Measurement of Oxidative Stress Index (OSI) in Penile Corpora Cavernosa and Peripheral Blood of Peyronie’s Disease Patients: A Report of 49 Cases
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Purpose of This Study
- -
- Evaluation of the OS index in healthy subjects without PD or other acute or chronic organic pathologies;
- -
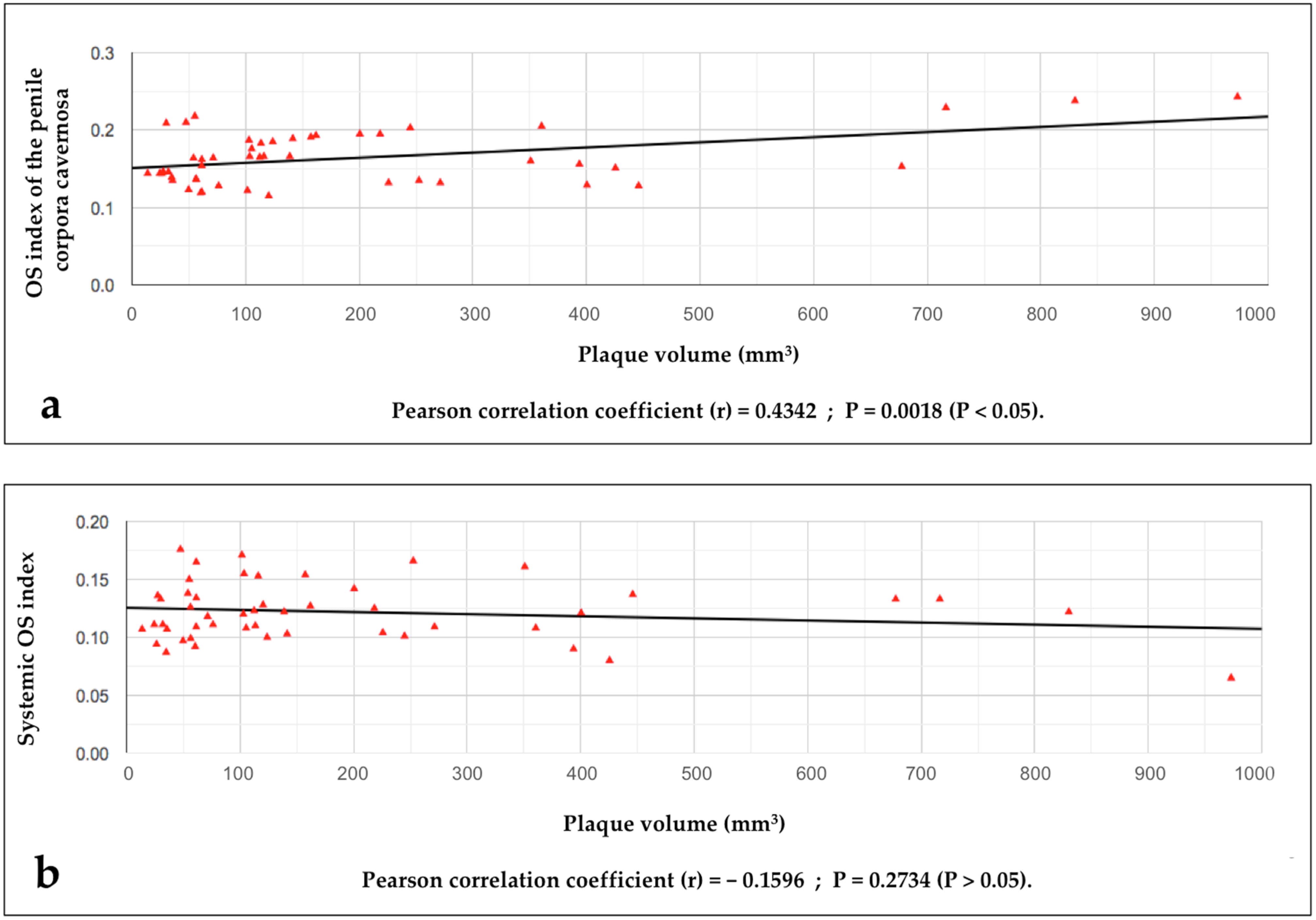
- The search for a possible relationship between the value of the OS index detected in the penile corpora cavernosa (penile OSI value) and the volume of the disease area (plaque);
- -
- The search for a possible relationship between the values of the systemic OSI (blood sampling from a peripheral vein) and the volumes of the disease area (plaque);
- -
- The search for a possible relationship between the values of systemic OSI and penile OSI;
- -
- The identification of penile OSI values indicative of regression of the disease area (PD plaque);
- -
- The search for a possible relationship between values of systemic OSI and ongoing chronic pathology;
- -
- The search for a possible relationship between penile OSI values and ongoing chronic pathology;
- -
- The search for a possible relationship between penile OSI values and an anxious–depressive state (and its prevalence);
- -
- The search for a possible relationship between systemic OSI values and an anxious–depressive state (and its prevalence).
