Abstract
Adenocarcinoma lung cancer is a type of non-small cell lung carcinoma (NSCLC), which accounts for 85% of lung cancer incidence globally. The therapies that are being applied, both conventional therapies and antibody-based treatments, are still found to have side effects. Several previous studies have demonstrated the ability of the ethanolic extract of Ocimum sanctum Linn. (EEOS) as an ethnomedicine with anti-tumor properties. The aim of this study was to determine the effect of Ocimum sanctum Linn. ethanolic extract in inhibiting the proliferation, angiogenesis, and migration of A549 cells (NSCLC). The adhesion as well as the migration assay was performed. Furthermore, enzyme-linked immunosorbent assay (ELISA) was used to measure the expression of αvβ3 integrins, α5β1 integrins, and VEGF. The cells were divided into the following treatment groups: control (non-treated/NT), positive control (AP3/inhibitor β3 80 µg/mL), cisplatin (9 µg/mL), and EEOS at concentrations of 50, 70, 100, and 200 µg/mL. The results showed that EEOS inhibits the adhesion ability and migration of A549 cells, with an optimal concentration of 200 µg/mL. ELISA testing showed that the group of A549 cells given EEOS 200 µg/mL presented a decrease in the optimal expression of integrin α5β1, integrin αvβ3, and VEGF.
1. Introduction
Lung cancer is one of the biggest causes of death around the world. The results of the International Agency for Research on Cancer Global Cancer Observatory related to Cancer Incidence and Mortality Worldwide in 2018 showed that 58.5% of the world’s lung cancer cases occurred in Asia. More than 85% of all lung cancers are non-small cell lung carcinoma (NSCLC). Adenocarcinoma is one type of NSCLC and is the most common type of lung cancer, in all patients and among non-smokers, globally [1]. Surgery, radiotherapy, and chemotherapy are conventional therapies that are still applied to reduce or delay deaths from NSCLC [2]. In the last few decades, despite advances in antibody-based NSCLC treatment technology, applied in combination with conventional therapies, such as pembrolizumab, nivolumab, and ipilimumab, the administration of therapy has not been optimal [3,4].
The side effects of therapy have prompted scientists to find innovative sources of new anti-cancer compounds from natural sources, including traditional herbal plants [1,5]. Herbal plants can provide beneficial effects through natural bioactive compounds found in tumor cases, including helping to overcome side effects or intrinsic radioresistance, preventing metastases, and improving quality of life and patient survival rates [6]. Holy basil (Ocimum sanctum Linn.) is a native Indonesian plant that is often found in the yards of houses, and it is widely consumed by the community as a complement to cuisine. Previous research on the ethanolic extract of Ocimum sanctum Linn. showed its ability to induce in vitro apoptosis in A-549 cells (human lung adenocarcinoma) [7,8] and inhibit angiogenesis [9]. In vivo, the ethanolic extract of Ocimum sanctum Linn. was shown to induce apoptosis [7] and inhibit metastasis [10] in Lewis lung carcinoma (LLC) cells.
2. Materials and Methods
2.1. Preparation of Ocimum sanctum Linn. Ethanolic Extract (EEOS)
The leaves of Ocimum sanctum Linn. simplisia were obtained from CV. Merapi Herbal, Yogyakarta, Indonesia, and the species was identified at the Department of Biology, Gadjah Mada University (Yogyakarta, Indonesia). The ethanolic extract was obtained by a maceration technique. A total of 4000 mL of 96% ethanol (Merck, Darmstadt, Germany) was added to 300 g simplicia Ocimum sanctum Linn. The filtration results were concentrated using a vacuum rotary evaporator (Heidolph, Schwabach, Germany), and 8.82% w/w of Ocimum sanctum Linn. ethanol extract was obtained in the form of a paste.
2.2. Cell Maintenance
A-549 cells were grown in DMEM high-glucose medium (Gibco, Oslo, Norway) with 10% FBS (Gibco, Oslo, Norway) supplementation, penicillin–streptomycin 2% (Gibco, Oslo, Norway), and amphotericin B 0.5% (Gibco, Oslo, Norway) in T25/T75 flasks (Greiner, Frickenhausen, Germany) and then stored in an incubator (Sanyo, Tokyo, Japan) at 37 °C, with 5% CO2. The medium was changed every three days and subcultured when in confluent conditions. Cells were harvested by accutase cell detachment (0.5 mM EDTA.4Na) (Gibco, Oslo, Norway) and grown in new flasks. Confluent cells that were not used for the experiment were stored frozen with a composition of 10% DMSO (Santa Cruz Biotechnology, Dallas, TX, USA) and 90% medium in a 1 mL cryo-vial, and then stored in a −80 °C freezer or cryotank.
