Dysphania ambrosioides as a Source of Antioxidant Candidates for Benign Prostatic Hyperplasia (BPH) and Prostatitis: A Critical Review and In Silico Prioritisation
Abstract
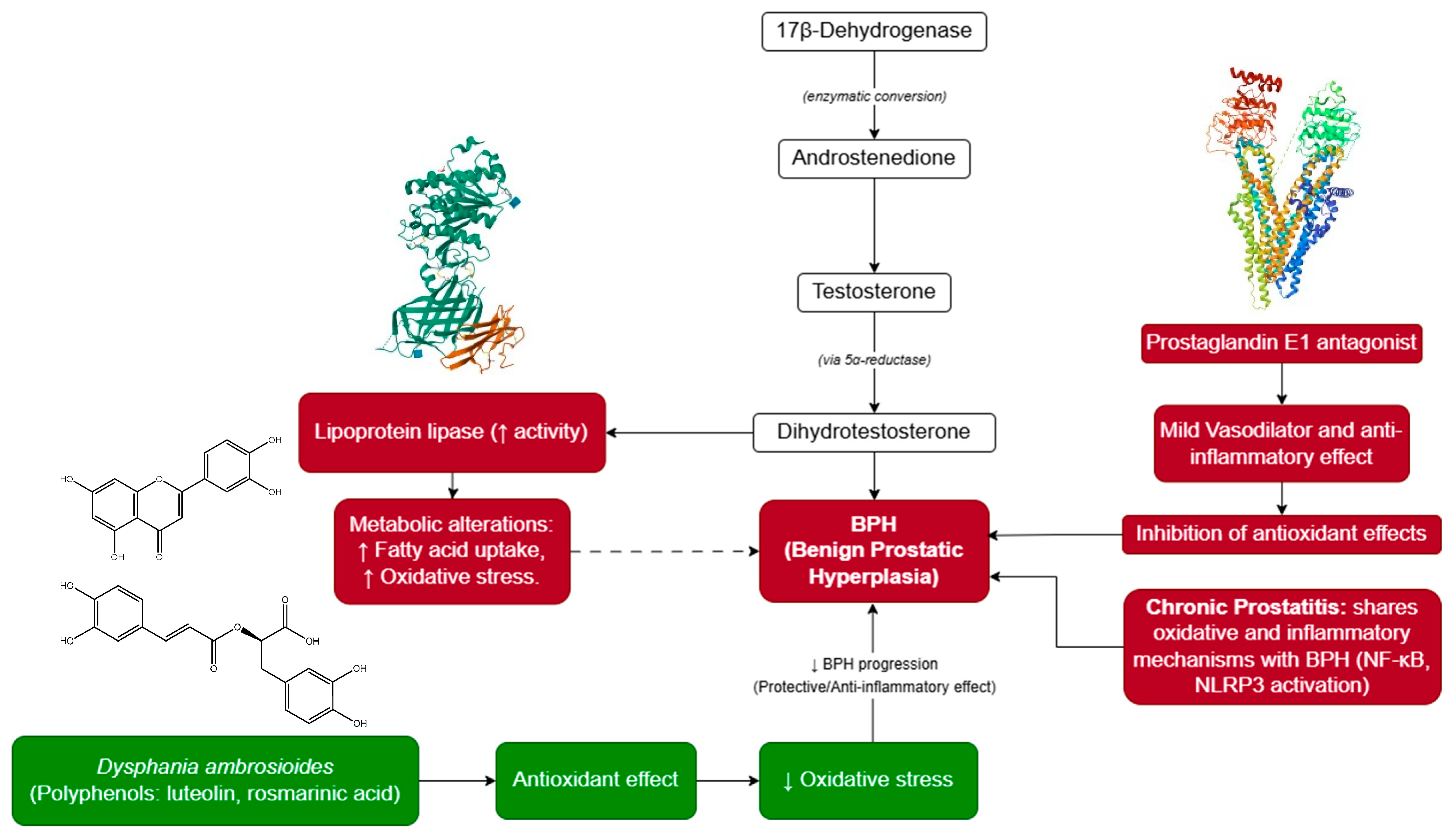
1. Introduction
2. Materials and Methods
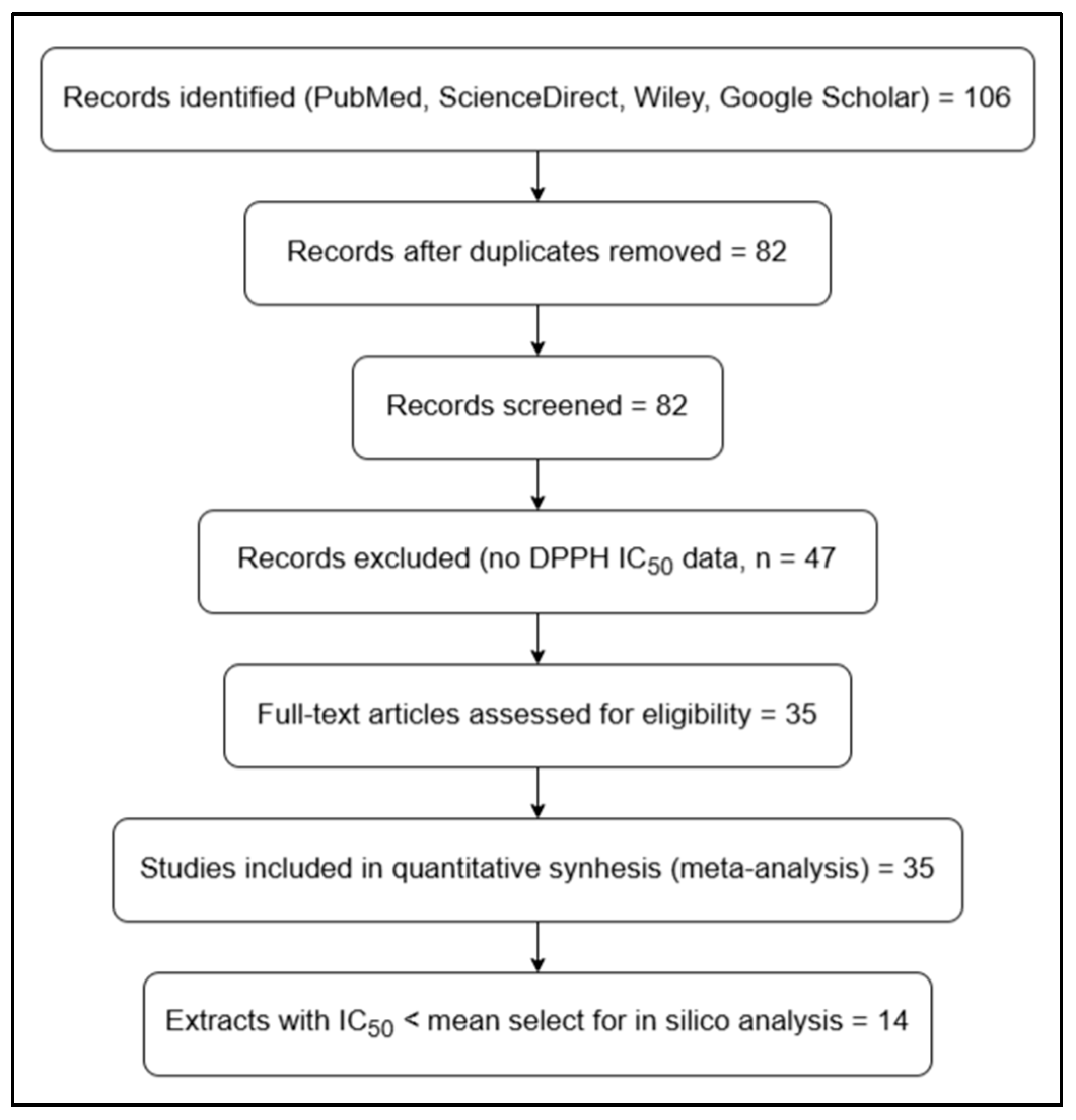
2.1. Search Strategy and Comparative Quantitative Analysis
2.2. Data Processing and Statistical Analysis
2.3. Retrieval and Standardisation of Chemical Structures
2.4. In Silico Prioritisation of Metabolites
2.5. Ligand Preparation
2.6. Target Protein Selection and Preparation
2.7. Molecular Docking Using CB-Dock2
2.8. Interaction Analysis
2.9. Statistical Analysis
