Studies on the Flavonoid Composition of Pleioblastus amarus Leaves and Shoots Based on Targeted Metabolomics
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material and Preparation of Extracts
2.3. Liquid Chromatography–Tandem Mass Spectrometry System
2.4. Data Preprocessing
2.5. Principal Component Analysis (PCA), Quality Assessment (QA), Quality Control (QC)
2.6. Statistical Analysis of Metabolite Content
3. Results
3.1. Determination of Flavonoids by Liquid Chromatography–Tandem Mass Spectrometry (LC-MS/MS)
3.2. Results and Analyses of Principal Component Analysis (PCA), Quality Assessment (QA) and Quality Control (QC)
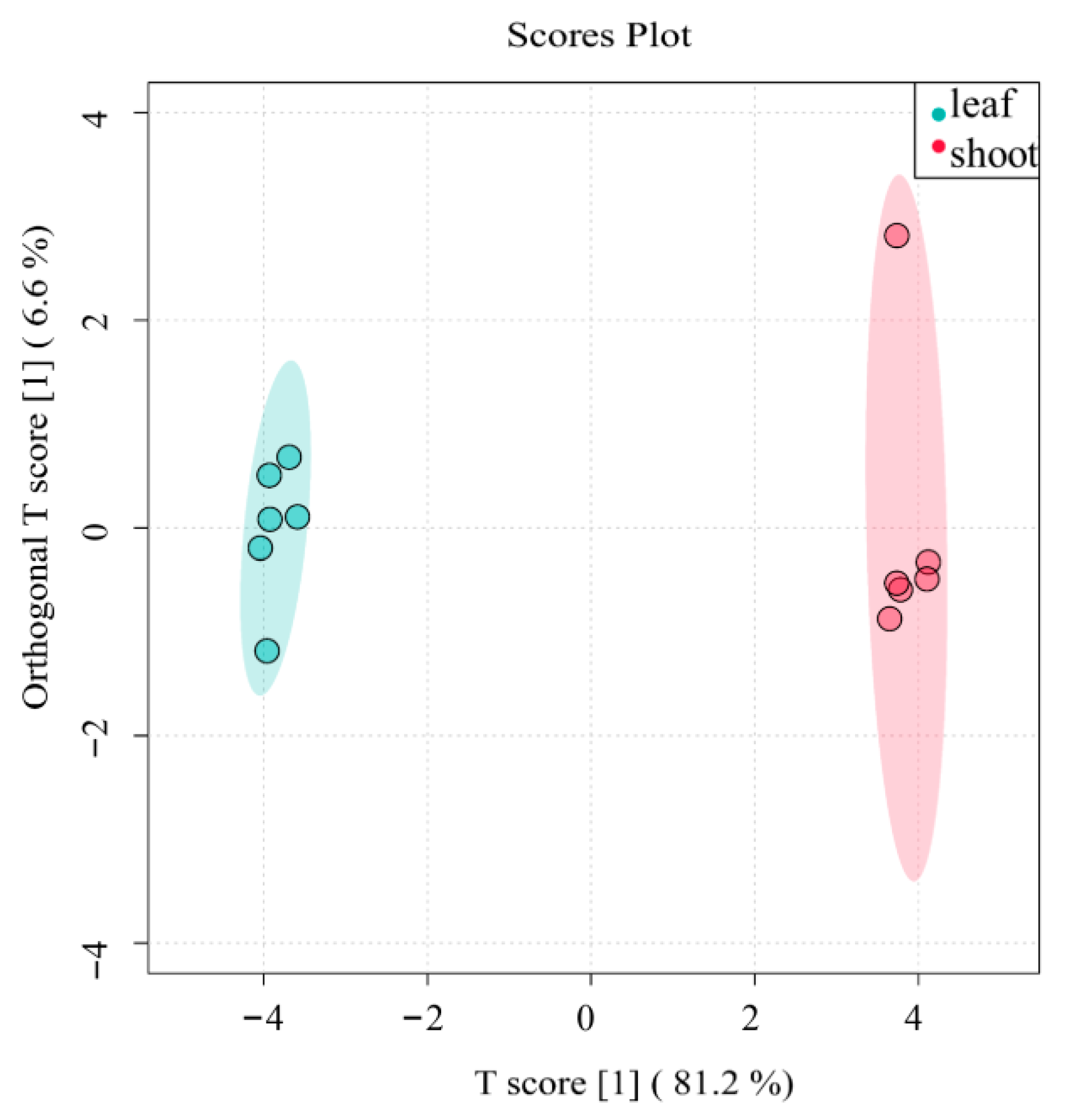
3.2.1. PCA Results and Analyses
3.2.2. QA and QC Results and Analyses
3.3. Statistical Analysis of Metabolite Content Results
3.3.1. Identification and Analysis of Flavonoids in P. amarus Leaves and Shoots
3.3.2. Structural Similarity Analysis of Flavonoid Compositions in P. amarus Leaves and Shoots
3.3.3. Differential Analysis of Flavonoids in P. amarus Leaves and Shoots
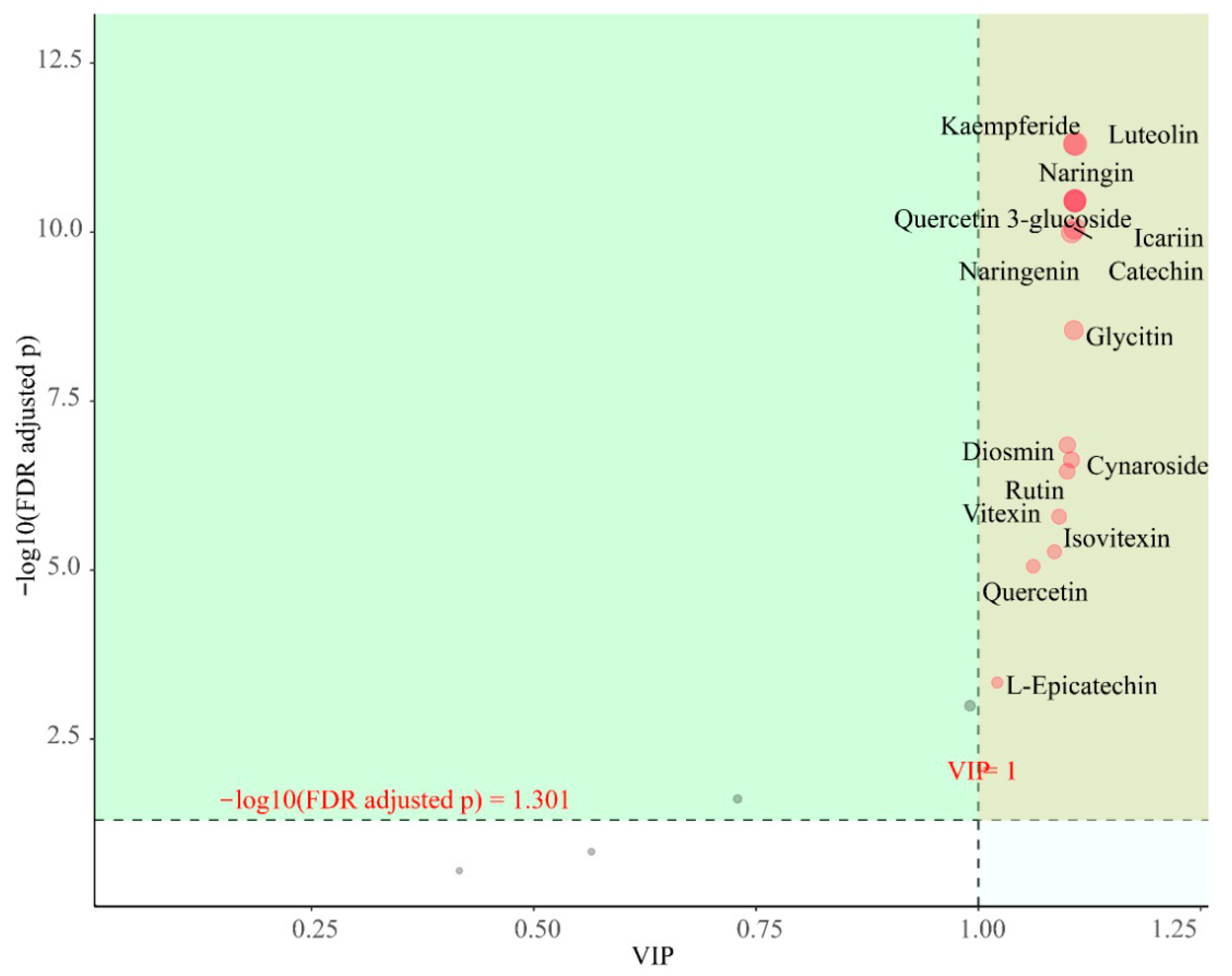
3.3.4. Screening of Characterized Flavonoids in P. amarus Leaves and Shoots
3.3.5. Correlation Analysis of Flavonoids in P. amarus Leaves and Shoots
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Flora of China Editorial Committee. Flora of China, Vol. 22 (Poaceae); Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MI, USA, 2006. [Google Scholar]
- Ohrnberger, D. The Bamboos of the World: Annotated Nomenclature and Literature of the Species and the Higher and Lower Taxa; Elsevier: Amsterdam, The Netherlands, 1999. [Google Scholar]
- Tzvelev, N.N. The system of grasses (Poaceae) and their evolution. Bot. Rev. 1989, 55, 141–203. [Google Scholar] [CrossRef]
- Yan, J.L.; Li, M.; Zhang, W.J.; Zhao, J.W.; Lu, P.; Zheng, J.; Zhao, D. Research progress on biological activity and applications of Pleioblastus species. J. Sichuan For. Sci. Technol. 2023, 44, 19–26. [Google Scholar]
