Resistant Potato Starch Supplementation Increases the Serum Levels of Choline and Sphingomyelins Without Affecting Trimethylamine Oxide Levels
Abstract
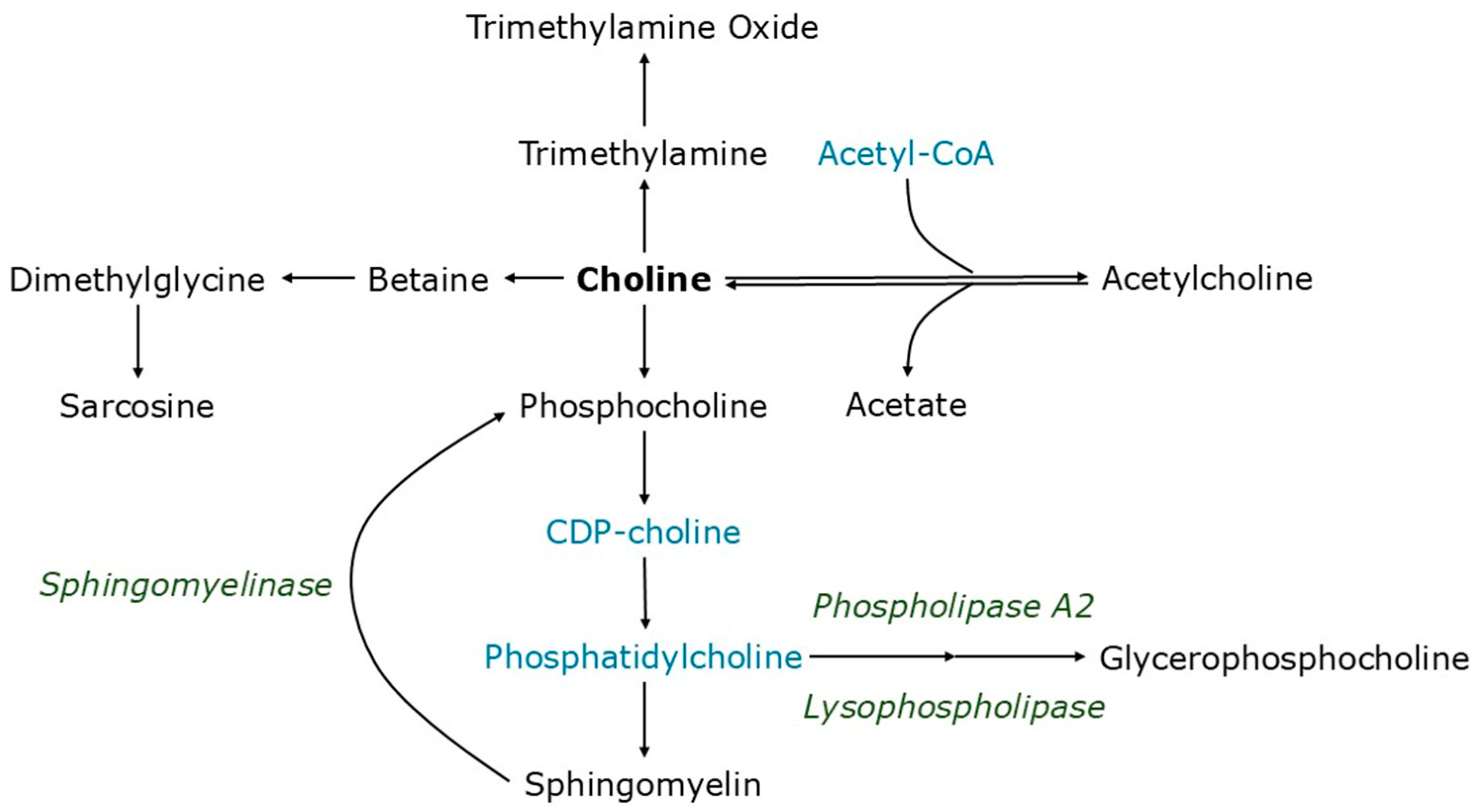
1. Introduction
2. Materials and Methods
2.1. Investigational Product
2.2. Study Design
2.3. Clinical Trial Conduct
2.4. Metabolomic Analysis
2.5. Statistical Analysis
3. Results
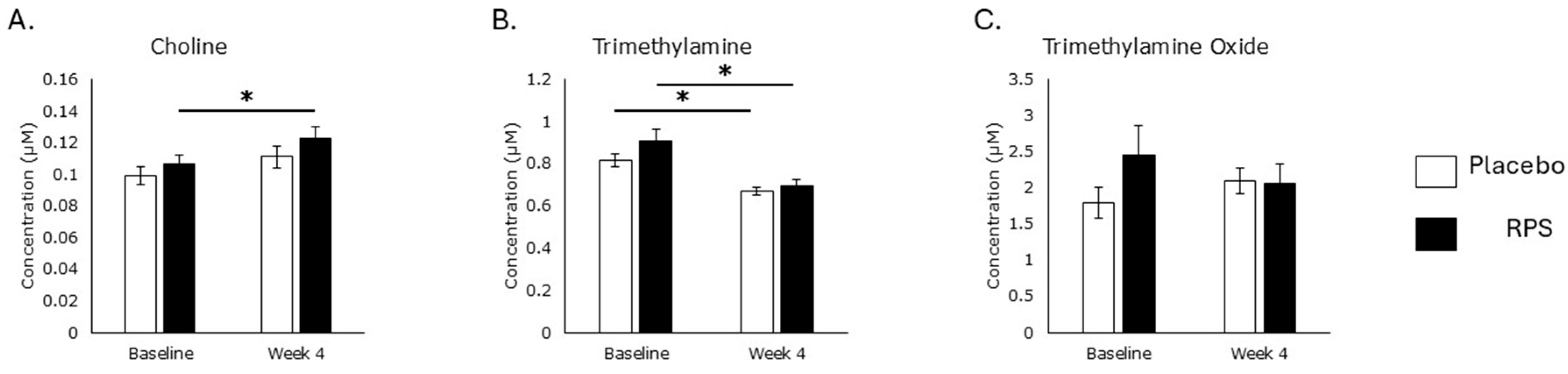
3.1. Serum Choline Levels
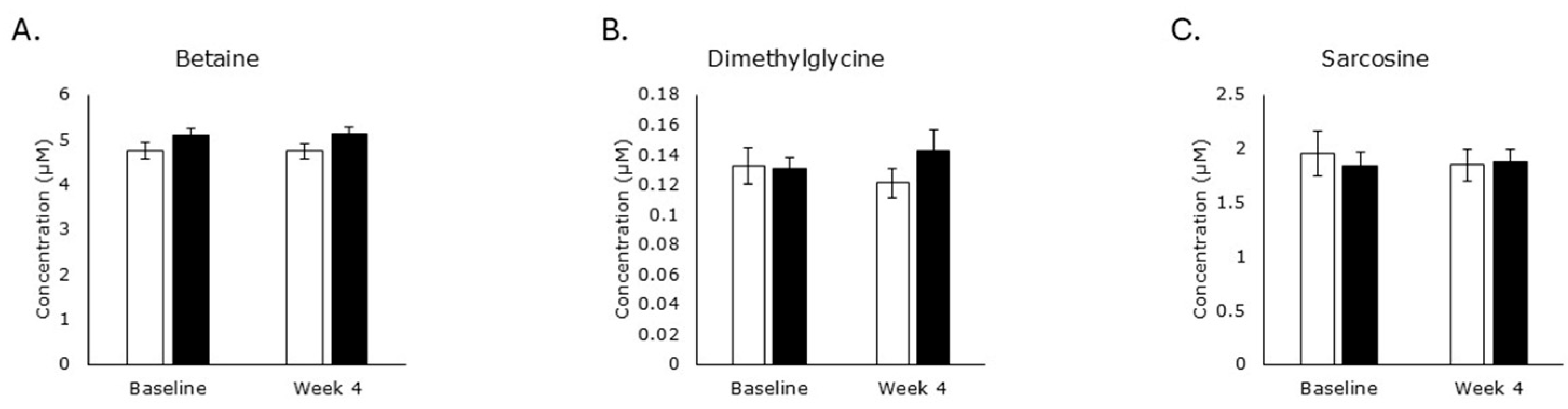
3.2. Choline Oxidation
3.3. Acetylcholine Metabolism
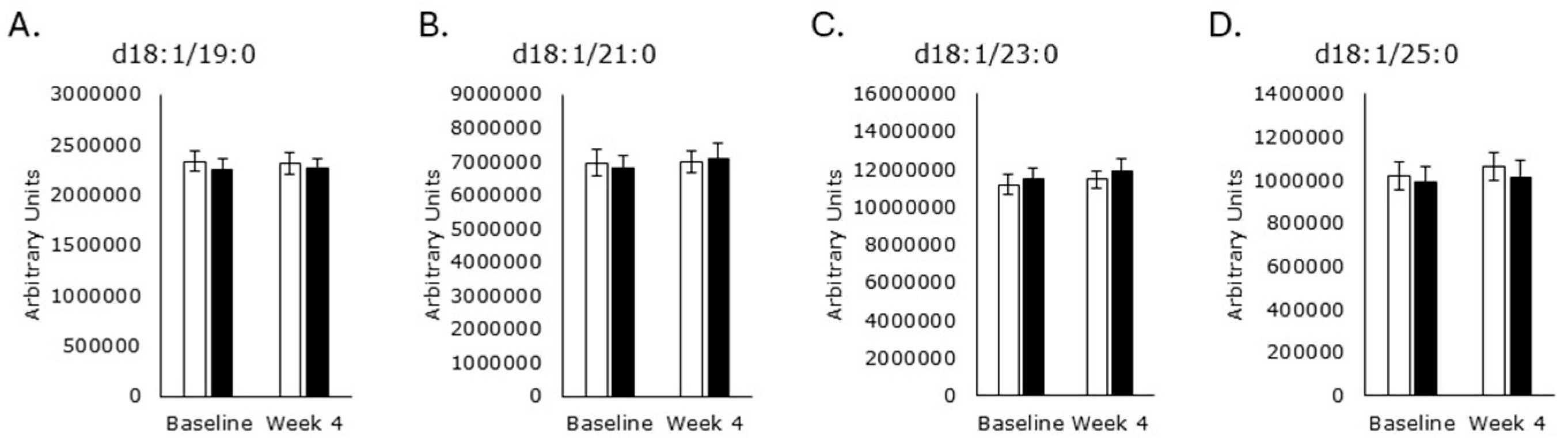
