Abstract
Secondary metabolites have been shown to possess a range of biological functions. Flavonoids, due to their ability to scavenge ROS, are famous antioxidants. The plants of Artemisia species are rich sources of flavonoids; however, the amount of these metabolites is less. In the current study, the flavonoid content was detected and then enhanced by genetically modifying the Artemisia carvifolia Buch with Agrobacterium tumefaciens strain GV3101 carrying rol A gene. The transformation of rol A gene was confirmed with PCR and the gene copy number was confirmed by Southern blot analysis. The HPLC analysis revealed the presence of catechin (3.19 ug/mg DW) and geutisic acid (2.22 ug/mg DW) in transformed plants, unlike wild-type plants. In transformed plants, all detected flavonoids (vanillic acid, rutin, catechine, gallic acid, syringic acid, caffeic acid, coumaric acid, geutisic acid, ferulic acid, and cinnamic acid) were increased up to several folds. Real-time qPCR revealed the higher expression levels of the genes for flavonoid biosynthesis enzymes phenylalanine ammonia-lyase (PAL) and chalcone synthase (CHS) in plants transformed with rol A genes, as the expression levels were increased up to 9–20-fold and 2–6-fold, respectively. The rol A transgenic lines T3 and T5 carrying two copies of rol A gene, particularly showed higher expression of both PAL and CHS gene, with the highest expression in T3 line. The transgenic lines demonstrated an average increase of 1.4-fold in the total phenolic content and 1–2-fold in the total flavonoid content as compared to wild-type plants. Total antioxidant capacity and total reducing power were increased up to an average of 1–2-fold and 1.5–2-fold respectively, along with increased free radical scavenging ability. Furthermore, the rol A gene transgenics were found to have much greater cytotoxic capacity than the A. carvifolia wild-type plant against the MCF7, HeLA, and HePG2 cancer cell lines. Current findings show that the rol A gene effectively increases the flavonoid content of A. carvifolia Buch, boosting the plant’s capacity as an antioxidant and an anticancer. This is the first-ever report, demonstrating the genetic transformation of Artemisia carvifolia Buch with rol A gene.
1. Introduction
Now a days naturally occurring compounds and their associated medicines are used to treat nearly 87% of human diseases, including different bacterial infections, cancer, and immunological disorders. It has been revealed that almost 25% of approved drugs around the world are obtained from plant origin. Furthermore, about 80% people in many developing countries use medicines obtained from plants for their health-related issues [1]. Artemisia L. is a diverse genus of fragrant small herbs and shrubs widely spread in northern temperate regions. The Artemisia L. genus comprises over 500 species, found in the northern hemisphere, in Asia, Europe, and North America. It belongs to the important family Compositae (Asteraceae) [2].
Plant extracts attained from Artemisia species have pharmacological properties and are used for the treatment of illnesses such as depression, anxiety, insomnia, epilepsy, stress, irritability and psychoneurosis. Additionally, plants from Artemisia species hold a wide range of biological functions such as antibacterial, antitumor, hepatoprotective, antimalarial, antiseptic, antispasmodic, and antirheumatic activities [1,3]. The primary cause of the intense and aromatic scent of some Artemisia species is high quantities of volatile terpenes, which are components of their essential oils and are found in particular in the leaves and flowers of these plants. Numerous species of the Artemisia genus from around the world have undergone extensive chemical analysis to determine the chemical makeup of essential oils. Numerous investigations have revealed that the terpene components of the essential oils produced by Artemisia species exhibit notable intraspecific differences [2].
A class of phenylpropanoids with low molecular weight compounds known as flavonoids are found in plant cells vacuoles where they act as water soluble pigments [4]. Due to their capacity to scavenge reactive oxygen species (ROS), flavonoids are regarded as antioxidants or phytoalexins [5]. Thus, they shield plants against both biotic and abiotic factors—including UV exposure, extreme cold temperatures, microbial contamination, and insect feeding—to prevent serious harm [6,7,8]. Additionally, serving as chemical messengers in plants, flavonoids draw pollinating insects and play a role in the metabolism of auxin [9]. The genus Artemisia is a plentiful supplier of flavonoids [10]. Although A. annua—from which over 50 flavonoids have been found—has received the most attention among the Artemisia species, other species, such as A. absinthium L. [11], A. asiatica [12], and A. herba-alba [13] have also been found to contain a number of flavonoids.
However, the amount of these flavonoids in plants, besides this, at different growth stages of a plant fluctuations or variability in flavonoids concentrations has been observed. For example, the highest concentrations of chrysoplenetin and casticin were discovered in the leaves and flowers of one cultivar of A. annua during blossom [13]. Similar to this, a recent study found that the antioxidant capacity varied between cultivars, indicating that the antioxidant components, particularly flavonoids, can vary in content [10].
In order to improve the synthesis of secondary metabolites in plants, genetic engineering has proven to be the most effective method among various approaches by altering the biochemical pathways that are involved in the synthesis of secondary plant chemicals [14]. It has been revealed in different studies that rol genes act as a powerful inducers or activators of secondary metabolite production in various plant families [15]. The rol A gene encodes a growth-stimulating protein that binds to DNA, while the rol B gene regulates the signaling pathway of auxin by acting as a tyrosine phosphatase [16,17]. Besides this, the transformation of Vitis amurensis with rol B gene has increased the resveratrol synthesis [18]. It was further found that the anthraquinones production was increased with rol B in Rubia cardifolia transformed calli [19]. In plants as well as cell cultures that have undergone transformation, the rol C genes cytokinin glucosidase activity can activate the creation of a wide collection of secondary compounds, including ginsenosides and various alkaloids [20,21,22,23,24].
In the present study, flavonoids were detected in the Artemisia carvifolia Buch wild type and then flavonoid content was enhanced by genetically modifying A. carvifolia with rol A gene. Aimed to correlate the levels of gene expression responsible for the production of flavonoids with metabolite concentrations, real-time qPCR was used to analyze their response. For this purpose, two genes that encode PAL and CHS of the phenylpropanoid route of flavonoid biosynthesis were investigated. Furthermore, HPLC analysis was used to quantify the flavonoids in transformed and untransformed plants. Moreover, antioxidant potential and cytotoxicity were also tested. This is the first-ever report, demonstrating the genetic transformation of Artemisia carvifolia Buch with rol A gene.
2. Materials and Methods
2.1. Seed Germination and DNA Barcoding
For the present research, seeds of Artemisia carvifolia were utilized. These seeds were procured from Astore located at 74.8500° E, 35.3667° N and an elevation of 8500 feet in Northern Pakistan. Using 70% ethanol, the gathered seed surfaces were sterilized. After sterilization, a half-strength MS medium was used to germinate seeds. Extraction of plant genomic DNA from germinating plantlets was carried out according to a predetermined protocol [25]. DNA barcoding was used to identify Artemisia carvifolia buch. For this purpose, a non-coding region (spacer) between the trnH and psbA genes of chloroplast. Using primers of psbA: 5′-GTTATGCATGAACGTAATGCTC-3′; and trnH: 5′-CGCGCATGGTGGATTCACAATC-3′, DNA was amplified. The PCR reaction was conducted using the previously stated reaction parameters [26]. For PCR product purification, the Rapid PCR Purification System 9700 (Marligen Biosciences, Ijamsville, MD, USA) was employed. The PCR product was sequenced by utilizing the dideoxy-chain termination method. The BioEdit sequence alignment program was then used to identify and align the sequences.
2.2. Bacterial Strains and Plasmids
The plasmid pPCV002-A was carried by the Agrobacterium tumefaciens strain GV3101 that was graciously provided by Dr. A. Spena [27]. Its T-DNA region—which also includes the NPTII gene (neomycin phosphotransferase gene), the NOS (nopaline synthase) promoter, and terminator sequences—enables the CaM35S promoter to regulate the rol A gene’s expression. Agrobacterium tumefaciens (strain GV3101 carrying pPCV002-A) were introduced into a flask with LB and bacterial cultures were then left in the shaker incubator for the night at 120 rpm and 28 °C. The bacterial culture was utilized to transform the plants after 24 h of growth had been attained and OD had been evaluated using a spectrophotometer when it was between 0.2 and 0.8.
2.3. Transformation and Regeneration
The genetic transformation was achieved according to previously described method [22]. One-month-old nodal explants initially precultured on shooting media with 200 µM acetosyringone and hormone supplements comprising 0.5 mg/L BAP and 0.1 mg/L NAA were used for the experiment. The explants were infected with an Agrobacterium tumefaciens culture bearing the required constructs after three days. The explants were then placed on filter paper that was already autoclaved, for 1–2 min to eliminate excess bacterial culture and after that, they were put on MS shooting media enriched with 200 µM acetosyringone. The explants were kept in darkness at 28 °C. After two days, the explants were given three antibiotic-based washes before being placed on a media known as a selection medium with the same hormones and antibiotics concentrations as those previously reported [22]. After 3–4 weeks, regeneration of explants occurred so they were transferred to fresh selection medium, after that subculturing was carried out after every two weeks. The explants were then shifted to rooting media [22] for root development. The entire plants were then regenerated on selection media after four selection cycles.
2.4. Molecular Analysis
To confirm the transformation of rol A gene, molecular analysis was performed. For this purpose, the CTAB technique was used to isolate genomic DNA from 7 to 8 weeks old transformed and untransformed Artemisia carvifolia plants [25], and the alkaline lysis method was used to isolate plasmid DNA from Agrobacterium strain GV3101. A programmed DNA thermal cycler was used to perform the PCR analysis. The rol A gene forward primer 5′-AGAATGGAATTAGCCGGACTA-3′ and reverse primer 5′-GTATTAATCCCGTAGG TTTGTT3′ were utilized. The PCR conditions have been previously described [28]. Agarose gel of 1.5% w/v was prepared for agarose gel electrophoresis to confirm and analyze the PCR product.
2.5. Southern Blotting
For Southern blot testing of rol A transformed plants, 3–5 μg of plant genomic DNA was digested with EcoRI and then electrophoresed on 0.7% agarose gel. The processed DNA fragments are transferred to a nylon membrane which is positively charged, in accordance with the standard procedure [29]. As directed by the manufacturer, the DIG High Prime DNA Labeling and Detection Starter Kit II (Roche cat. no. 11585614910) was utilized to conduct the Southern blot analysis. The probe was created using rol A gene PCR products from plasmids that were labeled with digoxigenin (DIG)-11-dUTP and DIG High Prime DNA Labeling reagents. The probe was then hybridized with a membrane bearing genomic DNA at a temperature of 42–44 °C. The membrane was then placed on X-ray film to conduct the immunological detection process.
