Abstract
Nerve cell death accounts for various neurodegenerative disorders, in which altered immunity to the integrated central nervous system (CNS) might have destructive consequences. This undesirable immune response often affects the progressive neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, schizophrenia and/or amyotrophic lateral sclerosis (ALS). It has been shown that commensal gut microbiota could influence the brain and/or several machineries of immune function. In other words, neurodegenerative disorders may be connected to the gut–brain–immune correlational system. The engrams in the brain could retain the information of a certain inflammation in the body which might be involved in the pathogenesis of neurodegenerative disorders. Tactics involving the use of probiotics and/or fecal microbiota transplantation (FMT) are now evolving as the most promising and/or valuable for the modification of the gut–brain–immune axis. More deliberation of this concept and the roles of gut microbiota would lead to the development of stupendous treatments for the prevention of, and/or therapeutics for, various intractable diseases including several neurodegenerative disorders.
1. Introduction
Neurodegenerative disorders are the most common factors of disability, which refer to the gradual decrease in function of the nerves of sensory, motor, and mental activity subsequent to the death of several neurons [1]. The nerve cell death accounts for the various neurological dysregulations of neurodegenerative disorders including Alzheimer’s disease, Parkinson’s disease, schizophrenia and amyotrophic lateral sclerosis (ALS) [2,3,4,5]. Similarly, some cases of autism and depression also result from nerve cell death [6,7,8]. Precise insight into the pathology of these diseases still remains elusive. Oxidative stress is defined as a condition of metabolic dysfunction facilitated by the discrepancy between the elevated production of reactive oxygen species (ROS) and the antioxidant defense activity in a body [9]. In one sense, a conceivable pathophysiology of neurodegenerative disorders might be recognized by the increased oxidative stress. For example, the elevated production of ROS has been hypothesized to play a key role in the development and poor outcome of schizophrenia patients [10]. Oxidative stress may also be increased in ALS patients, which may affect the mitochondrial dysfunction eventually leading to nerve cell damage and/or neuronal loss [11]. In particular, mitochondrial homeostasis is critical to maintain neuronal function and mitochondrial dysfunction is connected to neurodegeneration [12]. Neurons and glial cells are typically vulnerable to excess ROS because of comparatively insufficient antioxidant capabilities, which may increase vulnerability to neuronal damage and functional deficits [13]. It has been shown that these related mitochondrial disruptions of the oxidative pathways, several inflammatory cytokines, excess amounts of ROS, and altered microglia activities may bring harmful results to the process of nerve cell degeneration that eventually leads to nerve cell death [14] (Figure 1).
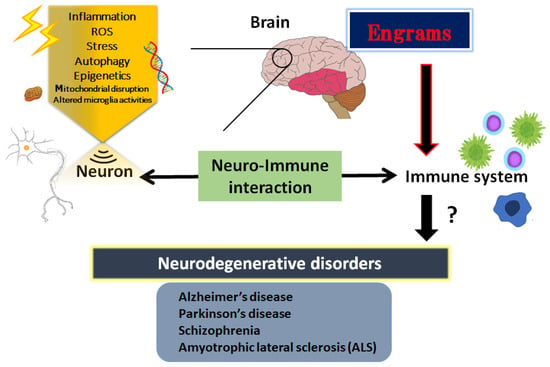
Figure 1.
Schematic illustration shows an introduction to the essential role of neuro-immune interaction in the pathogenesis of neurodegenerative disorders such as Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis (ALS) and schizophrenia. The immunity could generally communicate with the brain or CNS. Illustration of the involvement for the pathogenic roles of various stresses, inflammation, ROS, epigenetics, and engrams is shown. Note that several significant items have been omitted for clarity. Abbreviation: CNS, central nervous system; ROS, reactive oxygen species.
Increasing evidence indicates the complicated associations between gut microbiota, immunity and the central nervous system (CNS) [15]. Additionally, a variety of studies have shown the potential association between gut microbiota and neurodegenerative disorders including depression, autism, schizophrenia and Parkinson’s disease [16]. While regular gut microbiota could defend the CNS, the dysbiosis of microbiota might aggravate neurodegenerative and/or mental health disorders [17]. Hence, a good alteration of the microbiota could also support the inhibition and/or regulation of the development of neurodegenerative disorders. Although gut microbiota may play a critical role in the pathogenesis of ALS, for example, comprehensive studies implicating the intestinal changes in the pathology of neurodegenerative disorders are limited [18]. Recent studies could shed new light on the importance of disease-specific interactions between gut microbiota and neurodegenerative disorders [19]. Recently, we have suggested that immunological memory named “engrams” could restore the initial disease state in schizophrenia [20]. Based on this concept, innovative therapeutic strategies for several neurodegenerative disorders could be applied to the modification of gut microbiota. This review would emphasize the roles of the associations between gut microbiota, immunity and the central nervous system in the pathophysiology of neurodegenerative disorders, which could be modified by the alteration of gut microbiota as a hopeful treatment. This concept could also suggest supreme preventative and/or therapeutic strategies for the broader neurodegenerative disorders.
2. Inflammatory Neuro-Immune Responses
Inflammatory progression has a key role in various cellular processes and is suggested as the pathogenesis of neurodegenerative disorders [21]. Consistently, it has been revealed that neuro-inflammation triggered by bacterial or viral infections could induce schizophrenia in animal models [22]. In addition, it has been described as a reciprocal functional mechanism between the immune system and CNS [23]. For example, immune cells could modulate behavior and cognition of the host by direct interactions with the CNS [24]. A low-grade neuro-immune/inflammatory response is essential to keep the neurogenesis and/or the homeostasis of brain [5,7,25], suggesting that mild transient immune response might be employed as a restorative role in CNS. Consequently, an array of neuro-immune aberrations related to the chronic activated inflammatory reaction have been identified in patients with neurodegenerative disorders including schizophrenia [25]. Generally, an elevated level of inflammation markers in the blood and/or in cerebrospinal fluid (CSF) of the CNS has been detected in patients with neurodegenerative disorders [26]. Therefore, prospective treatment with anti-inflammatory medication has been suggested as a secondary treatment in patients with neurodegenerative disorders including schizophrenia or ALS [27]. It has been shown that extra prolonged stresses may be a robust risk factor for the development of some psychiatric diseases with a reduced number of mitochondria in the cortex [28].
Inflammatory oxidative stress may produce an excess amount of ROS which could be characterized as oxygen-comprising small molecules prone to react with several biological materials such as DNA [29]. In addition, an excess amount of ROS production could initiate an activation of autophagy in cells, suggesting an essential role for ROS in the activation of autophagy [30]. Generally, autophagy would play a protective role in cells; however, autophagy is also related to apoptotic cell death or necrosis in certain conditions. Additionally, autophagy could regulate the levels of several inflammations [31]. Hence, autophagy might be involved in the pathogenesis of neurodegenerative disorders. The significant effect of autophagy may be determined by the type of stimulus, cell types, the microenvironment, and/or other biological factors [32]. In intracellular signaling pathways, autophagy could be stimulated by the activated AMP-activated protein kinase (AMPK) during the situation of energy deficit in cells [33]. The activity of AMPK is also critical in the cells of the CNS for preserving neuronal integrity and for neuron survival against an excess amount of oxidative stresses [34]. Once activated, the consequently activated autophagy could overcome the inflammation by blocking the excretion of pro-inflammatory cytokines such as IL-1β and IL-18, which are an indispensable component of the autophagic mechanism responsible for the control of inflammatory immune response [35]. It is remarkable that damage in the neuro–immune interaction brings acute and/or chronic CNS pathologies, in which autophagy might be involved in neurons and/or glial cells [36].
