Abstract
Current mixotrophic culture systems for Dunaliella salina have technical limitations to achieve high growth and productivity. The purpose of this study was to optimize the mixotrophic conditions imposed by glycerol, light, and salinity that lead to the highest biomass and β-carotene yields in D. salina. The combination of 12.5 mM glycerol, 3.0 M salinity, and 50 μmol photons m−2 s−1 light intensity enabled significant assimilation of glycerol by D. salina and consequently enhanced growth (2.1 × 106 cell mL−1) and β-carotene accumulation (4.43 pg cell−1). The saline and light shock induced the assimilation of glycerol by this microalga. At last stage of growth, the increase in light intensity (300 μmol photons m−2 s−1) caused the β-carotene to reach values higher than 30 pg cell−1 and tripled the β-carotene values obtained from photoautotrophic cultures using the same light intensity. Increasing the salt concentration from 1.5 to 3.0 M NaCl (non-isosmotic salinity) produced higher growth and microalgal β-carotene than the isosmotic salinity 3.0 M NaCl. The mixotrophic strategy developed in this work is evidenced in the metabolic capability of D. salina to use both photosynthesis and organic carbon, viz., glycerol that leads to higher biomass and β-carotene productivity than that of an either phototrophic or heterotrophic process alone. The findings provide insights into the key role of exogenous glycerol with a strategic combination of salinity and light, which evidenced unknown roles of this polyol other than that in osmoregulation, mainly on the growth, pigment accumulation, and carotenogenesis of D. salina.
1. Introduction
Microalgae are one of the primary aquatic resources with the potential for exploitation and cultivation to obtain economically valuable compounds. The chlorophyte Dunaliella salina is the supreme and natural source of β-carotene, a carotenoid with bioactive roles such as provitamin A, colorant, antioxidant, antimicrobial, pro-apoptotic, anti-aging immunomodulator, and anticarcinogenic [1,2]. The biomass and β-carotene of this microalga are used extensively in the food, feed, pharmaceutical, biomedical, and nutraceutical and cosmeceutical industries [3,4]. Moreover, this halophilic microorganism is a promising source of other carotenoids (zeaxanthin, lutein, and α-carotene), vitamins, lipids, and proteins [3,5,6]. For a long time, the uniqueness of D. salina to thrive under extreme environmental conditions has been worldwide exploited at photoautotrophic production systems. However, yield and productivity under this practice are low due to photoinhibition, photo-oxidative damage, and self-shading of cultures intended to produce high cell densities [7]. Mixotrophic systems are attractive due to their low light requirements and large availability of carbon (inorganic and organic), contributing to improved growth rate, biomass, and extensive synthesis of metabolites. Mixotrophy involves the concomitant, simultaneous, and efficient metabolism between photosynthesis and respiration. Consequently, various metabolic pathways and sub-routes to incorporate and allocate carbon and energy are activated in microalgae [8,9]. Today, organic carbon nutrition and mixotrophy linked to metabolic pathways represent new scenarios in expanding knowledge and diversification of the metabolism and physiology of microalgae of environmental and economic importance.
Efforts to develop mixotrophic growth media for D. salina and many other microalgae are currently underway. The growth of D. salina on glucose [10,11,12] and acetate [10,13,14] supplemented media was reported, but they still exhibit predominantly low growth rates or a very low amount of β-carotene. Regarding substrates, glucose has the disadvantage of reducing photochemical O2 evolution, cellular affinity for CO2, and the synthesis of the Calvin cycle enzymes in microalgae [15]. This sugar is relatively expensive compared to other organic substrates [16]. Acetate can be toxic in high concentrations for various microorganisms [17] and sometimes is an inadequate substrate for β-carotene synthesis in microalgae [13]. In recent years, the use of glycerol for the mixotrophic production of biomass and carotenoids from microalgae, including D. salina, has been an important initiative by some groups of research [18,19,20,21]. Glycerol can be assimilated properly under respiratory conditions by photosynthetic microorganisms [22]. Moreover, this polyol lacks toxic effects on microalgal cells even at high concentrations [23]. This C3 molecule is the major by-product of the biodiesel industry (known as crude glycerol). This hypersaline waste could promote the growth and production of halophilic carotenogenic microorganisms [24]. Meanwhile, D. salina synthesizes glycerol to succeed in media and environments containing NaCl concentrations from about 0.05 M to saturation (around 5.5 M) [25,26]. Unlike other organic carbon substrates, the role of glycerol on the physiology and metabolism of D. salina may involve increased cell growth, β-carotene synthesis, and carotenogenesis.
Currently, entrepreneurs and companies have difficulties in implementing mixotrophic microalgal production under optimal culture conditions to maximize the yield and productivity in this biosystem [27]. Therefore, the substrate concentration and the environmental factors that best stimulate the mixotrophic metabolism of D. salina on glycerol must be optimized. Likewise, the role of glycerol on generating more biomass, β-carotene synthesis and carotenogenesis from D. salina must be explained. To investigate these gaps in knowledge of the induced mixotrophic metabolism, we assessed how glycerol, salinity, and light intensity under optimized conditions can stimulate the simultaneous and competitive production of biomass and β-carotene in this microalga under mixotrophic culture conditions. This paper presents the individual and combined effect of glycerol level, salinity, and light intensity on the nutritional and metabolic capacities of D. salina to maximize the cellular growth and carotenoids content under mixotrophy. Moreover, this research reveals for the first time the relationship between growth and carotenogenesis, especially of β-carotene in D. salina cultured mixotrophically on glycerol.
2. Results
2.1. Effect of Glycerol Concentration on Mixotrophic Culture of D. salina under 1.5 M NaCl Isosmotic Salinity
Due to the heterogeneity of both the salinities and glycerol levels, and the cell growth reported from previous studies [14,20,28], the effect of glycerol concentration on the culture of D. salina under better growth isosmotic conditions (1.5 M NaCl) [29] was evaluated. This saline condition and 12.5 mM glycerol promoted the best growth of this microalga (1.19 × 106 cells mL−1) (p < 0.05), even exceeding cell density obtained in photoautotrophic cultures (IM) (Figure 1). In addition, the higher concentrations of glycerol yielded the lowest cell growth. Table 1 shows the lower contents for Chl a, similar for Chl b, and higher for βCar in this alga under 12.5 mM glycerol compared to IM.
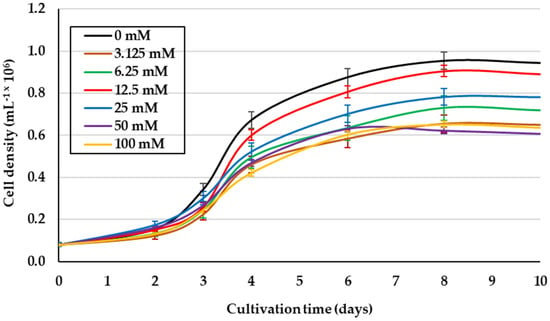
Figure 1.
Cell density of D. salina cultured with different concentrations of glycerol (Gly). Salinity and light intensity were 1.5 M NaCl and 50 μmol photons m−2 s−1, respectively. Values correspond to the mean ± standard deviation of three replicates.

Table 1.
Chl a, Chl b, BCar, and culture pH of D. salina grown under 12.5 mM Gly and photoautotrophic control. Efficient salinity for growth and light intensity were 1.5 M NaCl and 50 μmol photons m−2 s−1, respectively. Values in the same column with different superscript are significantly different.
