Abstract
The prevalence of overweight and obesity has risen dramatically in the last few years. This has led to an increase in both conditions in pregnant women. Obesity and overweight are associated with complications for both the mother and the newborn. The aim of this study is to determine the prevalence of obesity and its association with the risk of complications during pregnancy. Materials and Methods: We conducted a retrospective cohort study of pregnant women who delivered from 1 January 2012 to 31 December 2018. Results: A higher prevalence of obesity is observed in the group of women aged 35 or older. Women with a BMI > 25 present a higher risk of cesarean section (aOR 1.49, 95% CI: 1.37–1.61), preeclampsia (aOR 1.64, 95% CI: 1.19–2.26), high-risk pregnancy (aOR 2.34, 95% CI: 1.68–2.6), Apgar < 7 at one minute (aOR 1.53, 95% CI: 1.25–1.89) and macrosomia (aOR 2.08, 95% CI: 1.83–2.37). Maternal overweight and obesity are important determinants of the risk of complications for both the mother and the newborn.
1. Introduction
In recent decades, the prevalence of overweight and obesity (established as BMI 25–30 and BMI ≥ 30 kg/m2, respectively) has risen to epidemic proportions. In pregnant women, the prevalence of both conditions has also increased dramatically in both high- and middle-income countries [1], but the exact burden of overweight and obesity during pregnancy remains unclear. Some studies suggest that in 2025, more than 25% of all women in the world will suffer from obesity [2].
In Europe, estimations of the prevalence of overweight and obesity are alarming, given that most countries report figures approaching 20% in the general population [3]. In pre-pregnant women in Europe, the rates range between 26.8% and 54% [4]. In Spain, around 30% of women are overweight, and as many as 16.7% are obese [5].
Obesity during pregnancy is associated with short and long-term complications for both the mother and the newborn, such as gestational hypertension and diabetes, preeclampsia, preterm birth and increased risk of miscarriage or congenital defects [1]. Furthermore, obese pregnant women with a previous diagnosis of other diseases (such as diabetes or hypertension) have a higher risk of complications during pregnancy and childbirth [2]. For their part, children born to mothers with obesity have a greater risk of being overweight or obese both early in life and at later stages [6]. The epigenetic programming that occurs at conception and during pregnancy may predispose these children to various chronic metabolic conditions [7].
The aim of the present study was to evaluate the consequences of maternal obesity on pregnancy, childbirth and the state of the newborn in the health region of Lleida, Spain, over a 7-year period.
2. Materials and Methods
2.1. Study Design and Data Collection
From 2012 to 2018, pregnant women from the health region of Lleida participated in a retrospective observational cohort study.
Information on women who had delivered at the Arnau de Vilanova Hospital between 1 January 2012 and 31 December 2018 was obtained from the CMBD database, which compiles data on hospital discharges. Clinical histories were retrieved from the E-CAP database of the Catalan Institute of Health (ICS) for all eligible patients allocated to the primary care area, and data related to health prescriptions were also collected from the ICS database.
This article is part of the Iler Pregnancy Project carried out in the health region of Lleida, which was designed in order to evaluate the prevalence of chronic pathologies such as depression, obesity, diabetes mellitus and hypothyroidism during pregnancy and to assess adherence to the medication prescribed [8].
2.2. Study Population
All women who gave birth between 1 January 2012 and 31 December 2018 at the Arnau de Vilanova Hospital (HUAV) were included in this study. Patients who were not assigned to the area of Lleida or who were attended privately were excluded. Data were included from the date of last period until delivery (therefore, some data collected are from 2011 in the cases of women whose last period occurred before 2012).
To assess the representativeness of the sample, the number of births in our study was calculated as a percentage of the total number of births in the health region, extracted from the database of the Statistical Institute of Catalonia (Idescat) (Table 1).

Table 1.
Number of births registered in our sample compared to the total in the area of Lleida over the study period (by year).
Indicators under review were the following:
- -
- Perinatal: Age; body mass index (BMI) in kg/m2: normal weight (BMI between 18.5 and 24.9 kg/m2), overweight (BMI between 25 and 29.9 kg/m2) and obesity (BMI ≥ 30 kg/m2) [9]; number of pregnancies and multiple pregnancies; diabetes mellitus (code O24.9 in ICD-10); hypothyroidism (code EO3.9 and EO2 in ICD-10); arterial hypertension (code I10-I16 in ICD-10); dyslipidemia (code E78 in ICD-10); depression (codes F32.0-F32.9, F33.0-F33.3, F33.8, F33.9, F34.1, or F41.2 in ICD-10); and preeclampsia and level of risk during pregnancy.
