Subclinical Reactive Hypoglycemia with Low Glucose Effectiveness—Why We Cannot Stop Snacking despite Gaining Weight
Abstract
1. Introduction
2. Snacking and Obesity
3. Blood Glucose and Appetite
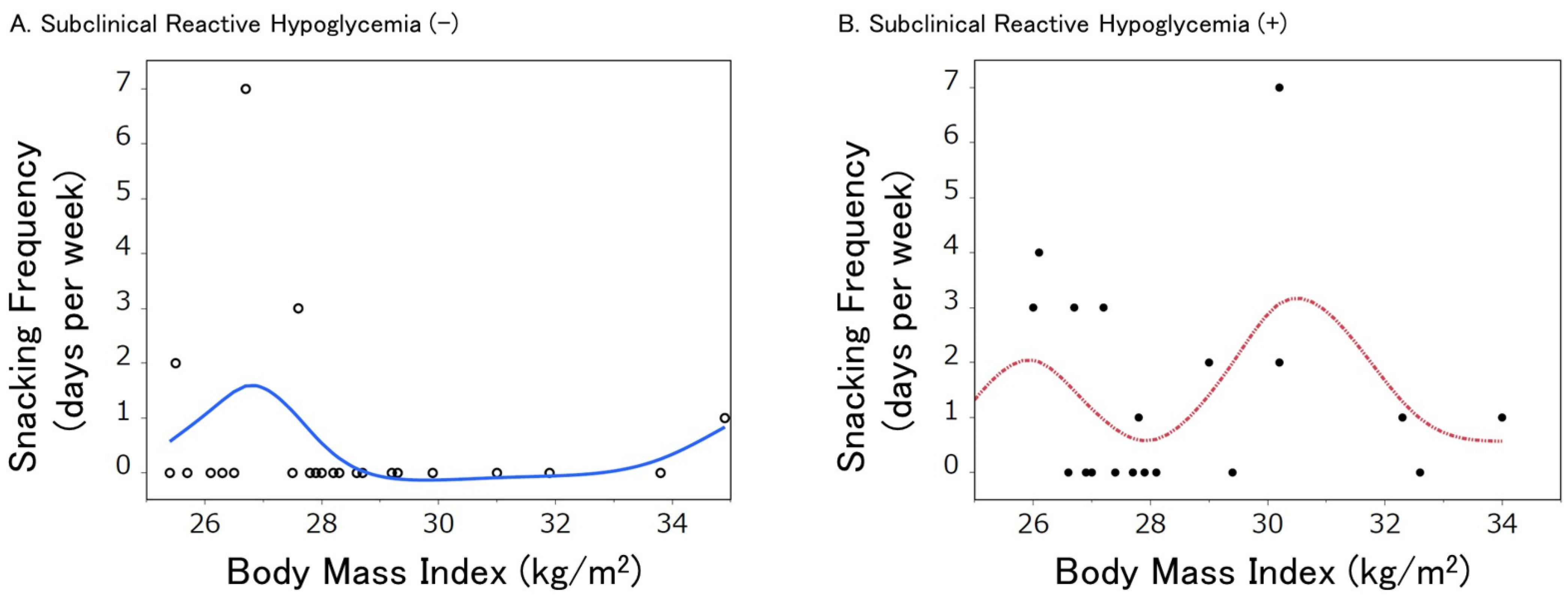
4. Reactive Hypoglycemia and Snacking Frequency in Obese/Overweight
5. Effect of SRH on the Relationship between Snacking Habits and Obesity
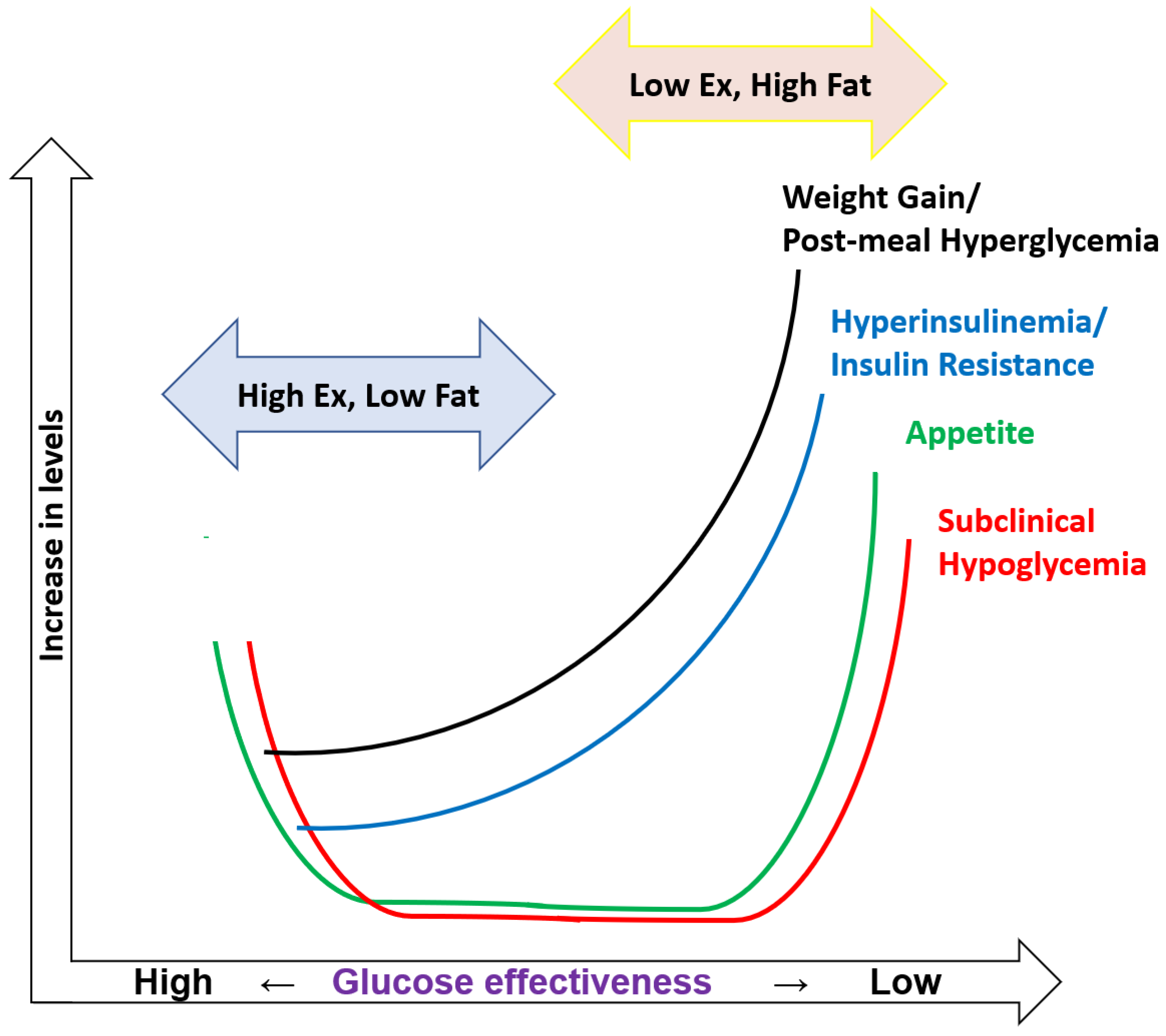
6. Biphasic Effect of Glucose Effectiveness on Reactive Hypoglycemia
7. SRH in Subjects with Higher and Lower Sg
8. Lifestyles Related to Sg
9. Historical Perspectives on SRH-Induced Appetite and Glucose Effectiveness
10. Childhood Lifestyles to Maintain Sg High
11. Possible Pharmacological Intervention
12. Brown Adipose Tissue (BAT) and Sg
13. Postprandial Inflammatory Response and Sg
14. Reactive Hypoglycemia and Inflammation
15. Conclusion and Future Directions
Funding
Conflicts of Interest
References
- World Health Organization. Obesity. Available online: https://www.who.int/health-topics/obesity/ (accessed on 12 June 2023).