2.2. Combined Effect of Glycerol, Light, and Salinity on Mixotrophic Culture of D. salina
Figure 2a–d shows the effect of lower glycerol level combined with different light intensities and salinities on the growth and pigments of D. salina. The highest cellular yields were obtained with 12.5 mM glycerol, 3.0 M non-isosmotic salinity, and 50 μmol photons m−2 s−1 light intensity (Figure 2c) (p < 0.05), which exceeded threefold and twofold the microalgal productivity of the controls (IM) under 3.0 and 1.5 M NaCl, respectively (Figure 2a,c). The remarkable increase in chlorophylls a and b, and βCar (Table 2) was observed when D. salina was grown under the optimal glycerol treatment (12.5 mM glycerol, 3.0 M non-isosmotic salinity and 50 μmol photons m−2 s−1 light intensity). The analysis of culture pH in D. salina under the optimum glycerol treatment tended to mild basicity (7.0–8.2) (Table 2).
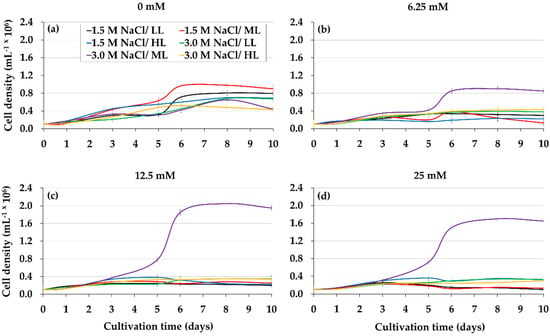
Figure 2.
Cell density of D. salina cultured with different combinations of glycerol, salinity, and light intensity. The following interaction is indicated: glycerol, 0 mM (a), 6.25 mM (b), 12.5 mM (c) and 25 mM (d) with 1.5 and 3.0 M NaCl as well as with 10, 50 and 100 μmol photons m−2 s−1 respectively. Values correspond to the mean ± standard deviation of three replicates.

Table 2.
Chl a, Chl b, BCar, and culture pH of D. salina grown under different levels of glycerol, non-isosmotic salinity, and light intensity. The initial pH range was 6.20–6.35, and the final pH corresponds to the 7th day of cultivation. Values in the same columns with different superscript are significantly different.
To stimulate the massive synthesis of β-carotene, D. salina cells that had reached their maximum at the seventh day of culture on inorganic medium enriched with glycerol were exposed to higher light intensity (from 50 to 300 µmol photons m−2 s−1) [30] for a period of three days. Figure 3 and Figure 4 show changes in the pigment content of D. salina under mixotrophic cultures at changed light intensity. In glycerol treatments under high light, chlorophyll a and b levels remained almost constant, whereas their levels of β-carotene and zeaxanthin increased compared to those treatments under low light.

Figure 3.
Cultures of D. salina on glycerol and controls. (a) IM, 1.5 M NaCl and 50 µmol m−2 s−1. (b) Gly, 3.0 M NaCl and 50 µmol photons m−2 s−1. (c) IM, 1.5 M NaCl and 300 µmol photons m−2 s−1. (d) Gly, 3.0 M NaCl and 300 µmol photons m−2 s−1. The images were captured on the seventh day of culture when the pigments were analyzed. (a) and (b) correspond to the original culture flasks, whereas (c) and (d) are recipients only used for photography.
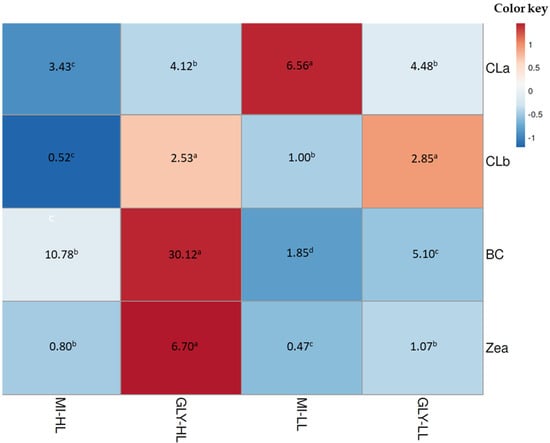
Figure 4.
Heat map colors correspond to lower (blue) and higher (red) abundance for the main pigments of D. salina cultured under mixotrophic conditions. The real concentrations of pigments (pg cell−1) are in the heat map figure. MI-HL and Gly-HL correspond to the inorganic medium and glycerol exposed to 300 µmol photons m−2 s−1, respectively. MI-LL and Gly-LL correspond to the inorganic medium and glycerol exposed to 50 µmol photons m−2 s−1, respectively. Pigments (CLa: chlorophyll a, CLb: chlorophyll b, BC: β-carotene, Zea: zeaxanthin) were separated and identified by HPLC. Different letters in the same row are significantly different between pigment content for each treatment.
Another set of experiments was developed to rule out whether the effect of glycerol by D. salina is dependent on change in salinity (1.5 to 3.0 M, non-isosmotic salinity) and not of the saline adaptation (3 M, isosmotic salinity). The microalga D. salina on 3 M isosmotic salinity exhibited intermediate cell growth. Chl a, Chl b, and BCar concentrations of microalgal cells exposed to this isosmotic salinity were lower than those obtained with non-isosmotic salinity, 3 M (Table 2 and Table 3). The culture pH under isosmotic salinity was maintained to be slightly acidic compared to the slightly basic pH of the microalgal culture under non-isosmotic salinity.

Table 3.
Cell density, Chl a, Chl b, BCar, and culture pH of D. salina grown under 12.5 mM Gly and IM control. Isosmotic salinity for growth and light intensity were 3.0 M NaCl and 50 μmol photons m−2 s−1, respectively. The initial pH range was 6.20–6.35, and the final pH corresponds to the 7th day of cultivation. Values in the same columns with different superscript are significantly different.
2.3. Extracellular and Intracellular Glycerol of D. salina
Another group of experiments was carried out in order to know the dynamics of uptake of glycerol by D. salina. A continuous and substantial decrease in the extracellular glycerol of D. salina is observed with 3.0 M salinity compared to 1.5 M (Figure 5b). At the same time, a tiny amount of intracellular glycerol was observed throughout the microalgal culture at 1.5 and 3.0 M saline condition (Figure 5d). Lower values of extracellular (Figure 5a) and intracellular (Figure 5c) glycerol were observed in the inorganic medium without glycerol for both saline conditions. From these results, the uptake of glycerol from the medium by D. salina was preliminarily demonstrated.
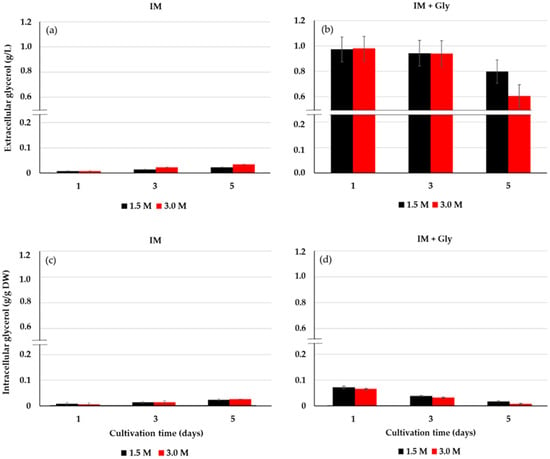
Figure 5.
Extracellular glycerol from IM (a) and IM + Gly (b) and intracellular glycerol from IM (c) and IM + Gly (d) in D. salina cultivated on 12.5 mM glycerol. Salinities: 1.5 and 3.0 M NaCl. Intracellular glycerol concentrations were normalized to dry cell weight (DW). Values correspond to the mean ± standard deviation of three replicates.