- Yang, Y.F.; Huang, C.L. Study on flavonoids in shoots of three Pleioblastus species. J. Bamboo Res. 2009, 28, 56–60. [Google Scholar]
- Sun, J.; Yue, Y.D.; Tang, F.; Guo, X.F.; Wang, J.; Yao, X. Flavonoids from the Leaves of Neosinocalamus affinis. Chem. Nat. Compd. 2013, 49, 822–825. [Google Scholar] [CrossRef]
- Guo, X.F.; Yue, Y.D.; Tang, F.; Wang, J.; Yao, X. Flavonoids from the leaves of Pleioblastus argenteastriatus. J. Asian Nat. Prod. Res. 2008, 10, 903–907. [Google Scholar] [CrossRef]
- Shi, W.M.; Wang, Y.; Zhang, Y.H. Optimization of extracting flavonoids from Matteuccia Struthiopteris (L.) Todaro by response surface methodology. J. Guangzhou City Polytech. 2016, 2, 11–16. [Google Scholar]
- Zhong, S.J.; He, C.M.; Lin, C. Optimization of extraction process of total flavonoids from Astragalus sinicus by response surface method. Fujian Agric. Sci. Technol. 2016, 9, 6–11. [Google Scholar]
- Liang, Q.; Yang, S.X.; Kuang, Y.; Liu, L. Chemical Constituents from the stems of Pleioblastus amarus. Chin. Tradit. Herb. Drugs 2015, 46, 1125–1128. [Google Scholar] [CrossRef]
- Li, X.; Tao, W.; Xun, H.; Yao, X.; Wang, J.; Sun, J.; Yue, Y.; Tang, F. Simultaneous determination of flavonoids from bamboo leaf extracts using liquid chromatography-tandem mass spectrometry. Rev. Bras. Farmacogn. 2021, 31, 347–352. [Google Scholar] [CrossRef]
- Chu, B.Q.; Pang, M.R.; Zhang, Y. Literature review on flavonoid abundance in bamboo leaves. Nat. Prod. Res. Dev. 2015, 27, 1308–1316. [Google Scholar]
- Hu, X.; Liao, H.; Chen, X.; Yin, P.; Liu, X. Study on extraction conditions and content comparison of total flavonoids from bitter bamboo shoots and sinocalamus affinis shoots. IOP Conf. Ser. Mater. Sci. Eng. 2018, 394, 022010. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, X.Y.; Zhao, X.D.; Zhong, Y. Research progress on the regulation of plant flavonoid biosynthesis. Sci. Technol. Food Ind. 2021, 42, 454–463. [Google Scholar]
- Tian, S.; Yang, Y.; Wu, T.; Luo, C.; Li, X.; Zhao, X.; Xi, W.; Liu, X.; Zeng, M. Functional Characterization of a Flavone Synthase That Participates in a Kumquat Flavone Metabolon. Front. Plant Sci. 2022, 13, 826780. [Google Scholar] [CrossRef]
