Metabolic–Nutritional Associations with Depression in Elderly Chronic Kidney Disease Patients: Hemodialysis Versus Non-Dialysis Populations
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection Tools
- -
- Personal Information Form: Developed by the researchers to collect sociodemographic and clinical data, including age, gender, marital status, education level, occupation, disease duration, smoking and alcohol use, and body mass index (BMI). BMI was categorized according to the World Health Organization (WHO) criteria: under-weight (<18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), and obesity (≥30.0 kg/m2) [48].
- -
- Mini Nutritional Assessment—Short Form (MNA-SF): This validated tool evaluates nutritional status using six parameters: recent weight loss, appetite, mobility, psychological stress, neuropsychological issues, and BMI. We selected the MNA-SF because it is specifically designed for geriatric populations and has been extensively validated as a practical and reliable screening instrument. The Turkish version of the MNA-SF has also been validated and shown to be reliable in elderly populations [49]. Although biomarker panels (e.g., prealbumin, transferrin, CRP) may provide additional objective insights, they are less feasible in routine geriatric care due to cost and accessibility. To strengthen our assessment, we also incorporated serum albumin, hemoglobin, and ALT values as complementary laboratory markers [50].
- -
- Geriatric Depression Scale—Short Form (GDS-SF): Developed to screen for depressive symptoms in older adults, this 15-item questionnaire includes yes/no responses. Each item is scored 0 or 1, with total scores classified as follows: 0–4 = normal, 5–8 = mild depression, 9–11 = moderate depression, and 12–15 = severe depression [51]. The Turkish version of the GDS-SF has been validated, demonstrating good reliability and internal consistency in elderly patients [52].
- -
- EQ-5D General Health Questionnaire: This tool was included to evaluate participants’ perceived quality of life across five domains: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression [53].
2.3. Statistical Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALT | Alanine Aminotransferase |
| BMI | Body Mass Index |
| CKD | Chronic Kidney Disease |
| GDS-SF | Geriatric Depression Scale–Short Form |
| Hb | Hemoglobin |
| MNA-SF | Mini Nutritional Assessment–Short Form |
References
- Dyakin, V.V.; Dyakina-Fagnano, N.V.; McIntire, L.B.; Uversky, V.N. Fundamental Clock of Biological Aging: Convergence of Molecular, Neurodegenerative, Cognitive and Psychiatric Pathways: Non-Equilibrium Thermodynamics Meet Psychology. Int. J. Mol. Sci. 2021, 23, 285. [Google Scholar] [CrossRef]