2.2.1. Inclusion and Exclusion Criteria
2.2.2. Data Collection
2.2.3. Sample Collection
2.2.4. Plasma Collection
2.2.5. d-ROMs and PAT Measurements
2.2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Herati, A.S.; Pastuszak, A.W. The Genetic Basis of Peyronie’s Disease: A Review. Sex Med. Rev. 2016, 4, 85–94. [Google Scholar] [CrossRef]
- Bias, W.B.; Nyberg, L.M., Jr.; Hochberg, M.C.; Walsh, P.C.; Opitz, J.M. Peyronie’s disease: A newly recognized autosomal-dominant trait. Am. J. Med. Genet. 1982, 12, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Schwarzer, U.; Sommer, F.; Klotz, T.; Braun, M.; Reifenrath, B.; Engelmann, U. The prevalence of Peyronie’s disease: Results of a large survey. BJU Int. 2001, 88, 727–730. [Google Scholar] [CrossRef]
- Stuntz, M.; Perlaky, A.; des Vignes, F.; Kyriakides, T.; Glass, D. The prevalence of Peyronie’s disease in the United States: A population-based study. PLoS ONE 2016, 11, e0150157. [Google Scholar] [CrossRef] [PubMed]
- La Pera, G.; Pescatori, E.S.; Calabrese, M.; Boffini, A.; Colombo, F.; Andriani, E.; Natali, A.; Vaggi, L.; Catuogno, C.; Giustini, M.; et al. Peyronie’s disease: Prevalence and association with cigarette smoking. A multicenter population-based study in men aged 50–69 years. Eur. Urol. 2001, 40, 525–530. [Google Scholar] [CrossRef] [PubMed]
- DiBenedetti, D.B.; Nguyen, D.; Zografos, L.; Ziemiecki, R.; Zhou, X. A Population-based study of peyronie’s disease: Prevalence and treatment patterns in the United States. Adv. Urol. 2011, 2011, 282503. [Google Scholar] [CrossRef]
- Hellstrom, W.J.; Bivalacqua, T.J. Peyronie’s disease: Etiology, medical, and surgical therapy. J. Androl. 2000, 21, 347–354. [Google Scholar] [CrossRef]
- Weidner, W.; Schroeder-Printzen, I.; Weiske, W.-H.; Vosshenrich, R. Sexual dysfunction in Peyronie’s disease: An analysis of 222 patients without previous local plaque therapy. J. Urol. 1997, 157, 325–328. [Google Scholar] [CrossRef]
- Pryor, J.P.; Ralph, D.J. Clinical presentations of Peyronie’s disease. Int. J. Impot. Res. 2002, 14, 414–417. [Google Scholar] [CrossRef]
- Nelson, C.J.; Diblasio, C.; Kendirci, M.; Hellstrom, W.; Guhring, P.; Mulhall, J.P. The chronology of depression and distress in men with Peyronie’s disease. J. Sex. Med. 2008, 5, 1985–1990. [Google Scholar] [CrossRef]
- Devine, C.J., Jr.; Somers, K.D.; Ladaga, L.E. Peyronie’s disease: Pathophysiology. Prog. Clin. Biol. Res. 1991, 370, 355–358. [Google Scholar]
- Devine, C.J., Jr.; Somers, K.D.; Jordan, G.H.; Schlossberg, S.M. Proposal: Trauma as a cause of Peyronie’s lesion. J. Urol. 1997, 157, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Jarow, J.P.; Lowe, F.C. Penile trauma: An etiologic factor in Peyronie’s disease and erectile dysfunction. J. Urol. 1997, 158, 1388–1390. [Google Scholar] [CrossRef] [PubMed]
- Somers, K.D.; Dawson, D.M. Fibrin deposition in Peyronie’s disease plaque. J. Urol. 1997, 157, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Sikka, S.C.; Hellstrom, W.J. Role of oxidative stress and antioxidants in Peyronie’s disease. Int. J. Impot. Res. 2002, 14, 353–360. [Google Scholar] [CrossRef]
- El-Sakka, A.I.; Salabas, E.; Dinçer, M.; Kadioglu, A. The pathophysiology of Peyronie’s disease. Arab. J. Urol. 2013, 11, 272–277. [Google Scholar] [CrossRef]
- Paulis, G.; Brancato, T. Inflammatory mechanisms and oxidative stress in Peyronie’s disease: Therapeutic “rationale” and related emerging treatment strategies. Inflamm. Allergy Drug Targets 2012, 11, 48–57. [Google Scholar] [CrossRef]
