Untargeted Metabolomics Reveals Distinct Serum Metabolic Profiles in Avian Influenza Occupational Exposure Populations
Abstract
1. Introduction
2. Experimental Section
2.1. Study Subjects and Inclusion Criteria
2.2. Sample Pretreatment
2.3. Instrument Analysis
2.4. Data Analysis and Mining
3. Results and Discussion
3.1. Clinical Characteristic of Subjects
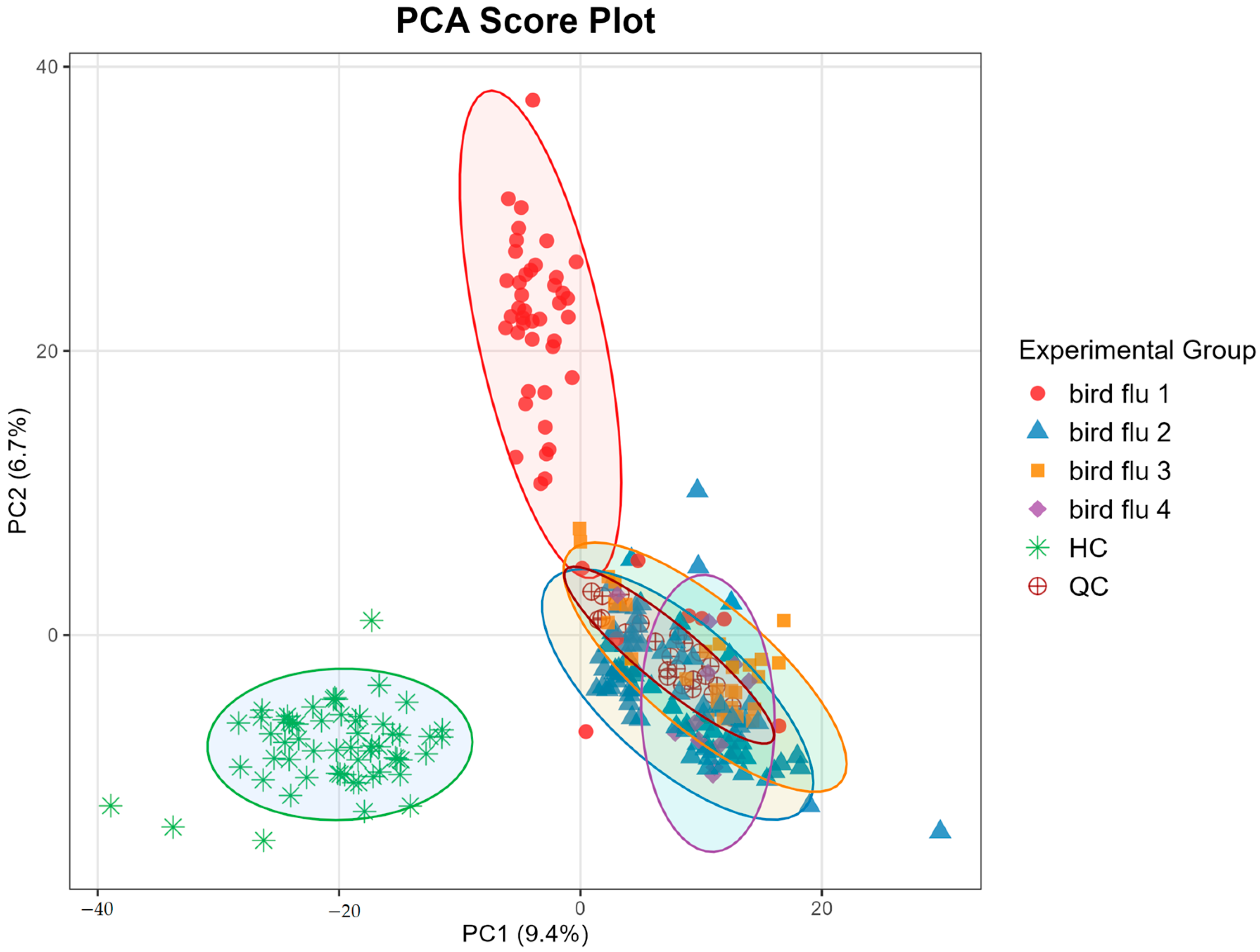
3.2. Metabolomic Analysis
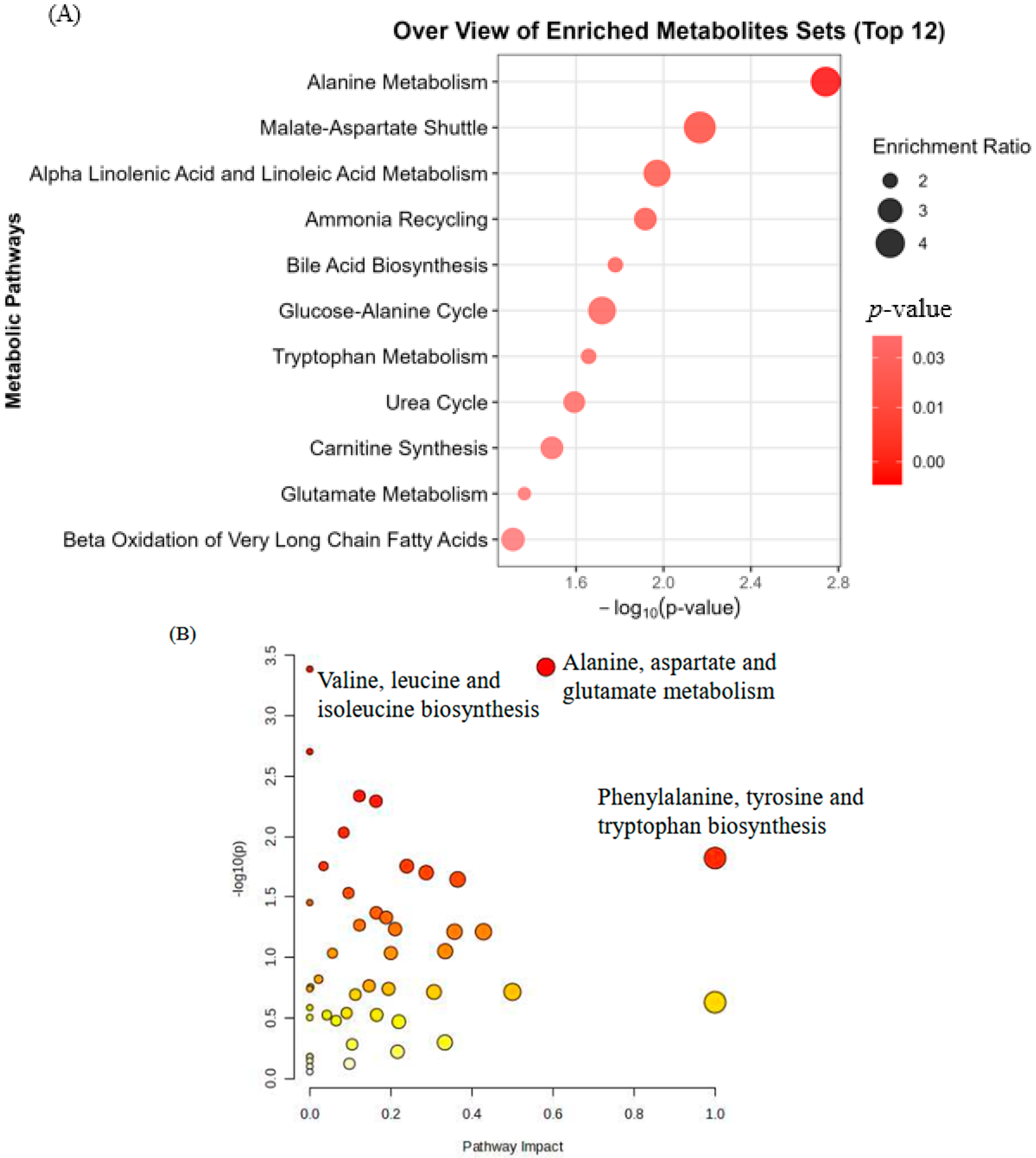
3.3. Enrichment and Pathway Analysis
3.4. Biological Pathway Insights
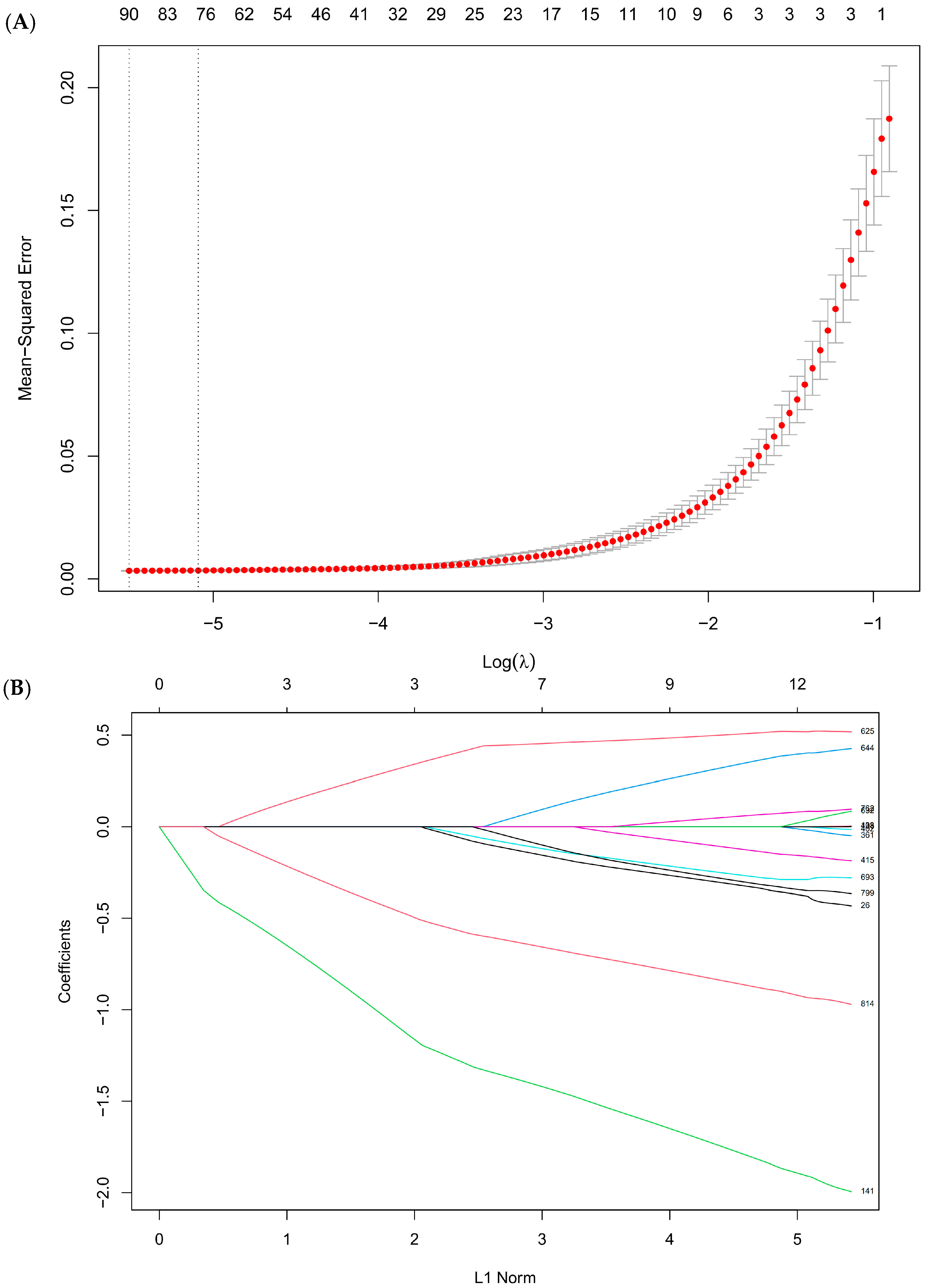
3.5. Key Metabolites Identification by Machine Learning
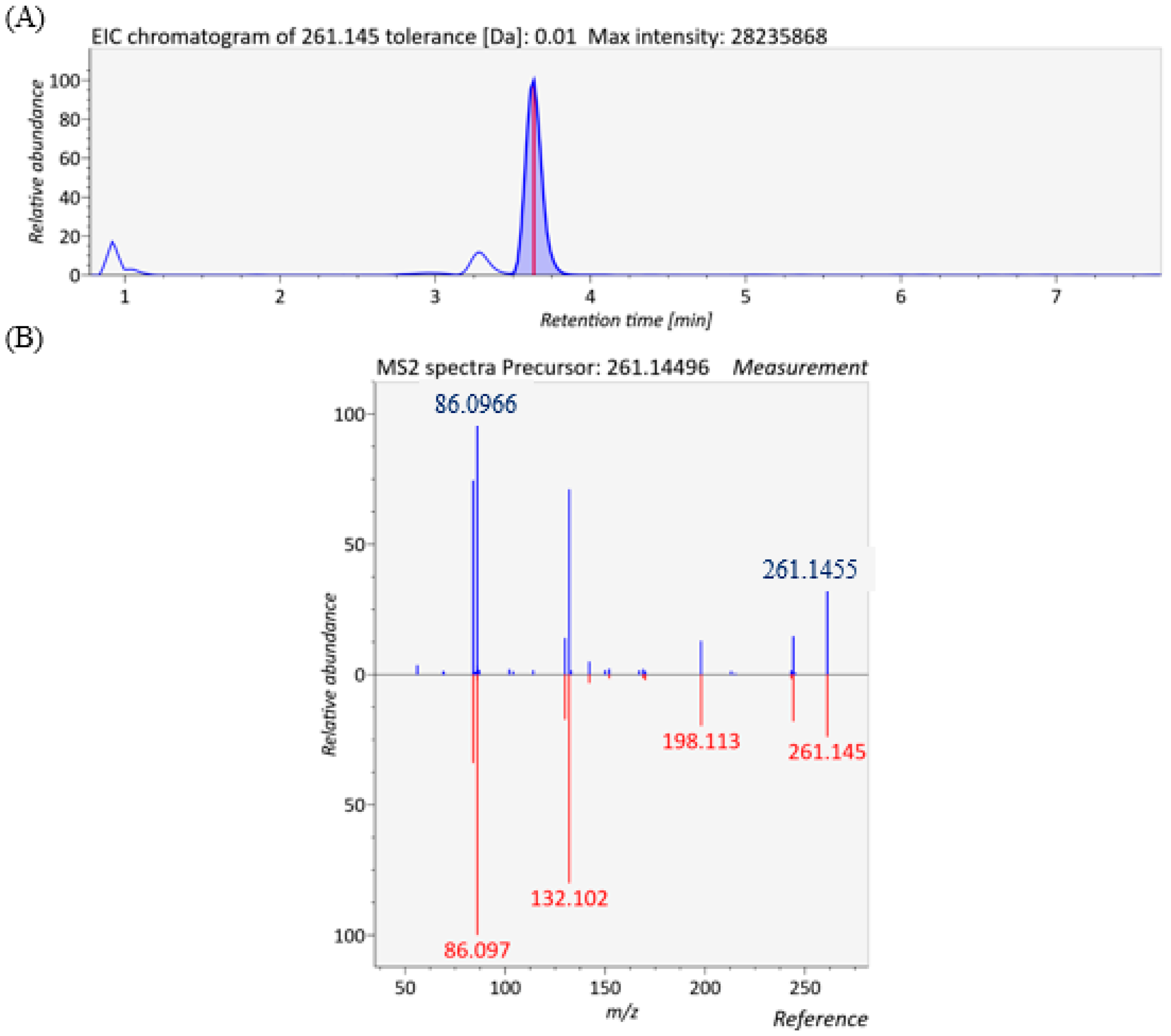
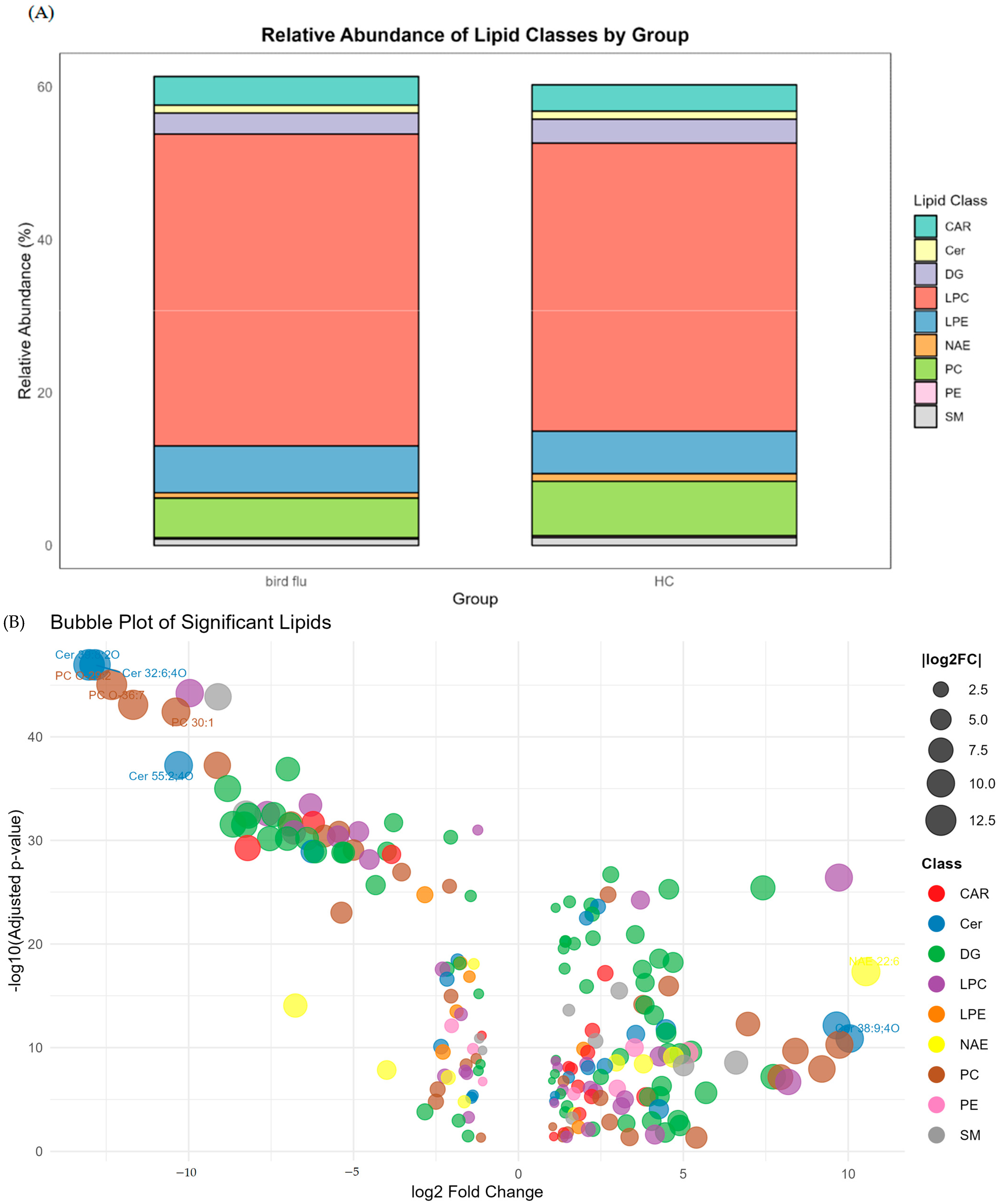
3.6. Lipidomic Analysis
4. Limitation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Declaration of Generative AI and AI-Assisted Technologies in the Writing Process
References
- Wong, S.S.; Yuen, K.Y. Avian influenza virus infections in humans. Chest 2006, 129, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Pardo-Roa, C.; Nelson, M.I.; Ariyama, N.; Aguayo, C.; Almonacid, L.I.; Gonzalez-Reiche, A.S.; Munoz, G.; Ulloa, M.; Avila, C.; Navarro, C.; et al. Cross-species and mammal-to-mammal transmission of clade 2.3.4.4b highly pathogenic avian influenza A/H5N1 with PB2 adaptations. Nat. Commun. 2025, 16, 2232. [Google Scholar] [CrossRef] [PubMed]