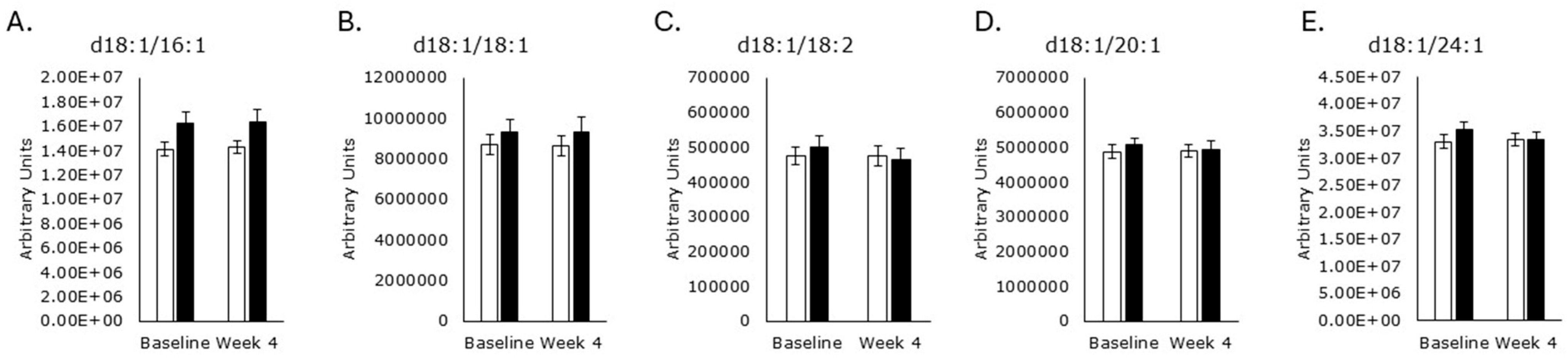
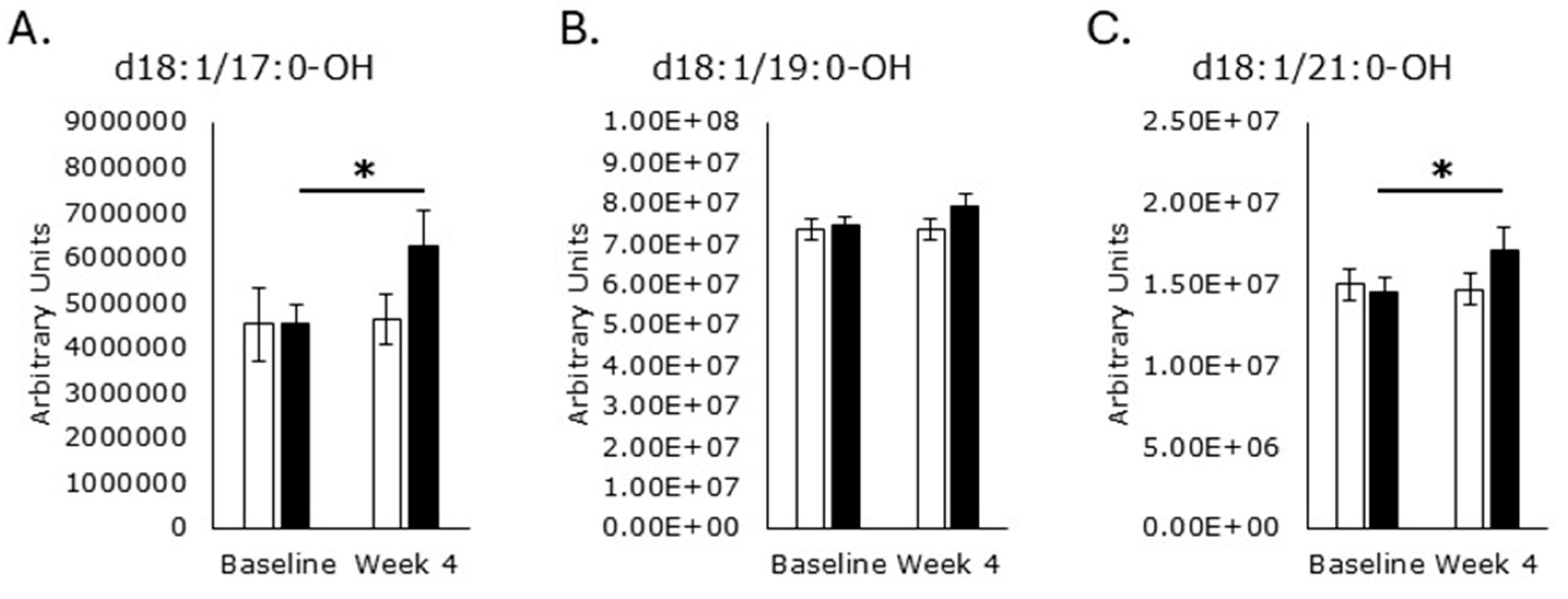
3.4. Sphingomyelin Synthesis
3.5. Sphingomyelinase and Phospholipase Activity
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| CVD | Cardiovascular disease |
| FFA | Free fatty acid |
| LC-MS/MS | Liquid chromatography coupled to tandem mass spectrometry |
| NAFLD | Non-alcoholic fatty liver disease |
| QC | Quality control |
| RPS | Resistant potato starch |
| RS | Resistant starch |
| RS2 | Resistant starch type 2 |
| SM | Sphingomyelin |
| SPT | Serine palmitoyltransferase |
