Influence of Ispaghula and Zein Coating on Ibuprofen-Loaded Alginate Beads Prepared by Vibration Technology: Physicochemical Characterization and Release Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Ibuprofen-Loaded Alginate Beads
2.3. Preparation of Ibuprofen-Loaded Alginate–Ispaghula Beads
2.4. Coating with Zein
2.5. Drug Loading and Entrapment Efficiency
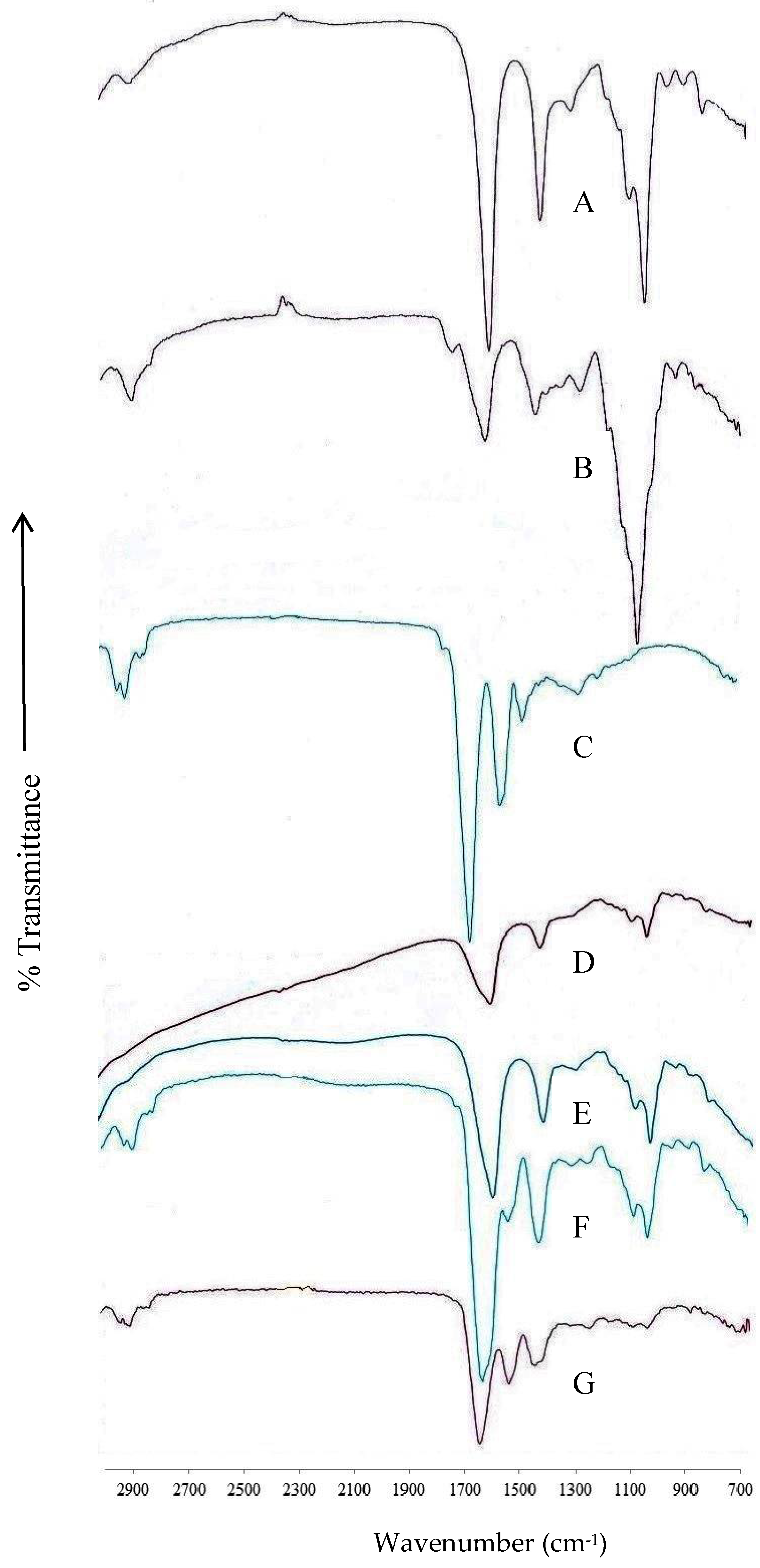
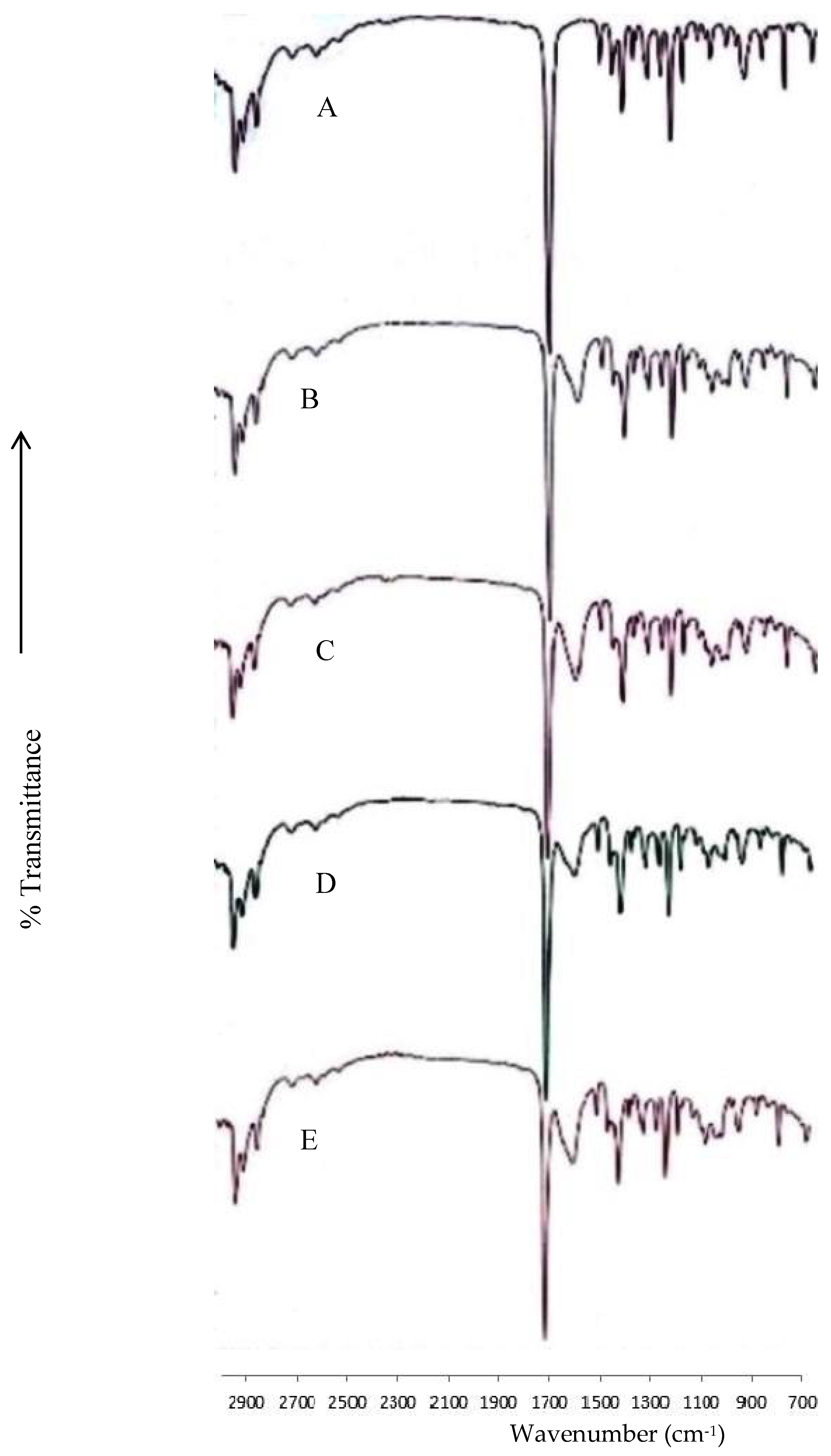
2.6. Fourier-Transform Infrared Spectroscopy
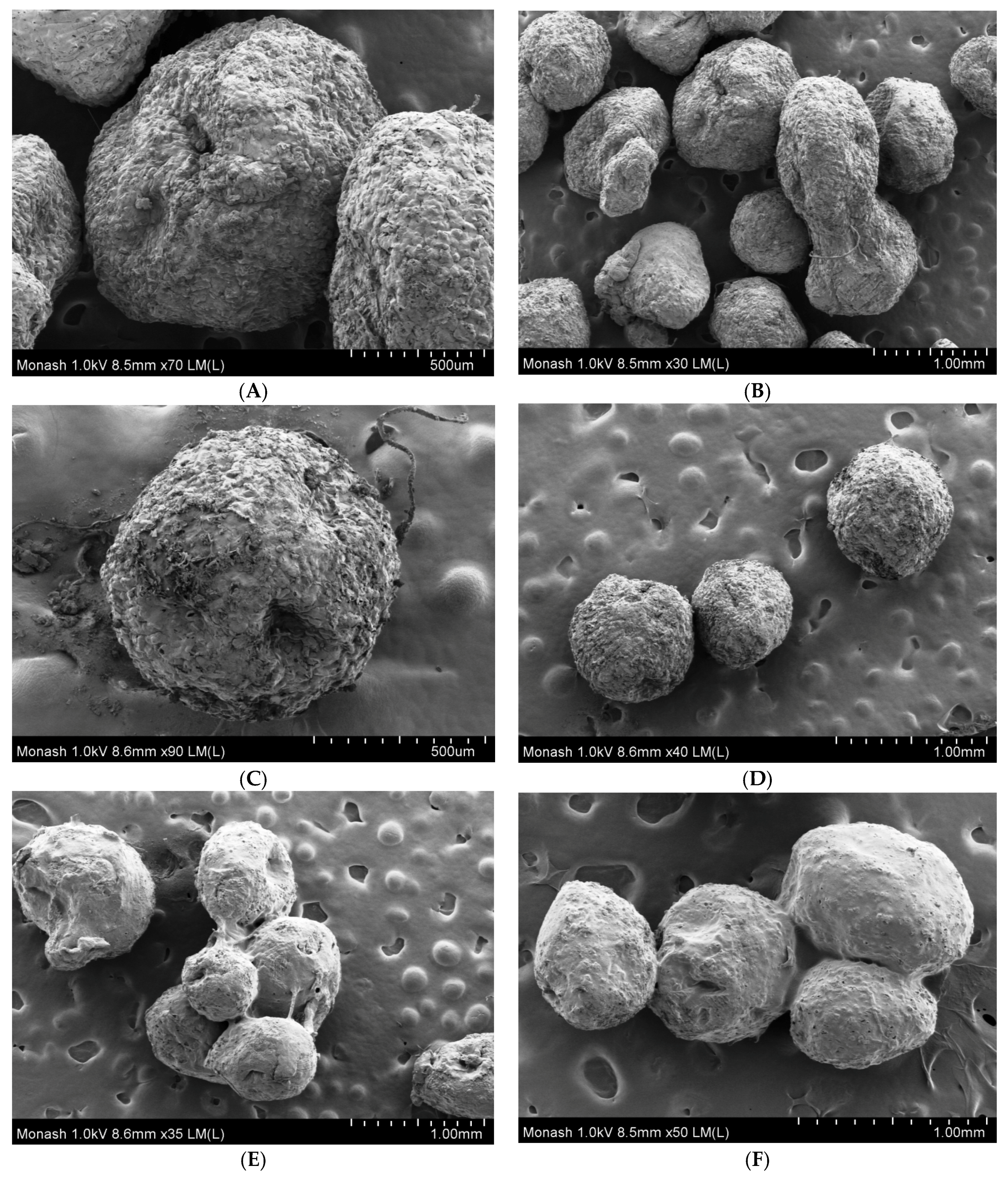
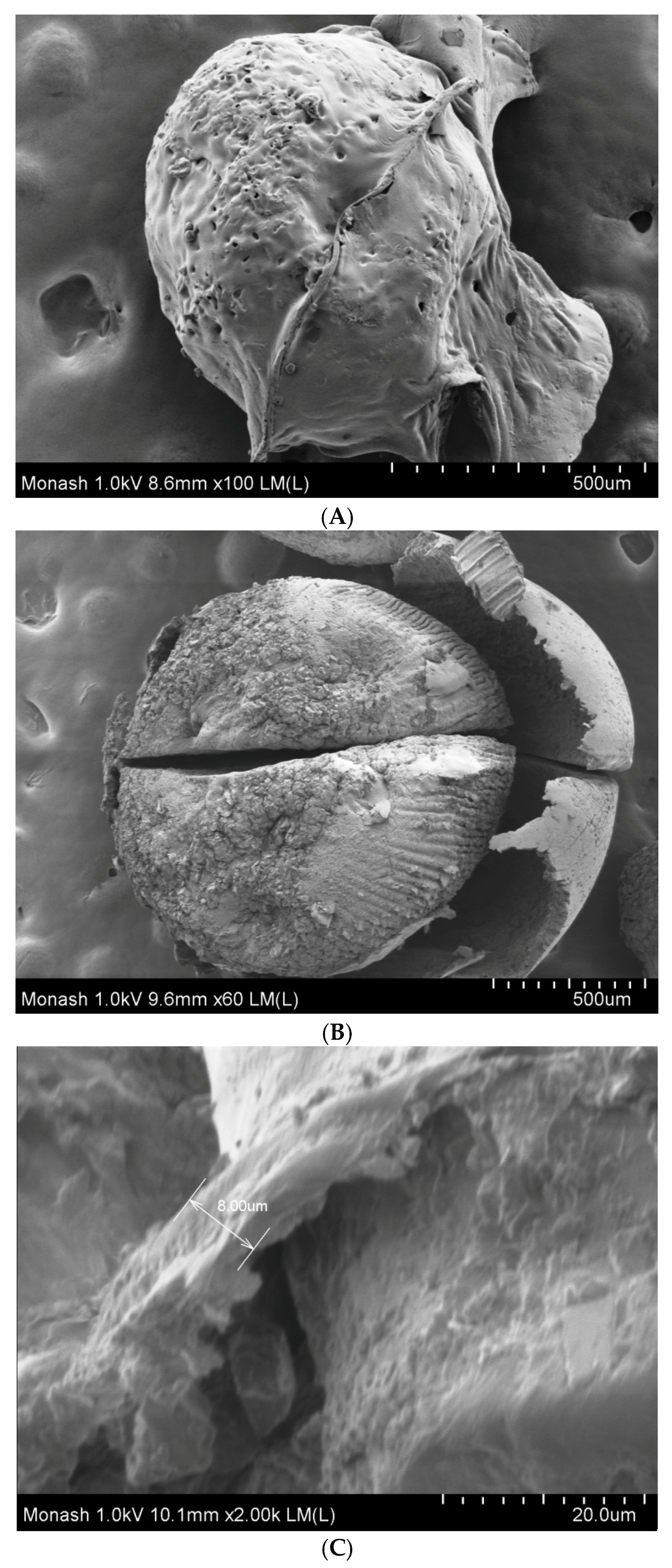
2.7. Field-Emission Scanning Electron Microscopy
2.8. Differential Scanning Calorimetry
2.9. In-Vitro Release
2.10. Statistical Analysis
3. Results and Discussion
3.1. Preparation and Entrapment Efficiency of Alginate Beads
3.2. Fourier-Transform Infrared Spectroscopy
3.3. Field-Emission Scanning Electron Microscopy