- Poovorawan, Y.; Pyungporn, S.; Prachayangprecha, S.; Makkoch, J. Global alert to avian influenza virus infection: From H5N1 to H7N9. Pathog. Glob. Health 2013, 107, 217–223. [Google Scholar] [CrossRef]
- De Marco, M.A.; Delogu, M.; Facchini, M.; Di Trani, L.; Boni, A.; Cotti, C.; Graziosi, G.; Venturini, D.; Regazzi, D.; Ravaioli, V.; et al. Serologic Evidence of Occupational Exposure to Avian Influenza Viruses at the Wildfowl/Poultry/Human Interface. Microorganisms 2021, 9, 2153. [Google Scholar] [CrossRef]
- Condor, A.M.; Kui, A.I.; Buduru, S.D.; Negucioiu, M.; Condor, D.C.; Lucaciu, P.O. Metabolomics Analysis as a Tool in Periodontitis Diagnosis: A Systematic Review. Clin. Exp. Dent. Res. 2025, 11, e70095. [Google Scholar] [CrossRef]
- Han, J.; Datla, R.; Chan, S.; Borchers, C.H. Mass spectrometry-based technologies for high-throughput metabolomics. Bioanalysis 2009, 1, 1665–1684. [Google Scholar] [CrossRef]
- Dunn, W.B.; Broadhurst, D.; Begley, P.; Zelena, E.; Francis-McIntyre, S.; Anderson, N.; Brown, M.; Knowles, J.D.; Halsall, A.; Haselden, J.N.; et al. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2011, 6, 1060–1083. [Google Scholar] [CrossRef] [PubMed]