3. Results
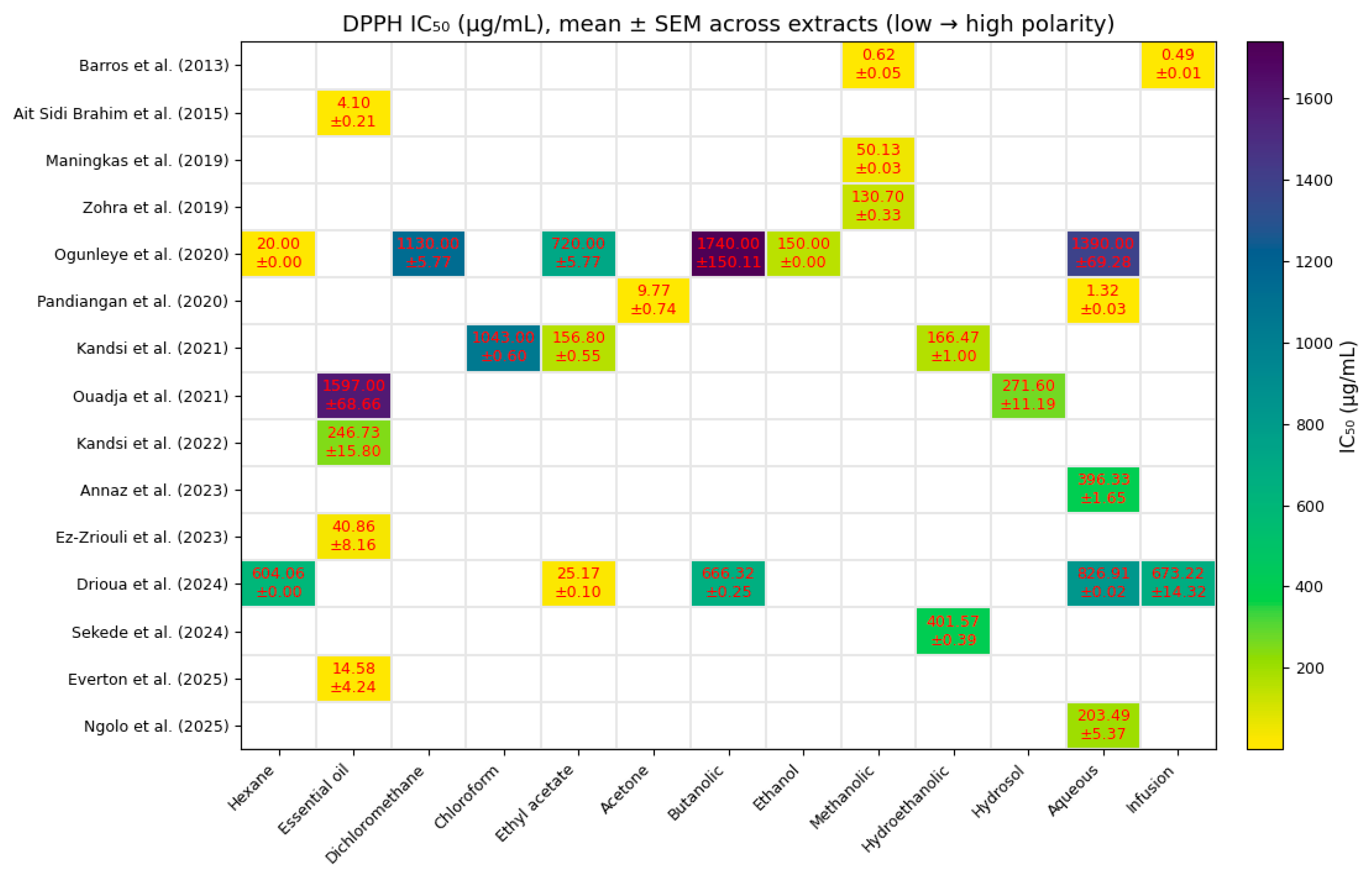
3.1. Comparative Quantitative Analysis of Antioxidant Activity
3.2. Pharmacoinformatic Evaluation and Prioritisation of Metabolites
- High probability of biological activity (Pa > 0.6 in PASS Online);
- Absence of significant toxicological alerts (green classification in OSIRIS);
- Favourable hepatic and renal safety predictions in ProTox 3.0.
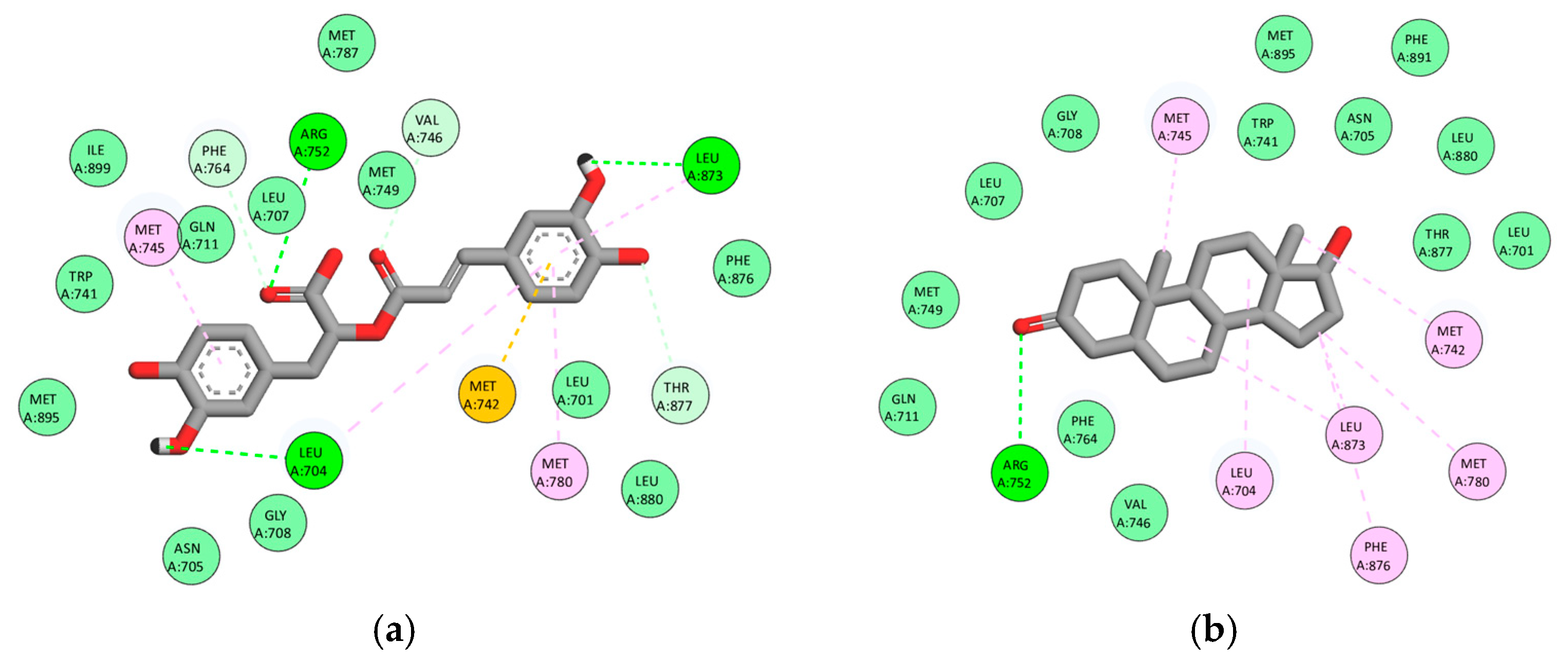
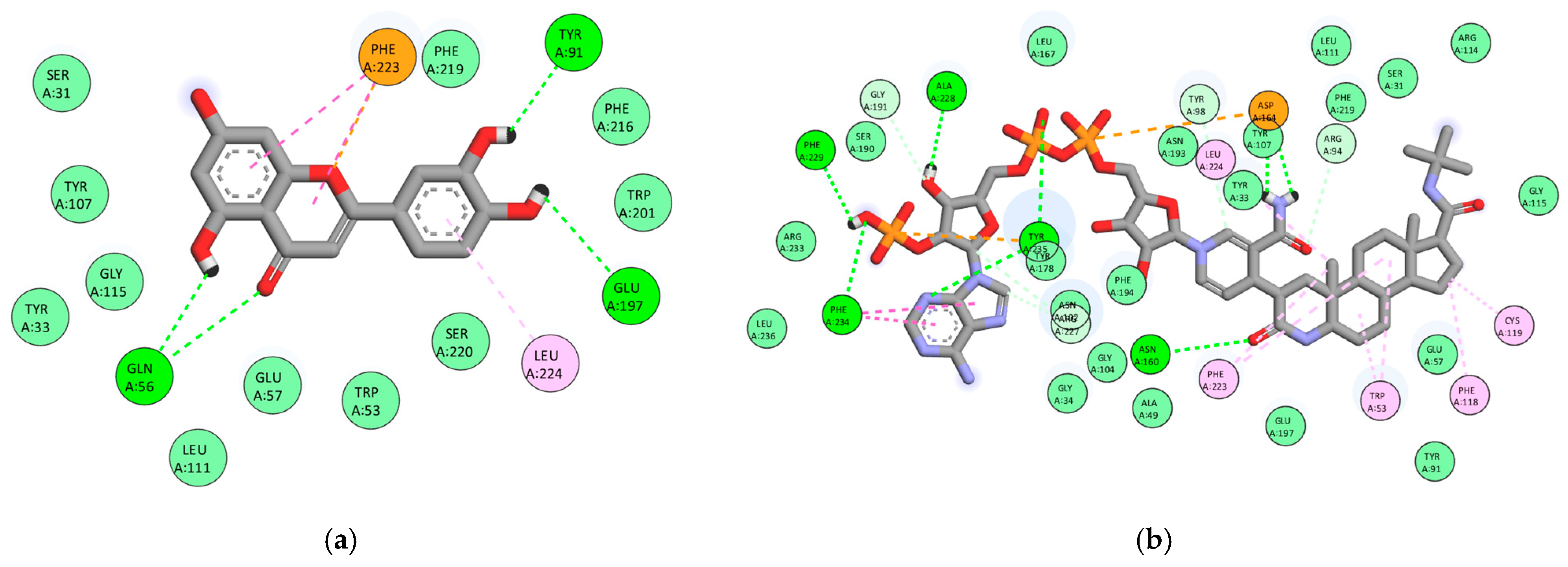
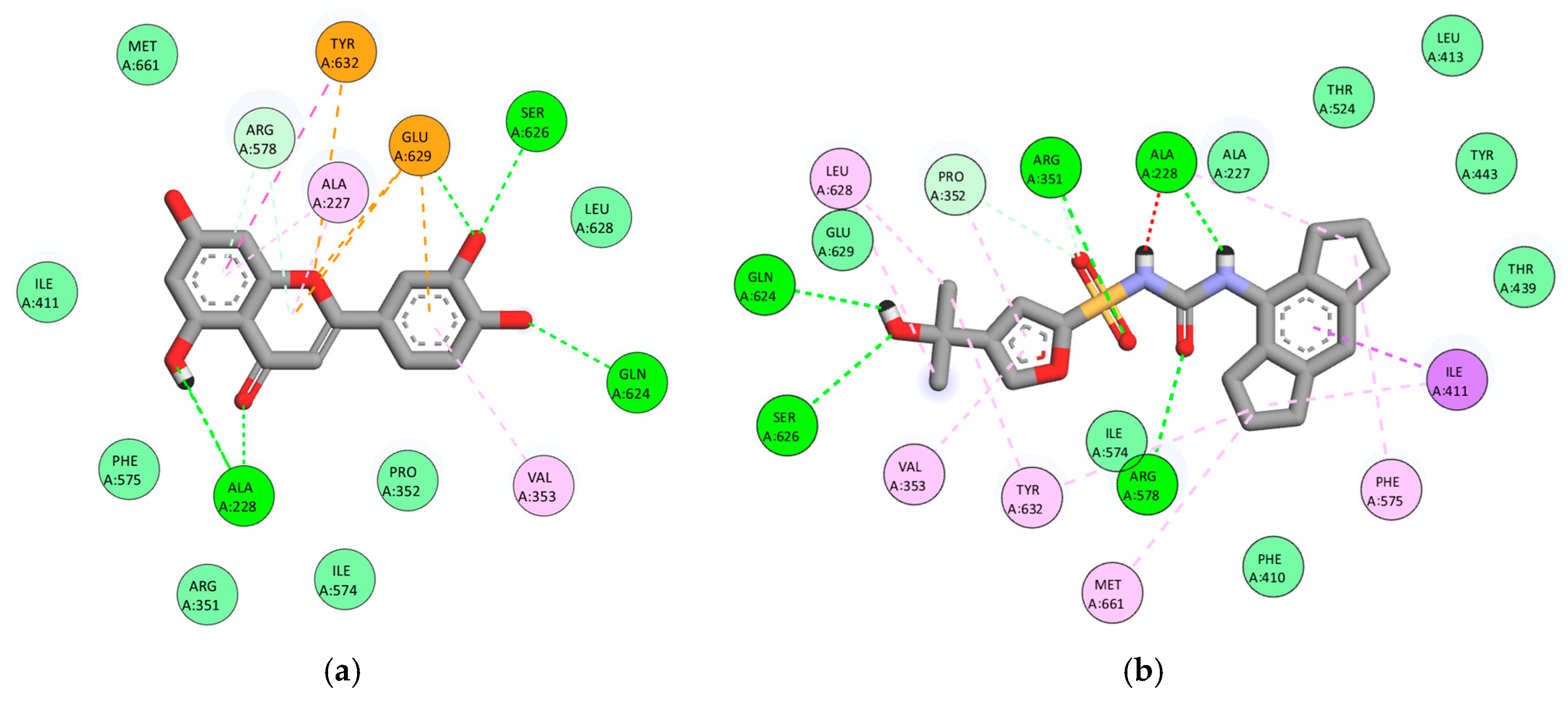
3.3. Multitarget Molecular Docking
3.3.1. Androgen Receptor (AR, PDB: 2AM9)
3.3.2. Steroid 5α-Reductase Type 2 (5AR2, PDB: 7BW1)
