Thiazole-Bearing 4-Thiazolidinones as New Anticonvulsant Agents
Abstract
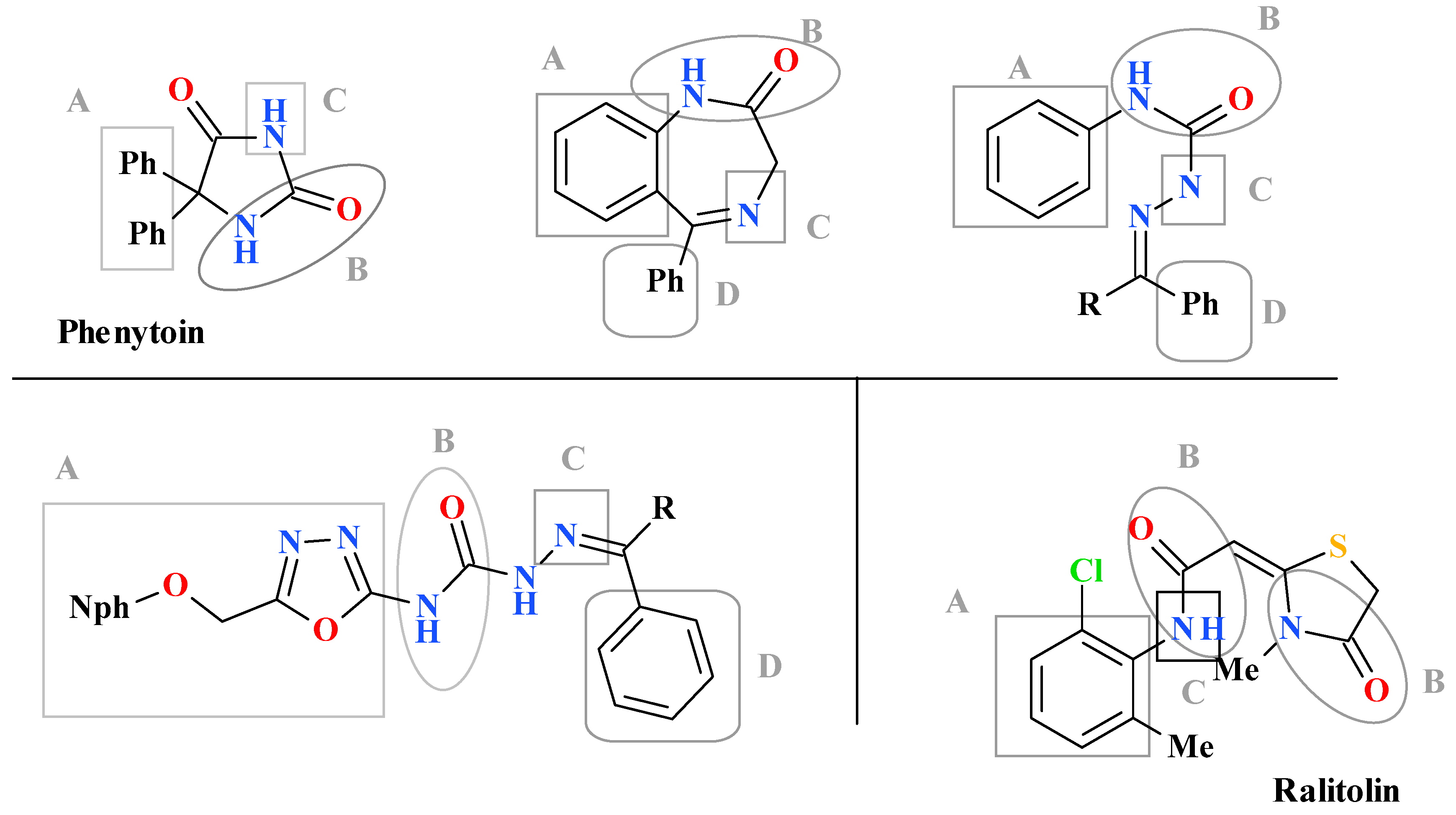
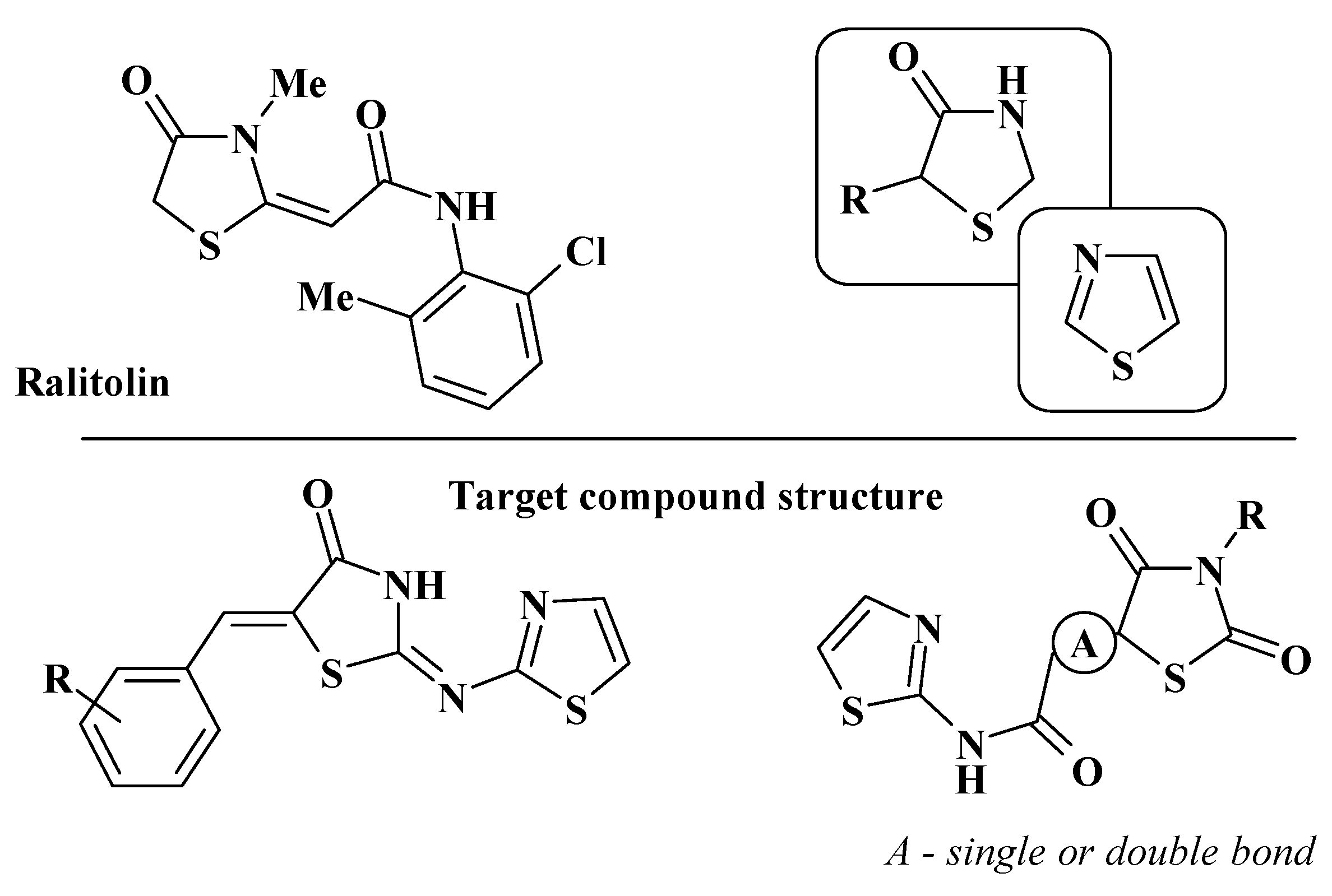
1. Introduction
2. Materials and Methods
2.1. Chemistry
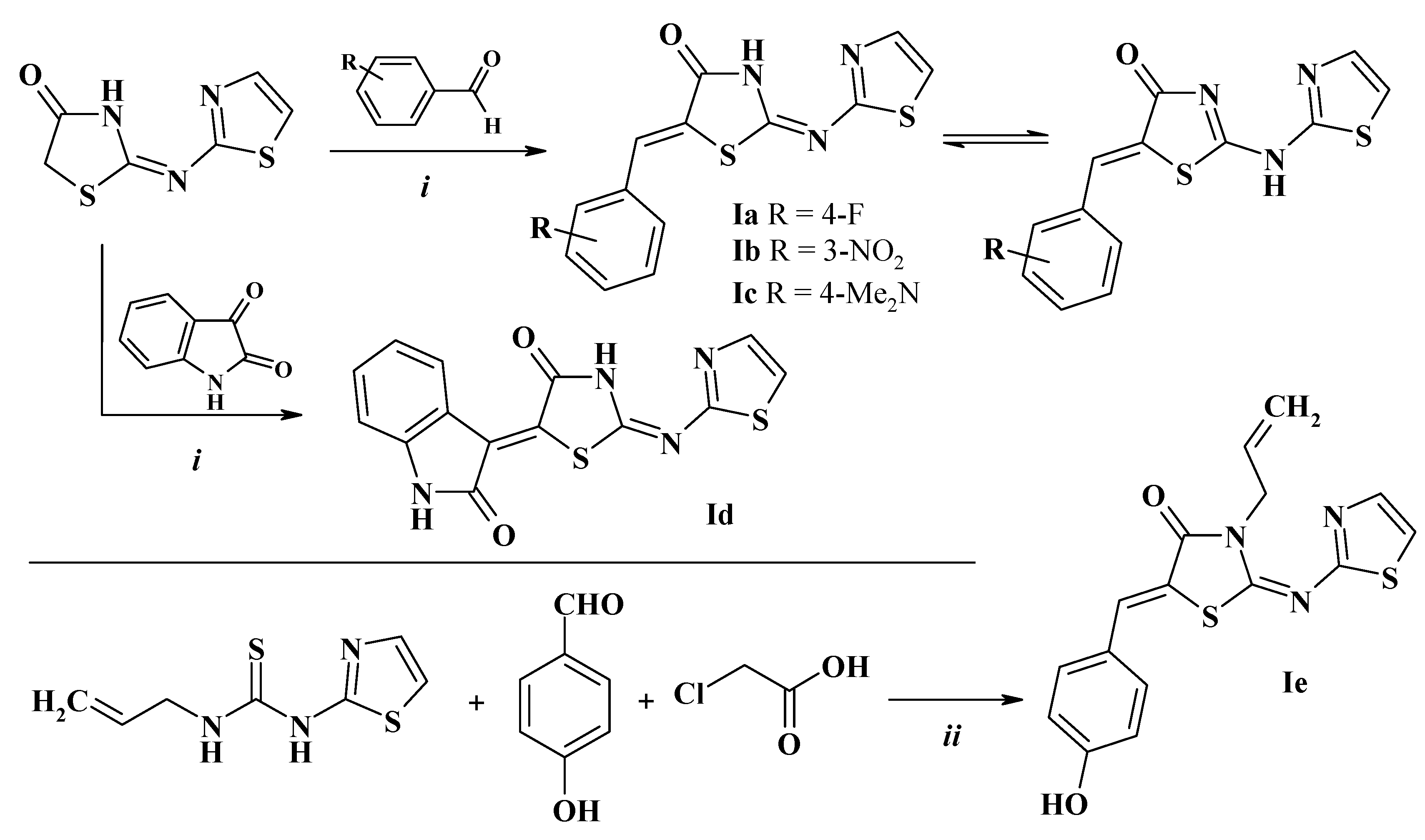
2.2. General Procedure for the 5-(Het)Arylidene-2-(Thiazol-2-yl)Amino(Imino)-4-Thiazolidinones Synthesis (I)