- Takeda, H.; Matsuzawa, Y.; Takeuchi, M.; Takahashi, M.; Nishida, K.; Harayama, T.; Todoroki, Y.; Shimizu, K.; Sakamoto, N.; Oka, T.; et al. MS-DIAL 5 multimodal mass spectrometry data mining unveils lipidome complexities. Nat. Commun. 2024, 15, 9903. [Google Scholar] [CrossRef]
- Pang, Z.; Lu, Y.; Zhou, G.; Hui, F.; Xu, L.; Viau, C.; Spigelman, A.F.; MacDonald, P.E.; Wishart, D.S.; Li, S.; et al. MetaboAnalyst 6.0: Towards a unified platform for metabolomics data processing, analysis and interpretation. Nucleic Acids Res. 2024, 52, W398–W406. [Google Scholar] [CrossRef]
- Chandel, N.S. Amino Acid Metabolism. Cold Spring Harb. Perspect. Biol. 2021, 13, a040584. [Google Scholar] [CrossRef]
- Palmer, C.S. Innate metabolic responses against viral infections. Nat. Metab. 2022, 4, 1245–1259. [Google Scholar] [CrossRef]
- Ye, Z.; Wang, S.; Zhang, C.; Zhao, Y. Coordinated Modulation of Energy Metabolism and Inflammation by Branched-Chain Amino Acids and Fatty Acids. Front. Endocrinol. 2020, 11, 617. [Google Scholar] [CrossRef]
- Nie, C.; He, T.; Zhang, W.; Zhang, G.; Ma, X. Branched Chain Amino Acids: Beyond Nutrition Metabolism. Int. J. Mol. Sci. 2018, 19, 954. [Google Scholar] [CrossRef]