2.3. Adhesion Assay Using Cell Counting Kit-8 Assay
The CCK test was carried out according to the manual of the CCK-8 Kit (Abbkine, Hubei, China). A quantity of 1.5 × 104 A-549 cells/100 mL was grown on a culture test 96-well plate (Greiner, Frickenhausen, Germany). Cells were incubated for 24 h, then divided into five groups, including the non-treated group (NT); AP3 80 µg/mL; ethanol extract of Ocimum sanctum Linn. (EEOS) at 50, 70, 100, or 200 g/mL; and cisplatin 9 g/mL. Each treatment was replicated three times. The treatments were incubated for 24 h, then 100 mL of water-soluble tetrazolium (WST-8) reagent was added to each sample and incubated for 4 h (in the dark). Then, the reaction was stopped by adding 100 mL/well of DMSO (Santa Cruz Biotechnology, Dallas, TX, USA). The results were read using an ELISA Reader (BioRad, Hercules, CA, USA) at a wavelength (λ) of 460 nm. The absorbance results obtained were then calculated using the following formula to obtain the percentage of viability.
The final data were analyzed via one-way ANOVA using GraphPad Prism 7 software (La Jolla, CA, USA).
2.4. A549 Cell Lysate Preparation
A-549 cell lysate preparation was carried out according to the kit manual (Biomol, Hamburg, Germany). A total of 5 × 105 A-549 cells/mL were grown in each well on a tissue culture test 6-well plate and then incubated for 1 hour. The treatments in each well consisted of non-treatment (NT); AP3 80 µg/mL; ethanol extract of Ocimum sanctum Linn. (EEOS) at 50, 70, 100, or 200 g/mL; or cisplatin 9 g/mL, all followed by incubation for 24 h. The medium was aspirated and the plate was washed using Dulbecco’s PBS (Gibco, Oslo, Norway), then 900 L of RIPA lysis buffer was added (Santa Cruz Biotechnology, Dallas, TX, USA), and the plate was shaken for 15 min. A cell scraper was used to remove the cells from the bottom of the plate. The lysate was transferred to 1.5 mL microtubes (Eppendorf, Hamburg, Germany). The lysate was centrifuged at 10,000× g for 10 min at 4 °C. The supernatant was transferred to 1.5 mL microtubes.
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
2.5.1. Integrin Human ITG αvβ3 dan VEGF
This test was carried out using sandwich ELISA for both human integrin αvβ3 and VEGF (Fine Test, Wuhan, China). The procedure was performed according to the manual in the kit (Fine Test, Wuhan, China). The plate was washed twice before adding the sample in the form of lysate, along with the negative control, to a 96-well plate. A total of 100 mL of sample and standard was added to each well and incubated for 90 min at 37 °C. The plate then washed for 2 times. A volume of 100 uL of biotin-labeled antibody was added to each well and incubated for 60 min at 37 °C. The plate then washed 3 times. Working solution of 100 mL HRP–streptavidin conjugate (SABC) was added to each well and incubated for 30 min at 37 °C, and the plate was then washed for 5 times. TMB substrate (90 mL) was added and incubated for 15–30 min at 37 °C. A 50 mL stop solution was added, and the well plates were immediately read at a wavelength of 450 nm (BioRad, Hercules, CA, USA).
2.5.2. Integrin Human ITG α5β1
The test was performed using competitive ELISA human integrin α5β1 (MyBiosource, San Diego, CA, USA). The procedure was carried out according to the manual in the kit (MyBiosource, San Diego, CA, USA). A total of 100 mL of standard and lysate sample was added to each, then 10 mL of balanced solution was added and homogenized; no bubbles were formed. A total of 50 mL of the conjugate was added in the well, then homogenized, and incubated at 37 °C for 60 min. After 60 min, we drained the liquid on the plate and washed it with wash buffer 5 times, for 1 minute each time. Volumes of 50 mL of substrates A and B were added to the wells. The plates were then closed tightly and incubated at 37 °C for 15 min. Stop solution (50 mL) was added, and the well plate was immediately read at a wavelength of 450 nm (BioRad, Hercules, CA, USA).
2.5.3. Enzyme-Linked Immunosorbent Assay (ELISA) Data Analysis
ELISA data analysis of human ITG αvβ3, human ITG α5β1, and vascular endothelial growth factor (VEGF) was carried out quantitatively using an ELISA reader to determine the optical density value of each test, then the concentration values were calculated based on the standard value. Data were analyzed via one-way ANOVA using the GraphPad Prism 7 software (La Jolla, CA, USA).
2.6. Scratch Wound Healing Assay
The scratch wound healing assay procedure was carried out based on [11]. A-549 cells at 2.5 × 104 cells/500 mL were grown on a culture test 12-well plate (Greiner, Frickenhausen, Germany). Cells were incubated for 24 h. Cells were rinsed with DMEM high-glucose three times. A sterile 200 mL pipette tip (Vertex, Boston, MA, USA) was used to make a scratch on the cell surface, then they were treated in groups, namely non-treated (NT); AP3 80 µg/mL; ethanol extract of Ocimum sanctum Linn. (EEOS) at graded concentrations of 50, 70, 100, and 200 g/mL; and cisplatin 9 g/mL. The treatments were incubated for 24 h in a CO2 incubator at 37 °C, then observed after 24 h via inverted microscopy. Data analysis of the scratch wound healing assay to determine cell migration was carried out by measuring the surface area of the treated cells. Areas were calculated using the free software ImageJ (https://imagej.nih.gov/ij/ accessed on 4 April 2022) (National institute of Health-NIH, Bethesda, MD, USA). We then calculated the percentage of the area covered according to the following formula:
The final data were analyzed via one-way ANOVA using GraphPad Prism 7 software (La Jolla, CA, USA).