- Paulis, G.; Romano, G.; Paulis, L.; Barletta, D. Recent Pathophysiological Aspects of Peyronie’s Disease: Role of Free Radicals, Rationale, and Therapeutic Implications for Antioxidant Treatment-Literature Review. Adv. Urol. 2017, 2017, 4653512. [Google Scholar] [CrossRef]
- Paulis, G.; De Giorgio, G.; Paulis, L. Role of Oxidative Stress in Peyronie’s Disease: Biochemical Evidence and Experiences of Treatment with Antioxidants. Int. J. Mol. Sci. 2022, 23, 15969. [Google Scholar] [CrossRef]
- Davila, H.H.; Magee, T.R.; Vernet, D.; Rajfer, J.; Gonzalez-Cadavid, N.F. Gene transfer of inducible nitric oxide synthase complementary DNA regresses the fibrotic plaque in an animal model of Peyronie’s disease. Biol. Reprod. 2004, 71, 1568–1577. [Google Scholar] [CrossRef]
- Bivalacqua, T.J.; Champion, H.C.; Hellstrom, W.J. Implications of nitric oxide synthase isoforms in the pathophysiology of Peyronie’s disease. Int. J. Impot. Res. 2002, 14, 345–352. [Google Scholar] [CrossRef]
- Gonzalez-Cadavid, N.F.; Magee, T.R.; Ferrini, M.; Qian, A.; Vernet, D.; Rajfer, J. Gene expression in Peyronie’s disease. Int. J. Impot. Res. 2002, 14, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Moreland, R.B.; Nehra, A. Pathophysiology of Peyronie’s disease. Int. J. Impot. Res. 2002, 14, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Mulhall, J.P.; Schiff, J.; Guhring, P. An analysis of the natural history of Peyronie’s disease. J. Urol. 2006, 175, 2115–2118. [Google Scholar] [CrossRef]
- Garaffa, G.; Trost, L.W.; Serefoglu, E.C.; Ralph, D.; Hellstrom, W.J.G. Understanding the course of Peyronie’s disease. Int. J. Clin. Pract. 2013, 67, 781–788. [Google Scholar] [CrossRef]
- Paulis, G.; Cavallini, G. Clinical evaluation of natural history of Peyronie’s disease: Our experience, old myths and new certainties. Inflamm. Allergy Drug Targets 2013, 12, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Jalkut, M.; Gonzalez-Cadavid, N.; Rajfer, J. Peyronie’s disease: A review. Rev. Urol. 2003, 5, 142–148. [Google Scholar]
- Levine, L.A.; Larsen, S. Diagnosis and management of Peyronie disease. In Campbell-Walsh Urology, 11th ed.; Wein, A.J., Kavoussi, L.R., Partin, A.W., Peters, C.A., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2015; pp. 722–748. [Google Scholar]
- Brimley, S.C.; Yafi, F.A.; Greenberg, J.; Hellstrom, W.J.; Nguyen, H.M.T.; Hatzichristodoulou, G. Review of management options for active-phase Peyronie’s disease. Sex. Med. Rev. 2019, 7, 329–337. [Google Scholar] [CrossRef]
- Capoccia, E.; Levine, L.A. Contemporary review of Peyronie’s disease treatment. Curr. Urol. Rep. 2018, 19, 51. [Google Scholar] [CrossRef]
- Gur, S.; Limin, M.; Hellstrom, W.J. Current status and new developments in Peyronie’s disease: Medical, minimally invasive and surgical treatment options. Expert Opin. Pharmacother. 2011, 12, 931–944. [Google Scholar] [CrossRef]
- Tsambarlis, P.; Levine, L.A. Nonsurgical management of Peyronie’s disease. Nat. Rev. Urol. 2019, 16, 172–186. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.B.W.; Sangkum, P.; Mitchell, G.C.; Hellstrom, W.J.G. Update on medical management of Peyronie’s disease. Curr. Urol. Rep. 2014, 15, 415. [Google Scholar] [CrossRef] [PubMed]