2.6. Evaluation of Flavonoids through an HPLC-DAD System
Polyphenols in extracts from both A. carvifolia plants of the transformed and wild types were measured using HPLC-DAD analysis. The protocol followed was reported earlier [30] with slight changes. For this experiment, dry sample extracts were diluted in methanol to provide a solution with 10,000 μg/mL concentration. Additionally, the stock solutions of 10 standards—including vanillic acid, rutin, catechine, gallic acid, syringic acid, caffeic acid, coumaric acid, geutisic acid, ferulic acid, and cinnamic acid—were prepared in methanol at 1000 μg/mL concentration. For the standard calibration curve, 10, 20, 50, 100, 150, and 200 μg/mL dilutions were made in series using these freshly prepared stock solutions. The amount injected was 20 μL, and constant flow rate of 1 mL/min was maintained. In mobile phase A, chemicals were combined with water in a ratio of 10:5:1:85 (methanol-acetonitrile-acetic acid-water), while in mobile phase B, only chemicals were used in a ratio of 60:40:1 (methanol-acetonitrile-acetic acid). In the gradient approach, the distribution of volume of B was as follows: 0–50% in 0–25 min, 50–100% in 25–30 min, 100% in 30–35 min. It was a room temperature HPLC analysis. Furthermore, UV retention periods, and absorption spectra of plant extracts were checked in comparison with standards in order to identify compounds. The column was always reconditioned for 10 minutes before the subsequent analysis began. By comparing retention rates of reference standards, peaks in the extracts were discovered. The analytes were identified using wavelengths and retention times that were specific for each metabolite as shown in Table 1.

Table 1.
Retention time of examined flavonoids with wavelength.
2.7. Analysis of Genes Involved in the Flavonoid Biosynthetic Pathway Using Real-Time qPCR
In order to check the expression level of rol A gene, a semi-quantitative reverse transcriptase-polymerase chain reaction was performed with rol A gene primers as reported previously [22], using 1 μL of cDNA reaction mixture as a template. Following a previously described procedure, real-time qPCR was used to assess the expression of genes of the flavonoid biosynthetic pathway [22]. For amplification reaction, primers that target a certain gene were utilized. For this, the PAL forward and reverse primers were 5′-ACACTCGGTTAGCTATTGCTGCAA-3′ and 5′-CCATGGCGATTTCTGCACCT-3′, respectively. The CHS forward and reverse primers were 5′-AGGCTAACAGAGGAGGGTA-3′ and 5′-CCAATTTACCGGCTTTCT-3′, respectively. The actin primers used were 5′-ATCAGCAATACCAGGGAACATAGT-3′ (forward) and 5′-AGGTGCCCTGAGGTCTTGTTCC-3′ (reverse).
2.8. Antioxidant Potential Measurement
The antioxidant ability of both untransformed and transformed plants was assessed using in vitro antioxidant assays. The methodology used was same as previously reported in which one-gram air-dried powder of plant shoots of each type were used to prepare methanolic extract by soaking in 3 mL of methanol [31].
2.8.1. Total Phenolic Content Measurement
Following a previously established procedure, plant extracts were measured for their total phenolic content (TPC) [32,33]. Gallic acid and DMSO were employed as positive and negative controls, respectively. TPC was represented as gallic acid equivalent.
2.8.2. Total Flavonoid Content Measurement
The colorimetric method using aluminum chloride was used to assess the TFC of the under-researched plant extracts by using the previously described method [33]. Quercetin served as the positive control, and the negative control was DMSO. TFC was represented as quercetin equivalent.
2.8.3. Total Antioxidant Capacity Measurement
Total antioxidant capacity (TAC) of the under-study plant extracts was calculated by using the reported methodology [34]. Ascorbic acid and DMSO were used as the positive and negative controls, respectively. TAC was represented as an ascorbic acid equivalent and determined by using the formula below:
Ascorbic Acid Equivalence = 100/2.651 × Absorbance of sample μg/mL
2.8.4. Total Reducing Power Measurement
The total reducing power of plant extracts from wild type and transgenic A. carvifolia was evaluated by following the reported methodology [35]. The appositive and negative controls were ascorbic acid and DMSO, respectively. The evaluation was carried out in triplicate. TRP was expressed as an ascorbic acid equivalent and quantified using the formula below:
Ascorbic Acid Equivalence = 100/2.7025 × Absorbance of sample μg/mL
2.9. DPPH Free Radical Scavenging Assay
All plant extracts DPPH levels were calculated based on the reported protocol [31]. The experiments were performed in triplicate and the absorbance was measured at 517 nm. The percentage of DPPH free radical scavenging for each concentration of plant extract was determined by the following formula.
Percentage scavenging (%) = [1 − absorbance of extract/absorbance of control] × 100
2.10. Measurement of Anticancerous Activity through MTT Assay
The MTT test was applied to evaluate the antiproliferative activity of the plant extracts under investigation. This colorimetric method relies on the fact that living cells have the capacity to convert the tetrazolium dye MTT via mitochondria into its insoluble formazan, a distinctive purple precipitate [36]. Three cancer cell lines—HePG2 (derived from hepatic carcinoma), HeLA (derived from cervical cancer cells), and MCF7 (derived from breast carcinoma)—were employed for that aim. The samples were prepared and assay was performed by following the reported protocol [34]. Briefly, 1 mL of methanol was used to extract 100 mg of dried powdered material from transformed and untransformed A. carvifolia plants for 1 h at room temperature. The sonication bath was used for this purpose. A rota-evaporator was used at 40 °C to dry the supernatant after centrifuging the extract. Then, for the analysis of all cancer, these dried samples were dissolved in a 100% (v/v) DMSO solution at a final concentration of 40 mg/mL. For cytotoxicity assay, cells of cell lines were seeded in a 12-well plate and after 24 h of growth of cells, plant extracts were added to the medium in each well at a concentration of 200 ppm, and the viability was assessed after 48 h. All the conditions were run in triplicate. A control without drug treatment and a control where the cells were only treated with the solvent (DMSO) were included in all the assays. The viability of the cells was determined by the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
2.11. Statistical Analysis
All the experiments were run in triplicate for analysis. The statistical significance level of the data were assessed by applying two-way ANOVA.
3. Results
3.1. DNA Barcoding for Identification of Plant
The chloroplast genomes psbA-trnH region, about 500 bp, was amplified successfully. To check the reliability of species-specific nucleotides, DNA samples were sequenced three times, with the same outcomes. For the purpose of confirming the plant species, the reference sequence with the GenBank Accession number (NCBI: FJ418751) was used. The sequence was identified as psbA-trnH of A. carvifolia after doing the CLUSTAL W in BioEdit program and BLAST in NCBI.
3.2. Transformation and Regeneration
A. carvifolia was successfully transformed with A. tumefaciens strain GV3101 carrying the rol A gene. A total of 400 explants were employed in each of the two separate transformation experiments. Although just five rol A transformants made it to maturity on the selection media, a 35% transformation efficiency was discovered. Morphological differences were depicted between transgenic plants carrying rol A gene and wild type plants in Figure 1. Dwarfness and harder texture of stems of transformed plants than wild type plants were also observed. Rol A transgenics had short, narrow, dark-colored leaves, and grew more quickly on the selection media.

Figure 1.
Genetic transformation of Artemisia carvifolia. (A) Seeds germination, (B) co-cultivation, (C) wild type plant, (D) rol A transgenic plant.
3.3. Molecular Analysis
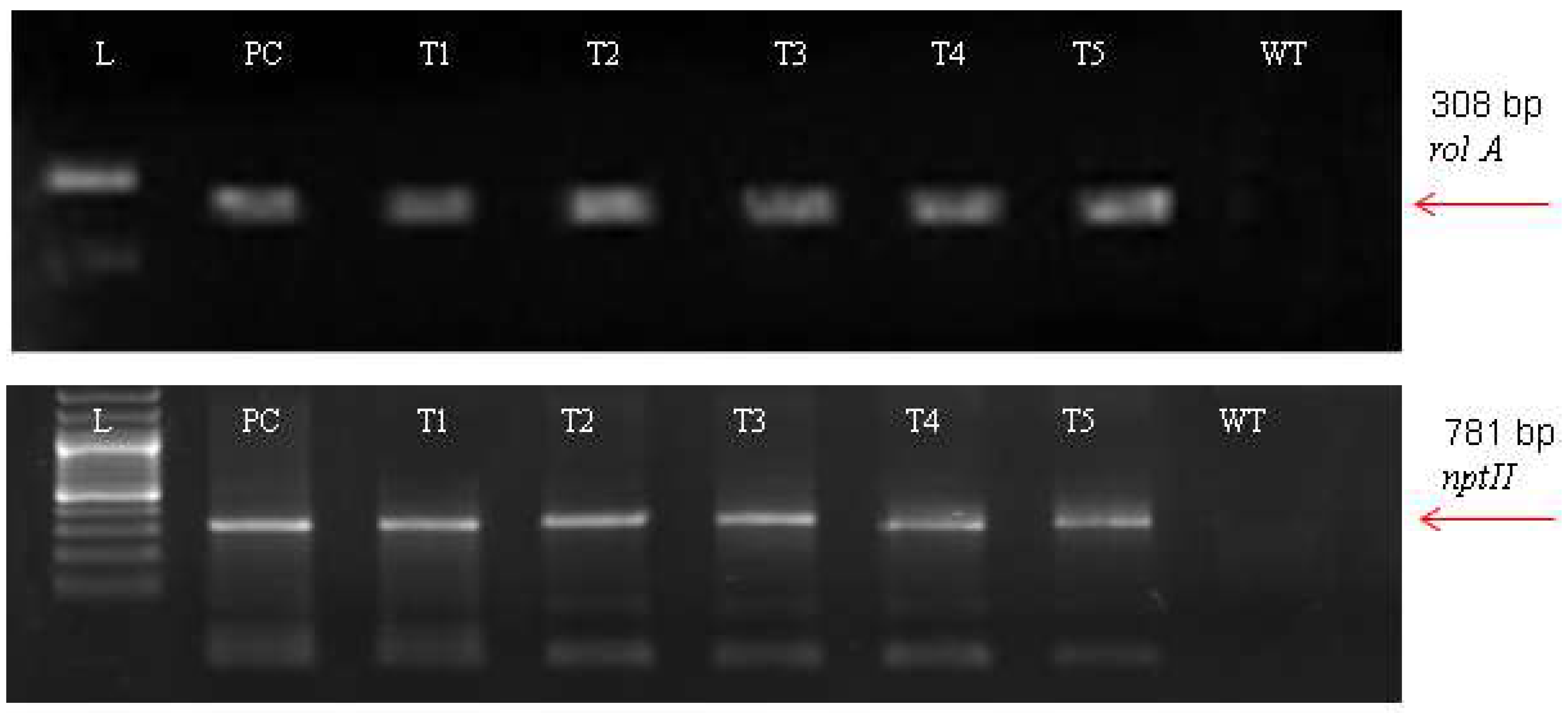
For the A. carvifolia rol gene transformants, PCR results revealed 308 bp for rol A gene and 781 bp for the nptII gene amplified products (Figure 2). The plasmid DNA of GV3101-PCV002-A yielded similar amplified products. Besides this, wild-type plants did not exhibit that these genes were present in their genome.
