Liquid Chromatography-Tandem Mass Spectrometry Method for Detection and Quantification of Meloxicam and 5′-Carboxymeloxicam in Oral Fluid Samples
Abstract
1. Introduction
2. Experimental Design
2.1. Chemicals and Reagents
2.2. Samples Preparation and Extraction
2.3. Standard Solutions and Analytical Validation
2.4. LC-MS/MS
2.5. Volunteers and Sample Collection for PK Analysis
- (a)
- Area under the curve from zero to the last quantifiable time (AUC0-t);
- (b)
- Expected total clearance (Cl/f);
- (c)
- Volume of distribution (Vd/F);
- (d)
- Drug elimination half-life (t1/2);
- (e)
- Elimination constant (Kel);
- (f)
- (Tmax);
- (g)
- Estimated maximum observed concentration value (Cmax).
2.6. Statistical Analysis
3. Results
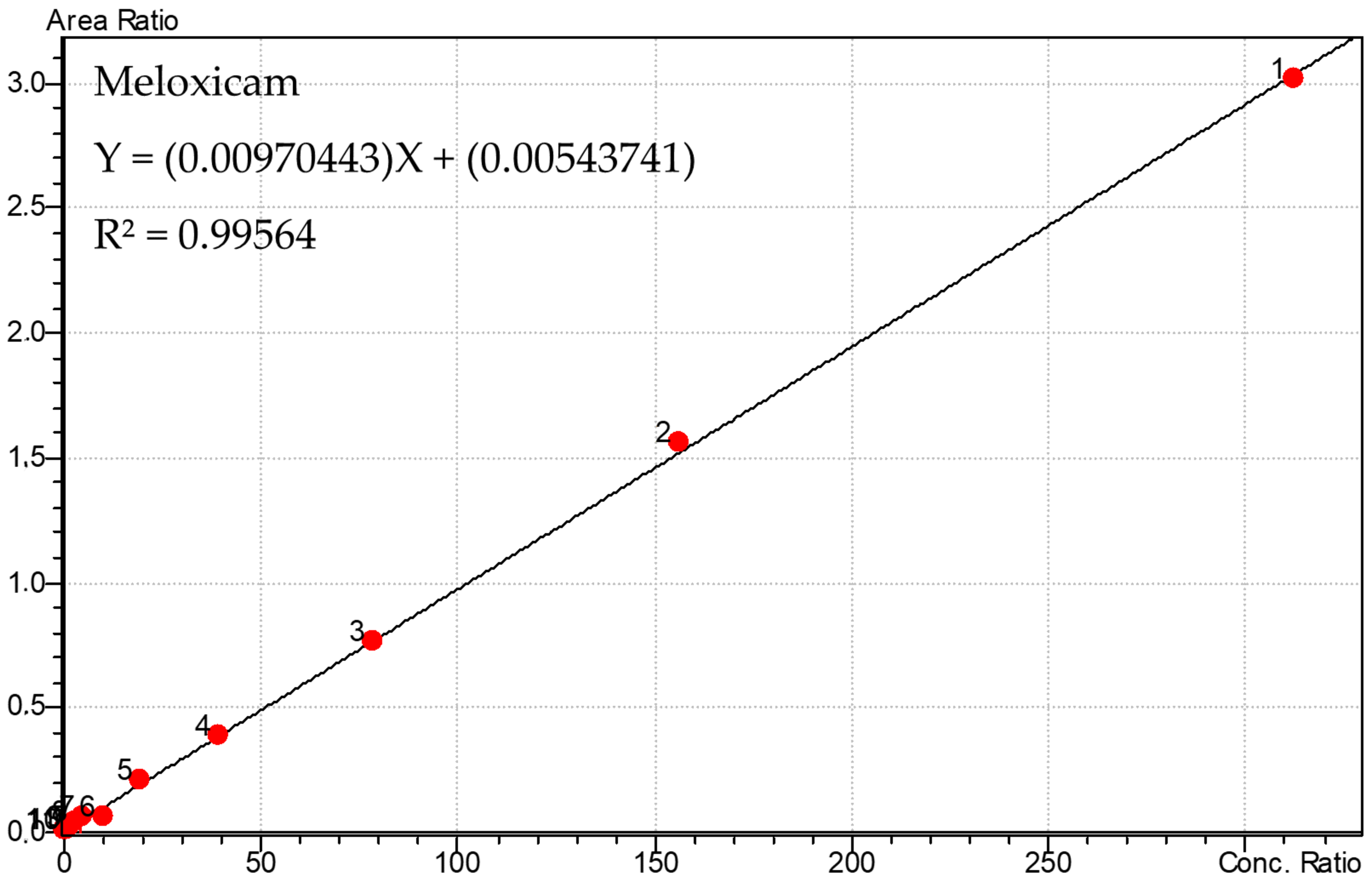
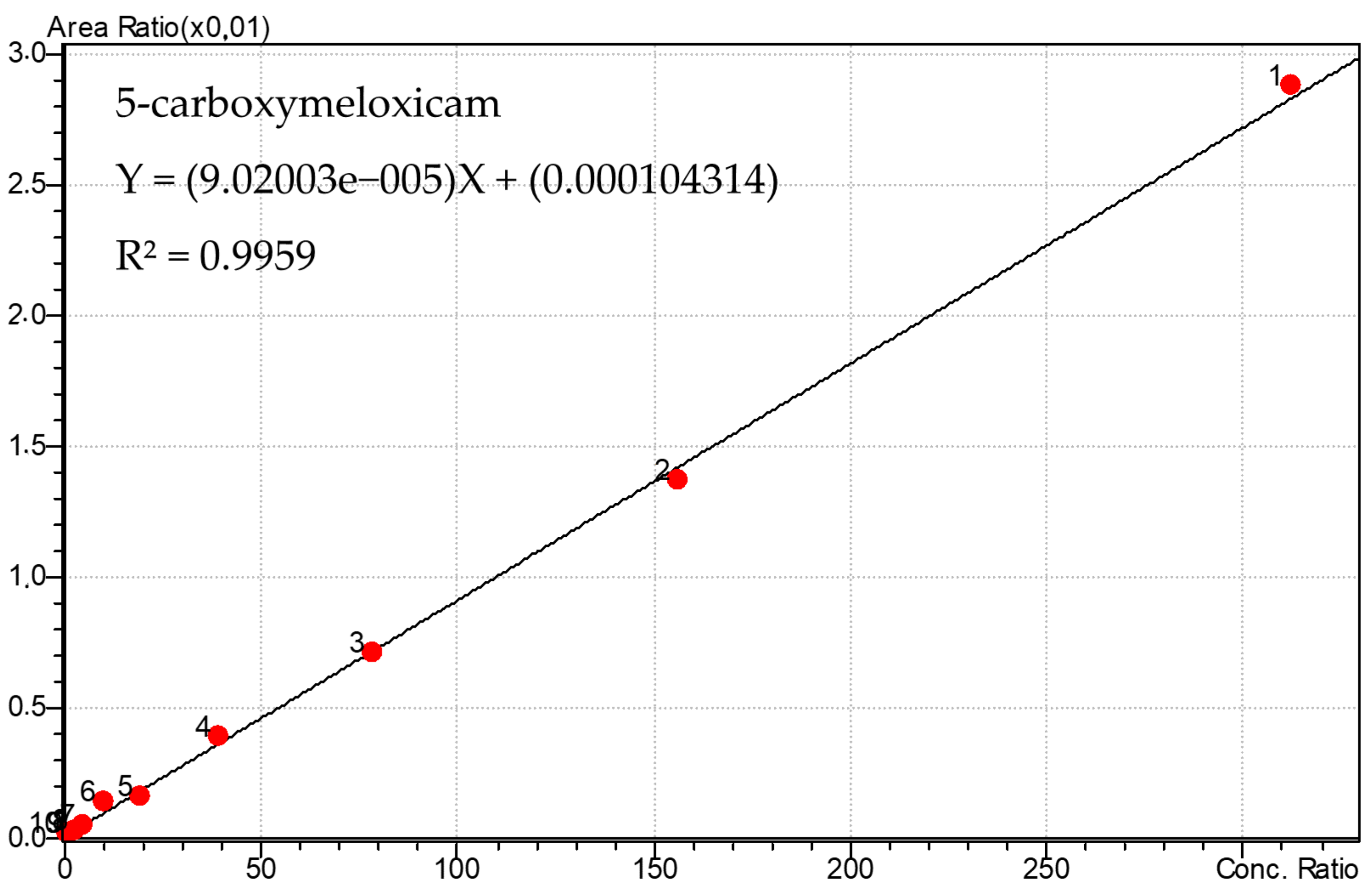
3.1. Calibration Curve of Meloxicam and 5′-Carboxymeloxicam and PGE2
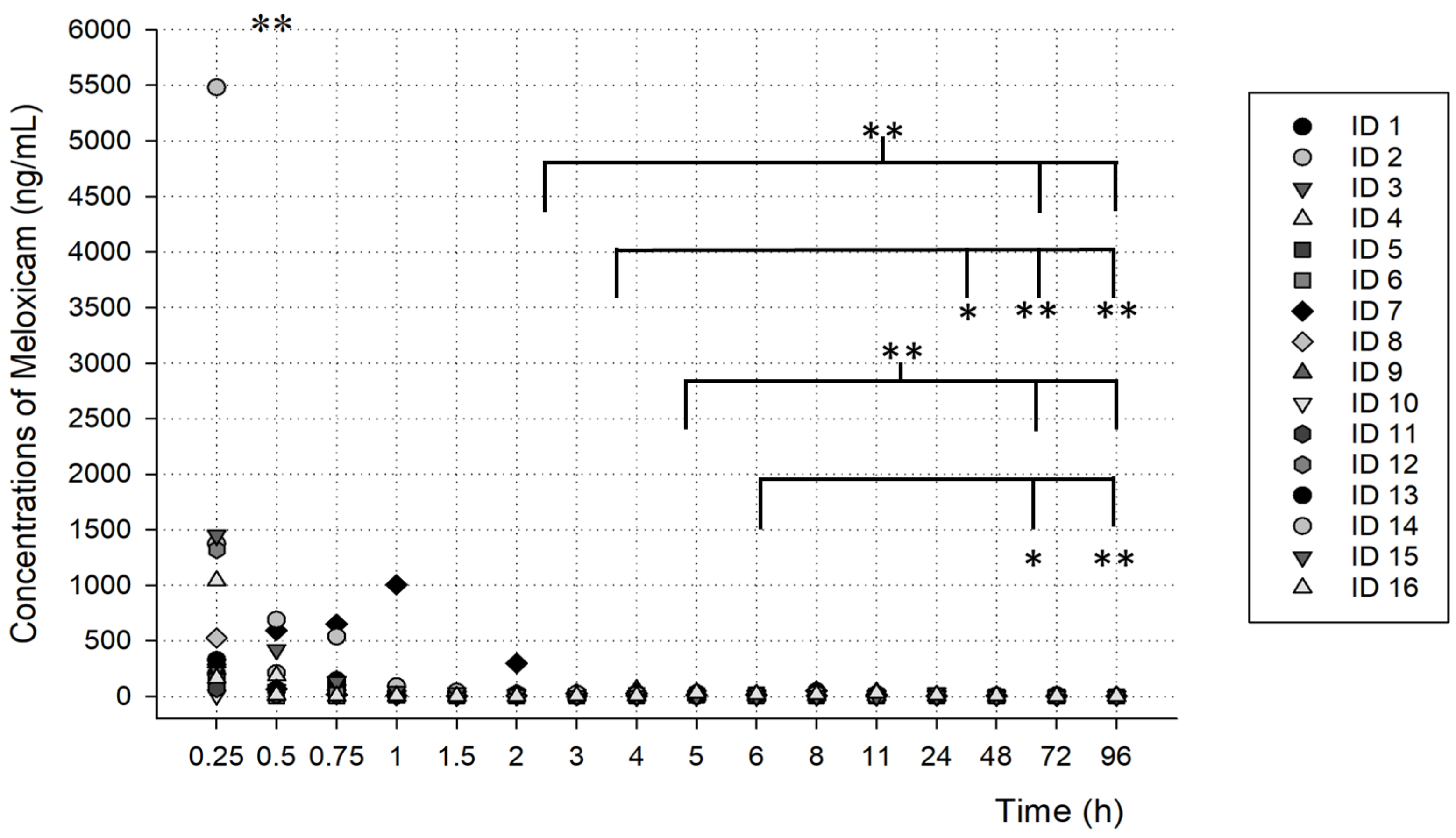
3.2. PK Analysis
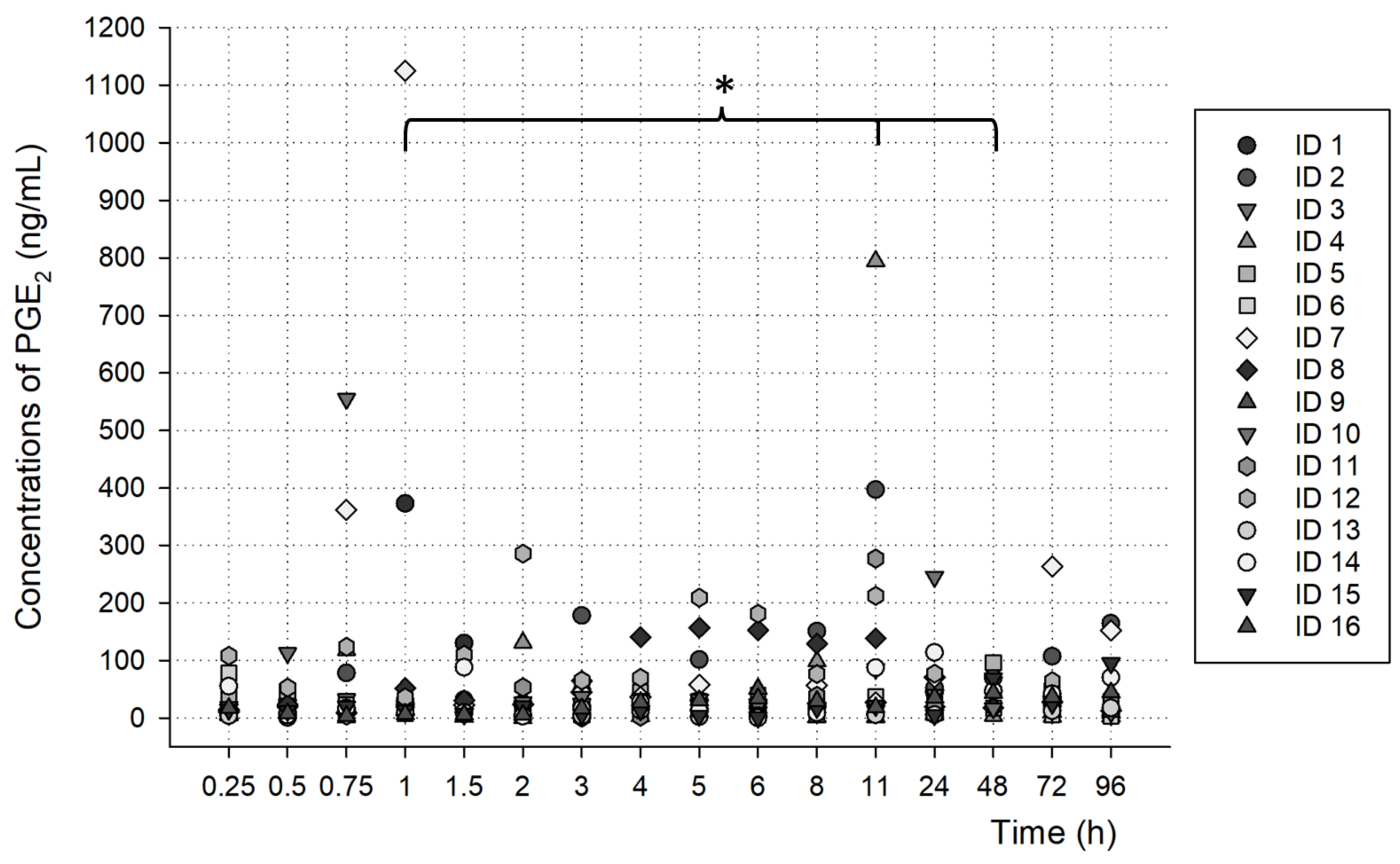
3.3. PGE2 Analysis
3.4. Method Validation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Katz, J.A. COX-2 inhibition: What we learned—A controversial update on safety data. Pain Med. 2013, 14 (Suppl. 1), S29–S34. [Google Scholar] [CrossRef] [PubMed]
- Türck, D.; Roth, W.; Busch, U. A review of the clinical pharmacokinetics of meloxicam. Br. J. Rheumatol. 1996, 35 (Suppl. 1), 13–16. [Google Scholar] [CrossRef] [PubMed]
- Bekker, A.; Kloepping, C.; Collingwood, S. Meloxicam in the management of post-operative pain: Narrative review. J. Anaesthesiol. Clin. Pharmacol. 2018, 34, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Khalil, N.Y.; Aldosari, K.F. Chapter Six—Meloxicam. In Profiles of Drug Substances, Excipients, and Related Methodology; Brittain Excipients and Related Methodology; Brittain, H.G., Ed.; Academic Press: Cambridge, MA, USA, 2020; Volume 45, pp. 159–197. ISBN 1871-5125. [Google Scholar]