- -
- Childbirth: Method of delivery (vaginal delivery vs. caesarean section); preterm birth (<37 weeks of pregnancy) or prolonged.
- -
- Neonatal: Birth weight (underweight < 2500 g, normal weight 2500–4000 g and macrosomia > 4000 g); Apgar test score at 1 min and at 5 min (good Apgar score if ≥7 points, poor Apgar score < 7 points).
2.3. Data Analysis
A descriptive analysis was performed using absolute and relative frequencies for categorical variables and means and standard deviations for numerical variables. To assess the differences between groups, the Chi-squared test was used for categorical variables and the Student t-test for numerical variables.
Multivariate logistic regression was used to assess the association between obesity and overweight and the perinatal, labour and neonatal complications, calculating odds ratios (OR) with the respective 95% confidence intervals. Statistical significance was defined as p < 0.05.
2.4. Ethical Aspects
This study received approval from the ethics and clinical research committee of the Jordi Gol Primary Care Research Institute (IDIAP), on 29 April 2020, code no. 19/195-P, and was executed in accordance with the tenets established in the Declaration of Helsinki. Data were obtained from electronic medical reports recorded in the E-CAP database and were collected by the Department of Health Evaluation and Research Management Unit. Informed consent was not required. Furthermore, the information from E-CAP was processed anonymously and confidentially as required by national legislation and in Regulation 2016/679 of the European Parliament and of the Council for the protection of individuals.
3. Results
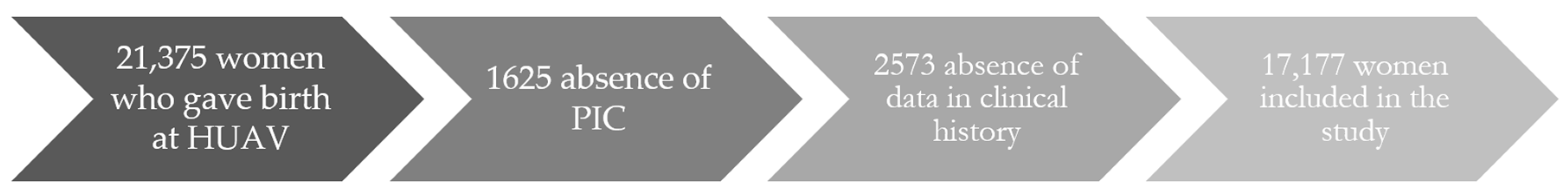
The initial sample comprised 21,375 women who had delivered a child at the HUAV from 2012 to 2018. Of these, 1625 patients were not included due to the absence of a personal identification code (PIC), and 2573 others were excluded because of the absence of data in their clinical history. The final sample thus comprised 17,177 patients (Figure 1).

Figure 1.
Study sample.
Table 2 shows that the presence of obesity and overweight was significantly affected by maternal age. Obesity was present in 10.7% of pregnant women under the age of 30 and in 13.2% of those aged 35 or older. Regarding the relationship between BMI and maternal complications, preeclampsia was recorded in 1.7% of patients compared to a rate of 0.8% in patients with BMI < 25 kg/m2. As for the effects on the neonate, caesarean delivery was performed in 15.3% of women with normal weight compared to a rate of 26.4% in the group with BMI > 30 kg/m2. Additionally, the percentage of pregnancies classified as high risk was 1.8% in patients with BMI < 25 as opposed to 3.3% in the group with BMI > 30.

Table 2.
Obstetric and socio-demographic characteristics and their association with pre-pregnancy BMI.
In reference to perinatal outcomes, fetal macrosomia was recorded in 5.1% of neonates born to women with normal weight in contrast to 11.4% of those born to women with overweight. Apgar scores one minute after birth were below 7 in 2.2% of neonates born to mothers with BMI < 25 compared to 4% in those born to mothers with BMI > 30 prior to pregnancy, and Apgar scores at five minutes were below 7 points in 0.6% of neonates of women with BMI < 25 and in 1.3% of those born to women with BMI > 30.