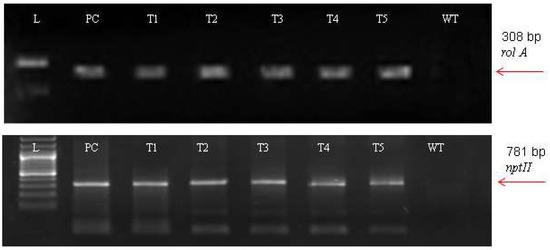
Figure 2.
PCR result of transgenic Artemisia carvifolia harbouring rol A genes showing 308 bp for rol A and 781 bp for the nptII gene.
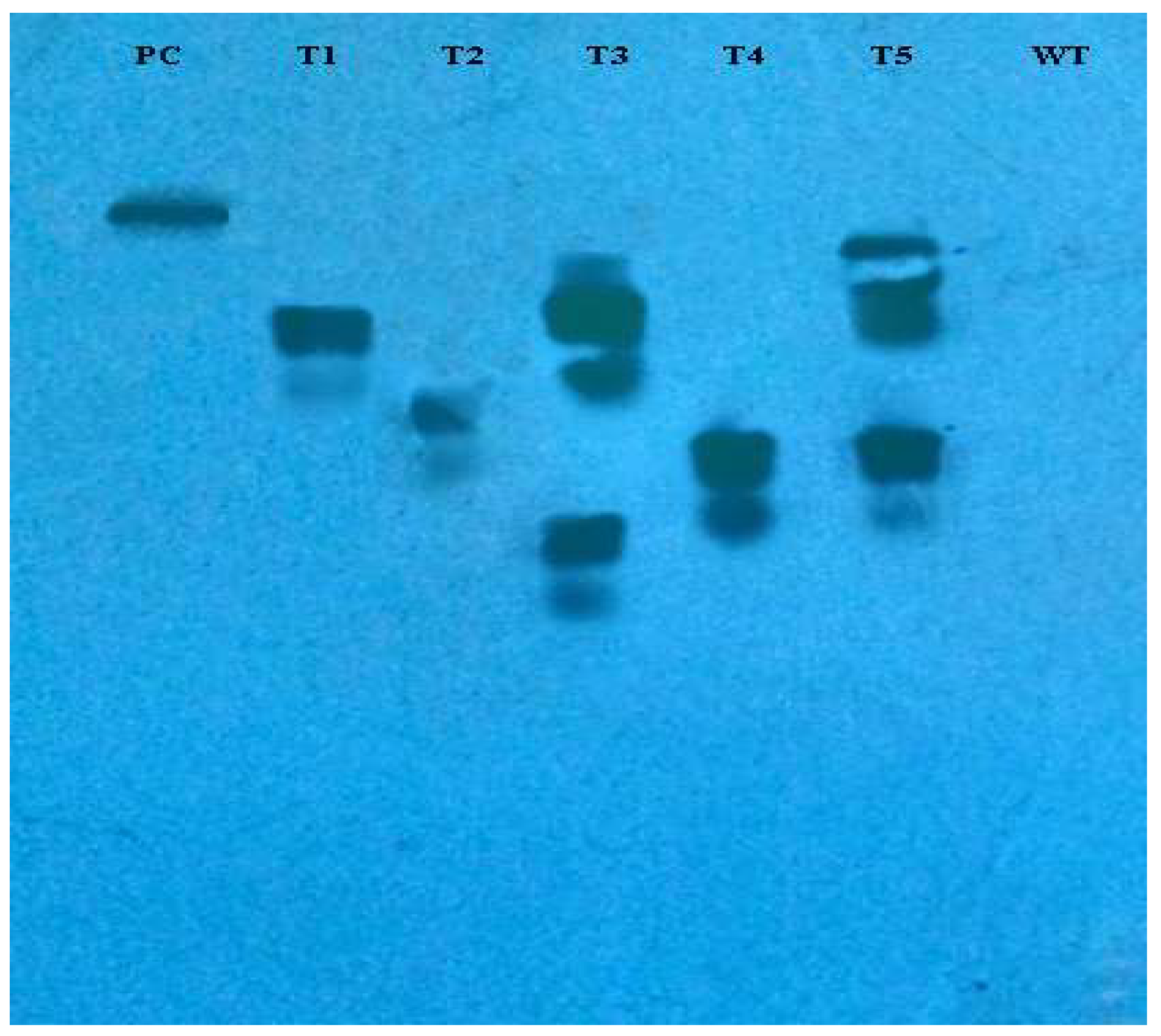
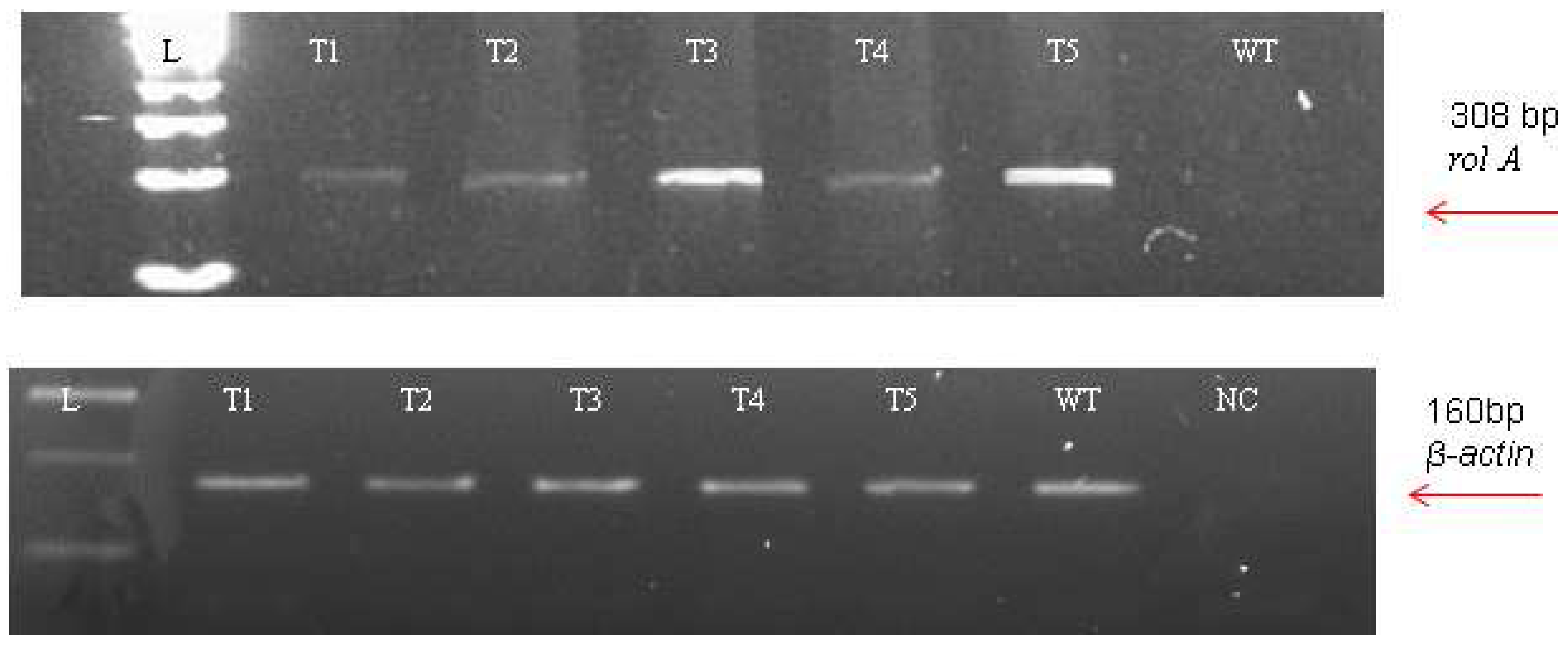
The incorporation of rol genes into the plant genome was confirmed by Southern blot analysis, which also provided information on the number of copies of each gene present in various transgenic lines. All five transgenic lines of Artemisia carvifolia showed the successful stable integration of rol A gene. One copy of rol A gene was seen in transgenic lines T1, T2, and T4. Whereas T3 and T5 showed two copies of the integrated gene as shown in Figure 3. Although there were differences in the expression level of these genes among studied lines, RT-PCR validated gene expression in all transformed lines. The rol A transgenic lines T3 and T5 in Figure 4 displayed the highest levels of expression due to double copy numbers as visible in Southern blot analysis results (Figure 3). β-actin was chosen as the positive control (housekeeping gene) which showed similar expression in all transgenic lines.
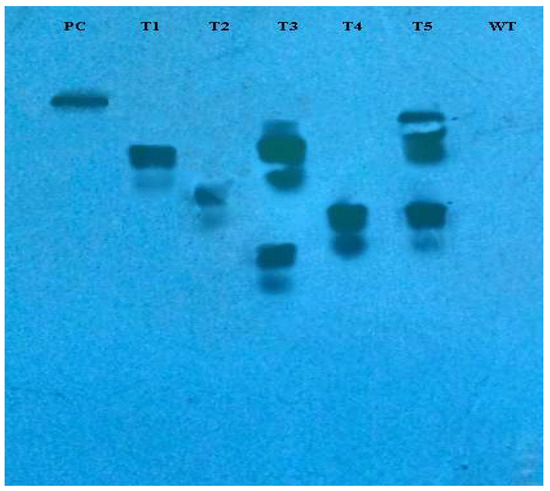
Figure 3.
