Cobalt and Titanium Alleviate the Methylglyoxal-Induced Oxidative Stress in Pennisetum divisum Seedlings under Saline Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Planting Site, Soil Preparation, and Seedling Transplant
2.2. Treatments
2.3. Parameters Studied
2.3.1. Morphological Parameters
2.3.2. Nutrients Uptake
2.3.3. Methylglyoxal Quantification Method
2.3.4. Gly I and Gly II Quantification/MG-Detoxification Mechanism/Biochemical Assays
2.3.5. Metabolites Extraction
2.4. Statistical Analysis
3. Results
3.1. Growth Indices
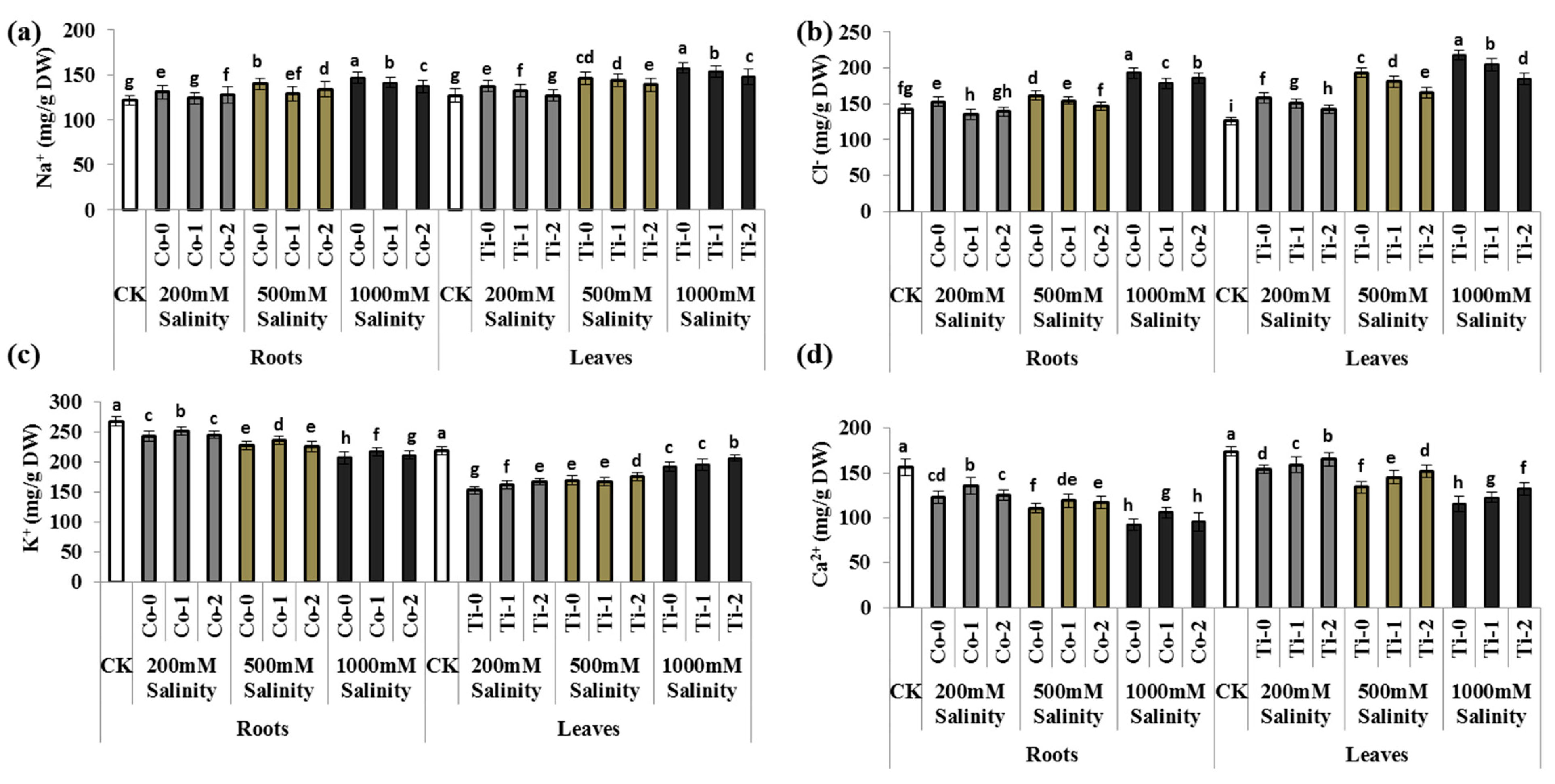
3.2. Nutritional Imbalance/Ionic Toxicity
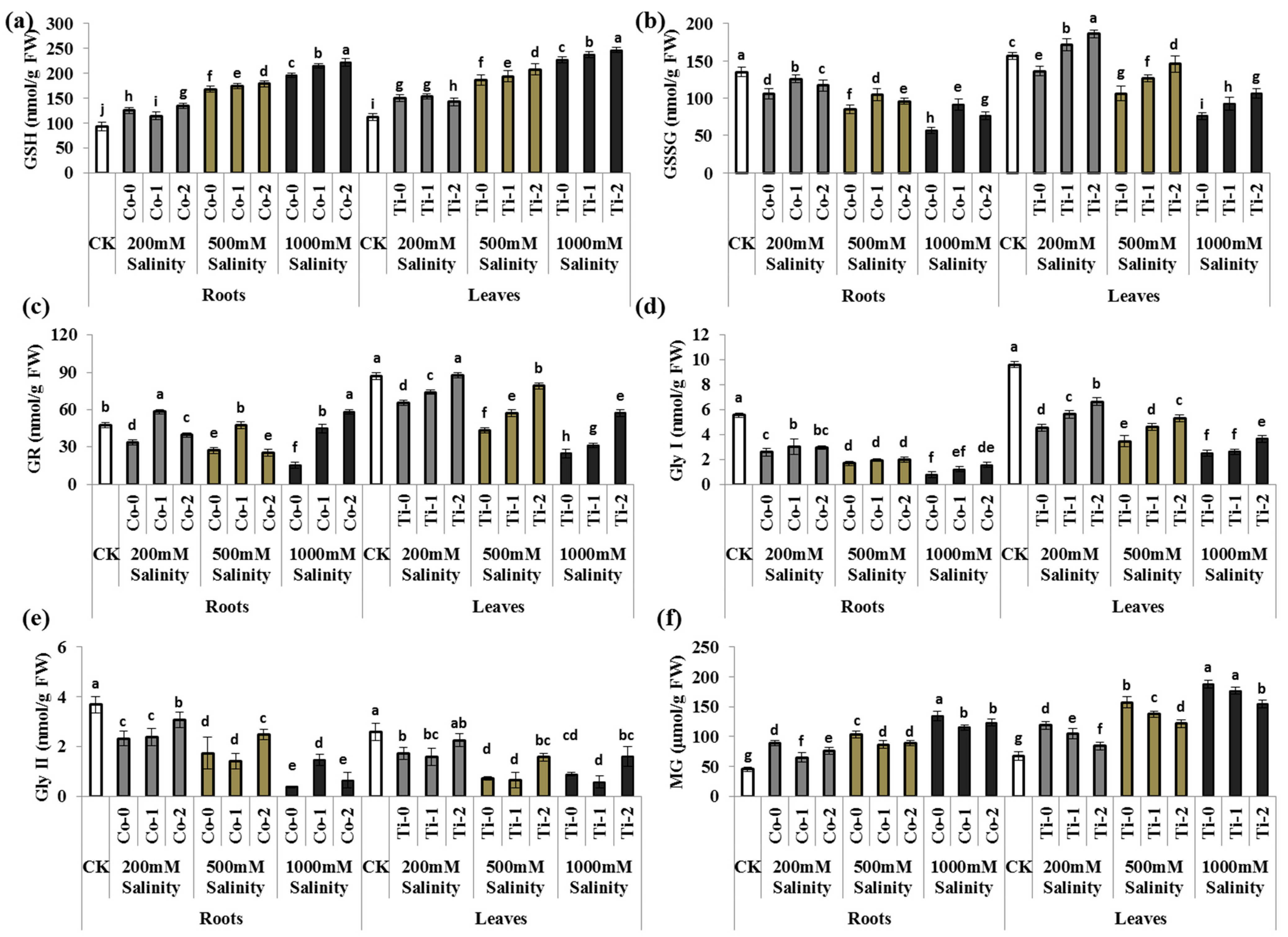
3.3. MG and Its Scavenging Enzymes
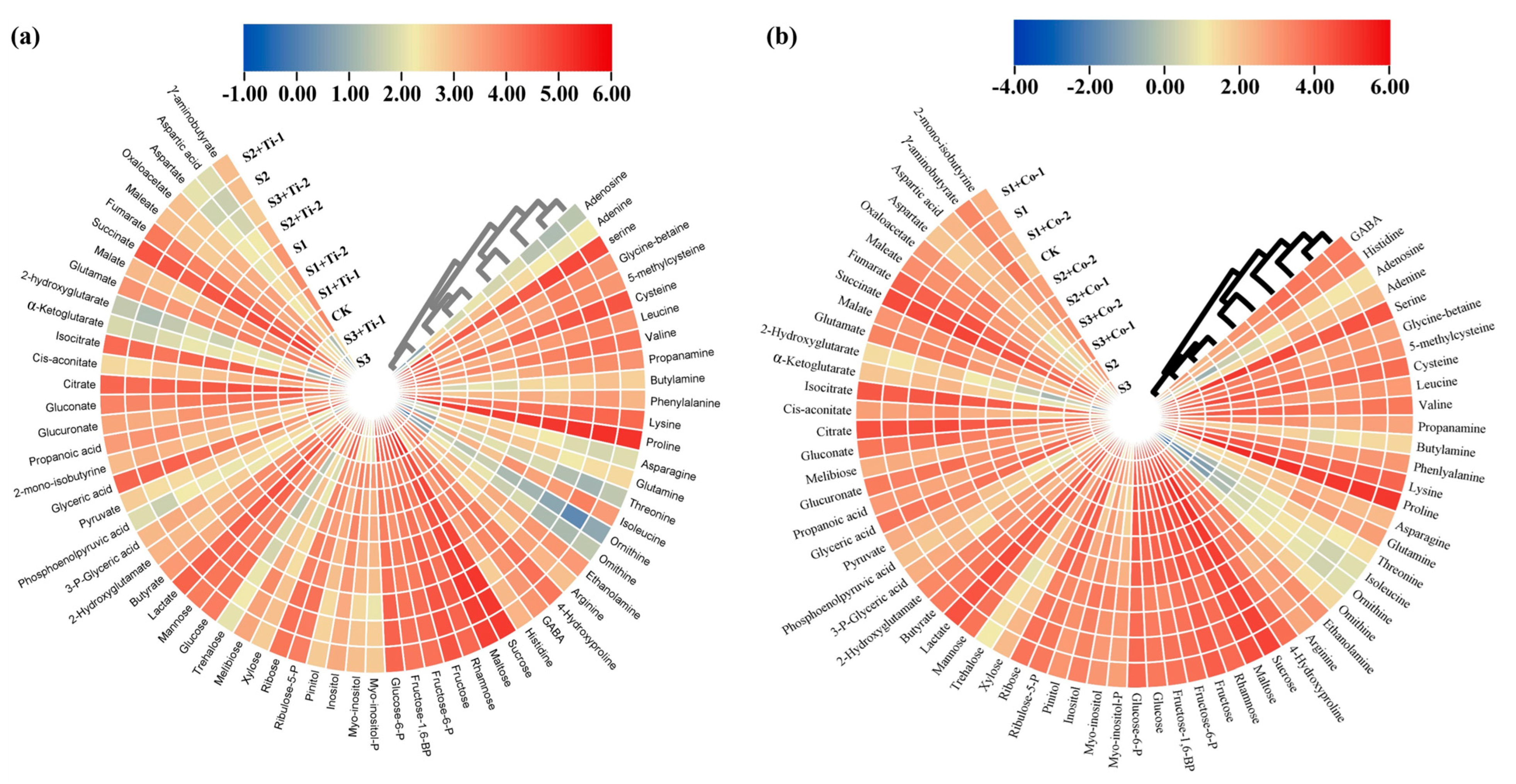
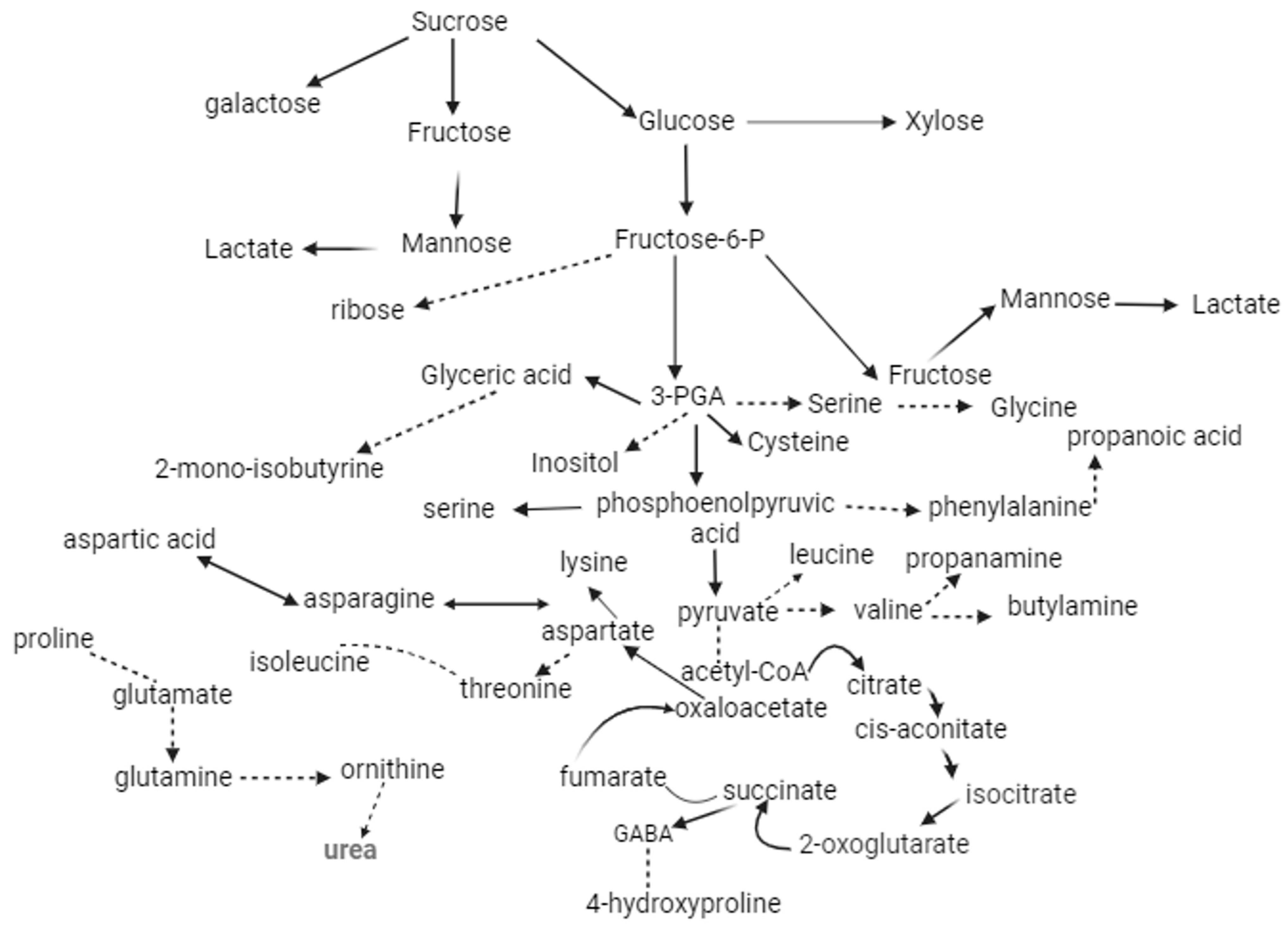
3.4. Metabolic Response
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dustgeer, Z.; Seleiman, M.F.; Khan, I.; Chattha, M.U.; Ali, E.F.; Alhammad, B.A.; Jalal, R.S.; Refay, Y.; Hassan, M.U. Glycine-betaine induced salinity tolerance in maize by regulating the physiological attributes, antioxidant defense system and ionic homeostasis. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 12248. [Google Scholar] [CrossRef]
- Pham, J.; Liu, J.; Bennett, M.H.; Mansfield, J.W.; Desikan, R. Arabidopsis Histidine Kinase 5 Regulates Salt Sensitivity and Resistance against Bacterial and Fungal Infection. New Phytol. 2012, 194, 168–180. [Google Scholar] [CrossRef]
- Rivero-Marcos, M.; Ariz, I. Can N Nutrition Lead to “Plant Diabetes”? The Perspective From Ammonium Nutrition and Methylglyoxal Accumulation. Front. Plant Sci. 2022, 13, 928876. [Google Scholar] [CrossRef] [PubMed]
- Borysiuk, K.; Ostaszewska-Bugajska, M.; Vaultier, M.-N.; Hasenfratz-Sauder, M.-P.; Szal, B. Enhanced Formation of Methylglyoxal-Derived Advanced Glycation End Products in Arabidopsis Under Ammonium Nutrition. Front. Plant Sci. 2018, 9, 667. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-G. Methylglyoxal. In Plant Signaling Molecules; Elsevier: Amsterdam, The Netherlands, 2019; pp. 219–233. ISBN 978-0-12-816451-8. [Google Scholar]
- Taha, R.S.; Seleiman, M.F.; Alhammad, B.A.; Alkahtani, J.; Alwahibi, M.S.; Mahdi, A.H. Activated Yeast extract enhances growth, anatomical structure, and productivity of Lupinus termis L. plants under actual salinity conditions. Agronomy 2021, 11, 74. [Google Scholar] [CrossRef]
- Taha, R.S.; Seleiman, M.F.; Shami, A.; Alhammad, B.A.; Mahdi, A.H.A. Integrated application of selenium and silicon enhances growth and anatomical structure, antioxidant defense system and yield of wheat grown in salt-stressed soil. Plants 2021, 10, 1040. [Google Scholar] [CrossRef]