- Winkel-Shirley, B. It takes a garden. How work on diverse plant species has contributed to an understanding of flavonoid metabolism. Plant Physiol. 2001, 127, 4, 1399–1404. [Google Scholar] [CrossRef]
- Azuma, A.; Yakushiji, H.; Koshita, Y.; Kobayashi, S. Flavonoid biosynthesis-related genes in grape skin are differentially regulated by temperature and light conditions. Planta 2012, 236, 1067–1080. [Google Scholar] [CrossRef]
- Chen, S.X.; Cui, C.J.; Wang, H.; Cao, Z.M.; Li, X. Effects of temperature on the growth and physicochemical composition of Nitzschia palea. Curr. Biotechnol. 2012, 2, 48–51. [Google Scholar]
- Zhou, M.; Shen, Y.G.; Zhu, L.Q.; Ai, X.W.; Zeng, J.; Yang, H.H. Research progress on biosynthesis, accumulation, and regulation of plant flavonoids. Food Res. Dev. 2016, 37, 216–221. [Google Scholar]
- Hao, G.; Du, X.; Zhao, F.; Shi, R.; Wang, J. Role of nitric oxide in UV-B-induced activation of PAL and stimulation of flavonoid biosynthesis in Ginkgo biloba callus. Plant Cell Tissue Organ Cult. (PCTOC) 2009, 97, 175–185. [Google Scholar] [CrossRef]
- Li, Y.; Sun, C.; Yao, D.; Cao, X.; Wei, X.; Qi, Y.; Liang, Y.; Ye, J. A review of MicroRNAs and flavonoids: New insights into plant secondary metabolism. Int. J. Biol. Macromol. 2025, 309, 142518. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.S.; Zhang, M.M.; Shi, J.; Yang, Y.; Meng, X.; Xue, J.P.; Sun, W.; Wan, H.H.; Sheng, W. Research progress on the regulatory mechanism of phytohormones on flavonoid metabolism. China J. Chin. Mater. Medica 2021, 46, 3806–3813. [Google Scholar]
- Wang, T.; Xiao, J.; Hou, H.; Li, P.; Yuan, Z.; Xu, H.; Liu, R.; Li, Q.; Bi, K. Development of an ultra-fast liquid chromatography–tandem mass spectrometry method for simultaneous determination of seven flavonoids in rat plasma: Application to a comparative pharmacokinetic investigation of Ginkgo biloba extract and single pure ginkgo flavonoids after oral administration. J. Chromatogr. B 2017, 1060, 173–181. [Google Scholar]