3. Results
3.1. EEOS Inhibited the Adhesion of A549 (Non-Small Cell Lung Carcinoma)
To analyze the ability of EEOS to inhibit cell attachment, A459 cells were cultured on a well plate and treated for 24 h with different concentrations of EEOS (50, 70, 100, and 200 µg/mL). Cisplatin was used as a positive control and to provide a comparison with commercial drugs. Our results show that EEOS inhibited the cell attachment of A549 cells in a dose-dependent manner. EEOS showed significant inhibition at the optimum concentration of 200 µg/mL, but the inhibition was not significant with 50 µg/mL of EEOS (Figure 1).
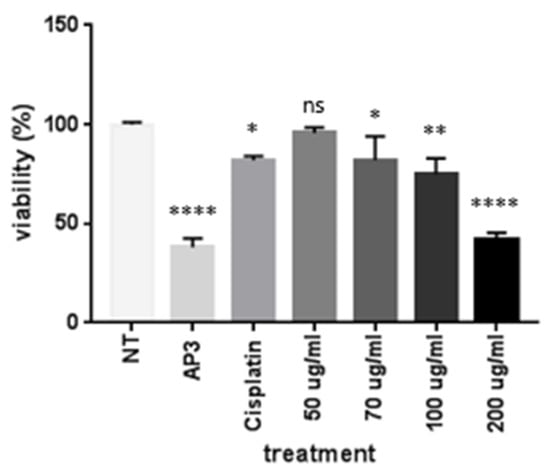
Figure 1.
The ethanolic extract of Ocimum sanctum Linn. (EEOS) inhibited the adhesion of A549 cells (non-small cell lung carcinoma). The cells were cultivated in the presence of an inhibitor (AP3) as the positive control, cisplatin as the commercial drug comparison, and EEOS at concentrations of 50, 70, 100, and 200 µg/mL. After 24 h, EEOS’s inhibitory effect was visualized using MTT reagent at a wavelength of 450 nm (NT: non-treated; * significant p = 0.0332; ** significant p = 0.026; **** significant p < 0.0001; ns = not significant).
3.2. EEOS Inhibited the Cell Migration of A549 (Non-Small Cell Lung Carcinoma) after 24 h of Treatment
The scratch wound healing assay is one of the most commonly used assays for assessing therapeutic impacts on cell migration. In this study, we found that EEOS significantly suppressed the cell migration of NSCLC (A549 cell line). We examined cell migration in response to the mechanical scratch wound. The cells were cultured in a well plate, and after confluence, the cells were treated with EEOS. After 24 h, the cell culture was observed under inverted microscopy. Images of scratch areas after 24 h (Figure 2) indicate that the untreated wounds were half closed within 24 h. To quantify the effects of putative migration inhibitors, the percentage of the open wound area after 24 h was determined (Figure 2). Our data clearly show that treatment with EEOS caused a significant inhibition of cell migration in a concentration-dependent manner.
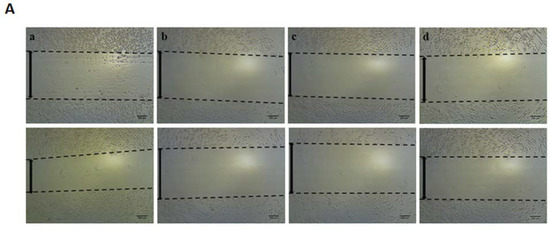
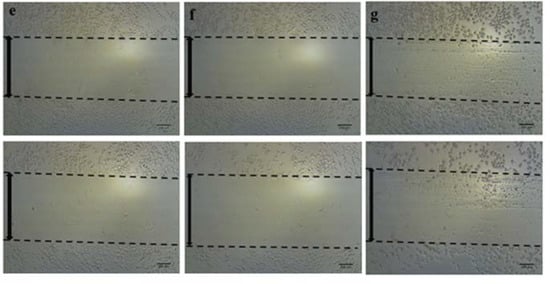
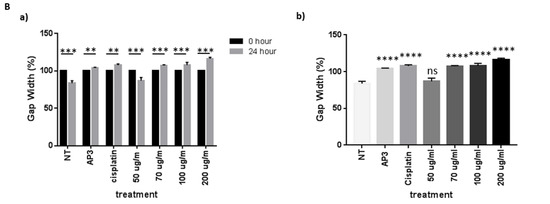
Figure 2.