2.4. PSII Activity from D. salina Cultivated on Glycerol
The maximum quantum efficiency Fv/Fm may be used to screen stressful or beneficial of organic substrates for the photosynthetic efficiency of D. salina under the mixotrophic growth conditions. In this work, the PSII activity of D. salina cultivated on glycerol is affected by the salinity level (Figure 6a,c). The Fv/Fm of D. salina cells cultivated on the optimized conditions of glycerol and light is greater for salinity 3 M than 1.5 M, but these are less than photoautotrophic cultures (Figure 6a,c). Microalgal cultures under glycerol and DCMU at 1.5 and 3 M NaCl showed a notable drop in Fv/Fm of D. salina cells for both salinities (Figure 6b,d). Furthermore, the different glycerol cultures and IM exposed to DCMU did not show microalgal growth.
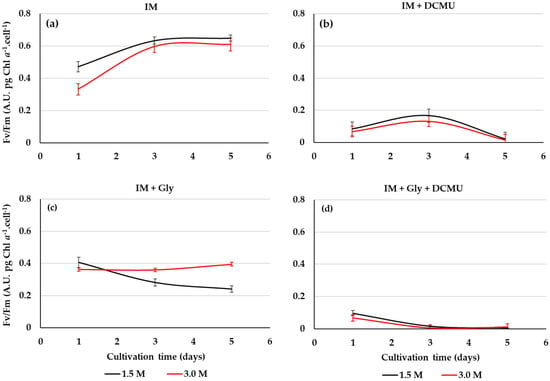
Figure 6.
Photosynthetic efficiency (Fv/Fm) of D. salina cultivated at different conditions: IM (a), IM + DCMU (b), IM + 12.5 mM Gly (c), and IM + 12.5 mM Gly + DCMU (d). Culture salinities: 1.5 and 3.0 M NaCl. Values correspond to the mean ± standard deviation of three replicates.
3. Discussion
In this study, the combined effect of glycerol level, light intensity, and salinity was assessed in order to optimize the mixotrophic culture conditions of D. salina. Our main aim was to contribute to develop a simple mixotrophic strategy to increase biomass and β-carotene production of this halophilic chlorophyte based exclusively on the optimization of the mixotrophic culture conditions with a non-fermentative organic carbon substrate, viz., glycerol. The proper growth of D. salina under low doses of glycerol is indicative of the efficient diffusion, active transport, or both of this substrate through the cell membranes. At higher concentrations, the glycerol could have an inhibitory effect on the growth and productivity of D. salina as reported [20]. Even high levels of glycerol may change the chloroplast ultrastructure, which reduces the excitation energy in PSII and decreases the photosynthetic activity and chlorophyll content [31]. Decreased concentrations of D. salina Chl a, Chl b, and β-Car in glycerol compared to photoautotrophic cultures (Control) may indicate that heterotrophic and myxotrophic culture conditions are progressing (Figure 5), as reported in Chlorella sorokiniana UTEX 1230 [32] and 1602 [33], respectively. These variations may respond to transition imbalances between photoautotrophy and heterotrophy, which cannot be discarded according to the experimental mixotrophic growth conditions, as recently reported [34,35]. Moreover, some organic substrates can change the pH of the medium and other properties such as viscosity, osmotic pressure, and gas–liquid transfer coefficient [36]. The pH close to neutral obtained from the microalgal mixotrophy assayed, may be demonstrative of a balanced system between photosynthesis (CO2 consumed and O2 released) and respiration (O2 consumed and CO2 released) fated to the glycerol assimilation for this chlorophyte [34].
The best results of the mixotrophic growth of this alga were obtained from low doses of glycerol (12.5 mM) and non-isosmotic salinity (3 M NaCl), whose interaction stimulates the uptake of glycerol by the microalgal cells [37]. Concerning light intensity, better results were achieved with 50 μmol photons m−2 s−1, which demonstrated that glycerol has broad performance under dim light in microalgae [38], and happened when D. salina was grown in mixotrophic conditions. Regarding to salinity, this green alga can grow in a range from 0.05 M to 5.5 M NaCl [25], but show maximal growth performance at 1.5–3.0 M NaCl [29]. Compared to cultures at 1.5 M NaCl, D. salina grown at 3.0 M NaCl favors higher growth and synthesis of β-carotene in this alga on glycerol due to its antioxidant ability [29]. The high cell density obtained at usual promoting salinities for the growth of D. salina demonstrated that exogenous glycerol might play a nutritional role in this alga besides its well-known osmoregulatory role. This advantage is supported by its lower energy cost for cell metabolism compared to the assimilation of endogenous glycerol from photosynthesis or starch degradation [39]. The conversion of glycerol into glyceraldehyde 3-phosphate (G3P) allows for the binding to precursors associated with the carbon metabolic pathways of photosynthesis and respiration (phosphoenolpyruvate or pyruvate) that can affect cell growth and the synthesis of cellular metabolites [40,41]. In the optimized conditions of this work, chlorophyll a level (4.58 pg. cell−1) was lower than the level of 11 pg cell−1 obtained from D. salina cultures with glycerol [20]. However, initial β-carotene levels (4.43 pg. cel−1) in this study were higher than β-carotene (0.8 pg cell−1) reported in this same work. Unlike the current knowledge on the mixotrophy of D. salina with glycerol, which is dominated by the photoautotrophic component, the optimized conditions of this work could induce glycerol balanced mixotrophy with codominance of both pigments.
In comparison to the straightforward mixotrophic production of biomass and β-carotene with different organic carbon substrates (glucose, acetate, and glycerol), our optimized cultivation condition with glycerol enhances suitable and productive balance between growth and carotenoids in this halophilic microalga (Table 4). In most initiatives on mixotrophic cultures [12,13,20], only microalgal growth is enhanced, but β-carotene concentration remains low. Unlike our work, most of the reported microalgal cultures with higher growth or pigment levels [10,12,13,20] are due to additional environmental and nutritional conditions that different authors carry out (e.g., higher substrate concentration, inoculum size, and high light intensity, longer cultivation periods, addition of other nutrients, and agitation or aeration systems), which affect microalgal production costs. Our approach seeks to develop a simple, practical, and inexpensive mixotrophic system. As a C3 molecule, glycerol can integrate into photoautotrophic and photoheterotrophic metabolism, but an efficient metabolic fit for its assimilation slightly favors photosynthesis over respiration that could be undergoing in this alga.

Table 4.
Comparison of yield parameters of D. salina grown in glucose and glycerol from the literature and in the best experimental conditions in this work.
In our case, the photosynthetic reactions in D. salina were able to continue producing chlorophyllian pigments, energy, CO2 fixation, and even the incorporated carbon from glycerol could have been used to synthesize compounds not directly involved with cellular respiration (e. g., amino acids synthesis) [42]. Moreover, since the oxidative metabolism of the organic substrate is operating simultaneously in mixotrophy, a part of C-glycerol may switch to the tricarboxylic acid (TCA) cycle, where citrate intermediates are expressed at the gene level to induce respiration via the alternative mitochondrial pathway [43]. In principle, this mechanism may facilitate the massive synthesis of carotenoids [43] as the increase in β-carotene observed in D. salina. (Table 2).
The slightly basic pH values with glycerol are quite different from the sharp values of acidity and basicity obtained with glucose and acetate, respectively (data no shown). The mixotrophic growth of D. salina with 12.5 mM glycerol, 3.0 M non-isosmotic salinity, and 50 μmol photons m−2 s−1 light intensity suggests a balance in the following conditions: CO2/O2 gases, photosynthesis and respiration, and pH [34] during organic and inorganic carbon metabolism. Lastly, the highest microalgal productivity obtained under a self-balanced culture pH could provide comparative advantages of mixotrophic cultures with glycerol compared to other organic substrates (e.g., glucose and acetate) for the massive and commercial production of this microalga.