Southern blot analysis of PCR-positive plants demonstrating the incorporation of rol A gene into the Artemisia carvifolia genome. Transgenic lines T3 and T4 showed two copies of integrated gene.
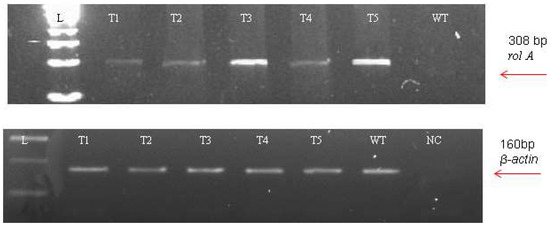
Figure 4.
Reverse transcriptase PCR using the β-actin (160 bp) amplification as an internal control.
3.4. HPLC-DAD-Based Quantification of Flavonoids
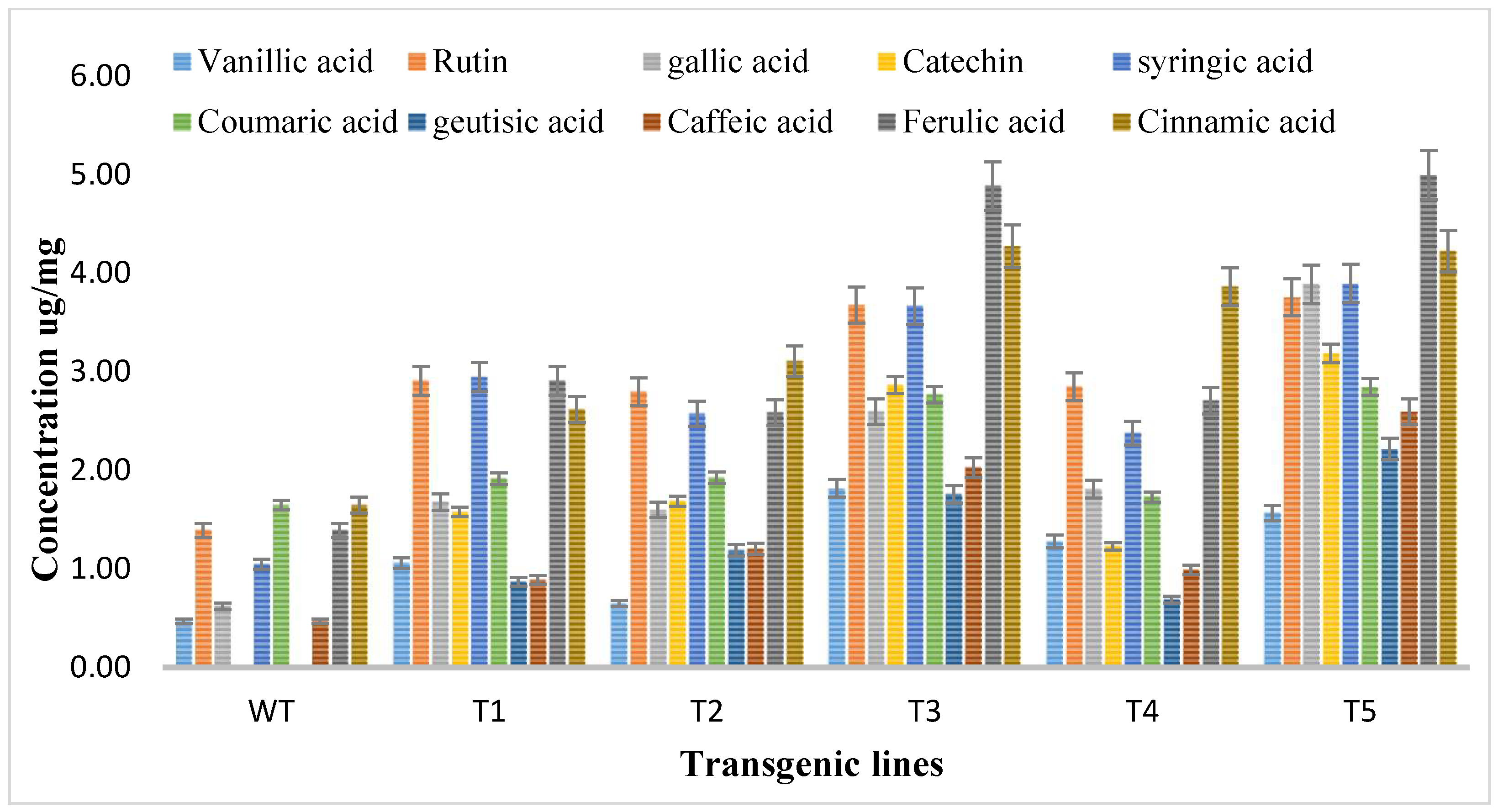
Methanolic extracts of shoots of both transgenic and wild type A. carvifolia plants were prepared and then an HPLC-DAD system was used for the detection and quantification of flavonoids. The HPLC profile obtained was evaluated against the absorption spectra and retention duration of 10 standard compounds or flavonoid markers including vanillic acid, rutin, catechine, gallic acid, syringic acid, caffeic acid, coumaric acid, geutisic acid, ferulic acid, and cinnamic acid. Vanillic acid, syringic acid, gallic acid, coumaric acid, ferulic acid, caffeic acid, and cinnamic acid were present in both wild type plants and transformed plants but the concentration of these phenolic compounds was enhanced in rol gene transformants. As illustrated in Figure 5, unlike the wild type plants, flavonoids catechin and geutisic acid were discovered in the transformed plants only.
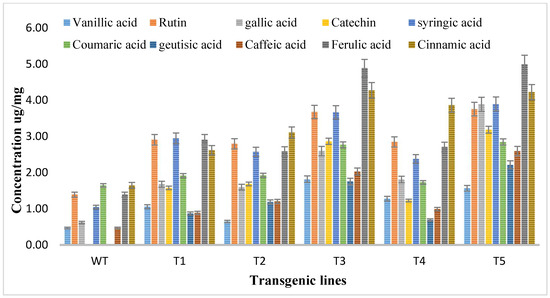
Figure 5.
Quantitative analysis of flavonoids by HPLC in A. carvifolia rol A transgenic lines and wild type plants.
The vanillic acid content in wild type plants was 0.47 ug/mg DW but in the transformed plants it reached the highest 1.82 ug/mg DW in T3, showing a 4-fold increase. Rutin levels in wild type plants were 1.39 ug/mg DW, highest increase was observed in T5, showing a 2.7-fold increase of rutin. The gallic acid concentration was 0.62 ug/mg DW in wild type plants, increasing to 3.89 ug/mg DW with up to 6.2-fold in rol A transformants. Syringic acid wild type content was 1.05 ug/mg DW, increasing up to 3.7-fold to (3.90 ug/mg DW) highest in T5 transformed plants. The concentration of coumaric acid in wild type plants was 1.65 ug/mg DW but in transformed plants, it reached 2.85 ug/mg DW, showing the 1.7-fold increase in transformed plants. The concentrations of caffeic acid, ferulic acid, and cinnamic acid in wild type plants were 0.47 ug/mg DW, 1.39 ug/mg DW, and 1.67 ug/mg DW respectively and in transformed plants, these reached 2.60 ug/mg DW, and 5 ug/mg DW, and 4.23 ug/mg DW respectively showing a 5.5-fold increase of caffeic acid, a 3.6-fold increase of ferulic acid, a 2.56-fold increase of cinnamic acid in transformed plants. The results of HPLC showed that all flavonoids were increased up to the highest level in T3 and T5 transformed plants due to the integration of a double copy number of rol A gene.
Catechin and geutisic acid were absent in wild type plants and the concentration of catechin in transformed plants was 3.19 ug/mg DW and the amount of geutisic acid was 2.22 ug/mg DW in transformed plants. Statistical analysis was conducted, where the production levels of investigated phenolic compounds in rol A transgenic plants exhibited an extremely significant difference (p < 0.0001) when compared to the wild type A. carvifolia plants (Table 2).

Table 2.
Two-way ANOVA for HPLC analysis.