| TMA | Trimethylamine |
| TMAO | Trimethylamine oxide |
References
- Office of Dietary Supplements, National Institutes of Health. Choline: Fact Sheet for Health Professionals. 2 June 2022. Available online: https://ods.od.nih.gov/factsheets/Choline-HealthProfessional/#:~:text=Choline%20is%20an%20essential%20nutrient,phospholipids%20vital%20for%20cell%20membranes (accessed on 27 August 2025).
- Kenny, T.C.; Scharenberg, S.; Abu-Remaileh, M.; Birsoy, K. Cellular and organismal function of choline metabolism. Nat. Metab. 2025, 7, 35–52. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H. Choline. In Modern Nutrition in Health and Disease, 11th ed.; Ross, A.C., Caballero, B., Cousins, R.J., Tucker, K.L., Ziegler, T.R., Eds.; Lippincott Williams & Wilkins: Baltimore, MD, USA, 2014; pp. 416–426. [Google Scholar]
- Patterson, K.Y.; Bhagwat, S.A.; Williams, J.R.; Howe, J.C.; Holden, J.M. USDA Database for the Choline Content of Common Foods, Release Two; United States Department of Agriculture (USDA): Washington, DC, USA, 2008; p. 1. Available online: https://www.ars.usda.gov/ARSUserFiles/80400525/data/choline/choln02.pdf (accessed on 27 August 2025).