In the case of D. tertiolecta cultivated under non-isosmotic conditions (0.5 to 4 mM NaCl), unlike the low assimilation rates of 5 mM glycerol (0.78 pg glycerol cell−1 min−1 for 5 min) under isosmotic conditions (0.5 mM NaCl), this alga could assimilate the substrate at a rate of 3.14 pg glycerol cell−1 min−1 for 15 min [37]. This phenomenon could also be occurring in our cultures with D. salina, where the cells grown under non-isosmotic salinity are receiving slight salt stress. This fact may trigger an osmoregulation process synthesizing the enzyme machinery involved with the uptake and assimilation of exogenous glycerol, fated to energy and carbon for osmotic regulation, growth, and cell pigments. Glycerol assimilation is species dependent; hence, some microalgae exhibit high growth in inorganic media supplemented with glycerol, while other species remain unaffected [44]. Unlike chlorophyte, photoheterotroph Dactylococcus dissociatus MT1 can totally consume the initial concentration of 6 mM of glycerol within 24 h [31]. According to our results, chlorophyte photoautotroph D. salina slowly and progressively consumes glycerol throughout the culture, and possibly according to its nutritional and osmoregulatory needs. Likewise, although the plasmatic membrane of D. salina is several orders of magnitude less permeable to glycerol osmolyte, the presence of this compound in the controls of our experiments may suggest that the leakage of glycerol through these membranes may be much more widely distributed than just the release of glycerol by death and cell lysis [45].
The entire fate of incorporated glycerol by D. salina is still unknown. In our work, the gradual increase in extracellular glycerol content was observed through the IM medium (5a), and increase and decrease in the glycerol of D. salina in the IM + Gly medium. We hypothesized that these fluctuations may obey to the maintenance of glycerol homeostasis by the microalgal cells. Regarding to the large difference between glycerol uptake and glycerol assimilated by cells for their osmoregulation, much glycerol is metabolized (through diffusion, active transport, or both) and incorporated into the networks and pathways involved in the production of biomass, β-carotene, and carotenogenesis in D. salina. However, further experiments would be required to confirm these assertions.
The maximum quantum efficiency Fv/Fm is an extremely sensitive, non-invasive index to assess the wellness of the microalgae photosynthetic apparatus via the photochemical efficiency of the PSII in response to energy metabolism and the interaction between carbon and nutrient assimilation [32,46]. Fv/Fm values obtained with glycerol (0.435 on average) were lower than those obtained in photoautotrophic cultures (IM), because the incorporation of this substrate linked to salinity could be generating different degrees of stress to the photosystems of D. salina. Contrary to the isosmotic condition (1.5 M), the non-isosmotic salinity (3 M) induces to the highest Fv/Fm of D. salina cells grown on this substrate under mixotrophy. Indeed, an increase in photosynthetic activity coupled to the use of glycerol for respiration could be produced by these saline changes as reported for other stressors (e.g., high light) [47].
A remarkable decrease in the Fv/Fmax index was registered when D. salina was cultured with glycerol and DCMU, reflecting a disruption in the energetic status of the photosynthetic intermediaries across the damage in PSII reaction centers from this chlorophyte during the combined action of photoautotrophy and mixotrophy. In some way, the exerted action by photosynthesis and glycerol metabolism allow to allocate carbon toward growth and metabolites synthesis [48]. In this mixotrophic process, energy-rich molecules such as G3P and dihydroxyacetone phosphate (DHAP) are generated from glycerol. These C3 molecules transform into pyruvate, which, in turn, is decarboxylated to convert into acetyl-CoA [49]. In microalgal mixotrophy, biomass (energy from TCA cycle) and metabolites (e.g., pigments and fatty acid) synthesis pathways compete for this key thioester substrate [50]. In this study, optimized mixotrophy conditions by glycerol suggest that acetyl CoA or another intermediate is appropriately channeled for mixotrophic biosynthetic processes that stimulate growth and β-carotene in D. salina. This is the first study that reveals an unknown role of glycerol in addition to that involved in osmoregulation, mainly the simultaneous and improved production of cellular biomass and pigments of D. salina, especially β-carotene. This metabolic pattern occurs when the particular mixotrophic conditions exerted by glycerol are optimized, particularly through a suitable and selective combination of salinity and light. The photoautotrophic cultures, such as in the case of Dunaliella, are characterized per se by the low capacity of microalgal photosystems to capture light energy, as well as inorganic carbon limitation in extreme and hypersaline environments and media. In contrast, the mixotrophic culture of D. salina when regulated by sodium chloride and glycerol may become a new culture strategy to increase D. salina production yields. This microalga cultured on glycerol exhibited maxima yields, viz., yield biomass/substrate (Yx/s), and yield product (β-carotene)/substrate (Yp/s) (Table 4). The bioproduction processes and parameters of microalgae and particularly of Dunaliella salina under mixotrophic growth conditions, still require of specific optimization approaches. Our mixotrophic cultures with glycerol did not receive CO2, any other source of nutrients (only the inorganic culture medium), or continuous mechanical agitation. In mixotrophic cultures, microalgal growth is affected by CO2/O2 availability from agitation [51]. Despite this, our Yx/s and Yp/s (β-carotene) results are superior to yields obtained by some reports on mixotrophic cultures in this species. (Table 4).
Microalgal cultures with the highest cellular growth (3.0 M NaCl and Gly) progressively reach high levels of β-carotene, which occurred in cellular cultures after three days of being subjected to a high light level. In D. salina CCAP 19/30 cells, this effect was attributed to an increase in light intensity (50 to 200 µmol photons m−2 s−1), which caused the stabilization of the photosynthetic apparatus and of the chlorophylls [47].
The increase in carotenoids (β-carotene and zeaxanthin) is related to their protective action against oxidative stress such as hypersalinity and high light intensity [52]. The microalga Chlamydomonas acidophila grown on glycerol seems to enhance the β-carotene pathway compared to its photoautotrophic metabolism [53]. Under these conditions, the acetyl-CoA and NADPH production required to increase the β-carotene content should come from the energy excess of glycerol catabolism as reported in another microorganism [54]. In addition to the bioconversion of exogenous glycerol into key intermediates of general metabolism, it could have directly impacted the synthesis of carotenogenesis. The effect of high intensity light has been related with the transcriptional activation of carotenogenic genes in response to stimulation by phytohormones [55]. Our results, in accordance with recent reports on high growth rates and β-carotene content in D. salina MCCS-001, are associated with the effect of indole-3-acetic acid [56] that could be derived from exogenous glycerol [57], which may support our results in cellular performance.
In most cases of photoautotrophic and mixotrophic cultures of D. salina, cellular density can improve and the content of β-carotene remains low, and vice versa (Table 5). Our culture system is more suitable and efficient for the production of biomass and β-carotene in D. salina compared with the current literature. Sohrabi et al. [20] obtained biomass yields higher than ours; however, their cultivation conditions consisted of 2× concentrations for the glycerol and inocula compared to our experimental approach.

Table 5.
Biomass and β-carotene of D. salina grown with various carbon substrates reported by the literature and in this work.
Until quite recently, it was generally believed that D. salina was an obligate photoautotroph. This assigned nutrition mode limited the development of the great potential of this model alga in many applications that remained unexplored and unexploited. Our results indicated that the synergy of photosynthesis and glycerol uptake under mixotrophic culture conditions induced higher yields of biomass and β-carotene when compared to the photoautrophic and heterotrophic metabolism of D. salina. In this research, glycerol, besides its main role in osmoregulation, plays a strategic role as a metabolic substrate particularly valuable for the profitable production of biomass and carotenoids, mainly β-carotene, at low cost, and opens new avenues for future applications inserting D. salina in the cost-effective production of various bioproducts, bioprocesses, and services. This approach makes mixotrophy in this alga advantageous in many marine, saline, and industrial production processes that are carried out in the presence of high concentrations of NaCl. Particularly, the advantages of this mixotrophy initiative represent a particular interest in halophiles, such as D. salina, for biofuel production in coastal–marine wastewater treatment and bioremediation that, when coupled to the mixotrophic production of low-cost biomass and β-carotene, represent a profitable and affordable approaches leading to biorefineries. The production of high-value nutraceuticals, antioxidants, pro-vitamin A production, food and feed pigments, and wastewater treatment and bioremediation can greatly benefit from this.