- Lei, Z.; Sumner, B.W.; Bhatia, A.; Sarma, S.J.; Sumner, L.W. UHPLC-MS analyses of plant flavonoids. Curr. Protoc. Plant Biol. 2019, 4, e20085. [Google Scholar] [CrossRef]
- de Villiers, A.; Venter, P.; Pasch, H. Recent advances and trends in the liquid-chromatography mass spectrometry analysis of flavonoids. J. Chromatogr. A 2016, 1430, 16–78. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Xia, J. MetaboAnalystR: An R package for flexible and reproducible analysis of metabolomics data. Bioinformatics 2018, 34, 4313–4314. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Guo, S. Comparative analysis of flavonoid metabolites from different parts of Hemerocallis citrina. BMC Plant Biol. 2023, 23, 491. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Chen, W.; Wang, W.; Zhang, H.; Liu, X.; Luo, J. Comprehensive profiling and natural variation of flavonoids in rice. J. Integr. Plant Biol. 2014, 56, 876–886. [Google Scholar] [CrossRef]
- Karak, P. Biological activities of flavonoids: An overview. Int. J. Pharm. Sci. Res. 2019, 10, 1567–1574. [Google Scholar] [CrossRef]
- Sun, J.; Wang, Z.; Chen, L.; Sun, G. Hypolipidemic effects and preliminary mechanism of chrysanthemum flavonoids, its main components luteolin and luteoloside in hyperlipidemia rats. Antioxidants 2021, 10, 1309. [Google Scholar] [CrossRef]
- Tao, M.; Li, R.; Zhang, Z.; Wu, T.; Xu, T.; Zogona, D.; Huang, Y.; Pan, S.; Xu, X. Vitexin and Isovitexin Act through Inhibition of Insulin Receptor to Promote Longevity and Fitness in Caenorhabditis elegans. Mol. Nutr. Food Res. 2022, 66, 2100845. [Google Scholar] [CrossRef]
- Stabrauskiene, J.; Kopustinskiene, D.M.; Lazauskas, R.; Bernatoniene, J. Naringin and Naringenin: Their mechanisms of action and the potential anticancer activities. Biomedicines 2022, 10, 1686. [Google Scholar] [CrossRef]
- Kang, Y.L.; Pei, J.; Cai, W.L.; Liu, W. Research progress on metabolic synthesis pathways and related functional genes of flavonoids in medicinal plants. Chin. Tradit. Herb. Drugs 2014, 45, 1336–1341. [Google Scholar]