3. Engrams and Neuro-Immune Responses in the Pathogenesis of Neurodegenerative Disorders
The CNS and the immune system might collaborate on various levels in a body; however, the mechanisms of holding the specific immune-challenge have remained vague. Very lately, it has been clearly shown that the brain keeps the facts of certain inflammation such as inflammatory bowel syndrome occurred in the body [37]. This specific memory seems to be an immunological remembrance called “engrams” [38]. Here, we would like to use this word “engrams” as the meaning of immunological remembrance matching to the meaning of “memory-traces”. The concept of engrams has been fairly hypothetical for the basic units of memory. Now, neuronal assemblies that hold the specific disease engrams have been known in the amygdala, hippocampus, and/or cortex, which may suggest that engrams are distributed among multiple brain regions functionally linking each other as an integrated engrams organization [39]. Associations of these engrams are thought to determine the situation of the host, either of health or disease, by engram arrangements, which may be frequently dependent on several environmental conditions [40]. Consequently, the immunological engrams could restore the initial inflammatory disease condition, if rebooted [38]. Created by stressful and/or repetitive inflammatory occasions, the engrams might commit to a slow progression of chronic diseases including neurodegenerative disorders [41]. Epigenetic changes such as DNA methylation or acetylation within the cells of the neuronal assemblies might be important mechanisms of the engram formation [42], which is also a significant factor for the fine-tuning of the function in the healthy brain [43]. Epigenetics may also stabilize engrams for the effective recovery of fear memory [44]. Therefore, engrams and/or epigenetic changes could be related to the immune consequences in the pathogenesis of various neurodegenerative disorders [45] (Figure 1). The synergistic arrangement of engrams might bring in the solid progression of several diseases, which involves the concept that any complex neurological and/or immunological consequences could result from the interaction of these engrams with immunity. Additionally, frequent subtle immune challenges might result in the stable formation of multiple engrams positioning independent information [46]. Synaptic variations might validate the specific development of engrams during memorizing for supporting memory. Maintenance of the memory might be achieved by a meta-plasticity mechanism that raises the change in neurons within an engram, which may be further encouraged by epigenetic regulators such as histone deacetylases (HDACs) [47]. In fact, the HDACs-related signaling pathways have been significantly associated with the alternative expression of several genes related to neurodegenerative disorders [48]. Consistently, some kinds of epigenetic regulators modified by environmental factors have been suggested as playing a crucial role in the pathogenesis of various neurodegenerative disorders [49]. In short, the brain could hold several specific inflammatory responses as information of pathological neuronal images called “engrams”. This concept could correctly elucidate the pathogenesis of various neurodegenerative disorders and the related CNS disorders, which might contribute to establishing a new strategy for the therapeutic interventions.
4. How to Modulate the Engrams
Some engrams could potentially trigger and/or exacerbate the conditions of neurodegenerative disorders [50]. Therefore, clearing the bad memory of “engrams” might be favorable for the prevention and/or treatment of neurodegenerative disorders. In the experiment of dextran sulfate sodium-induced colitis, the authors applied the chemo-genetic procedure of the designer receptor exclusively activated by designer drugs (DREADD) system for the inhibition of engram activity [37]. However, it seems to be impossible to currently use this system in the clinical treatment of humans. Now, is it possible to clear the memory of engrams without neuronal cell death and/or any brain damage? This is the point for therapeutic interventions. In one possible way, synaptic removal could be achieved by microglia capable of initiating the oblivion of memories with engram cells [51]. It is considered that microglia can make synapse elimination a mechanism for forgetting memory retentions [51]. In addition, it has been reported that microglia are related to synapse density, learning, and/or memory [52]. There are significant associations between gut microbiota and demyelination by the microglia in the brain, suggesting that the crosstalk of gut-microbiota and brain-microglia might play a key role for the clearance of engrams [53]. It has been shown that regulation of the microbiota might be connected to the possible therapies of neurodegenerative disorders [54]. A gut–brain axis indicates a bidirectional connection between gut microbiota and brain, which is a vital assembly in the pathophysiology of several neurodegenerative disorders [55]. This concept might include the associations between gut microbiota and more broad CNS disorders. For example, it has been shown that the composition of gut microbiota might be associated with narcolepsy type 1 [56]. Changes in the conformation of gut microbiota may be accepted by the sympathetic vagal afferent nerve transmitting to the CNS via the microglial action, which in turn could produce and/or modulate the responses of engrams. Studies have proven that some species of bacteria could produce catecholamines and/or acetylcholine, which might contribute to the responses of the sympathetic nerve [57]. Some of vagal neurons in the sympathetic pathway usually have an afferent role for the microbiota-mediated adjustment of brain [58]. Convincing evidence has demonstrated the roles of gut microbiota in the pathogenesis of Alzheimer’s disease and/or Parkinson’s disease, which are partly mediated by modified microglial activity in the brain [59]. In fact, microglial dysfunction has been detected in a variety of neurodegenerative disorders including Alzheimer’s disease, Parkinson’s disease and/or ALS [59]. Possibly, the gut-microbiota–glia-brain–immune axis might be influenced by the production of inflammatory cytokines and/or by the reduction of favorable substances such as short-chain fatty acids (SCFAs), modifying the regulation of the sympathetic afferent nerve and glial cells [60]. For example, butyric acid, a key SCFA, might be connected with a favorable response in the treatment of schizophrenia, suggesting an important role in the gut microbiota–brain axis [61]. SCFAs can cross the blood–brain barrier (BBB) and could interact with microglia to regulate their functions [62]. Gut microbiota could also communicate with the brain through intricate communication systems, which incorporate the intestinal function with the cognitive and/or emotional brain via the neuro-immuno-endocrine mediators [63]. At least, some of the potential effectors in the gut could actually stimulate the sympathetic nerve pathway [58]. It has been demonstrated by reproducible and translatable findings that the efficacy of intervention could be achieved with microbial-derived metabolites for modulating the disease progression in ALS [64]. In addition, the impact of gut microbiota on brain function might be also related to brain cognition and/or perception. Therefore, several brain inflammations and/or neurodegeneration in the brain might be related to the action of the gut–brain axis [65], in which the immunity-linked processes might be associated with the neuronal responses to memory engrams [66]. Furthermore, there might be wide-ranging reciprocal connections between gut microbiota and immune-inflammatory responses with engrams, which have a critical significance in the function of the healthy brain and in the pathogenesis of various neurodegenerative disorders [67].
5. Utilization of Gut–Brain Axis for the Treatment of Neurodegenerative Disorders
The dynamic residency of microbes in the gut may play a fundamental role in managing host physiology. In addition, recent advances have emphasized the significance of gut microbiota in neurodevelopment with considerable associations with the onset and/or the progression of neurodegenerative disorders [68,69]. Furthermore, it has been shown that the dysbiosis of gut microbiota might worsen the symptoms of various neurodegenerative disorders [70]. Alterations in the composition of gut microbiota, termed gut dysbiosis, with an increased number of potentially pathological organisms might play a prominent role in the pathogenesis of CNS-related disorders. For example, ALS patients may often demonstrate some changes in their gut microbial communities compared to the paired healthy controls [71]. Furthermore, increasing gut dysbiosis has been shown to worsen the symptoms with ALS [72]. Evolving evidence also connects the gut dysbiosis to the exacerbation of impaired autophagy in the immune-mediated chronic neuroinflammation [73]. Interestingly, it has been reported that a pleiotropic drug modulating AMPK and/or autophagy signaling, such as metformin, could alter the gut microbiota and its metabolic processes [74]. Consequently, dietary approach to alter the gut microbiota could be advantageous for the treatment of neurodegenerative disorders [75]. Gut microbiota could regulate and/or inhibit the production of ROS to retain the host’s brain health [76]. It might be important to diminish the levels of ROS for neuroregeneration with neuronal stem cells [77,78]. In addition to the unfavorable effects for the stem cells, ROS might skew the function of microglia with the oxidized mitochondria in glial cells (Figure 1) [79]. Inflammatory factors, oxidative stress, and/or the alteration of microglia are known to limit neuroplasticity in the CNS [80]. In these ways, certain gut microbiota with the inhibition of ROS could probably prevent the incidence and/or attenuate the symptoms of neurodegenerative disorders by regulating the production of ROS and by clearing engram memory via the alteration of functional microglia in the brain (Figure 2).