3.3.3. Cyclooxygenase-2 (COX-2, PDB: 3LN1)
3.3.4. NLRP3 (PDB: 7ALV)
3.3.5. α1A-Adrenergic Receptor (PDB: 7YMJ)
3.4. Global Comparison of Binding Affinities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garraway, W.M.; Collins, G.N.; Lee, R.J. High Prevalence of Benign Prostatic Hypertrophy in the Community. Lancet 1991, 338, 469–471. [Google Scholar] [CrossRef]
- Kaltsas, A.; Giannakas, T.; Stavropoulos, M.; Kratiras, Z.; Chrisofos, M. Oxidative Stress in Benign Prostatic Hyperplasia: Mechanisms, Clinical Relevance and Therapeutic Perspectives. Diseases 2025, 13, 53. [Google Scholar] [CrossRef]
- Choi, Y.-J.; Wedamulla, N.E.; Kim, S.-H.; Oh, M.; Seo, K.S.; Han, J.S.; Lee, E.J.; Park, Y.H.; Park, Y.J.; Kim, E.-K. Salvia Miltiorrhiza Bunge Ameliorates Benign Prostatic Hyperplasia through Regulation of Oxidative Stress via Nrf-2/HO-1 Activation. J. Microbiol. Biotechnol. 2024, 34, 1059–1072. [Google Scholar] [CrossRef]
- El-Sherbiny, M.; El-Shafey, M.; El-din El-Agawy, M.S.; Mohamed, A.S.; Eisa, N.H.; Elsherbiny, N.M. Diacerein Ameliorates Testosterone-Induced Benign Prostatic Hyperplasia in Rats: Effect on Oxidative Stress, Inflammation and Apoptosis. Int. Immunopharmacol. 2021, 100, 108082. [Google Scholar] [CrossRef] [PubMed]
- Kuzmenko, A.V.; Gyaurgiev, T.A.; Kuzmenko, V.V.; Kuzmenko, G.A. The use of antioxidants in combination therapy of chronic prostatitis. Urologiia 2024, 1, 162–167. [Google Scholar] [CrossRef]
- Pereira, W.S.; Ribeiro, B.P.; Sousa, A.I.P.; Serra, I.C.P.B.; Mattar, N.S.; Fortes, T.S.; Reis, A.S.; Silva, L.A.; Barroqueiro, E.S.B.; Guerra, R.N.M.; et al. Evaluation of the Subchronic Toxicity of Oral Treatment with Chenopodium ambrosioides in Mice. J. Ethnopharmacol. 2010, 127, 602–605. [Google Scholar] [CrossRef]
- Rain-Tree Nutrition, Inc. Epazote (Dysphania ambrosioides)—Worldwide Ethnomedical Uses; Rain-Tree Nutrition, Inc.: Carson City, NV, USA, 2025; Available online: https://www.rain-tree.com/epazote.htm (accessed on 3 October 2025).