- Mustafiz, A.; Sahoo, K.K.; Singla-Pareek, S.L.; Sopory, S.K. Metabolic Engineering of Glyoxalase Pathway for Enhancing Stress Tolerance in Plants. In Plant Stress Tolerance; Sunkar, R., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2010; Volome 639, pp. 95–118. ISBN 978-1-60761-701-3. [Google Scholar]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Anee, T.I.; Parvin, K.; Nahar, K.; Mahmud, J.A.; Fujita, M. Regulation of Ascorbate-Glutathione Pathway in Mitigating Oxidative Damage in Plants under Abiotic Stress. Antioxidants 2019, 8, 384. [Google Scholar] [CrossRef]
- Batth, R.; Jain, M.; Kumar, A.; Nagar, P.; Kumari, S.; Mustafiz, A. Zn2+ Dependent Glyoxalase I Plays the Major Role in Methylglyoxal Detoxification and Salinity Stress Tolerance in Plants. PLoS ONE 2020, 15, e0233493. [Google Scholar] [CrossRef]
- Brengi, S.H.; Khedr, A.A.E.M.; Abouelsaad, I.A. Effect of Melatonin or Cobalt on Growth, Yield and Physiological Responses of Cucumber (Cucumis sativus L.) Plants under Salt Stress. J. Saudi Soc. Agric. Sci. 2022, 21, 51–60. [Google Scholar] [CrossRef]
- Gómez-Merino, F.C.; Trejo-Téllez, L.I. The Role of Beneficial Elements in Triggering Adaptive Responses to Environmental Stressors and Improving Plant Performance. In Biotic and Abiotic Stress Tolerance in Plants; Vats, S., Ed.; Springer: Singapore, 2018; pp. 137–172. ISBN 978-981-10-9028-8. [Google Scholar]
- Gad, N.; Hassan, N.M.; Sayed, S. Influence of Cobalt on Tolerating Climatic Change (Salinity) in Onion Plant with Reference to Physiological and Chemical Approach. Plant Arch. 2020, 20, 1496–1500. [Google Scholar]
- Akeel, A.; Jahan, A. Role of Cobalt in Plants: Its Stress and Alleviation. In Contaminants in Agriculture; Naeem, M., Ansari, A.A., Gill, S.S., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 339–357. ISBN 978-3-030-41551-8. [Google Scholar]
- Lyu, S.; Wei, X.; Chen, J.; Wang, C.; Wang, X.; Pan, D. Titanium as a Beneficial Element for Crop Production. Front. Plant Sci. 2017, 8, 597. [Google Scholar] [CrossRef] [PubMed]
- Kleiber, T.; Markiewicz, B. Application of “Tytanit” in Greenhouse Tomato Growing. Acta Sci. Pol. Hortorum Cultus 2013, 12, 117–126. [Google Scholar]
- Hu, X.; Wei, X.; Ling, J.; Chen, J. Cobalt: An Essential Micronutrient for Plant Growth? Front. Plant Sci. 2021, 12, 768523. [Google Scholar] [CrossRef]
- Osman, D.; Cooke, A.; Young, T.R.; Deery, E.; Robinson, N.J.; Warren, M.J. The Requirement for Cobalt in Vitamin B12: A Paradigm for Protein Metalation. Biochim. Et Biophys. Acta (BBA)-Molecul. Cell Res. 2021, 1868, 118896. [Google Scholar] [CrossRef]
