Cocrystal of Ibuprofen–Nicotinamide: Solid-State Characterization and In Vivo Analgesic Activity Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Drugs and Chemicals
2.2. Preparation of Physical Mixtures and Cocrystals by Slow Evaporation
2.3. Solid-State Characterization
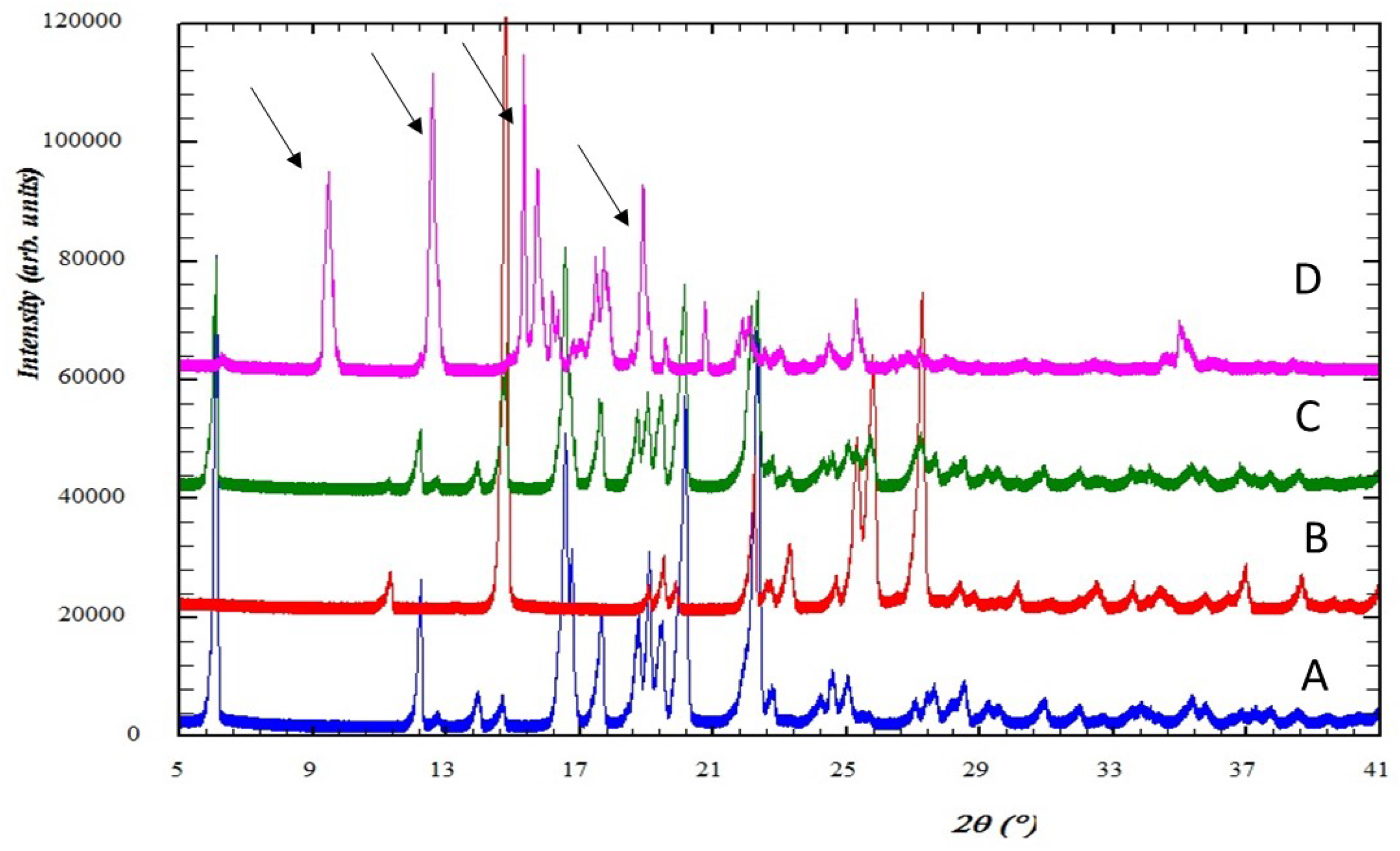
2.3.1. Powder X-ray Diffraction (PXRD) Analysis
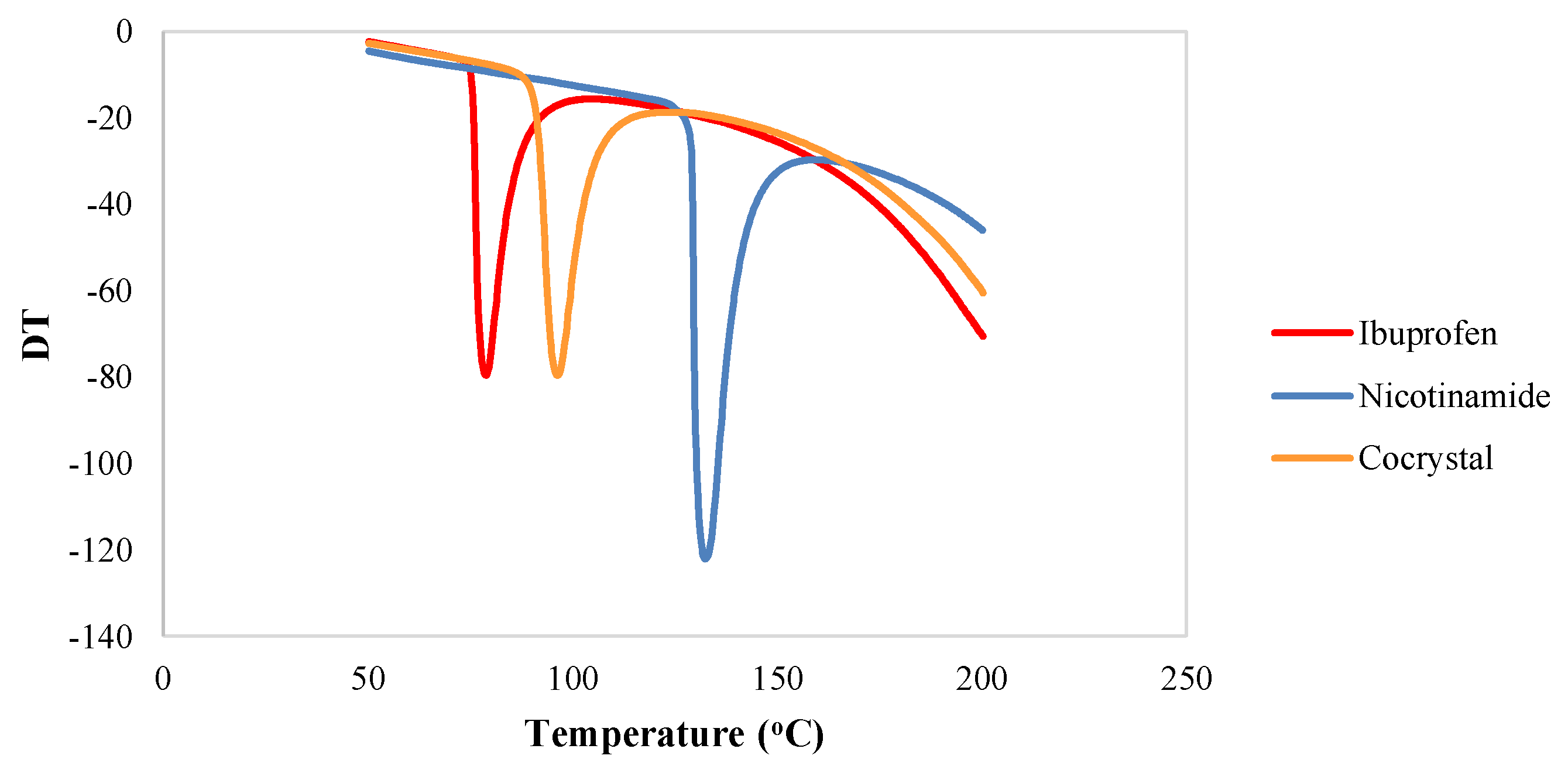
2.3.2. Differential Thermal Analysis (DTA)
2.3.3. Microscopic Analysis
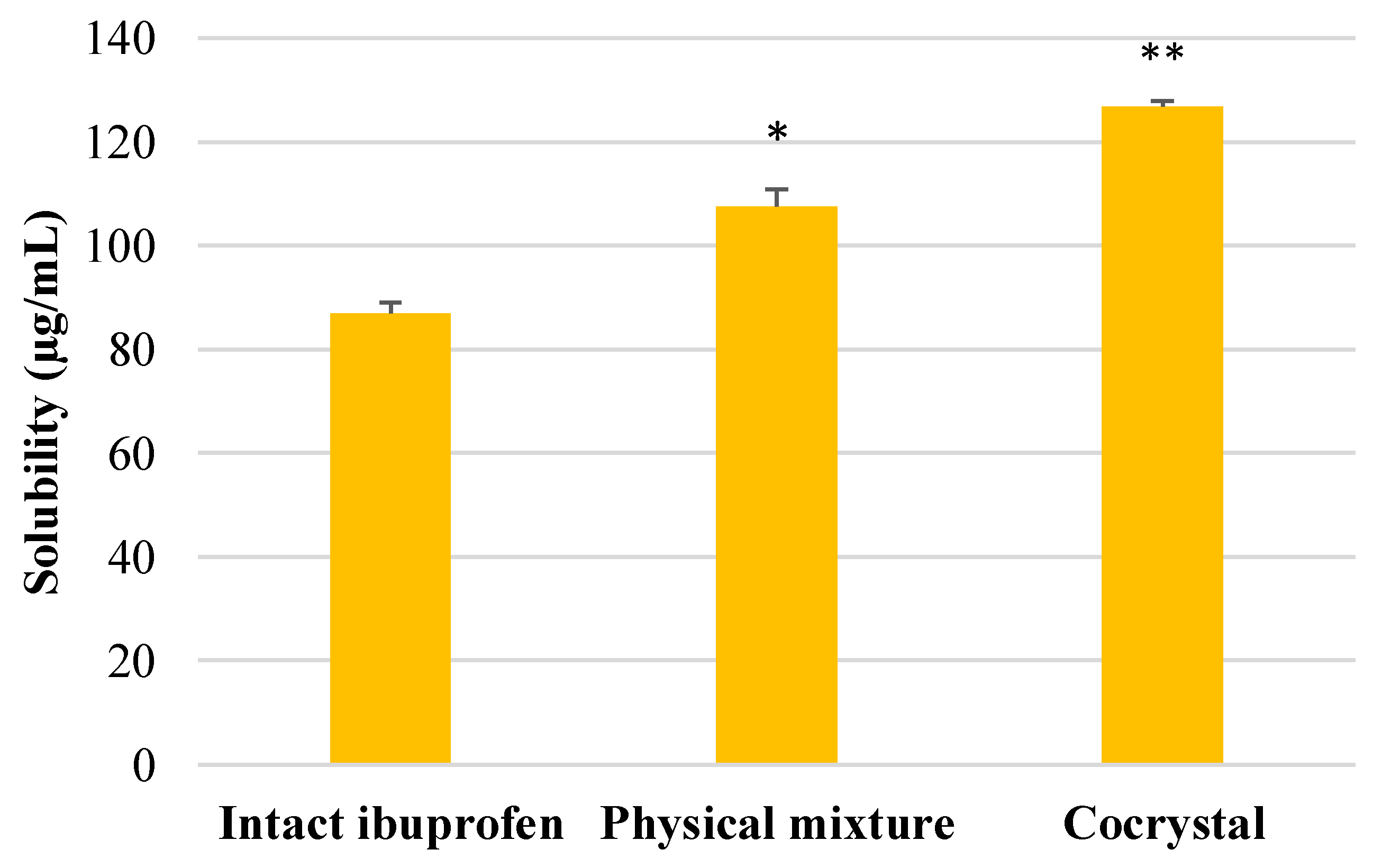
2.4. Solubility Test
2.5. In Vivo Evaluation of Analgesic Activity
2.5.1. Animal Preparation
2.5.2. Analgesic Activity Evaluation
- Wc = Number of writhes in control group
- Wt = Number of writhes in test group
2.5.3. Statistical Analysis
3. Results
3.1. Solid State Characterization
3.1.1. Powder X-ray Diffraction (PXRD) Patterns
3.1.2. Differential Thermal Analysis (DTA) Thermogram
3.1.3. Scanning Electron Micrographs
3.2. Solubility Measurement
3.3. In vivo Analgesic Activity of Cocrystal Ibuprofen
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Laska, E.M.; Sunshine, A.; Marrero, I.; Olson, N.; Siegel, C.; McCormick, N. The correlation between blood levels of ibuprofen and clinical analgesic response. Clin. Pharmacol. Ther. 1986, 40, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Newa, M.; Bhandari, K.H.; Li, D.X.; Kwon, T.H.; Kim, J.A.; Yoo, B.K.; Woo, J.S.; Lyoo, W.S.; Yong, C.S.; Choi, H.G. Preparation, characterization and in vivo evaluation of ibuprofen binary solid dispersions with poloxamer 188. Int. J. Pharm. 