(A) Photomicroscopic images of A549 cells during the scratch wound healing assay. The cells were cultivated under normal conditions as non-treated cells (a), in the presence of an inhibitor (AP3) as the positive control (b), with cisplatin as the commercial drug comparison (c), and with EEOS at concentrations of 50 (d), 70 (e), 100 (f), and 200 g/mL (e). The wound healing was observed at the 0th hour and after 24 h. (B) Ethanolic extract of Ocimum sanctum Linn. reduced the migration ability of non-small cell lung carcinoma (A549), as shown by the scratch wound assay. The cells were cultivated in the presence of an inhibitor (AP3) as the positive control, cisplatin as the commercial drug comparison, and EEOS at concentrations of 50, 70, 100, and 200 g/mL. (a). The wound healing was observed at 0 h and after 24 h. (b). The wound healing after 24 h. Statistical analysis was performed via one-way ANOVA, followed by post hoc Tukey test (NT: non-treated; ** significant p < 0.0060; *** significant p < 0.0009; **** significant p < 0.0001; ns = not significant).
3.3. EEOS Inhibited Cell Migration of the A549 Cell Line (Non-Small Cell Line Carcinoma) by Suppressing the Concentrations of Integrin αvβ3, Integrin α5β1, and Vascular Endothelial Growth Factor (VEGF)
To strengthen the evidence regarding the effect of EEOS on cell migration, we performed ELISA on A549 cell lysates. The representative parameters observed were integrin αvβ3, integrin α5β1, and VEGF. The untreated A549 cells produced the highest concentration of integrin αvβ3, integrin α5β1, and VEGF. Additional treatment of A549 with EEOS diminished the integrin αvβ3, integrin α5β1, and VEGF concentrations in a dose-dependent manner. The integrin αvβ3 (Figure 3A), integrin α5β1 (Figure 3B), and VEGF (Figure 3C) concentrations were significantly suppressed under the optimum concentration of EEOS (200 µg/mL) and under cisplatin.
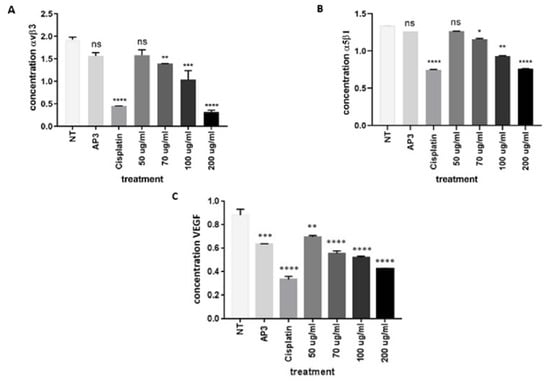
Figure 3.
(A) The ethanolic extract of Ocimum sanctum Linn. decreased expression of non-small cell lung cancer (A549) integrin αvβ3, as shown by sandwich ELISA. The cells were cultivated in the presence of an inhibitor (AP3) as the positive control, cisplatin as the commercial drug comparison, and EEOS at concentrations of 50, 70, 100, and 200 g/mL for 24 h. A549 cells were then lysed and analyzed via ELISA for the concentration of αvβ3 integrin (ug/mL). Statistical analysis was performed via one-way ANOVA, followed by post hoc Tukey test (NT: non-treated; **, ***, and **** indicate statistically significant values for the non-treated group as a negative control compared with treatment, with p-values of 0.0085, 0.0004, and <0.0001, respectively; ns = not significant). (B) Ethanolic extract of Ocimum sanctum Linn. decreased expression of integrin α5β1 in non-small cell lung cancer (A549), as shown by competitive ELISA. The cells were cultivated in the presence of an inhibitor (AP3) as the positive control, cisplatin as the commercial drug comparison, and EEOS at concentrations of 50, 70, 100, and 200 g/mL for 24 h. A549 cells were then lysed and analyzed via ELISA for the concentration of α5β1 integrin (ug/mL). Statistical analysis was performed via one-way ANOVA, followed by post hoc Tukey test (NT: non-treated; *, **, and **** indicate statistically significant values for the non-treated group as a negative control compared with treatment, with p-values of 0.0348, 0.0027, and <0.0001, respectively; ns = not significant). (C) Ethanolic extract of Ocimum sanctum Linn. decreased expression of the non-small cell lung cancer (A549) integrin VEGF, as shown by sandwich ELISA. The cells were cultivated in the presence of an inhibitor (AP3) as the positive control, cisplatin as the commercial drug comparison, and EEOS at concentrations of 50, 70, 100, and 200 g/mL for 24 h. A549 cells were then lysed and analyzed via ELISA for the concentration of VEGF ((ug/mL). Statistical analysis was performed via one-way ANOVA, followed by post hoc Tukey test (NT: non-treated; **, *** and **** indicate statistical significance of the non-treated group as a negative control compared with treatment, with p-values of 0.0012, 0.0002, and <0.0001, respectively; ns = not significant).