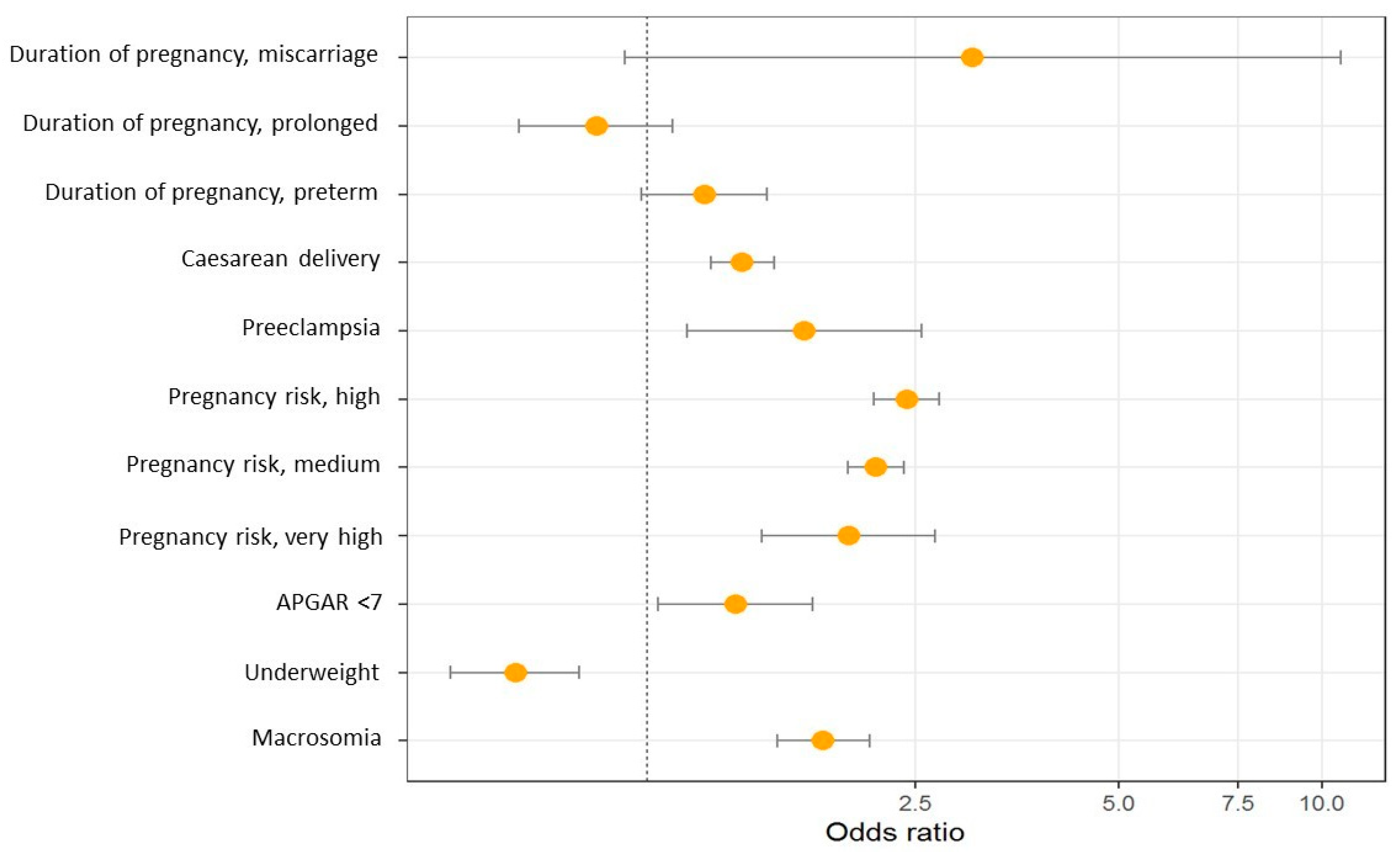
Figure 2 and Table 3 show the results of the multinomial logistic regression between obstetric characteristics and maternal BMI. Pre-pregnancy obesity is associated with an increased risk of caesarean delivery, preeclampsia, higher risk during pregnancy, macrosomia and lower Apgar score.
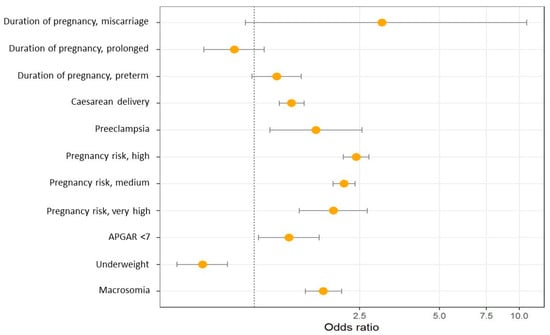
Figure 2.
Odds ratio graph between obstetric characteristics and maternal BMI.

Table 3.
Multinomial logistic regression between obstetric characteristics and maternal BMI.