2007, 343, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Potthast, H.; Dressman, J.B.; Junginger, H.E.; Midha, K.K.; Oeser, H.; Shah, V.P.; Vogelpoel, H.; Barends, D.M. Biowaiver monographs for immediate release solid oral dosage forms: Ibuprofen. J. Pharm. Sci. 2005, 94, 2121–2131. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Kwon, R.; Quan, Q.Z.; Oh, D.H.; Kim, J.O.; Hwang, M.R.; Koo, Y.B.; Woo, J.S.; Yong, C.S.; Choi, H.G. Development of novel ibuprofen-loaded solid dispersion with improved bioavailability using aqueous solution. Arch. Pharm. Res. 2009, 32, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Salústio, P.J.; Cabral-Marques, H.M.; Costa, P.C.; Pinto, J.F. Comparison of ibuprofen release from minitablets and capsules containing ibuprofen: β-Cyclodextrin complex. Eur. J. Pharm. Biopharm. 2011, 78, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.C.; Ng, W.K.; Chia, L.; Dong, Y.C.; Tan, R.B.H. Stabilized amorphous state of ibuprofen by co-spray drying with mesoporous SBA-15 to enhance dissolution properties. J. Pharm. Sci. 2010, 99, 1997–2007. [Google Scholar] [CrossRef] [PubMed]
- Serrano, D.R.; Walsh, D.; O’Connell, P.; Mugheirbi, N.A.; Worku, Z.A.; Bolas-Fernandez, F.; Galiana, C.; Dea-Ayuela, M.A.; Healy, A.M. Optimising the in vitro and in vivo performance of oral cocrystal formulations via spray coating. Eur. J. Pharm. Biopharm. 