- Paulis, G.; Brancato, T.; D’ascenzo, R.; De Giorgio, G.; Nupieri, P.; Orsolini, G.; Alvaro, R. Efficacy of vitamin E in the conservative treatment of Peyronie’s disease: Legend or reality? A controlled study of 70 cases. Andrology 2013, 1, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Paulis, G.; Cavallini, G.; De Giorgio, G.; Quattrocchi, S.; Brancato, T.; Alvaro, R. Long-term multimodal therapy (verapamil associated with propolis, blueberry, vitamin E and local diclofenac) on patients with Peyronie’s disease (chronic inflammation of the tunica albuginea). Results of a controlled study. Inflamm. Allergy Drug Targets 2013, 12, 403–409. [Google Scholar] [CrossRef]
- Paulis, G.; Barletta, D.; Turchi, P.; Vitarelli, A.; Dachille, G.; Fabiani, A.; Gennaro, R. Efficacy and safety evaluation of pentoxifylline associated with other antioxidants in medical treatment of Peyronie’s disease: A case-control study. Res. Rep. Urol. 2016, 8, 1–10. [Google Scholar]
- Gennaro, R.; Barletta, D.; Paulis, G. Intralesional hyaluronic acid: An innovative treatment for Peyronie’s disease. Int. Urol. Nephrol. 2015, 47, 1595–1602. [Google Scholar] [CrossRef]
- Zucchi, A.; Costantini, E.; Cai, T.; Cavallini, G.; Liguori, G.; Favilla, V.; De Grande, G.; D’Achille, G.; Silvani, M.; Franco, G.; et al. Intralesional Injection of Hyaluronic Acid in Patients Affected with Peyronie’s Disease: Preliminary Results from a Prospective, Multicenter, Pilot Study. Sex. Med. 2016, 4, e85–e90. [Google Scholar] [CrossRef]
- Kendirci, M.; Hellstrom, W.J. Critical analysis of surgery for Peyronie’s disease. Curr. Opin. Urol. 2004, 6, 381–388. [Google Scholar] [CrossRef]
- Levine, L.A.; Burnett, A.L. Standard operating procedures for Peyronie’s disease. J. Sex. Med. 2013, 10, 230–244. [Google Scholar] [CrossRef]
- Kadioglu, A.; Akman, T.; Sanli, O.; Gurkan, L.; Cakan, M.; Celtik, M. Surgical treatment of Peyronie’s disease: A critical analysis. Eur. Urol. 2006, 50, 235–248. [Google Scholar] [CrossRef]
- Rice, P.G.; Somani, B.K.; Rees, R.W. Twenty Years of Plaque Incision and Grafting for Peyronie’s Disease: A Review of Literature. Sex. Med. 2019, 7, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Ralph, D.; Gonzalez-Cadavid, N.; Mirone, V.; Perovic, S.; Sohn, M.; Usta, M.; Levine, L. The management of Peyronie’s disease: Evidence-based 2010 guidelines. J. Sex. Med. 2010, 7, 2359–2374. [Google Scholar] [CrossRef] [PubMed]
- Hatzimouratidis, K.; Eardley, I.; Giuliano, F.; Hatzichristou, D.; Moncada, I.; Salonia, A.; Vardi, Y.; Wespes, E. EAU guidelines on penile curvature. Eur. Urol. 2012, 62, 543–552. [Google Scholar] [CrossRef]
- Nehra, A.; Alterowitz, R.; Culkin, D.J.; Faraday, M.M.; Hakim, L.S.; Heidelbaugh, J.J.; Khera, M.; Kirkby, E.; McVary, K.T.; Miner, M.M.; et al. Peyronie’s disease: AUA guideline. J. Urol. 2015, 194, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Bella, A.J.; Lee, J.C.; Grober, E.D.; Carrier, S.; Benard, F.; Brock, G.B. Canadian Urological Association guideline for Peyronie’s disease and congenital penile curvature. Can. Urol. Assoc. J. 2018, 12, E197–E209. [Google Scholar] [CrossRef] [PubMed]
- Levine, L.; Rybak, J.; Corder, C.; Farrel, M.R. Peyronie’s disease plaque calcification--prevalence, time to identification, and development of a new grading classification. J. Sex. Med. 2013, 10, 3121–3128. [Google Scholar] [CrossRef] [PubMed]
- Paulis, G.; Paulis, A. Calcification in Peyronie’s disease: Its role and clinical influence on the various symptoms and signs of the disease, including psychological impact. Our study of 551 patients. Arch. Ital. Urol. Androl. 2023, 95, 11549. [Google Scholar] [CrossRef] [PubMed]
