Effect of Cycling Exercise Resisting Electrically Stimulated Antagonist Muscle Contractions in Healthy Males
Abstract
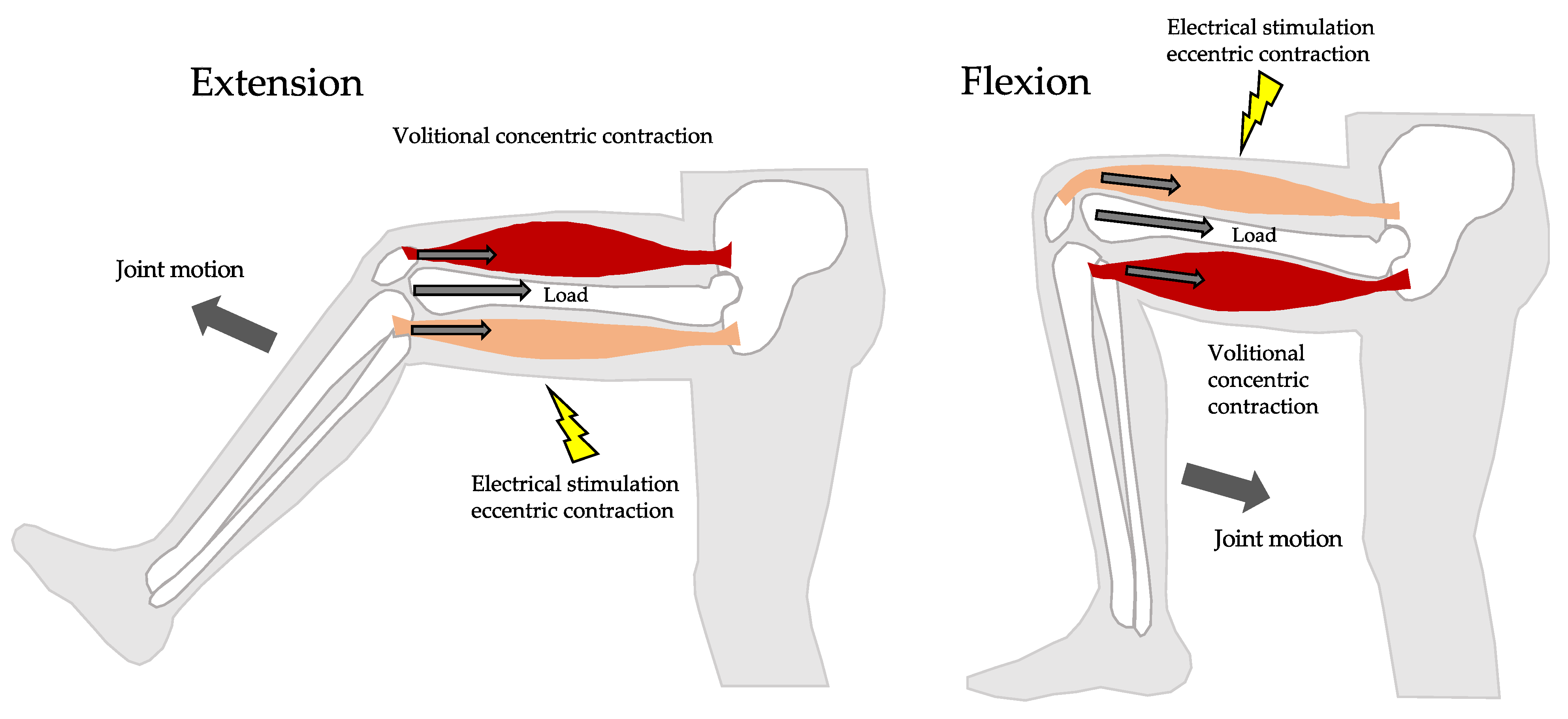
1. Introduction
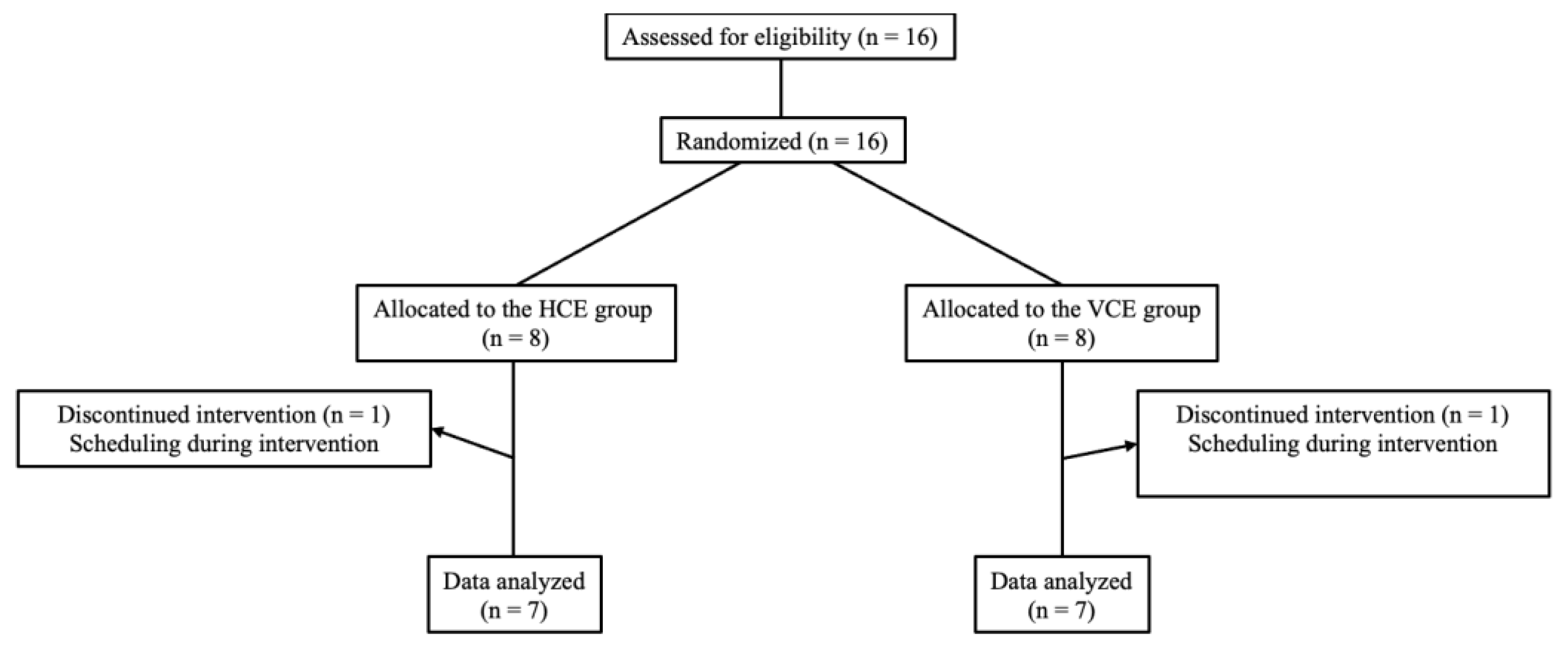
2. Participants and Methods
2.1. Participants
2.2. Training Protocol
2.3. VCE Group Protocol
2.4. HCE Group Protocol
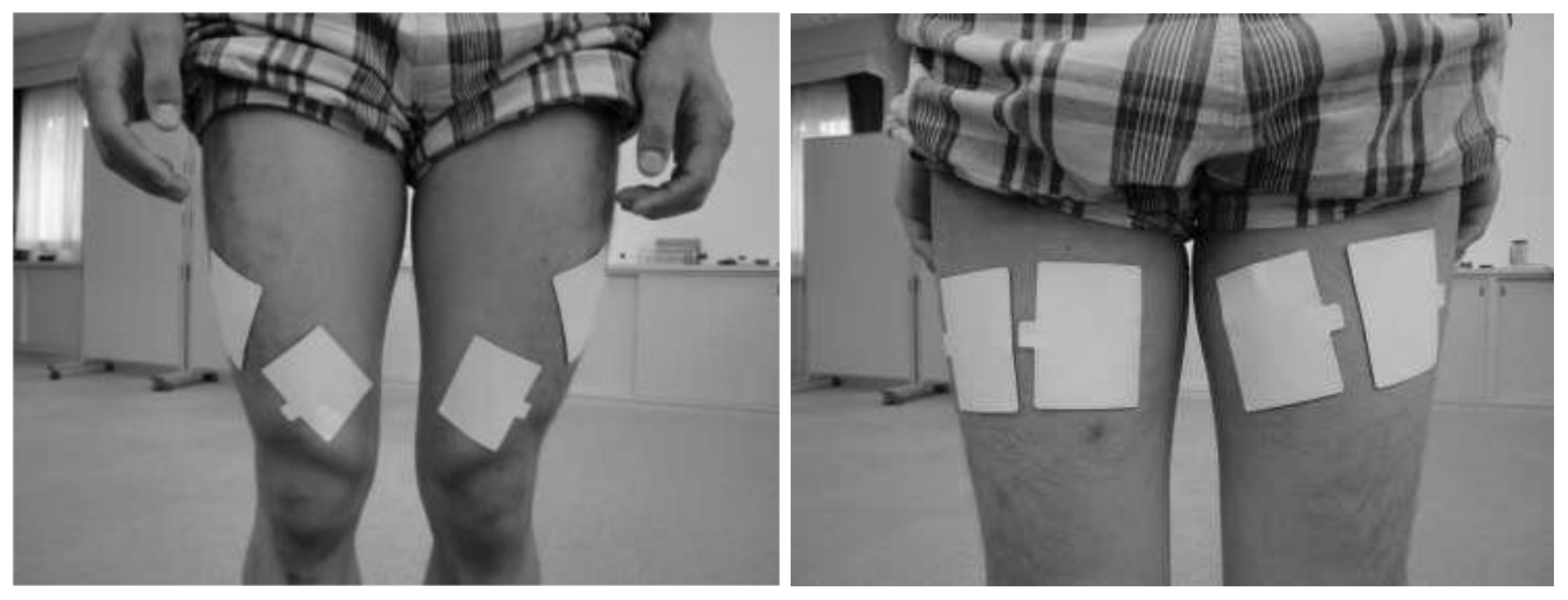
2.5. Electrical Stimulation Protocol
2.6. Evaluations
2.7. Blood Lactate Concentration
2.8. Statical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Muscle Strength
3.3. Muscle Mass
3.4. Peak Oxygen Uptake (O2peak)
3.5. Anaerobic Threshold
3.6. Peak Blood Lactate
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Natalia, T.-S.; Amanda, V.-L.; Kenneth, V.; João, L.Q.D.; Dominique, H.; Gerson, C., Jr. High-intensity interval training versus progressive high-intensity circuit resistance training on endothelial function and cardiorespiratory fitness in heart failure: A preliminary randomized controlled trial. PLoS ONE 2021, 16, e0257607. [Google Scholar]
- Bocalini, D.S.; Lima, L.S.; de Andrade, S.; Madureira, A.; Rica, R.L.; dos Santos, R.N.; Serra, A.J.; Silva, J.A., Jr.; Rodriguez, D.; Figueira, A., Jr.; et al. Effects of circuit-based exercise programs on the body composition of elderly obese women. Clin. Interv. Aging 2012, 7, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Fyfe, J.J.; Bishop, D.J.; Stepto, N.K. Interference between concurrent resistance and endurance exercise: Molecular bases and the role of individual training variables. Sports Med. Adis Int. Ltd. 2014, 44, 743–762. [Google Scholar] [CrossRef] [PubMed]
- Macaluso, A.; Young, A.; Gibb, K.S.; Rowe, D.A.; de Vito, G. Cycling as a novel approach to resistance training increases muscle strength, power, and selected functional abilities in healthy older women. J. Appl. Physiol. 2003, 95, 2544–2553. [Google Scholar] [CrossRef] [PubMed]