- Bak, L.K.; Obel, L.F.; Walls, A.B.; Schousboe, A.; Faek, S.A.; Jajo, F.S.; Waagepetersen, H.S. Novel model of neuronal bioenergetics: Postsynaptic utilization of glucose but not lactate correlates positively with Ca2+ signalling in cultured mouse glutamatergic neurons. ASN Neuro. 2012, 4, AN20120004. [Google Scholar] [CrossRef]
- Reitzer, L. Biosynthesis of Glutamate, Aspartate, Asparagine, L-Alanine, and D-Alanine. EcoSal Plus 2004, 1. [Google Scholar] [CrossRef]
- Li, K.; Wei, X.; Jiao, X.; Deng, W.; Li, J.; Liang, W.; Zhang, Y.; Yang, J. Glutamine Metabolism Underlies the Functional Similarity of T Cells between Nile Tilapia and Tetrapod. Adv. Sci. 2023, 10, e2201164. [Google Scholar] [CrossRef]
- Patel, D.; Menon, D.; Bernfeld, E.; Mroz, V.; Kalan, S.; Loayza, D.; Foster, D.A. Aspartate Rescues S-phase Arrest Caused by Suppression of Glutamine Utilization in KRas-driven Cancer Cells. J. Biol. Chem. 2016, 291, 9322–9329. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz, M.; Solaymani-Mohammadi, F.; Namdari, H.; Arjeini, Y.; Mousavi, M.J.; Rezaei, F. Metabolic host response and therapeutic approaches to influenza infection. Cell. Mol. Biol. Lett. 2020, 25, 15. [Google Scholar] [CrossRef]