- Corbin, K.D.; Zeisel, S.H. Choline metabolism provides novel insights into nonalcoholic fatty liver disease and its progression. Curr. Opin. Gastroenterol. 2018, 28, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Brunst, K.J.; Wright, R.O.; DiGioia, K.; Enlow, M.B.; Fernandez, H.; Wright, R.J.; Kannan, S. Racial/ethnic and sociodemographic factors associated with micronutrient intakes and inadequacies among pregnant women in an urban US population. Public Health Nutr. 2014, 17, 1960–1970. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.; Oktayani, P.P.I.; Lee, S.-D.; Huang, L.-C. Choline in pregnant women: A systematic review and meta-analysis. Nutr. Rev. 2025, 83, e273–e289. [Google Scholar] [CrossRef]
- Da Costa, K.-A.; Kozyreva, O.G.; Song, J.; Galanko, J.A.; Fischer, L.M.; Zeisel, S.H. Common genetic polymorphisms affect the human requirement for the nutrient choline. FASEB J. 2006, 20, 1336–1344. [Google Scholar] [CrossRef]
- Wortmann, S.B.; Mayr, J.A. Choline-related-inherited metabolic diseases-A mini review. J. Inherit. Metab. Dis. 2019, 42, 237–242. [Google Scholar] [CrossRef]
- Ganz, A.B.; Klatt, K.C.; Caudill, M.A. Common Genetic Variants Alter Metabolism and Influence Dietary Choline Requirements. Nutrients 2017, 9, 837. [Google Scholar] [CrossRef]
- Vanek, V.W.; Borum, P.; Buchman, A.; Fessler, T.A.; Howard, L.; Jeejeebhoy, K.; Kochevar, M.; Shenkin, A.; Valentine, C.J.; Novel Nutrient Task Force, Parenteral Multi-Vitamin and Multi–Trace Element Working Group; et al. A.S.P.E.N. position paper: Recommendations for changes in commercially available parenteral multivitamin and multi-trace element products. Nutr. Clin. Pract. 2012, 27, 440–449. [Google Scholar] [CrossRef]
- Nurk, E.; Refsum, H.; Bjelland, I.; Drevon, C.A.; Tell, G.S.; Ueland, P.M.; Vollset, S.E.; Engedal, K.; Nygaard, H.A.; Smith, D.A. Plasma free choline, betaine and cognitive performance: The Hordaland Health Study. Br. J. Nutr. 2013, 109, 511–519. [Google Scholar] [CrossRef]
- Eslami, M.; Alibabaei, F.; Babaeizad, A.; Banihashemian, S.Z.; Mazandarani, M.; Hoseini, A.; Ramezankhah, M.; Oksenych, V.; Yousefi, B. The Importance of Gut Microbiota on Choline Metabolism in Neurodegenerative Diseases. Biomolecules 2024, 14, 1345. [Google Scholar] [CrossRef]
- Buchman, A.L.; Dubin, M.D.; Moukarzel, A.A.; Jenden, D.J.; Roch, M.; Rice, K.M.; Gornbein, J.; Ament, M.E. Choline deficiency: A cause of hepatic steatosis during parenteral nutrition that can be reversed with intravenous choline supplementation. Hepatology 1995, 22, 1399–1403. [Google Scholar] [CrossRef]
- Cochrane, K.M.; Hutcheon, J.A.; Karakochuk, C.D. Supplementation practices among pregnant women and those trying to conceive: A population-representative survey in Vancouver, Canada. Appl. Physiol. Nutr. Metab. 2024, 49, 1495–1506. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; DuGar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.-M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Zhen, J.; Zhou, Z.; He, M.; Han, H.-X.; Lv, E.-H.; Wen, P.-B.; Liu, X.; Wang, Y.-T.; Cai, X.-C.; Tian, J.-Q.; et al. The gut microbial metabolite trimethylamine N-oxide and cardiovascular diseases. Front. Endocrinol. 2023, 14, 1085041. [Google Scholar] [CrossRef] [PubMed]
