Identification and Analysis of Antimicrobial Activities from a Model Moss Ceratodon purpureus
Abstract
1. Introduction
2. Materials and Methods
2.1. Moss Strains, Growth Conditions and Exudate Collection
2.2. Tests for Antibacterial Activity
2.3. Evaluation of Minimal Bactericidal Concentration
2.4. Size Fractionation of Extracellular Metabolites
2.5. Exudate Metabolite Stability and Sensitivity Tests
2.6. Data Analysis
3. Results
3.1. Identification of Antibacterial Activity in C. purpureus exudates
3.2. Quantitative Analysis of Antimicrobial Activity from C. purpureus R40 Exudate
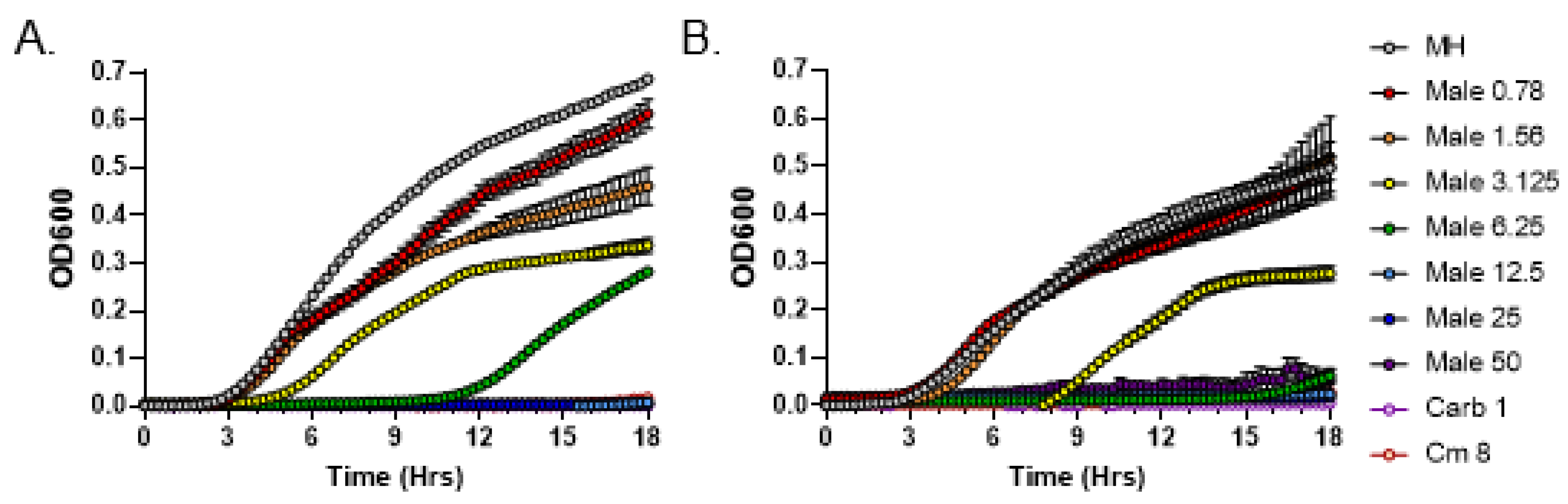
3.3. C. purpureus R40 Exudates Display Bactericidal Mode of Action
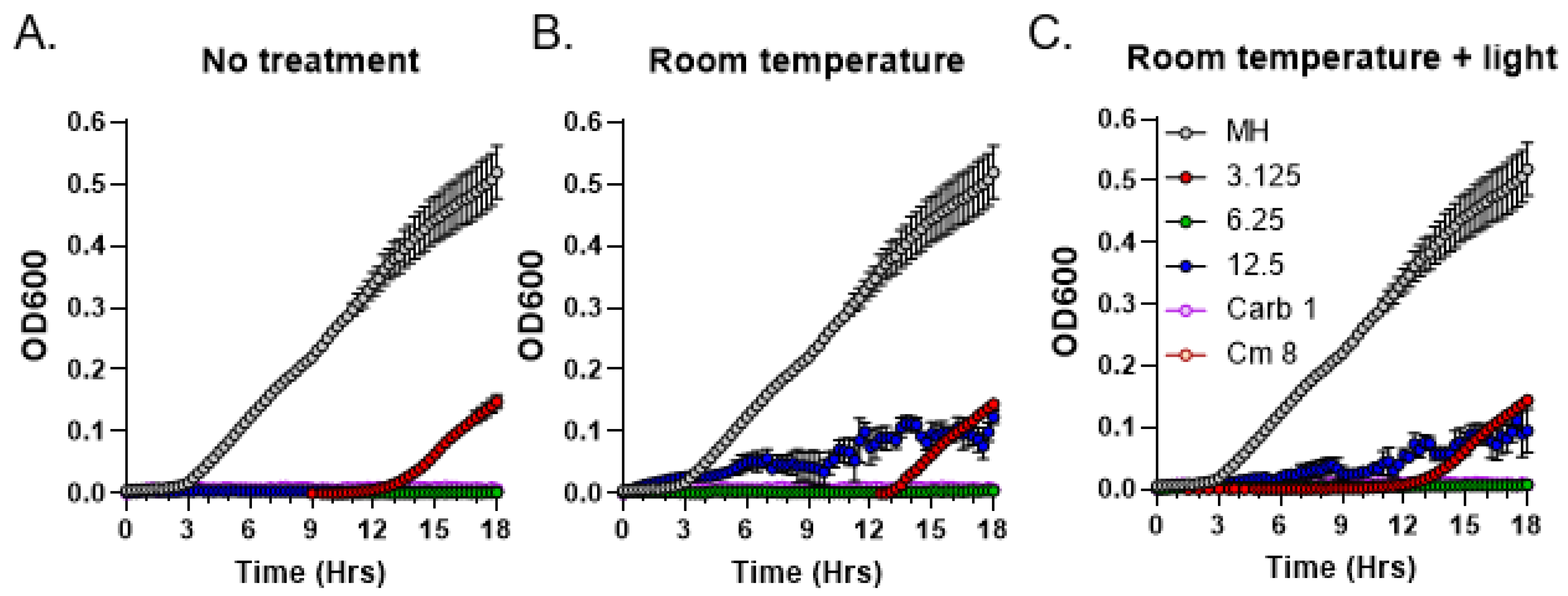
3.4. Light Sensitivity of Antibacterial Compounds Present in C. purpureus Exudates
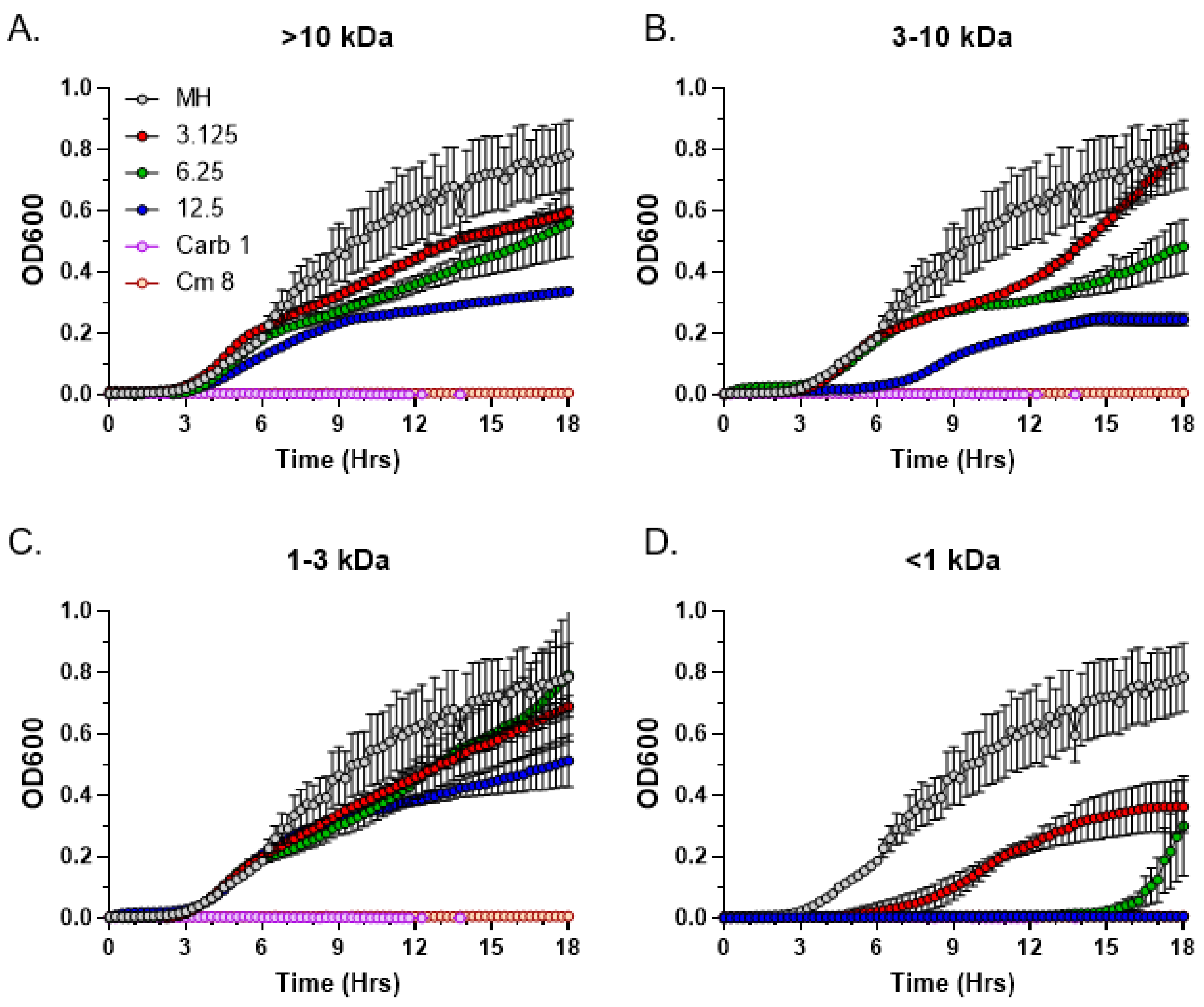
3.5. Size Fractionation of Bioactive C. purpureus Exudate Components
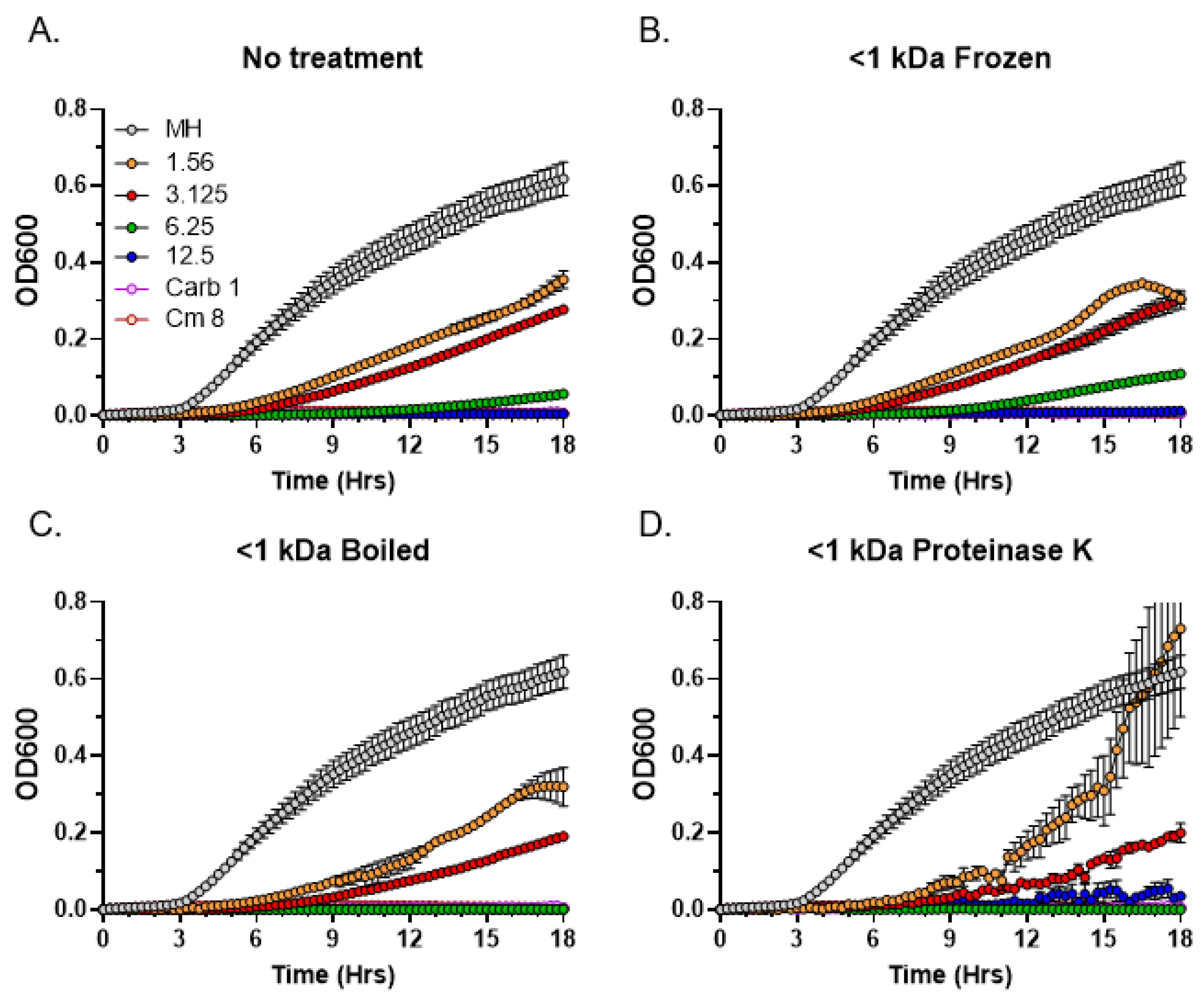
3.6. Thermostability and Sensitivity to Proteinase K Treatment
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2019 Antimicrobial Resistance Collaborators. Global mortality associated with 33 bacterial pathogens in 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2022, 400, 2221–2248. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Harant, A.; Fernandes, G.; Mwamelo, A.J.; Hein, W.; Dekker, D.; Sridhar, D. Measuring the global response to antimicrobial resistance, 2020–2021: A systematic governance analysis of 114 countries. Lancet Infect. Dis. 2023, 202, 1–13. [Google Scholar] [CrossRef]