- Meldrum, D.R.; Morris, M.A.; Gambone, J.C. Obesity pandemic: Causes, consequences, and solutions-but do we have the will? Fertil. Steril. 2017, 107, 833–839. [Google Scholar] [CrossRef]
- Bellisle, F. Meals and snacking, diet quality and energy balance. Physiol. Behav. 2014, 134, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Skoczek-Rubińska, A.; Bajerska, J. The consumption of energy-dense snacks and some contextual factors of snacking may contribute to higher energy intake and body weight in adults. Nutr. Res. 2021, 96, 20–36. [Google Scholar] [CrossRef] [PubMed]
- Vatanparast, H.; Islam, N.; Masoodi, H.; Shafiee, M.; Patil, R.P.; Smith, J.; Whiting, S.J. Time, location and frequency of snack consumption in different age groups of Canadians. Nutr. J. 2020, 19, 85. [Google Scholar] [CrossRef] [PubMed]
- González-Monroy, C.; Gómez-Gómez, I.; Olarte-Sánchez, C.M.; Motrico, E. Eating Behaviour Changes during the COVID-19 Pandemic: A Systematic Review of Longitudinal Studies. Int. J. Environ. Res. Public Health 2021, 18, 11130. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, I.; Ohashi, A. Subclinical Reactive Hypoglycemia Is Associated with Higher Eating and Snacking Frequencies in Obese or Overweight Men without Diabetes. Endocrines 2022, 3, 530–537. [Google Scholar] [CrossRef]
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 12 June 2023).
- Zizza, C.; Siega-Riz, A.M.; Popkin, B.M. Significant Increase in Young Adults’ Snacking between 1977–1978 and 1994–1996 Represents a Cause for Concern! Prev. Med. 2001, 32, 303–310. [Google Scholar] [CrossRef]
- Murakami, K.; Livingstone, M.B.E. Associations between meal and snack frequency and overweight and abdominal obesity in US children and adolescents from National Health and Nutrition Examination Survey (NHANES) 2003–2012. Br. J. Nutr. 2016, 115, 1819–1829. [Google Scholar] [CrossRef]
- Poorolajal, J.; Sahraei, F.; Mohamdadi, Y.; Doosti-Irani, A.; Moradi, L. Behavioral factors influencing childhood obesity: A systematic review and meta-analysis. Obes. Res. Clin. Pract. 2020, 14, 109–118. [Google Scholar] [CrossRef]
- Tripicchio, G.L.; Kachurak, A.; Davey, A.; Bailey, R.L.; Dabritz, L.J.; Fisher, J.O. Associations between Snacking and Weight Status among Adolescents 12-19 Years in the United States. Nutrients 2019, 11, 1486. [Google Scholar] [CrossRef]
- Keast, D.R.; Nicklas, T.A.; O’Neil, C.E. Snacking is associated with reduced risk of overweight and reduced abdominal obesity in adolescents: National Health and Nutrition Examination Survey (NHANES) 1999–2004. Am. J. Clin. Nutr. 2010, 92, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Kerver, J.M.; Yang, E.J.; Obayashi, S.; Bianchi, L.; Song, W.O. Meal and snack patterns are associated with dietary intake of energy and nutrients in US adults. J. Am. Diet. Assoc. 2006, 106, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Ovaskainen, M.-L.; Reinivuo, H.; Tapanainen, H.; Hannila, M.-L.; Korhonen, T.; Pakkala, H. Snacks as an element of energy intake and food consumption. Eur. J. Clin. Nutr. 2006, 60, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Nudge-It. Snacking—A Cause of Weight Gain? Available online: https://www.nudge-it.eu/topics/snacking-a-cause-of-weight-gain.html (accessed on 12 June 2023).