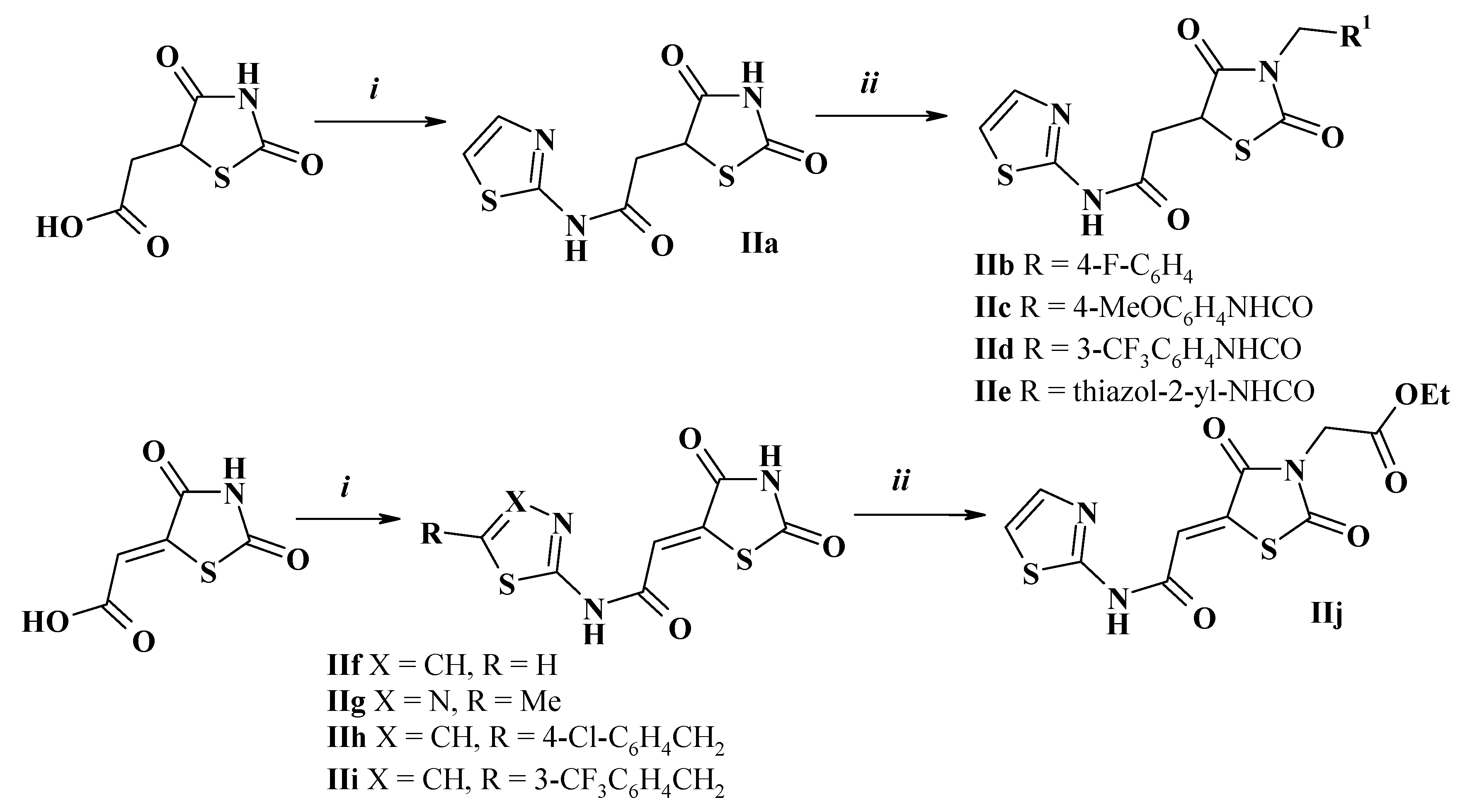
2.3. General Method for Amides of 2,4-Dioxothiazolidine-5-Carboxylic Acids Synthesis (II)
2.4. General Procedure for N3-Substituted 2,4-Dioxothiazolidine-5-Carboxamides Synthesis
2.5. Pharmacology
3. Results and Discussion
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Forsgren, L.; Beghi, E.; Oun, A.; Sillanpää, M. The epidemiology of epilepsy in Europe—A systematic review. Eur. J. Neurol. 2005, 12, 245–253. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Fact Sheets: Epilepsy. Available online: http://www.who.int/ru/news-room/fact-sheets/detail/epilepsy (accessed on 27 December 2019).