4. Materials and Methods
4.1. Strain and Culture Conditions
The Mexican strain, Dunaliella salina BC02 used in this work, was obtained from the microalgae collection of the Department of Marine Biotechnology from CICESE (Ensenada, Mexico). Microalgal cells were grown in 250 mL Erlenmeyer flasks containing 100 mL of inorganic culture medium (IM) designed for Dunaliella [60]. The inoculated culture flasks were placed in an environmental chamber built ex professo both to maintain the temperature of the culture (22 ± 02 °C) [61] and to manage the different light intensities with 40 watts cool white fluorescent lamps, unless otherwise indicated. Photoautotrophic pre-cultures were grown in IM at 50 µmol photons m−2 s−1 light, 1.5 M salinity, pH in the 7.5–8.0 range, and without aeration. Once at exponential phase, inocula precultures (10%) were set up at 0.1 × 106 cells ml−1 for later inoculate each mixotrophic culture for D. salina with glycerol (Gly) and the controls.
4.2. Mixotrophic Growth Conditions Set Up
To improve the β-carotene and microalgal growth, the proposed inorganic medium was optimized to the most suitable conditions of glycerol, light intensity, and salinity for the mixotrophic culture. The proper concentrations of 1.5 and 3.0 M NaCl used in the experimental units served to assess changes in cell growth by salinity according to Farhat et al. [29]. The light intensities were set according to Sforza et al. [62] and were set up as follows: heterotrophy in light (10 µmol photons m−2 s−1), mixotrophy (50 µmol photons m−2 s−1), photoautotrophy (100 µmol photons m−2 s−1), and carotenogenic light (300 µmol photons m−2 s−1) were measured with a light meter (VWR, Catalog Number 21800-014). A Hanna meter (HI98130) recorded the influence of pH on the cell growth in glycerol. The microalgal cultures were gently hand-shaken (two times daily for 2 min) [14], and CO2 unfed. The mixotrophic growth of D. salina was compared only to the photoautotrophic control, because no cellular growth on glycerol was observed under heterotrophic conditions without light. For mixotrophic experiments, glycerol (Sigma Aldrich, Mexico) was added in the inorganic growth medium (IM) to give the following concentrations: 3.12, 6.25, 12.5, 25, 50, and 100 mM, respectively.
4.3. Cell Growth and Dry Cell Weight
The cellular density of D. salina was evaluated by direct counting for each experimental treatment and control by the Neubauer chamber and an Olympus compound microscope model BX60. With the obtained data, a growth curve was made, which exponential phase was selected, in order to calculate the specific growth rate (µ) as suggested [63] from Equation (1):
where N1 and N0 are the final and initial cell density (cells number per mL), respectively; T1 and T0 are the final and initial cultivation time (days).
The initial and final biomass were determined gravimetrically to calculate the dry cell weight as recommended [64]. Aliquots of 10 mL of microalgal culture at each experimental condition were filtered through preweighed, and precombusted (450 °C for two hours) glass-fiber filters (Whatman GF/F, 47 mm, pore size 0.7 µm) using a vacuum pump (at 35 to 55 mm Hg). Then, the filters were washed three times, with 20 mL 0.5 M ammonium formate to remove the remaining salt. Finally, filters were dried and weighted to obtain dry biomass at constant weight.
4.4. Pigment Content
4.4.1. Spectrophotometric Determination
For pigment extraction, 2 mL of microalgal suspension were placed in microtubes and centrifuged at 5000 rpm for 10 min at 4 °C in an Eppendorf refrigerate centrifuge. Then, the culture medium was removed, and 1 mL pure acetone was added to dissolve the residual pellet [65]. The samples were vortexed and stored at 4 °C in the dark for at least 5 h. Afterward, the homogenates were filtered onto 0.2 µm filters (Whatman) and the extracts were immediately used for spectrophotometric measurements. The analysis of chlorophyll a (Chl a), chlorophyll b (Chl b), and β-carotene (BCar) was carried out in a 2 mL quartz cuvette by a Hach DR 5000 spectrophotometer. The scan spectra from 200 to 700 nm, with a resolution of 1.0 nm, were digitally recorded and processed. The Equations (2)–(4) describe the calculated pigment concentrations as previously reported [66]: Later, these values were standardized using the cell concentration (pg cell−1) of each treatment.
Chl a = 11.75A662 − 2.35A645 (in µg mL of extract−1)
Chl b = 18.61A645 − 3.96A662 (in µg mL of extract−1)
4.4.2. HPLC Analysis
Pigment profile analysis and the quantitative estimation of chlorophylls and carotenoids were carried out using an Agilent 1260 HPLC system equipped with a Zorbax XDB-C8 reversed phase column (4.6 × 150 mm, 3.5 μm pore size and 1 mL min−1 flow-rate [67,68]. Pigments were extracted from 1 mL of microalgal culture using 1 mL of 100% acetone. The samples were placed at −20 °C overnight in darkness [68]. Prior to the HPLC analysis, samples were centrifuged at 5000 rpm for 5 min at 4 °C. The supernatant was filtered with 0.22 μm filter into amber vials. The pigments extracted in acetone were directly injected to the HPLC column. The column temperature was set at 60 °C and the injection volume was 20 µL. Mobile phase: solvent A, methanol and 28 mM tetrabutyl ammonium acetate, 70:30 (v:v); solvent B, methanol. Solvent delivery gradient was in % B (min): 5%, 0 min; 5%, 5 min; 95%, 22 min; 95%, 27 min; 5%, 30 min as reported [67]. Pigments were eluted at a flow rate of 1 mL min−1 and detected at an absorbance range of 360–700 nm, specifically at 436 nm to separate properly chlorophylls and carotenoids. The identification of the pigments was based on the comparison of the retention times of samples with those of pure standard pigments obtained from DHI Laboratory Products (Hørsholm, Denmark) as previously described [69].
4.5. Extracellular and Intracellular Glycerol of D. salina
Extra and intracellular glycerol was assayed enzymatically with a commercial glycerol determination kit (SKU, 12812, BioSystems Reagents & Instruments, Barcelona, Spain). For sample preparation, 1 mL aliquots of microalgal culture were placed into microtubes to determine extracellular and total glycerol [70]. A set of tubes was directly centrifuged at 5000 rpm for 15 min at 4 °C and the supernatants used to estimate extracellular glycerol according to manufacturer´s specifications. Other tubes with microalgal culture were incubated for 10 min at 100 °C. After, the homogenates were centrifuged and supernatants were used for total glycerol determination. Intracellular glycerol was calculated from the difference between total glycerol and extracellular glycerol. Glycerol concentrations were normalized to dry cells weight.
4.6. Photosynthetic Efficiency Measurement
The Chl a fluorescence index, represented by the equation: Fv/Fm = (Fm − F)/Fm [46] was applied to assess the changes in photosynthetic activity, viz., the maximum quantum efficiency of PSII in microalgal cells when exposed to glycerol. The Fv/Fm was determined on cell cultures of D. salina exposed to the best treatment (12.5 mM Gly, 50 µmol photons m−2 s−1 and 3.0 M NaCl), DCMU (3-(3,4-dichlorophenyl)-1,1-dimethylurea), and controls, respectively. The registered fluorescence was carried out in samples previously adapted to dim light for 15 min, at 25 °C, using a Pulse-amplitude modulated fluorometer (DUAL-PAM 100, Heinz Walz, Effeltrich, Germany). Triplicate measurements for each sample and five saturating pulses at intervals of 30 s were sufficient to obtain a stationary level of maximum fluorescence (Fm) of D. salina cells. The fluorescence signal was normalized to chlorophyll a content of the microalgal cells as recommended [46]. To evaluate the changes on PSII activity by glycerol, intact cells of D. salina were exposed to 20 mM DCMU [71].