- Mayer, J. Glucostatic mechanism of regulation of food intake. N. Engl. J. Med. 1953, 249, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, R.K. Hunger—A common symptom of hypoglycemia. Diabetes Care 1993, 16, 1049. [Google Scholar] [CrossRef] [PubMed]
- Ahima, R.S.; Antwi, D.A. Brain regulation of appetite and satiety. Endocrinol. Metab. Clin. N. Am. 2008, 37, 811–823. [Google Scholar] [CrossRef]
- Lewis, D.M.; Oser, T.K.; Wheeler, B.J. Continuous glucose monitoring. BMJ 2023, 380, e072420. [Google Scholar] [CrossRef]
- Kim, J.; Lam, W.; Wang, Q.; Parikh, L.; Elshafie, A.; Sanchez-Rangel, E.; Schmidt, C.; Li, F.; Hwang, J.; Belfort-DeAguiar, R. In a Free-Living Setting, Obesity is Associated with Greater Food Intake in Response to a Similar Pre-Meal Glucose Nadir. J. Clin. Endocrinol. Metab. 2019, 104, 3911–3919. [Google Scholar] [CrossRef]
- Wyatt, P.; Berry, S.E.; Finlayson, G.; O’Driscoll, R.; Hadjigeorgiou, G.; Drew, D.A.; Khatib, H.A.; Nguyen, L.H.; Linenberg, I.; Chan, A.C.; et al. Postprandial glycaemic dips predict appetite and energy intake in healthy individuals. Nat. Metab. 2021, 3, 523–529. [Google Scholar] [CrossRef]
- Sivakumar, T.; Sivakumar, S.; Chaychi, L.; Comi, R.J. A Review of the Use of Acarbose for the Treatment of Post-prandial Syndrome (Reactive Hypoglycemia). Endocrinol. Metabol. Syndr. 2012, S1, 010. [Google Scholar] [CrossRef]
- Nguyen, Q.; Pandya, S.; Chin, K.; Parkin, C.G. Use of Continuous Glucose Monitoring in Detecting Reactive Hypoglycemia in Individuals Without Diabetes. J. Diabetes Sci. Technol. 2018, 12, 1244–1245. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, I.; Ohashi, A. Hyperglycemia During Continuous Glucose Monitoring in Obese/Overweight Male Individuals without Diabetes. J. Diabetes Sci. Technol. 2021, 15, 1198–1199. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, I.; Ohashi, A. Impact of Lifestyle Behaviors on Postprandial Hyperglycemia during Continuous Glucose Monitoring in Adult Males with Overweight/Obesity but without Diabetes. Nutrients 2021, 13, 3092. [Google Scholar] [CrossRef]
- Kishimoto, I.; Ohashi, A. Lower Glucose Effectiveness Is Associated with Postprandial Hyperglycemia in Obese/Overweight Men, Independently of Insulin Secretion. Metabolites 2022, 12, 1022. [Google Scholar] [CrossRef] [PubMed]
- Best, J.D.; Kahn, S.E.; Ader, M.; Watanabe, R.M.; Ni, T.C.; Bergman, R.N. Role of glucose effectiveness in the determination of glucose tolerance. Diabetes Care 1996, 19, 1018–1030. [Google Scholar] [CrossRef]
- Edgerton, D.S.; Cardin, S.; Neal, D.; Farmer, B.; Lautz, M.; Pan, C.; Cherrington, A.D. Effects of hyperglycemia on hepatic gluconeogenic flux during glycogen phosphorylase inhibition in the conscious dog. Am. J. Physiol. Endocrinol. Metab. 2004, 286, E510–E522. [Google Scholar] [CrossRef]
- Petersen, K.F.; Laurent, D.; Rothman, D.L.; Cline, G.W.; Shulman, G.I. Mechanism by which glucose and insulin inhibit net hepatic glycogenolysis in humans. J. Clin. Investig. 1998, 101, 1203–1209. [Google Scholar] [CrossRef]
- Hawkins, M.; Tonelli, J.; Kishore, P.; Stein, D.; Ragucci, E.; Gitig, A.; Reddy, K. Contribution of elevated free fatty acid levels to the lack of glucose effectiveness in type 2 diabetes. Diabetes 2003, 52, 2748–2758. [Google Scholar] [CrossRef]
- Tonelli, J.; Kishore, P.; Lee, D.E.; Hawkins, M. The regulation of glucose effectiveness: How glucose modulates its own production. Curr. Opin. Clin. Nutr. Metab. Care 2005, 8, 450–456. [Google Scholar] [CrossRef]
- Nagasaka, S.; Kusaka, I.; Yamashita, K.; Funase, Y.; Yamauchi, K.; Katakura, M.; Ishibashi, S.; Aizawa, T. Index of glucose effectiveness derived from oral glucose tolerance test. Acta Diabetol. 2012, 49, S195–S204. [Google Scholar] [CrossRef]
- Kishimoto, I.; Ohashi, A. Lower Glucose Effectiveness Is Associated with Subclinical Reactive Hypoglycemia, Snacking Habits, and Obesity. Metabolites 2023, 13, 238. [Google Scholar] [CrossRef] [PubMed]