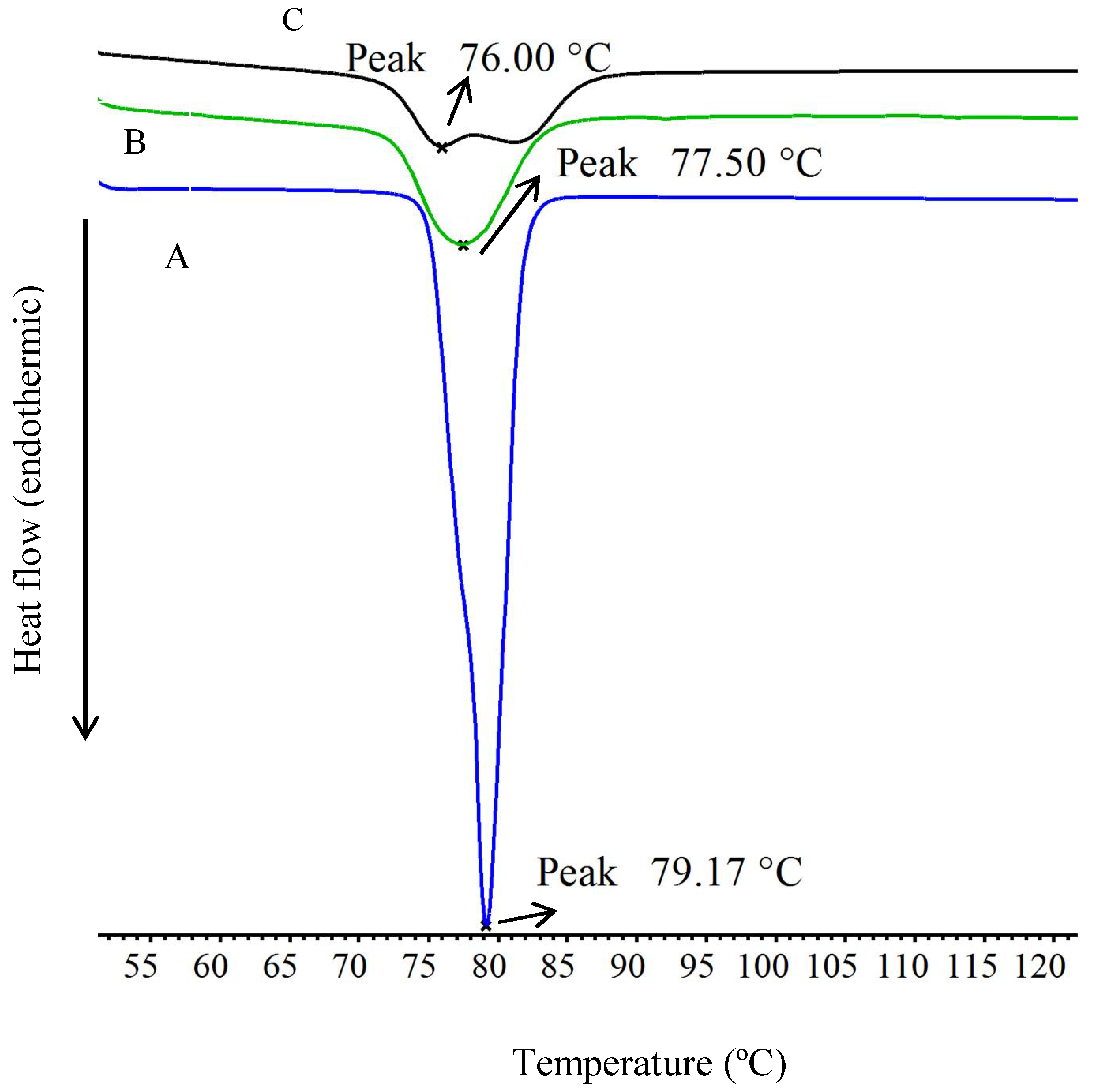
3.4. Differential Scanning Calorimetry
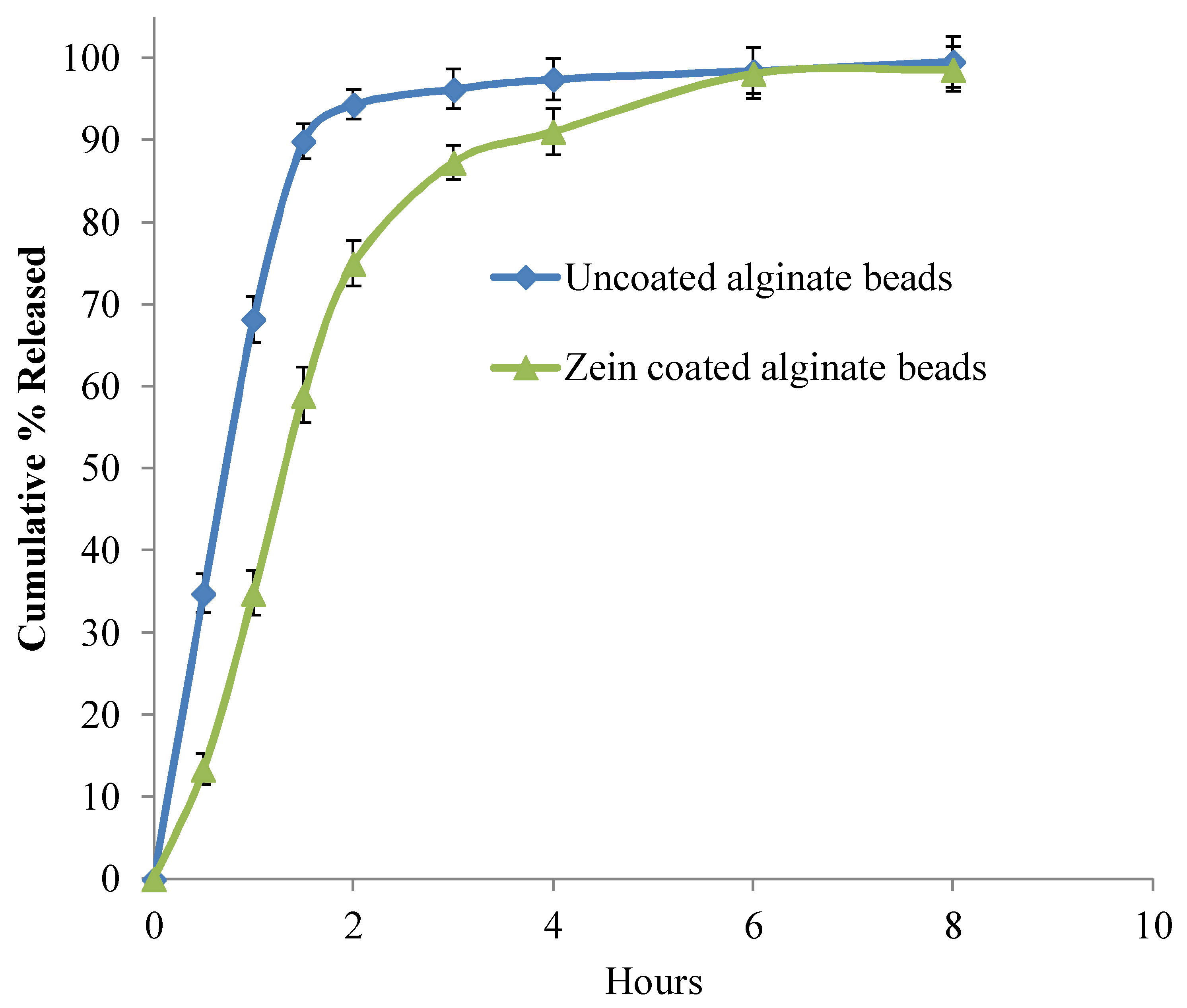
3.5. In-Vitro Release Studies
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Jain, D.; Bar-Shalom, D. Alginate drug delivery systems: Application in context of pharmaceutical and biomedical research. Drug Dev. Ind. Pharm. 2014, 40, 1576–1584. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.J.; Rhee, G.J.; Lee, K.M.; Oh, K.H.; Kim, C.K. Release characteristics of ibuprofen from excipient loaded alginate gel beads. Int. J. Pharm. 1995, 116, 125–128. [Google Scholar] [CrossRef]
- Agarwal, T.; Narayana, S.; Pal, K.; Pramanik, K.; Giri, S.; Banerjee, I. Calcium alginate-carboxymethyl cellulose beads for colon-targeted drug delivery. Int. J. Biol. Macromol. 