- Fang, Y.M.; Cui, M.Y.; Liu, J.; Pei, T.L.; Wei, K.Y.; Zhao, Q. Research advances in flavonoid biosynthesis in Scutellaria species. China J. Chin. Mater. Medica 2020, 45, 4819–4826. [Google Scholar]
- Mao, Y.; Luo, J.; Cai, Z. Biosynthesis and Regulatory Mechanisms of Plant Flavonoids: A Review. Plants 2025, 14, 1847. [Google Scholar] [CrossRef]
- Dubos, C.; Le Gourrierec, J.; Baudry, A.; Huep, G.; Lanet, E.; Debeaujon, I.; Routaboul, J.M.; Alboresi, A.; Weisshaar, B.; Lepiniec, L. MYBL2 is a new regulator of flavonoid biosynthesis in Arabidopsis thaliana. Plant J. 2008, 55, 940–953. [Google Scholar] [CrossRef] [PubMed]
- Matsui, K.; Umemura, Y.; Ohme-Takagi, M. AtMYBL2, a protein with a single MYB domain, acts as a negative regulator of anthocyanin biosynthesis in Arabidopsis. Plant J. 2008, 55, 954–967. [Google Scholar] [CrossRef] [PubMed]
- Gou, J.Y.; Felippes, F.F.; Liu, C.J.; Weigel, D.; Wang, J.W. Negative regulation of anthocyanin biosynthesis in Arabidopsis by a miR156-targeted SPL transcription factor. Plant Cell 2011, 23, 1512–1522. [Google Scholar] [CrossRef]
- Wen, D.; Wu, L.; Wang, M.; Yang, W.; Wang, X.; Ma, W.; Sun, W.; Chen, S.; Xiang, L.; Shi, Y. CRISPR/Cas9-Mediated Targeted Mutagenesis of FtMYB45 Promotes Flavonoid Biosynthesis in Tartary Buckwheat (Fagopyrum tataricum). Front. Plant Sci. 2022, 13, 879390. [Google Scholar] [CrossRef]








| Number | Name | Precursor Ion (m/z) | Quantitative Product Ion (m/z) | DP (V) | EP (V) | CE (V) | CXP (V) |
|---|---|---|---|---|---|---|---|
| 1 | Chrysin | 252.977 | 63.100 | −105 | −10 | −52 | −9 |
| 2 | Daidzein | 253.028 | 91.100 | −98 | −10 | −52 | −7 |
| 3 | Liquiritigenin | 255.102 | 119.100 | −85 | −10 | −30 | −1 |
| 4 | Formononetin | 266.985 | 252.100 | −105 | −10 | −28 | −5 |
| 5 | Apigenin | 268.898 | 117.100 | −105 | −10 | −44 | −1 |
| 6 | Genistein | 268.919 | 133.200 | −105 | −10 | −42 | −1 |
| 7 | Naringenin | 271.028 | 151.100 | −75 | −10 | −26 | −1 |