- Calvo, A.M.; Sakai, V.T.; Giglio, F.P.M.; Modena, K.C.S.; Colombini, B.L.; Benetello, V.; Sakamoto, F.C.; Freire, T.M.S.; Dionísio, T.J.; Lauris, J.R.P.; et al. Analgesic and anti-inflammatory dose-response relationship of 7.5 and 15 mg meloxicam after lower third molar removal: A double-blind, randomized, crossover study. Int. J. Oral Maxillofac. Surg. 2007, 36, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Whirl-Carrillo, M.; Huddart, R.; Gong, L.; Sangkuhl, K.; Thorn, C.F.; Whaley, R.; Klein, T.E. An Evidence-Based Framework for Evaluating Pharmacogenomics Knowledge for Personalized Medicine. Clin. Pharmacol. Ther. 2021, 110, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Whirl-Carrillo, M.; McDonagh, E.M.; Hebert, J.M.; Gong, L.; Sangkuhl, K.; Thorn, C.F.; Altman, R.B.; Klein, T.E. Pharmacogenomics knowledge for personalized medicine. Clin. Pharmacol. Ther. 2012, 92, 414–417. [Google Scholar] [CrossRef]
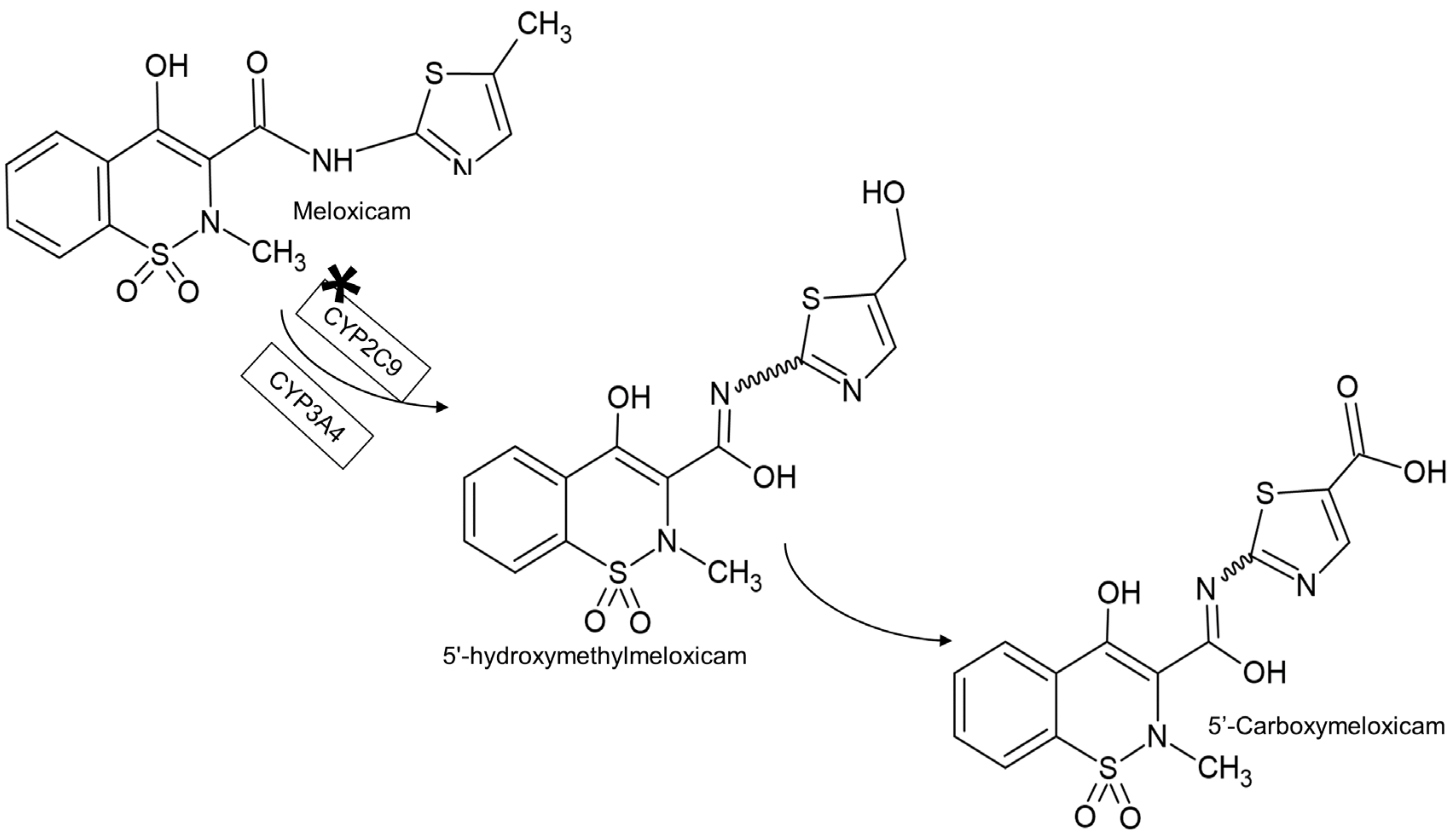
- Prasad, G.S.; Srisailam, K.; Sashidhar, R.B. Metabolic inhibition of meloxicam by specific CYP2C9 inhibitors in Cunninghamella blakesleeana NCIM 687: In silico and in vitro studies. Springerplus 2016, 5, 166. [Google Scholar] [CrossRef]
- Chesné, C.; Guyomard, C.; Guillouzo, A.; Schmid, J.; Ludwig, E.; Sauter, T. Metabolism of Meloxicam in human liver involves cytochromes P4502C9 and 3A4. Xenobiotica 1998, 28, 1–13. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z.; et al. DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids Res. 2018, 46, D1074–D1082. [Google Scholar] [CrossRef]
- Ludwig, E.; Schmid, J.; Beschke, K.; Ebner, T. Activation of human cytochrome P-450 3A4-catalyzed meloxicam 5′-methylhydroxylation by quinidine and hydroquinidine in vitro. J. Pharmacol. Exp. Ther. 1999, 290, 1–8. [Google Scholar]
- Cho, C.; Park, H.-J.; Kang, P.; Moon, S.; Lee, Y.J.; Bae, J.; Jang, C.-G.; Lee, S.-Y. Physiologically based pharmacokinetic (PBPK) modeling of meloxicam in different CYP2C9 genotypes. Arch. Pharm. Res. 2021, 44, 1076–1090. [Google Scholar] [CrossRef] [PubMed]
- Rollason, V.; Samer, C.; Piguet, V.; Dayer, P.; Desmeules, J. Pharmacogenetics of analgesics: Toward the individualization of prescription. Pharmacogenomics 2008, 9, 905–933. [Google Scholar] [CrossRef] [PubMed]