4. Discussion
The results of this retrospective cohort study show that the prevalence of pre-pregnancy overweight and obesity in pregnant women is 22% and 11.7%, respectively. Women with pre-pregnancy obesity presented a higher risk of maternal and neonatal complications, including caesarean delivery (aOR 1.49, 95% CI: 1.37–1.61), preeclampsia (aOR 1.64, 95% CI: 1.19–2.26), higher risk pregnancy (aOR 2.34, 95% CI: 1.68–2.6), Apgar score < 7 at one minute (aOR 1.53, 95% CI: 1.25–1.89) and macrosomia (aOR 2.08, 95% CI: 1.83–2.37). In contrast, women with obesity had a lower risk of low birth weight (aOR 0.63, 95% CI: 0.51–0.79).
González-Plaza et al. [10] reported pre-pregnancy overweight in 18.9% of pregnant women in their study and obesity in 8.4%, while Melchor et al. [5] recorded respective figures of 25% and 13.3%, similar to those presented in our study. The European Perinatal Report found that Poland (7.1%), Slovenia (9.0%) and France (9.9%) had the lowest levels of obesity in pregnant women, while Scotland (20.7%) had the highest. Most other European countries had figures between 12 and 14%, which is in line with our study [11].
The risk of complications during pregnancy is high. Kim et al. [12] estimated the overall risk of complications in obese women to be between 18% and 47%. The meta-analysis carried out by Santos et al., with individual data from 39 cohorts, estimated that maternal overweight/obesity accounted for 23.9% of any pregnancy complication [13]. A retrospective cohort study using publicly available data from a high-income population found that the risk of fetal, neonatal or infant death increased with maternal BMI both above and below the 21–22 kg/m2 range, which is the lowest risk [14].
González-Plaza et al. [10] reported a higher frequency of maternal complications such as preeclampsia and gestational diabetes in overweight and obese pregnant women. Similar results have been published by other studies [8,15,16,17]. In an analysis of complications related to the degree of obesity, Kim et al. [12] found a four-fold increase in preeclampsia in women with grade III obesity compared to those with normal weight. Finally, a historical cohort study analysing the Spanish population reported a higher risk of preeclampsia in obese than in normal-weight women (aOR 2.199, 95% CO: 1.46–3.29) [18]. These data are consistent with the results observed in our study.
In the study by Sunder et al. [19], obese women were older, more likely to be multiparous and had a higher rate of spontaneous abortions than non-obese women. Our results do not show a significant increase in miscarriage among women with obesity, but there was a greater association with age and multiparity.
In our study, women with BMI > 25 were significantly more likely to be classified as high-risk pregnancies compared with those of normal weight. Our results corroborate those recorded in the study by Melchor et al. [5] (aOR 2.755, 95% CI: 2.46–3.08). Furthermore, weight gain during pregnancy by overweight or obese women could result in aggravating conditions for developing non-communicable diseases.
González-Plaza et al. [10] reported cesarean section in 43.9% of obese women, a percentage higher than our rate of 26.4%. Other studies record figures that range between 18.7% and 35% [20]. An analysis of the possible adverse effects of obesity and gestational diabetes mellitus showed that both (either alone or combined) were associated with a higher rate of macrosomia and a higher rate of caesarean delivery [21]. That study recorded a progressive increase in neonatal weight in the following order: lowest weight in the group without obesity or gestational diabetes; second lowest in the group with gestational diabetes but no obesity; second highest in patients with obesity but no gestational diabetes; and highest in the group with both obesity and gestational diabetes. These results may account for the relatively low percentage of underweight newborns in our study compared with previous reports [22].
Obesity is known as determinant for failured induction of labor, for emergency caesarean section and for perinatal complications [15,23,24,25]. Zhu et al. [26] reported a higher risk of Apgar score below 7 at one minute after birth in newborns of overweight women. This systematic review also demonstrated associations between Apgar < 7 at 5 min and maternal BMI: overweight (OR 1.13; 95% CI, 1.08–1.20), obesity (OR 1.40; 95% CI, 1.27–1.54) and morbid obesity (OR 1.71; 95% CI, 1.55–1.89). No association was observed between maternal BMI and umbilical cord pH. Our study showed an increased risk of Apgar < 7 at both one and five minutes.