- Chu, W.-M.; Tsai, H.-B.; Chen, Y.-C.; Hung, K.-Y.; Cheng, S.-Y.; Lin, C.-P. Palliative Care for Adult Patients Undergoing Hemodialysis in Asia: Challenges and Opportunities. J. Hosp. Palliat. Care 2024, 27, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, R.A.C.; Nishita, Y.; Tange, C.; Zhang, S.; Tateishi, M.; Furuya, K.; Kubota, S.; Sewo Sampaio, P.Y.; Shimokata, H.; Arai, H.; et al. High hand-grip strength asymmetry and mortality risk in community-dwelling Japanese middle-aged and older adults: Results from the National Institute for Longevity Sciences-Longitudinal Study of Aging. Arch. Gerontol. Geriatr. 2025, 138, 105969. [Google Scholar] [CrossRef] [PubMed]
- Aydemir, T. Mortality Rate and Care in Elderly Hemodialysis Patients. J. Nephrol. Nurs. 2025, 20, 61–70. [Google Scholar] [CrossRef]
- Maresova, P.; Javanmardi, E.; Barakovic, S.; Barakovic Husic, J.; Tomsone, S.; Krejcar, O.; Kuca, K. Consequences of chronic diseases and other limitations associated with old age—A scoping review. BMC Public Health 2019, 19, 1431. [Google Scholar] [CrossRef]
- Triposkiadis, F.; Xanthopoulos, A.; Parissis, J.; Butler, J.; Farmakis, D. Pathogenesis of chronic heart failure: Cardiovascular aging, risk factors, comorbidities, and disease modifiers. Heart Fail. Rev. 2022, 27, 337–344. [Google Scholar] [CrossRef]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef]
- Garcia-Dominguez, M. Chronic pain in the elderly: Exploring cellular and molecular mechanisms and therapeutic perspectives. Front. Aging 2024, 5, 1477017. [Google Scholar] [CrossRef]
- Guo, J.; Huang, X.; Dou, L.; Yan, M.; Shen, T.; Tang, W.; Li, J. Aging and aging-related diseases: From molecular mechanisms to interventions and treatments. Signal Transduct. Target. Ther. 2022, 7, 391. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Garagnani, P.; Morsiani, C.; Conte, M.; Santoro, A.; Grignolio, A.; Monti, D.; Capri, M.; Salvioli, S. The Continuum of Aging and Age-Related Diseases: Common Mechanisms but Different Rates. Front. Med. 2018, 5, 61. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Z.; Ren, Y.; Wang, Y.; Fang, J.; Yue, H.; Ma, S.; Guan, F. Aging and age-related diseases: From mechanisms to therapeutic strategies. Biogerontology 2021, 22, 165–187. [Google Scholar] [CrossRef]
- Gianfredi, V.; Nucci, D.; Pennisi, F.; Maggi, S.; Veronese, N.; Soysal, P. Aging, longevity, and healthy aging: The public health approach. Aging Clin. Exp. Res. 2025, 37, 125. [Google Scholar] [CrossRef]
- MacNeil-Vroomen, J.L.; Thompson, M.; Leo-Summers, L.; Marottoli, R.A.; Tai-Seale, M.; Allore, H.G. Health-care use and cost for multimorbid persons with dementia in the National Health and Aging Trends Study. Alzheimer’s Dement. 2020, 16, 1224–1233. [Google Scholar] [CrossRef]
- Noronha, I.L.; Santa-Catharina, G.P.; Andrade, L.; Coelho, V.A.; Jacob-Filho, W.; Elias, R.M. Glomerular filtration in the aging population. Front. Med. 2022, 9, 769329. [Google Scholar] [CrossRef]
- Chen, D.; Jiang, L.; Ma, M.; Wan, X. Association of Blood Selenium Level with Estimated Glomerular Filtration Rate in the Aging Population: A Cross-sectional Study. Biol. Trace Elem. Res. 2023, 201, 2258–2265. [Google Scholar] [CrossRef]