4. Discussion
Cancer has properties such as evading cell death, sustaining proliferation, inducing vasculature, and activating invasion and metastasis [12]. In the process of malignancy, tumor cells will migrate to other organs through blood vessels and lymph vessels and grow in the appropriate organs; this process is called metastasis. Cell–cell and cell–extracellular matrix (ECM) adhesions play a fundamental role in governing the structural integrity of healthy tissue and in regulating cellular morphology, migration, proliferation, survival, and differentiation events [13]. In the classic view of malignant transformation in the epithelium, cells lose their dependence on integrin-mediated interactions with the extracellular matrix and the resulting signaling [14]. In the process of metastasis, tumor cells will migrate to find the best place to maintain their function. Cell migration, invasion, and adhesion are pivotal steps in this process [15,16].
In this study, we observed the ability of EEOS to prevent the adhesion of the A549 cell line. The CCK-8 test chart showed a decrease in the adhesion ability of A549 cells treated with EEOS (Figure 1). The results of this study add to the information from previous studies that EEOS can reduce the adhesion ability of A549 cells, as shown via adhesion assay [8]. We also performed scratch wound healing assay to investigate the migration ability of the A549 cell line. Our data show that EEOS also has the ability to inhibit A549 cell migration (Figure 2A,B). The ability of tumor cells to adhere and migrate is closely related to the process of tumor progression and metastasis, which is responsible for 90% of cancer-related deaths [17]. The phytochemical compounds in EEOS were previously dialyzed using thin-layer chromatography (TLS) and UV–vis spectrophotometry. The results of the analysis showed that EEOS contains several active compounds, such as flavonoids, phenols, saponins, alkaloids, tannins, terpenoids, and steroids [18]. Flavonoids and phenols have important roles as anti-cancer and cytotoxic agents, inducing apoptosis in cancer cells [19]. In silico molecular docking was also performed to predict the chemical binding between active compounds and protein. In silico molecular docking analysis of the flavonoid compounds (quercetin) and flavonoids (eugenol) showed that these active compounds can bind to the active site of integrins and VEGF, thereby inhibiting the activity of integrins and VEGF for adhesion, cell spread, and blood vessel formation [20]. The inhibition of active compounds with integrin complexes will have an impact on the inhibition of the extracellular matrix (ECM) adhesion process and result in a decrease in tumor cell invasion. In vitro results on the cell line A549 also showed consistent results that the content of active compounds in EEOS can reduce the viability of the A549 cell line.
To elucidate this mechanism, we also examined the expression of integrin αvβ3, integrin α5β1, and VEGF as biochemical cues for blood vessel formation, adhesion, and migration of cancer cells. We found that EEOS reduced the concentrations of integrin αvβ3, integrin α5β1, and VEGF in the A549 cell line (Figure 3A–C). Integrins are transmembrane adhesion receptors for the extracellular matrix (ECM) and have essential roles, including sensing and adhering to the extracellular environment to maintain global tissue architecture and multicellularity [21]. Integrins are the major class of receptors in adhesive events, acting by bi-directionally (inside-out and outside-in) transducing biochemical signals and mechanical force across the plasma membrane [22]. Integrins play a key role in single-cell migration and act via conformational changes in the extracellular matrix (outside-in) or intracellular protein that are triggered by altering the affinity of integrins (inside-out). These changes recruit cytoskeletal linker proteins to remodel nascent or focal adhesions and generate tension; these adherent structures generate forces of cellular movement. There are several pathways by which integrin can mediate cell spreading and migration and one of them involves focal adhesion kinase and the capacity of tyrosine-protein kinase Src to up-regulate integrin expression [23].
The integrins αvβ3 and α5β1 have roles as adhesion molecules in cell-to-cell interactions and motility-supporting roles that promote cell migration during nervous system development, and they also promote metastatic spread [24]. Integrin αvβ3 is mostly expressed on angiogenic endothelial cells in remodeling and pathological tissues. Expression of the αvβ3 integrin by endothelial cells promotes cell adhesion to the ECM, cell migration, and angiogenesis, along with angiogenic growth factors, including VEGF/VEGFR [25]. The α5β1 integrin is also overexpressed in, and closely related with, metastatic events. In normal endothelial cells, α5β1 will be expressed at very low levels, but this expression will be significantly increased in endothelial cells during cancer cell angiogenesis [26]. Integrin expression and activation directly influence human malignancies. Due to their broad impact in malignant transformations, they are considered potential targets for cancer therapy [27]. Integrins are considered as pharmacological targets for drugs by inhibiting several key processes in cancer development, such as cell proliferation, survival, and migration. Targeting integrins to enhance the delivery of anti-tumor agents or to delineate cancerous lesions is a new and promising approach. Integrin-inhibiting anticancer drugs have been conceived for their ability to impair ligand binding [28].