- Cameron, S.J.; Li, X.S.; Benson, T.W.; Conrad, K.A.; Wang, Z.; Fleifil, S.; Maegdefessel, L.; Mani, K.; Björck, M.; Scalise, A.; et al. Circulating Trimethylamine N-Oxide and Growth Rate of Abdominal Aortic Aneurysms and Surgical Risk. JAMA Cardiol. 2025, 10, 1000–1009. [Google Scholar] [CrossRef]
- Budoff, M.J.; de Oliveira Otto, M.C.; Li, X.S.; Lee, Y.; Wang, M.; Lai, H.T.M.; Lemaitre, R.N.; Pratt, A.; Tang, W.H.W.; Psaty, B.M.; et al. Trimethylamine-N-oxide (TMAO) and risk of incident cardiovascular events in the multi ethnic study of Atherosclerosis. Sci. Rep. 2025, 15, 23362. [Google Scholar] [CrossRef]
- Quinville, B.M.; Deschenes, N.M.; Ryckman, A.E.; Walia, J.S. A Comprehensive Review: Sphingolipid Metabolism and Implications of Disruption in Sphingolipid Homeostasis. Int. J. Mol. Sci. 2021, 22, 5793. [Google Scholar] [CrossRef]
- Li, S.; Xie, T.; Liu, P.; Wang, L.; Gong, X. Structural insights into the assembly and substrate selectivity of human SPT–ORMDL3 complex. Nat. Struct. Mol. Biol. 2021, 28, 249–257. [Google Scholar] [CrossRef]
- Park, K.-H.; Ye, A.-W.; Zhang, J.; Hammad, S.M.; Townsend, D.M.; Rockey, D.C.; Kim, S.-H. 3-ketodihydrosphingosine reductase mutation induces steatosis and hepatic injury in zebrafish. Sci. Rep. 2019, 9, 1138. [Google Scholar] [CrossRef]
- McMaster, C.R. From yeast to humans—Roles of the Kennedy pathway for phosphatidylcholine synthesis. FEBS Lett. 2018, 592, 1256–1272. [Google Scholar] [CrossRef]
- Zöller, I.; Meixner, M.M.; Hartmann, D.; Büssow, H.; Meyer, R.; Gieselmann, V.; Eckhardt, M. Absence of 2-hydroxylated sphingolipids is compatible with normal neural development but causes late-onset axon and myelin sheath degeneration. J. Neurosci. 2008, 28, 9741–9754. [Google Scholar] [CrossRef]
- Li, C.-Z.; Wu, L.-M.; Zhu, C.-X.; Du, H.-Y.; Chen, G.-X.; Yang, F. The impacts of dietary sphingomyelin supplementation on metabolic parameters of healthy adults: A systematic review and meta-analysis of randomized controlled trials. Front. Nutr. 2024, 11, 1363077. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Venkataraman, A.; Sieber, J.R.; Schmidt, A.W.; Waldron, C.; Theis, K.R.; Schmidt, T.M. Variable responses of human microbiomes to dietary supplementation with resistant starch. Microbiome 2016, 4, 33. [Google Scholar] [CrossRef] [PubMed]
- Alfa, M.J.; Strang, D.; Tappia, P.S.; Graham, M.; Van Domselaar, G.; Forbes, J.D.; Laminman, V.; Olson, N.; DeGagne, P.; Bray, D.; et al. A randomized trial to determine the impact of a digestion resistant starch composition on the gut microbiome in older and mid-age adults. Clin. Nutr. 2018, 37, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Bush, J.R.; Baisley, J.; Harding, S.V.; Alfa, M.J. Consumption of Solnul Resistant Potato Starch Produces a Prebiotic Effect in a Randomized, Placebo-Controlled Clinical Trial. Nutrients 2023, 15, 1582. [Google Scholar] [CrossRef]
- Bush, J.R.; Han, J.; Deehan, E.C.; Harding, S.V.; Maiya, M.; Baisley, J.; Schibli, D.; Goodlett, D.R. Resistant potato starch supplementation reduces serum histamine levels in healthy adults with links to attenuated intestinal permeability. J. Funct. Foods 2023, 108, 105740. [Google Scholar] [CrossRef]