- Kobayashi, A.; Hirano, K.; Okuda, T.; Ikenoue, T.; Yamada, Y.; Yokoo, T.; Fukuma, S. Comprehensive Conservative Kidney Management among Older Population: A Nationwide Administrative Claims Database Analysis. Kidney360 2025, 7, 1–9. [Google Scholar] [CrossRef]
- Chanvillard, L.; Mason, T.; Ferenbach, D.A. Understanding and targeting senescence in kidney disease. Clin. Kidney J. 2025, 18, sfaf190. [Google Scholar] [CrossRef] [PubMed]
- Rafferty, Q.; Stafford, L.K.; Vos, T.; Thomé, F.S.; Aalruz, H.; Abate, Y.H.; Abbafati, C.; ElHafeez, S.A.; Abedi, A.; Abiodun, O.O.; et al. Global, regional, and national prevalence of kidney failure with replacement therapy and associated aetiologies, 1990–2023: A systematic analysis for the Global Burden of Disease Study 2023. Lancet Glob. Health 2025, 13, e1378–e1395. [Google Scholar] [CrossRef] [PubMed]
- Carriazo, S.; Ortiz, A. European East–West divide in kidney disease: The need to understand the drivers of chronic kidney disease outcomes. Clin. Kidney J. 2021, 14, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Sever, M.Ş.; Jager, K.J.; Vanholder, R.; Stengel, B.; Harambat, J.; Finne, P.; Tesař, V.; Barbullushi, M.; Bumblytė, I.A.; Zakharova, E.; et al. A roadmap for optimizing chronic kidney disease patient care and patient-oriented research in the Eastern European nephrology community. Clin. Kidney J. 2021, 14, 23–35. [Google Scholar] [CrossRef]
- Seyahi, N.; Koçyiğit, İ.; Eren, N.; Tonbul, H.Z.; Tatar, E.; Yılmaz, Z.; Gök Oğuz, E.; Türkmen, E.; Ateş, K. Current Status of Kidney Replacement Therapy in Türkiye: A Summary of 2023 Turkish Society of Nephrology Registry Report. Turk. J. Nephrol. 2025, 34, 141–148. [Google Scholar] [CrossRef]
- Adejumo, O.A.; Edeki, I.R.; Sunday Oyedepo, D.; Falade, J.; Yisau, O.E.; Ige, O.O.; Adesida, A.O.; Daniel Palencia, H.; Sabri Moussa, A.; Abdulmalik, J.; et al. Global prevalence of depression in chronic kidney disease: A systematic review and meta-analysis. J. Nephrol. 2024, 37, 2455–2472. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Zhao, J.; Ma, Y.; He, L.; Ren, Z.; Yang, K.; Tang, J.; Liu, J.; Luo, J.; Zhang, H. Association of cognitive impairment with the interaction between chronic kidney disease and depression: Findings from NHANES 2011–2014. BMC Psychiatry 2024, 24, 312. [Google Scholar] [CrossRef]
- Seyahi, N.; Kocyigit, İ.; Eren, N.; Tonbul, H.Z.; Tatar, E.; Yilmaz, Z.; Oguz, E.G.; Turkmen, E.; Ates, K. Current Status of Kidney Replacement Therapy in Türkiye: A Summary of 2022 Turkish Society of Nephrology Registry Report. Turk. J. Nephrol. 2024, 33, 134–139. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, C. Recent Advances in the Management of Diabetic Kidney Disease: Slowing Progression. Int. J. Mol. Sci. 2024, 25, 3086. [Google Scholar] [CrossRef]