3.5. Expression Analysis of Flavonoid Biosynthetic Pathway Genes through Real-Time qPCR
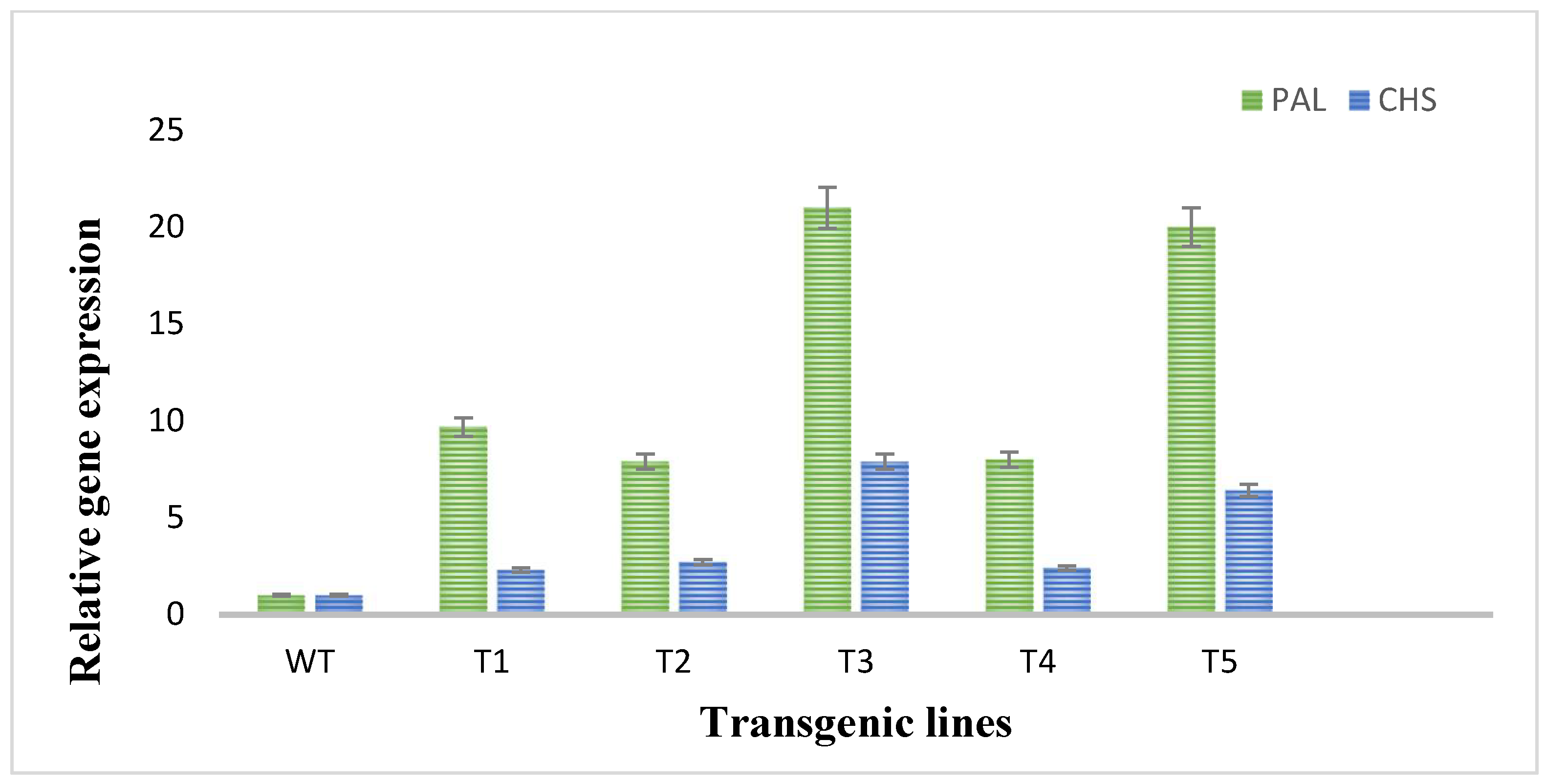
To evaluate the expression of two flavonoid biosynthetic genes (PAL and CHS), RT-qPCR was performed. In RT-qPCR, significant changes and higher expression degrees of the investigated genes of flavonoids biosynthetic pathway in plants transformed with rol A gene were observed. The expression of PAL and CHS genes was much low in wild type plants as shown in Figure 6. The PAL gene was highly expressed in the transformed plants as compared to CHS gene expression in transformed plants as an expression of PAL gene was 9–20-folds higher and expression of CHS gene was 2–6-fold higher in transformed plants. The rol A transgenic lines T3 and T5, which both carrying two copies of rol A gene, particularly showed higher expression of both PAL and CHS gene, with the highest expression in T3 line. These results showed that rol A gene plays role in inducing flavonoid biosynthesis by enhancing the expression of their biosynthetic genes, i.e., PAL and CHS.
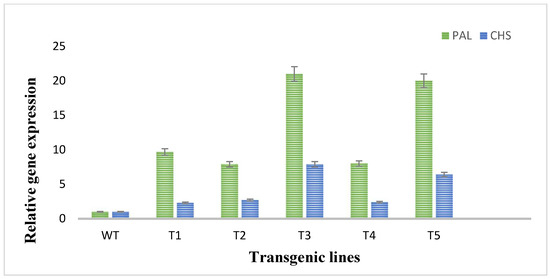
Figure 6.
Quantitative real-time PCR analysis of flavonoid biosynthetic pathway genes. PAL and CHS stands for phenylalanine ammonia lyase and chalcone synthase respectively.
3.6. Analyzation of the Antioxidant Potential of Rol Gene Transformed and Untransformed A. carvifolia
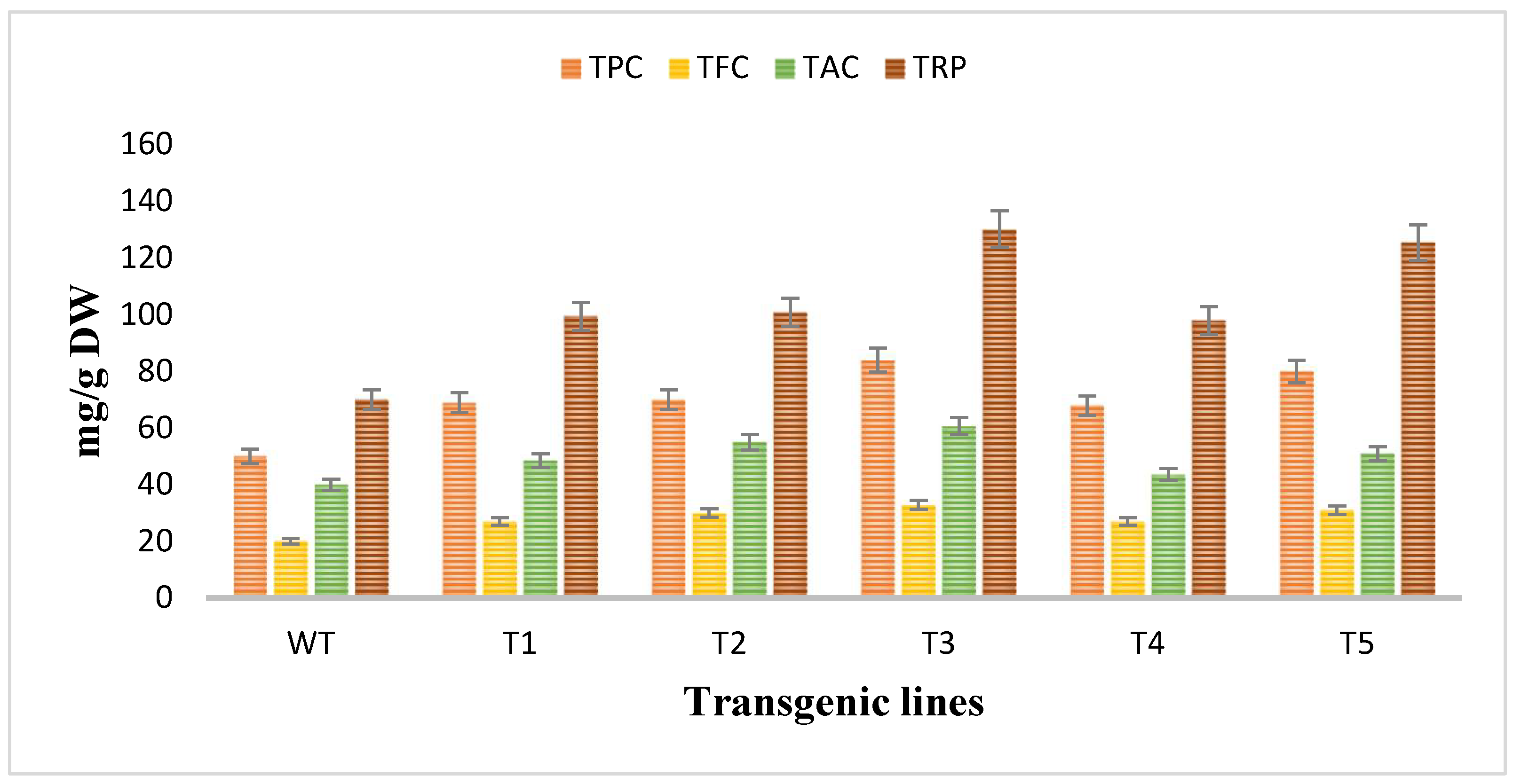
The antioxidant potential of Artemisia carvifolia rol A transgenic plants and wild type plants was assessed by using antioxidant assays. The total phenolic content and total flavonoid content was calculated as the equivalent of gallic acid (mg/g of DW) and quercetin (mg/g of DW) respectively, so TPC in wild type plants was 50 mg/g whereas rol A transgenic lines demonstrated an average increase of 1.4-fold in the phenolic content and the level of phenolic content was highest in line T3 (84 mg/g) followed by T5 (80 mg/g) as shown in Figure 7. TFC in wild type was 20 mg/g compared to 1–2-fold increase in rol A transgenic lines. Total antioxidant capacity was determined in terms of ascorbic acid equivalence (mg/g of the DW). TAC was increased up to an average 1–2-fold, as in wild type TAC was measured 40 mg/g but in transformed plants it was enhanced in all transgenic lines with highest level in T3 (60.7 mg/g) and then in T5 (50.9 mg/g). Similarly, total reducing power was also significantly increased in rol A transformed plants. In wild type plants, total reducing power was 70 mg/g. In transgenic line T1, T2, T3, T4, and T5 the TRP was measured as 99.4 mg/g, 100 mg/g, 130 mg/g, 97 mg/g, and 125 mg/g respectively, showing an average increase of 1.5–2-fold in total reducing power. Rol A gene showed extremely significant effect (p < 0.0001) on the antioxidant capacity of the A. carvifolia plants (Table 3 and Table 4). All these results indicated that the transgenic plant’s antioxidant capacity was increased by the incorporation of the rol A gene.
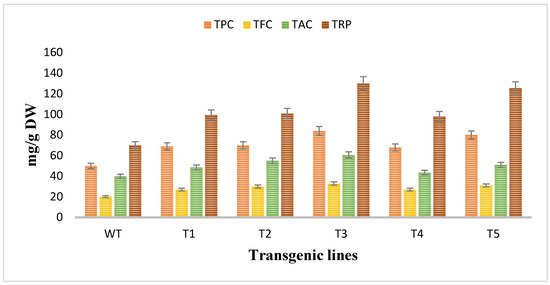
Figure 7.
Evaluation of antioxidant potent through different antioxidant assays. TPC (total phenolic content, TFC (total flavonoid content), TAC (total antioxidant capacity), and TRP (total reducing power).

Table 3.
Two-way ANOVA for antioxidant assays.

Table 4.
Two-way ANOVA for DPPH free radical scavenging assay.