- Vianna-Jorge, R.; Perini, J.A.; Rondinelli, E.; Suarez-Kurtz, G. CYP2C9 genotypes and the pharmacokinetics of tenoxicam in Brazilians. Clin. Pharmacol. Ther. 2004, 76, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Perini, J.A.; Viannajorge, R.; Brogliato, A.; Suarezkurtz, G. Influence of genotypes on the pharmacokinetics and pharmacodynamics of piroxicam. Clin. Pharmacol. Ther. 2005, 78, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Lu, C. PBPK modeling and simulation in drug research and development. Acta Pharm. Sin. B 2016, 6, 430–440. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Kang, P.; Cho, C.; Jung, E.H.; Park, H.-J.; Lee, Y.J.; Bae, J.; Jang, C.-G.; Lee, S.-Y. Physiologically based pharmacokinetic (PBPK) modeling for prediction of celecoxib pharmacokinetics according to CYP2C9 genetic polymorphism. Arch. Pharm. Res. 2021, 44, 713–724. [Google Scholar] [CrossRef]
- Meibohm, B.; Derendorf, H. Basic concepts of pharmacokinetic/pharmacodynamic (PK/PD) modelling. Int. J. Clin. Pharmacol. Ther. 1997, 35, 401–413. [Google Scholar]
- Zou, H.; Banerjee, P.; Leung, S.S.Y.; Yan, X. Application of Pharmacokinetic-Pharmacodynamic Modeling in Drug Delivery: Development and Challenges. Front. Pharmacol. 2020, 11, 997. [Google Scholar] [CrossRef]
- Shin, D.; Lee, S.J.; Ha, Y.-M.; Choi, Y.-S.; Kim, J.-W.; Park, S.-R.; Park, M.K. Pharmacokinetic and pharmacodynamic evaluation according to absorption differences in three formulations of ibuprofen. Drug Des. Dev. Ther. 2017, 11, 135–141. [Google Scholar] [CrossRef]
- Oliveira, G.M.; Dionísio, T.J.; Weckwerth, G.M.; Siqueira-Sandrin, V.S.; Polanco, N.L.D.H.; Faria, F.A.C.; Santos, C.F.; Calvo, A.M. Detection and quantification of prostaglandin E2 in saliva by liquid chromatography-tandem mass spectrometry using microextraction by packed sorbent. Prostaglandins Other Lipid Mediat. 2022, 163, 106672. [Google Scholar] [CrossRef]
- Horning, M.G.; Brown, L.; Nowlin, J.; Lertratanangkoon, K.; Kellaway, P.; Zion, T.E. Use of saliva in therapeutic drug monitoring. Clin. Chem. 1977, 23, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Idkaidek, N.; Arafat, T. Saliva versus Plasma Pharmacokinetics: Theory and Application of a Salivary Excretion Classification System. Mol. Pharm. 2012, 9, 2358–2363. [Google Scholar] [CrossRef] [PubMed]