2018, 124, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Chow, S.F.; Chen, M.; Shi, L.; Chow, A.H.L.; Sun, C.C. Simultaneously improving the mechanical properties, dissolution performance, and hygroscopicity of ibuprofen and flurbiprofen by cocrystallization with nicotinamide. Pharm. Res. 2012, 29, 1854–1865. [Google Scholar] [CrossRef] [PubMed]
- Aitipamula, S.; Banerjee, R.; Bansal, A.K.; Biradha, K.; Cheney, M.L.; Choudhury, A.R.; Desiraju, G.R.; Dikundwar, A.G.; Dubey, R.; Duggirala, N.; et al. Polymorphs, salts, and cocrystals: What’s in a name? Cryst. Growth Des. 2012, 12, 2147–2152. [Google Scholar] [CrossRef]
- Hiendrawan, S.; Veriansyah, B.; Widjojokusumo, E.; Soewandhi, S.N.; Wikarsa, S.; Tjandrawinata, R.R. Physicochemical and mechanical properties of paracetamol cocrystal with 5-nitroisophthalic acid. Int. J. Pharm. 2016, 497, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.S.; Kim, J.S.; Kim, M.S.; Alhalaweh, A.; Cho, W.; Hwang, S.J.; Velaga, S.P. Bioavailability of indomethacin-saccharin cocrystals. J. Pharm. Pharmacol. 2010, 62, 1560–1568. [Google Scholar] [CrossRef] [PubMed]
- Putra, O.D.; Umeda, D.; Nugraha, Y.P.; Furuishi, T.; Nagase, H.; Fukuzawa, K.; Uekusa, H.; Yonemochi, E. Solubility improvement of epalrestat by layered structure formation via cocrystallization. Cryst. Eng. Commun. 2017, 19, 2614–2622. [Google Scholar] [CrossRef]
- Jones, W.; Motherwell, W.D.S.; Trask, A.V. Pharmaceutical cocrystals: An emerging approach to physical property enhancement. MRS Bull. 2006, 31, 875–879. [Google Scholar] [CrossRef]