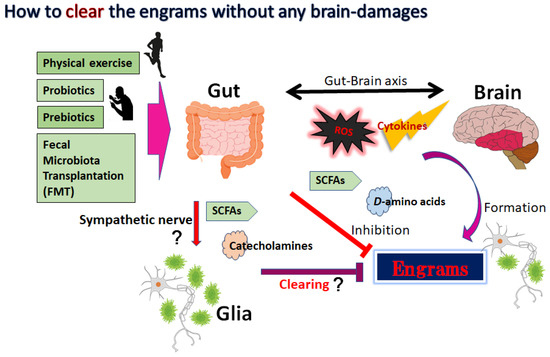
Figure 2.
The gut microbiota could support favorable action against disease progression of neurodegenerative disorders by affecting the engrams and/or brain–immune axis, which may include the inhibition or production of cytokines, ROS, SCFAs, certain D-amino acids, and catecholamines. Mild physical exercise, probiotics, prebiotics, and fecal microbiota transplantation (FMT) might potentially be more successful than conventional symptomatic therapy for the treatment of neurodegenerative disorders. Arrowhead indicates stimulation whereas hammerhead shows inhibition. Note that several important activities such as cytokine induction or anti-inflammatory reaction have been omitted for clarity. Abbreviations: FMT, fecal microbiota transplantation; SCFAs, short-chain fatty acids; ROS, reactive oxygen species.
Innovative treatments for the neurodegenerative disorders including schizophrenia and/or Parkinson’s disease are progressing. Some methods for action that might efficiently influence the composition of gut microbiota may include fecal-microbiota transplantation (FMT) (Figure 2). By transferring the gut microbiota from a healthy donor, there have been promising signs of improving the capability of the gut microbiota for the treatment of neurodegenerative disorders [81]. In particular, the transplantation of microbiota containing Faecalibacterium prausnitzii (F. prausnitzii) could repair the structure of gut microbiota. For example, transplantation of F. prausnitzii has been utilized as an intervention method to treat dysbiosis of the gut microbiota connected to the inflammation preceding autoimmune diseases and/or diabetes [82]. In addition, it has been shown that patients with Parkinson’s disease have a considerably decreased number of F. prausnitzii compared to the control patients [83]. Moreover, the amount of F. prausnitzii may also work as a diagnostic and/or analytic biomarker for the successful procedure of FMT [84]. Consistently, the transplantation of fecal microbiota from patients with schizophrenia has triggered behavior alterations such as impaired learning and/or hyperactivity in the recipient animal [85]. Investigations with animal models suggest that the FMT is also valuable for the treatment of Parkinson’s disease [86]. Similarly, the administration of prebiotics and/or probiotics might be applicable to prevent and/or restore neurodegenerative disorders. The prebiotics are particular plant fibers which may stimulate the growth of healthy bacteria in the gut. The probiotics usually contain specific live organisms, which directly increase the populations of healthy microbes in the gut. Certain gut microbiota with prebiotics and/or probiotics have been shown to contribute to the treatment of ALS, suggesting that gut microbiota might be a new strategy for ALS treatment [87]. Furthermore, it has been shown that mild physical exercise has a cooperative effect on the gut microbiota with higher diversity [88], which might also improve the symptoms in schizophrenia and/or in major depression [89,90] (Figure 2).
6. Next Perspectives
With no current cure for the various neurodegenerative disorders, therapeutics seem to have been concentrated on attempting to decelerate the progression of the disease and provide symptomatic treatments to maintain patient quality of life (QOL). Therapeutic exercise and/or rehabilitation are also recommended for patients to slow symptomatic progression [91]. Furthermore, multidisciplinary teams for therapy are known to improve patient QOL and prolong patient survival [92]. However, there is still no cure that could reverse the progression of these disorders. For example, at present, riluzole and edaravone may be two major disease-modifying drugs for the treatment of ALS [93,94]. The most broadly used drug, showing little beneficial effect on patient survival [95], riluzole, might have a complex mechanism of biochemical action [96]. Riluzole may prolong the survival of ALS patients by up to 20 months [97]. In the experimental study, enhanced mTOR levels and/or attenuated autophagic activity might have increased the survival of motor neurons, suggesting that the downregulation of autophagy might proffer a therapeutic procedure for the treatment of ALS [98]. Riluzole may show antioxidant capabilities against oxidative stress [99]. Another drug, edaravone, is also an antioxidant compound anticipated to reduce oxidative stress and remove lipid peroxidation [100]. Edaravone has been detected to have a therapeutic effect in ALS patients, exhibiting a decreased functional loss of several neurons [101]. Edaravone has been shown to remove hydroxyl radicals for the protection of neurons in ALS [102]. In addition, edaravone could also reduce excessive ROS, as a free radical scavenger, to prevent brain damage [103]. Interestingly, it has been shown that edaravone could ameliorate chronic stress-induced depressive symptoms in mice by regulating the gut microbiota [104]. The rather unsatisfactory efficacy of these conventional drugs might imply that new strategies are immediately needed to articulate therapeutic development for the treatment of ALS. The autophagic signaling pathway may be a crucial therapeutic target [105]. New therapeutic strategies for the ALS community are also mandatory in the struggle against an exponentially rising epidemiology of this disease [106].
Microbial fermentation-derived metabolites could introduce their effects via immunological and neuroendocrine mechanisms [107]. In particular, microbial neurochemicals such as amines, amino acids, and SCFAs, could contribute to the harmonious interactions between the intestinal microbial consortium, systemic immune cells, and the CNS [108], probably in part via the epigenetic mechanism. Notably, the gut microbiota–brain communication is bidirectional. This conversation might stabilize the physical and/or mental health condition, which could otherwise cause serious physical and/or mental health problems [109]. These psychobiotic treatments have exhibited favorable effects on neurodegenerative disorders by altering gut microbiota [110]. In addition, a probiotic supplement has been shown to amend the cognition of the recipients with Alzheimer’s disease [111]. It has been revealed that probiotics including B. bifidum, and/or B. longum supplementation in patients with Alzheimer’s disease could improve the cognitive function [112]. Therefore, the modulation of gut microbiota may be an encouraging therapeutic option to prevent Alzheimer’s disease [113]. Furthermore, such probiotics could inhibit many harmful effects of aging that are the recognized aggravators of various neurodegenerative disorders [114]. Interestingly, SCFAs generated by gut microbiota may improve the synaptic plasticity by reducing neuro-inflammation and epigenetically suppressing the accumulation of β-amyloid via the inhibition of HDACs in the mouse model of Alzheimer’s disease [115], which may be reminiscent of engram modulation via the interaction with microglia, as mentioned in Section 4. Psychobiotic treatments could be an encouraging strategy to improve the QOL for the patients who suffer from neurodegenerative disorders. With an intricate etiology and no current cure for many neurodegenerative disorders, broadening the understanding of the disease pathology is required to progress with patient care [116]. Through the modulation of functional pathways related to the brain–immune communication axis, the gut microbiota could influence the pathophysiology of neurodegenerative disorders. However, there is only sparse evidence on the precise role of the gut microbiota on the programming of immune cells in the underlying neurobiological pathways of neurodegenerative disorders. The microbiota metabolic pathways in the gut might be related to the secretion of inflammatory cytokines [117]. It is uncertain whether gut microbiota could decrease the risks of causing neurodegenerative disorders as a consequence of inhibiting the critical pathological processes. Comprehension of the precise relationship between gut microbial metabolic pathways and the clinical consequences would contribute a great deal to the progression of treatment for valuable interventions in neurodegenerative disorders. These tactics might be applicable for exploring the splendid function of gut microbiota. Therefore, prospective exploration is mandatory to understand the intricate interactions between brain engrams, certain immunity, and gut microbial communities. A systematized consideration of the roles of specific gut microbiota towards the development of various neurodegenerative disorders could confidently provide novel insight into the procedure of probiotics and/or FMT at least as a substitute approach for preventing and/or treating such diseases. It has been suggested that several neurodegenerative disorders have similar aspects to those of autoimmune diseases with the key pathogenic process mediating autoreactive T cells [118]. Biomarkers and possible therapeutic targets in neurodegenerative disorders may also overlap with those of several autoimmune diseases [119,120]. Hence, we here and now believe that the application of the gut–brain axis could expand for the superior treatment of autoimmune diseases and/or the related inflammatory diseases. Subsequently, forthcoming research should focus on the identification of disease-specific engram retention over time during the latent period of those diseases. The large number of researchers need to be united to comprehend the molecular mechanisms with better clarity to obtain superior therapeutic interventions for these intractable diseases.