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Asokan, G.V.; Ramadhan, T.; Ahmed, E.; Sanad, H. WHO Global Priority Pathogens List: A Bibliometric Analysis of Medline-PubMed for Knowledge Mobilization to Infection Prevention and Control Practices in Bahrain. Oman Med. J. 2019, 34, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Mattingly, J.M.; Dunham, C.M. ESKAPE velocity: Total synthesis platforms promise to increase the pace and diversity of antibiotic development. Nat. Struct. Mol. Biol. 2022, 29, 3–9. [Google Scholar] [CrossRef]
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Bazzaz, B.S.F. Review on plant antimicrobials: A mechanistic viewpoint. Antimicrobial Resistance and Infection Control. BMC 2019, 8, 118. [Google Scholar] [CrossRef]
- Shen, B. A New Golden Age of Natural Products Drug Discovery. Cell 2015, 163, 1297–1300. [Google Scholar] [CrossRef] [PubMed]
- Horn, A.; Pascal, A.; Lončarević, I.; Marques, R.; Lu, Y.; Miguel, S.; Bourgaud, F.; Thorsteinsdóttir, M.; Cronberg, N.; Becker, J.D.; et al. Natural Products from Bryophytes: From Basic Biology to Biotechnological Applications. Crit. Rev. Plant Sci. 2021, 40, 191–217. [Google Scholar] [CrossRef]
- Waterman, M.J.; Nugraha, A.S.; Hendra, R.; Ball, G.E.; Robinson, S.A.; Keller, P.A. Antarctic Moss Biflavonoids Show High Antioxidant and Ultraviolet-Screening Activity. J. Nat. Prod. 2017, 80, 2224–2231. [Google Scholar] [CrossRef]
- Kang, S.J.; Kim, S.H.; Liu, P.; Jovel, E.; Towers, G.H. Antibacterial activities of some mosses including Hylocomium splendens from South Western British Columbia. Fitoterapia 2007, 78, 373–376. [Google Scholar] [CrossRef]