- Duggirala, N.K.; Perry, M.L.; Almarsson, Ö.; Zaworotko, M.J. Pharmaceutical cocrystals: Along the path to improved medicines. Chem. Commun. 2016, 52, 640–655. [Google Scholar] [CrossRef] [PubMed]
- Alshahateet, S.F. Synthesis and X-ray crystallographic analysis of pharmaceutical model rac-ibuprofen cocrystal. J. Chem. Crystallogr. 2011, 41, 276–279. [Google Scholar] [CrossRef]
- Kelly, A.L.; Gough, T.; Dhumal, R.S.; Halsey, S.A.; Paradkar, A. Monitoring ibuprofen-nicotinamide cocrystal formation during solvent free continuous cocrystallization (SFCC) using near infrared spectroscopy as a PAT tool. Int. J. Pharm. 2012, 426, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Soares, F.L.F.; Carneiro, R.L. Green synthesis of ibuprofen-nicotinamide cocrystals and in-line evaluation by Raman spectroscopy. Cryst. Growth Des. 2013, 13, 1510–1517. [Google Scholar] [CrossRef]
- Schleyerbach, R.; Weithmann, K.U.; Bartlett, R.R. Analgesic, anti-inflammatory, and anti-pyretic activity. In Drug Discovery and Evaluation: Pharmacological Assays, 4th ed.; Hock, F.J., Ed.; Springer: Berlin, Germany, 2015; ISBN 9783319053929. [Google Scholar]
- Thayer, A.M.; Houston, E.N. Finding Solutions: Custom manufacturers take on drug solubility issues to help pharmaceutical firms move products through development. Chemical & Engineering News, 31 May 2010; 13–18. [Google Scholar]