- Seo, S.K.; Kwon, B. Immune regulation through tryptophan metabolism. Exp. Mol. Med. 2023, 55, 1371–1379. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.W.; Feng, G.S. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB J. 1991, 5, 2516–2522. [Google Scholar] [CrossRef] [PubMed]
- Schultz, D.; Cuypers, F.; Skorka, S.B.; Rockstroh, J.; Gesell Salazar, M.; Krieger, J.; Albrecht, D.; Volker, U.; Hammerschmidt, S.; Lalk, M.; et al. Bioactive lipid screening during respiratory tract infections with bacterial and viral pathogens in mice. Metabolomics 2022, 18, 39. [Google Scholar] [CrossRef]
- Schultz, D.; Surabhi, S.; Stelling, N.; Rothe, M.; Group, K.S.; Methling, K.; Hammerschmidt, S.; Siemens, N.; Lalk, M. 16HBE Cell Lipid Mediator Responses to Mono and Co-Infections with Respiratory Pathogens. Metabolites 2020, 10, 113. [Google Scholar] [CrossRef]
- Shi, X.; Hua, S.; Chen, Z.; Cao, W.; Xiao, M.; Pei, W.; Cao, Z.; Zhang, Z.; Yang, H.; Shao, X.; et al. Characterization of serum metabolome and respiratory microbiota in children with influenza A virus infection. Front. Cell. Infect. Microbiol. 2024, 14, 1478876. [Google Scholar] [CrossRef]
- Yue, Y.; Li, Q.; Chen, C.; Yang, J.; Song, W.; Zhou, C.; Cui, Y.; Wei, Z.; He, Q.; Wang, C.; et al. Purine nucleoside phosphorylase dominates Influenza A virus replication and host hyperinflammation through purine salvage. Signal Transduct. Target. Ther. 2025, 10, 191. [Google Scholar] [CrossRef]