7. Conclusions
The essential role of the gut-microbiota–neuron–immunity interaction in the pathogenesis of neurodegenerative disorders such as Alzheimer’s disease, Parkinson’s disease, ALS, and schizophrenia has been shown here. In particular, that immunity can generally communicate with the engrams in the brain. Therefore, gut microbiota could provide support by taking favorable action via the modulation of engrams against the disease progression of several neurodegenerative disorders as well as probably several autoimmune diseases.
Author Contributions
S.Y., K.T. and S.M. contributed to the conception of the study. Each author (S.Y., K.T., H.S., Y.I., A.T., S.M.) has participated sufficiently in this work in drafting the article and/or revising the article for the important rational content. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare there are no conflict of interest.
Abbreviations
| ALS | amyotrophic lateral sclerosis |
| AMP | adenosine mono-phosphate |
| AMPK | AMP-activated protein kinase |
| BBB | blood–brain barrier |
| CNS | central nervous system |
| CSF | cerebrospinal fluid |
| DREADD | designer receptor exclusively activated by designer drugs |
| FMT | fecal microbiota transplantation |
| GABA | gamma amino butyric acid |
| HDACs | histone deacetylases |
| IL | interleukin |
| ROS | reactive oxygen species |
| SCFAs | short-chain fatty acids |
References
- Heneka, M.T.; McManus, R.M.; Latz, E. Inflammasome signalling in brain function and neurodegenerative disease. Nat. Rev. Neurosci. 2018, 19, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Gilodi, M.; Lisi, S.; Dudás, E.F.; Fantini, M.; Puglisi, R.; Louka, A.; Marcatili, P.; Cattaneo, A.; Pastore, A. Selection and Modelling of a New Single-Domain Intrabody Against TDP-43. Front. Mol. Biosci. 2022, 8, 773234. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Nagase, N.; Tsuji, A.; Kitagishi, Y.; Matsuda, S. Neuroprotection by dipeptidyl-peptidase-4 inhibitors and glucagon-like peptide-1 analogs via the modulation of AKT-signaling pathway in Alzheimer’s disease. World. J. Biol. Chem. 2021, 12, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Ogino, M.; Ichimura, M.; Nakano, N.; Minami, A.; Kitagishi, Y.; Matsuda, S. Roles of PTEN with DNA Repair in Parkinson’s Disease. Int. J. Mol. Sci. 2016, 17, 954. [Google Scholar] [CrossRef]
- Kitagishi, Y.; Nakanishi, A.; Ogura, Y.; Matsuda, S. Dietary regulation of PI3K/AKT/GSK-3β pathway in Alzheimer’s disease. Alzheimers. Res. Ther. 2014, 6, 35. [Google Scholar] [CrossRef]
- Abbaszadeh, F.; Fakhri, S.; Khan, H. Targeting apoptosis and autophagy following spinal cord injury: Therapeutic approaches to polyphenols and candidate phytochemicals. Pharmacol. Res. 2020, 160, 105069. [Google Scholar] [CrossRef]
- Matsuda, S.; Ikeda, Y.; Murakami, M.; Nakagawa, Y.; Tsuji, A.; Kitagishi, Y. Roles of PI3K/AKT/GSK3 Pathway Involved in Psychiatric Illnesses. Diseases 2019, 7, 22. [Google Scholar] [CrossRef]
- Kitagishi, Y.; Minami, A.; Nakanishi, A.; Ogura, Y.; Matsuda, S. Neuron membrane trafficking and protein kinases involved in autism and ADHD. Int. J. Mol. Sci. 2015, 16, 3095–3115. [Google Scholar] [CrossRef]
- Singh, A.; Kukretim, R.; Saso, L.; Kukreti, S. Mechanistic Insight into Oxidative Stress-Triggered Signaling Pathways and Type 2 Diabetes. Molecules 2022, 27, 950. [Google Scholar] [CrossRef]
- Hitzeroth, A.; Niehaus, D.J.; Koen, L.; Botes, W.C.; Deleuze, J.F.; Warnich, L. Association between the MnSOD Ala-9Val polymorphism and development of schizophrenia and abnormal involuntary movements in the Xhosa population. Prog. Neuropsychopharmacol. Biol. Psychiatry 2007, 31, 664–672. [Google Scholar] [CrossRef]
- Le Gall, L.; Anakor, E.; Connolly, O.; Vijayakumar, U.G.; Duddy, W.J.; Duguez, S. Molecular and Cellular Mechanisms Affected in ALS. J. Pers. Med. 2020, 10, 101. [Google Scholar] [CrossRef] [PubMed]
- Godoy, J.A.; Rios, J.A.; Picón-Pagès, P.; Herrera-Fernández, V.; Swaby, B.; Crepin, G.; Vicente, R.; Fernández-Fernández, J.M.; Muñoz, F.J. Mitostasis, Calcium and Free Radicals in Health, Aging and Neurodegeneration. Biomolecules 2021, 11, 1012. [Google Scholar] [CrossRef]
- Lee, K.H.; Cha, M.; Lee, B.H. Neuroprotective Effect of Antioxidants in the Brain. Int. J. Mol. Sci. 2020, 21, 7152. [Google Scholar] [CrossRef]
- Fakhri, S.; Abbaszadeh, F.; Jorjanim, M. On the therapeutic targets and pharmacological treatments for pain relief following spinal cord injury: A mechanistic review. Biomed. Pharmacother. 2021, 139, 111563. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.R.; Kuang, Q.; Zhang, F.; Chen, B.; Zhong, Z.G. Functional roles of the microbiota-gut-brain axis in Alzheimer’s disease: Implications of gut microbiota-targeted therapy. Transl. Neurosci. 2021, 12, 581–600. [Google Scholar] [CrossRef]
- Dinan, T.G.; Cryan, J.F. Gut instincts: Microbiota as a key regulator of brain development, ageing and neurodegeneration. J. Physiol. 2017, 595, 489–503. [Google Scholar] [CrossRef] [PubMed]
- Bruce-Keller, A.J.; Salbaum, J.M.; Berthoud, H.R. Harnessing Gut Microbes for Mental Health: Getting from Here to There. Biol. Psychiatry 2018, 83, 214–223. [Google Scholar] [CrossRef]
- Martin, S.; Battistini, C.; Sun, J. A Gut Feeling in Amyotrophic Lateral Sclerosis: Microbiome of Mice and Men. Front. Cell. Infect. Microbiol. 2022, 12, 839526. [Google Scholar] [CrossRef]
- Cox, L.M.; Calcagno, N.; Gauthier, C.; Madore, C.; Butovsky, O.; Weiner, H.L. The microbiota restrains neurodegenerative microglia in a model of amyotrophic lateral sclerosis. Microbiome 2022, 10, 47. [Google Scholar] [CrossRef]
- Sawamura, H.; Taniguchi, K.; Ikeda, Y.; Tsuji, A.; Kitagishi, Y.; Matsuda, S. Gut microbiota could modulate the effects of neuro-immune responses and memory traces via the gut-brain-immune axis in schizophrenia. Explor. Neuroprot. Ther. 2022, 2, 74–86. [Google Scholar] [CrossRef]
- Noss, M.M.; Millwood, S.N.; Kuhlman, K.R. Women with lower systemic inflammation demonstrate steeper cognitive decline with age: Results from a large prospective, longitudinal sample. Brain. Behav. Immun. Health 2022, 22, 100465. [Google Scholar] [CrossRef] [PubMed]