In addition to integrin expression, VEGF expression has been confirmed to be a critical pathological factor in the occurrence of NSCLC by increasing vascular permeability and increasing angiogenesis [29]. This study confirmed that EEOS has the ability to inhibit A549 cell angiogenesis by inhibiting tube formation, as shown through the angiogenesis assay [9] and reducing VEGF concentrations. During angiogenesis, VEGF has an associated mechanism with integrins, as integrins are overexpressed on the endothelial cell surface to facilitate the growth and survival of new vessels [25]. The supply of oxygen and nutrients to cells through blood vessels is the most important aspect in the survival of cells, including cancer cells. Vascular endothelial growth factor (VEGF) is a homodimeric glycoprotein from the endothelial growth factor family and is an important factor in the formation and regulation of angiogenesis processes [30]; in addition, VEGF has biological roles in the regulation of vascular permeability, metabolism, immune system, inflammation, and neurological function [31]. Tumors can generate their own vascular system. VEGF acts as an angiogenic factor by promoting their proliferation, migration, adhesion, and survival. VEGF may, thus, play a role in vascular invasion [32]. Furthermore, VEGF also play role in targeting other cells in the tumor microenvironment, as well as initiating the function of growth factors and integrin, mainly svb3 and a5b1 [33,34,35]. In recent years, the inhibition expression of VEGF has been utilized in tumor-targeted therapy [34].
Taken together, our findings underline the ability of the ethanolic extract of Ocimum sanctum Linn. to prevent the migration and metastasis of human lung adenocarcinoma cells (A549); however, more research and discussion are required, since our research was limited only to the role of integrin αvβ3, integrin α5β1, and VEGF. Moreover, the data derived from the in vitro analysis demonstrate the direct impact on the cells (Figure 4). Furthermore, in vivo experiments are needed as basic data to complete the preclinical phase of this analysis.
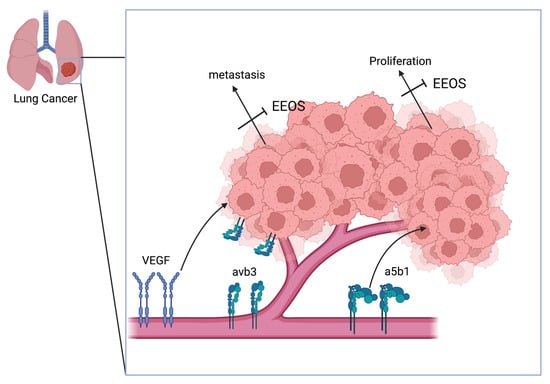
Figure 4.
Schematic overview of the mechanism of Ocimum sanctum Linn. ethanolic extract, inhibiting the adhesion, proliferation, and migration of A549 human lung adenocarcinoma, mediated by the downregulation of αvβ3, α5β1, and VEGF. Overall, inhibition by EEOS will mitigate angiogenesis and metastasis of human lung adenocarcinoma cells.
5. Conclusions
Our findings demonstrate that EEOS disturbed the proliferation, angiogenesis, and migration of A549 cells, which may result from the disruption of cell adhesion and migration, as shown by the CCK-8 assay and scratch wound healing assay, as a consequence of the downregulation of αvβ3 integrins, α5β1 integrins, and VEGF. As a result, EEOS may represent a good therapeutic candidate for the treatment of lung adenocarcinoma. Further studies using in vivo methods are required to fully validate our findings in human lung adenocarcinoma growth.
Author Contributions
Conceptualization, H.W. and D.L.K.; Methodology, H.W. and D.L.K.; Software, U.K.; Validation, H.W. and U.K.; formal analysis, U.K., H.W. and S.K.; Investigation, U.K., H.W. and D.A.A.N.; Resources, H.W., D.L.K. and D.A.A.N.; Data Curation, U.K., D.L.K. and H.W.; Writing—Original Draft Preparation, H.W. and U.K.; Writing—Review and Editing, H.W., S.E., S.K., D.A.A.N. and D.L.K.; Visualization, H.W.; Supervision, H.W. and D.L.K. Project Administration, H.W. and D.L.K.; Funding Acquisition, H.W. All authors have read and agreed to the published version of the manuscript.
Funding
The research was funded by the Ministry of Education, Culture, Research and Technology for the PDUPT Grant Universitas Gadjah Mada, with the grant number 1703/UN1/DITLIT/DIT-LIT/PT.01.02/2022.