- Yesilirmak, N.; Bukan, N.; Kurt, B.; Yuzbasioglu, S.; Zhao, M.; Rodrigues-Braz, D.; Aktas, A.; Behar-Cohen, F.; Bourges, J.-L. Evaluation of Ocular and Systemic Oxidative Stress Markers in Ocular Rosacea Patients. Investig. Opthalmol. Vis. Sci. 2023, 64, 22. [Google Scholar] [CrossRef]
- Dinç, G.; Fentoğlu, Ö.; Doğru, A.; İlhan, I.; Kırzıoğlu, F.Y.; Orhan, H. The evaluation of salivary oxidative stress in patients with familial Mediterranean fever and chronic periodontitis. J. Periodontol. 2018, 89, 1112–1120. [Google Scholar] [CrossRef]
- Valtuille, R.A.; Rossi, G.; Gimenez, E. Protective Effect of Autologous Arteriovenous Fistulae Against Oxidative Stress in Hemodialyzed Patients. Cureus 2021, 13, e15398. [Google Scholar] [CrossRef]
- Serena, B.; Primiterra, M.; Catalani, S.; Finco, A.; Canestrari, F.; Cornelli, U. Performance evaluation of the innovative PAT test, comparison with the common BAP test and influence of interferences on the evaluation of the plasma antioxidant capacity. Clin. Lab. 2013, 59, 1091–1097. [Google Scholar] [PubMed]
- Harma, M.; Harma, M.; Erel, O. Increased oxidative stress in patients with hydatidiform mole. Swiss. Med. Wkly. 2003, 133, 563–566. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, M.A.; Mendoza-Núñez, V.M. Oxidative Stress Indexes for Diagnosis of Health or Disease in Humans. Oxidative Med. Cell. Longev. 2019, 2019, 4128152. [Google Scholar] [CrossRef] [PubMed]
- Demirbag, R.; Gur, M.; Yilmaz, R.; Kunt, A.S.; Erel, O.; Andac, M.H. Influence of oxidative stress on the development of collateral circulation in total coronary occlusions. Int. J. Cardiol. 2007, 116, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, S.; Ozgu-Erdinc, A.S.; Demirtas, C.; Ozturk, G.; Erkaya, S.; Uygur, D. The oxidative stress index increases among patients with hyperemesis gravidarum but not in normal pregnancies. Redox Rep. 2015, 20, 97–102. [Google Scholar] [CrossRef] [PubMed]
- The FRAS System. 2023. Available online: https://hedsrl.it/en/fras-5/ (accessed on 24 December 2023).
- Coaccioli, S.; Panaccione, A.; Biondi, R.; Sabatini, C.; Landucci, P.; Del Giorno, R.; Fantera, M.; Mondo, A.M.; Di Cato, L.; Paladini, A.; et al. Evaluation of oxidative stress in rheumatoid and psoriatic arthritis and psoriasis. Clin. Ter. 2009, 160, 467–472. [Google Scholar] [PubMed]
- Mukhopadhyay, K.; De, S.; Kundu, S.; Ghosh, P.; Chatterjee, S.; Chatterjee, M. Evaluation of levels of oxidative stress as a potential biomarker in patients with rheumatoid arthritis. J. Fam. Med. Prim. Care 2021, 10, 1981–1986. [Google Scholar] [CrossRef] [PubMed]
- Karaagac, L.; Koruk, S.T.; Koruk, I.; Aksoy, N. Decreasing oxidative stress in response to treatment in patients with brucellosis: Could it be used to monitor treatment? Int. J. Infect. Dis. 2011, 15, e346–e349. [Google Scholar] [CrossRef]
- Motor, S.; Ozturk, S.; Ozcan, O.; Gurpinar, A.B.; Can, Y.; Yuksel, R.; Yenin, J.Z.; Seraslan, G.; Ozturk, O.H. Evaluation of total antioxidant status, total oxidant status and oxidative stress index in patients with alopecia areata. Int. J. Clin. Exp. Med. 2014, 7, 1089–1093. [Google Scholar]
- Yalcin, S.; Ulas, T.; Eren, M.A.; Aydogan, H.; Camuzcuoglu, A.; Kucuk, A.; Yuce, H.H.; Demir, M.E.; Vural, M.; Aksoy, N. Relationship between oxidative stress parameters and cystatin C levels in patients with severe preeclampsia. Medicina 2013, 49, 19. [Google Scholar] [CrossRef]