- Lavisiony, H.; Giovanni, D.; Kenjiro, T.; Carlos, C.; Sahamastuti, A.A.T. Review of Botany, Phytochemical, and Pharmacological Effects of Dysphania ambrosioides. Indones. J. Life Sci. 2020, 2, 70–82. [Google Scholar] [CrossRef]
- Kandsi, F.; Lafdil, F.Z.; El Hachlafi, N.; Jeddi, M.; Bouslamti, M.; El Fadili, M.; Seddoqi, S.; Gseyra, N. Dysphania ambrosioides (L.) Mosyakin and Clemants: Bridging Traditional Knowledge, Photochemistry, Preclinical Investigations, and Toxicological Validation for Health Benefits. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 397, 969–1001. [Google Scholar] [CrossRef]
- Pandiangan, D.; Taghulihi, Y.; Nainggolan, N.; Yordan, G.; Nainggolan, I.C.; Nainggolan, E.A.; Tampubolon, P.Y.; Gobel, A.R.V.; Nainggolan, V.P.G.; Maliangkay, H.P.; et al. Pasote Plant Tea Bags (Dysphania ambrosioides L.) Have Kidney Creatinine Level Reduction Activity. AIP Conf. Proc. 2023, 2694, 050006. [Google Scholar] [CrossRef]
- Metri, N.A.; Mandl, A.; Paller, C.J. Harnessing Nature’s Therapeutic Potential: A Review of Natural Products in Prostate Cancer Management. Urol. Oncol. Semin. Orig. Investig. 2025, 43, 221–243. [Google Scholar] [CrossRef]
- Marvin, version 25.3.4; Marvin Was Used for Drawing Substructures; Chemaxon: Budapest, Hungary, 2025. Available online: https://www.chemaxon.com (accessed on 3 October 2025).
- Filimonov, D.A.; Lagunin, A.A.; Gloriozova, T.A.; Rudik, A.V.; Druzhilovskii, D.S.; Pogodin, P.V.; Poroikov, V.V. Prediction of the Biological Activity Spectra of Organic Compounds Using the PASS Online Web Resource. Chem. Heterocycl. Compd. 2014, 50, 444–457. [Google Scholar] [CrossRef]
- Banerjee, P.; Eckert, A.O.; Schrey, A.K.; Preissner, R. ProTox-II: A Webserver for the Prediction of Toxicity of Chemicals. Nucleic Acids Res. 2018, 46, W257–W263. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An Advanced Semantic Chemical Editor, Visualization, and Analysis Platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef]
- Liu, Y.; Grimm, M.; Dai, W.; He, X.; He, C.; Wang, S.; Zhang, Y. CB-Dock2: Improved Protein-Ligand Blind Docking by Integrating Cavity Detection, Docking and Homologous Template Fitting. Nucleic Acids Res. 2022, 50, W159–W164. [Google Scholar] [CrossRef]
- Barros, L.; Pereira, E.; Calhelha, R.C.; Dueñas, M.; Carvalho, A.M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Bioactivity and Chemical Characterization in Hydrophilic and Lipophilic Compounds of Chenopodium ambrosioides L. J. Funct. Foods 2013, 5, 1732–1740. [Google Scholar] [CrossRef]
- Ait Sidi Brahim, M.; Fadli, M.; Hassani, L.; Boulay, B.; Markouk, M.; Bekkouche, K.; Abbad, A.; Ait Ali, M.; Larhsini, M. Chenopodium ambrosioides var. ambrosioides Used in Moroccan Traditional Medicine Can Enhance the Antimicrobial Activity of Conventional Antibiotics. Ind. Crops Prod. 2015, 71, 37–43. [Google Scholar] [CrossRef]
- Zohra, T.; Ovais, M.; Khalil, A.T.; Qasim, M.; Ayaz, M.; Shinwari, Z.K. Extraction Optimization, Total Phenolic, Flavonoid Contents, HPLC-DAD Analysis and Diverse Pharmacological Evaluations of Dysphania ambrosioides (L.) Mosyakin & Clemants. Nat. Prod. Res. 2019, 33, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Ogunleye, G.S.; Fagbohun, O.F.; Babalola, O.O. Chenopodium ambrosioides var. ambrosioides Leaf Extracts Possess Regenerative and Ameliorative Effects against Mercury-Induced Hepatotoxicity and Nephrotoxicity. Ind. Crops Prod. 2020, 154, 112723. [Google Scholar] [CrossRef]
- Ouadja, B.; Katawa, G.; Toudji, G.A.; Layland, L.; Gbekley, E.H.; Ritter, M.; Anani, K.; Ameyapoh, Y.; Karou, S.D. Anti-Inflammatory, Antibacterial and Antioxidant Activities of Chenopodium ambrosioides L. (Chenopodiaceae) Extracts. J. Appl. Biosci. 2021, 162, 16764–16794. [Google Scholar] [CrossRef]
- Ez-Zriouli, R.; ElYacoubi, H.; Imtara, H.; Mesfioui, A.; ElHessni, A.; Al Kamaly, O.; Zuhair Alshawwa, S.; Nasr, F.A.; Benziane Ouaritini, Z.; Rochdi, A. Chemical Composition, Antioxidant and Antibacterial Activities and Acute Toxicity of Cedrus atlantica, Chenopodium ambrosioides and Eucalyptus camaldulensis Essential Oils. Molecules 2023, 28, 2974. [Google Scholar] [CrossRef]