- Wolski, G.J.; Sadowska, B.; Fol, M.; Podsędek, A.; Kajszczak, D.; Kobylińska, A. Cytotoxicity, antimicrobial and antioxidant activities of mosses obtained from open habitats. PLoS ONE 2021, 16, e0257479. [Google Scholar] [CrossRef]
- Olofin, T.A.; Akande, A.O.; Oyetayo, V.O. Assessment of the antimicrobial properties of fractions obtained from bryophytes. J. Microbiol. Antimicrob. 2013, 5, 50–54. [Google Scholar] [CrossRef]
- Mishra, R.; Pandey, V.K.; Chandra, R. Potential of Bryophytes as therapeutics. IJPSR 2014, 5, 3584–3593. [Google Scholar]
- Seitz, V.A.; McGivern, B.B.; Daly, R.A.; Chaparro, J.M.; Borton, M.A.; Sheflin, A.M.; Kresovich, S.; Shields, L.; Schipanski, M.E.; Wrighton, K.C.; et al. Variation in Root Exudate Composition Influences Soil Microbiome Membership and Function. Appl. Environ. Microbiol. 2022, 88, e00226-22. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Robert, C.A.M.; Cadot, S.; Zhang, X.; Ye, M.; Li, B.; Manzo, D.; Chervet, N.; Steinger, T.; van der Heijden, M.G.A.; et al. Root exudate metabolites drive plant-soil feedbacks on growth and defense by shaping the rhizosphere microbiota. Nat. Comm. 2022, 9, 2738. [Google Scholar] [CrossRef] [PubMed]
- Samaddar, S.; Karp, D.S.; Schmidt, R.; Devarajan, N.; McGarvey, J.A.; Pires, A.F.A.; Scow, K. Role of soil in the regulation of human and plant pathogens: Soils’ contributions to people. Phil. Trans. R. Soc. B 2021, 376, 20200179. [Google Scholar] [CrossRef] [PubMed]
