Associations between Smoking and Smoking Cessation during Pregnancy and Newborn Metabolite Concentrations: Findings from PRAMS and INSPIRE Birth Cohorts
Abstract
:1. Introduction
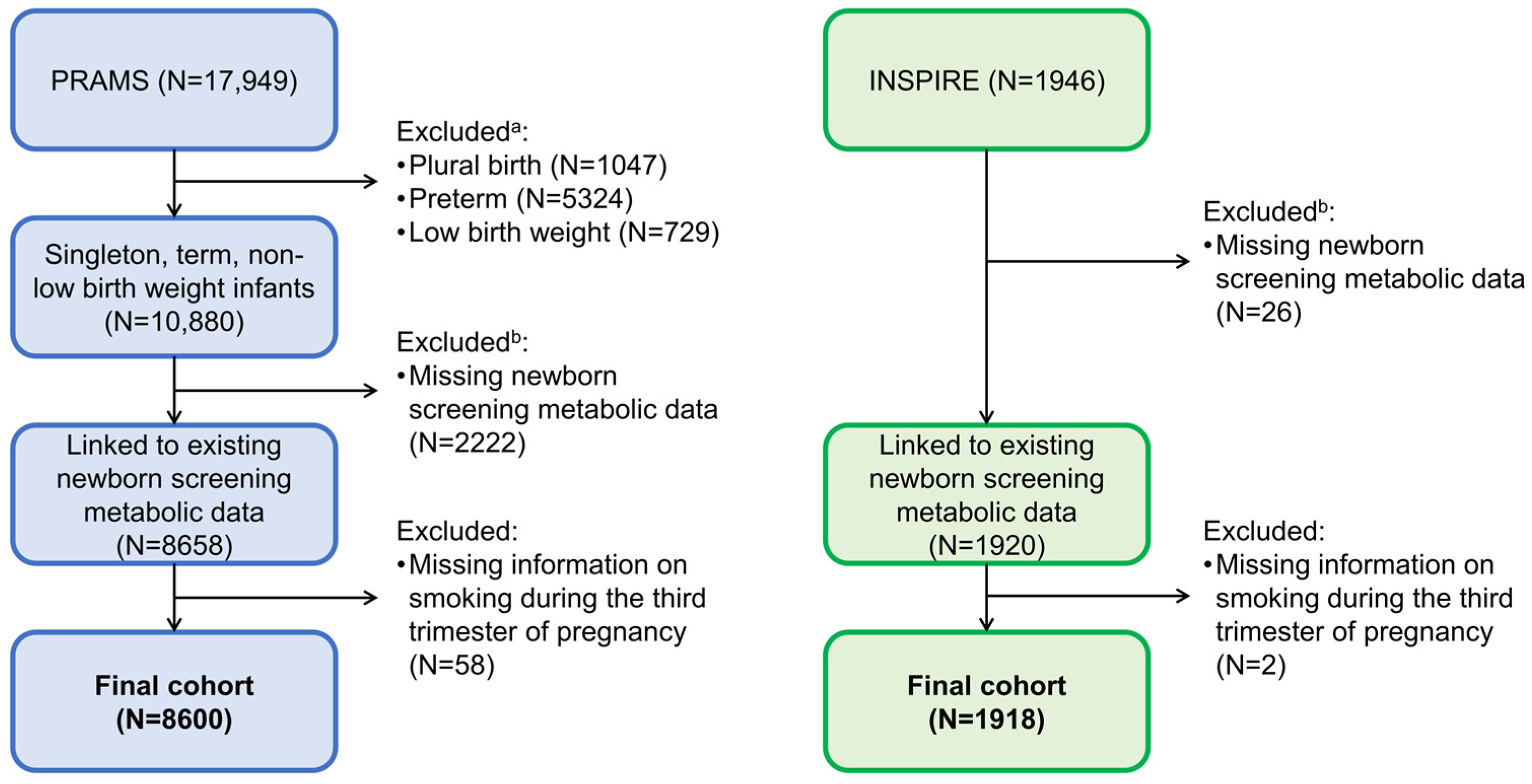
2. Materials and Methods
2.1. Study Design and Populations
2.2. Smoking Ascertainment
2.3. Newborn Screening Metabolic DATA Collection
2.4. Covariate Ascertainment
2.5. Statistical Analysis
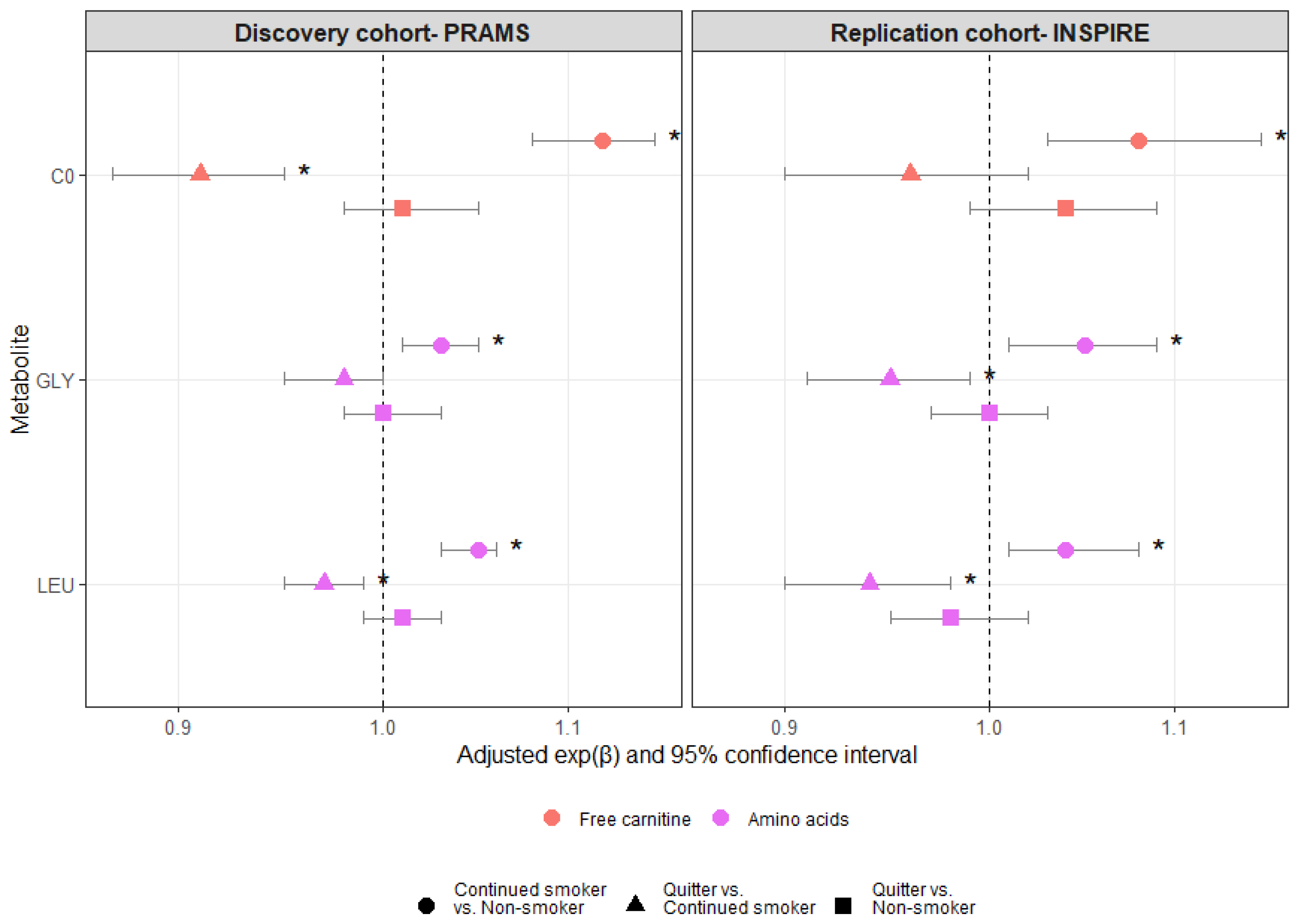
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lamm, S.H.; Ferdosi, H.; Boroje, I.J.; Afari-Dwamena, N.A.; Qian, L.; Dash, E.D.; Li, J.; Chen, R.; Feinleib, M. Maternal tobacco use: A third-trimester risk factor for small-for-gestational-age pregnancy outcome. Prev. Med. Rep. 2020, 18, 101080. [Google Scholar] [CrossRef] [PubMed]
- Azagba, S.; Manzione, L.; Shan, L.; King, J. Trends in smoking during pregnancy by socioeconomic characteristics in the United States, 2010–2017. BMC Pregnancy Childbirth 2020, 20, 52. [Google Scholar] [CrossRef]
- Martin, J.A.; Osterman, M.J.K.; Driscoll, A.K. Declines in Cigarette Smoking During Pregnancy in the United States, 2016–2021. NCHS Data Brief 2023, 458, 1–8. [Google Scholar]
- UpToDate. Cigarette and Tobacco Products in Pregnancy: Impact on Pregnancy and the Neonate. Available online: https://www.uptodate.com/contents/cigarette-and-tobacco-products-in-pregnancy-impact-on-pregnancy-and-the-neonate (accessed on 20 September 2023).