- Galiana, G.L.; Gauthier, A.C.; Mattson, R.H. Eslicarbazepine acetate: A new improvement on a classic drug family for the treatment of partial-onset seizures. Drugs R D 2017, 17, 329–339. [Google Scholar] [CrossRef]
- Frampton, J.E. Perampanel: A review in drug-resistant epilepsy. Drugs 2015, 75, 1657–1668. [Google Scholar] [CrossRef] [PubMed]
- Gunthorpe, M.J.; Large, C.H.; Sankar, R. The mechanism of action of retigabine (ezogabine), a first-in-class K+ channel opener for the treatment of epilepsy. Epilepsia 2012, 53, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Kwan, P.; Arzimanoglou, A.; Berg, A.T.; Brodie, M.J.; Allen Hauser, W.; Mathern, G.; French, J. Definition of drug resistant epilepsy: Consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2010, 51, 1069–1077. [Google Scholar] [CrossRef]
- Devinsky, O. Patients with refractory seizures. N. Engl. J. Med. 1999, 340, 1565–1570. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, F.; Teng, W.; Hao, F.; Zhang, J.; Yin, M.; Wang, N. Efficacy and safety of antiepileptic drugs for refractory partial-onset epilepsy: A network meta-analysis. J. Neurol. 2018, 265, 1–11. [Google Scholar] [CrossRef]
- Perucca, P.; Gilliam, F.G. Adverse effects of antiepileptic drugs. Lancet Neurol. 2012, 11, 792–802. [Google Scholar] [CrossRef]
- Kaminskyy, D.; Kryshchyshyn, A.; Lesyk, R. Recent developments with rhodanine as a scaffold for drug discovery. Expert Opin. Drug Dis. 2017, 12, 1233–1252. [Google Scholar] [CrossRef]
- Kaminskyy, D.; Kryshchyshyn, A.; Lesyk, R. 5-Ene-4-thiazolidinones—An efficient tool in medicinal chemistry. Eur. J. Med. Chem. 2017, 140, 542–594. [Google Scholar] [CrossRef]
- Gong, G.H.; Wang, D.; Zhang, J.F.; Wei, C.X.; Quan, Z.S. Anticonvulsant activity of 2-(substituted-imino)thiazolidin-4-ones. Drug Res. 2014, 64, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Senthilraja, M.; Alagarsamy, V. Synthesis and pharmacological investigation of 2-(4-dimethylaminophenyl)-3,5-disubstituted thiazolidin-4-ones as anticonvulsants. Arch. Pharm. 2012, 345, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Thenmozhiyal, J.C.; Wong, P.T.; Chui, W.K. Anticonvulsant activity of phenylmethylenehydantoins: A structure-activity relationship study. J. Med. Chem. 2004, 47, 1527–1535. [Google Scholar] [CrossRef] [PubMed]
- Taroua, M.; Ribuot, C.; Pera, M.H.; Taillandier, G.; Fatome, M.; Laval, J.D.; Leclerc, G. New α, β and γ semicarbazone and thiosemicarbazone 1,3-dithiolanes as radioprotectors. Anticonvulsant activity. Eur. J. Med. Chem. 1996, 31, 589–595. [Google Scholar] [CrossRef]
- Pandeya, S.N.; Yogeeswari, P.; Stables, J.P. Synthesis and anticonvulsant activity of 4-bromophenyl substituted aryl semicarbazones. Eur. J. Med. Chem. 2000, 35, 879–886. [Google Scholar] [CrossRef]
- Sun, Q.; Tafesse, L.; Limberis, J.T.; Islam, K.; Kyle, D.J. Parallel synthesis of a biased library of thiazolidinones as novel sodium channel antagonists. Comb. Chem. High Throughput Screen 2003, 6, 481–488. [Google Scholar] [CrossRef]
- Amin, K.M.; Rahman, D.E.A.; Al-Eryani, Y.A. Synthesis and preliminary evaluation of some substituted coumarins as anticonvulsant agents. Bioorg. Med. Chem. 2008, 16, 5377–5388. [Google Scholar] [CrossRef]
- Ghogare, J.G.; Bhandari, S.V.; Bothara, K.G.; Madgulkar, A.R.; Parashar, G.A.; Sonawane, B.G.; Inamdar, P.R. Design, synthesis and pharmacological screening of potential anticonvulsant agents using hybrid approach. Eur. J. Med. Chem. 2010, 45, 857–863. [Google Scholar] [CrossRef]