- Kelâmi, A. Autophotography in evaluation of functional penile disorders. Urology 1983, 21, 628–629. [Google Scholar] [CrossRef] [PubMed]
- Eri, L.M.; Thomassen, H.; Brennhovd, B.; Håheim, L.L. Accuracy and repeatability of prostate volume measurements by transrectal ultrasound. Prostate Cancer Prostatic Dis. 2002, 5, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Chung, B.H. Transrectal ultrasound versus magnetic resonance imaging in the estimation of prostate volume as compared with radical prostatectomy specimens. Urol. Int. 2007, 78, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Rosen, R.C.; Riley, A.; Wagner, G.; Osterloh, I.H.; Kirkpatrick, J.; Mishra, A. The international index of erectile function (IIEF): A multidimensional scale for assessment of erectile dysfunction. Urology 1997, 49, 822–830. [Google Scholar] [CrossRef]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, R.L.; Kroenke, K.; Williams, J.B.; Löwe, B. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch. Intern. Med. 2006, 166, 1092–1097. [Google Scholar] [CrossRef]
- Kahl, C.; Cleland, J.A. Visual analogue scale, numeric pain rating scale and the McGill pain Questionnaire: An overview of psychometric properties. Phys. Ther. Rev. 2005, 10, 123–128. [Google Scholar] [CrossRef]
- Mitsui, Y.; Yamabe, F.; Hori, S.; Uetani, M.; Kobayashi, H.; Nagao, K.; Nakajima, K. Molecular Mechanisms and Risk Factors Related to the Pathogenesis of Peyronie’s Disease. Int. J. Mol. Sci. 2023, 24, 10133. [Google Scholar] [CrossRef]
- Özdemir, E.Ç.; Erciyas, K.; Ünsal, B.; Sezer, U.; Taysi, S.; Araz, M. The Effects of Chronic Periodontitis and Obesity on Total Antioxidant/Oxidant Status and Oxidative Stress Index. Acta Endocrinol. 2022, 18, 294–300. [Google Scholar] [CrossRef]
- Paulis, G.; De Giorgio, G. Complete Plaque Regression in Patients with Peyronie’s Disease after Multimodal Treatment with Antioxidants: A Report of 2 Cases. Am. J. Case Rep. 2022, 23, e936146. [Google Scholar] [CrossRef]
- Paulis, G.; De Giorgio, G. Full Regression of Peyronie’s Disease Plaque Following Combined Antioxidant Treatment: A Three-Case Report. Antioxidants 2022, 11, 1661. [Google Scholar] [CrossRef]
- Paulis, G.; De Giorgio, G. Patients with Peyronie’s disease achieve complete plaque regression after multimodal treatment with antioxidants: A case series. J. Med. Case Rep. 2022, 16, 359. [Google Scholar] [CrossRef]
- Paulis, G.; De Giorgio, G. Disappearance of Plaque Following Treatment with Antioxidants in Peyronie’s Disease Patients—A Report of 3 Cases. Clin. Pract. 2022, 12, 1020–1033. [Google Scholar] [CrossRef]
- Terrier, J.E.; Nelson, C.J. Psychological aspects of Peyronie’s disease. Transl. Androl. Urol. 2016, 5, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.F.; Walsh, T.J.; Conti, S.L.; Turek, P.; Lue, T. Risk factors for emotional and relationship problems in Peyronie’s disease. J. Sex. Med. 2008, 5, 2179–2184. [Google Scholar] [CrossRef] [PubMed]
- Nelson, C.J.; Mulhall, J.P. Psychological impact of Peyronie’s disease: A review. J. Sex. Med. 2013, 10, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Cilio, S.; Fallara, G.; Capogrosso, P.; Candela, L.; Belladelli, F.; Pozzi, E.; Corsini, C.; Raffo, M.; Schifano, N.; Boeri, L.; et al. The symptomatic burden of Peyronie’s disease at presentation according to patient age: A critical analysis of the Peyronie’s disease questionnaire (PDQ) domains. Andrology 2023, 11, 501–507. [Google Scholar] [CrossRef]