3.7. DPPH Free Radical Scavenging Assay
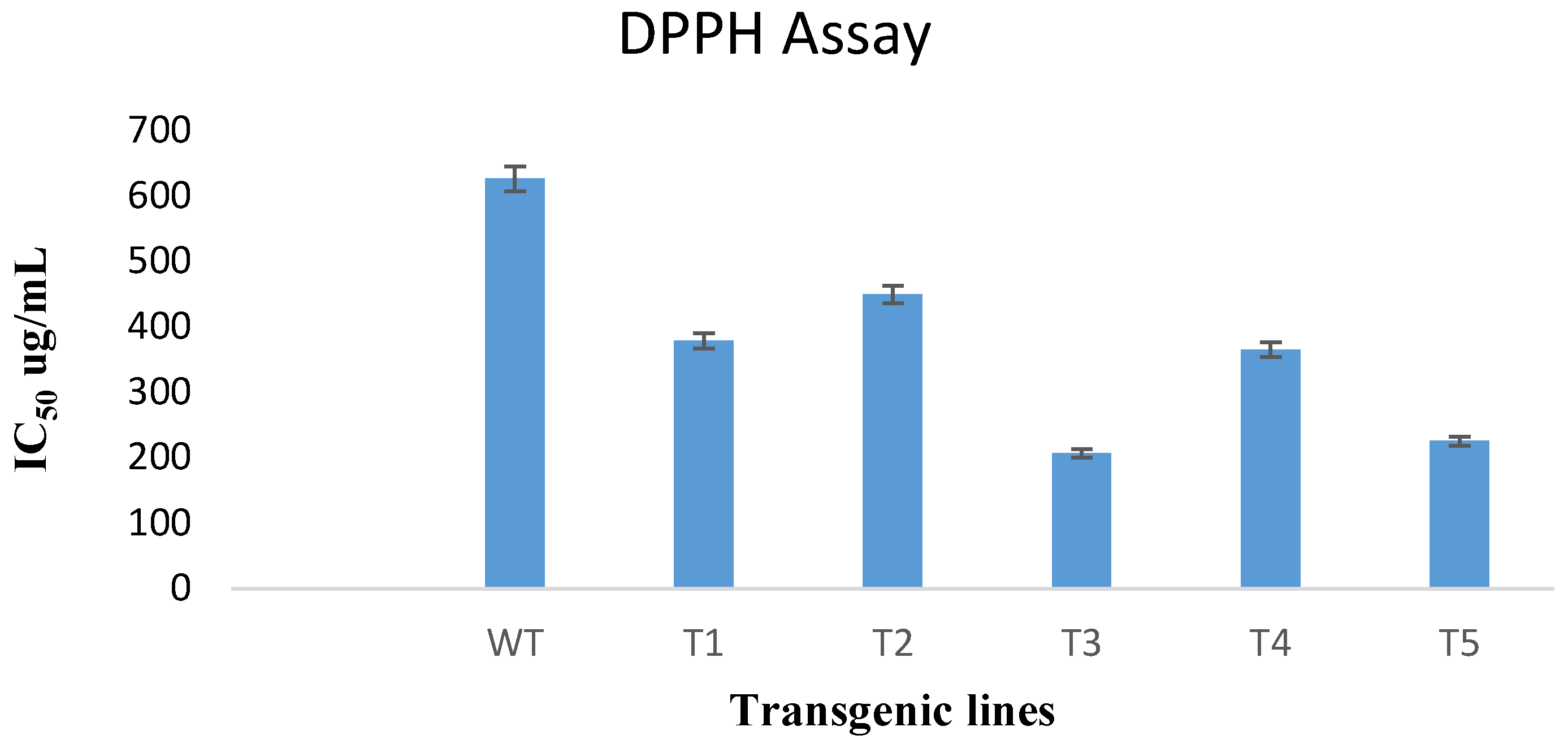
DPPH free radical scavenging is an efficient and widespread technique for determining the antioxidant activity of plant extracts. In this study, the DPPH assay was used to test the ability of transformed and untransformed plants to scavenge free radicals. According to results obtained, the rol A gene transformed plants showed significant increase in antioxidant potential as compared to untransformed wild type plants as shown in Figure 8. The extract of rol A transgenic line T3 displayed the maximum radical scavenging ability with an IC50 value of 206.9 ug/mL compared to the wild type plants having IC50 of 627 ug/mL. Similarly, the extracts of T1, T2, T4, and T5 exhibited more efficacy with less IC50 values (379, 450, 365.7, and 225.65 ug/mL respectively) as compared to wild type plants.
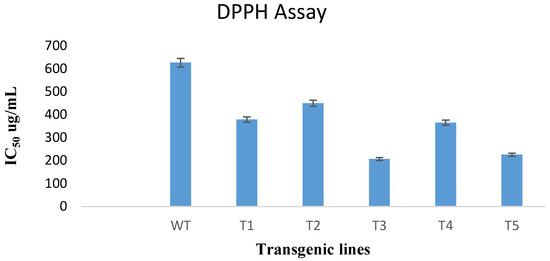
Figure 8.
Results of the DPPH assay of extract of five rol A integrated transgenic lines and wild type plant extract (WT).
3.8. Measurement of Anticancerous Activity against Cancer Cell Lines through MTT Assay
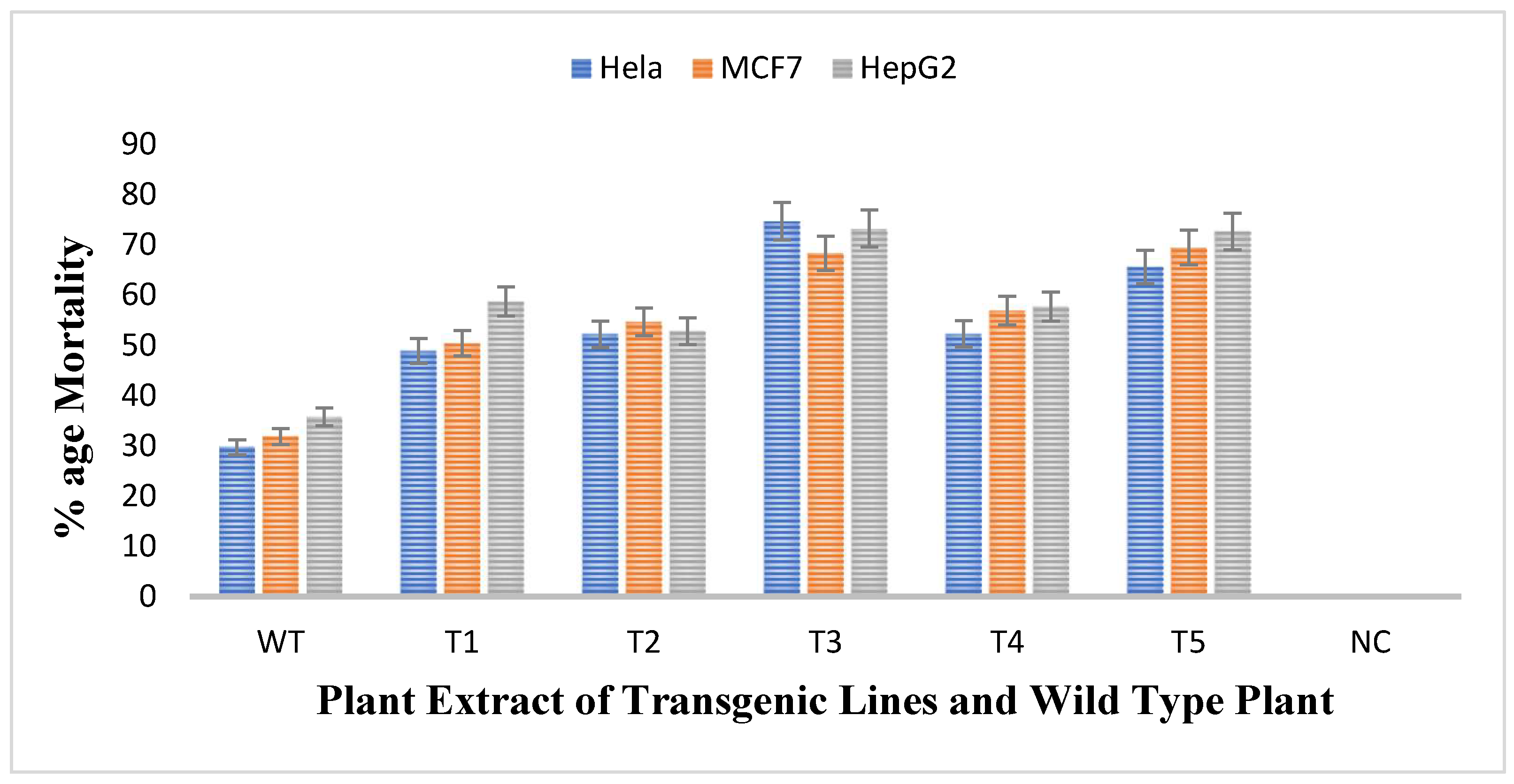
The antiproliferative activity of untransformed and transgenic plants of rol A gene were checked by treating three different cell lines namely HeLA, MCF7, and HePG2 with the methanolic extracts of plants and thus the cell viability of all cell lines was evaluated. The results obtained showed that all transgenic lines were more effective against all cell lines as compared to wild type plant as shown in Figure 9. Mortality rate of Hela cells after treatment with untransformed A. carvifolia extract was 30% but after treating with rol A transgenic extracts, it increases up to 75%. Similarly, the mortality rate of MCF7 and HePG2 cells after treating with wild type plant extract was 32% and 36% respectively. After treatment with transgenic cell lines, the mortality rate of MCF7 and HePG2 was increased up to 70% and 74% respectively. Transgenic line T3 and T5 shows maximum effect on the viability of cancer cells. Thus, rol A gene showed extremely significant effect (p < 0.0001) on the enhancement of anticancerous properties of the plants under study (Table 5).
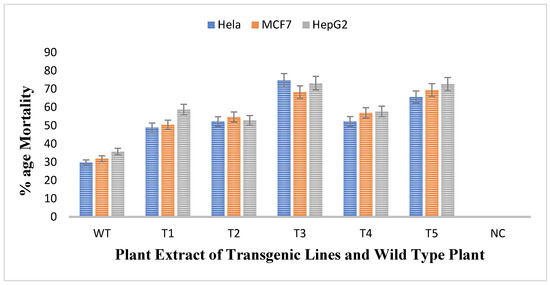
Figure 9.
MTT assay for determination of cytotoxic activity of plant extracts (40 mg/mL) against HeLA, MCF7, and HePG2 cancer cell lines.

Table 5.
Two-way ANOVA for MTT assay.