- Sabra, S.; Gratacós, E.; Gómez Roig, M.D. Smoking-Induced Changes in the Maternal Immune, Endocrine, and Metabolic Pathways and Their Impact on Fetal Growth: A Topical Review. Fetal Diagn. Ther. 2017, 41, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Behl, M.; Rao, D.; Aagaard, K.; Davidson, T.L.; Levin, E.D.; Slotkin, T.A.; Srinivasan, S.; Wallinga, D.; White, M.F.; Walker, V.R.; et al. Evaluation of the association between maternal smoking, childhood obesity, and metabolic disorders: A national toxicology program workshop review. Environ. Health Perspect. 2013, 121, 170–180. [Google Scholar] [CrossRef]
- Rotroff, D.M.; Joubert, B.R.; Marvel, S.W.; Håberg, S.E.; Wu, M.C.; Nilsen, R.M.; Ueland, P.M.; Nystad, W.; London, S.J.; Motsinger-Reif, A. Maternal smoking impacts key biological pathways in newborns through epigenetic modification in Utero. BMC Genom. 2016, 17, 976. [Google Scholar] [CrossRef] [PubMed]
- Fiehn, O. Metabolomics—The link between genotypes and phenotypes. Plant Mol. Biol. 2002, 48, 155–171. [Google Scholar] [CrossRef]
- Snyder, B.M.; Gebretsadik, T.; Rohrig, N.B.; Wu, P.; Dupont, W.D.; Dabelea, D.M.; Fry, R.C.; Lynch, S.V.; McEvoy, C.T.; Paneth, N.S.; et al. The Associations of Maternal Health Characteristics, Newborn Metabolite Concentrations, and Child Body Mass Index among US Children in the ECHO Program. Metabolites 2023, 13, 510. [Google Scholar] [CrossRef]
- de Nava, A.S.L.; Raja, A. Physiology, Metabolism; StatPearls [Internet] StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK546690/ (accessed on 20 September 2023).
- McCann, M.R.; George De la Rosa, M.V.; Rosania, G.R.; Stringer, K.A. L-Carnitine and Acylcarnitines: Mitochondrial Biomarkers for Precision Medicine. Metabolites 2021, 11, 51. [Google Scholar] [CrossRef]
- Bröer, S.; Bröer, A. Amino acid homeostasis and signalling in mammalian cells and organisms. Biochem. J. 2017, 474, 1935–1963. [Google Scholar] [CrossRef]
- Xu, T.; Holzapfel, C.; Dong, X.; Bader, E.; Yu, Z.; Prehn, C.; Perstorfer, K.; Jaremek, M.; Roemisch-Margl, W.; Rathmann, W.; et al. Effects of smoking and smoking cessation on human serum metabolite profile: Results from the KORA cohort study. BMC Med. 2013, 11, 60. [Google Scholar] [CrossRef]
- Shulman, H.B.; D’Angelo, D.V.; Harrison, L.; Smith, R.A.; Warner, L. The Pregnancy Risk Assessment Monitoring System (PRAMS): Overview of Design and Methodology. Am. J. Public Health 2018, 108, 1305–1313. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. PRAMS. Available online: https://www.cdc.gov/prams/index.htm (accessed on 20 September 2023).