- Malawska, B. New anticonvulsant agents. Curr. Top. Med. Chem. 2005, 5, 69–85. [Google Scholar] [CrossRef]
- Mullick, P.; Khan, S.A.; Verma, S.; Alam, O. Thiadiazole derivatives as potential anticonvulsant agents. Bull. Korean Chem. Soc. 2011, 32, 1011–1016. [Google Scholar] [CrossRef]
- Myronenko, S.I.; Kaminskyy, D.V.; Nektegayev, I.O.; Pinyazko, O.R.; Lesyk, R.B. Search of new anticonvulsant agents among 4-thiazolidinones and related heterocyclic systems. Clin. Pharm. Pharmacother. Med. Standart 2012, 1–2, 124–131. [Google Scholar]
- Rajak, H.; Deshmukh, R.; Veerasamy, R.; Sharma, A.K.; Mishra, P.; Kharya, M.D. Novel semicarbazones based 2,5-disubstituted-1,3,4-oxadiazoles: One more step towards establishing four binding site pharmacophoric model hypothesis for anticonvulsant activity. Bioorg. Med. Chem. Lett. 2010, 20, 4168–4172. [Google Scholar] [CrossRef] [PubMed]
- Angelova, V.T.; Voynikov, Y.; Andreeva-Gateva, P.; Surcheva, S.; Vassilev, N.; Pencheva, T.; Tchekalarova, J. In vitro and in silico evaluation of chromene based aroylhydrazones as anticonvulsant agents. Med. Chem. Res. 2017, 26, 1884–1896. [Google Scholar] [CrossRef]
- Al-Otaibi, F. An overview of structurally diversified anticonvulsant agents. Acta Pharm. 2019, 69, 321–344. [Google Scholar] [CrossRef]
- Kumar, A.; Mishra, A.K. Design, molecular docking, synthesis, characterization, biological activity evaluation (against MES model), in-silico biological activity spectrum (PASS analysis), toxicological and predicted oral rat LD 50 studies of novel sulphonamide derivatives. Front. Biol. 2018, 13, 425–451. [Google Scholar] [CrossRef]
- Shiradkar, M.R.; Ghodake, M.; Bothara, K.G.; Bhandari, S.V.; Nikalje, A.; Akula, K.C.; Desai, N.C.; Burange, P.J. Synthesis and anticonvulsant activity of clubbed thiazolidinone–barbituric acid and thiazolidinone-triazole derivatives. Arkivoc 2007, 14, 58–74. [Google Scholar] [CrossRef]
- Faizi, M.; Jahani, R.; Ebadi, S.A.; Tabatabai, S.A.; Rezaee, E.; Lotfaliei, M.; Amini, M.; Almasirad, A. Novel 4-thiazolidinone derivatives as agonists of benzodiazepine receptors: Design, synthesis and pharmacological evaluation. EXCLI J. 2017, 16, 52–62. [Google Scholar] [CrossRef]
- Nikalje, A.P.G.; Shaikh, A.N.; Shaikh, S.I.; Khan, F.A.K.; Sangshetti, J.N.; Shinde, D.B. Microwave assisted synthesis and docking study of N-(2-oxo-2-(4-oxo-2-substituted thiazolidin-3ylamino)ethyl)benzamide derivatives as anticonvulsant agents. Bioorg. Med. Chem. Lett. 2014, 24, 5558–5562. [Google Scholar] [CrossRef]
- Nikalje, A.P.G.; Shaikh, S.I.; Mulay, A.; Khan, F.A.; Sangshetti, J.N.; Shaikh, S. Ultrasound-assisted synthesis, anticonvulsant activity, and docking study of indole-appended thiazolidin-4-ones. Arch. Pharm. 2014, 347, 756–767. [Google Scholar] [CrossRef]
- Alagarsamy, V.; Senthilraja, M.; Raja Solomon, V. Design and synthesis of 3-substituted-thiazolyl-2-iminothiazolidin-4-ones as a new class of anticonvulsants. J. Heterocyclic Chem. 2016, 53, 1635–1639. [Google Scholar] [CrossRef]
- Siddiqui, N.; Ahsan, W. Triazole incorporated thiazoles as a new class of anticonvulsants: Design, synthesis and in vivo screening. Eur. J. Med. Chem. 2010, 45, 1536–1543. [Google Scholar] [CrossRef] [PubMed]