- Bhatt, A.; Batista-Silva, W.; Gallacher, D.J.; Pompelli, M.F. Germination of Cenchrus Ciliaris, Pennisetum Divisum, and Panicum Turgidum Is Seasonally Dependent. Botany 2020, 98, 449–458. [Google Scholar] [CrossRef]
- Bhatt, A.; de Moura Souza-Filho, P.R.; Gallacher, D. Germination Response of Four Arabian Desert Grasses to Low Water Potential. Nord. J. Bot. 2020, 38. [Google Scholar] [CrossRef]
- Yong, J.W.H.; Ng, Y.F.; Tan, S.N.; Chew, A.Y.L. Effect of Fertilizer Application on Photosynthesis and Oil Yield of Jatropha Curcas L. Photosynthetica 2010, 48, 208–218. [Google Scholar] [CrossRef]
- Hewitt, E.J.; Smith, T.A. Plant Mineral Nutrition; English Universities Press Ltd.: London, UK, 1974. [Google Scholar]
- Rahman, A.; Nahar, K.; Hasanuzzaman, M.; Fujita, M. Calcium Supplementation Improves Na+/K+ Ratio, Antioxidant Defense and Glyoxalase Systems in Salt-Stressed Rice Seedlings. Front. Plant Sci. 2016, 7, 609. [Google Scholar] [CrossRef]
- Parvin, K.; Nahar, K.; Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Mohsin, S.M.; Fujita, M. Exogenous Vanillic Acid Enhances Salt Tolerance of Tomato: Insight into Plant Antioxidant Defense and Glyoxalase Systems. Plant Physiol. Biochem. 2020, 150, 109–120. [Google Scholar] [CrossRef]
- Li, Z.-G.; Xu, Y.; Bai, L.-K.; Zhang, S.-Y.; Wang, Y. Melatonin Enhances Thermotolerance of Maize Seedlings (Zea mays L.) by Modulating Antioxidant Defense, Methylglyoxal Detoxification, and Osmoregulation Systems. Protoplasma 2019, 256, 471–490. [Google Scholar] [CrossRef]
- Foyer, C.H.; Halliwell, B. The Presence of Glutathione and Glutathione Reductase in Chloroplasts: A Proposed Role in Ascorbic Acid Metabolism. Planta 1976, 133, 21–25. [Google Scholar] [CrossRef]
- Griffith, O.W. Determination of Glutathione and Glutathione Disulfide Using Glutathione Reductase and 2-Vinylpyridine. Anal. Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Gödde, V.; Niehaus, K.; Zörb, C. Metabolic Adaptations of White Lupin Roots and Shoots under Phosphorus Deficiency. Front. Plant Sci. 2015, 6, 1014. [Google Scholar] [CrossRef] [PubMed]
- Kopka, J.; Schauer, N.; Krueger, S.; Birkemeyer, C.; Usadel, B.; Bergmuller, E.; Dormann, P.; Weckwerth, W.; Gibon, Y.; Stitt, M.; et al. GMD@CSB.DB: The Golm Metabolome Database. Bioinformatics 2005, 21, 1635–1638. [Google Scholar] [CrossRef] [PubMed]
- Fiehn, O.; Kopka, J.; Dörmann, P.; Altmann, T.; Trethewey, R.N.; Willmitzer, L. Metabolite Profiling for Plant Functional Genomics. Nat. Biotechnol. 2000, 18, 1157–1161. [Google Scholar] [CrossRef] [PubMed]
- Seleiman, M.F.; Semida, W.M.; Rady, M.M.; Mohamed, G.F.; Hemida, K.A.; Alhammad, B.A.; Hassan, M.M.; Shami, A. Sequential application of antioxidants rectifies ion imbalance and strengthens antioxidant systems in salt-stressed cucumber. Plants 2020, 9, 1783. [Google Scholar] [CrossRef] [PubMed]