- Mucklow, J.C.; Bending, M.R.; Kahn, G.C.; Dollery, C.T.; Mueklow, J.C.; Bending, M.R.; Kahn, G.C.; Dollery, C.T. Drug concentration in saliva. Clin. Pharmacol. Ther. 1978, 24, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Feller, K.; le Petit, G. On the distribution of drugs in saliva and blood plasma. Int. J. Clin. Pharmacol. Biopharm. 1977, 15, 468–469. [Google Scholar] [PubMed]
- Bolani, B.; Oliveira, G.M.; Dionísio, T.J.; Faria, F.A.C.; Fernandes, M.H.R.; Santos, C.F.; Calvo, A.M. Pharmacogenetic and Pharmacokinetic Assays from Saliva Samples Can Guarantee Personalized Drug Prescription. Braz. Dent. J. 2021, 32, 3–8. [Google Scholar] [CrossRef]
- Oliveira, G.M.; Dionísio, T.J.; Siqueira-Sandrin, V.S.; Ferrari, L.A.d.L.; Bolani, B.; Parisi, V.A.; Polanco, N.L.D.H.; Colombini-Ishikiriama, B.L.; Faria, F.A.C.; Santos, C.F.; et al. CYP2C9 Polymorphism Influence in PK/PD Model of Naproxen and 6-O-Desmethylnaproxen in Oral Fluid. Metabolites 2022, 12, 1106. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, G.M.; Dionísio, T.J.; Polanco, N.L.D.H.; Siqueira-Sandrin, V.S.; Faria, F.A.C.; Santos, C.F.; Calvo, A.M. Simultaneous separation of naproxen and 6-O-desmethylnaproxen metabolite in saliva samples by liquid chromatography-tandem mass spectrometry: Pharmacokinetic study of naproxen alone and associated with esomeprazol-Results. PLoS ONE 2022, 17, e0278411. [Google Scholar] [CrossRef]
- Calvo, A.M.; Prado, M.T.d.O.; Dionísio, T.J.; Marques, M.P.; Brozoski, D.T.; Lanchote, V.L.; Faria, F.A.C.; Santos, C.F. Effective method for the detection of piroxicam in human plasma using HPLC. Braz. Oral Res. 2016, 30, e58. [Google Scholar] [CrossRef]
- Calvo, A.M.; Santos, G.M.; Dionísio, T.J.; Marques, M.P.; Brozoski, D.T.; Lanchote, V.L.; Fernandes, M.H.R.; Faria, F.A.C.; Santos, C.F. Quantification of piroxicam and 5′-hydroxypiroxicam in human plasma and saliva using liquid chromatography–tandem mass spectrometry following oral administration. J. Pharm. Biomed. Anal. 2016, 120, 212–220. [Google Scholar] [CrossRef]