Institutional Review Board Statement
The experimental procedures were approved by the Ethical Committee of the Faculty of Veterinary Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia (approval number 00057/EC-FKH/Int./2021).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank the Ministry of Education, Culture, Research and Technology for the research funding (Basic Research) with the grant number 1703/UN1/DITLIT/DIT-LIT/PT.01.02/2022. The authors also thank the Pharmacology Laboratory, Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada, and Mosa Rini Nurul H. for their excellent technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wulandari, L.; Febriani, A.; Fatmawati, F.; Soegiarto, G. Evaluation of Patients with Lung Cancer Treated with Epidermal Growth Factor Receptor–Tyrosine Kinase Inhibitor. Asian J. Oncol. 2018, 4, 48–53. [Google Scholar] [CrossRef]
- Saba, N.; Khuri, F. The Role of Bisphosphonates in the Management of Advanced Cancer with a Focus on Non-Small-Cell Lung Cancer—Part I: Mechanisms of Action, Role of Biomarkers and Preclinical Applications. Oncology 2005, 68, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, M.D.; Paz-Ares, L.; Bernabe Caro, R.; Zurawski, B.; Kim, S.-W.; Carcereny Costa, E.; Park, K.; Alexandru, A.; Lupinacci, L.; de la Mora Jimenez, E.; et al. Nivolumab plus Ipilimumab in Advanced Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2019, 381, 2020–2031. [Google Scholar] [CrossRef]
- Park, J.; Jeong, D.; Song, M.; Kim, B. Antioxidants Recent Advances in Anti-Metastatic Approaches of Herbal Medicines in 5 Major Cancers: From Traditional Medicine to Modern Drug Discovery. Antioxidants 2021, 10, 527. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Oliveira, P.; Fraga-Corral, M.; Pereira, A.G.; Lourenço-Lopes, C.; Jimenez-Lopez, C.; Prieto, M.A.; Simal-Gandara, J. Scientific Basis for the Industrialization of Traditionally Used Plants of the Rosaceae Family. Food Chem. 2020, 330, 127197. [Google Scholar] [CrossRef]
- Redondo-Blanco, S.; Fernández, J.; Gutiérrez-del-Río, I.; Villar, C.J.; Lombó, F. New Insights toward Colorectal Cancer Chemotherapy Using Natural Bioactive Compounds. Front. Pharmacol. 2017, 8, 109. [Google Scholar] [CrossRef]
- Magesh, V.; Lee, J.-C.; Ahn, K.S.; Lee, H.-J.; Lee, H.-J.; Lee, E.-O.; Jung, H.J.; Kim, J.S.; Kim, D.K.; Choi, S.-H.; et al. Ocimum sanctum Induces Apoptosis in A549 Lung Cancer Cells and Suppresses the In Vivo Growth of Lewis Lung Carcinoma Cells. Phytother. Res. 2009, 23, 1385–1391. [Google Scholar] [CrossRef]
- Wihadmadyatami, H.; Karnati, S.; Hening, P.; Tjahjono, Y.; Rizal; Maharjanti, F.; Kusindarta, D.L.; Triyono, T.; Supriatno. Ethanolic Extract Ocimum sanctum Linn. Induces an Apoptosis in Human Lung Adenocarcinoma (A549) Cells. Heliyon 2019, 5, e02772. [Google Scholar] [CrossRef]
- Wihadmadyatami, H.; Hening, P.; Kustiati, U.; Kusindarta, D.L.; Triyono, T.; Supriatno, S. Ocimum sanctum Linn. Ethanolic Extract Inhibits Angiogenesis in Human Lung Adenocarcinoma (A549) Cells. Vet. World 2020, 13, 2028–2032. [Google Scholar] [CrossRef]
- Kim, S.C.; Magesh, V.; Jeong, S.J.; Lee, H.J.; Ahn, K.S.; Lee, H.J.; Lee, E.O.; Kim, S.H.; Lee, M.H.; Kim, J.H.; et al. Ethanol Extract of Ocimum sanctum Exerts Anti-Metastatic Activity through Inactivation of Matrix Metalloproteinase-9 and Enhancement of Anti-Oxidant Enzymes. Food Chem. Toxicol. 2010, 48, 1478–1482. [Google Scholar] [CrossRef]
- Martinotti, S.; Ranzato, E. Scratch Wound Healing Assay. In Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2020; Volume 2109, pp. 225–229. [Google Scholar]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Abduljauwad, S.N.; Ahmed, H.u.R. Enhancing Cancer Cell Adhesion with Clay Nanoparticles for Countering Metastasis. Sci. Rep. 2019, 9, 5935. [Google Scholar] [CrossRef] [PubMed]
- Janiszewska, M.; Primi, M.C.; Izard, T. Cell Adhesion in Cancer: Beyond the Migration of Single Cells. J. Biol. Chem. 2020, 295, 2495–2505. [Google Scholar] [CrossRef] [PubMed]
- Pijuan, J.; Barceló, C.; Moreno, D.F.; Maiques, O.; Sisó, P.; Marti, R.M.; Macià, A.; Panosa, A. In Vitro Cell Migration, Invasion, and Adhesion Assays: From Cell Imaging to Data Analysis. Front. Cell Dev. Biol. 2019, 7, 107. [Google Scholar] [CrossRef]