- Alshahateet, S.F. Synthesis and supramolecularity of hydrogen-bonded cocrystals of pharmaceutical model rac-ibuprofen with pyridine derivatives. Mol. Cryst. Liq. Cryst. 2010, 533, 152–161. [Google Scholar] [CrossRef]
- Harmsen, B.; Leyssens, T. Dual-Drug Chiral Resolution: Enantiospecific Cocrystallization of (S)-Ibuprofen Using Levetiracetam. Cryst. Growth Des. 2018, 18, 441–448. [Google Scholar] [CrossRef]
- Zaini, E.; Sumirtapura, Y.C.; Halim, A.; Fitriani, L.; Soewandhi, S.N. Formation and characterization of sulfamethoxazole-trimethoprim cocrystal by milling process. J. Appl. Pharm. Sci. 2017, 7, 169–173. [Google Scholar] [CrossRef]
- Berry, D.J.; Seaton, C.C.; Clegg, W.; Harrington, R.W.; Coles, S.J.; Horton, P.N.; Hursthouse, M.B.; Storey, R.; Jones, W.; Friščić, T.; et al. Applying hot-stage microscopy to co-crystal screening: A study of nicotinamide with seven active pharmaceutical ingredients. Cryst. Growth Des. 2008, 8, 1697–1712. [Google Scholar] [CrossRef]
- Haleblian, J.K. Characterization of habits and crystalline modification of solids and their pharmaceutical applications. J. Pharm. Sci. 1975, 64, 1269–1288. [Google Scholar] [CrossRef] [PubMed]
- Zaini, E.; Sumirtapura, Y.C.; Soewandhi, S.N.; Halim, A.; Uekusa, H.; Fujii, K. Cocrystalline phase transformation of binary mixture of trimethoprim and sulfamethoxazole by slurry technique. Asian J. Pharm. Clin. Res. 2010, 3, 26–29. [Google Scholar]