- Esposito, P.; Picciotto, D.; Cappadona, F.; Costigliolo, F.; Russo, E.; Maccio, L.; Viazzi, F. Multifaceted relationship between diabetes and kidney diseases: Beyond diabetes. World J. Diabetes 2023, 14, 1450–1462. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Yuan, M.; Li, L.; Zhou, S.; Huang, L.; Su, L.; Bai, Y. Association of Neutrophil Percentage-to-Albumin Ratio with All-Cause and Cardiovascular Mortality in Maintenance Hemodialysis Patients: A Retrospective Study. J. Inflamm. Res. 2025, 18, 9935–9949. [Google Scholar] [CrossRef]
- Chisavu, L.; Mihaescu, A.; Bob, F.; Motofelea, A.; Schiller, O.; Marc, L.; Dragota-Pascota, R.; Chisavu, F.; Schiller, A. Trends in mortality and comorbidities in hemodialysis patients between 2012 and 2017 in an East-European Country: A retrospective study. Int. Urol. Nephrol. 2023, 55, 2579–2587. [Google Scholar] [CrossRef]
- Bellanti, F.; Lo Buglio, A.; Quiete, S.; Vendemiale, G. Malnutrition in Hospitalized Old Patients: Screening and Diagnosis, Clinical Outcomes, and Management. Nutrients 2022, 14, 910. [Google Scholar] [CrossRef]
- Borkent, J.W.; Van Hout, H.P.J.; Feskens, E.J.M.; Naumann, E.; de van der Schueren, M.A.E. Diseases, Health-Related Problems, and the Incidence of Malnutrition in Long-Term Care Facilities. Int. J. Env. Res. Public Health 2023, 20, 3170. [Google Scholar] [CrossRef]
- Lu, Y.; Nyunt, M.S.Z.; Gao, Q.; Gwee, X.; Chua, D.Q.; Yap, K.B.; Pan, F.; Ng, T.P. Malnutrition Risk and Kidney Function and Decline in Community-Dwelling Older Adults. J. Ren. Nutr. 2022, 32, 560–568. [Google Scholar] [CrossRef]
- Moldovan, D.; Kacso, I.; Avram, L.; Bondor, C.; Rusu, C.; Potra, A.; Tirinescu, D.; Ticala, M.; Condor, A.; Crisan, D.; et al. Malnutrition in Elderly Patients with Chronic Kidney Disease-The Role of Albuminuria. Life 2025, 15, 898. [Google Scholar] [CrossRef] [PubMed]
- Sahathevan, S.; Khor, B.H.; Ng, H.M.; Gafor, A.H.A.; Mat Daud, Z.A.; Mafra, D.; Karupaiah, T. Understanding Development of Malnutrition in Hemodialysis Patients: A Narrative Review. Nutrients 2020, 12, 3147. [Google Scholar] [CrossRef] [PubMed]
- Dorner, B.; Friedrich, E.K. Position of the Academy of Nutrition and Dietetics: Individualized Nutrition Approaches for Older Adults: Long-Term Care, Post-Acute Care, and Other Settings. J. Acad. Nutr. Diet. 2018, 118, 724–735. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Liao, Z.; Zhou, Y.; Gao, Z.; Mao, Y. Non-linear relationship of serum albumin-to-globulin ratio and cognitive function in American older people: A cross-sectional national health and nutrition examination survey 2011–2014 (NHANES) study. Front. Public Health 2024, 12, 1375379. [Google Scholar] [CrossRef]
- Corona, L.P.; de Oliveira Duarte, Y.A.; Lebrao, M.L. Markers of nutritional status and mortality in older adults: The role of anemia and hypoalbuminemia. Geriatr. Gerontol. Int. 2018, 18, 177–182. [Google Scholar] [CrossRef]
- Li, Y.; Chen, L.; Yang, X.; Cui, H.; Li, Z.; Chen, W.; Shi, H.; Zhu, M. Dynamic association of serum albumin changes with inflammation, nutritional status and clinical outcomes: A secondary analysis of a large prospective observational cohort study. Eur. J. Med. Res. 2025, 30, 679. [Google Scholar] [CrossRef]