A national analysis of preterm infants by a Swedish study demonstrates a correlation between the incidence of severe neonatal hypoxia and the mother’s rising BMI. Mothers with BMI > 35 presented a higher risk of low Apgar scores at 5 and 10 min, neonatal seizures and intraventricular haemorrhage grade 1–4 compared to those with BMI < 25. A greater risk of low Apgar scores at 5 and 10 min was also associated with the group of BMI 30 to 35 [27]. In a retrospective cohort study conducted in the USA with a population of 9,282,486 pairs of mothers and children, an increased risk of low Apgar score was also observed in cases of overweight (OR (95% CI) of 1.12 (1.11–1.14)), as well as in grade 1 obesity (1.21 (1.19–1.23)), grade 2 obesity (1.34 (1.31–1.36)) and grade 3 obesity (1.55 (1.51–1.58)) [28].
In another study by Vinturache et al. [29], an increased risk of macrosomia was observed in children of overweight and obese women compared to those born to women of normal weight. Similar results were reported by Gaudet et al. [30], with a prevalence of fetal macrosomia of 15.8% compared to 9.3% in those born to mothers with normal weight [31].
The results of a meta-analysis including 21 studies found that pre-pregnancy overweight and obesity increased the risk of macrosomia (OR, 1.67; 95% CI, 1.42–1.97; and OR, 3.23; 95% CI, 2.39–4.37) and subsequent offspring overweight and obesity (OR, 1.95; 95% CI, 1.77–2.13; and OR, 3.06; 95% CI, 2.68–3.49) [32]. Maternal overweight/obesity and gestational diabetes mellitus were considered in different studies as the main determinants of fetal macrosomia [33]. In a study that evaluates the possible influence of gestational diabetes mellitus as a potential mediator, the risk of macrosomia in overweight women is estimated at 40% [34].
We found that maternal pre-pregnancy overweight and obesity only increased the odds of macrosomia rather than low birthweight (aOR 0.63, 95% CI: 0.51–0.79), which was consistent with the conclusions of some meta-analyses [32,35]. Unlike our results, in other studies, both macrosomia and low birth weight of the newborn are related to maternal obesity [35,36], and large-for-gestational-age or small-for-gestational-age is also related to maternal obesity [37]. Different characteristics of the population, sample size or different covariates may account for the differences observed in our study. A prospective Polish cohort of 912 pregnant women was conducted, and newborn weight was analysed based on pre-pregnancy body mass index (BMI). Compared to women with normal BMI, women with maternal obesity were at a three-fold higher risk of macrosomia (AOR = 3.21 (1.69–6.1), p < 0.001). In this study, the prevalence of macrosomia was 10.6%, higher than the prevalence of low birth weight, which was found to be 6.6%. Higher maternal BMI values were associated with macrosomia, low birth weight and fetal growth restriction [22].
The mechanisms that cause complications in obese mothers during pregnancy are not well known. Factors such as endothelial dysfunction, lipotoxicity, inflammation or infection, insulin resistance or oxidative stress have been explored [23,38]. The excessive fat accumulation in obesity is caused by an energy imbalance that generates signalling through the TNF family cytokines that mediate cell death and inflammation within adipose tissue, eventually resulting in lipid leakage, glucotoxicity and insulin resistance [39]. In addition, elevated levels of these mediators (such as tumour necrosis factor or interleukin 6) cause placental inflammation, with detrimental effects on fetal metabolism, and increase the risk of metabolic syndrome and obesity in the long term [40].
The limitations of this study include the absence of data regarding obesity risk factors such as diet, physical activity or the social environment, which would have allowed us to refine our analysis. Other maternal factors that were not included in the study but might have influenced the gestational outcomes would be age, race or ethnicity, gestational weight gain or multiparity.
Another limitation of this study is the lack of information on some of the participants in our study, for example, newborns who were not weighed or Apgar-tested or women whose pregnancies were privately monitored. However, due to the Spanish National Health System’s universal coverage, the estimated representation of these participants is approximately 2.2% of the total. Therefore, they are unlikely to have influenced the study results.
5. Conclusions
Maternal obesity prior to pregnancy is associated with an increase in complications for both the mother and the newborn. Preventive strategies for promoting a healthy BMI before pregnancy can help to reduce the burden of complications in pregnancy and, ultimately, the morbidity for the mother and the newborn. Detecting overweight and obesity prior to pregnancy can help to implement health strategies, such as advising on healthy lifestyles or appropriate levels of physical activity, with interventions adapted to each individual characteristic.
Further studies are needed to evaluate public health and healthcare interventions that can help women achieve optimal health conditions during pregnancy and postpartum and thus prevent complications. Moreover, additional studies should be carried out to investigate the multifactorial origin of obesity and all the variables that may influence body weight, such as socio-demographic factors, religion or lifestyle.