- Arsenault, D.; Coulombe, K.; Zhu, A.; Gong, C.; Kil, K.E.; Choi, J.K.; Poutiainen, P.; Brownell, A.L. Loss of Metabotropic Glutamate Receptor 5 Function on Peripheral Benzodiazepine Receptor in Mice Prenatally Exposed to LPS. PLoS ONE 2015, 10, e0142093. [Google Scholar] [CrossRef] [PubMed]
- Chavan, S.S.; Pavlov, V.A.; Tracey, K.J. Mechanisms and Therapeutic Relevance of Neuro-immune Communication. Immunity 2017, 46, 927–942. [Google Scholar] [CrossRef]
- Clark, S.M.; Vaughn, C.N.; Soroka, J.A.; Li, X.; Tonelli, L.H. Neonatal adoptive transfer of lymphocytes rescues social behaviour during adolescence in immune-deficient mice. Eur. J. Neurosci. 2018, 47, 968–978. [Google Scholar] [CrossRef] [PubMed]
- Theodoropoulou, S.; Spanakos, G.; Baxevanis, C.N.; Economou, M.; Gritzapis, A.D.; Papamichail, M.P.; Stefanis, C.N. Cytokine serum levels, autologous mixed lymphocyte reaction and surface marker analysis in never medicated and chronically medicated schizophrenic patients. Schizophr. Res. 2001, 47, 13–25. [Google Scholar] [CrossRef]
- Lupaescu, A.V.; Iavorschi, M.; Covasa, M. The Use of Bioactive Compounds in Hyperglycemia- and Amyloid Fibrils-Induced Toxicity in Type 2 Diabetes and Alzheimer’s Disease. Pharmaceutics 2022, 14, 235. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.C.; Kwak, S.G.; Park, J.S.; Park, D. The effectiveness of nonsteroidal anti-inflammatory drugs and acetaminophen in reduce the risk of amyotrophic lateral sclerosis? A meta-analysis. Sci. Rep. 2020, 10, 14759. [Google Scholar] [CrossRef] [PubMed]
- Csabai, D.; Sebők-Tornai, A.; Wiborg, O.; Czéh, B. A Preliminary Quantitative Electron Microscopic Analysis Reveals Reduced Number of Mitochondria in the Infralimbic Cortex of Rats Exposed to Chronic Mild Stress. Front. Behav. Neurosci. 2022, 16, 885849. [Google Scholar] [CrossRef]
- Karmakar, J.; Mukherjee, K.; Mandal, C. Siglecs Modulate Activities of Immune Cells Through Positive and Negative Regulation of ROS Generation. Front. Immunol. 2021, 12, 758588. [Google Scholar] [CrossRef]
- Scherz-Shouval, R.; Elazar, Z. Regulation of autophagy by ROS: Physiology and pathology. Trends. Biochem. Sci. 2011, 36, 30–38. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, L. New Insights into the Interplay Among Autophagy, the NLRP3 Inflammasome and Inflammation in Adipose Tissue. Front. Endocrinol. 2022, 13, 739882. [Google Scholar] [CrossRef] [PubMed]
- Marcucci, F.; Bellone, M.; Caserta, C.A.; Corti, A. Pushing tumor cells towards a malignant phenotype: Stimuli from the microenvironment, intercellular communications and alternative roads. Int. J. Cancer. 2014, 135, 1265–1276. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.S.; Zhang, S.F.; Luo, G.; Cheng, B.C.; Zhang, C.; Wang, Y.W.; Qiu, X.Y.; Zhou, X.H.; Wang, Q.G.; Song, X.L.; et al. Schisandrin B mitigates hepatic steatosis and promotes fatty acid oxidation by inducing autophagy through AMPK/mTOR signaling pathway. Metabolism 2022, 131, 155200. [Google Scholar] [CrossRef]
- Nagy, S.; Maurer, G.W.; Hentze, J.L.; Rose, M.; Werge, T.M.; Rewitz, K. AMPK signaling linked to the schizophrenia-associated 1q21.1 deletion is required for neuronal and sleep maintenance. PLoS Genet. 2018, 14, e1007623. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, T.; Fujita, N.; Jang, M.H.; Uematsu, S.; Yang, B.G.; Satoh, T.; Omori, H.; Noda, T.; Yamamoto, N.; Komatsu, M.; et al. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature 2008, 456, 264–268. [Google Scholar] [CrossRef]
- De Luca, C.; Colangelo, A.M.; Alberghina, L.; Papa, M. Neuro-Immune Hemostasis: Homeostasis and Diseases in the Central Nervous System. Front. Cell Neurosci. 2018, 12, 459. [Google Scholar] [CrossRef]
- Koren, T.; Yifa, R.; Amer, M.; Krot, M.; Boshnak, N.; Ben-Shaanan, T.L.; Azulay-Debby, H.; Zalayat, I.; Avishai, E.; Hajjo, H.; et al. Insular cortex neurons encode and retrieve specific immune responses. Cell 2021, 184, 5902–5915.e17. [Google Scholar] [CrossRef]
- Gogolla, N. The brain remembers where and how inflammation struck. Cell 2021, 184, 5851–5853. [Google Scholar] [CrossRef]
- Roy, D.S.; Park, Y.G.; Kim, M.E.; Zhang, Y.; Ogawa, S.K.; DiNapoli, N.; Gu, X.; Cho, J.H.; Choi, H.; Kamentsky, L.; et al. Brain-wide mapping reveals that engrams for a single memory are distributed across multiple brain regions. Nat. Commun. 2022, 13, 1799. [Google Scholar] [CrossRef]
- Sakaguchi, M.; Hayashi, Y. Catching the engram: Strategies to examine the memory trace. Mol. Brain. 2012, 5, 32. [Google Scholar] [CrossRef]
- Gebicke-Haerter, P.J. Engram formation in psychiatric disorders. Front. Neurosci. 2014, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Ramos, M.; Alaiz-Noya, M.; Barco, A. Transcriptome and epigenome analysis of engram cells: Next-generation sequencing technologies in memory research. Neurosci. Biobehav. Rev. 2021, 127, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, S.; Linde, J.; Bell, M.; Spehr, M.; Zempel, H.; Zimmer-Bensch, G. DNA Methyltransferase 1 (DNMT1) Shapes Neuronal Activity of Human iPSC-Derived Glutamatergic Cortical Neurons. Int. J. Mol. Sci. 2021, 22, 2034. [Google Scholar] [CrossRef] [PubMed]
- Gulmez Karaca, K.; Kupke, J.; Brito, D.V.C.; Zeuch, B.; Thome, C.; Weichenhan, D.; Lutsik, P.; Plass, C.; Oliveira, A.M.M. Neuronal ensemble-specific DNA methylation strengthens engram stability. Nat. Commun. 2020, 11, 639. [Google Scholar] [CrossRef]
- Niemi, M.B.; Härting, M.; Kou, W.; Del Rey, A.; Besedovsky, H.O.; Schedlowski, M.; Pacheco-López, G. Taste-immunosuppression engram: Reinforcement and extinction. J. Neuroimmunol. 2007, 188, 74–79. [Google Scholar] [CrossRef]
- Pacheco-López, G.; Niemi, M.B.; Kou, W.; Baum, S.; Hoffman, M.; Altenburger, P.; del Rey, A.; Besedovsky, H.O.; Schedlowski, M. Central blockade of IL-1 does not impair taste-LPS associative learning. Neuroimmunomodulation 2007, 14, 150–156. [Google Scholar] [CrossRef]