- Alkharabsheh, H.M.; Seleiman, M.F.; Hewedy, O.A.; Battaglia, M.L.; Jalal, R.S.; Alhammad, B.A.; Schillaci, C.; Ali, N.; Al-Doss, A. Field crop responses and management strategies to mitigate soil salinity in modern agriculture: A Review. Agronomy 2021, 11, 2299. [Google Scholar] [CrossRef]
- Wang, H.; Liang, L.; Liu, S.; An, T.; Fang, Y.; Xu, B.; Zhang, S.; Deng, X.; Palta, J.A.; Siddique, K.H. Maize Genotypes with Deep Root Systems Tolerate Salt Stress Better than Those with Shallow Root Systems during Early Growth. J. Agron. Crop Sci. 2020, 206, 711–721. [Google Scholar] [CrossRef]
- Zhang, M.; Cao, Y.; Wang, Z.; Wang, Z.; Shi, J.; Liang, X.; Song, W.; Chen, Q.; Lai, J.; Jiang, C. A Retrotransposon in an HKT1 Family Sodium Transporter Causes Variation of Leaf Na+ Exclusion and Salt Tolerance in Maize. New Phytol. 2018, 217, 1161–1176. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Y.; Liang, X.; Lu, M.; Lai, J.; Song, W.; Jiang, C. A Teosinte-derived Allele of an HKT1 Family Sodium Transporter Improves Salt Tolerance in Maize. Plant Biotech. J. 2023, 21, 97–108. [Google Scholar] [CrossRef]
- Gao, Y.; Lu, Y.; Wu, M.; Liang, E.; Li, Y.; Zhang, D.; Yin, Z.; Ren, X.; Dai, Y.; Deng, D.; et al. Ability to Remove Na+ and Retain K+ Correlates with Salt Tolerance in Two Maize Inbred Lines Seedlings. Front. Plant Sci. 2016, 7, 1716. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.A.; Sarkhosh, A.; Khan, N.; Balal, R.M.; Ali, S.; Rossi, L.; Gómez, C.; Mattson, N.; Nasim, W.; Garcia-Sanchez, F. Insights into the Physiological and Biochemical Impacts of Salt Stress on Plant Growth and Development. Agronomy 2020, 10, 938. [Google Scholar] [CrossRef]
- Shabala, S.; Pottosin, I. Regulation of Potassium Transport in Plants under Hostile Conditions: Implications for Abiotic and Biotic Stress Tolerance. Physiol Plant. 2014, 151, 257–279. [Google Scholar] [CrossRef] [PubMed]
- Shabala, S. Signalling by Potassium: Another Second Messenger to Add to the List? J. Exp. Bot. 2017, 68, 4003–4007. [Google Scholar] [CrossRef] [PubMed]
- Mandujano-Piña, M.; Colinas-León, M.T.; Castillo-González, A.M.; Alia-Tejacal, I.; Valdez-Aguilar, L.A. Cobalt as senescence retardant in postharvest of oriental hybrid Lilium. Rev. Chapingo Ser. Hortic. 2012, 18, 239–252. [Google Scholar] [CrossRef]
- Kazemi, M. Effect of Ni, CO, SA and Sucrose on Extending the Vase-Life of Lily Cut Flower. Iran. J. Energy Environ. 2012, 3, 162–166. [Google Scholar] [CrossRef]