- Cabric, M.; Appell, H.J.; Resic, A. Effects of electrical stimulation of different frequencies on the myonuclei and fiber size in human muscle. Int. J. Sports Med. 1987, 8, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Gondin, J.; Guette, M.; Ballay, Y.; Martin, A. Electromyostimulation training effects on neural drive and muscle architecture. Med. Sci. Sports Exerc. 2005, 37, 1291–1299. [Google Scholar] [CrossRef] [PubMed]
- Maffiuletti, N.A.; Dugnani, S.; Folz, M.; Pierno, D.; Mauro, F. Effect of combined electrostimulation and plyometric training on vertical jump height. Med. Sci. Sports Exerc. 2002, 34, 1638–1644. [Google Scholar] [CrossRef]
- Benavent-Caballer, V.; Rosado-Calatayud, P.; Segura-Ortí, E.; Amer-Cuenca, J.J.; Lisón, J.F. Effects of three different low-intensity exercise interventions on physical performance, muscle CSA and activities of daily living: A randomized controlled trial. Exp. Gerontol. 2014, 58, 159–165. [Google Scholar] [CrossRef]
- Paillard, T. Combined Application of Neuromuscular Electrical Stimulation and Voluntray Muscular Contractions. Sports Med. 2008, 38, 161–177. [Google Scholar] [CrossRef]
- Shiba, N.; Matsuse, H.; Takano, Y.; Yoshimitsu, K.; Omoto, M.; Hashida, R.; Tagawa, Y.; Inada, T.; Yamada, S.; Ohshima, H. Electrically stimulated antagonist muscle contraction increased muscle mass and bone mineral density of one astronaut-Initial verification on the International Space Station. PLoS ONE 2015, 10, e0134736. [Google Scholar]
- Yanagi, T.; Shiba, N.; Maeda, T.; Iwasa, K.; Umezu, Y.; Tagawa, Y.; Matsuo, S.; Nagata, K.; Yamamoto, T.; Basford, J.R. Agonist contractions against electrically stimulated antagonists. Arch. Phys. Med. Rehabil. 2003, 84, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Takano, Y.; Haneda, Y.; Maeda, T.; Sakai, Y.; Matsuse, H.; Kawaguchi, T.; Tagawa, Y.; Shiba, N. Increasing muscle strength and mass of thigh in elderly people with the hybrid-training method of electrical stimulation and volitional contraction. Tohoku J. Exp. Med. 2010, 221, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Taniguchi, Y.; Moritani, T. Metabolic and cardiovascular responses during voluntary pedaling exercise with electrical muscle stimulation. Eur. J. Appl. Physiol. 