4.7. Yield Parameters
Representative parameters of microalgal yield, such as: biomass/substrate yield (Yx/s) (g g−1) and β-carotene/substrate yield (Yp/s) (mg g−1) were calculated from Equations (5) and (6) as proposed [72]. The biomass productivity was calculated at maximum microalgal growth (seventh day), as follows:
4.8. Statistical Analysis
All experimental designs accomplished the criteria of randomness to ensure regular and reproducible results as suggested [73]. The data correspond to the mean ± standard deviation from three independent cultures. One-way analysis of variance (ANOVA) at p-value < 0.05 was calculated to detect significant differences in microalgal growth and pigments under different concentrations of glycerol using the STATISTICA 12.0 program. A Tukey’s test was applied to assess differences among treatments means. The combined effect among glycerol concentration, salinity, and light intensity on cell growth, pigments, and culture pH were pondered by a factorial ANOVA test.
5. Conclusions
The photosystems of D. salina can choose other options than obligate photoautotrophy, viz., facultative mixotrophy. This microalga possesses the physiometabolic and productive capacity to assimilate flexible and highly reduced organic substrates as glycerol under optimized culture conditions. This condition induced by glycerol, light, and salinity serve to maximize β-carotene accumulation without compromising the high biomass production through dichotomous role of glycerol in photosynthesis and respiration under mixotrophy. In particular, the uptake of glycerol by D. salina represents more energy and carbon to promote growth, carotenoid synthesis, and carotenogenesis. This optimized mixotrophic culture system could be expanded to transfer strains and substrates, and increase production yields in a low-cost and sustainable way. In this research, glycerol, besides its main role in osmoregulation, plays a strategic role as a metabolic substrate in this microalga, and is particularly valuable for the profitable and affordable production of biomass and carotenoids, mainly β-carotene. It also opens new avenues for future applications inserting D. salina in the cost-effective production of various bioproducts, bioprocesses, and services. The synergy of photosynthesis and glycerol metabolism makes mixotrophy in this alga advantageous in many marine, saline, and industrial production processes that are carried out in the presence of high concentrations of NaCl. These advantages are of particular interest in halophiles, such as D. salina, for biofuel production in coastal–marine wastewater treatment and bioremediation that, when coupled to the mixotrophic production of biomass and B-carotene, represent profitable and affordable approaches. Particularly, this research may greatly impact the production of high-value nutraceuticals, antioxidants, pro-vitamin A production, the concept of marine algal biofuels and biorefineries, and wastewater treatment and bioremediation.
Author Contributions
Investigation, W.C.-R. and J.d.J.P.-M.; methodology, E.G.-M.; writing—original draft, W.C.-R. and J.d.J.P.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by CICESE internal funding 682101. The APC was funded in part by CICESE funding number 682101.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cezare-Gomes, E.A.; Mejia-da-Silva, L.; Pérez-Mora, L.S.; Matsudo, M.; Ferreira-Camargo, L.; Singh, A.; Monteiro de Carvalho, J.C. Potential of microalgae carotenoids for industrial application. Appl. Biochem. Biotech. 2019, 188, 602–634. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Z.; Jiang, H.; Mao, X. Biotechnology advances in β-carotene production by microorganisms. Trends Food Sci. Technol. 2021, 111, 322–332. [Google Scholar] [CrossRef]
- Mehariya, S.; Goswami, R.K.; Karthikeysan, O.P.; Verma, P. Microalgae for high-value products: A way towards green nutraceutical and pharmaceutical compounds. Chemosphere. 2021, 280, 130553. [Google Scholar] [CrossRef]
- Molino, A.; Mehariya, S.; Di Sanzo, G.; Larocca, V.; Martino, M.; Leone, G.P.; Marino, T.; Chianese, S.; Balducchi, R.; Musmarra, D. Recent developments in supercritical fluid extraction of bioactive compounds from microalgae: Role of key parameters, technological achievements and challenges. J. CO2 Util. 2020, 36, 196–209. [Google Scholar] [CrossRef]
- Ejike, E.C.C.; Collins, S.A.; Balasuriya, N.; Swanson, A.K.; Mason, B.; Udenigwe, C.C. Prospects of microalgae proteins in producing peptide-based functional foods for promoting cardiovascular health. Trends Food Sci. Technol. 2017, 59, 30–36. [Google Scholar] [CrossRef]
- Monte, J.; Ribeiro, C.; Parreira, C.; Costa, L.; Brive, L.; Casal, S.; Brazinha, C.; Crespo, J.G. Biorefinery of Dunaliella salina: Sustainable recovery of carotenoids, polar lipids and glycerol. Bioresour. Technol. 2020, 297, 122509. [Google Scholar] [CrossRef]
- Patel, A.K.; Singhania, R.R.; Sim, S.J.; Di Dong, C. Recent advancements in mixotrophic bioprocessing for production of high value microalgal products. Bioresour. Technol. 2021, 320, 124421. [Google Scholar] [CrossRef] [PubMed]
- Poddar, N.; Sen, R.; Martin, G. Glycerol and nitrate utilisation by marine microalgae Nannochloropsis salina and Chlorella sp. and associated bacteria during mixotrophic and heterotrophic growth. Algal Res. 2018, 33, 298–309. [Google Scholar] [CrossRef]
- Sun, H.; Zhao, W.; Mao, X.; Li, Y.; Wu, T.; Chen, F. High-value biomass from microalgae production platforms: Strategies and progress based on carbon metabolism and energy conversion. Biotechnol. Biofuels 2018, 11, 227. [Google Scholar] [CrossRef] [Green Version]
- Chavoshi, Z.Z.; Shariati, M. Lipid production in Dunaliella salina under autotrophic, heterotrophic, and mixotrophic conditions. Biologia 2019, 74, 1579–1590. [Google Scholar] [CrossRef]
- Gonabadi, E.; Samadlouie, H.R.; Zenoozian, M.S. Optimization of culture conditions for enhanced Dunaliella salina productions in mixotrophic culture. Prep. Biochem. Biotechnol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Morowvat, M.; Ghasemi, Y. Culture medium optimization for enhanced β-carotene and biomass production by Dunaliella salina in mixotrophic culture. Biocatal. Agric. Biotechnol. 2016, 7, 217–223. [Google Scholar] [CrossRef]
- Mojaat, M.; Pruvost, J.; Foucault, A.; Legrand, J. Effect of organic carbon sources and Fe2+ ions on growth and β-carotene accumulation by Dunaliella salina. Biochem. Eng. J. 2008, 39, 177–184. [Google Scholar] [CrossRef] [Green Version]
- Keerthi, S.; Koduru, U.; Sarma, N. A nutrient medium for development of cell dense inoculum in mixotrophic mode to seed mass culture units of Dunaliella salina. Algol. Stud. 2015, 147, 7–28. [Google Scholar] [CrossRef]