- Garekani, H.A.; Sadeghi, F.; Badiee, A.; Mostafa, S.A.; Rajabi-Siahboomi, A.R.; Rajabi-Siahboomi, A.R. Crystal habit modifications of ibuprofen and their physicomechanical characteristics. Drug Dev. Ind. Pharm. 2001, 27, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Dressman, J.B.; Vertzoni, M.; Goumas, K.; Reppas, C. Estimating drug solubility in the gastrointestinal tract. Adv. Drug Deliv. Rev. 2007, 59, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Sunada, H. Mechanistic studies on hydrotropic solubilization of nifedipine in nicotinamide solution. Chem. Pharm. Bull. 1998, 46, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.-F.; Tong, W.-Q. Impact of solid state properties on developability assessment of drug candidates. Adv. Drug Deliv. Rev. 2004, 56, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Pawar, Y.B.; Shete, G.; Popat, D.; Bansal, A.K. Phase behavior and oral bioavailability of amorphous Curcumin. Eur. J. Pharm. Sci. 2012, 47, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, B.; Gao, Y.; Zhang, J.; Shi, L. Baicalein-nicotinamide cocrystal with enhanced solubility, dissolution, and oral bioavailability. J. Pharm. Sci. 2014, 103, 2330–2337. [Google Scholar] [CrossRef] [PubMed]
- Dressman, J.B.; Reppas, C. In vitro-in vivo correlations for lipophilic, poorly water-soluble drugs. Eur. J. Pharm. Sci. 2000, 11, S73–S80. [Google Scholar] [CrossRef]
- Tasleem, F.; Azhar, I.; Ali, S.N.; Perveen, S.; Mahmood, Z.A. Analgesic and anti-inflammatory activities of Piper nigrum L. Asian Pac. J. Trop. Med. 2014, 7, S461–S468. [Google Scholar] [CrossRef]