2014, 114, 1801–1807. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Kang, S.Y.; Im, S.H.; Kim, B.R.; Kim, S.M.; Yoon, H.M.; Han, E.Y. The effects of assisted ergometer training with a functional electrical stimulation on exercise capacity and functional ability in subacute stroke patients. Ann. Rehabil. Med. 2013, 37, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Masayuki, O.; Matsuse, H.; Takano, Y.; Yamada, S.; Ohshima, H.; Tagawa, Y.; Shiba, N. Oxygen Uptake during Aerobic Cycling Exercise Simultaneously Combined with Neuromuscular Electrical Stimulation of Antagonists. J. Nov. Physiother. 2013, 3, 185. [Google Scholar] [CrossRef]
- Hultman, E.; Spriet, L.L. With 1 text-figure skeletal muscle metabolism, contraction force and glycogen utilization during prolonged electrical stimulation in humans. J. Physiol. 1986, 374, 493–501. [Google Scholar] [CrossRef]
- Omoto, M.; Matsuse, H.; Hashida, R.; Takano, Y.; Yamada, S.; Ohshima, H.; Tagawa, Y.; Shiba, N. Cycling exercise with electrical stimulation of antagonist muscles increases plasma growth hormone and IL-6. Tohoku J. Exp. Med. 2015, 237, 209–217. [Google Scholar] [CrossRef]
- Tomoaki, M.; Rina, S.; Kiyoji, T.; Chiaki, M. High-intensity interval aerobic exercise training (HIAT) in occupational health. J. Phys. Fit. Sports Med. 2021, 10, 145–150. [Google Scholar]
- McCleary, R.W.; Andersen, J.C. Test-Retest Reliability of Reciprocal Isokinetic Knee Extension and Flexion Peak Torque Measurements. J. Athl. Train. 1992, 27, 362–365. [Google Scholar]
- Tanner, R.K.; Fuller, K.L.; Ross, M.L.R. Evaluation of three portable blood lactate analysers: Lactate Pro, Lactate Scout and Lactate Plus. Eur. J. Appl. Physiol. 2010, 109, 551–559. [Google Scholar] [CrossRef]