Author Contributions
All the authors included (M.O., M.L., M.C.S., J.S., D.P. and B.S.-R.) contributed to conceiving and designing the study. Materials were prepared and data was collected and analysed by M.O., M.L., J.S., D.P. and M.C.S. The manuscript was first drafted by M.L. and M.O. All authors provided comments on subsequent versions. All authors have read and agreed to the published version of the manuscript.
Funding
Jordi Gol and Gurina Foundation funded the APC of this study, grant no. 2401, awarded on 10 January 2023.
Institutional Review Board Statement
This study received approval from the ethics and clinical research committee of the Jordi Gol Primary Care Research Institute (IDIAP), code no. 19/195-P (11 January 2024), and was executed in accordance with the tenets established in the Declaration of Helsinki.
Informed Consent Statement
The pseudonymised retrospective descriptive cross-sectional survey complies with additional requirement 17.2.d of the LOPD-GDD for research purposes, with no informed consent required from the data holders. The research team and the pseudonymiser were technically and functionally separated. Only the research team had access to the data, and technical measures were taken to avoid re-identification and access by others via the CMBD (“Conjunt Minim de Base de Dades”), the E-CAP computerised medical history and the Catalan Health Service databases.
Data Availability Statement
The data used in this study are only available for the participating researchers, in accordance with current European and national laws. Thus, the distribution of the data is not allowed. However, researchers from public institutions can request data from SIDIAP. Further information is available online (https://www.sidiap.org/index.php/en/solicituds-en (accessed on 11 January 2024)).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chen, C.; Xu, X.; Yan, Y. Estimated global overweight and obesity burden in pregnant women based on panel data model. PLoS ONE 2018, 13, e0202183. [Google Scholar] [CrossRef]
- Poston, L.; Caleyachetty, R.; Cnattingius, S.; Corvalán, C.; Uauy, R.; Herring, S.; Gillman, M.W. Preconceptional and maternal obesity: Epidemiology and health consequences. Lancet Diabetes Endocrinol. 2016, 4, 1025–1036. [Google Scholar] [CrossRef] [PubMed]
- Stival, C.; Lugo, A.; Odone, A.; van den Brandt, P.A.; Fernandez, E.; Tigova, O.; Soriano, J.B.; José López, M.; Scaglioni, S.; Gallus, S.; et al. Prevalence and Correlates of Overweight and Obesity in 12 European Countries in 2017–2018. Obes. Facts 2022, 15, 655–665. [Google Scholar] [CrossRef]
- Devlieger, R.; Benhalima, K.; Damm, P.; Van Assche, A.; Mathieu, C.; Mahmood, T.; Dunne, F.; Bogaerts, A. Maternal obesity in Europe: Where do we stand and how to move forward? A scientific paper commissioned by the European Board and College of Ob-stetrics and Gynaecology (EBCOG). Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 201, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Melchor, I.; Burgos, J.; Del Campo, A.; Aiartzaguena, A.; Gutiérrez, J.; Melchor, J.C. Effect of maternal obesity on pregnancy outcomes in women delivering singleton babies: A historical cohort study. J. Perinat. Med. 2019, 47, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, R.; Steegers, E.A.; Duijts, L.; Felix, J.F.; Hofman, A.; Franco, O.H.; Jaddoe, V.W. Childhood cardiometabolic outcomes of maternal obe-sity during pregnancy: The Generation R Study. Hypertension 2014, 63, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.J.; Ryckman, K.K. Epigenetic and developmental influences on the risk of obesity, diabetes, and metabolic syndrome. Diabetes Metab. Syndr. Obes. Targets Ther. 2015, 8, 295. [Google Scholar]
- Siscart, J.; Orós, M.; Serna, M.C.; Perejón, D.; Galván, L.; Ortega, M. Adherence to treatment for hypothyroidism in pregnancy and relationship with thyrotropin control: A retrospective observational cohort study. BMC Pregnancy Childbirth 2022, 22, 168. [Google Scholar] [CrossRef]
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 11 January 2024).