- Larkin, E.K.; Gebretsadik, T.; Moore, M.L.; Anderson, L.J.; Dupont, W.D.; Chappell, J.D.; Minton, P.A.; Peebles, R.S., Jr.; Moore, P.E.; Valet, R.S.; et al. Objectives, design and enrollment results from the Infant Susceptibility to Pulmonary Infections and Asthma Following RSV Exposure Study (INSPIRE). BMC Pulm. Med. 2015, 15, 45. [Google Scholar] [CrossRef] [PubMed]
- Snyder, B.M.; Gebretsadik, T.; Turi, K.N.; McKennan, C.; Havstad, S.; Jackson, D.J.; Ober, C.; Lynch, S.; McCauley, K.; Seroogy, C.M.; et al. Association of citrulline concentration at birth with lower respiratory tract infection in infancy: Findings from a multi-site birth cohort study. Front. Pediatr. 2022, 10, 979777. [Google Scholar] [CrossRef]
- Textor, J.; van der Zander, B.; Gilthorpe, M.S.; Liskiewicz, M.; Ellison, G.T. Robust causal inference using directed acyclic graphs: The R package ‘dagitty’. Int. J. Epidemiol. 2016, 45, 1887–1894. [Google Scholar] [CrossRef] [PubMed]
- Rolle-Kampczyk, U.E.; Krumsiek, J.; Otto, W.; Röder, S.W.; Kohajda, T.; Borte, M.; Theis, F.; Lehmann, I.; von Bergen, M. Metabolomics reveals effects of maternal smoking on endogenous metabolites from lipid metabolism in cord blood of newborns. Metabolomics 2016, 12, 76. [Google Scholar] [CrossRef]
- Cajachagua-Torres, K.N.; Blaauwendraad, S.M.; El Marroun, H.; Demmelmair, H.; Koletzko, B.; Gaillard, R.; Jaddoe, V.W.V. Fetal Exposure to Maternal Smoking and Neonatal Metabolite Profiles. Metabolites 2022, 12, 1101. [Google Scholar] [CrossRef] [PubMed]
- Caliri, A.W.; Tommasi, S.; Besaratinia, A. Relationships among smoking, oxidative stress, inflammation, macromolecular damage, and cancer. Mutat. Res. Rev. Mutat. Res. 2021, 787, 108365. [Google Scholar] [CrossRef]
- Suter, M.A.; Aagaard, K.M. The impact of tobacco chemicals and nicotine on placental development. Prenat. Diagn. 2020, 40, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Aycicek, A.; Varma, M.; Ahmet, K.; Abdurrahim, K.; Erel, O. Maternal active or passive smoking causes oxidative stress in placental tissue. Eur. J. Pediatr. 2011, 170, 645–651. [Google Scholar] [CrossRef]
- Dittrich, R.; Schibel, A.; Hoffmann, I.; Mueller, A.; Beckmann, M.W.; Cupisti, S. Influence of maternal smoking during pregnancy on oxidant status in amniotic fluid. In Vivo 2012, 26, 813–818. [Google Scholar]
- Noakes, P.S.; Thomas, R.; Lane, C.; Mori, T.A.; Barden, A.E.; Devadason, S.G.; Prescott, S.L. Association of maternal smoking with increased infant oxidative stress at 3 months of age. Thorax 2007, 62, 714–717. [Google Scholar] [CrossRef] [PubMed]
- Chełchowska, M.; Gajewska, J.; Ambroszkiewicz, J.; Mazur, J.; Ołtarzewski, M.; Maciejewski, T.M. Influence of Oxidative Stress Generated by Smoking during Pregnancy on Glutathione Status in Mother-Newborn Pairs. Antioxidants 2021, 10, 1866. [Google Scholar] [CrossRef] [PubMed]
- Rua, E.d.A.O.; Porto, M.L.; Ramos, J.P.L.; Nogueira, B.V.; dos Santos Meyrelles, S.; Vasquez, E.C.; Pereira, T.d.M.C. Effects of tobacco smoking during pregnancy on oxidative stress in the umbilical cord and mononuclear blood cells of neonates. J. Biomed. Sci. 2014, 21, 105. [Google Scholar] [CrossRef] [PubMed]