- Dorel, S.; Couturier, A.; Hug, F. Intra-session repeatability of lower limb muscles activation pattern during pedaling. J. Electromyogr. Kinesiol. 2008, 18, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Hortobágyi, T.; Maffiuletti, N.A. Neural adaptations to electrical stimulation strength training. Eur. J. Appl. Physiol. 2011, 111, 2439–2449. [Google Scholar] [CrossRef] [PubMed]
- Gladden, L.B. Lactate metabolism: A new paradigm for the third millennium. J. Physiol. 2004, 558, 5–30. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.L.; Wu, H.Y.; Tian, Z.X.; Luo, Z.; Wu, Y.F.; Zhao, J. Electrical stimulation induces mitochondrial autophagy via activating oxidative stress and Sirt3 signaling pathway. Chin. Med. J. 2021, 134, 628–630. [Google Scholar] [CrossRef] [PubMed]
- Terence, E.R.; Melissa, L.E.; Young, H.-J.; Kevin, K.M. Case Report: Endurance Electrical Stimulation Training Improves Skeletal Muscle Oxidative Capacity in Chronic Spinal Cord Injury. Arch. Phys. Med. Rehabil. 2013, 94, 2559–2561. [Google Scholar]
- Craig, D.M.; Ashcroft, S.P.; Belew, M.Y.; Stocks, B.; Currell, K.; Baar, K.; Philp, A. Utilizing small nutrient compounds as enhancers of exercise-induced mitochondrial biogenesis. Front. Physiol. 2015, 6, 296. [Google Scholar] [CrossRef]
| HCE | VCE | ||||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p | |||
| Height (cm) | 168.57 | ± | 4.04 | 171.43 | ± | 11.49 | 0.55 # |
| Body mass (Kg) | 59.29 | ± | 4.54 | 65.00 | ± | 10.88 | 0.22 # |
| Knee extension muscle strength at 60°/s (Nm/kg) | 137.61 | ± | 34.75 | 156.64 | ± | 35.48 | 0.33 # |
| Knee extension muscle strength at 180°/s (Nm/kg) | 76.49 | ± | 22.23 | 104.43 | ± | 17.92 | 0.02 # |
| Knee flexion muscle strength at 60°/s (Nm/kg) | 78.74 | ± | 13.71 | 92.53 | ± | 17.22 | 0.12 # |
| Knee flexion muscle strength at 180°/s (Nm/kg) | 44.97 | ± | 10.63 | 67.97 | ± | 11.82 | 0.12 # |
| Resting blood lactate (mmol/L) | 2.24 | ± | 0.81 | 2.14 | ± | 1.12 | 0.85 # |
| Peak blood lactate (mmol/L) | 8.86 | ± | 3.18 | 8.36 | ± | 3.46 | 0.57 † |