- Alfa, M.J.; Strang, D.; Tappia, P.S.; Olson, N.; DeGagne, P.; Bray, D.; Murray, B.-L.; Hiebert, B. A Randomized Placebo Controlled Clinical Trial to Determine the Impact of Digestion Resistant Starch MSPrebiotic on Glucose, Insulin, and Insulin Resistance in Elderly and Mid-Age Adults. Front. Med. 2018, 4, 260. [Google Scholar] [CrossRef]
- Bush, J.R.; Iwuamadi, I.; Han, J.; Schibli, D.J.; Goodlett, D.R.; Deehan, E.C. Resistant Potato Starch Supplementation Reduces Serum Free Fatty Acid Levels and Influences Bile Acid Metabolism. Metabolites 2024, 14, 536. [Google Scholar] [CrossRef]
- Bergeron, N.; Williams, P.T.; Lamendella, R.; Faghihnia, N.; Grube, A.; Li, X.; Wang, Z.; Knight, R.; Jansson, J.K.; Hazen, S.L.; et al. Diets high in resistant starch increase plasma levels of trimethylamine-N-oxide, a gut microbiome metabolite associated with CVD risk. Br. J. Nutr. 2016, 116, 2020–2029. [Google Scholar] [CrossRef] [PubMed]
- Kemp, J.A.; Dos Santos, H.F.; de Jesus, H.E.; Esgalhado, M.; de Paiva, B.R.; Azevedo, R.; Stenvinkel, P.; Bergman, P.; Lindholm, B.; Ribeiro-Alves, M.; et al. Resistant Starch Type-2 Supplementation Does Not Decrease Trimethylamine N-Oxide (TMAO) Plasma Level in Hemodialysis Patients. J. Am. Nutr. Assoc. 2022, 41, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Baxter, N.T.; Schmidt, A.W.; Venkataraman, A.; Kim, K.S.; Waldron, C.; Schmidt, T.M. Dynamics of Human Gut Microbiota and Short-Chain Fatty Acids in Response to Dietary Interventions with Three Fermentable Fibers. mBio 2019, 10, e02566-18. [Google Scholar] [CrossRef] [PubMed]
- Deehan, E.C.; Yang, C.; Perez-Muñoz, E.M.; Nguyen, N.K.; Cheng, C.C.; Triador, L.; Zhang, Z.; Bakal, J.A.; Walter, J. Precision Microbiome Modulation with Discrete Dietary Fiber Structures Directs Short-Chain Fatty Acid Production. Cell Host Microbe 2020, 27, 389–404. [Google Scholar] [CrossRef]
- Bush, J.R.; Alfa, M.J. Consumption of resistant potato starch produces changes in gut microbiota that correlate with improvements in abnormal bowel symptoms: A secondary analysis of a clinical trial. BMC Nutr. 2024, 10, 152. [Google Scholar] [CrossRef]
- Mo, C.; Lou, X.; Xue, J.; Shi, Z.; Zhao, Y.; Wang, F.; Chen, G. The influence of Akkermansia muciniphila on intestinal barrier function. Gut Pathog. 2024, 16, 41. [Google Scholar] [CrossRef]
- Zhu, Z.; Gregory, J.C.; Org, E.; Buffa, J.A.; Gupta, N.; Wang, Z.; Li, L.; Fu, X.; Wu, Y.; Mehrabian, M.; et al. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell 2016, 165, 111–124. [Google Scholar] [CrossRef]
- Kawashima, K.; Fujii, T. Basic and clinical aspects of non-neuronal acetylcholine: Overview of non-neuronal cholinergic systems and their biological significance. J. Pharmacol. Sci. 2008, 106, 167–173. [Google Scholar] [CrossRef]
- Genoni, A.; Christophersen, C.T.; Lo, J.; Coghlan, M.; Boyce, M.C.; Bird, A.R.; Lyons-Wall, P.; Devine, A. Long-term Paleolithic diet is associated with lower resistant starch intake, different gut microbiota composition and increased serum TMAO concentrations. Eur. J. Nutr. 2020, 59, 1845–1858. [Google Scholar] [CrossRef]
- Genoni, A.; Lo, J.; Lyons-Wall, P.; Boyce, M.C.; Christophersen, C.T.; Bird, A.; Devine, A. A Paleolithic diet lowers resistant starch intake but does not affect serum trimethylamine-N-oxide concentrations in healthy women. Br. J. Nutr. 2019, 121, 322–329. [Google Scholar] [CrossRef]