- Karstoft, K.; Clark, M.A.; Jakobsen, I.; Knudsen, S.H.; van Hall, G.; Pedersen, B.K.; Solomon, T.P.J. Glucose effectiveness, but not insulin sensitivity, is improved after short-term interval training in individuals with type 2 diabetes mellitus: A controlled, randomised, crossover trial. Diabetologia 2017, 60, 2432–2442. [Google Scholar] [CrossRef] [PubMed]
- Araújo-Vilar, D.; Osifo, E.; Kirk, M.; García-Estévez, D.A.; Cabezas-Cerrato, J.; Hockaday, T.D. Influence of moderate physical exercise on insulin-mediated and non-insulin-mediated glucose uptake in healthy subjects. Metabolism 1997, 46, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Nishida, Y.; Higaki, Y.; Tokuyama, K.; Fujimi, K.; Kiyonaga, A.; Shindo, M.; Sato, Y.; Tanaka, H. Effect of mild exercise training on glucose effectiveness in healthy men. Diabetes Care 2001, 24, 1008–1013. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.W.; Hirshman, M.F.; Gervino, E.V.; Ocel, J.V.; Forse, R.A.; Hoenig, S.J.; Aronson, D.; Goodyear, L.J.; Horton, E.S. Acute exercise induces GLUT4 translocation in skeletal muscle of normal human subjects and subjects with type 2 diabetes. Diabetes 1999, 48, 1192–1197. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Hirshman, M.F.; Kurth, E.J.; Winder, W.W.; Goodyear, L.J. Evidence for 5′ AMP-activated protein kinase mediation of the effect of muscle contraction on glucose transport. Diabetes 1998, 47, 1369–1373. [Google Scholar] [PubMed]
- Ahrén, B.; Pacini, G.J. Glucose effectiveness: Lessons from studies on insulin-independent glucose clearance in mice. Diabetes Investig. 2021, 12, 675–685. [Google Scholar] [CrossRef]
- Hari, A.; Fealy, C.; Solomon, T.P.J.; Haus, J.M.; Kelly, K.R.; Barkoukis, H.; Kirwan, J.P. Exercise-induced improvements in glucose effectiveness are blunted by a high glycemic diet in adults with prediabetes. Acta Diabetol. 2019, 56, 211–217. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Historical Background and Evolution of Physical Activity Recommendations. Available online: https://www.cdc.gov/nccdphp/sgr/intro2.htm (accessed on 12 June 2023).
- Woessner, M.N.; Tacey, A.; Levinger-Limor, A.; Parker, A.G.; Levinger, P.; Levinger, I. The Evolution of Technology and Physical Inactivity: The Good, the Bad, and the Way Forward. Front. Public Health 2021, 9, 55491. [Google Scholar] [CrossRef]
- Smilowitz, J.T.; German, J.B.; Zivkovic, A.M. Food Intake and Obesity: The Case of Fat. In Fat Detection: Taste, Texture, and Post Ingestive Effects; Montmayeur, J.P., le Coutre, J., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2010; Chapter 22. [Google Scholar]
- Muskiet, F.A.J. Pathophysiology and Evolutionary Aspects of Dietary Fats and Long-Chain Polyunsaturated Fatty Acids across the Life Cycle. In Fat Detection: Taste, Texture, and Post Ingestive Effects; Montmayeur, J.P., le Coutre, J., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2010; Chapter 2. [Google Scholar]
- University of Cambridge. Why We Just Can’t Stop Eating. Available online: https://www.cam.ac.uk/cantstopeating (accessed on 12 June 2023).
- Centers for Disease Control and Prevention Adult Obesity Map. Available online: https://www.cdc.gov/obesity/data/prevalence-maps.html (accessed on 12 June 2023).
- Centers for Disease Control and Prevention Adult Obesity Facts. Available online: https://www.cdc.gov/obesity/data/adult.html (accessed on 12 June 2023).
- Centers for Disease Control and Prevention State Prevalence Obesity Map. 2011. Available online: https://www.cdc.gov/obesity/downloads/dnpao_state_obesity_prevalence_map_2011_508.pdf (accessed on 12 June 2023).
- Amador, C.; Xia, C.; Nagy, R.; Campbell, A.; Porteous, D.; Smith, B.H.; Hastie, N.; Vitart, V.; Hayward, C.; Navarro, P.; et al. Regional variation in health is predominantly driven by lifestyle rather than genetics. Nat. Commun. 2017, 8, 801. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Youth Obesity Maps (2003–2019). Available online: https://www.cdc.gov/healthyschools/obesity/obesity-youth.htm (accessed on 12 June 2023).