- Riviati, N.; Legiran; Indrajaya, T.; Saleh, I.; Ali, Z.; Irfannuddin; Probosuseno; Indra, B. Serum Albumin as Prognostic Marker for Older Adults in Hospital and Community Settings. Gerontol. Geriatr. Med. 2024, 10, 23337214241249914. [Google Scholar] [CrossRef]
- Tsai, W.C.; Hsu, S.P.; Chiu, Y.L.; Wu, H.Y.; Luan, C.C.; Yang, J.Y.; Pai, M.F.; Lin, C.J.; Lin, W.Y.; Sun, W.H.; et al. Short-Term Effects of a Therapeutic Diet on Biochemical Parameters in Hemodialysis Patients: A Randomized Crossover Trial. J. Ren. Nutr. 2023, 33, 731–739. [Google Scholar] [CrossRef]
- Costacurta, M.; Basilicata, M.; Marrone, G.; Di Lauro, M.; Campolattano, V.; Bollero, P.; Docimo, R.; Di Daniele, N.; Noce, A. The Impact of Chronic Kidney Disease on Nutritional Status and Its Possible Relation with Oral Diseases. Nutrients 2022, 14, 2002. [Google Scholar] [CrossRef]
- Alshelleh, S.; Alhouri, A.; Taifour, A.; Abu-Hussein, B.; Alwreikat, F.; Abdelghani, M.; Badran, M.; Al-Asa’d, Y.; Alhawari, H.; Oweis, A.O. Prevelance of depression and anxiety with their effect on quality of life in chronic kidney disease patients. Sci. Rep. 2022, 12, 17627. [Google Scholar] [CrossRef]
- Butt, M.D.; Ong, S.C.; Butt, F.Z.; Sajjad, A.; Rasool, M.F.; Imran, I.; Ahmad, T.; Alqahtani, F.; Babar, Z.U. Assessment of Health-Related Quality of Life, Medication Adherence, and Prevalence of Depression in Kidney Failure Patients. Int. J. Env. Res. Public Health 2022, 19, 15266. [Google Scholar] [CrossRef] [PubMed]
- Zis, P.; Daskalaki, A.; Bountouni, I.; Sykioti, P.; Varrassi, G.; Paladini, A. Depression and chronic pain in the elderly: Links and management challenges. Clin. Interv. Aging 2017, 12, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Norman, K.; Haß, U.; Pirlich, M. Malnutrition in Older Adults—Recent Advances and Remaining Challenges. Nutrients 2021, 13, 2764. [Google Scholar] [CrossRef]
- CindogLu, Ç.; BeyazgüL, B. Nutritional status and anxiety-depression relationship in hemodialysis patients. J. Surg. Med. 2021, 5, 429–432. [Google Scholar] [CrossRef]
- de Alencar, S.B.V.; Dias, L.D.A.; Dias, V.D.A.; de Lima, F.M.; Montarroyos, U.R.; de Petribu, K.C.L. Quality of life may be a more valuable prognostic factor than depression in older hemodialysis patients. Qual. Life Res. 2020, 29, 1829–1838. [Google Scholar] [CrossRef]
- Wang, W.L.; Liang, S.; Zhu, F.L.; Liu, J.Q.; Wang, S.Y.; Chen, X.M.; Cai, G.Y. The prevalence of depression and the association between depression and kidney function and health-related quality of life in elderly patients with chronic kidney disease: A multicenter cross-sectional study. Clin. Interv. Aging 2019, 14, 905–913. [Google Scholar] [CrossRef]
- World Health Organization. Obesity: Preventing and Managing the Global Epidemic; Report of a WHO Consultation; World Health Organization Technical Report Series; WHO: Geneva, Switzerland, 2000; Volume 894, pp. 1–253. [Google Scholar]