- Amidon, G.L.; Lennernas, H.; Shah, V.P.; Crison, J.R. A theoretical basis for a Biopharmaceutic Drug Classification: The correlation of in-vitro drug product dissolution and in-vivo bioavailability. Pharm. Res. 1995, 12, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Suleiman, M.S.; Najib, N.M.; Hassan, M.A.; Abdel-Hamid, M.E. Physico-chemical characterization of a new salt of ibuprofen. J. Pharm. Biomed. Anal. 1990, 8, 321–327. [Google Scholar] [CrossRef]
- Motola, S.; Branfman, A.R.; Agisim, G.R.; Quirk, D.J. Acid Addition Salt of Ibuprofen and Meglumine. U.S. Patent No 5028625, 20 June 1989. [Google Scholar]
- Lee, T.; Wang, Y.W. Initial salt screening procedures for manufacturing ibuprofen. Drug Dev. Ind. Pharm. 2009, 35, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, P.; Black, P.; Papageorge, M.; Norwood, T.; Shen, D.D.; Norris, L.; Ardia, A. Ibuprofen arginate provides effective relief from postoperative dental pain with a more rapid onset of action than ibuprofen. Eur. J. Clin. Pharmacol. 2002, 58, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Geisslinger, G.; Dietzel, K.; Bezler, H.; Nuernberg, B.; Brune, K. Therapeutically relevant differences in the pharmacokinetical and pharmaceutical behavior of ibuprofen lysinate as compared to ibuprofen acid. Int. J. Clin. Pharmacol. Ther. Toxicol. 1989, 27, 324–328. [Google Scholar] [PubMed]

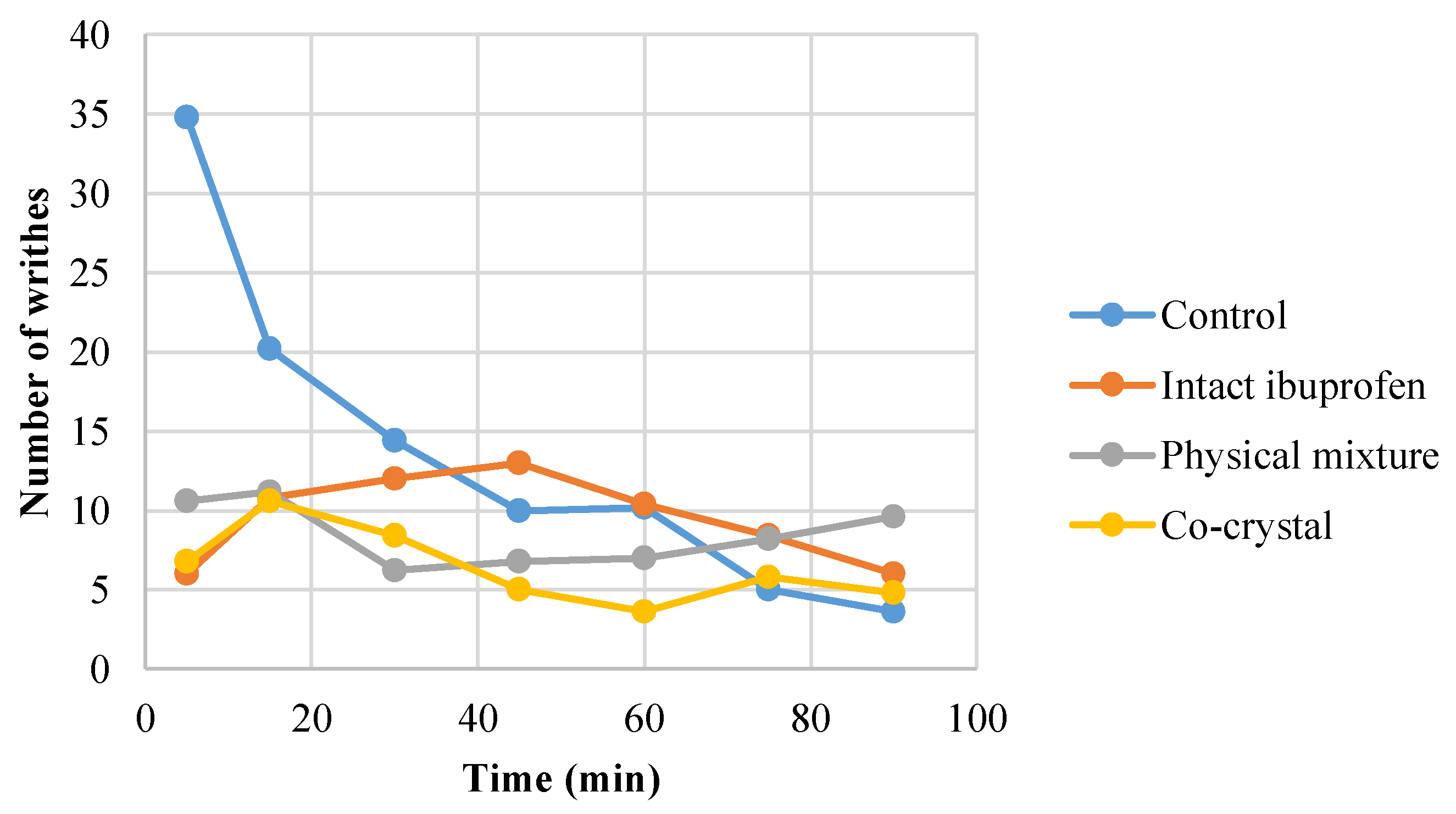
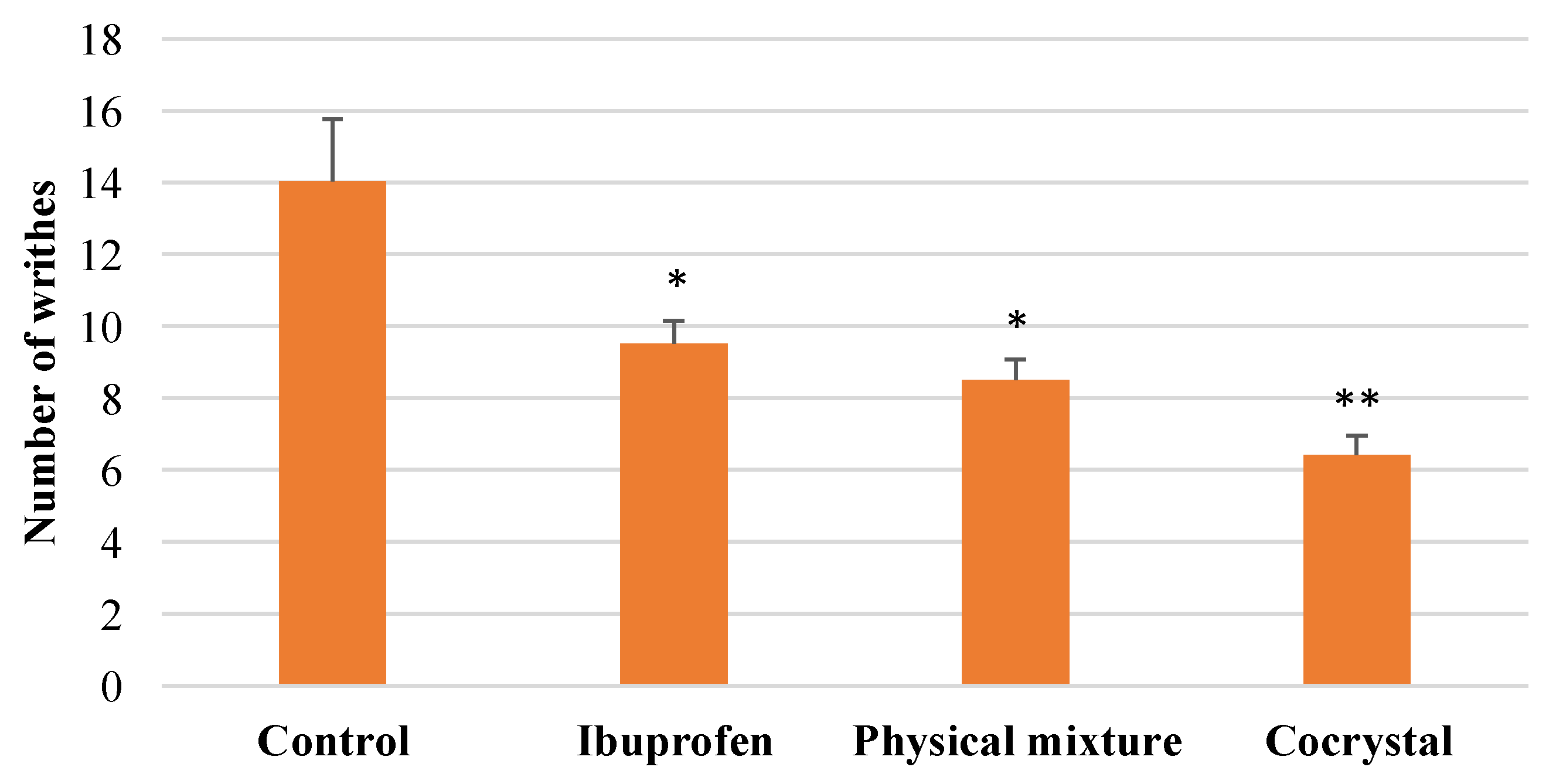