- Kishimoto, I. Trunk-to-Leg Fat Ratio—An Emerging Early Marker of Childhood Adiposity, and Future Cardiometabolic Risks. Circ. J. 2016, 80, 1707–1709. [Google Scholar] [CrossRef] [PubMed]
- Melore, C. Most People Think Their Parents, Culture Largely Influence Their Snacking Habits. Available online: https://studyfinds.org/parents-culture-snacking-habits/ (accessed on 12 June 2023).
- The Washington Post. How Japan’s Revolutionary School Lunches Helped Slow the Rise of Child Obesity. Available online: https://www.washingtonpost.com/news/worldviews/wp/2013/01/28/how-japans-revolutionary-school-lunches-helped-slow-the-rise-of-child-obesity/ (accessed on 12 June 2023).
- Mori, N.; Armada, F.; Willcox, D.C. Walking to school in Japan and childhood obesity prevention: New lessons from an old policy. Am. J. Public Health 2012, 102, 2068–2073. [Google Scholar] [CrossRef] [PubMed]
- Knop, F.K.; Vilsbøll, T.; Madsbad, S.; Holst, J.J.; Krarup, T. Inappropriate suppression of glucagon during OGTT but not during isoglycaemic i.v. glucose infusion contributes to the reduced incretin effect in type 2 diabetes mellitus. Diabetologia 2007, 50, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Plamboeck, A.; Veedfald, S.; Deacon, C.F.; Hartmann, B.; Wettergren, A.; Svendsen, L.B.; Meisner, S.; Hovendal, C.; Knop, F.K.; Vilsbøll, T.; et al. Characterisation of oral and i.v. glucose handling in truncally vagotomised subjects with pyloroplasty. Eur. J. Endocrinol. 2013, 169, 187–201. [Google Scholar] [CrossRef]
- Kimberley, E.L.; Campbell, J.E. The role of GIP in α-cells and glucagon secretion. Peptides 2020, 125, 170213. [Google Scholar]
- Seino, Y.; Fukushima, M.; Daisuke Yabe, D. GIP and GLP-1, the two incretin hormones: Similarities and differences. J. Diabetes Investig. 2010, 1, 8–23. [Google Scholar] [CrossRef] [PubMed]
- Samms, R.J.; Coghlan, M.P.; Sloop, K.W. How May GIP Enhance the Therapeutic Efficacy of GLP-1? Trends Endocrinol. Metab. 2020, 31, 410–421. [Google Scholar] [CrossRef]
- Coskun, T.; Sloop, K.W.; Loghin, C.; Alsina-Fernandez, J.; Urva, S.; Bokvist, K.B.; Cui, X.; Briere, D.A.; Cabrera, O.; Roell, W.C.; et al. LY3298176, a novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: From discovery to clinical proof of concept. Mol. Metab. 2018, 18, 3–14. [Google Scholar] [CrossRef]
- Jastreboff, A.M.; Aronne, L.J.; Ahmad, N.N.; Wharton, S.; Connery, L.; Alves, B.; Kiyosue, A.; Zhang, S.; Liu, B.; Bunck, M.C.; et al. Tirzepatide Once Weekly for the Treatment of Obesity. N. Engl. J. Med. 2022, 387, 205–216. [Google Scholar] [CrossRef]
- O’Mara, A.E.; Johnson, J.W.; Linderman, J.D.; Brychta, R.J.; McGehee, S.; Fletcher, L.A.; Fink, Y.A.; Kapuria, D.; Cassimatis, T.M.; Kelsey, N.; et al. Chronic mirabegron treatment increases human brown fat, HDL cholesterol, and insulin sensitivity. J. Clin. Investig. 2020, 130, 2209–2219. [Google Scholar] [CrossRef]
- NOAA Climate.gov. What’s the Coldest the Earth’s Ever Been? Available online: https://www.climate.gov/news-features/climate-qa/whats-coldest-earths-ever-been (accessed on 12 June 2023).