- Culha, M.G.; Erkan, E.; Cay, T.; Yücetaş, U. The Effect of Platelet-Rich Plasma on Peyronie’s Disease in Rat Model. Urol. Int. 2019, 102, 218–223. [Google Scholar] [CrossRef]
- Bivalacqua, T.J.; Diner, E.K.; Novak, T.E.; Vohra, Y.; Sikka, S.C.; Champion, H.C.; Kadowitz, P.J.; Hellstrom, W.J. A rat model of Peyronie’s disease associated with a decrease in erectile activity and an increase in inducible nitric oxide synthase protein expression. J. Urol. 2000, 163, 1992–1998. [Google Scholar] [CrossRef]
- Sohn, D.W.; Bae, W.J.; Kim, H.S.; Kim, S.W.; Kim, S.W. The anti-inflammatory and antifibrosis effects of anthocyanin extracted from black soybean on a Peyronie disease rat model. Urology 2014, 84, 1112–1116. [Google Scholar] [CrossRef]


| All n. 49 PD Patients | n. 25 PD Patients with Plaque Elimination | n. 24 PD Patients with Partial Plaque Elimination | Statistical Analysis 25 PD p. versus 24 PD p. p-Value (t-Test) | |
|---|---|---|---|---|
| Mean age (years) (SD) | 49.65 (±11.01) | 46.68 (±11.29) | 50.66 (±10.85) | 0.214 * |
| Plaque volume (mm3) (SD) | 194.33 (±218.55) | 152.64 (±192.30) | 237.75 (±239.30) | 0.175 * |
| n. 25 PD patients with plaque elimination | n. 24 PD patients with partial plaque elimination | |||
| Patient no. | Plaque volume (mm3) | Plaque volume (mm3) | ||
| 1. | 34.9 | 113.4 | ||
| 2. | 26.4 | 76.2 | ||
| 3. | 13.7 | 350.8 | ||
| 4. | 35.6 | 101.5 | ||
| 5. | 24.3 | 138.8 | ||
| 6. | 123.8 | 112.3 | ||
| 7. | 105.3 | 200.5 | ||
| 8. | 27.4 | 71.4 | ||
| 9. | 716.4 | 102.9 | ||
| 10. | 120.2 | 973.0 | ||
| 11. | 393.7 | 445.9 | ||
| 12. | 56.3 | 425.4 | ||
| 13. | 60.4 | 830.0 | ||
| 14. | 54.0 | 30.0 | ||
| 15. | 61.4 | 400.4 | ||
| 16. | 61.4 | 271.3 | ||
| 17. | 56.4 | 225.7 | ||
| 18. | 157.4 | 116.0 | ||
| 19. | 103.5 | 61.3 | ||
| 20. | 49.7 | 252.5 | ||
| 21. | 244.8 | 141.4 | ||
| 22. | 677.5 | 161.9 | ||
| 23. | 218.3 | 47.4 | ||
| 24. | 360.4 | 55.2 | ||
| 25. | 31.9 | - | ||
| Group of PD Patients (n. 49) Mean Age 49.65 Years (SD ± 11.01) | Control Group (n. 50) Mean Age 49.94 Years (SD ± 11.42) | Statistical Analysis Group-PD versus Control Group p-Value (t-Test) 0.912 | |||
|---|---|---|---|---|---|
| Demographic characteristics | N. patients (out 49) (%) | N. patients (out 50) (%) | p-value (chi-square test) | ||
| Race | |||||
| Caucasian | 49 (100) | 50 (100) | 1.000 | ||
| Age Range | - | - | - | ||
| From 20 to 30 years | 2 (4.08) | 3 (6.0) | 0.713 | ||
| From 31 to 40 years | 10 (20.4) | 9 (18.0) | 0.762 | ||
| From 41 to 50 years | 13 (26.53) | 8 (16.0) | 0.202 | ||
| From 51 to 60 years | 14 (28.57) | 20 (40.0) | 0.233 | ||
| From 61 to 70 years | 10 (20.4) | 10 (20.0) | 0.959 | ||
| Type of school education | - | - | |||
| elementary school | 2 (4.08) | 1 (2.0) | 0.547 | ||
| secondary school | 38 (77.55) | 39 (78.0) | 0.957 | ||
| university degree | 9 (18.36) | 10 (20.0) | 0.837 | ||
| Clinical condition associated in PD patients | N. patients (out 49) | N. patients (out 50) | Statistical analysis Group-PD versus Control group p-value (t-test) | ||
| Anxiety | 40 | Mean GAD-7 score = 16.4 | 42 | Mean GAD-7 score = 16.2 | 0.806 |
| Depression | 29 | Mean PHQ-9 score = 15.2 | 32 | Mean PHQ-9 score = 14.4 | 0.383 |
| Penile curvature | 45 | Average penile curvature angle (degrees) = 35.1° | 0 | Average penile curvature angle (degrees) = 0° | <0.0001 |