- Garrabou, G.; Hernàndez, A.S.; Catalán García, M.; Morén, C.; Tobías, E.; Córdoba, S.; López, M.; Figueras, F.; Grau, J.M.; Cardellach, F. Molecular basis of reduced birth weight in smoking pregnant women: Mitochondrial dysfunction and apoptosis. Addict. Biol. 2016, 21, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Ribas, G.S.; Vargas, C.R.; Wajner, M. l-carnitine supplementation as a potential antioxidant therapy for inherited neurometabolic disorders. Gene 2014, 533, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Silva-Adaya, D.; Pérez-De La Cruz, V.; Herrera-Mundo, M.N.; Mendoza-Macedo, K.; Villeda-Hernández, J.; Binienda, Z.; Ali, S.F.; Santamaría, A. Excitotoxic damage, disrupted energy metabolism, and oxidative stress in the rat brain: Antioxidant and neuroprotective effects of L-carnitine. J. Neurochem. 2008, 105, 677–689. [Google Scholar] [CrossRef]
- Petrillo, T.; Battipaglia, C.; Virmani, M.A.; Genazzani, A.R.; Genazzani, A.D. Neuroendocrine Effects of Carnitines on Reproductive Impairments. Int. J. Mol. Sci. 2021, 22, 10781. [Google Scholar] [CrossRef]
- Pillich, R.T.; Scarsella, G.; Risuleo, G. Reduction of apoptosis through the mitochondrial pathway by the administration of acetyl-L-carnitine to mouse fibroblasts in culture. Exp. Cell Res. 2005, 306, 1–8. [Google Scholar] [CrossRef]
- Abdelrazik, H.; Sharma, R.; Mahfouz, R.; Agarwal, A. L-carnitine decreases DNA damage and improves the in vitro blastocyst development rate in mouse embryos. Fertil. Steril. 2009, 91, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Wheeler, M.D.; Li, X.; Froh, M.; Schemmer, P.; Yin, M.; Bunzendaul, H.; Bradford, B.; Lemasters, J.J. L-Glycine: A novel antiinflammatory, immunomodulatory, and cytoprotective agent. Curr. Opin. Clin. Nutr. Metab. Care 2003, 6, 229–240. [Google Scholar] [CrossRef]
- Zhang, S.; Zeng, X.; Ren, M.; Mao, X.; Qiao, S. Novel metabolic and physiological functions of branched chain amino acids: A review. J. Anim. Sci. Biotechnol. 2017, 8, 10. [Google Scholar] [CrossRef]
- Wu, S.; Liu, X.; Cheng, L.; Wang, D.; Qin, G.; Zhang, X.; Zhen, Y.; Wang, T.; Sun, Z. Protective Mechanism of Leucine and Isoleucine against H(2)O(2)-Induced Oxidative Damage in Bovine Mammary Epithelial Cells. Oxid. Med. Cell Longev. 2022, 2022, 4013575. [Google Scholar] [CrossRef]
- Mao, H.; Wang, C.; Yu, Z. Dietary leucine supplementation enhances the health of early weaned Hu lambs. Anim. Feed. Sci. Technol. 2019, 247, 248–254. [Google Scholar] [CrossRef]
- Sun, B.; Sun, Y.; Han, X.; Ma, Q.; Meng, Q. Leucine Supplementation Alleviates Immune and Antioxidant Function Damage in Adult Rats Induced by Early Weaning. J. Nutr. 2023, 153, 1607–1617. [Google Scholar] [CrossRef]
- Chen, X.; Xiang, L.; Jia, G.; Liu, G.; Zhao, H.; Huang, Z. Effects of dietary leucine on antioxidant activity and expression of antioxidant and mitochondrial-related genes in longissimus dorsi muscle and liver of piglets. Anim. Sci. J. 2019, 90, 990–998. [Google Scholar] [CrossRef] [PubMed]
- Cruzat, V.; Macedo Rogero, M.; Noel Keane, K.; Curi, R.; Newsholme, P. Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation. Nutrients 2018, 10, 1564. [Google Scholar] [CrossRef] [PubMed]
- Bonvini, A.; Coqueiro, A.Y.; Tirapegui, J.; Calder, P.C.; Rogero, M.M. Immunomodulatory role of branched-chain amino acids. Nutr. Rev. 2018, 76, 840–856. [Google Scholar] [CrossRef]