- Oliveira Pedrosa, M.D.; Duarte da Cruz, R.M.; Oliveira Viana, J.; de Moura, R.O.; Ishiki, H.M.; Bardosa, F.J.M.; Diniz, M.F.F.M.; Scotti, M.T.; Scotti, L.; Bezzera, M.F.J. Hybrid compounds as direct multitarget ligands: A review. Curr. Top. Med. Chem. 2017, 17, 1044–1079. [Google Scholar] [CrossRef] [PubMed]
- Song, M.X.; Deng, X.Q. Recent developments on triazolenucleus in anticonvulsant compounds: A review. J. Enzym. Inhib. Med. Chem. 2018, 33, 453–478. [Google Scholar] [CrossRef] [PubMed]
- Rohini, R.M.; Manjunath, M. Synthesis and anticonvulsant activity of triazothiole/thiazolylthiazolidinone derivatives of indole. Der. Pharm. Chem. 2012, 4, 2438–2441. [Google Scholar]
- Kaur, H.; Kumar, S.; Vishwakarma, P.; Sharma, M.; Saxena, K.K.; Kumar, A. Synthesis and antipsychotic and anticonvulsant activity of some new substituted oxa/thiadiazolylazetidinonyl/thiazolidinonylcarbazoles. Eur. J. Med. Chem. 2010, 45, 2777–2783. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, V.K.; Kumar, A. Synthesis of newer thiadiazolyl and thiazolidinonyl quinazolin-4(3H)-ones as potential anticonvulsant agents. Eur. J. Med. Chem. 2002, 37, 873–882. [Google Scholar] [CrossRef]
- Tripathi, A.C.; Gupta, S.J.; Fatima, G.N.; Sonar, P.K.; Verma, A.; Saraf, S.K. 4-Thiazolidinones: The advances continue. Eur. J. Med. Chem. 2014, 72, 52–77. [Google Scholar] [CrossRef]
- Myronenko, S.; Pinyazhko, O.; Lesyk, R. Effects of 4-thiazolidinone derivatives les-2658 and les-1205 on sleep-wakefulness cycle in kindled rats. Eureka Life Sci. 2017, 1, 51–56. [Google Scholar] [CrossRef][Green Version]
- Zimenkovsky, B.; Kutsyk, R.; Lesyk, R.; Matiychuk, V.; Obushak, N.; Klyufinska, T. Synthesis and antimicrobial activityof 2,4-dioxothiazolidine-5-acetic acid amides. Pharm. Chem. J. 2006, 40, 303–306. [Google Scholar] [CrossRef]
- Deghenghi, R.; Daneault, G. Orotic acid and its analogues: Part II. On the alkaline rearrangement of 5-carboxymethylidenehydantoin. Can. J. Chem. 1960, 38, 1255–1260. [Google Scholar] [CrossRef]
- Vicini, P.; Amoretti, L.; Chiavarini, M.; Impicciatore, M. Synthesis and local anesthetic activity of alkylaminoacyl derivatives of 3-amino-1,2-benzisothiazoles. Farmaco 1990, 45, 933–943. [Google Scholar] [PubMed]
- Vicini, P.; Geronikaki, A.; Anastasia, K.; Incerti, M.; Zani, F. Synthesis and antimicrobial activity of novel 2-thiazolylimino-5-arylidene-4-thiazolidinones. Bioorg. Med. Chem. 2006, 14, 3859–3864. [Google Scholar] [CrossRef]
- Golovenko, M.Y.; Gromov, L.O. Pre-clinical Study of the Specific Activity of the Potential Anticonvulsants: Methodical Recommendations; DC Ministry of Healthcare of Ukraine: Kyiv, Ukraine, 2003; 46p. (In Ukrainian) [Google Scholar]
- Mironov, A.N.; Bunyatyan, N.D.; Vasilyev, A.N. (Eds.) Guidelines for Conducting Preclinical Studies of Drugs; Part 1; Grif & Co: Moscow, Russia, 2012; 944p. (In Russian) [Google Scholar]
- Litchfield, J.T.; Wilcoxon, F. A simplified method of evaluating dose-effect experiments. J. Pharmacol. Exp. Ther. 1949, 96, 99–113. [Google Scholar] [PubMed]
- Smith, W.G. Pharmacological screening tests. Prog. Med. Chem. 1961, 1, 1–33. [Google Scholar] [CrossRef]
- Glanc, C. Medical-Biological Statistics; Practica: Moscow, Russian, 1998; 459p. [Google Scholar]
- Mishra, C.B.; Kumari, S.; Tiwari, M. Thiazole: A promising heterocycle for the development of potent CNS active agents. Eur. J. Med. Chem. 2015, 92, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Ayati, A.; Emami, S.; Asadipour, A.; Shafiee, A.; Foroumadi, A. Recent applications of 1,3-thiazole core structure in the identification of new lead compounds and drug discovery. Eur. J. Med. Chem. 2015, 97, 699–718. [Google Scholar] [CrossRef]