- Ichino, T.; Yazaki, K. Modes of secretion of plant lipophilic metabolites via ABCG transporter-dependent transport and vesicle-mediated trafficking. Curr. Opin. Plant Biol. 2022, 66, 102184. [Google Scholar] [CrossRef]
- Romani, F.; Banić, E.; Florent, S.N.; Kanazawa, T.; Goodger, J.Q.D.; Mentink, R.A.; Dierschke, T.; Zachgo, S.; Ueda, T.; Bowman, J.L.; et al. Oil body formation in Marchantia polymorpha is controlled by MpC1HDZ and serves as a defense against arthropod herbivores. Curr. Biol. 2020, 30, 2815–2828. [Google Scholar] [CrossRef]
- Fesenko, I.; Azarkina, R.; Kirov, I.; Kniazev, A.; Filippova, A.; Grafskaia, E.; Lazarev, V.; Zgoda, V.; Butenko, I.; Bukato, O.; et al. Phytohormone treatment induces generation of cryptic peptides with antimicrobial activity in the Moss Physcomitrella Patens. BMC Plant Biol. 2019, 19, 9. [Google Scholar] [CrossRef]
- Valeeva, L.R.; Dague, A.L.; Hall, M.H.; Tikhonova, A.E.; Sharipova, M.R.; Valentovic, M.A.; Bogomolnaya, L.M.; Shakirov, E.V. Antimicrobial Activities of Secondary Metabolites from Model Mosses. Antibiotics 2022, 11, 1004. [Google Scholar] [CrossRef]
- Biersma, E.M.; Convey, P.; Wyber, R.; Robinson, S.A.; Dowton, M.; van de Vijver, B.; Linse, K.; Griffiths, H.; Jackson, J.A. Latitudinal Biogeographic Structuring in the Globally Distributed Moss Ceratodon Purpureus. Front. Plant Sci. 2020, 11, 502359. [Google Scholar] [CrossRef]
- Carey, S.B.; Jenkins, J.; Lovell, J.T.; Maumus, F.; Sreedasyam, A.; Payton, A.C.; Shu, S.; Tiley, G.P.; Fernandez-Pozo, N.; Healey, A.; et al. Gene-rich UV sex chromosomes harbor conserved regulators of sexual development. Sci. Adv. 2021, 7, eabh2488. [Google Scholar] [CrossRef]