- Ryckman, K.K.; Berberich, S.L.; Dagle, J.M. Predicting gestational age using neonatal metabolic markers. Am. J. Obstet. Gynecol. 2016, 214, 515.e511–515.e513. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Newborn Screening by Tandem Mass Spectrometry, 2nd ed.; CLSI Guideline NBS04; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017. [Google Scholar]
- De Jesús, V.R.; Mei, J.V.; Cordovado, S.K.; Cuthbert, C.D. The Newborn Screening Quality Assurance Program at the Centers for Disease Control and Prevention: Thirty-five Year Experience Assuring Newborn Screening Laboratory Quality. Int. J. Neonatal. Screen. 2015, 1, 13–26. [Google Scholar] [CrossRef]
- Orsso, C.E.; Colin-Ramirez, E.; Field, C.J.; Madsen, K.L.; Prado, C.M.; Haqq, A.M. Adipose Tissue Development and Expansion from the Womb to Adolescence: An Overview. Nutrients 2020, 12, 2735. [Google Scholar] [CrossRef] [PubMed]
- Clemmons, D.R. Metabolic actions of insulin-like growth factor-I in normal physiology and diabetes. Endocrinol. Metab. Clin. N. Am. 2012, 41, 425–443. [Google Scholar] [CrossRef] [PubMed]
- Kvalvik, L.G.; Nilsen, R.M.; Skjærven, R.; Vollset, S.E.; Midttun, O.; Ueland, P.M.; Haug, K. Self-reported smoking status and plasma cotinine concentrations among pregnant women in the Norwegian Mother and Child Cohort Study. Pediatr. Res. 2012, 72, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Mattsson, K.; Källén, K.; Rignell-Hydbom, A.; Lindh, C.H.; Jönsson, B.A.; Gustafsson, P.; Olofsson, P.; Ivarsson, S.A.; Rylander, L. Cotinine Validation of Self-Reported Smoking During Pregnancy in the Swedish Medical Birth Register. Nicotine Tob. Res. 2016, 18, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Addicott, M.A.; Sutfin, E.L.; Reynolds, L.M.; Donny, E.C.; Matich, E.K.; Hsu, P.C. Biochemical validation of self-reported electronic nicotine delivery system and tobacco heaviness of use. Exp. Clin. Psychopharmacol. 2023, 31, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Boykan, R.; Messina, C.R.; Chateau, G.; Eliscu, A.; Tolentino, J.; Goniewicz, M.L. Self-Reported Use of Tobacco, E-cigarettes, and Marijuana Versus Urinary Biomarkers. Pediatrics 2019, 143, e20183531. [Google Scholar] [CrossRef]

| Maternal Characteristic | PRAMS | INSPIRE | p-Value a |
|---|---|---|---|
| Sample size, n (%) | 8600 | 1918 | |
| Race and ethnicity, n (%) | <0.001 * | ||
| Non-Hispanic White | 5448 (63) | 1312 (68) | |
| Non-Hispanic Black | 1928 (22) | 366 (19) | |
| Hispanic | 797 (9) | 130 (7) | |
| Other b | 410 (5) | 110 (6) | |
| Missing, n (%) | 17 (0) | 0 (0) | |
| Education (years), n (%) | <0.001 * | ||
| <12 | 1480 (17) | 152 (8) | |
| 12 | 2550 (30) | 523 (27) | |
| 13–15 | 2549 (30) | 573 (30) | |
| ≥16 | 1995 (23) | 669 (35) | |
| Missing, n (%) | 26 (0) | 1 (0) | |
| Marital status, n (%) | <0.001 * | ||
| Married | 4538 (53) | 1102 (57) | |
| Other c | 4062 (47) | 816 (43) | |
| Missing, n (%) | 0 (0) | 0 (0) | |
| Age at delivery (years), n (%) | <0.001 * | ||
| <20 | 881 (10) | 149 (8) | |
| 20–24 | 2314 (27) | 548 (29) | |
| 25–29 | 2533 (29) | 555 (29) | |
| 30–34 | 1887 (22) | 487 (25) | |
| ≥35 | 985 (11) | 179 (9) | |
| Missing, n (%) | 0 (0) | 0 (0) | |
| Delivery method, n (%) | 0.006 * | ||
| Vaginal | 6178 (72) | 1318 (69) | |
| Cesarean section | 2422 (28) | 600 (31) | |
| Missing, n (%) | 0 (0) | 0 (0) | |
| Insurance, n (%) | <0.001 * | ||
| Government | 4525 (53) | 1041 (54) | |
| Private | 3546 (41) | 855 (45) | |
| Other d | 217 (3) | 20 (1) | |
| Missing, n (%) | 312 (4) | 2 (0) | |