- Valdes, A.; Moreno, L.O.; Rello, S.R.; Orduna, A.; Bernardo, D.; Cifuentes, A. Metabolomics study of COVID-19 patients in four different clinical stages. Sci. Rep. 2022, 12, 1650. [Google Scholar] [CrossRef] [PubMed]
- Knuplez, E.; Marsche, G. An Updated Review of Pro- and Anti-Inflammatory Properties of Plasma Lysophosphatidylcholines in the Vascular System. Int. J. Mol. Sci. 2020, 21, 4501. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Ohto, T.; Kita, Y. Cytosolic phospholipase A2: Biochemical properties and physiological roles. IUBMB Life 2006, 58, 328–333. [Google Scholar] [CrossRef]
- Delcheva, G.; Stefanova, K.; Stankova, T. Ceramides-Emerging Biomarkers of Lipotoxicity in Obesity, Diabetes, Cardiovascular Diseases, and Inflammation. Diseases 2024, 12, 195. [Google Scholar] [CrossRef] [PubMed]
| Clinical Characteristics | Bird Flu (n = 177) | Health Control (n = 59) | p-Value |
|---|---|---|---|
| Age (years) | 44.94 ± 11.03 | 46.44 ± 9.22 | 0.307 |
| Male/female | 99/78 | 35/24 | 0.762 |
| ID | Metabolite Name | Fold Change | Regulation | p Value | Ontology |
|---|---|---|---|---|---|
| neg11330 | 9-HODE | 0.06625251 | ↓ | 2.60 × 10−25 | Lineolic acids and derivatives |