- Liu, X.; Duan, S.; Li, A.; Xu, N.; Cai, Z.; Hu, Z. Effects of organic carbon sources on growth, photosynthesis, and respiration of Phaeodactylum tricornutum. J. Appl. Phycol. 2009, 21, 239–246. [Google Scholar] [CrossRef]
- Kim, H.S.; Park, W.K.; Lee, B.; Seon, G.; Suh, W.I.; Moon, M.; Chang, Y.K. Optimization of heterotrophic cultivation of Chlorella sp. HS2 using screening, statistical assessment, and validation. Sci. Rep. 2019, 9, 19383. [Google Scholar] [CrossRef] [Green Version]
- Chalima, A.; Oliver, L.; Fernández de Castro, L.; Karnaouri, A.; Dietrich, T.; Topakas, A. Utilization of Volatile Fatty Acids from Microalgae for the Production of High Added Value Compounds. Fermentation 2017, 3, 54. [Google Scholar] [CrossRef] [Green Version]
- Baldisserotto, C.; Sabia, A.; Guerrini, A.; Demaria, S.; Maglie, M.; Ferroni, L.; Pancaldi, S. Mixotrophic cultivation of Thalassiosira pseudonana with pure and crude glycerol: Impact on lipid profile. Algal Res. 2021, 54, 102194. [Google Scholar] [CrossRef]
- Rajendran, L.; Nagarajan, N.G.; Karuppan, M. Enhanced biomass and lutein production by mixotrophic cultivation of Scenedesmus sp. using crude glycerol in an airlift photobioreactor. Biochem. Eng. J. 2020, 161, 107684. [Google Scholar] [CrossRef]
- Sohrabi, D.; Jazini, M.H.; Shariati, M. Mixotrophic cultivation of Dunaliella salina on crude glycerol obtained from calcinated fatty acid production process. Russ. J. Mar. Biol. 2019, 45, 470–480. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, C.; Liu, J.; Yang, N. A strategy for stimulating astaxanthin and lipid production in Haematococcus pluvialis by exogenous glycerol application under low light. Algal Res. 2020, 46, 101779. [Google Scholar] [CrossRef]
- Clomburg, J.M.; Gonzalez, R. Anaerobic fermentation of glycerol: A platform for renewable fuels and chemicals. Trends Biotechnol. 2013, 31, 20–28. [Google Scholar] [CrossRef] [Green Version]
- Taghavi, N.; Robinson, G. Improving the optimum yield and growth of Chlamydomonas reinhardtii CC125 and CW15 using various carbon sources and growth regimes. Afr. J. Biotechnol. 2016, 15, 1083–1100. [Google Scholar]
- Bindea, M.; Rusu, B.; Rusu, A.; Trif, M.; Leopold, L.F.; Dulf, F.; Vodnar, D.C. Valorification of crude glycerol for pure fractions of docosahexaenoic acid and β-carotene production by using Schizochytrium limacinum and Blakeslea trispora. Microb. Cell Fact. 2018, 17, 97. [Google Scholar] [CrossRef]
- Borowitzka, M.; Borowitzka, L. Dunaliella. In Micro-Algal Biotechnology; Borowitzka, M., Borowitzka, L., Eds.; Cambridge University Press: New York, NY, USA, 1988; pp. 27–58. [Google Scholar]
- Chen, H.; Jiang, J. Osmotic Responses of Dunaliella to the Changes of Salinity. J. Cell. Physiol. 2009, 219, 251–258. [Google Scholar] [CrossRef]
- D’Imporzano, G.; Salati, S.; Veronesi, D.; Scaglia, B.; Adani, F. Microalgae Mixotrophic Growth: Opportunity for Stream Depuration and Carbon Recovery. In Prospects and Challenges in Algal Biotechnology; Tripathi, B.N., Kumar, D., Eds.; Springer Nature: Singapore, 2017; pp. 141–177. [Google Scholar]
- Suarez, G.; Romero, T.; Borowitzka, M. Cultivo de Dunaliella salina en medio orgánico. Boletín Cent. Investig. Biológicas Maracaibo 1999, 33, 211–225. [Google Scholar]
- Farhat, N.; Rabhi, M.; Falleh, H.; Jouini, J.; Abdelly, C.; Smaoui, A. Optimization of salt concentrations for a higher carotenoid production in Dunaliella salina (chlorophyceae). J. Phycol. 2011, 47, 1072–1077. [Google Scholar] [CrossRef]
- Guevara, M.; Lodeiros, C.; Gómez, O.; Lemus, N.; Núñez, P.; Romero, L.; Vásquez, A.; Rosales, N. Carotenogénesis de cinco cepas del alga Dunaliella sp. (Chlorophyceae) aisladas de lagunas hipersalinas de Venezuela. Rev. Biol. Trop. 2005, 53, 331–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grama, B.S.; Agathos, S.N.; Jeffryes, C.S. Balancing photosynthesis and respiration increase microalgal biomass productivity during photoheterotrophy on glycerol. ACS Sustain. Chem. Eng. 2016, 4, 1611–1618. [Google Scholar] [CrossRef]
- Cecchin, M.; Benfatto, B.; Griggio, F.; Mori, A.; Cazzaniga, S.; Vitulo, N.; Delledonne, M.; Ballottari, M. Molecular basis of autotrophic vs mixotrophic growth in Chlorella sorokiniana. Sci. Rep. 2018, 8, 6465. [Google Scholar] [CrossRef]
- Marchello, A.E.; Dos Santos, A.C.; Lombardi, A.T.; Oliveira de Souza, C.W.; Montanhim, G.C. Physiological and ecological aspects of Chlorella sorokiniana (Trebouxiophyceae) under photoautotrophic and mixotrophic conditions. Microb. Ecol. 2018, 76, 791–800. [Google Scholar] [CrossRef]
- Abiusi, F.; Wijffels, R.H.; Janssen, M. Doubling of microalgae productivity by oxygen balanced mixotrophy. ACS Sustain. Chem. Eng. 2020, 8, 6065–6074. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.K.P.; Hughes, A.D.; McEvoy, L.; Thornton, B.; Day, J.G. The carbon partitioning of glucose and DIC in mixotrophic, heterotrophic and photoautotrophic cultures of Tetraselmis suecica. Biotechnol. Lett. 2021, 43, 729–743. [Google Scholar] [CrossRef] [PubMed]
- Pérez-García, O.; Bashan, Y. Microalgal heterotrophic and mixotrophic culturing for bio-refining: From metabolic routes to techno-economics. In Algal Biorefineries: Products and Refinery Design; Prokop, A., Bajpai, R., Zappi, M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 61–131. [Google Scholar]
- Lin, H.; Fang, L.; Low, C.S.; Chow, Y.; Lee, Y.K. Occurrence of glycerol uptake in Dunaliella tertiolecta under hyperosmotic stress. FEBS J. 2013, 280, 1064–1072. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, T.; Mori, N.; Sato, N. Activation of oxidative carbon metabolism by nutritional enrichment by photosynthesis and exogenous organic compounds in the red alga Cyanidioschyzon merolae: Evidence for heterotrophic growth. SpringerPlus 2015, 4, 559. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, A.; Schreiber, U.; Avron, M. Salt-induced metabolic changes in Dunaliella salina. Plant Physiol. 1980, 65, 810–813. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Jiang, J.; Wu, G. Effects of salinity changes on the growth of Dunaliella salina and its isozyme activities of Glycerol-3-phosphate dehydrogenase. J. Agric. Food Chem. 2009, 57, 6178–6182. [Google Scholar] [CrossRef]
- Yazdani, S.S.; González, R. Anaerobic fermentation of glycerol: A path to economic viability for the biofuels industry. Curr. Opin. Biotechnol. 2007, 18, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhuge, J.; Fang, H.; Prior, B.A. Glycerol production by microbial fermentation: A review. Biotechnol. Adv. 2001, 19, 201–223. [Google Scholar] [CrossRef]
- Han, D.; Li, Y.; Hu, Q. Astaxanthin in microalgae: Pathways, functions and biotechnological implications. Algae 2013, 28, 131–147. [Google Scholar] [CrossRef]