- González-Plaza, E.; Bellart, J.; Martínez-Verdú, M.Á.; Arranz, Á.; Luján-Barroso, L.; Seguranyes, G. Pre-pregnancy overweight and obesity prevalence and relation to maternal and perinatal outcomes. Enferm. Clin. 2021. [Google Scholar] [CrossRef]
- Peristat, E.; Macfarlane, A.J. Euro-Peristat Project. European Perinatal Health Report. In Core Indicators of the Health and Care of Pregnant Women and Babies in Europe in 2015; University of London: Lond, UK, 2018. [Google Scholar]
- Kim, S.S.; Zhu, Y.; Grantz, K.L.; Hinkle, S.N.; Chen, Z.; Wallace, M.E.; Smarr, M.M.; Epps, N.M.B.; Mendola, P. Obstetric and neonatal risks among obese women without chronic disease. Obstet. Gynecol. 2016, 128, 104–112. [Google Scholar]
- Santos, S.; Voerman, E.; Amiano, P.; Barros, H.; Beilin, L.; Bergström, A.; Charles, M.; Chatzi, L.; Chevrier, C.; Chrousos, G.; et al. Impact of maternal body mass index and gestational weight gain on pregnancy compli-cations: An individual participant data meta-analysis of European, North American and Australian cohorts. BJOG 2019, 126, 984–995. [Google Scholar] [CrossRef] [PubMed]
- Thornton, H.V.; Cornish, R.P.; Lawlor, D.A. Non-linear associations of maternal pre-pregnancy body mass index with risk of still-birth, infant, and neonatal mortality in over 28 million births in the USA: A retrospective cohort study. EClinicalMedicine 2023, 66, 102351. [Google Scholar] [CrossRef]
- Vats, H.; Saxena, R.; Sachdeva, M.P.; Walia, G.K.; Gupta, V. Impact of maternal pre-pregnancy body mass index on maternal, fetal and neonatal adverse outcomes in the worldwide populations: A systematic review and meta-analysis. Obes. Res. Clin. Pract. 2021, 15, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Simko, M.; Totka, A.; Vondrova, D.; Samohyl, M.; Jurkovicova, J.; Trnka, M.; Cibulkova, A.; Stofko, J.; Argalasova, L. Maternal body mass index and gestational weight gain and their association with pregnancy complications and perinatal conditions. Int. J. Environ. Res. Public Health 2019, 16, 1751. [Google Scholar] [CrossRef] [PubMed]
- Chaemsaithong, P.; Leung, T.Y.; Sahota, D.; Cheng, Y.K.Y.; Leung, W.C.; Lo, T.K.; Poon, L.C.Y. Body mass index at 11–13 weeks’ gestation and pregnancy complications in a Southern Chinese population: A retrospective cohort study. J. Matern. Neonatal Med. 2019, 32, 2056–2068. [Google Scholar] [CrossRef]
- Bautista-Castaño, I.; Henriquez-Sanchez, P.; Alemán-Perez, N.; Garcia-Salvador, J.J.; Gonzalez-Quesada, A.; García-Hernández, J.A.; Serra-Majem, L. Maternal obesity in early pregnancy and risk of adverse outcomes. PLoS ONE 2013, 8, e80410, Erratum in: PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Sunder, A.; Varghese, B.; Darwish, B.; Shaikho, N.M.; AlSada, A.; Albuainain, H.; Alrowaijeh, S.; Al-Khalifa, S.A.; Khalid Bughamar, A.; Dayoub, N. Maternal Obesity: An Obstetric Risk. Cureus 2022, 14, e29345. [Google Scholar] [CrossRef]
- Ballesta-Castillejos, A.; Gómez-Salgado, J.; Rodríguez-Almagro, J.; Ortiz-Esquinas Hernández-Martínez, A. Relationship between maternal body mass index and obstetric and perinatal complications. J. Clin. Med. 2020, 9, 707. [Google Scholar]
- Alfadhli, E.M. Maternal obesity influences Birth Weight more than gestational Diabetes author. BMC Pregnancy Childbirth 2021, 21, 111. [Google Scholar] [CrossRef]
- Lewandowska, M. Maternal Obesity and Risk of Low Birth Weight, Fetal Growth Restriction, and Macrosomia: Multiple Anal-yses. Nutrients 2021, 13, 1213. [Google Scholar] [CrossRef]
- Marchi, J.; Berg, M.; Dencker, A.; Olander, E.K.; Begley, C. Risks associated with obesity in pregnancy, for the mother and baby: A systematic review of reviews. Obes. Rev. 2015, 16, 621–628+630. [Google Scholar] [PubMed]