- Sheikhalipour, M.; Esmaielpour, B.; Gohari, G.; Haghighi, M.; Jafari, H.; Farhadi, H.; Kulak, M.; Kalisz, A. Salt Stress Mitigation via the Foliar Application of Chitosan-Functionalized Selenium and Anatase Titanium Dioxide Nanoparticles in Stevia (Stevia rebaudiana Bertoni). Molecules 2021, 26, 4090. [Google Scholar] [CrossRef]
- Yan, G.; Fan, X.; Peng, M.; Yin, C.; Xiao, Z.; Liang, Y. Silicon Improves Rice Salinity Resistance by Alleviating Ionic Toxicity and Osmotic Constraint in an Organ-Specific Pattern. Front. Plant Sci. 2020, 11, 260. [Google Scholar] [CrossRef]
- Mahmood, T.; Khalid, S.; Abdullah, M.; Ahmed, Z.; Shah, M.K.N.; Ghafoor, A.; Du, X. Insights into Drought Stress Signaling in Plants and the Molecular Genetic Basis of Cotton Drought Tolerance. Cells 2019, 9, 105. [Google Scholar] [CrossRef]
- Gupta, A.; Mishra, R.; Rai, S.; Bano, A.; Pathak, N.; Fujita, M.; Kumar, M.; Hasanuzzaman, M. Mechanistic Insights of Plant Growth Promoting Bacteria Mediated Drought and Salt Stress Tolerance in Plants for Sustainable Agriculture. Int. J. Mol. Sci. 2022, 23, 3741. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Alamri, S.; Alsubaie, Q.D.; Ali, H.M. Melatonin and Gibberellic Acid Promote Growth and Chlorophyll Biosynthesis by Regulating Antioxidant and Methylglyoxal Detoxification System in Tomato Seedlings Under Salinity. J. Plant Growth Regul. 2020, 39, 1488–1502. [Google Scholar] [CrossRef]
- Badshah, I.; Mustafa, N.; Khan, R.; Mashwani, Z.-R.; Raja, N.I.; Almutairi, M.H.; Aleya, L.; Sayed, A.A.; Zaman, S.; Sawati, L.; et al. Biogenic Titanium Dioxide Nanoparticles Ameliorate the Effect of Salinity Stress in Wheat Crop. Agronomy 2023, 13, 352. [Google Scholar] [CrossRef]
- Mustafa, N.; Raja, N.I.; Ilyas, N.; Abasi, F.; Ahmad, M.S.; Ehsan, M.; Mehak, A.; Badshah, I.; Proćków, J. Exogenous Application of Green Titanium Dioxide Nanoparticles (TiO2 NPs) to Improve the Germination, Physiochemical, and Yield Parameters of Wheat Plants under Salinity Stress. Molecules 2022, 27, 4884. [Google Scholar] [CrossRef] [PubMed]
- Akram, W.; Yasin, N.A.; Shah, A.A.; Khan, W.U.; Li, G.; Ahmad, A.; Ahmed, S.; Hussaan, M.; Rizwan, M.; Ali, S. Exogenous Application of Liquiritin Alleviated Salt Stress and Improved Growth of Chinese Kale Plants. Sci. Hortic. 2022, 294, 110762. [Google Scholar] [CrossRef]
- Mushtaq, N.U.; Alghamdi, K.M.; Saleem, S.; Tahir, I.; Bahieldin, A.; Henrissat, B.; Alghamdi, M.K.; Rehman, R.U.; Hakeem, K.R. Exogenous Zinc Mitigates Salinity Stress by Stimulating Proline Metabolism in Proso Millet (Panicum miliaceum L.). Front. Plant Sci. 2023, 14, 1053869. [Google Scholar] [CrossRef]
- Conde, A.; Chaves, M.M.; Gerós, H. Membrane Transport, Sensing and Signaling in Plant Adaptation to Environmental Stress. Plant Cell Physiol. 2011, 52, 1583–1602. [Google Scholar] [CrossRef]