- Ekici, E.; Vatansever, N.; Çolak, M.; Kozan, E.H. Validity and Reliability of Turkish Self Mini Nutritional Assessment Scale. Elder. Health J. 2021, 7, 32–38. [Google Scholar] [CrossRef]
- Luo, T.; Zhang, W.; Yuan, Y.; Zhao, Y.; Yang, M.; Wang, G. Nutritional status according to the short-form mini nutritional assessment (MNA-SF) as predictors of perioperative myocardial injury among older hip fracture surgery patients: A prospective cohort study. Curr. Probl. Surg. 2025, 69, 101838. [Google Scholar] [CrossRef]
- Sultana, N.; Nguyen, T.T.P.; Hossain, A.; Asaduzzaman, M.; Nguyen, M.H.; Jahan, I.; Nguyen, K.T.; Duong, T.V. Psychometric Properties of the Short-Form Geriatric Depression Scale (GDS-SF) and Its Associated Factors among the Elderly in Bangladesh. Int. J. Env. Res. Public Health 2022, 19, 7935. [Google Scholar] [CrossRef]
- Durmaz, B.; Soysal, P.; Ellidokuz, H.; Isik, A.T. Validity and Reliability of Geriatric Depression Scale-15 (Short Form) in Turkish older adults. North. Clin. Istanb. 2018, 5, 216–220. [Google Scholar] [CrossRef]
- Ock, M.; Pyo, J.; Jo, M.W.; Herdman, M.; Luo, N. Perceptions of the General Public About Health-related Quality of Life and the EQ-5D Questionnaire: A Qualitative Study in Korea. J. Prev. Med. Public Health 2022, 55, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, F.; Wang, Y.; Wu, D. Prevalence and association of depression with uremia in dialysis population: A retrospective cohort analysis. Medicine 2020, 99, e20401. [Google Scholar] [CrossRef] [PubMed]
- Guenzani, D.; Buoli, M.; Caldiroli, L.; Carnevali, G.S.; Serati, M.; Vezza, C.; Armelloni, S.; Messa, P.; Vettoretti, S. Malnutrition and inflammation are associated with severity of depressive and cognitive symptoms of old patients affected by chronic kidney disease. J. Psychosom. Res. 2019, 124, 109783. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Rhee, C.M.; Chou, J.; Ahmadi, S.F.; Park, J.; Chen, J.L.; Amin, A.N. The Obesity Paradox in Kidney Disease: How to Reconcile it with Obesity Management. Kidney Int. Rep. 2017, 2, 271–281. [Google Scholar] [CrossRef]

| Category | Subgroup | Hemodialysis (n = 78), n (%) | Non-Hemodialysis (n = 74), n (%) | p-Value |
|---|---|---|---|---|
| Gender | Female | 40 (51.3%) | 42 (56.8%) | 0.607 |
| Male | 38 (48.7%) | 32 (43.2%) | ||
| Marital Status | Married | 58 (74.4%) | 55 (74.3%) | 1.000 |
| Single | 20 (25.6%) | 19 (25.7%) | ||
| Divorced/Widowed | 28 (35.9%) | 44 (59.5%) | ||
| Education | Illiterate | 32 (41.0%) | 27 (36.5%) | 0.008 |
| Primary school | 10 (12.8%) | 2 (2.7%) | ||
| Secondary/High school | 8 (10.3%) | 1 (1.3%) | ||
| Bachelor’s and above | 20 (25.6%) | 17 (23.0%) | ||
| Smoking | Yes | 58 (74.4%) | 57 (77.0%) | 0.726 |
| No | 6 (7.7%) | 3 (4.1%) | ||
| Alcohol Use | Yes | 72 (92.3%) | 71 (95.9%) | 0.493 |
| No | 2 (2.6%) | 2 (2.7%) | ||
| BMI | Underweight | 31 (39.7%) | 23 (31.1%) | 0.001 |
| Normal weight | 30 (38.5%) | 27 (36.5%) | ||
| Overweight | 15 (19.2%) | 22 (29.7%) | ||