| 8 | Glycitein | 283.042 | 268.200 | −95 | −10 | −26 | −5 |
| 9 | Biochanin A | 283.065 | 268.100 | −95 | −10 | −30 | −5 |
| 10 | Fisetin | 284.890 | 134.800 | −105 | −10 | −30 | −7 |
| 11 | Kaempferol | 284.976 | 93.100 | −115 | −10 | −54 | −7 |
| 12 | Luteolin | 285.025 | 133.100 | −100 | −10 | −44 | −13 |
| 13 | Catechin | 289.012 | 245.100 | −80 | −10 | −32 | −5 |
| 14 | L-Epicatechin | 289.044 | 109.200 | −95 | −10 | −36 | −9 |
| 15 | Kaempferide | 299.010 | 284.000 | −110 | −10 | −30 | −5 |
| 16 | Kaempferide | 301.001 | 151.000 | −95 | −10 | −30 | −13 |
| 17 | Taxifolin | 302.997 | 285.100 | −70 | −10 | −16 | −5 |
| 18 | Myricetin | 16.872 | 150.800 | −75 | −10 | −34 | −9 |
| 19 | Dihydromyricetin | 318.957 | 192.700 | −75 | −10 | −14 | −3 |
| 20 | Puerarin | 415.101 | 295.200 | −100 | −10 | −30 | −5 |
| 21 | lsovitexin | 431.027 | 311.100 | −95 | −10 | −30 | −7 |
| 22 | Vitexin | 431.047 | 311.100 | −105 | −10 | −30 | −7 |
| 23 | Genistin | 431.076 | 268.100 | −130 | −10 | −42 | −5 |
| 24 | Baicalin | 445.151 | 269.200 | −75 | −10 | −30 | −5 |
| 25 | Glycitin | 445.162 | 283.100 | −55 | −10 | −12 | −5 |
| 26 | Astragalin | 447.089 | 284.000 | −105 | −10 | −36 | −5 |
| 27 | Quercitrin | 447.122 | 300.100 | −95 | −10 | −36 | −7 |
| 28 | Cynaroside | 447.127 | 285.000 | −120 | −10 | −36 | −5 |
| 29 | Daidzin | 461.104 | 253.100 | −65 | −10 | −22 | −5 |
| 30 | Quercetin 3-glucoside | 463.130 | 300.100 | −120 | −10 | −36 | −5 |
| 31 | Silybin | 481.107 | 125.100 | −110 | −10 | −36 | −11 |
| 32 | Leariin | 513.213 | 366.100 | −160 | −10 | −38 | −9 |
| 33 | Naringin | 579.260 | 271.200 | −135 | −10 | −46 | −5 |
| 34 | Diosmin | 607.260 | 299.100 | −110 | −10 | −36 | −7 |
| 35 | Rutin | 609.138 | 300.000 | −165 | −10 | −52 | −7 |
| Number | Flavonoids | Retention Time (min) | Linear Equation (Math.) | Correlation Coefficient (r) | Linear Range (ng/mL) | Limit of Quantification (LOQ) (ng/mL) | RSD (%) |
|---|---|---|---|---|---|---|---|
| 1 | Chrysin | 18.57 | y = 2.1 × 100.005x + 1.04 × 104 | 0.9919 | 0.2–100 | 0.2 | 2.95 |