- Wang, Z.; Wang, P.; Liu, H.; He, X.; Zhang, J.; Yan, H.; Xu, D.; Wang, B. Maternal adiposity as an independent risk factor for pre-eclampsia: A meta-analysis of prospective cohort studies. Obes. Rev. 2013, 14, 508–521. [Google Scholar] [PubMed]
- Chu, S.Y.; Kim, S.Y.; Schmid, C.H.; Dietz, P.M.; Callaghan, W.M.; Lau, J.; Curtis, K.M. Maternal obesity and risk of cesarean delivery: A me-ta-analysis. Obes. Rev. 2007, 8, 385–394. [Google Scholar] [PubMed]
- Zhu, T.; Tang, J.; Zhao, F.; Qu, Y.; Mu, D. Association between maternal obesity and offspring Apgar score or cord pH: A systematic review and meta-analysis. Sci. Rep. 2015, 5, 18386. [Google Scholar] [CrossRef] [PubMed]
- Mitha, A.; Chen, R.; Johansson, S.; Razaz, N.; Cnattingius, S. Maternal body mass index in early pregnancy and severe asphyxia-related complications in preterm infants. Int. J. Epidemiol. 2020, 49, 1647–1660. [Google Scholar] [CrossRef]
- Zong, X.; Wang, H.; Yang, L.; Guo, Y.; Zhao, M.; Magnussen, C.G.; Xi, B. Maternal Pre-pregnancy Body Mass Index Categories and Infant Birth Outcomes: A Population-Based Study of 9 Million Mother-Infant Pairs. Front. Nutr. 2022, 9, 789833. [Google Scholar] [CrossRef]
- Vinturache, A.E.; McDonald, S.; Slater, D.; Tough, S. Perinatal outcomes of maternal overweight and obesity in term infants: A pop-ulation-based cohort study in Canada. Sci. Rep. 2015, 5, 9334. [Google Scholar] [CrossRef]
- Gaudet, L.; Ferraro, Z.M.; Wen, S.W.; Walker, M. Maternal obesity and occurrence of fetal macrosomia: A systematic review and meta-analysis. Biomed. Res. Int. 2014, 2014, 640291. [Google Scholar] [CrossRef]
- Khashu, M.; Pelligra, G.; Bhargava, S.; Smyth, J.A. Perinatal Morbidity in Macrosomic Infants; Pediatric Academy of Sciences: Toronto, ON, Canada, 2005. [Google Scholar]
- Yu, Z.; Han, S.; Zhu, J.; Sun, X.; Ji, C.; Guo, X. Pre-pregnancy body mass index in relation to infant birth weight and offspring over-weight/obesity: A systematic review and meta-analysis. PLoS ONE 2013, 8, e61627. [Google Scholar] [CrossRef]
- Wang, D.; Hong, Y.; Zhu, L.; Wang, X.; Lv, Q.; Zhou, Q.; Ruan, M.; Chen, C. Risk factors and outcomes of macrosomia in China: A multicentric survey based on birth data. J. Matern. Neonatal Med. 2017, 30, 623–627. [Google Scholar] [CrossRef]
- Song, X.; Shu, J.; Zhang, S.; Chen, L.; Diao, J.; Li, J.; Li, Y.; Wei, J.; Liu, Y.; Sun, M.; et al. Pre-Pregnancy Body Mass Index and Risk of Macrosomia and Large for Gestational Age Births with Gestational Diabetes Mellitus as a Mediator: A Prospective Co-hort Study in Central China. Nutrients 2022, 14, 1072. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Xu, L.; Wang, Y.; Zhang, Y.; Du, Y.; Sun, Y.; Wang, Z. Association between perinatal outcomes and maternal pre-pregnancy body mass index. Obes. Rev. 2016, 17, 1091–1102. [Google Scholar] [CrossRef] [PubMed]
- Nucci, D.; Chiavarini, M.; Duca, E.; Pieroni, L.; Salmasi, L.; Minelli, L. Pre-pregnancy body mass index, gestational weight gain and adverse birth outcomes: Some evidence from Italy. Ann. Ig. 2018, 30, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Li, L.; Chen, W.; Liu, Z.B.; Ma, L.; Gao, X.X.; He, J.L.; Wang, H.; Zhao, M.; Yang, Y.Y.; et al. Pre-pregnancy underweight and obesity are positively associated with small-for-gestational-age infants in a Chinese population. Sci. Rep. 2019, 9, 15544. [Google Scholar] [CrossRef] [PubMed]
- Catalano, P.M.; Shankar, K. Obesity and pregnancy: Mechanisms of short term and long term adverse consequences for mother and child. BMJ 2017, 356, j1. [Google Scholar]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: The linking mechanism and the complica-tions. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef]
- Dunkerton, S.; Aiken, C. Impact of the intrauterine environment on future reproductive and metabolic health. Obstet. Gynaecol. 2022, 24, 93–100. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).