- Kollar, L.M.; Kiel, S.; James, A.J.; Carnley, C.T.; Scola, D.N.; Clark, T.N.; Khanal, T.; Rosenstiel, T.N.; Gall, E.T.; Grieshop, K.; et al. The genetic architecture of sexual dimorphism in the moss Ceratodon purpureus. Proc. Biol. Sci. 2021, 288, 20202908. [Google Scholar] [CrossRef]
- Rosenstiel, T.N.; Shortlidge, E.E.; Melnychenko, A.N.; Pankow, J.F.; Eppley, S.M. Sex-specific volatile compounds influence microarthropod-mediated fertilization of moss. Nature 2012, 489, 431–433. [Google Scholar] [CrossRef]
- Brennan, D.L.; Kollar, L.M.; Kiel, S.; Deakova, T.; Laguerre, A.; McDaniel, S.F.; Eppley, S.M.; Gall, E.T.; Rosenstiel, T.N. Measuring volatile emissions from moss gametophytes: A review of methodologies and new applications. Appl. Plant Sci. 2022, 10, e11468. [Google Scholar] [CrossRef] [PubMed]
- Sala-Carvalho, W.R.; Montessi-Amaral, F.P.; Esposito, M.P.; Campestrini, R.; Rossi, M.; Peralta, D.F.; Furlan, C.M. Metabolome of Ceratodon purpureus (Hedw.) Brid.; a cosmopolitan moss: The influence of seasonality. Planta 2022, 255, 77. [Google Scholar] [CrossRef]
- Ashton, N.V.; Cove, D.J. The Isolation and Preliminary Characterization of Auxotrophic and Analogue Resistant Mutants of the Moss, Physcomitrella patens. Molec. Gen. Genet. 1977, 154, 87–95. [Google Scholar] [CrossRef]