| neg12434 | FA 18:2+2O | 0.10958917 | ↓ | 2.87 × 10−21 | Lineolic acids and derivatives |
| neg12973 | 9-HETE | 0.02030262 | ↓ | 3.53 × 10−44 | Hydroxyeicosatetraenoic acids |
| neg1780 | Hypoxanthine | 5.11259294 | ↑ | 3.82 × 10−28 | Hypoxanthines |
| neg2115 | Glutamine | 2.7646163 | ↑ | 1.01 × 10−66 | Alpha amino acids |
| neg21888 | LPE 18:1 | 2.15101375 | ↑ | 0.03280666 | 1-acyl-sn-glycero-3-phosphoethanolamines |
| neg2332 | Xanthine | 2.25017518 | ↑ | 2.42 × 10−12 | Xanthines |
| pos13087 | gamma-Glutamylleucine | 0.1499749 | ↓ | 2.11 × 10−30 | Dipeptides |
| pos14218 | FA 18:3+1O | 0.09900637 | ↓ | 2.86 × 10−20 | Medium-chain fatty acids |
| pos15560 | Androstane-3,17-diol | 0.04531475 | ↓ | 6.27 × 10−16 | Androgens and derivatives |
| pos19515 | LAUROYLCARNITINE | 11.1916456 | ↑ | 4.82 × 10−20 | Acyl carnitines |
| pos21869 | Sphinganine 1-phosphate | 0.46320622 | ↓ | 7.09 × 10−31 | Phosphosphingolipids |
| pos24058 | GLYCOCHENODEOXYCHOLATE | 2.43241955 | ↑ | 7.22 × 10−11 | Glycinated bile acids and derivatives |
| pos4781 | Methionine | 3.46770679 | ↑ | 1.06 × 10−69 | Methionine and derivatives |
| pos6206 | 4-ACETAMIDOBUTANOATE | 0.13766022 | ↓ | 1.66 × 10−08 | Gamma amino acids and derivatives |