- Maathuis, F.J.M.; Ahmad, I.; Patishtan, J. Regulation of Na+ Fluxes in Plants. Front. Plant Sci. 2014, 5, 467. [Google Scholar] [CrossRef]
- Woodrow, P.; Ciarmiello, L.F.; Annunziata, M.G.; Pacifico, S.; Iannuzzi, F.; Mirto, A.; D’Amelia, L.; Dell’Aversana, E.; Piccolella, S.; Fuggi, A.; et al. Durum Wheat Seedling Responses to Simultaneous High Light and Salinity Involve a Fine Reconfiguration of Amino Acids and Carbohydrate Metabolism. Physiol Plant. 2017, 159, 290–312. [Google Scholar] [CrossRef] [PubMed]
- Saito, R.; Yamamoto, H.; Makino, A.; Sugimoto, T.; Miyake, C. Methylglyoxal Functions as Hill Oxidant and Stimulates the Photoreduction of O2 at Photosystem I: A Symptom of Plant Diabetes: Methylglyoxal Stimulates O2 Photoreduction at PSI. Plant Cell Environ. 2011, 34, 1454–1464. [Google Scholar] [CrossRef]
- Takagi, D.; Inoue, H.; Odawara, M.; Shimakawa, G.; Miyake, C. The Calvin Cycle Inevitably Produces Sugar-Derived Reactive Carbonyl Methylglyoxal During Photosynthesis: A Potential Cause of Plant Diabetes. Plant Cell Physiol. 2014, 55, 333–340. [Google Scholar] [CrossRef]
- Shimakawa, G.; Suzuki, M.; Yamamoto, E.; Saito, R.; Iwamoto, T.; Nishi, A.; Miyake, C. Why Don’t Plants Have Diabetes? Systems for Scavenging Reactive Carbonyls in Photosynthetic Organisms. Biochem. Soc. Trans. 2014, 42, 543–547. [Google Scholar] [CrossRef]
- Talaat, N.B.; Mostafa, A.A.; El-Rahman, S.N.A. A Novel Plant Growth–Promoting Agent Mitigates Salt Toxicity in Barley (Hordeum vulgare L.) by Activating Photosynthetic, Antioxidant Defense, and Methylglyoxal Detoxification Machineries. J. Soil Sci. Plant Nutr. 2023, 23, 308–324. [Google Scholar] [CrossRef]
- Mohsin, S.M.; Hasanuzzaman, M.; Parvin, K.; Fujita, M. Pretreatment of Wheat (Triticum aestivum L.) Seedlings with 2,4-D Improves Tolerance to Salinity-Induced Oxidative Stress and Methylglyoxal Toxicity by Modulating Ion Homeostasis, Antioxidant Defenses, and Glyoxalase Systems. Plant Physiol. Biochem. 2020, 152, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Raihan, M.R.H.; Khojah, E.; Samra, B.N.; Fujita, M.; Nahar, K. Biochar and Chitosan Regulate Antioxidant Defense and Methylglyoxal Detoxification Systems and Enhance Salt Tolerance in Jute (Corchorus olitorius L.). Antioxidants 2021, 10, 2017. [Google Scholar] [CrossRef] [PubMed]