- Lee, G.; Choi, S.; Chang, J.; Choi, D.; Son, J.S.; Kim, K.; Kim, S.M.; Jeong, S.; Park, S.M. Association of L-α Glycerylphosphorylcholine With Subsequent Stroke Risk After 10 Years. JAMA Netw. Open 2021, 4, e2136008. [Google Scholar] [CrossRef]
- Onono, F.O.; Morris, A.J. Phospholipase D and Choline Metabolism. Lipid Signal. Hum. Dis. 2020, 259, 205–218. [Google Scholar] [CrossRef]
- Bush, J.R.; Han, J.; Goodlett, D.R. Resistant Potato Starch Supplementation Increases Serum Antioxidant Levels in a Randomized Trial. Metabolites 2025, 15, 661. [Google Scholar] [CrossRef]
- Gault, C.R.; Obeid, L.M.; Hannun, Y.A. Chalfant, C., Del Poeta, M., Eds.; An Overview of Sphingolipid Metabolism: From Synthesis to Breakdown. In Sphingolipids as Signaling and Regulatory Molecules, 1st ed.; Springer: New York, NY, USA, 2010; Volume 688, pp. 1–23. [Google Scholar] [CrossRef]
- Harada, S.; Taketomi, Y.; Aiba, T.; Kawaguchi, M.; Hirabayashi, T.; Uranbileg, B.; Kurano, M.; Yatomi, Y.; Murakami, M. The Lysophospholipase PNPLA7 Controls Hepatic Choline and Methionine Metabolism. Biomolecules 2023, 13, 471. [Google Scholar] [CrossRef]
- Staellberg-Stenhagen, S.; Svennerholm, L. Fatty acid composition of human brain sphingomyelins: Normal variation with age and changes during myelin disorders. J Lipid Res 1965, 6, 146–155. [Google Scholar]
- Potter, K.A.; Kern, M.J.; Fullbright, G.; Bielawski, J.; Scherer, S.S.; Yum, S.W.; Li, J.J.; Cheng, H.; Han, X.; Venkata, J.K.; et al. Central nervous system dysfunction in a mouse model of FA2H deficiency. Glia 2011, 59, 1009–1021. [Google Scholar] [CrossRef] [PubMed]
- Padilha, M.; Ferreira, A.L.L.; Normando, P.; Schincaglia, R.M.; Freire, S.R.; Keller, V.N.; Figueiredo, A.C.C.; Yin, X.; Brennan, L.; Kac, G. Maternal serum amino acids and hydroxylated sphingomyelins at pregnancy are associated with anxiety symptoms during pregnancy and throughout the first year after delivery. J. Affect. Disord. 2024, 351, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Czysz, A.H.; South, C.; Gadad, B.S.; Arning, E.; Soyombo, A.; Bottiglieri, T.; Trivedi, M.H. Can targeted metabolomics predict depression recovery? Results from the CO-MED trial. Transl. Psychiatry 2019, 9, 11. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bush, J.R.; Han, J.; Goodlett, D.R. Resistant Potato Starch Supplementation Increases the Serum Levels of Choline and Sphingomyelins Without Affecting Trimethylamine Oxide Levels. Metabolites 2025, 15, 662. https://doi.org/10.3390/metabo15100662
Bush JR, Han J, Goodlett DR. Resistant Potato Starch Supplementation Increases the Serum Levels of Choline and Sphingomyelins Without Affecting Trimethylamine Oxide Levels. Metabolites. 2025; 15(10):662. https://doi.org/10.3390/metabo15100662
Chicago/Turabian StyleBush, Jason R., Jun Han, and David R. Goodlett. 2025. "Resistant Potato Starch Supplementation Increases the Serum Levels of Choline and Sphingomyelins Without Affecting Trimethylamine Oxide Levels" Metabolites 15, no. 10: 662. https://doi.org/10.3390/metabo15100662
APA StyleBush, J. R., Han, J., & Goodlett, D. R. (2025). Resistant Potato Starch Supplementation Increases the Serum Levels of Choline and Sphingomyelins Without Affecting Trimethylamine Oxide Levels. Metabolites, 15(10), 662. https://doi.org/10.3390/metabo15100662