| Penile pain | 26 | Mean VAS score = 4.6 | 0 | Mean VAS score = 0 | <0.0001 |
| Erectile dysfunction | 19 | Mean IIEF score = 21.9 | 50 | Mean IIEF score = 22.4 | 0.469 |
| Cigarette smoking | 16 | Mean No. of cigarettes per day = 9.3 | 17 | Mean No. of cigarettes per day = 8.76 | 0.808 |
| Clinical Condition Associated in PD Patients | N. Patients (out 49) | Type of Statistical Analysis Used and (p-Value) | Correlation with Penile OS Index Values (YES or NO) | Type of Statistical Analysis Used and (p-Value) | Correlation with Systemic OS Index Values (YES or NO) | ||
|---|---|---|---|---|---|---|---|
| Anxiety | 40 | t-test (p = 0.153) | Pearson correlation coefficient (p = 0.094) | NO | t-test (p = 0.281) | Pearson correlation coefficient (p = 0.524) | NO |
| Depression | 29 | t-test (p = 0.781) | Pearson correlation coefficient (p = 0.901) | NO | t-test (p = 0.184) | Pearson correlation coefficient (p = 0.171) | NO |
| Penile pain | 26 | t-test (p = 0.221) | Pearson correlation coefficient (p = 0.081) | NO | t-test (p = 0.676) | Pearson correlation coefficient (p = 0.834) | NO |
| Penile curvature | 45 | t-test (p = 0.920) | Pearson correlation coefficient (p = 0.704) | NO | t-test (p = 0.796) | Pearson correlation coefficient (p = 0.290) | NO |
| Erectile dysfunction | 19 | t-test (p = 0.753) | Pearson correlation coefficient (p = 0.774) | NO | t-test (p = 0.611) | Pearson correlation coefficient (p = 0.655) | NO |
| Cigarette smoking | 16 | t-test (p = 0.488) | Pearson correlation coefficient (p = 0.380) | NO | t-test (p = 0.564) | Pearson correlation coefficient (p = 0.381) | NO |
| PHQ-9 Score Range | No. Total Cases (%) | |
|---|---|---|
| No depression | 0 | 0 |
| Minimal or mild depression | 1–9 | 20 (40.8) |
| Moderate–Severe depression | 10–27 | 29 (59.1) |
| - Moderate depression | 10–14 | 16 (32.6) |
| - Moderately severe depression | 15–19 | 11 (22.4) |
| - Severe depression | 20–27 | 2 (4.08) |
| TOTAL | 49 | |
| GAD-7 score range | No. total cases (%) | |
| No anxiety | 0 | 0 |
| Minimal or mild anxiety | 1–9 | 6 (12.2) |
| Moderate–Severe anxiety | 10–21 | 40 (81.6) |
| - Moderate anxiety | 10–14 | 20 (40.8) |
| - Severe anxiety | 15–21 | 20 (40.8) |
| TOTAL | 49 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paulis, G.; Paulis, A.; De Giorgio, G.; Quattrocchi, S. Measurement of Oxidative Stress Index (OSI) in Penile Corpora Cavernosa and Peripheral Blood of Peyronie’s Disease Patients: A Report of 49 Cases. Metabolites 2024, 14, 55. https://doi.org/10.3390/metabo14010055
Paulis G, Paulis A, De Giorgio G, Quattrocchi S. Measurement of Oxidative Stress Index (OSI) in Penile Corpora Cavernosa and Peripheral Blood of Peyronie’s Disease Patients: A Report of 49 Cases. Metabolites. 2024; 14(1):55. https://doi.org/10.3390/metabo14010055
Chicago/Turabian StylePaulis, Gianni, Andrea Paulis, Giovanni De Giorgio, and Salvatore Quattrocchi. 2024. "Measurement of Oxidative Stress Index (OSI) in Penile Corpora Cavernosa and Peripheral Blood of Peyronie’s Disease Patients: A Report of 49 Cases" Metabolites 14, no. 1: 55. https://doi.org/10.3390/metabo14010055
APA StylePaulis, G., Paulis, A., De Giorgio, G., & Quattrocchi, S. (2024). Measurement of Oxidative Stress Index (OSI) in Penile Corpora Cavernosa and Peripheral Blood of Peyronie’s Disease Patients: A Report of 49 Cases. Metabolites, 14(1), 55. https://doi.org/10.3390/metabo14010055