2015, 75, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Maurya, D.P.; Sultana, Y.; Aqil, M.; Panda, B.P.; Ali, A. Formulation and Optimization of Alkaline Extracted Ispaghula Husk Microscopic Reservoirs of Isoniazid by Box-Behnken Statistical Design. J. Dispers. Sci. Technol. 2011, 32, 424–432. [Google Scholar] [CrossRef]
- Bulut, E.; Dilek, M. Development and characterization of pH-sensitive locust bean gum-alginate microspheres for controlled release of ibuprofen. J. Drug Deliv. Sci. Technol. 2014, 24, 613–619. [Google Scholar] [CrossRef]
- Nayak, A.K.; Pal, D. Formulation optimization and evaluation of jackfruit seed starch-alginate mucoadhesive beads of metformin HCl. Int. J. Biol. Macromol. 2013, 59, 264–272. [Google Scholar] [CrossRef] [PubMed]
- El-Kamel, A.H.; Al-Gohary, O.M.; Hosny, E.A. Alginate-diltiazem hydrochloride beads: Optimization of formulation factors, in vitro and in vivo availability. J. Microencapsul. 2003, 20, 211–225. [Google Scholar] [CrossRef] [PubMed]
- Raja, D.; Poornima, D.; Bhaskar, K.; Saravanan, M. Ispaghula husk based extended release tablets: Formulation, evaluation and in vitro release studies. Trop. J. Pharm. Res. 2014, 13, 205–210. [Google Scholar] [CrossRef]
- Maurya, D.P.; Sultana, Y.; Aqil, M.; Kumar, D.; Chuttani, K.; Ali, A.; Mishra, A.K. Formulation and optimization of alkaline extracted ispaghula husk microparticles of isoniazid—In vitro and in vivo assessment. J. Microencapsul. 2011, 28, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.K.; Pal, D.; Santra, K. Plantago ovata F. Mucilage-Alginate Mucoadhesive Beads for Controlled Release of Glibenclamide: Development, Optimization, and In Vitro-In Vivo Evaluation. J. Pharm. 2013, 11. [Google Scholar] [CrossRef]
- Muller, V.; Piai, J.F.; Fajardo, A.R.; Favaro, S.L.; Rubira, A.F.; Muniz, E.C. Preparation and Characterization of Zein and Zein-Chitosan Microspheres with Great Prospective of Application in Controlled Drug Release. J. Nanomater. 2011, 6. [Google Scholar] [CrossRef]