- Kaminskyy, D.; Den Hartog, G.J.; Wojtyra, M.; Lelyukh, M.; Gzella, A.; Bast, A.; Lesyk, R. Antifibrotic and anticancer action of 5-ene amino/iminothiazolidinones. Eur. J. Med. Chem. 2016, 112, 180–195. [Google Scholar] [CrossRef]
- Subtel’na, I.; Atamanyuk, D.; Szymanska, E.; Kiec-Kononowicz, K.; Zimenkovsky, B.; Vasylenko, O.; Gzella, A.; Lesyk, R. Synthesis of 5-arylidene-2-amino-4-azolones and evaluation of their anticancer activity. Bioorg. Med. Chem. 2010, 14, 5090–5102. [Google Scholar] [CrossRef]
- Nowaczyk, A.; Kowiel, M.; Gzella, A.; Fijałkowski, Ł.; Horishny, V.; Lesyk, R. Conformational space and vibrational spectra of 2-[(2,4-dimethoxyphenyl)amino]-1,3-thiazolidin-4-one. J. Mol. Model. 2014, 20, 1–9. [Google Scholar] [CrossRef]
- Kaminskyy, D.; Zimenkovsky, B.; Lesyk, R. Synthesis and in vitro anticancer activity of 2,4-azolidinedione-acetic acids derivatives. Eur. J. Med. Chem. 2009, 44, 3627–3636. [Google Scholar] [CrossRef]
- Stables, J.P.; Kupferberg, H.J. The NIH Anticonvulsant Drug Development (ADD) Program: Preclinical anticonvulsant screening project. In Molecular and Cellular Targets for Anti-Epileptic Drugs; Avarzini, G., Tanganelli, P., Avoli, M., Eds.; John Libbey& Co.: London, UK, 1997; pp. 191–198. [Google Scholar]
- Mula, M. GABAergic drugs in the treatment of epilepsy: Modern or out moded? Future Med. Chem. 2011, 3, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Wlaź, P.; Roliński, Z.; Kleinrok, Z.; Czuczwar, S.J. Anticonvulsant activity of carbamazepine and diphenylhydantoin against maximal electroshock in mice chronically treated with aminophylline. J. Neur. Trans. 1992, 89, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Vicini, P.; Geronikaki, A.; Incerti, M.; Zani, F.; Dearden, J.; Hewitt, M. 2-Heteroarylimino-5-benzylidene-4-thiazolidinones analogues of 2-thiazolylimino-5-benzylidene-4-thiazolidinones with antimicrobial activity: Synthesis and structure–activity relationship. Bioorg. Med. Chem. 2008, 16, 3714–3724. [Google Scholar] [CrossRef] [PubMed]
- Geronikaki, A.; Eleftheriou, P.; Vicini, P.; Alam, I.; Dixit, A.; Saxena, A.K. 2-Thiazolylimino/heteroarylimino-5-arylidene-4-thiazolidinones as new agents with SHP-2 inhibitory action. J. Med. Chem. 2008, 51, 5221–5228. [Google Scholar] [CrossRef]
- Löscher, W. Strategies for antiepileptogenesis: Antiepileptic drugs versus novel approaches evaluated in post-status epilepticus models of temporal lobe epilepsy. In Jasper’s Basic Mechanisms of the Epilepsies, 4th ed.; National Center for Biotechnology Information: Bethesda, MA, USA, 2012. Available online: https://www.ncbi.nlm.nih.gov/books/NBK98148/ (accessed on 1 March 2020).
- Lesyk, R.B.; Pinyazko, O.R.; Myronenko, S.I.; Nektegayev, I.O. Patent UA112347 U Ethyl-2-[2,4-dioxo-5-[2-oxo-2-(Thiazol-2-Ylamino)ethyl]Thiazolidin-3-yl]Acetate, Which Possessed Anticonvulsant Activity. Date of Application 17.06.2016; Published 12.12.2016, Bul.№ 23. Available online: https://sis.ukrpatent.org/uk/search/detail/822102/ (accessed on 1 March 2020). (In Ukrainian).
| Group | n | Latent Period, min | Number of Clonic-Tonic Seizures per Mouse | % of Mice with Seizures | Severity of Seizures, Points | Period of Seizures, min | Time to Death, min | Mortality, % | |
|---|---|---|---|---|---|---|---|---|---|
| Clonic | Tonic | ||||||||