- Shirshikova, T.V.; Sierra-Bakhshi, C.G.; Kamaletdinova, L.K.; Matrosova, L.E.; Khabipova, N.N.; Evtugyn, V.G.; Khilyas, I.V.; Danilova, I.V.; Mardanova, A.M.; Sharipova, M.R.; et al. The ABC-Type Efflux Pump MacAB is Involved in Protection of Serratia marcescens against Aminoglycoside Antibiotics, Polymyxins, and Oxidative Stress. mSphere 2021, 6, e00033-21. [Google Scholar] [CrossRef] [PubMed]
- Pankey, G.A.; Sabath, L.D. Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of Gram-positive bacterial infections. Clin. Infect. Dis. 2004, 38, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Wald-Dicker, N.; Holtom, P.; Spellberg, B. Busting the myth of “static vs. cidal”: A systemic literature review. Clin. Infect. Dis. 2018, 66, 1470–1474. [Google Scholar] [CrossRef]
- Motyl, M.; Dorso, K.; Barrett, J.; Giacobbe, R. Basic Microbiological Techniques for Antibacterial Drug Discovery. Curr. Protoc. Pharmacol. 2003, 13A.3 (Suppl. S31), 1–22. [Google Scholar] [CrossRef] [PubMed]
- Badri, D.V.; Vivanco, J.M. Regulation and function of root exudates. Plant Cell Environ. 2009, 32, 666–681. [Google Scholar] [CrossRef]
- Lyapina, I.; Filippova, A.; Kovalchuk, S.; Ziganshin, R.; Mamaeva, A.; Lazarev, V.; Latsis, I.; Mikhalchik, E.; Panasenko, O.; Ivanov, O.; et al. Possible role of small secreted peptides (SSPs) in immune signaling in bryophytes. Plant Mol. Biol. 2021, 106, 123–143. [Google Scholar] [CrossRef] [PubMed]
- Slate, M.L.; Rosenstiel, T.N.; Eppley, S.M. Sex-specific morphological and physiological differences in the moss Ceratodon purpureus (Dicranales). Ann. Bot. 2017, 120, 845–854. [Google Scholar] [CrossRef]