| 2 | Daidzein | 12.27 | y = 5.47 × 104x + 5.37 × 103 | 0.9958 | 0.8–400 | 0.8 | 1.39 |
| 3 | Liquiritigenin | 10.28 | y = 1.74 × 105x + 1.54 × 104 | 0.9905 | 0.4–400 | 0.4 | 1.92 |
| 4 | Formononetin | 17.41 | y = 2.43 × 105x + 3.74 × 103 | 0.9905 | 0.02–40 | 0.02 | 0.85 |
| 5 | Apigenin | 16.43 | y = 1.35 × 105x + 3.74 × 103 | 0.9953 | 0.1–200 | 0.1 | 0.83 |
| 6 | Genistein | 14.96 | y = 5.19 × 104x − 238 | 0.9957 | 0.4–400 | 0.4 | 2.26 |
| 7 | Naringenin | 14.14 | y = 1.72 × 105x + 1.35 × 104 | 0.9951 | 0.2–200 | 0.2 | 3.54 |
| 8 | Glycitein | 4.85 | y = 6.6 × 105x + 1.35 × 104 | 0.9942 | 0.2–12.5 | 0.2 | 1.41 |
| 9 | Biochanin A | 18.51 | y = 1.48 × 106x + 7.88 × 103 | 0.9922 | 0.02–20 | 0.02 | 2.50 |
| 10 | Fisetin | 9.40 | y = 1.84 × 104x − 5.83 × 103 | 0.9907 | 0.8–4000 | 0.8 | 0.89 |
| 11 | Kaempferol | 16.13 | y = 2.44 × 104x + 4.96 × 103 | 0.9943 | 2–2000 | 2 | 1.23 |
| 12 | Luteolin | 14.51 | y = 1.23 × 105x + 1.52 × 104 | 0.9942 | 1–1000 | 1 | 3.68 |
| 13 | Catechin | 3.47 | y = 2.99 × 103x + 896 | 0.9945 | 2–4000 | 2 | 2.18 |
| 14 | L-Epicatechin | 4.11 | y = 1.65 × 104x + 6.04 × 103 | 0.9960 | 2–500 | 2 | 1.98 |
| 15 | Kaempferide | 18.88 | y = 2.2 × 105x + 239 | 0.9940 | 0.1–200 | 0.1 | 4.63 |
| 16 | Quercetin | 13.48 | y = 7.81 × 104x − 1.31 × 104 | 0.9945 | 1–2000 | 1 | 2.39 |
| 17 | Taxifolin | 5.20 | y = 2.68 × 104x + 3.25 × 103 | 0.9950 | 0.4–800 | 0.4 | 1.01 |
| 18 | Myricetin | 8.28 | y = 1.09 × 104x − 6.81 × 104 | 0.9910 | 10–10,000 | 10 | 1.96 |
| 19 | Dihydromyricetin | 4.05 | y = 6.52 × 103x + 0.419 | 0.9954 | 0.8–1600 | 0.8 | 1.84 |
| 20 | Puerarin | 4.17 | y = 1.31 × 105x + 2.01 × 103 | 0.9929 | 0.2–100 | 0.2 | 1.67 |
| 21 | lsovitexin | 6.21 | y = 6.46 × 104x + 4.32 × 103 | 0.9966 | 0.4–400 | 0.4 | 1.68 |
| 22 | Vitexin | 5.64 | y = 9.91 × 104x + 6.64 × 103 | 0.9929 | 0.2–400 | 0.2 | 1.05 |
| 23 | Genistin | 7.56 | y = 1.51 × 105x + 1.39 × 104 | 0.9914 | 0.4–400 | 0.4 | 2.85 |
| 24 | Baicalin | 12.60 | y = 1.38 × 104x − 2.29 × 103 | 0.9947 | 2–10,000 | 2 | 4.13 |