| Control | 12 | 5.63 ± 0.76 | 3.42 ± 0.47 | 100 | 91.67 | 5.58 ± 0.29 | 9.68 ± 1.96 | 15.71 ± 2.70 | 83.33 |
| SV | 12 | 34.06 ± 7.87 ** | 1.42 ± 0.53 * | 50 ** | 33.33 ** | 2.00 ± 0.64 ** | 5.10 ± 2.24 * | 20.9 | 8.33 ** |
| Ia | 6 | 9.58 ± 2.11 | 1.50 ± 0.34 ** | 100 ## | 83.33 | 5.50 ± 0.50 ## | 2.92 ± 2.62 * | 12.61 ± 3.32 | 83.33 ## |
| Ib | 6 | 43.59 ± 10.58 * | 0.50 ± 0.34 ** | 33.33 ** | 16.67 ** | 1.50 ± 1.03 ** | 1.40 ± 1.38 ** | 26.98 | 16.67 ** |
| Ic | 6 | 20.26 ± 8.57 * | 1.50 ± 0.34 ** | 83.33 | 66.67 | 4.17 ± 0.98 | 4.66 ± 2.99 | 11.72 ± 4.15 | 50.00 |
| Id | 6 | 3.89 ± 1.38 ## | 2.17 ± 0.31 | 100 ## | 100 ## | 5.67 ± 0.33 ## | 6.87 ± 2.37 | 10.76 ± 2.31 | 83.33 ## |
| Ie | 6 | 12.73 ± 9.46 # | 2.67 ± 0.88 | 83.33 | 66.67 | 4.17 ± 0.98 | 7.97 ± 2.92 | 9.51 ± 3.10 | 50.00 |
| IIb | 6 | 15.55 ± 9.20 | 1.50 ± 0.56 * | 83.33 | 83.33 | 4.67 ± 0.99 # | 4.41 ± 3.65 * | 10.35 ± 5.46 | 66.67 ## |
| IIc | 6 | 5.51 ± 2.30 # | 2.17 ± 0.75 | 100 ## | 100 ## | 5.33 ± 0.42 ## | 2.25 ± 1.36 * | 4.91 ± 1.62 * | 66.67 ## |
| IId | 6 | 16.92 ± 8.90 | 2.33 ± 0.76 | 83.33 | 50.00 * | 3.67 ± 0.88 * | 7.64 ± 2.79 | 9.23 | 16.67 ** |
| IIe | 6 | 13.33 ± 9.37 | 2.17 ± 0.70 | 83.33 | 66.67 | 4.17 ± 0.98 | 4.64 ± 1.86 | 12.22 ± 2.79 | 50.00 |
| IIf | 6 | 4.29 ± 0.66 ## | 1.83 ± 0.31 * | 100 ## | 83.33 | 5.17 ± 0.54 # | 7.38 ± 2.63 | 11.74 ± 3.22 | 66.67 ## |
| IIg | 6 | 3.63 ± 1.17 ## | 2.67 ± 0.92 | 100 ## | 66.67 | 4.67 ± 0.62 # | 8.39 ± 2.74 | 10.59 ± 4.42 | 50.00 |
| IIh | 6 | 3.43 ± 0.46 ## | 2.00 ± 0.52 | 100 ## | 66.67 | 5.00 ± 0.63 # | 7.06 ± 5.33 | 13.72 ± 7.55 | 66.67 ## |
| IIi | 6 | 14.81 ± 9.17 | 2.50 ± 0.76 | 83.33 | 83.33 | 4.50 ± 0.96 # | 8.45 ± 3.99 | 15.34 ± 5.02 | 50.00 |
| IIj | 6 | 6.37 ± 2.05 # | 2.00 ± 0.52 | 100 ## | 83.33 | 4.50 ± 0.50 # | 2.93 ± 1.99 * | 11.38 ± 4.18 | 33.33 * |
| Group | n | % of Mice with Seizures | Severity of Seizures, Points | Period of Seizures, min | Recovery Period (Lateral Position), min | Ttime to Death, min | Mortality, % | |
|---|---|---|---|---|---|---|---|---|
| Clonic | Tonic | |||||||
| Control | 17 | 100 | 100 | 5.53 ± 0.13 | 0.24 ± 0.03 | 0.80 ± 0.16 | 0.30 ± 0.03 | 52.94 |
| CM | 14 | 100 | 92.86 | 4.78 ± 0.28 * | 0.10 ± 0.03 ** | 0.41 ± 0.14 ** | 0.32 ± 0.02 | 35.71 |
| Ib | 14 | 100 | 78.57 ** | 4.57 ± 0.33 * | 0.08 ± 0.03 ** | 0.27 ± 0.27 ** | 0.28 ± 0.03 | 35.71 |
| IId | 14 | 100 | 85.71 * | 5.00 ± 0.33 | 0.13 ± 0.03 * | 0.23 ± 0.03 ** | 0.30 ± 0.02 | 57.14 |
| IIj | 14 | 100 | 78.57 ** | 4.71 ± 0.34 | 0.10 ± 0.03 ** | 0.32 ± 0.10 ** | 0.26 ± 0.01 | 42.86 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mishchenko, M.; Shtrygol, S.; Kaminskyy, D.; Lesyk, R. Thiazole-Bearing 4-Thiazolidinones as New Anticonvulsant Agents. Sci. Pharm. 2020, 88, 16. https://doi.org/10.3390/scipharm88010016
Mishchenko M, Shtrygol S, Kaminskyy D, Lesyk R. Thiazole-Bearing 4-Thiazolidinones as New Anticonvulsant Agents. Scientia Pharmaceutica. 2020; 88(1):16. https://doi.org/10.3390/scipharm88010016
Chicago/Turabian StyleMishchenko, Mariia, Sergiy Shtrygol, Danylo Kaminskyy, and Roman Lesyk. 2020. "Thiazole-Bearing 4-Thiazolidinones as New Anticonvulsant Agents" Scientia Pharmaceutica 88, no. 1: 16. https://doi.org/10.3390/scipharm88010016
APA StyleMishchenko, M., Shtrygol, S., Kaminskyy, D., & Lesyk, R. (2020). Thiazole-Bearing 4-Thiazolidinones as New Anticonvulsant Agents. Scientia Pharmaceutica, 88(1), 16. https://doi.org/10.3390/scipharm88010016