- Annaz, H.; Abdelaal, S.; Mandour, D.A.; Mahdi, I.; Mahmoud, M.F.; Sobeh, M. Mexican Tea (Dysphania ambrosioides (L.) Mosyakin & Clemants) Seeds Attenuate Tourniquet-Induced Hind Limb Ischemia–Reperfusion Injury by Modulating ROS and NLRP3 Inflammasome Pathways. J. Funct. Foods 2023, 108, 105712. [Google Scholar] [CrossRef]
- Drioua, S.; El-Guourrami, O.; Assouguem, A.; Ameggouz, M.; Kara, M.; Ullah, R.; Bari, A.; Zahidi, A.; Skender, A.; Benzeid, H.; et al. Phytochemical Study, Antioxidant Activity, and Dermoprotective Activity of Chenopodium ambrosioides (L.). Open Chem. 2024, 22, 20230194. [Google Scholar] [CrossRef]
- Sekede, K.D.; Dermane, A.; Motto, E.A.; Kpoyizoun, P.K.; Metowogo, K.; Eklu-Gadegbeku, K. In Vitro Antioxidant, Anti-Inflammatory and Ex Vivo Nephroprotective Activities of Chenopodium ambrosioides. Int. J. Pharm. Phys. Chem. Nutr. Anal. (IJPPNA) 2024, 1, 9–15. [Google Scholar] [CrossRef]
- Ngolo, L.M.; Faraja, F.M.; Ngandu, O.K.; Kapepula, P.M.; Mutombo, S.M.; Tshitenge, T.B. Phytochemical Screening, UPLC Analysis, Evaluation of Synergistic Antioxidant and Antibacterial Efficacy of Three Medicinal Plants Used in Kinshasa, D.R. Congo. Sci. Rep. 2025, 15, 10083. [Google Scholar] [CrossRef]
- Everton, G.O.; Serejo, A.P.M.; Arruda, M.O.; Mattos, M.C.A.B.; Gomes, P.R.B.; Marinho, S.C.; Luz, D.A.; da Glória Almeida Bandeira, M.; Dantas, A.R.; Mouchrek Filho, V.E. Antioxidant and Anticancer Activity In Vitro of Chenopodium ambrosioides L. Essential Oil. In Open Science Research; Editora Científica: São Paulo, Brazil, 2025; Volume 19, pp. 48–63. ISBN 978-65-5360-944-0. [Google Scholar]
- Maningkas, P.; Pandiangan, D.; Kandou, F. Uji Antikanker Dan Antioksidan Ekstrak Metanol Daun Pasote (Dysphania ambrosioides L.) Anticancer and Antioxidant Test of Methanol Extract of Epazote Leaves (Dysphania ambrosioides L.). J. BIOS Logos 2019, 9, 102–110. [Google Scholar] [CrossRef]
- Pandiangan, D. Product Quality Test of Pasote Tea Bags Leaves Pasote (Dysphania ambrosioides): Comparison of Antioxidant Activities of Water Extract with Acetone Extract. Eur. J. Mol. Clin. Med. 2020, 7, 10. [Google Scholar]
- Kandsi, F.; Conte, R.; Marghich, M.; Lafdil, F.Z.; Alajmi, M.F.; Bouhrim, M.; Mechchate, H.; Hano, C.; Aziz, M.; Gseyra, N. Phytochemical Analysis, Antispasmodic, Myorelaxant, and Antioxidant Effect of Dysphania ambrosioides (L.) Mosyakin and Clemants Flower Hydroethanolic Extracts and Its Chloroform and Ethyl Acetate Fractions. Molecules 2021, 26, 7300. [Google Scholar] [CrossRef]
- Kandsi, F.; Elbouzidi, A.; Lafdil, F.Z.; Meskali, N.; Azghar, A.; Addi, M.; Hano, C.; Maleb, A.; Gseyra, N. Antibacterial and Antioxidant Activity of Dysphania ambrosioides (L.) Mosyakin and Clemants Essential Oils: Experimental and Computational Approaches. Antibiotics 2022, 11, 482. [Google Scholar] [CrossRef]
- Al-Zobaidy, M.J.; Al-Hussaniy, H.A.; Al-Tameemi, Z.S. In Silico Comparison between the Mutated and Wild-Type Androgen Receptors and Their Influence on the Selection of Optimum Androgenic Receptor Blockers for the Treatment of Prostate Cancer. F1000Research 2022, 11, 516. [Google Scholar] [CrossRef]
- Xiao, Q.; Wang, L.; Supekar, S.; Shen, T.; Liu, H.; Ye, F.; Huang, J.; Fan, H.; Wei, Z.; Zhang, C. Structure of Human Steroid 5α-Reductase 2 with the Anti-Androgen Drug Finasteride. Nat. Commun. 2020, 11, 5430. [Google Scholar] [CrossRef] [PubMed]
- Amos-Tautua, B.M.; Ajoko, I.T.; Bunu, S.J. Molecular Docking Studies of Phytochemicals from Triumfetta cordifolia and Spondias mombin against COX-1 and COX-2 Enzymes. Asian J. Trop. Biotechnol. 2025, 22, 30–40. [Google Scholar] [CrossRef]