| 25 | Glycitin | 4.83 | y = 196x + 648 | 0.9923 | 20–5000 | 20 | 2.64 |
| 26 | Astragalin | 9.29 | y = 6.62 × 104x + 6.26 × 103 | 0.9931 | 0.5–1000 | 0.5 | 3.51 |
| 27 | Quercitrin | 9.06 | y = 5.18 × 104x + 1.93 × 103 | 0.9973 | 0.5–1000 | 0.5 | 3.41 |
| 28 | Cynaroside | 6.37 | y = 6.72 × 104x + 1.93 × 103 | 0.9952 | 0.4–800 | 0.4 | 1.71 |
| 29 | Daidzin | 4.61 | y = 3.29 × 104x + 885 | 0.9909 | 0.5–1000 | 0.5 | 1.80 |
| 30 | Quercetin 3-glucoside | 6.80 | y = 7.11 × 104x + 7.24 × 103 | 0.9917 | 0.5–1000 | 0.5 | 1.91 |
| 31 | Silybin | 15.08 | y = 5.58 × 104x + 5.04 × 103 | 0.9915 | 0.5–1000 | 0.5 | 1.46 |
| 32 | Icariin | 17.35 | y = 1.67 × 104x + 1.74 × 103 | 0.9930 | 1–1000 | 1 | 1.32 |
| 33 | Naringin | 7.42 | y = 3.58 × 104x + 7.59 × 103 | 0.9915 | 1–2000 | 1 | 2.36 |
| 34 | Diosmin | 9.57 | y = 7.99 × 104x + 6.10 × 103 | 0.9916 | 0.4–800 | 0.4 | 2.56 |
| 35 | Rutin | 6.69 | y = 3.90 × 104x + 3.55 × 103 | 0.9965 | 1–1000 | 1 | 2.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Wei, Q.; Wang, J.; Huang, C.; Wang, Z.; Liu, Y. Studies on the Flavonoid Composition of Pleioblastus amarus Leaves and Shoots Based on Targeted Metabolomics. Metabolites 2025, 15, 709. https://doi.org/10.3390/metabo15110709
Chen Y, Wei Q, Wang J, Huang C, Wang Z, Liu Y. Studies on the Flavonoid Composition of Pleioblastus amarus Leaves and Shoots Based on Targeted Metabolomics. Metabolites. 2025; 15(11):709. https://doi.org/10.3390/metabo15110709
Chicago/Turabian StyleChen, Yongmei, Qingwen Wei, Jing Wang, Chuanyao Huang, Zhenghao Wang, and Yuchun Liu. 2025. "Studies on the Flavonoid Composition of Pleioblastus amarus Leaves and Shoots Based on Targeted Metabolomics" Metabolites 15, no. 11: 709. https://doi.org/10.3390/metabo15110709
APA StyleChen, Y., Wei, Q., Wang, J., Huang, C., Wang, Z., & Liu, Y. (2025). Studies on the Flavonoid Composition of Pleioblastus amarus Leaves and Shoots Based on Targeted Metabolomics. Metabolites, 15(11), 709. https://doi.org/10.3390/metabo15110709