- Kyrke-Smith, M.; Williams, J.M. Bridging Synaptic and Epigenetic Maintenance Mechanisms of the Engram. Front. Mol. Neurosci. 2018, 11, 369. [Google Scholar] [CrossRef]
- Manea, S.A.; Vlad, M.L.; Fenyo, I.M.; Lazar, A.G.; Raicu, M.; Muresian, H.; Simionescu, M.; Manea, A. Pharmacological inhibition of histone deacetylase reduces NADPH oxidase expression, oxidative stress and the progression of atherosclerotic lesions in hypercholesterolemic apolipoprotein E-deficient mice; potential implications for human atherosclerosis. Redox Biol. 2020, 28, 101338. [Google Scholar] [CrossRef]
- Qing, L.; Liu, L.; Zhou, L.; Zhang, F.; Gao, C.; Hu, L.; Nie, S. Sex-dependent association of mineralocorticoid receptor gene (NR3C2) DNA methylation and schizophrenia. Psychiatry Res. 2020, 292, 113318. [Google Scholar] [CrossRef]
- Bostancıklıoğlu, M. An update on memory formation and retrieval: An engram-centric approach. Alzheimers. Dement. 2020, 16, 926–937. [Google Scholar] [CrossRef]
- Wang, C.; Yue, H.; Hu, Z.; Shen, Y.; Ma, J.; Li, J.; Wang, X.D.; Wang, L.; Sun, B.; Shi, P.; et al. Microglia mediate forgetting via complement-dependent synaptic elimination. Science 2020, 367, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Deng, Y.S.; Dai, S.K.; Mi, T.W.; Li, R.Y.; Liu, P.P.; Liu, C.; He, B.D.; He, X.C.; Du, H.Z.; et al. Loss of microglial EED impairs synapse density, learning, and memory. Mol. Psychiatry 2022, 27, 2999–3009. [Google Scholar] [CrossRef]
- Wang, X.; Chang, L.; Wan, X.; Tan, Y.; Qu, Y.; Shan, J.; Yang, Y.; Ma, L.; Hashimoto, K. (R)-ketamine ameliorates demyelination and facilitates remyelination in cuprizone-treated mice: A role of gut-microbiota-brain axis. Neurobiol. Dis. 2022, 165, 105635. [Google Scholar] [CrossRef]
- Ghezzi, L.; Cantoni, C.; Pinget, G.V.; Zhou, Y.; Piccio, L. Targeting the gut to treat multiple sclerosis. J. Clin. Investig. 2021, 131, e143774. [Google Scholar] [CrossRef] [PubMed]
- Klann, E.M.; Dissanayake, U.; Gurrala, A.; Farrer, M.; Shukla, A.W.; Ramirez-Zamora, A.; Mai, V.; Vedam-Mai, V. The Gut-Brain Axis and Its Relation to Parkinson’s Disease: A Review. Front. Aging Neurosci. 2022, 13, 782082. [Google Scholar] [CrossRef] [PubMed]
- Lecomte, A.; Barateau, L.; Pereira, P.; Paulin, L.; Auvinen, P.; Scheperjans, F.; Dauvilliers, Y. Gut microbiota composition is associated with narcolepsy type 1. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, e896. [Google Scholar] [CrossRef] [PubMed]
- Wiley, N.C.; Cryan, J.F.; Dinan, T.G.; Ross, R.P.; Stanton, C. Production of Psychoactive Metabolites by Gut Bacteria. Mod. Trends Psychiatry 2021, 32, 74–99. [Google Scholar] [PubMed]
- Muller, P.A.; Schneeberger, M.; Matheis, F.; Wang, P.; Kerner, Z.; Ilanges, A.; Pellegrino, K.; Del Mármol, J.; Castro, T.B.R.; Furuichi, M.; et al. Microbiota modulate sympathetic neurons via a gut-brain circuit. Nature 2020, 583, 441–446. [Google Scholar] [CrossRef]
- Janssens, Y.; Debunne, N.; De Spiegeleer, A.; Wynendaele, E.; Planas, M.; Feliu, L.; Quarta, A.; Claes, C.; Van Dam, D.; De Deyn, P.P.; et al. PapRIV, a BV-2 microglial cell activating quorum sensing peptide. Sci. Rep. 2021, 11, 10723. [Google Scholar] [CrossRef]
- Welcome, M.O. Gut Microbiota Disorder, Gut Epithelial and Blood-Brain Barrier Dysfunctions in Etiopathogenesis of Dementia: Molecular Mechanisms and Signaling Pathways. Neuromolecular. Med. 2019, 21, 205–226. [Google Scholar] [CrossRef]
- Li, X.; Fan, X.; Yuan, X.; Pang, L.; Hu, S.; Wang, Y.; Huang, X.; Song, X. The Role of Butyric Acid in Treatment Response in Drug-Naive First Episode Schizophrenia. Front. Psychiatry 2021, 12, 724664. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, T.J.; Gates, E.J.; Ranger, A.L.; Klegeris, A. Short-chain fatty acids (SCFAs) alone or in combination regulate select immune functions of microglia-like cells. Mol. Cell. Neurosci. 2020, 105, 103493. [Google Scholar] [CrossRef] [PubMed]
- Kesika, P.; Suganthy, N.; Sivamaruthi, B.S.; Chaiyasut, C. Role of gut-brain axis, gut microbial composition, and probiotic intervention in Alzheimer’s disease. Life Sci. 2021, 264, 118627. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Huang, T.; Debelius, J.W.; Fang, F. Gut microbiome and amyotrophic lateral sclerosis: A systematic review of current evidence. J. Intern. Med. 2021, 290, 758–788. [Google Scholar] [CrossRef] [PubMed]
- Mou, Y.; Du, Y.; Zhou, L.; Yue, J.; Hu, X.; Liu, Y.; Chen, S.; Lin, X.; Zhang, G.; Xiao, H.; et al. Gut Microbiota Interact with the Brain Through Systemic Chronic Inflammation: Implications on Neuroinflammation, Neurodegeneration, and Aging. Front. Immunol. 2022, 13, 796288. [Google Scholar] [CrossRef]
- Håvik, B.; Røkke, H.; Dagyte, G.; Stavrum, A.K.; Bramham, C.R.; Steen, V.M. Synaptic activity-induced global gene expression patterns in the dentate gyrus of adult behaving rats: Induction of immunity-linked genes. Neuroscience 2007, 148, 925–936. [Google Scholar] [CrossRef]
- Rudzki, L.; Maes, M. From “Leaky Gut” to Impaired Glia-Neuron Communication in Depression. Adv. Exp. Med. Biol. 2021, 1305, 129–155. [Google Scholar]
- Caputi, V.; Popov, J.; Giron, M.C.; O’Mahony, S. Gut Microbiota as a Mediator of Host Neuro-Immune Interactions: Implications in Neuroinflammatory Disorders. Mod. Trends. Psychiatry 2021, 32, 40–57. [Google Scholar]
- Benakis, C.; Martin-Gallausiaux, C.; Trezzi, J.P.; Melton, P.; Liesz, A.; Wilmes, P. The microbiome-gut-brain axis in acute and chronic brain diseases. Curr. Opin. Neurobiol. 2020, 61, 1–9. [Google Scholar] [CrossRef]
- Castanon, N.; Luheshi, G.; Layé, S. Role of neuroinflammation in the emotional and cognitive alterations displayed by animal models of obesity. Front. Neurosci. 2015, 9, 229. [Google Scholar] [CrossRef]
- Hertzberg, V.S.; Singh, H.; Fournier, C.N.; Moustafa, A.; Polak, M.; Kuelbs, C.A.; Torralba, M.G.; Tansey, M.G.; Nelson, K.E.; Glass, J.D. Gut microbiome differences between amyotrophic lateral sclerosis patients and spouse controls. Amyotroph. Lateral. Scler. Frontotemporal. Degener. 2022, 23, 91–99. [Google Scholar] [CrossRef]