- Shortlidge, E.E.; Carey, S.B.; Payton, A.C.; McDaniel, S.F.; Rosenstiel, T.N.; Eppley, S.M. Microarthropod contributions to fitness variation in the common moss Ceratodon purpureus. Proc. Biol. Sci. 2021, 288, 20210119. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Pang, Z.; Yuan, Z.; Fallah, N.; Jia, H.; Ming, R. Sex-based metabolic and microbiota differences in roots and rhizosphere soils of dioecious papaya (Carica papaya L.). Front. Plant Sci. 2022, 13, 991114. [Google Scholar] [CrossRef]
- Fesenko, I.; Shabalina, S.A.; Mamaeva, A.; Knyazev, A.; Glushkevich, A.; Lyapina, I.; Ziganshin, R.; Kovalchuk, S.; Kharlampieva, D.; Lazarev, V.; et al. A vast pool of lineage-specific microproteins encoded by long non-coding RNAs in plants. Nucleic Acids Res. 2021, 49, 10328–10346. [Google Scholar] [CrossRef]
- Fesenko, I.; Khazigaleeva, R.; Kirov, I.; Kniazev, A.; Glushenko, O.; Babalyan, K.; Arapidi, G.; Shashkova, T.; Butenko, I.; Zgoda, V.; et al. Alternative splicing shapes transcriptome but not proteome diversity in Physcomitrella patens. Sci. Rep. 2017, 7, 2698. [Google Scholar] [CrossRef]
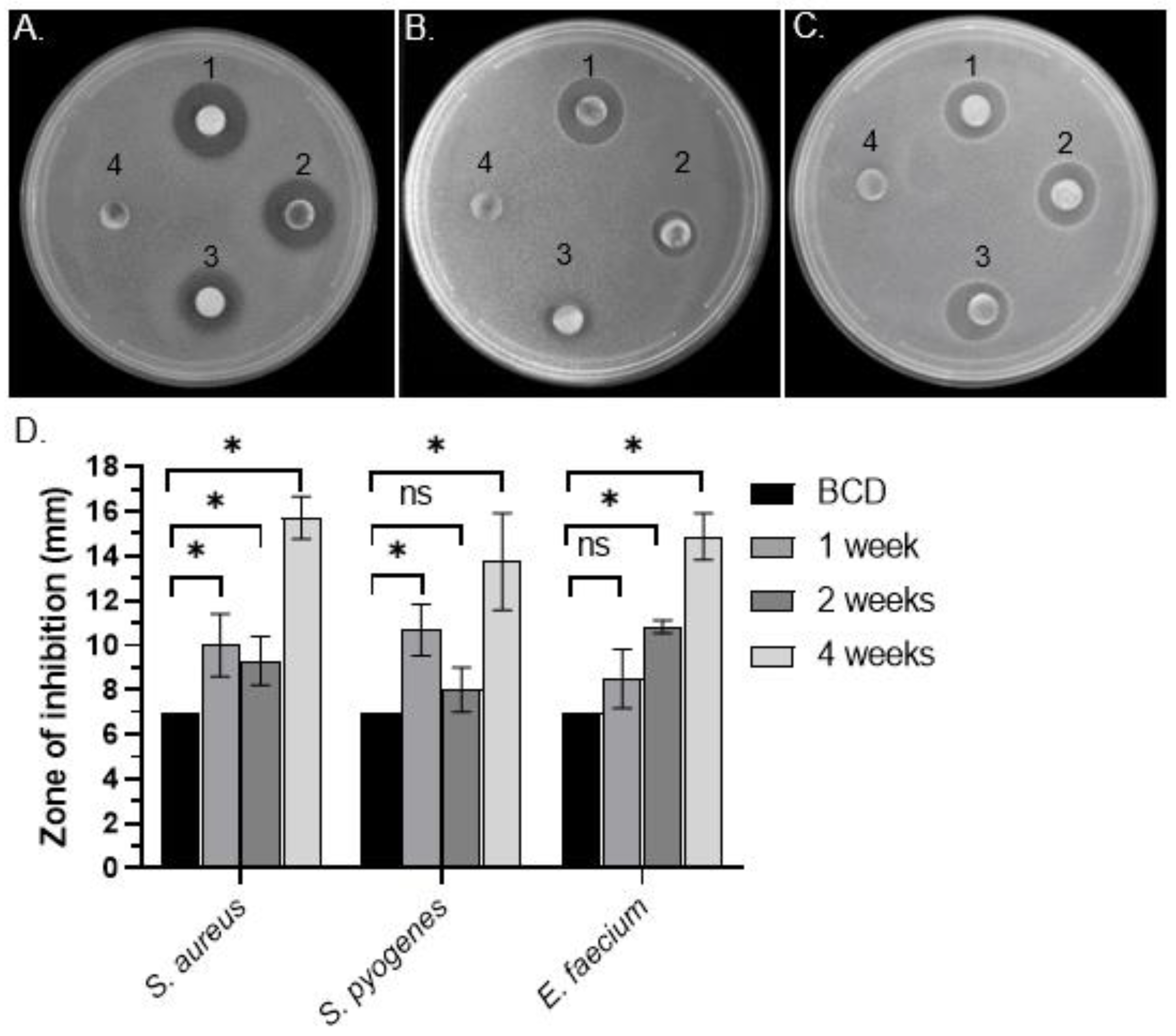


| Bacterial Growth Inhibition Zone in DDM Assays, in mm | MIC Values, mg/mL | MBC, mg/mL | ||||||
|---|---|---|---|---|---|---|---|---|
| C. purpureus strain | Bacteria | No Exudate Control a | 1-Week-Old Moss Exudate | 2-Week-Old Moss Exudate | 4-Week-Old Moss Exudate | 2-Week-Old Moss Exudate | 4-Week-Old Moss Exudate | 4-Week-Old Moss Exudate |
| R40 | S. aureus | 7 | 10.00 ± 1.41 * | 9.31 ± 1.09 ** | 15.71 ± 0.95 ** | 12.5 | 6.25 | 6.25 |
| S. pyogenes | 7 | 10.67 ± 1.16 * | 8.00 ± 1.00 | 13.75 ± 2.17 ** | - | 50 | - | |