| No | Compound | Total Number of Writhes | Pain Inhibition (%) |
|---|---|---|---|
| 1 | Control | 98.2 ± 4.76 | 0 |
| 2 | Intact ibuprofen | 66.6 ± 2.63 | 32.18 |
| 3 | Physical mixture | 59.6 ± 2.7 | 39.31 |
| 4 | Cocrystalline phase | 45.0 ± 5.34 | 54.17 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuliandra, Y.; Zaini, E.; Syofyan, S.; Pratiwi, W.; Putri, L.N.; Pratiwi, Y.S.; Arifin, H. Cocrystal of Ibuprofen–Nicotinamide: Solid-State Characterization and In Vivo Analgesic Activity Evaluation. Sci. Pharm. 2018, 86, 23. https://doi.org/10.3390/scipharm86020023
Yuliandra Y, Zaini E, Syofyan S, Pratiwi W, Putri LN, Pratiwi YS, Arifin H. Cocrystal of Ibuprofen–Nicotinamide: Solid-State Characterization and In Vivo Analgesic Activity Evaluation. Scientia Pharmaceutica. 2018; 86(2):23. https://doi.org/10.3390/scipharm86020023
Chicago/Turabian StyleYuliandra, Yori, Erizal Zaini, Syofyan Syofyan, Wenny Pratiwi, Lidiya Novita Putri, Yuti Sahra Pratiwi, and Helmi Arifin. 2018. "Cocrystal of Ibuprofen–Nicotinamide: Solid-State Characterization and In Vivo Analgesic Activity Evaluation" Scientia Pharmaceutica 86, no. 2: 23. https://doi.org/10.3390/scipharm86020023
APA StyleYuliandra, Y., Zaini, E., Syofyan, S., Pratiwi, W., Putri, L. N., Pratiwi, Y. S., & Arifin, H. (2018). Cocrystal of Ibuprofen–Nicotinamide: Solid-State Characterization and In Vivo Analgesic Activity Evaluation. Scientia Pharmaceutica, 86(2), 23. https://doi.org/10.3390/scipharm86020023