- Kim, H.S.; Son, J.; Lee, D.; Tsai, J.; Wang, D.; Chocron, E.S.; Jeong, S.; Kittrell, P.; Murchison, C.F.; Kennedy, R.E.; et al. Gut- and oral-dysbiosis differentially impact spinal- and bulbar-onset ALS, predicting ALS severity and potentially determining the location of disease onset. BMC. Neurol. 2022, 22, 62. [Google Scholar] [CrossRef] [PubMed]
- Chidambaram, S.B.; Essa, M.M.; Rathipriya, A.G.; Bishir, M.; Ray, B.; Mahalakshmi, A.M.; Tousif, A.H.; Sakharkar, M.K.; Kashyap, R.S.; Friedland, R.P.; et al. Gut dysbiosis, defective autophagy and altered immune responses in neurodegenerative diseases: Tales of a vicious cycle. Pharmacol. Ther. 2022, 231, 107988. [Google Scholar] [CrossRef] [PubMed]
- Trujillo-Del Río, C.; Tortajada-Pérez, J.; Gómez-Escribano, A.P.; Casterá, F.; Peiró, C.; Millán, J.M.; Herrero, M.J.; Vázquez-Manrique, R.P. Metformin to treat Huntington disease: A pleiotropic drug against a multi-system disorder. Mech. Ageing Dev. 2022, 204, 111670. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, S.; Nakagawa, Y.; Amano, K.; Ikeda, Y.; Tsuji, A.; Kitagishi, Y. By using either endogenous or transplanted stem cells, which could you prefer for neural regeneration? Neural. Regen. Res. 2018, 13, 1731–1732. [Google Scholar] [CrossRef]
- Taniguchi, K.; Ikeda, Y.; Nagase, N.; Tsuji, A.; Kitagishi, Y.; Matsuda, S. Implications of Gut-Brain axis in the pathogenesis of Psychiatric disorders. AIMS. Bioeng. 2021, 8, 243–256. [Google Scholar] [CrossRef]
- Matsuda, S.; Nakagawa, Y.; Kitagishi, Y.; Nakanishi, A.; Murai, T. Reactive Oxygen Species, Superoxide Dimutases, and PTEN-p53-AKT-MDM2 Signaling Loop Network in Mesenchymal Stem/Stromal Cells Regulation. Cells 2018, 7, 36. [Google Scholar] [CrossRef]
- Ikeda, Y.; Taniguchi, K.; Nagase, N.; Tsuji, A.; Kitagishi, Y.; Matsuda, S. Reactive oxygen species may influence on the crossroads of stemness, senescence, and carcinogenesis in a cell via the roles of APRO family proteins. Explor. Med. 2021, 2, 443–454. [Google Scholar] [CrossRef]
- Zhang, L.; Qian, Y.; Li, J.; Zhou, X.; Xu, H.; Yan, J.; Xiang, J.; Yuan, X.; Sun, B.; Sisodia, S.S.; et al. BAD-mediated neuronal apoptosis and neuroinflammation contribute to Alzheimer’s disease pathology. iScience 2021, 24, 102942. [Google Scholar] [CrossRef]
- Acosta, S.; Jernberg, J.; Sanberg, C.D.; Sanberg, P.R.; Small, B.J.; Gemma, C.; Bickford, P.C. NT-020, a natural therapeutic approach to optimize spatial memory performance and increase neural progenitor cell proliferation and decrease inflammation in the aged rat. Rejuvenation Res. 2010, 13, 581–588. [Google Scholar] [CrossRef]
- Fond, G.B.; Lagier, J.C.; Honore, S.; Lancon, C.; Korchia, T.; Sunhary De Verville, P.-L.; Llorca, P.M.; Auquier, P.; Guedj, E.; Boyer, L. Microbiota-Orientated Treatments for Major Depression and Schizophrenia. Nutrients 2020, 12, 1024. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Chung, S.K.; Vanamala, J.; Xu, B. Causal Relationship between Diet-Induced Gut Microbiota Changes and Diabetes: A Novel Strategy to Transplant Faecalibacterium prausnitzii in Preventing Diabetes. Int. J. Mol. Sci. 2018, 19, 3720. [Google Scholar] [CrossRef] [PubMed]
- Grün, D.; Zimmer, V.C.; Kauffmann, J.; Spiegel, J.; Dillmann, U.; Schwiertz, A.; Faßbender, K.; Fousse, M.; Unger, M.M. Impact of oral COMT-inhibitors on gut microbiota and short chain fatty acids in Parkinson’s disease. Parkinsonism. Relat. Disord. 2020, 70, 20–22. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.T.; Huang, H.L.; Xu, H.M.; Luo, Q.L.; He, J.; Li, Y.Q.; Zhou, Y.L.; Nie, Y.Q.; Zhou, Y.J. Fecal microbiota transplantation ameliorates active ulcerative colitis. Exp. Ther. Med. 2020, 19, 2650–2660. [Google Scholar] [CrossRef]
- Zhu, F.; Guo, R.; Wang, W.; Ju, Y.; Wang, Q.; Ma, Q.; Sun, Q.; Fan, Y.; Xie, Y.; Yang, Z.; et al. Transplantation of microbiota from drug-free patients with schizophrenia causes schizophrenia-like abnormal behaviors and dysregulated kynurenine metabolism in mice. Mol. Psychiatry 2020, 25, 2905–2918. [Google Scholar] [CrossRef]
- Dong, S.; Sun, M.; He, C.; Cheng, H. Brain-gut-microbiota axis in Parkinson’s disease: A historical review and future perspective. Brain Res. Bull. 2022, 183, 84–93. [Google Scholar] [CrossRef]
- Casani-Cubel, J.; Benlloch, M.; Sanchis-Sanchis, C.E.; Marin, R.; Lajara-Romance, J.M.; de la Rubia Orti, J.E. The Impact of Microbiota on the Pathogenesis of Amyotrophic Lateral Sclerosis and the Possible Benefits of Polyphenols. An Overview. Metabolites 2021, 11, 120. [Google Scholar] [CrossRef]
- Torquati, L.; Gajanand, T.; Cox, E.R.; Willis, C.; Zaugg, J.; Keating, S.E.; Coombes, J.S. Effects of exercise intensity on gut microbiome composition and function in people with type 2 diabetes. Eur. J. Sport. Sci. 2022, 23, 1–12. [Google Scholar] [CrossRef]
- Nocera, A.; Nasrallah, H.A. The Association of the Gut Microbiota with Clinical Features in Schizophrenia. Behav. Sci. 2022, 12, 89. [Google Scholar] [CrossRef]
- Xu, Y.; Cai, Z.; Fang, C.; Zheng, J.; Shan, J.; Yang, Y. Impact of aerobic exercise on cognitive function in patients with schizophrenia during daily care: A meta-analysis. Psychiatry Res. 2022, 312, 114560. [Google Scholar] [CrossRef]
- Jopowicz, A.; Wiśniowska, J.; Tarnacka, B. Cognitive and Physical Intervention in Metals’ Dysfunction and Neurodegeneration. Brain. Sci. 2022, 12, 345. [Google Scholar] [CrossRef] [PubMed]
- Aridegbe, T.; Kandler, R.; Walters, S.J.; Walsh, T.; Shaw, P.J.; McDermott, C.J. The natural history of motor neuron disease: Assessing the impact of specialist care. Amyotroph. Lateral. Scler. Frontotemporal. Degener. 2013, 14, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Van Es, M.A.; Hardiman, O.; Chio, A.; Al-Chalabi, A.; Pasterkamp, R.J.; Veldink, J.H.; van den Berg, L.H. Amyotrophic lateral sclerosis. Lancet 2017, 390, 2084–2098. [Google Scholar] [CrossRef]
- Scott, A. Drug therapy: On the treatment trail for ALS. Nature 2017, 550, S120–S121. [Google Scholar] [CrossRef]