| Quadricep muscle cross-sectional area (mm2) | 6866.24 | ± | 613.32 | 8021.56 | ± | 655.04 | 0.20 # |
| Hamstring muscle cross-sectional area (mm2) | 6310.02 | ± | 1291.11 | 7169.85 | ± | 568.80 | 0.57 † |
| Peak oxygen uptake (mL/kg/min) | 40.50 | ± | 4.08 | 39.27 | ± | 3.51 | 0.56 # |
| Anaerobic thresholds (mL/kg/min) | 18.40 | ± | 1.58 | 18.93 | ± | 1.84 | 0.58 # |
| HCE | VCE | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | p | Before | After | p | |||||||||
| Knee extension muscle strength at 60°/s (Nm/kg) | 137.61 | ± | 34.75 | 156.66 | ± | 27.81 | 0.16 ## | 156.64 | ± | 35.48 | 173.21 | ± | 32.02 | 0.18 ## |
| Knee extension muscle strength at 180°/s (Nm/kg) | 76.49 | ± | 22.23 | 95.41 | ± | 18.33 * | 0.049 ## | 104.43 | ± | 17.92 | 106.20 | ± | 21.10 | 0.77 ## |
| Knee flexion muscle strength at 60°/s (Nm/kg) | 78.74 | ± | 13.71 | 94.30 | ± | 15.63 * | 0.04 ## | 92.53 | ± | 17.22 | 105.54 | ± | 14.21 | 0.06 ## |
| Knee flexion muscle strength at 180°/s (Nm/kg) | 44.97 | ± | 10.63 | 67.54 | ± | 20.06 * | 0.01 ## | 67.97 | ± | 11.82 | 74.14 | ± | 14.80 | 0.36 ## |
| Resting blood lactate (mmol/L) | 2.24 | ± | 0.81 | 1.97 | ± | 0.88 | 0.60 ## | 2.14 | ± | 1.12 | 2.37 | ± | 1.78 | 0.82 ## |
| Peak blood lactate (mmol/L) | 8.86 | ± | 3.18 | 5.29 | ± | 1.48 * | 0.02 †† | 8.36 | ± | 3.46 | 6.39 | ± | 2.39 | 0.23 †† |
| Quadricep muscle cross-sectional area (mm2) | 6866.24 | ± | 613.32 | 7587.55 | ± | 812.02 * | 0.01 ## | 8021.56 | ± | 655.04 | 8222.35 | ± | 743.07 | 0.10 ## |
| Hamstring muscle cross-sectional area (mm2) | 6310.02 | ± | 1291.11 | 6670.20 | ± | 1509.56 * | 0.03 †† | 7169.85 | ± | 568.80 | 7459.84 | ± | 499.84 * | 0.03 †† |
| Peak oxygen uptake (mL/kg/min) | 40.50 | ± | 4.10 | 44.17 | ± | 5.50 * | 0.02 ## | 39.27 | ± | 3.51 | 39.17 | ± | 2.60 | 0.93 ## |
| Anaerobic thresholds (mL/kg/min) | 18.40 | ± | 1.60 | 22.99 | ± | 2.36 * | 0.01 ## | 18.93 | ± | 1.84 | 19.43 | ± | 2.23 | 0.46 ## |
| Difference of Means (HCE-VCE) | 95%CI | p | |
|---|---|---|---|