| Obese | 37 (24.4%) | |||
| Hemodialysis Duration | 3–11 months | 8 (10.3%) | ||
| 1–2 years | 18 (23.1%) | |||
| 3–5 years | 21 (26.9%) | |||
| ≥6 years | 31 (39.7%) | |||
| Weekly Dialysis Sessions | 2 times/week | 19 (24.4%) | ||
| 3 times/week | 59 (75.6%) |
| Variable | Hemodialysis (n = 78), n (%) | Non-Hemodialysis (n = 74), n (%) | t-Value | p-Value |
|---|---|---|---|---|
| Age (years) | 70.13 ± 7.76 | 77.07 ± 8.61 | −5.222 | 0.001 |
| Height (cm) | 166.83 ± 7.60 | 164.16 ± 6.52 | 2.328 | 0.021 |
| Weight (kg) | 69.83 ± 12.05 | 74.95 ± 11.34 | −2.701 | 0.008 |
| BMI | 25.18 ± 4.75 | 28.44 ± 4.70 | −4.252 | 0.001 |
| Albumin (g/dL) | 3.50 ± 0.465 | 2.53 ± 0.67 | 9.973 | 0.001 |
| Hemoglobin (g/dL) | 11.16 ± 1.92 | 12.44 ± 2.03 | 3.979 | 0.001 |
| ALT (U/L) | 8.53 ± 4.31 | 17.16 ± 13.06 | −5.532 | 0.001 |
| Scale | Hemodialysis (n = 78), n (%) | Non-Hemodialysis (n = 74), n (%) | t-Value | p-Value |
|---|---|---|---|---|
| MNA-SF—Normal | 51 (65.4%) | 38 (51.4%) | 2.150 | 0.033 |
| MNA-SF—At Risk | 20 (25.6%) | 21 (28.3%) | ||
| MNA-SF—Malnutrition | 7 (9.0%) | 15 (20.3%) | ||
| GDS-SF—Normal | 49 (62.8%) | 35 (47.2%) | −1.703 | 0.091 |
| GDS-SF—Mild Depression | 17 (21.8%) | 17 (23.0%) | ||
| GDS-SF—Moderate Depression | 7 (9.0%) | 21 (28.4%) | ||
| GDS-SF—Severe Depression | 5 (6.4%) | 1 (1.4%) |
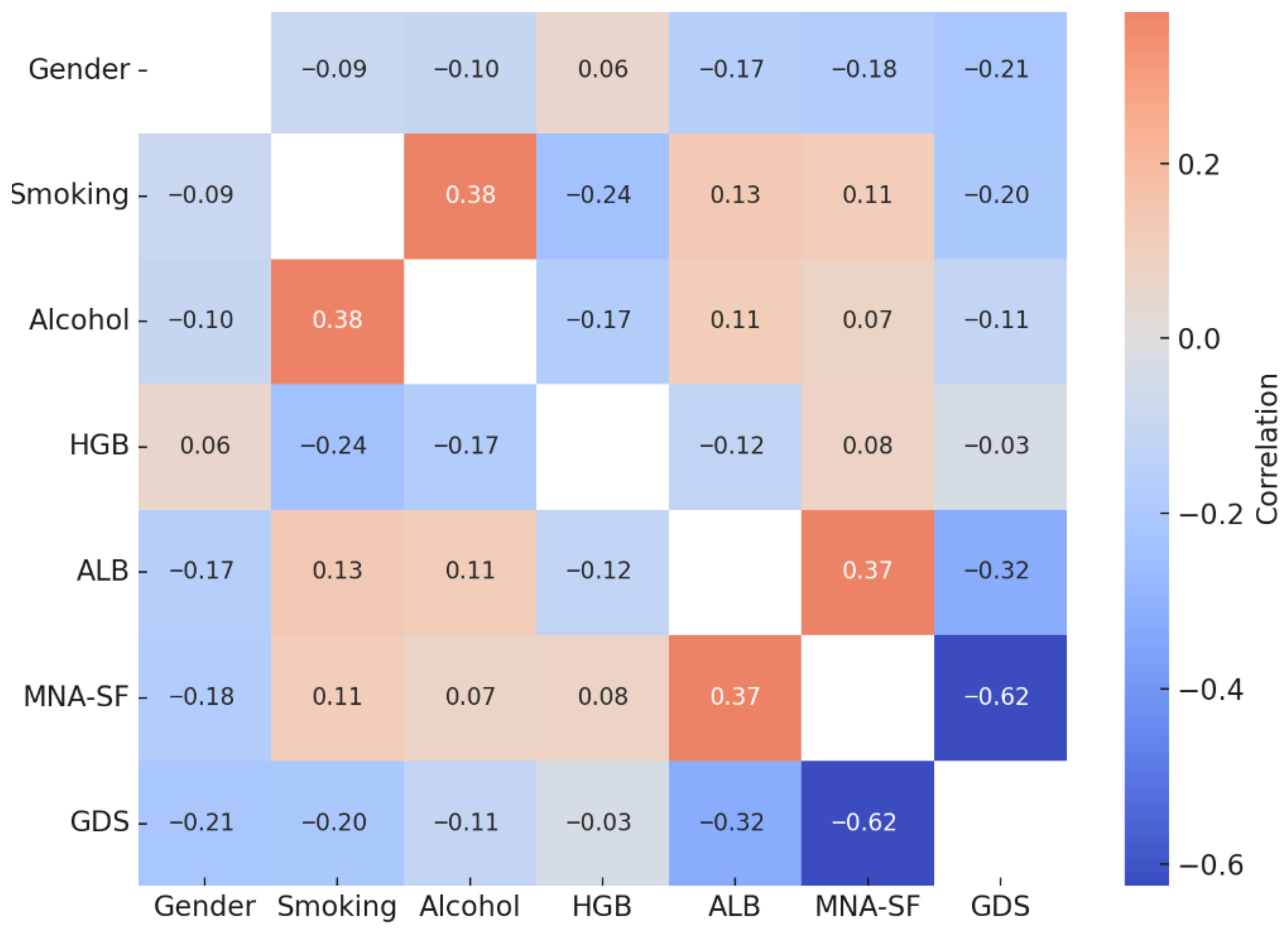
| Variables | Gender (r) | Smoking (r) | Alcohol (r) | HGB (r) | ALB (r) | MNA-SF (r) | GDS-SF (r) |
|---|---|---|---|---|---|---|---|
| Gender | – | ||||||
| Smoking | −0.091 | – | |||||
| Alcohol Use | −0.104 | 0.377 ** | – | ||||
| Hemoglobin (HGB) | 0.058 | −0.236 ** | −0.173 * | – | |||
| Albumin (ALB) | −0.165 | 0.131 | 0.112 | −0.124 | – | 0.374 ** | −0.323 ** |
| MNA-SF | −0.178 * | 0.111 | 0.075 | 0.078 | 0.374 ** | – | −0.625 ** |
| GDS-SF | −0.209 ** | −0.199 * | −0.112 | −0.033 | −0.323 ** | −0.625 ** | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozdemir, S.; Cakici, A.E.; Bilir, İ. Metabolic–Nutritional Associations with Depression in Elderly Chronic Kidney Disease Patients: Hemodialysis Versus Non-Dialysis Populations. Metabolites 2025, 15, 710. https://doi.org/10.3390/metabo15110710
Ozdemir S, Cakici AE, Bilir İ. Metabolic–Nutritional Associations with Depression in Elderly Chronic Kidney Disease Patients: Hemodialysis Versus Non-Dialysis Populations. Metabolites. 2025; 15(11):710. https://doi.org/10.3390/metabo15110710
Chicago/Turabian StyleOzdemir, Sedat, Aynur Ekren Cakici, and İbrahim Bilir. 2025. "Metabolic–Nutritional Associations with Depression in Elderly Chronic Kidney Disease Patients: Hemodialysis Versus Non-Dialysis Populations" Metabolites 15, no. 11: 710. https://doi.org/10.3390/metabo15110710
APA StyleOzdemir, S., Cakici, A. E., & Bilir, İ. (2025). Metabolic–Nutritional Associations with Depression in Elderly Chronic Kidney Disease Patients: Hemodialysis Versus Non-Dialysis Populations. Metabolites, 15(11), 710. https://doi.org/10.3390/metabo15110710