| pos6263 | 4-ureidobutanoic acid | 2.28864439 | ↑ | 3.17 × 10−39 | Gamma amino acids and derivatives |
| pos6904 | 3-Indoleacetic acid | 6.39444246 | ↑ | 5.03 × 10−15 | Indole-3-acetic acid derivatives |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, S.; Wang, L.; Su, J.; Long, C.; Mahe, M.; Gao, Z.; Liu, J. Untargeted Metabolomics Reveals Distinct Serum Metabolic Profiles in Avian Influenza Occupational Exposure Populations. Metabolites 2025, 15, 663. https://doi.org/10.3390/metabo15100663
Mao S, Wang L, Su J, Long C, Mahe M, Gao Z, Liu J. Untargeted Metabolomics Reveals Distinct Serum Metabolic Profiles in Avian Influenza Occupational Exposure Populations. Metabolites. 2025; 15(10):663. https://doi.org/10.3390/metabo15100663
Chicago/Turabian StyleMao, Shuoqin, Lei Wang, Jing Su, Caihua Long, Muti Mahe, Zhenguo Gao, and Jia Liu. 2025. "Untargeted Metabolomics Reveals Distinct Serum Metabolic Profiles in Avian Influenza Occupational Exposure Populations" Metabolites 15, no. 10: 663. https://doi.org/10.3390/metabo15100663
APA StyleMao, S., Wang, L., Su, J., Long, C., Mahe, M., Gao, Z., & Liu, J. (2025). Untargeted Metabolomics Reveals Distinct Serum Metabolic Profiles in Avian Influenza Occupational Exposure Populations. Metabolites, 15(10), 663. https://doi.org/10.3390/metabo15100663