| E. faecium | 7 | 8.50 ± 1.32 | 11.00 ± 0.01 ** | 14.88 ± 1.05 ** | - | 12.5 | - | |
| S. marcescens | 7 | 7 | 7 | 7 | - | - | - | |
| S. enterica ser. Typhimurium | 7 | 7 | 7 | 7 | - | - | - | |
| GG1 | S. aureus | 7 | 7 | 7 | 7 | 50 | 50 | - |
| S. marcescens | 7 | 7 | 7 | 7 | - | - | - | |
| S. enterica ser. Typhimurium | 7 | 7 | 7 | 7 | - | - | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dague, A.L.; Valeeva, L.R.; McCann, N.M.; Sharipova, M.R.; Valentovic, M.A.; Bogomolnaya, L.M.; Shakirov, E.V. Identification and Analysis of Antimicrobial Activities from a Model Moss Ceratodon purpureus. Metabolites 2023, 13, 350. https://doi.org/10.3390/metabo13030350
Dague AL, Valeeva LR, McCann NM, Sharipova MR, Valentovic MA, Bogomolnaya LM, Shakirov EV. Identification and Analysis of Antimicrobial Activities from a Model Moss Ceratodon purpureus. Metabolites. 2023; 13(3):350. https://doi.org/10.3390/metabo13030350
Chicago/Turabian StyleDague, Ashley L., Lia R. Valeeva, Natalie M. McCann, Margarita R. Sharipova, Monica A. Valentovic, Lydia M. Bogomolnaya, and Eugene V. Shakirov. 2023. "Identification and Analysis of Antimicrobial Activities from a Model Moss Ceratodon purpureus" Metabolites 13, no. 3: 350. https://doi.org/10.3390/metabo13030350
APA StyleDague, A. L., Valeeva, L. R., McCann, N. M., Sharipova, M. R., Valentovic, M. A., Bogomolnaya, L. M., & Shakirov, E. V. (2023). Identification and Analysis of Antimicrobial Activities from a Model Moss Ceratodon purpureus. Metabolites, 13(3), 350. https://doi.org/10.3390/metabo13030350