4. Discussion
Numerous investigations have demonstrated that rol genes are potent inducers or activators of the production of secondary metabolites in a variety of plant families [15]. After transformation with rol A gene, the morphological differences between transgenic plants carrying rol A gene and wild type plants were found. Rol A transgenics had short, narrow, dark-colored leaves and grew more quickly on the selection media. Similar leaf morphological changes were also reported in Ajuga bracteosa plant transformed with rol A gene in which leaves also showed moderate to severe wrinkling with epinasty [37]. Similarly, changes in leaf morphology due to rol A gene were also reported in tobacco plant [38,39,40,41]. Stunted growth of transformed plants than wild type plants was also observed which was similar to the findings already reported by researchers that rol A gene induce dwarfness in transformed plants of Ajuga bracteosa, potato, tomato, tobacco, and soybean plants [37,38,39,40,41]. All rol A soybean transformants showed significant variation in plant phenotype, primarily in terms of plant height, plant character, and leaf morphology. Additionally, a change in leaf morphology was noticed. The soybean plants that had undergone rol A transformation had elliptical-shaped leaves [42]. Similar findings were reported in which Artemisia annua plants that were transformed with rol B and C genes exhibited stunted growth and were shorter than those in the control group. These findings showed that while control plants had broad leaves, transformed plants had extremely short and narrow leaves. Compared to the control plants’ delicate stems, transgenic plant stems had a harder texture. In transformed plants, more branching was seen, but this had no effect on how quickly they grew overall [30]. Significant variations in growth and morphology were seen. Agrobacterium tumefacians mediated genetic transformation of Artemisia carvifolia plants with rol B and rol C genes have been reported but not with rol A gene. Morphological differences between wild type and rol genes transformants were reported by another group who used rol B and rol C genes for the genetic transformation of Artemisia carvifolia plants. They clearly mentioned the morphological heterogeneity between wild type or untransformed plants and plants transformed with rol genes. Broader leaves and more inflorescence were characteristics of rol B transformed plants, which developed more quickly on the selection media, as opposed to rol C transformed plants, which were resistant to regeneration and displayed a narrow leaf blade and shorter internodes [22]. In another study, Lettuce was genetically transformed with Agrobacterium tumefaciens strain GV3101 containing rol C gene. The construct was used to transform about 300 explants, with a transformation efficiency estimate of 65–75%; however, only three transgenic lines matured. All altered plants showed phenotypic changes in comparison to the control, including decreased leaf area, internodal lengths, stem heights, and inflorescence [43]. Additionally, A. annua and A. dubia plants that were genetically transformed with combined rol ABC genes also showed increased height and broad leaves [44], probably as a result of the combined action of rol genes. These morphological alterations may be the result of the hormonal imbalance brought on by the rol genes, which have been shown to induce abnormality in the ratio of auxin and cytokinin that favors cytokinins [45].
According to Southern blot experiment, transgenic lines T1, T2, and T4 all had one copy of the rol A gene. Contrarily, T3 and T4 displayed two copies of the integrated rol A gene. The results were similar to a study in which rol A gene transformants show single and double copy numbers [37]. HPLC analysis showed that all flavonoids are increased up to several folds and some flavonoids—such as catechin and geutisic acid—were absent in wild type plants but present in transformed plants. Similar effects of rol genes were observed previously [33] when the rol B and rol C transgenic plants were examined. A considerable rise in the content of identified flavonoids was seen. Rutin and caffeic acid levels increased up to 3-fold, quercetin levels increased by 4-fold and isoquercetin levels increased by 6-fold in the rol B transgenic plants. Contrarily, a threefold rise in rutin and quercetin, a 5-fold increase in isoquercetin, and a 2.6-fold increase in caffeic acid were seen in transgenics of the rol C gene [33]. Rol genes have been described as strong inducers of plant’s secondary metabolism [46]. Real time PCR confirmed that rol A gene play role in inducing flavonoid biosynthesis by enhancing the expression levels of PAL and CHS. The results correspond to the earlier reports in which the expression level of these both genes were higher in rol genes transformed Brassica rapa [47], Artemisia carvifolia [31], and Lactuca serriola [48] plants as compared to normal plants. Various reports describe that expression of PAL and CHS is directly related to the accumulation of flavonoids in the plant tissue. PAL enzyme catalyzes the flux of primary metabolites into the biosynthetic pathway of flavonoids through the phenylpropanoid pathway and hence performs a key role in flavonoid biosynthesis. CHS, the first enzyme of the flavonoid pathway, is an acyltransferase catalyzing the condensation of 4-coumaroyl CoA to the first flavonoid, naringenin chalcone, which is reported to be a rate-limiting step in flavonoid biosynthesis in different plants [33].
The antioxidant capacity of plants transformed with rol A gene were increased up to several folds. Similar findings were also shown in different reported researches on Artemisia carvifolia [31], Artemisia annua [30], Lactuca serriola L. [48], and Lactuca sativa L. [43] in which rol genes transformation boost the antioxidant potential in transformed plants. The transgenic lines of rol A gene displayed increase in the radical scavenging ability. The results are in accordance with the reported findings in which the rol genes transformation increased the antioxidant capacity of transformed Artemisia carvifolia plants.
Among secondary metabolites, flavonoids are famous for preventing or delaying cancer because it can prevent DNA mutations that takes place in important genes, such as oncogenes or tumor suppressor genes [49]. The results obtained were similar to the reported findings in which the viability of HeLA and MCF7 was 60% when treated with wild type A. annua extract and then decreased up to 40% and 35% respectively when treated with rol B transgenics [33]. Similarly, in previously reported research articles, some Artemisia species were found to be efficacious against the cancer cell lines like HeLA, P388 murine leukemia, and molt-4-human leukemia [50,51,52]. Moreover, cytotoxicity of Artemisia annua against MCF7, HepG2, and HelA cell lines was found significantly increased as compared to the wild type plant when transformed with rol B and rol C gene [33].
5. Conclusions
Secondary metabolites, especially flavonoids are the chemical building blocks of medicinal plants with clinically curative effects. To reap the amazing benefits of these metabolites on human wellbeing, genetic transformation plays very important role. The transformation of Artemisia carvifolia with rol A gene successfully improved the flavonoids content in the plant enhancing the antioxidant and anticancer potential of the plant.
Author Contributions
Conceptualization, E.D.; Formal analysis, A.N.K.; Methodology, A.N.K.; Project administration, E.D.; Supervision, E.D.; Writing—review and editing, A.N.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data is not publicly available due to privacy.
Acknowledgments
We are thankful to Capital University of Science and Technology Islamabad for providing a platform to conduct the current research work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hussain, A. The genus Artemisia (Asteraceae): A review on its ethnomedicinal prominence and taxonomy with emphasis on foliar anatomy, morphology, and molecular phylogeny. Proc. Pak. Acad. Sci. B Life Environ. Sci. 2020, 57, 1–28. [Google Scholar]
- Abad Martínez, M.J.; Bedoya del Olmo, L.M.; Apaza Ticona, L.N.; Bermejo Benito, P. The Artemisia L. Genus: A Review of Bioactive Essential Oils. Molecules 2012, 17, 2542–2566. [Google Scholar] [CrossRef]
- Hussain, A.; Hayat, M.Q.; Sahreen, S.; ul Ain, Q.; Bokhari, S.A. Pharmacological promises of genus Artemisia (Asteraceae): A review: Pharmacological promises of genus Artemisia. Proc. Pak. Acad. Sci. B Life Environ. Sci. 2017, 54, 265–287. [Google Scholar]
- Dong, N.Q.; Lin, H.X. Contribution of phenylpropanoid metabolism to plant development and plant–environment interactions. J. Integr. Plant Biol. 2021, 63, 180–209. [Google Scholar] [CrossRef] [PubMed]
- Cavaiuolo, M.; Cocetta, G.; Ferrante, A. The antioxidants changes in ornamental flowers during development and senescence. Antioxidants 2013, 2, 132–155. [Google Scholar] [CrossRef]
- Iwashina, T. Flavonoid function and activity to plants and other organisms. Biol. Sci. Space 2003, 17, 24–44. [Google Scholar] [CrossRef]
- Pourcel, L.; Routaboul, J.-M.; Cheynier, V.; Lepiniec, L.; Debeaujon, I. Flavonoid oxidation in plants: From biochemical properties to physiological functions. Trends Plant Sci. 2007, 12, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Du, H.; Wang, J.; Pu, Y.; Yang, C.; Yan, R.; Yang, H.; Cheng, H.; Yu, D. Multiplex CRISPR/Cas9-mediated metabolic engineering increases soya bean isoflavone content and resistance to soya bean mosaic virus. Plant Biotechnol. J. 2020, 18, 1384–1395. [Google Scholar] [CrossRef]