- Xu, L.; Gordon, R.; Farmer, R.; Pattanayak, A.; Binkowski, A.; Huang, X.; Avram, M.; Krishna, S.; Voll, E.; Pavese, J.; et al. Precision Therapeutic Targeting of Human Cancer Cell Motility. Nat. Commun. 2018, 9, 2454. [Google Scholar] [CrossRef]
- Campbell, K.; Rossi, F.; Adams, J.; Pitsidianaki, I.; Barriga, F.M.; Garcia-Gerique, L.; Batlle, E.; Casanova, J.; Casali, A. Collective Cell Migration and Metastases Induced by an Epithelial-to-Mesenchymal Transition in Drosophila Intestinal Tumors. Nat. Commun. 2019, 10, 2311. [Google Scholar] [CrossRef]
- Kustiati, U.; Wihadmadyatami, H.; Kusindarta, D.L. Dataset of Phytochemical and Secondary Metabolite Profiling of Holy Basil Leaf (Ocimum sanctum Linn) Ethanolic Extract Using Spectrophotometry, Thin Layer Chromatography, Fourier Transform Infrared Spectroscopy, and Nuclear Magnetic Resonance. Data Brief 2022, 40, 107774. [Google Scholar] [CrossRef]
- Abotaleb, M.; Samuel, S.M.; Varghese, E.; Varghese, S.; Kubatka, P.; Liskova, A.; Büsselberg, D. Flavonoids in Cancer and Apoptosis. Cancers 2019, 11, 28. [Google Scholar] [CrossRef]
- Kustiati, U.; Dewi Ratih, T.S.; Dwi Aris Agung, N.; Kusindarta, D.L.; Wihadmadyatami, H. In Silico Molecular Docking and in Vitro Analysis of Ethanolic Extract Ocimum sanctum Linn.: Inhibitory and Apoptotic Effects against Non-Small Cell Lung Cancer. Vet. World 2021, 14, 3175–3187. [Google Scholar] [CrossRef]
- Kadry, Y.A.; Calderwood, D.A. Chapter 22: Structural and Signaling Functions of Integrins. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183206. [Google Scholar] [CrossRef]
- Bachmann, M.; Kukkurainen, S.; Hytönen, V.P.; Wehrle-Haller, B. Cell adhesion by integrins. Physiol. Rev. 2019, 99, 1655–1699. [Google Scholar] [CrossRef] [PubMed]
- de Pascalis, C.; Etienne-Manneville, S. Single and Collective Cell Migration: The Mechanics of Adhesions. Mol. Biol. Cell 2017, 28, 1833–1846. [Google Scholar] [CrossRef] [PubMed]
- Sökeland, G.; Schumacher, U. The Functional Role of Integrins during Intra- and Extravasation within the Metastatic Cascade. Mol. Cancer 2019, 18, 12. [Google Scholar] [CrossRef]
- Weis, S.M.; Cheresh, D.A. Av Integrins in Angiogenesis and Cancer. Cold Spring Harb. Perspect. Med. 2011, 1, a006478. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, A.; Li, S.; Tao, X.; Sheng, B.; Chetry, M.; Zhu, X. Predictive Role of Galectin-1 and Integrin A5β1 in Cisplatin-Based Neoadjuvant Chemotherapy of Bulky Squamous Cervical Cancer. Biosci. Rep. 2017, 37, BSR20170958. [Google Scholar] [CrossRef] [PubMed]
- Harjunpää, H.; Asens, M.L.; Guenther, C.; Fagerholm, S.C. Cell Adhesion Molecules and Their Roles and Regulation in the Immune and Tumor Microenvironment. Front. Immunol. 2019, 10, 1078. [Google Scholar] [CrossRef]
- Valdembri, D.; Serini, G. The Roles of Integrins in Cancer. Fac. Rev. 2021, 10, 45. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Zhu, C.; He, J.; Chen, J.; Liang, Y.; Yang, F.; Wu, X.; Ma, X. Prognostic Role of Vascular Endothelial Growth Factor in Cervical Cancer: A Meta-Analysis. Oncotarget 2017, 8, 24797–24803. [Google Scholar] [CrossRef]
- Chen, Y.; Mathy, N.W.; Lu, H. The Role of VEGF in the Diagnosis and Treatment of Malignant Pleural Effusion in Patients with Non-Small Cell Lung Cancer (Review). Mol. Med. Rep. 2018, 17, 8019–8030. [Google Scholar] [CrossRef]
- Yang, Y.; Cao, Y. The Impact of VEGF on Cancer Metastasis and Systemic Disease. Semin. Cancer Biol. 2022, 86, 251–261. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.N.; Tang, J.M.; Kong, X.; Yang, J.Y.; Zheng, F.; Guo, L.Y.; Huang, Y.Z.; Zhang, L.; Tian, L.; et al. VEGF Is Essential for the Growth and Migration of Human Hepatocellular Carcinoma Cells. Mol. Biol. Rep. 2012, 39, 5085–5093. [Google Scholar] [CrossRef] [PubMed]
- Lino, R.; Dos Santos, L.B.; Pisani, P.K.; Altei, G.F.D.; Cominetti, W.F.; Selistre-de-Araújo, H.S. Alphavbeta3 integrin blocking inhibits apoptosis and induces autophagy in murine breast tumor cells. Biochimica et Biophysica Acta (BBA)—Molecular Cell Research. Biochim. Biophys. Acta Mol. Cell Res. 2019, 12, 118536. [Google Scholar] [CrossRef] [PubMed]
- Goel, H.L.; Mercurio, A.M. VEGF targets the tumour cell. Nat. Rev. Cancer 2013, 12, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Yan, D.; Liu, Y.; Huang, P.; Cui, H. The Roles of Integrin α5β1 in Human Cancer. Onco Targets Ther. 2020, 13, 13329–13344. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).