- Alcantara, A.C.S.; Aranda, P.; Darder, M.; Ruiz-Hitzky, E. Bionanocomposites based on alginate-zein/layered double hydroxide materials as drug delivery systems. J. Mater. Chem. 2010, 20, 9495–9504. [Google Scholar] [CrossRef]
- Muniyandy, S.; Sathasivam, T.; Veeramachineni, A.K.; Janarthanan, P. Dual crosslinked carboxymethyl sago pulp-gelatine complex coacervates for sustained drug delivery. Polymers 2015, 7, 1088–1105. [Google Scholar] [CrossRef]
- Belscak-Cvitanovic, A.; Komes, D.; Karlovic, S.; Djakovic, S.; Spoljaric, I.; Mrsic, G.; Jezek, D. Improving the controlled delivery formulations of caffeine in alginate hydrogel beads combined with pectin, carrageenan, chitosan and psyllium. Food Chem. 2015, 167, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, M.; Bhaskar, K.; Srinivasa Rao, G.; Dhanaraju, M.D. Ibuprofen loaded ethylcellulose/polystyrene microspheres: An approach to get prolonged drug release with reduced burst effect and low ethylcellulose content. J. Microencapsul. 2003, 20, 289–302. [Google Scholar] [PubMed]

| Batch No | Alginate (g) | Ispaghula (g) | Ibuprofen (g) | Drug Loading % (w/w) | Entrapment Efficiency * (w/w) | |
|---|---|---|---|---|---|---|
| Theoretical | Actual * | |||||
| 1 | 5 | 0 | 5 | 50 | 35.2 ± 1.6 | 70.3 ± 3.3 |
| 2 | 4.5 | 0.5 | 5 | 50 | 41.8 ± 1.2 | 83.7 ± 2.4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyn Heng, J.J.; Teng, J.H.; Saravanan, M.; Pushpamalar, J. Influence of Ispaghula and Zein Coating on Ibuprofen-Loaded Alginate Beads Prepared by Vibration Technology: Physicochemical Characterization and Release Studies. Sci. Pharm. 2018, 86, 24. https://doi.org/10.3390/scipharm86020024
Lyn Heng JJ, Teng JH, Saravanan M, Pushpamalar J. Influence of Ispaghula and Zein Coating on Ibuprofen-Loaded Alginate Beads Prepared by Vibration Technology: Physicochemical Characterization and Release Studies. Scientia Pharmaceutica. 2018; 86(2):24. https://doi.org/10.3390/scipharm86020024
Chicago/Turabian StyleLyn Heng, Jillian Jin, Jia Hao Teng, Muniyandy Saravanan, and Janarthanan Pushpamalar. 2018. "Influence of Ispaghula and Zein Coating on Ibuprofen-Loaded Alginate Beads Prepared by Vibration Technology: Physicochemical Characterization and Release Studies" Scientia Pharmaceutica 86, no. 2: 24. https://doi.org/10.3390/scipharm86020024
APA StyleLyn Heng, J. J., Teng, J. H., Saravanan, M., & Pushpamalar, J. (2018). Influence of Ispaghula and Zein Coating on Ibuprofen-Loaded Alginate Beads Prepared by Vibration Technology: Physicochemical Characterization and Release Studies. Scientia Pharmaceutica, 86(2), 24. https://doi.org/10.3390/scipharm86020024