- Saleem, K.; Asghar, M.A.; Raza, A.; Javed, H.H.; Farooq, T.H.; Ahmad, M.A.; Rahman, A.; Ullah, A.; Song, B.; Du, J.; et al. Biochar-Mediated Control of Metabolites and Other Physiological Responses in Water-Stressed Leptocohloa fusca. Metabolites 2023, 13, 511. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Hoque, T.S.; Zaid, A.; Wani, S.H.; Mostofa, M.G.; Henry, R. Targeting the Ascorbate-Glutathione Pathway and the Glyoxalase Pathway for Genetic Engineering of Abiotic Stress-Tolerance in Rice. In Molecular Breeding for Rice Abiotic Stress Tolerance and Nutritional Quality; Hossain, M.A., Hassan, L., Ifterkharuddaula, K.M., Kumar, A., Henry, R., Eds.; Wiley: Hoboken, NJ, USA, 2021; pp. 398–427. ISBN 978-1-119-63317-4. [Google Scholar]
- Borysiuk, K.; Ostaszewska-Bugajska, M.; Kryzheuskaya, K.; Gardeström, P.; Szal, B. Glyoxalase I Activity Affects Arabidopsis Sensitivity to Ammonium Nutrition. Plant Cell Rep. 2022, 41, 2393–2413. [Google Scholar] [CrossRef]
- Tahjib-Ul-Arif, M.; Zahan, M.I.; Karim, M.M.; Imran, S.; Hunter, C.T.; Islam, M.S.; Mia, M.A.; Hannan, M.A.; Rhaman, M.S.; Hossain, M.A.; et al. Citric Acid-Mediated Abiotic Stress Tolerance in Plants. Int. J. Mol. Sci. 2021, 22, 7235. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, Z.; Xiang, Z.; Yang, Z. Exogenous Application of Citric Acid Ameliorates the Adverse Effect of Heat Stress in Tall Fescue (Lolium arundinaceum). Front. Plant Sci. 2016, 7, 179. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Parvin, K.; Bardhan, K.; Nahar, K.; Anee, T.I.; Masud, A.A.C.; Fotopoulos, V. Biostimulants for the Regulation of Reactive Oxygen Species Metabolism in Plants under Abiotic Stress. Cells 2021, 10, 2537. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ma, L.; Li, Y.; Wang, L.; Zhang, L. Endophyte Infection Enhances Accumulation of Organic Acids and Minerals in Rice under Pb2+ Stress Conditions. Ecotoxicol. Environ. Saf. 2019, 174, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Jamali, F.; Etemad, V. Investigating Different Levels of Salinity and Drought Stress on the Germination and Growth of Pennisetum divisum. Iran. J. Seed Sci. Tech. 2022, 11, 89–102. [Google Scholar]
| SR | Treatment | Treatment Explanation |
|---|---|---|
| 1. | T0 | CK (control) |
| 2. | T1 | S1 (200 mM salt) |
| 3. | T2 | S1 + C0-1 |
| 4. | T3 | S1 + Co-2 |
| 5. | T4 | S1 + Ti-1 |
| 6. | T5 | S1 + Ti-2 |
| 7. | T6 | S2 (500 mM salt) |
| 8. | T7 | S2 + Co-1 |
| 9. | T8 | S2 + Co-2 |
| 10. | T9 | S2 + Ti-1 |
| 11. | T10 | S2 + Ti-2 |
| 12. | T11 | S3 (1000 mM salt) |
| 13. | T12 | S3 + Co-1 |
| 14. | T13 | S3 + Co-2 |
| 15. | T14 | S3 + Ti-1 |
| 16. | T15 | S3 + Ti-2 |
| LN/Plant | LA (cm2) | RFW (mg) | RDW (mg) | RL (cm) | SFW (g) | SDW (mg) | SL (cm) | |
|---|---|---|---|---|---|---|---|---|
| CK | 14.6 b | 25.71 ab | 15.73 a | 10.62 a | 23.72 a | 37.86 a | 21.81 a | 56.54 a |
| 200 mM Salinity (S1) | 14.2 bc | 23.69 d | 13.47 c | 8.62 c | 20.22 d | 34.58 d | 19.65 d | 53.55 d |
| S1 + Co-1 | 15.57 a | 24.65 c | 15.66 a | 9.18 b | 21.85 b | 35.65 c | 20.6 c | 54.03 d |