- Tan, H.; Man, C.; Xie, Y.; Yan, J.; Chu, J.; Huang, J. A crucial role of GA-regulated flavonol biosynthesis in root growth of Arabidopsis. Mol. Plant 2019, 12, 521–537. [Google Scholar] [CrossRef]
- Ferreira, J.F.; Luthria, D.L.; Sasaki, T.; Heyerick, A. Flavonoids from Artemisia annua L. as antioxidants and their potential synergism with artemisinin against malaria and cancer. Molecules 2010, 15, 3135–3170. [Google Scholar] [CrossRef]
- Singh, R.; Verma, P.K.; Singh, G. Total phenolic, flavonoids and tannin contents in different extracts of Artemisia absinthium. J. Complement. Med. Res. 1970, 1, 101. [Google Scholar] [CrossRef]
- Hajdú, Z.; Martins, A.; Orbán-Gyapai, O.; Forgo, P.; Jedlinszki, N.; Máthé, I.; Hohmann, J. Xanthine oxidase-inhibitory activity and antioxidant properties of the methanol extract and flavonoids of Artemisia asiatica. Rec. Nat. Prod. 2014, 8, 299–302. [Google Scholar]
- Qnais, E.; Raad, D.; Bseiso, Y. Analgesic and anti-inflammatory effects of an extract and flavonoids from Artemisia Herba-Alba and their mechanisms of action. Neurophysiology 2014, 46, 238–246. [Google Scholar] [CrossRef]
- Benedito, V.A.; Modolo, L.V. Introduction to Metabolic Genetic Engineering for the Production of Valuable Secondary Metabolites in in vivo and in vitro Plant Systems. Recent Pat. Biotechnol. 2014, 8, 61–75. [Google Scholar] [CrossRef]
- Bulgakov, V.P. Functions of rol genes in plant secondary metabolism. Biotechnol. Adv. 2008, 26, 318–324. [Google Scholar] [CrossRef]
- Filippini, F.; Rossi, V.; Marin, O.; Trovato, M.; Costantino, P.; Mark Downey, P.; Lo Schiavo, F.; Terzi, M. A plant oncogene as a phosphatase. Nature 1996, 379, 499–500. [Google Scholar] [CrossRef]
- Filippini, F.; Schiavo, F.L.; Terzi, M.; Costantino, P.; Trovato, M. The plant oncogene rolB alters binding of auxin to plant cell membranes. Plant Cell Physiol. 1994, 35, 767–771. [Google Scholar] [CrossRef]
- Kiselev, K.; Dubrovina, A.; Veselova, M.; Bulgakov, V.; Fedoreyev, S.; Zhuravlev, Y.N. The rolB gene-induced overproduction of resveratrol in Vitis amurensis transformed cells. J. Biotechnol. 2007, 128, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Shkryl, Y.N.; Veremeichik, G.N.; Bulgakov, V.P.; Tchernoded, G.K.; Mischenko, N.P.; Fedoreyev, S.A.; Zhuravlev, Y.N. Individual and combined effects of the rolA, B, and C genes on anthraquinone production in Rubia cordifolia transformed calli. Biotechnol. Bioeng. 2008, 100, 118–125. [Google Scholar] [CrossRef]
- Bulgakov, V.; Tchernoded, G.; Mischenko, N.; Shkryl, Y.N.; Glazunov, V.; Fedoreyev, S.; Zhuravlev, Y.N. Effects of Ca2+ channel blockers and protein kinase/phosphatase inhibitors on growth and anthraquinone production in Rubia cordifolia callus cultures transformed by the rolB and rolC genes. Planta 2003, 217, 349–355. [Google Scholar] [CrossRef]
- Bulgakov, V.P.; Veselova, M.; Tchernoded, G.; Kiselev, K.; Fedoreyev, S.; Zhuravlev, Y.N. Inhibitory effect of the Agrobacterium rhizogenes rolC gene on rabdosiin and rosmarinic acid production in Eritrichium sericeum and Lithospermum erythrorhizon transformed cell cultures. Planta 2005, 221, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Dilshad, E.; Cusido, R.M.; Estrada, K.R.; Bonfill, M.; Mirza, B. Genetic transformation of Artemisia carvifolia Buch with rol genes enhances artemisinin accumulation. PLoS ONE 2015, 10, e0140266. [Google Scholar] [CrossRef] [PubMed]
- Dilshad, E.; Cusido, R.M.; Palazon, J.; Estrada, K.R.; Bonfill, M.; Mirza, B. Enhanced artemisinin yield by expression of rol genes in Artemisia annua. Malar. J. 2015, 14, 424. [Google Scholar] [CrossRef] [PubMed]
- Palazón, J.; Cusidó, R.; Roig, C.; Pinol, M. Expression of the rolC gene and nicotine production in transgenic roots and their regenerated plants. Plant Cell Rep. 1998, 17, 384–390. [Google Scholar]
- Ahmed, I.; Islam, M.; Arshad, W.; Mannan, A.; Ahmad, W.; Mirza, B. High-quality plant DNA extraction for PCR: An easy approach. J. Appl. Genet. 2009, 50, 105–107. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Yunheng, J. psbA-trnH sequence analysis from chloroplast on medicinal plants of Artemisia. Chin. Agric. Sci. Bull. 2009, 25, 46–49. [Google Scholar]
- Spena, A.; Schmülling, T.; Koncz, C.; Schell, J. Independent and synergistic activity of rol A, B and C loci in stimulating abnormal growth in plants. EMBO J. 1987, 6, 3891–3899. [Google Scholar] [CrossRef]
- Kiani, B.H.; Safdar, N.; Mannan, A.; Mirza, B. Comparative Artemisinin analysis in Artemisia dubia transformed with two different Agrobacteria harbouring rol ABC genes. Plant Omics 2012, 5, 386–391. [Google Scholar]
- Sanbrook, J.; Fritsch, E.; Maniatis, T. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory: Laurel Hollow, NY, USA, 1989; Volume 11, p. 31. [Google Scholar]
- Zafar, S.; Dilshad, E.; Ismail, H.; Rizvi, C.B.; Mirza, B. Rol genes enhance content of artemisinin and other secondary metabolites in Shennong hybrid of Artemisia annua. Chin. Herb. Med. 2019, 11, 209–215. [Google Scholar] [CrossRef]
- Dilshad, E.; Ismail, H.; Haq, I.-u.; Cusido, R.M.; Palazon, J.; Ramirez-Estrada, K.; Mirza, B. Rol genes enhance the biosynthesis of antioxidants in Artemisia carvifolia Buch. BMC Plant Biol. 2016, 16, 125. [Google Scholar] [CrossRef]
- Ul-Haq, I.; Ullah, N.; Bibi, G.; Kanwal, S.; Ahmad, M.S.; Mirza, B. Antioxidant and cytotoxic activities and phytochemical analysis of Euphorbia wallichii root extract and its fractions. Iran. J. Pharm. Res. IJPR 2012, 11, 241. [Google Scholar] [PubMed]
- Dilshad, E.; Zafar, S.; Ismail, H.; Waheed, M.T.; Cusido, R.M.; Palazon, J.; Mirza, B. Effect of rol genes on polyphenols biosynthesis in Artemisia annua and their effect on antioxidant and cytotoxic potential of the plant. Appl. Biochem. Biotechnol. 2016, 179, 1456–1468. [Google Scholar] [CrossRef] [PubMed]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reaction--antioxidative activities of products of browning reaction prepared from glucosamine. Eiyogaku Zasshi Jpn. J. Nutr. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Rani, R. Agrobacterium Mediated Transformation and Secondary Metabolite Analysis of Ajuga bracteosa Wall. ex. Benth. Ph.D. Thesis, Quaid-I-Azam University, Islamabad, Pakistan, 2015. [Google Scholar]
- Schmülling, T.; Schell, J.; Spena, A. Promoters of the rolA, B, and C genes of Agrobacterium rhizogenesare differentially regulated in transgenic plants. Plant Cell 1989, 1, 665–670. [Google Scholar] [CrossRef]
- Sinkar, V.P.; Pythoud, F.; White, F.F.; Nester, E.W.; Gordon, M.P. rolA locus of the Ri plasmid directs developmental abnormalities in transgenic tobacco plants. Genes Dev. 1988, 2, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Michael, T.; Spena, A. The plant oncogenes rolA, B, and C from Agrobacterium rhizogenes. In Agrobacterium Protocols; Springer: Berlin/Heidelberg, Germany, 1995; pp. 207–222. [Google Scholar]
- Dehio, C.; Grossmann, K.; Schell, J.; Schmülling, T. Phenotype and hormonal status of transgenic tobacco plants overexpressing the rolA gene of Agrobacterium rhizogenes T-DNA. Plant Mol. Biol. 1993, 23, 1199–1210. [Google Scholar] [CrossRef]
- Zia, M.; Mirza, B.; Malik, S.A.; Chaudhary, M.F. Expression of rol genes in transgenic soybean (Glycine max L.) leads to changes in plant phenotype, leaf morphology, and flowering time. Plant Cell Tissue Organ Cult. (PCTOC) 2010, 103, 227–236. [Google Scholar] [CrossRef]
- Ismail, H.; Dilshad, E.; Waheed, M.T.; Sajid, M.; Kayani, W.K.; Mirza, B. Transformation of Lactuca sativa L. with rol C gene results in increased antioxidant potential and enhanced analgesic, anti-inflammatory and antidepressant activities in vivo. 3 Biotech 2016, 6, 215. [Google Scholar] [CrossRef]
- Kiani, B.H. Transfer of Rol Genes and Evaluation of Artemisinin Synthesis in Transgenic Artemisia annua L. and Artemisia Dubia Wall. Ph.D. Thesis, Quaid-i-Azam University, Islamabad, Pakistan, 2012. [Google Scholar]
- Schmülling, T.; Fladung, M.; Grossmann, K.; Schell, J. Hormonal content and sensitivity of transgenic tobacco and potato plants expressing single rol genes of Agrobacterium rhizogenes T-DNA. Plant J. 1993, 3, 371–382. [Google Scholar] [CrossRef]
- Bensaddek, L.; Villarreal, M.L.; Fliniaux, M.-A. Induction and growth of hairy roots for the production of medicinal compounds. Electron. J. Integr. Biosci. 2008, 3, 2–9. [Google Scholar]
- Chung, I.-M.; Rekha, K.; Rajakumar, G.; Thiruvengadam, M. Production of glucosinolates, phenolic compounds and associated gene expression profiles of hairy root cultures in turnip (Brassica rapa ssp. rapa). 3 Biotech 2016, 6, 175. [Google Scholar] [CrossRef] [PubMed]
- El-Esawi, M.A.; Elkelish, A.; Elansary, H.O.; Ali, H.M.; Elshikh, M.; Witczak, J.; Ahmad, M. Genetic transformation and hairy root induction enhance the antioxidant potential of Lactuca serriola L. Oxidative Med. Cell. Longev. 2017, 2017, 5604746. [Google Scholar] [CrossRef] [PubMed]
- Nijveldt, R.J.; Van Nood, E.; Van Hoorn, D.E.; Boelens, P.G.; Van Norren, K.; Van Leeuwen, P.A. Flavonoids: A review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Spies, L.; Koekemoer, T.; Sowemimo, A.; Goosen, E.; Van de Venter, M. Caspase-dependent apoptosis is induced by Artemisia afra Jacq. ex Willd in a mitochondria-dependent manner after G2/M arrest. S. Afr. J. Bot. 2013, 84, 104–109. [Google Scholar] [CrossRef]
- Tan, K.L.; Charles, S.V. Antioxidant, antibacterial and cytotoxic activities of essential oils and ethanol extracts of selected South East Asian herbs. J. Med. Plants Res. 2011, 5, 5284–5290. [Google Scholar]
- Singh, N.P.; Ferreira, J.F.; Park, J.S.; Lai, H.C. Cytotoxicity of ethanolic extracts of Artemisia annua to Molt-4 human leukemia cells. Planta Med. 2011, 77, 1788–1793. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).