- Dionísio, T.J.; Oliveira, G.M.; Morettin, M.; Faria, F.C.; Santos, C.F.; Calvo, A.M. Simultaneous separation of naproxen and 6-O-desmethylnaproxen metabolite in saliva samples by liquid chromatography–tandem mass spectrometry: Pharmacokinetic study of naproxen alone and associated with esomeprazole. PLoS ONE 2020, 15, e0236297. [Google Scholar] [CrossRef]
- Zhan, C.; Wang, C.; Wang, Y.; Xie, H.; Chu, J.; Zhang, R.; Hu, R.; Shen, J.; Jia, Y. Using a stable isotope-labeled internal standard for liquid chromatography-tandem mass spectrometry quantitation of meloxicam in human plasma. Biomed. Chromatogr. 2021, 35, e5217. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.-W.; Kim, M.-J.; Jang, C.-G.; Lee, S.-Y. Determination of meloxicam in human plasma using a HPLC method with UV detection and its application to a pharmacokinetic study. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 859, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Chen, X.; Zhong, D. Determination of meloxicam in human plasma by liquid chromatography-tandem mass spectrometry following transdermal administration. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 852, 650–654. [Google Scholar] [CrossRef]
- Laaks, J.; Jochmann, M.A.; Schmidt, T.C. Solvent-free microextraction techniques in gas chromatography. Anal. Bioanal. Chem. 2012, 402, 565–571. [Google Scholar] [CrossRef]
- Siqueira Sandrin, V.S.; Oliveira, G.M.; Weckwerth, G.M.; Polanco, N.L.D.H.; Faria, F.A.C.; Santos, C.F.; Calvo, A.M. Analysis of Different Methods of Extracting NSAIDs in Biological Fluid Samples for LC-MS/MS Assays: Scoping Review. Metabolites 2022, 12, 751. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rehim, A.; Abdel-Rehim, M. Screening and determination of drugs in human saliva utilizing microextraction by packed sorbent and liquid chromatography-tandem mass spectrometry. Biomed. Chromatogr. 2013, 27, 1188–1191. [Google Scholar] [CrossRef]
- Abdel-Rehim, M.; Altun, Z.; Blomberg, L. Microextraction in packed syringe (MEPS) for liquid and gas chromatographic applications. Part II—Determination of ropivacaine and its metabolites in human plasma samples using MEPS with liquid chromatography/tandem mass spectrometry. J. Mass Spectrom. 2004, 39, 1488–1493. [Google Scholar] [CrossRef]
