematosus" Metabolites 15, no. 11: 738. https://doi.org/10.3390/metabo15110738
APA StyleSchmitt, F., Nguyen, S., Claßen, P. C., Meineck, M., Hagen, M., Weinmann-Menke, J., & Schmidlin, T. (2025). Disease and Medication Context Shape Ex Vivo Metabolite Stability: A Pilot Study in Systemic Lupus Erythematosus. Metabolites, 15(11), 738. https://doi.org/10.3390/metabo15110738