- Nguyen, T.U.; Kwon, S.J.; Hurh, S.; Kale, A.; Cho, J.M.; Nada, H.; Kim, C.S.; Induvadana, P.; Park, B.J.; Lee, K.; et al. LMT2368 (1-(4-Chlorophenyl)-3-(3-Fluoro-5-(Trifluoromethyl)Phenyl)Urea) Negatively Regulates Inflammation by Inhibiting NLRP3 Inflammasome Activation. Pharmaceutics 2025, 17, 1241. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, Y.; Zhu, A.; Kong, F.; Shan, S.; Zhao, J.; Wang, N.; Sun, X.; Zhang, L.; Yan, C.; Kobilka, B.K.; et al. Structural Basis of α1A-Adrenergic Receptor Activation and Recognition by an Extracellular Nanobody. Nat. Commun. 2023, 14, 3655. [Google Scholar] [CrossRef]
- Ćavar Zeljković, S.; Šišková, J.; Komzáková, K.; De Diego, N.; Kaffková, K.; Tarkowski, P. Phenolic Compounds and Biological Activity of Selected Mentha Species. Plants 2021, 10, 550. [Google Scholar] [CrossRef]
- Topal, M.; Gulcin, İ. Evaluation of the in Vitro Antioxidant, Antidiabetic and Anticholinergic Properties of Rosmarinic Acid from Rosemary (Rosmarinus officinalis L.). Biocatal. Agric. Biotechnol. 2022, 43, 102417. [Google Scholar] [CrossRef]


| No. | Compound | OSIRIS Property Explorer | PASS Online | ProTox 3.0 | Composite Score |
|---|---|---|---|---|---|
| 1 | (+)-4-Carene | 0.340 | 0.569 | 1.000 | 0.636 |
| 2 | (1R,2R,3R,5S)-(-)-Isopinocampheol | 0.745 | 0.763 | 1.000 | 0.279 |
| 3 | (E)-Palmitoleic acid | 0.640 | 0.851 | 1.000 | 0.277 |
| 4 | Linolenic acid, ethyl ester | 0.610 | 0.699 | 1.000 | 0.257 |
| 5 | (9Z,12Z)-9,12-Octadecadienoic acid | 0.445 | 0.840 | 1.000 | 0.254 |
| 6 | Ethyl hexadecanoate | 0.610 | 0.647 | 1.000 | 0.251 |
| 7 | 1,4-Dihydroxy-p-menth-2-ene | 0.745 | 0.478 | 1.000 | 0.247 |
| 8 | 2,3-Dihydrobenzofuran | 0.815 | 0.381 | 1.000 | 0.244 |
| 9 | Isoascaridol | 0.740 | 0.436 | 1.000 | 0.242 |
| 10 | Phytol | 0.615 | 0.555 | 1.000 | 0.241 |
| 11 | Palmitic acid | 0.295 | 0.792 | 1.000 | 0.232 |
| 12 | m-Cymene | 0.525 | 0.494 | 1.000 | 0.224 |
| 13 | Luteolin | 0.920 | 0.338 | 0.690 | 0.216 |
| 14 | p-Cymene | 0.418 | 0.502 | 1.000 | 0.213 |
| 15 | Rosmarinic acid | 0.745 | 0.451 | 0.680 | 0.208 |
| 16 | Syringic acid | 0.658 | 0.538 | 0.670 | 0.207 |
| Compound | Site (CurPocket) | AR (2AM9) | 5AR2 (7BW1) | COX-2 (3LN1) | NLRP3 (7ALV) | α1A (7MYJ) |
|---|---|---|---|---|---|---|
| Dihydrotestosterone | C1 | −10.9 | - | - | - | - |
| Finasteride | C1 | - | −13.4 | - | - | - |
| Celecoxib | C1 | - | - | −11.4 | - | - |
| MCC950 | C1 | - | - | - | −10.6 | - |
| Tamsulosin | C1 | - | - | - | - | −7.3 |
| Rosmarinic acid | C1 | −9.1 | −9.3 | −9.3 | −8.4 | −7.7 |
| Luteolin | C1 | −8.3 | −9.4 | −9.5 | −8.7 | −7.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Österreichische Pharmazeutische Gesellschaft. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Ferrer, E.; Abarca-Salgado, T.; Vargas-Radilla, A.A.; de Jesús Flores-Melgar, J.; Abarca-Vargas, R. Dysphania ambrosioides as a Source of Antioxidant Candidates for Benign Prostatic Hyperplasia (BPH) and Prostatitis: A Critical Review and In Silico Prioritisation. Sci. Pharm. 2025, 93, 57. https://doi.org/10.3390/scipharm93040057
Jiménez-Ferrer E, Abarca-Salgado T, Vargas-Radilla AA, de Jesús Flores-Melgar J, Abarca-Vargas R. Dysphania ambrosioides as a Source of Antioxidant Candidates for Benign Prostatic Hyperplasia (BPH) and Prostatitis: A Critical Review and In Silico Prioritisation. Scientia Pharmaceutica. 2025; 93(4):57. https://doi.org/10.3390/scipharm93040057
Chicago/Turabian StyleJiménez-Ferrer, Enrique, Tania Abarca-Salgado, Azamar Aarón Vargas-Radilla, José de Jesús Flores-Melgar, and Rodolfo Abarca-Vargas. 2025. "Dysphania ambrosioides as a Source of Antioxidant Candidates for Benign Prostatic Hyperplasia (BPH) and Prostatitis: A Critical Review and In Silico Prioritisation" Scientia Pharmaceutica 93, no. 4: 57. https://doi.org/10.3390/scipharm93040057
APA StyleJiménez-Ferrer, E., Abarca-Salgado, T., Vargas-Radilla, A. A., de Jesús Flores-Melgar, J., & Abarca-Vargas, R. (2025). Dysphania ambrosioides as a Source of Antioxidant Candidates for Benign Prostatic Hyperplasia (BPH) and Prostatitis: A Critical Review and In Silico Prioritisation. Scientia Pharmaceutica, 93(4), 57. https://doi.org/10.3390/scipharm93040057