- Sorribes-Soriano, A.; Verdeguer, J.; Pastor, A.; Armenta, S.; Esteve-Turrillas, F.A. Determination of Third-Generation Synthetic Cannabinoids in Oral Fluids. J. Anal. Toxicol. 2021, 45, 331–336. [Google Scholar] [CrossRef]
- Abdel-Rehim, M.; Pedersen-Bjergaard, S.; Abdel-Rehim, A.; Lucena, R.; Moein, M.M.; Cárdenas, S.; Miró, M. Microextraction approaches for bioanalytical applications: An overview. J. Chromatogr. A 2020, 1616, 460790. [Google Scholar] [CrossRef]
- FDA-2013-D-1020; Bioanalytical Method Validation Guidance for Industry. U.S. FDA: Silver Spring, MD, USA, 2018.
- Araujo, P.; Mengesha, Z.; Lucena, E.; Grung, B. Development and validation of an extraction method for the determination of pro-inflammatory eicosanoids in human plasma using liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2014, 1353, 57–64. [Google Scholar] [CrossRef]
- DailyMed. Meloxicam Tablet. Available online: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=5fe76337-eae5-4e5c-96ef-6cfa97983c6e (accessed on 1 June 2023).
- Guo, C.; Li, D.; Liu, C.; Guo, Z.; Chen, Y. Facile derivatization of ultratrace carboxylic acids in saliva for quantification by HPLC-MS/MS. Anal. Bioanal. Chem. 2018, 410, 4293–4300. [Google Scholar] [CrossRef] [PubMed]
- Higashi, T.; Ichikawa, T.; Inagaki, S.; Min, J.Z.; Fukushima, T.; Toyo’oka, T. Simple and practical derivatization procedure for enhanced detection of carboxylic acids in liquid chromatography-electrospray ionization-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2010, 52, 809–818. [Google Scholar] [CrossRef]
- Modesto Rigato, H.; Duarte Mendes, G.; Carter do Carmo Borges, N.; Moreno, R.A. Meloxicam determination in human plasma by high-performance liquid chromatography coupled with tandem mass spectrometry (LC-MS-MS) in Brazilian bioequivalence studies. Int. J. Clin. Pharmacol. Ther. 2006, 44, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Filippou, O.; Bitas, D.; Samanidou, V. Green approaches in sample preparation of bioanalytical samples prior to chromatographic analysis. J. Chromatogr. B 2017, 1043, 44–62. [Google Scholar] [CrossRef] [PubMed]

| MEPS Procedure Step | The Optimized Parameters |
|---|---|
| Conditioning | Methanol (100 μL) and water (50 μL) |