| Knee extension muscle strength at 60°/s (Nm/kg) | 2.47 | −32.65, 37.59 | 0.70 α |
| Knee extension muscle strength at 180°/s (Nm/kg) | 17.16 | −3.83, 38.15 | 0.14 α |
| Knee flexion muscle strength at 60°/s (Nm/kg) | 2.54 | −14.94, 20.02 | 0.76 β |
| Knee flexion muscle strength at 180°/s (Nm/kg) | 16.40 | −0.98, 33.78 | 0.06 β |
| Peak blood lactate (mmol/L) | −1.59 | −5.32, 2.14 | 0.31 β |
| Quadricep muscle cross-sectional area (mm2) | 520.51 | 217.88, 823.15 | 0.01 β |
| Hamstring muscle cross-sectional area (mm2) | 70.19 | −284.33, 424.71 | 0.67 β |
| Peak oxygen uptake (mL/kg/min) | 3.77 | 0.24, 7.31 | 0.04 β |
| Anaerobic thresholds (mL/kg/min) | 4.09 | 1.60, 6.57 | 0.01 α |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omoto, M.; Tsukada, Y.; Hashida, R.; Matsuse, H.; Tajima, H.; Iwanaga, S.; Takano, Y.; Nago, T.; Tagawa, Y.; Shiba, N. Effect of Cycling Exercise Resisting Electrically Stimulated Antagonist Muscle Contractions in Healthy Males. Metabolites 2023, 13, 604. https://doi.org/10.3390/metabo13050604
Omoto M, Tsukada Y, Hashida R, Matsuse H, Tajima H, Iwanaga S, Takano Y, Nago T, Tagawa Y, Shiba N. Effect of Cycling Exercise Resisting Electrically Stimulated Antagonist Muscle Contractions in Healthy Males. Metabolites. 2023; 13(5):604. https://doi.org/10.3390/metabo13050604
Chicago/Turabian StyleOmoto, Masayuki, Yuya Tsukada, Ryuki Hashida, Hiroo Matsuse, Hiroshi Tajima, Sohei Iwanaga, Yoshio Takano, Takeshi Nago, Yoshihiko Tagawa, and Naoto Shiba. 2023. "Effect of Cycling Exercise Resisting Electrically Stimulated Antagonist Muscle Contractions in Healthy Males" Metabolites 13, no. 5: 604. https://doi.org/10.3390/metabo13050604
APA StyleOmoto, M., Tsukada, Y., Hashida, R., Matsuse, H., Tajima, H., Iwanaga, S., Takano, Y., Nago, T., Tagawa, Y., & Shiba, N. (2023). Effect of Cycling Exercise Resisting Electrically Stimulated Antagonist Muscle Contractions in Healthy Males. Metabolites, 13(5), 604. https://doi.org/10.3390/metabo13050604