- Maliszewska, K.; Kretowski, A. Brown Adipose Tissue and Its Role in Insulin and Glucose Homeostasis. Int. J. Mol. Sci. 2021, 22, 1530. [Google Scholar] [CrossRef] [PubMed]
- Meessen, E.C.E.; Warmbrunn, M.V.; Nieuwdorp, M.; Soeters, M.R. Human Postprandial Nutrient Metabolism and Low-Grade Inflammation: A Narrative Review. Nutrients 2019, 11, 3000. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, J.F.; Shah, R.Y.; Shah, R.; Mehta, N.N.; Rickels, M.R.; Reilly, M.P. Activation of innate immunity modulates insulin sensitivity, glucose effectiveness, and pancreatic β-cell function in both African ancestry and European ancestry healthy humans. Metabolism 2015, 64, 513–520. [Google Scholar] [CrossRef]
- Li, M.; Li, L.; Li, B.; Hambly, C.; Wang, G.; Wu, Y.; Jin, Z.; Wang, A.; Niu, C.; Wolfrum, C.; et al. Brown adipose tissue is the key depot for glucose clearance in microbiota depleted mice. Nat. Commun. 2021, 12, 4725. [Google Scholar] [CrossRef]
- Villarroya, F.; Cereijo, R.; Gavaldà-Navarro, A.; Villarroya, J.; Giralt, M. Inflammation of brown/beige adipose tissues in obesity and metabolic disease. J. Intern. Med. 2018, 284, 492–504. [Google Scholar] [CrossRef]
- Galloway, P.J.; Thomson, G.A.; Fisher, B.M.; Semple, C.G. Insulin-induced hypoglycemia induces a rise in C-reactive protein (Letter). Diabetes Care 2000, 23, 861. [Google Scholar] [CrossRef]
- Dotson, S.; Freeman, R.; Failing, H.J.; Adler, G.K. Hypoglycemia increases serum interleukin-6 levels in healthy men and women. Diabetes Care 2008, 31, 1222–1223. [Google Scholar] [CrossRef]
- Wright, R.J.; Newby, D.E.; Stirling, D.; Ludlam, C.A.; Macdonald, I.A.; Frier, B.M. Effects of acute insulin-induced hypoglycemia on indices of inflammation: Putative mechanism for aggravating vascular disease in diabetes. Diabetes Care 2010, 33, 1591–1597. [Google Scholar] [CrossRef]
- Wright, R.J.; Frier, B.M. Vascular disease and diabetes: Is hypoglycaemia an aggravating factor? Diabetes Metab. Res. Rev. 2008, 24, 353–363. [Google Scholar] [CrossRef]
- Ciftci, F.C.; Tugcu, A.U.; Ciftci, O. Sub-clinic atherosclerosis in patients with postprandial reactive hypoglycemia. Ann. Med. Res. 2019, 26, 2941–2947. [Google Scholar] [CrossRef]
- Hopkins, S.J.; Rothwell, N.J. Cytokines and the Nervous System. I: Expression and Recognition. Trends Neurosci. 1995, 18, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jiang, Q.; Wang, L. Appetite Regulation of TLR4-Induced Inflammatory Signaling. Front. Endocrinol. 2021, 12, 777997. [Google Scholar] [CrossRef] [PubMed]
- Kalin, S.; Heppner, F.L.; Bechmann, I.; Prinz, M.; Tschop, M.H.; Yi, C.X. Hypothalamic Innate Immune Reaction in Obesity. Nat. Rev. Endocrinol. 2015, 11, 339–351. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kishimoto, I. Subclinical Reactive Hypoglycemia with Low Glucose Effectiveness—Why We Cannot Stop Snacking despite Gaining Weight. Metabolites 2023, 13, 754. https://doi.org/10.3390/metabo13060754
Kishimoto I. Subclinical Reactive Hypoglycemia with Low Glucose Effectiveness—Why We Cannot Stop Snacking despite Gaining Weight. Metabolites. 2023; 13(6):754. https://doi.org/10.3390/metabo13060754
Chicago/Turabian StyleKishimoto, Ichiro. 2023. "Subclinical Reactive Hypoglycemia with Low Glucose Effectiveness—Why We Cannot Stop Snacking despite Gaining Weight" Metabolites 13, no. 6: 754. https://doi.org/10.3390/metabo13060754
APA StyleKishimoto, I. (2023). Subclinical Reactive Hypoglycemia with Low Glucose Effectiveness—Why We Cannot Stop Snacking despite Gaining Weight. Metabolites, 13(6), 754. https://doi.org/10.3390/metabo13060754