| Extraction | Draw-ejected, 100 μL of oral fluid |
| Washing | Milli-Q water (50 μL) |
| Elution | 10 mM ammonium acetate + methanol (80 v: 20 v) |
| Washing solution | 100 µL–Methanol |
| Compound | Precursor (m/z) | Product (m/z) | RT (min) | Collision Energy (CE) | Q1 pre-Bias (V) | Q3 pre-Bias (V) | Dwell Time | |
|---|---|---|---|---|---|---|---|---|
| Meloxicam | negative | 350.20 | 286.05 | 0.886 | 14.0 | 27 | 19 | 21 |
| 146.25 | 23.0 | 17 | 13 | |||||
| 5′-carboxymeloxicam | positive | 381.9 | 144.95 | 0.722 | −20 | −14 | −27 | 21 |
| 170.95 | −22 | −19 | −18 | |||||
| Meloxicam-D3 (IS) | negative | 353.10 | 289.25 149.20 | 0.888 | 14 20 | 14 | 19 15 | 21 |
| Prostaglandin E2 | negative | 351.40 | 271.20 | 0.949 | 18 | 27 | 12 | 21 |
| 315.20 | 12 | 21 |
| Female (n) | Male (n) | Age—Years (Mean ± SD) | Body Weight—kg (Mean ± SD) | Height—m (Mean ± SD) |
|---|---|---|---|---|
| 12 | 4 | 31.4 ± 9.9 | 75.8 ± 21.5 | 1.7 ± 0.1 |
| Mean ± SD | ||
|---|---|---|
| PK Parameters | Meloxicam | 5′-Carboxymeloxicam |
| AUC0-t (h× ng/mL) | 532.26 ± 815.55 | 134,733.7 ± 350,579.04 |
| Cl/F (L/h) | 101,938.84 ± 102,796.59 | 48,055.38 ± 61,627.98 |
| Cmax (ng/mL) | 2145.22 ± 4220.51 | 105.07 ± 233.15 |
| Kel (1/h) | 31.57 ± 20.81 | 4.95 ± 10.45 |
| T1/2 (h) | 2.48 ± 6.89 | 47.26 ± 144.04 |
| Tmax (h) | 1.41 ± 4.77 | 81.76 ± 236.18 |
| Vd/F (L) | 167.55 ± 415.67 | 21,065.43 ± 3398.62 |
| Meloxicam | 5′-Carboxymeloxicam | |||
|---|---|---|---|---|
| Linearity | ||||
| r2 | 0.99564 | 0.9959 | ||
| Equation of the line | Y = (0.00970443) X + (0.00543741) | Y = (9.02003 × 10−5) X + (0.000104314) | ||
| Low-quality control (ng/mL) | 2.44 | 2.44 | ||
| Precision (CV%; n = 10) | 7.98 | 13.99 | ||
| Accuracy (%) | −2.37 | 0.4 | ||
| Precision (CV%) and Accuracy (RE%) | ||||
| Intra-assay (n = 3) | CV | RE | CV | RE |
| LLOQ (0.6103 ng/mL) | 9.69 | −0.38 | 9.81 | 5.63 |
| LQC (2.44 ng/mL) | 8.78 | −3.63 | 13.17 | 5.41 |
| MQC (78.12 ng/mL) | 11.63 | −9.67 | 6.09 | 4.11 |
| HQC (156.25 ng/mL) | 13.17 | 12.19 | 12.64 | 6.86 |
| DQC (1250 ng/mL; 1:5) | 12.02 | −1.17 | 12.56 | −6.07 |
| Inter-assay (n = 8) | ||||
| LLOQ (0.6103 ng/mL) | 11.38 | 7.43 | 10.84 | 10.87 |