- Wobst, H.J.; Mack, K.L.; Brown, D.G.; Brandon, N.J.; Shorter, J. The clinical trial landscape in amyotrophic lateral sclerosis-Past, present, and future. Med. Res. Rev. 2020, 40, 1352–1384. [Google Scholar] [CrossRef] [PubMed]
- Bellingham, M.C. A review of the neural mechanisms of action and clinical efficiency of riluzole in treating amyotrophic lateral sclerosis: What have we learned in the last decade? CNS Neurosci. Ther. 2011, 17, 4–31. [Google Scholar] [CrossRef]
- Andrews, J.A.; Jackson, C.E.; Heiman-Patterson, T.D.; Bettica, P.; Brooks, B.R.; Pioro, E.P. Real-world evidence of riluzole effectiveness in treating amyotrophic lateral sclerosis. Amyotroph. Lateral. Scler. Frontotemporal. Degener. 2020, 21, 509–518. [Google Scholar] [CrossRef]
- West, R.J.H.; Ugbode, C.; Fort-Aznar, L.; Sweeney, S.T. Neuroprotective activity of ursodeoxycholic acid in CHMP2BIntron5 models of frontotemporal dementia. Neurobiol. Dis. 2020, 144, 105047. [Google Scholar] [CrossRef]
- Sala, G.; Arosio, A.; Conti, E.; Beretta, S.; Lunetta, C.; Riva, N.; Ferrarese, C.; Tremolizzo, L. Riluzole Selective Antioxidant Effects in Cell Models Expressing Amyotrophic Lateral Sclerosis Endophenotypes. Clin. Psychopharmacol. Neurosci. 2019, 17, 438–442. [Google Scholar] [CrossRef]
- Rothstein, J.D. Edaravone: A new drug approved for ALS. Cell 2017, 171, 725. [Google Scholar] [CrossRef]
- Sawada, H. Clinical efficacy of edaravone for the treatment of amyotrophic lateral sclerosis. Expert Opin. Pharmacother. 2017, 18, 735–738. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Wu, H.T.; Li, X.X.; Yu, Y.; Gu, R.Z.; Lan, R.; Qin, X.Y. Edaravone protects rat astrocytes from oxidative or neurotoxic inflammatory insults by restoring Akt/Bcl-2/Caspase-3 signaling axis. IBRO Rep. 2020, 8, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, S.; Murozono, M.; Kanazawa, M.; Nara, T.; Ozawa, T.; Watanabe, Y. Edaravone and cyclosporine A as neuroprotective agents for acute ischemic stroke. Acute Med. Surg. 2018, 5, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Bu, F.; Chen, T.; Shi, G.; Yuan, X.; Feng, Z.; Duan, Z.; Wang, R.; Zhang, S.; Wang, Q.; et al. A next-generation probiotic: Akkermansia muciniphila ameliorates chronic stress-induced depressive-like behavior in mice by regulating gut microbiota and metabolites. Appl. Microbiol. Biotechnol. 2021, 105, 8411–8426. [Google Scholar] [CrossRef]
- Xu, X.; Shen, D.; Gao, Y.; Zhou, Q.; Ni, Y.; Meng, H.; Shi, H.; Le, W.; Chen, S.; Chen, S. A perspective on therapies for amyotrophic lateral sclerosis: Can disease progression be curbed? Transl. Neurodegener. 2021, 10, 29. [Google Scholar] [CrossRef]
- Arthur, K.C.; Calvo, A.; Price, T.R.; Geiger, J.T.; Chiò, A.; Traynor, B.J. Projected increase in amyotrophic lateral sclerosis from 2015 to 2040. Nat. Commun. 2016, 7, 12408. [Google Scholar] [CrossRef]
- Oleskin, A.V.; Shenderov, B.A. Neuromodulatory effects and targets of the SCFAs and gasotransmitters produced by the human symbiotic microbiota. Microb. Ecol. Health Dis. 2016, 27, 30971. [Google Scholar] [CrossRef]
- Oleskin, A.V.; Shenderov, B.A.; Rogovsky, V.S. Role of Neurochemicals in the Interaction between the Microbiota and the Immune and the Nervous System of the Host Organism. Probiotics Antimicrob. Proteins 2017, 9, 215–234. [Google Scholar] [CrossRef]
- Oleskin, A.V.; Shenderov, B.A. Probiotics and Psychobiotics: The Role of Microbial Neurochemicals. Probiotics Antimicrob. Proteins 2019, 11, 1071–1085. [Google Scholar] [CrossRef]
- Cheng, L.H.; Liu, Y.W.; Wu, C.C.; Wang, S.; Tsai, Y.C. Psychobiotics in mental health, neurodegenerative and neurodevelopmental disorders. J Food Drug Anal. 2019, 27, 632–648. [Google Scholar] [CrossRef]
- Akbari, E.; Asemi, Z.; Daneshvar Kakhaki, R.; Bahmani, F.; Kouchaki, E.; Tamtaji, O.R.; Hamidi, G.A.; Salami, M. Effect of Probiotic Supplementation on Cognitive Function and Metabolic Status in Alzheimer’s Disease: A Randomized, Double-Blind and Controlled Trial. Front Aging Neurosci. 2016, 8, 256. [Google Scholar] [CrossRef] [PubMed]
- Tamtaji, O.R.; Heidari-Soureshjani, R.; Mirhosseini, N.; Kouchaki, E.; Bahmani, F.; Aghadavod, E.; Tajabadi-Ebrahimi, M.; Asemi, Z. Probiotic and selenium co-supplementation, and the effects on clinical, metabolic and genetic status in Alzheimer’s disease: A randomized, double-blind, controlled trial. Clin Nutr. 2019, 38, 2569–2575. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Liu, X.; Jiang, R.; Yan, X.; Ling, Z. Roles and Mechanisms of Gut Microbiota in Patients With Alzheimer’s Disease. Front Aging Neurosci. 2021, 13, 650047. [Google Scholar] [CrossRef] [PubMed]
- Westfall, S.; Lomis, N.; Kahouli, I.; Dia, S.Y.; Singh, S.P.; Prakash, S. Microbiome, probiotics and neurodegenerative diseases: Deciphering the gut brain axis. Cell Mol Life Sci. 2017, 74, 3769–3787. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, K.; Li, X.; Xu, L.; Yang, Z. Sodium butyrate ameliorates the impairment of synaptic plasticity by inhibiting the neuroinflammation in 5XFAD mice. Chem. Biol. Interact. 2021, 341, 109452. [Google Scholar] [CrossRef] [PubMed]
- Roberts, B.; Theunissen, F.; Mastaglia, F.L.; Akkari, P.A.; Flynn, L.L. Synucleinopathy in Amyotrophic Lateral Sclerosis: A Potential Avenue for Antisense Therapeutics? Int. J. Mol. Sci. 2022, 23, 9364. [Google Scholar] [CrossRef]
- Agorastos, A.; Bozikas, V.P. Gut microbiome and adaptive immunity in schizophrenia. Psychiatriki 2019, 30, 189–192. [Google Scholar] [CrossRef]
- Wang, L.; Wei, X. T Cell-Mediated Autoimmunity in Glaucoma Neurodegeneration. Front. Immunol. 2021, 12, 803485. [Google Scholar] [CrossRef]
- Taniguchi, K.; Sawamura, H.; Ikeda, Y.; Tsuji, A.; Kitagishi, Y.; Matsuda, S. D-Amino Acids as a Biomarker in Schizophrenia. Diseases 2022, 10, 9. [Google Scholar] [CrossRef]
- Varma-Doyle, A.V.; Lukiw, W.J.; Zhao, Y.; Lovera, J.; Devier, D. A hypothesis-generating scoping review of miRs identified in both multiple sclerosis and dementia, their protein targets, and miR signaling pathways. J. Neurol. Sci. 2021, 420, 117202. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).