- Cheah, W.Y.; Show, P.L.; Juan, J.C.; Chang, J.; Ling, T.C. Enhancing biomass and lipid productions of microalgae in palm oil mil effluent using carbon and nutrient supplementation. Energy Convers. Manag. 2018, 164, 188–197. [Google Scholar] [CrossRef]
- Bardavid, R.E.; Khristo, P.; Oren, A. Interrelationships between Dunaliella and halophilic prokaryotes in saltern crystallizer ponds. Extremophiles 2008, 12, 5–14. [Google Scholar] [CrossRef]
- White, S.; Anandraj, A.; Bux, F. PAM fluorometry as a tool to assess microalgal nutrient stress and monitor cellular neutral lipids. Bioresour. Technol. 2011, 102, 1675–1682. [Google Scholar] [CrossRef]
- Xu, Y.; Ibrahim, I.M.; Harvey, P.J. The influence of photoperiod and light intensity on the growth and photosynthesis of Dunaliella salina (chlorophyta) CCAP 19/30. Plant Physiol. Biochem. 2016, 106, 305–315. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zhang, M.M.; Liu, S.F.; Xu, R.L.; Mou, J.H.; Qin, Z.H.; Zhou, Z.G.; Li, H.Y.; Lin, C.S.; Sun, Z. Synergistic bioconversion of lipids and carotenoids from food waste by Dunaliella salina with fulvic acid via a two-stage cultivation strategy. Energy Convers. Manag. 2021, 234, 113908. [Google Scholar] [CrossRef]
- Xu, S.; Elsayed, M.; Ismail, G.A.; Li, C.; Wang, S.; Abomohra, A.E. Evaluation of bioethanol and biodiesel production from Scenedesmus obliquus grown in biodiesel waste glycerol: A sequential integrated route for enhanced energy recovery. Energy Convers. Manag. 2019, 197, 111907. [Google Scholar] [CrossRef]
- Tan, K.W.; Lee, Y.K. The dilemma for lipid productivity in green microalgae: Importance of substrate provision in improving oil yield without sacrificing growth. Biotechnol Biofuels 2016, 9, 255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bashir, K.M.I.; Mansoor, S.; Kim, N.R.; Grohmann, F.R.; Shah, A.A.; Cho, M.G. Effect of organic carbon sources and environmental factors on cell growth and lipid content of Pavlova lutheri. Ann. Microbiol. 2019, 69, 353–368. [Google Scholar] [CrossRef]
- Guedes, A.C.; Amaro, H.M.; Malcata, F.X. Microalgae as Sources of Carotenoids. Mar. Drugs 2011, 9, 625–644. [Google Scholar] [CrossRef]
- Cuaresma, M.; Casal, C.; Forján, E.; Vílchez, C. Productivity and selective accumulation of carotenoids of the novel extremophile microalga Chlamydomonas acidophila grown with different carbon sources in batch systems. J. Ind. Microbiol. Biotechnol. 2011, 38, 167–177. [Google Scholar] [CrossRef] [Green Version]
- Mantzouridou, F.; Naziri, E.; Tsimidou, M.Z. Industrial glycerol as a supplementary carbon source in the production of beta-carotene by Blakeslea trispora. J. Agric. Food Chem. 2008, 56, 2668–2675. [Google Scholar] [CrossRef]
- Lao, Y.M.; Xiao, L.; Luo, L.X.; Jiang, J.G. Hypoosmotic expression of Dunaliella bardawil ζ-carotene desaturase is attributed to a hypoosmolarity-responsive element different from other key carotenogenic genes. Plant Physiol. 2014, 165, 359–372. [Google Scholar] [CrossRef] [Green Version]
- Mousavi, P.; Morowvat, M.H.; Montazeri-Najafabady, N.; Abolhassanzadeh, Z.; Mohagheghzadeh, A.; Hamidi, M.; Niazi, A.; Ghasemi, Y. Investigating the effects of phytohormones on growth and β-carotene production in a naturally isolates stain of Dunaliella salina. J. Appl. Pharm. Sci. 2016, 6, 164–171. [Google Scholar] [CrossRef] [Green Version]
- Kasahara, H. Current aspects of auxin biosynthesis in plants. Biosci. Biotechnol. Biochem. 2016, 80, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes, A.S.; Gonzalez, M.; Conejeros, M.; Dellarossa, V.; Parra, O. Growth and carotenogenesis in eight strains of Dunaliella salina Teodoresco from Chile. J. Appl. Phycol. 1992, 4, 111–118. [Google Scholar] [CrossRef]
- Pourkarimi, S.; Hallajisani, A.; Alizadehdakhel, A.; Nouralishahi, A.; Golzary, A. Factors affecting production of beta-carotene from Dunaliella salina microalgae. Biocatal. Agric. Biotechnol. 2020, 29, 101771. [Google Scholar] [CrossRef]
- Ben-Amotz, A.; Avron, M. On the factors which determine massive β-carotene accumulation in the halotolerant alga Dunaliella bardawil. Plant Physiol. 1983, 72, 593–597. [Google Scholar] [CrossRef] [Green Version]
- Imamoglu, E.; Demirel, Z.; Dalay, M.C. Evaluation of culture conditions of locally isolated Dunaliella salina strain EgeMacc-024. Biochem. Eng. J. 2014, 92, 22–27. [Google Scholar] [CrossRef]
- Sforza, E.; Pastore, M.; Barbera, E.; Bertucco, A. Respirometry as a tool to quantify kinetic parameters of microalgal mixotrophic growth. Bioprocess Biosyst. Eng. 2019, 42, 839–851. [Google Scholar] [CrossRef]
- Guillard, R.R.L. Division rates. In Handbook of Phycological Methods. Culture Methods and Growth Measurements; Stein, J.R., Ed.; Cambridge University Press: Cambridge, UK, 1973; pp. 289–313. [Google Scholar]
- Zhu, C.J.; Lee, Y.K. Determination of biomass dry weight of marine microalgae. J. Appl. Phycol. 1997, 9, 189–194. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determination of total carotenoids and chlorophylls a and b of leaf in different solvents. Biochem. Soc. Trans. 1985, 11, 591–592. [Google Scholar] [CrossRef] [Green Version]
- Van Heukelem, L.; Thomas, C.S. Computer-assisted high-performance liquid chromatography meted development with applications to the isolation and analysis of phytoplankton pigments. J. Chromatogr. 2001, 910, 31–49. [Google Scholar] [CrossRef]
- Almazán-Becerril, A.; García-Mendoza, E. Maximum efficiency of charge separation of photosystem II of the phytoplankton community in the Eastern Tropical North Pacific of Mexico: A nutrient stress diagnostic tool? Cienc. Mar. 2008, 34, 29–43. [Google Scholar] [CrossRef]
- Wright, S.; Montoura, R.F.C. Guidelines for collection and pigment analysis of marine samples. In Phytoplankton Pigments in Oceanography: Guidelines to Modern Methods; Jeffrey, S.W., Mantoura, R.F.C., Wright, S., Eds.; UNESCO Publishing: Paris, France, 1997; pp. 429–445. [Google Scholar]
- Petrovic, U.; Plemenitas, A. Determination of intra-and extracellular concentration of glycerol in the halophilic black yeast Hortaea werneckii grown at different environmental salinities. In Non-Conventional Yeasts in Genetics, Biochemistry and Biotechnology; Wolf, K., Breunig, K., Barth, G., Eds.; Springer: New York, NY, USA, 2003; pp. 127–130. [Google Scholar]
- Guenther, J.E.; Melis, A. Dynamics of Photosystem II heterogeneity in Dunaliella salina (green algae). Photosynth. Res. 1990, 23, 195–203. [Google Scholar] [CrossRef]
- Gupta, S.; Pawar, S.B. Strategic mixed substrate cultivation of microalgae: Productivity, respiration, yield, and lipid quality. J. Appl. Phycol. 2019, 31, 1573–1588. [Google Scholar] [CrossRef]
- Montgomery, D. Diseño y Análisis de Experimentos; LIMUSA Wiley: Mexico, D.F., Mexico, 2004; p. 692. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).