| LQC (2.44 ng/mL) | 8.06 | 9.32 | 11.87 | −2.77 |
| MQC (78.12 ng/mL) | 13.88 | −6.32 | 5.79 | −3.18 |
| HQC (156.25 ng/mL) | 4.75 | 0.59 | 6.02 | −1.7 |
| Stabilities (n = 3) | ||||
| Short-term stability (12 h at 23 °C) | ||||
| LQC (2.44 ng/mL) | 9.8 | 3.78 | ||
| HQC (156.25 ng/mL) | 11.6 | 11.82 | ||
| Post-processing stability (12 h at 4 °C) | ||||
| LQC (2.44 ng/mL) | 13.04 | 7.8 | ||
| HQC (156.25 ng/mL) | 8.3 | 13.99 | ||
| Freeze/thaw cycle stability (−70 °C) | ||||
| LQC (2.44 ng/mL) | −8.7 | −6.53 | ||
| HQC (156.25 ng/mL) | 5.2 | 10.25 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, G.M.; Dionísio, T.J.; Siqueira-Sandrin, V.S.; Ferrari, L.A.d.L.; Colombini-Ishikiriama, B.L.; Faria, F.A.C.; Santos, C.F.; Calvo, A.M. Liquid Chromatography-Tandem Mass Spectrometry Method for Detection and Quantification of Meloxicam and 5′-Carboxymeloxicam in Oral Fluid Samples. Metabolites 2023, 13, 755. https://doi.org/10.3390/metabo13060755
Oliveira GM, Dionísio TJ, Siqueira-Sandrin VS, Ferrari LAdL, Colombini-Ishikiriama BL, Faria FAC, Santos CF, Calvo AM. Liquid Chromatography-Tandem Mass Spectrometry Method for Detection and Quantification of Meloxicam and 5′-Carboxymeloxicam in Oral Fluid Samples. Metabolites. 2023; 13(6):755. https://doi.org/10.3390/metabo13060755
Chicago/Turabian StyleOliveira, Gabriela Moraes, Thiago José Dionísio, Viviane Silva Siqueira-Sandrin, Leticia Alves de Lima Ferrari, Bella Luna Colombini-Ishikiriama, Flávio Augusto Cardoso Faria, Carlos Ferreira Santos, and Adriana Maria Calvo. 2023. "Liquid Chromatography-Tandem Mass Spectrometry Method for Detection and Quantification of Meloxicam and 5′-Carboxymeloxicam in Oral Fluid Samples" Metabolites 13, no. 6: 755. https://doi.org/10.3390/metabo13060755
APA StyleOliveira, G. M., Dionísio, T. J., Siqueira-Sandrin, V. S., Ferrari, L. A. d. L., Colombini-Ishikiriama, B. L., Faria, F. A. C., Santos, C. F., & Calvo, A. M. (2023). Liquid Chromatography-Tandem Mass Spectrometry Method for Detection and Quantification of Meloxicam and 5′-Carboxymeloxicam in Oral Fluid Samples. Metabolites, 13(6), 755. https://doi.org/10.3390/metabo13060755