| S1 + Co-2 | 13.66 cd | 23.62 d | 14.27 b | 8.34 c | 20.69 d | 34.86 d | 18.3 e | 53.96 d |
| S1 + Ti-1 | 15.3 a | 25.78 a | 13.65 c | 8.29 c | 21.25 c | 35.82 c | 20.73 c | 54.73 c |
| S1 + Ti-2 | 15.71 a | 25.28 b | 14.22 b | 9.07 b | 22.26 b | 36.41 b | 21.27 b | 55.54 b |
| 500 mM Salinity (S2) | 12.31 f | 19.71 g | 10.65 f | 6.65 g | 17.52 gh | 30.33 h | 16.65 g | 48.72 h |
| S2 + Co-1 | 13.15 de | 21.61 e | 12.16 d | 7.58 d | 18.7 e | 32.61 f | 18.63 e | 50.73 f |
| S2 + Co-2 | 11.58 g | 20.72 f | 11.37 e | 7.15 ef | 17.88 fg | 31.7 g | 15.6 h | 49.71 g |
| S2 + Ti-1 | 13.71 c | 21.77 e | 10.64 f | 6.83 fg | 17.68 g | 33.71 e | 18.74 e | 50.73 f |
| S2 + Ti-2 | 14.34 b | 22.05 e | 11.69 e | 7.55 de | 18.3 ef | 31.59 g | 17.78 f | 51.39 e |
| 1000 mM Salinity (S3) | 10.75 h | 17.55 i | 8.57 j | 5.58 i | 15.3 j | 25.37 l | 13.62 j | 46.19 j |
| S3 + Co-1 | 11.71 g | 19.67 g | 10.21 gh | 6.71 g | 17.11 hi | 27.68 j | 14.68 i | 48.92 h |
| S3 + Co-2 | 10.23 h | 17.98 i | 9.36 i | 6.21 h | 16.84 i | 26.05 k | 13.34 j | 47.62 i |
| S3 + Ti-1 | 11.63 g | 18.56 h | 9.83 h | 6.08 h | 16.71 i | 28.86 i | 15.79 h | 48.54 h |
| S3 + Ti-2 | 12.99 e | 20.54 f | 10.5 fg | 6.66 g | 18.61 e | 30.75 h | 14.75 i | 49.67 g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhammad, B.A.; Saleem, K.; Asghar, M.A.; Raza, A.; Ullah, A.; Farooq, T.H.; Yong, J.W.H.; Xu, F.; Seleiman, M.F.; Riaz, A. Cobalt and Titanium Alleviate the Methylglyoxal-Induced Oxidative Stress in Pennisetum divisum Seedlings under Saline Conditions. Metabolites 2023, 13, 1162. https://doi.org/10.3390/metabo13111162
Alhammad BA, Saleem K, Asghar MA, Raza A, Ullah A, Farooq TH, Yong JWH, Xu F, Seleiman MF, Riaz A. Cobalt and Titanium Alleviate the Methylglyoxal-Induced Oxidative Stress in Pennisetum divisum Seedlings under Saline Conditions. Metabolites. 2023; 13(11):1162. https://doi.org/10.3390/metabo13111162
Chicago/Turabian StyleAlhammad, Bushra Ahmed, Khansa Saleem, Muhammad Ahsan Asghar, Ali Raza, Abd Ullah, Taimoor Hassan Farooq, Jean W. H. Yong, Fei Xu, Mahmoud F. Seleiman, and Aamir Riaz. 2023. "Cobalt and Titanium Alleviate the Methylglyoxal-Induced Oxidative Stress in Pennisetum divisum Seedlings under Saline Conditions" Metabolites 13, no. 11: 1162. https://doi.org/10.3390/metabo13111162
APA StyleAlhammad, B. A., Saleem, K., Asghar, M. A., Raza, A., Ullah, A., Farooq, T. H., Yong, J. W. H., Xu, F., Seleiman, M. F., & Riaz, A. (2023). Cobalt and Titanium Alleviate the Methylglyoxal-Induced Oxidative Stress in Pennisetum divisum Seedlings under Saline Conditions. Metabolites, 13(11), 1162. https://doi.org/10.3390/metabo13111162