| Residence, n (%) | <0.001 * | ||
| Urban | 3810 (44) | 1448 (75) | |
| Rural | 3953 (46) | 453 (24) | |
| Missing, n (%) | 837 (10) | 17 (1) | |
| Pre-pregnancy BMI, n (%) | 0.007 * | ||
| <18.5 | 460 (5) | 66 (3) | |
| 18.5–24.9 | 3843 (45) | 846 (44) | |
| 25.0–29.9 | 2011 (23) | 468 (24) | |
| ≥30.0 | 2067 (24) | 466 (24) | |
| Missing, n (%) | 219 (3) | 72 (4) | |
| Pregnancy weight gain (kgs), median (IQR) | 14 (10–18) | 15 (10–19) | <0.001 * |
| Pregnancy weight gain (lbs), median (IQR) | 31 (21–40) | 32 (23–42) | <0.001 * |
| Missing, n (%) | 397 (5) | 86 (4) | |
| Pregnancy hypertension, n (%) | 651 (8) | 153 (8) | <0.001 * |
| Missing, n (%) | 0 (0) | 755 (39) | |
| Gestational diabetes, n (%) | 458 (5) | 126 (7) | 0.03 * |
| Missing, n (%) | 0 (0) | 0 (0) | |
| Participated in PRAMS survey e | 4718 (55) | N/A | N/A |
| Missing, n (%) | 0 (0) |
| Infant Characteristic | PRAMS | INSPIRE | p-Value a |
|---|---|---|---|
| Sample size, n (%) | 8600 | 1918 | |
| Gender, n (%) | 0.009 * | ||
| Female | 4369 (51) | 911 (47) | |
| Male | 4231 (49) | 1007 (53) | |
| Missing, n (%) | 0 (0) | 0 (0) | |
| Birth weight (grams), median (IQR) | 3260 (2835–3572) | 3405 (3120–3740) | <0.001 * |
| Missing, n (%) | 0 (0) | 0 (0) | |
| Gestational age (weeks), median (IQR) | 39 (38–39) | 39 (39–40) | <0.001 * |
| Missing, n (%) | 0 (0) | 0 (0) | |
| Ever breastfed, n (%) | 6130 (71) | 1469 (77) | 0.01 * |
| Missing, n (%) | 286 (3) | 0 (0) | |
| Birth year, n (%) | <0.001 * | ||
| 2009 | 566 (7) | N/A | |
| 2010 | 1092 (13) | N/A | |
| 2011 | 1124 (13) | N/A | |
| 2012 | 699 (8) | 844 (44) | |
| 2013 | 644 (7) | 1074 (56) | |
| 2014 | 169 (2) | N/A | |
| 2015 | 439 (5) | N/A | |
| 2016 | 1065 (12) | N/A | |
| 2017 | 1135 (13) | N/A | |
| 2018 | 1064 (12) | N/A | |
| 2019 | 603 (7) | N/A | |
| Missing, n (%) | 0 (0) | 0 (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Snyder, B.M.; Nian, H.; Miller, A.M.; Ryckman, K.K.; Li, Y.; Tindle, H.A.; Ammar, L.; Ramesh, A.; Liu, Z.; Hartert, T.V.; et al. Associations between Smoking and Smoking Cessation during Pregnancy and Newborn Metabolite Concentrations: Findings from PRAMS and INSPIRE Birth Cohorts. Metabolites 2023, 13, 1163. https://doi.org/10.3390/metabo13111163
Snyder BM, Nian H, Miller AM, Ryckman KK, Li Y, Tindle HA, Ammar L, Ramesh A, Liu Z, Hartert TV, et al. Associations between Smoking and Smoking Cessation during Pregnancy and Newborn Metabolite Concentrations: Findings from PRAMS and INSPIRE Birth Cohorts. Metabolites. 2023; 13(11):1163. https://doi.org/10.3390/metabo13111163
Chicago/Turabian StyleSnyder, Brittney M., Hui Nian, Angela M. Miller, Kelli K. Ryckman, Yinmei Li, Hilary A. Tindle, Lin Ammar, Abhismitha Ramesh, Zhouwen Liu, Tina V. Hartert, and et al. 2023. "Associations between Smoking and Smoking Cessation during Pregnancy and Newborn Metabolite Concentrations: Findings from PRAMS and INSPIRE Birth Cohorts" Metabolites 13, no. 11: 1163. https://doi.org/10.3390/metabo13111163
APA StyleSnyder, B. M., Nian, H., Miller, A. M., Ryckman, K. K., Li, Y., Tindle, H. A., Ammar, L., Ramesh, A., Liu, Z., Hartert, T. V., & Wu, P. (2023). Associations between Smoking and Smoking Cessation during Pregnancy and Newborn Metabolite Concentrations: Findings from PRAMS and INSPIRE Birth Cohorts. Metabolites, 13(11), 1163. https://doi.org/10.3390/metabo13111163







