Dietary Fiber Intake Was Inversely Associated with All-Cause Mortality but Not with Cancer and Cardiovascular Disease Mortalities in the US
Abstract
1. Introduction
2. Methods
2.1. Study Design and Sample Derivation
2.2. Outcome Variable: Mortality
2.3. Exposure Variable: Dietary Fiber Intake
2.4. Covariates
2.5. Data Analysis
3. Results
3.1. Sample Characteristics
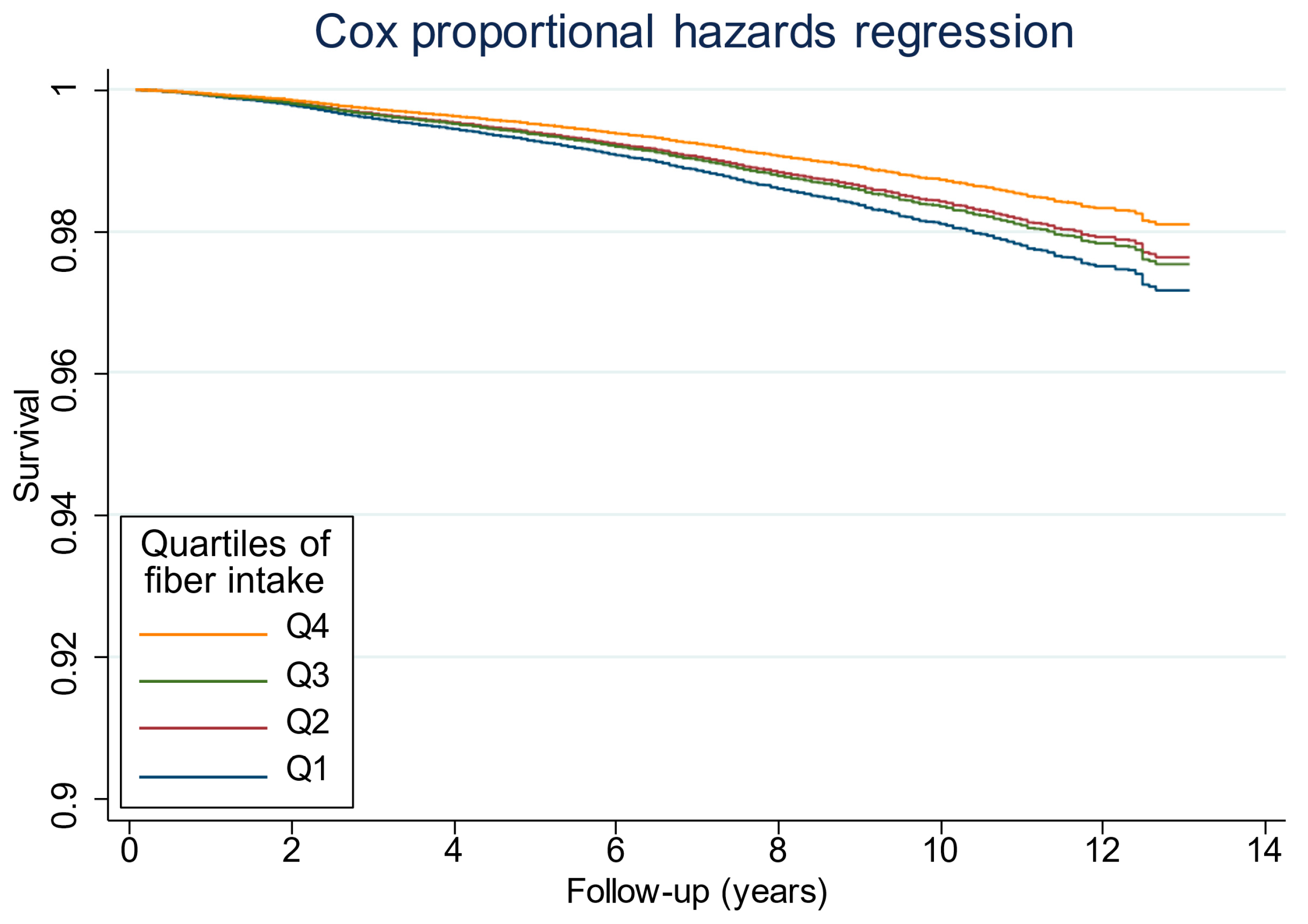
3.2. Associations Between Fiber Intake and Mortality
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef] [PubMed]
- Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2019 Results. Institute for Health Metrics and Evaluation. Available online: https://vizhub.healthdata.org/gbd-results/ (accessed on 24 December 2023).
- Budreviciute, A.; Damiati, S.; Sabir, D.K.; Onder, K.; Schuller-Goetzburg, P.; Plakys, G.; Katileviciute, A.; Khoja, S.; Kodzius, R. Management and Prevention Strategies for Non-communicable Diseases (NCDs) and Their Risk Factors. Front. Public Health 2020, 8, 574111. [Google Scholar] [CrossRef]
- Micha, R.; Khatibzadeh, S.; Shi, P.; Andrews, K.G.; Engell, R.E.; Mozaffarian, D.; on behalf of the Global Burden of Diseases Nutrition and Chronic Diseases Expert Group (NutriCoDE). Global, regional and national consumption of major food groups in 1990 and 2010: A systematic analysis including 266 country-specific nutrition surveys worldwide. BMJ Open 2015, 5, e008705. [Google Scholar] [CrossRef]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The Health Benefits of Dietary Fibre. Nutrients 2020, 12, 3209. [Google Scholar] [CrossRef]
- Lichtenstein, A.H.; Appel, L.J.; Brands, M.; Carnethon, M.; Daniels, S.; Franch, H.A.; Franklin, B.; Kris-Etherton, P.; Harris, W.S.; Howard, B.; et al. Summary of American Heart Association Diet and Lifestyle Recommendations Revision 2006. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2186–2191. [Google Scholar] [CrossRef]
- Stephen, A.M.; Champ, M.M.-J.; Cloran, S.J.; Fleith, M.; van Lieshout, L.; Mejborn, H.; Burley, V.J. Dietary fibre in Europe: Current state of knowledge on definitions, sources, recommendations, intakes and relationships to health. Nutr. Res. Rev. 2017, 30, 149–190. [Google Scholar] [CrossRef]
- Afshin, A.; Sur, P.J.; Fay, K.A.; Cornaby, L.; Ferrara, G.; Salama, J.S.; Mullany, E.C.; Abate, K.H.; Abbafati, C.; Abebe, Z.; et al. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef]
- Belle, F.N.; Kampman, E.; McTiernan, A.; Bernstein, L.; Baumgartner, K.; Baumgartner, R.; Ambs, A.; Ballard-Barbash, R.; Neuhouser, M.L. Dietary fiber, carbohydrates, glycemic index, and glycemic load in relation to breast cancer prognosis in the HEAL cohort. Cancer Epidemiol. Biomark. Prev. 2011, 20, 890–899. [Google Scholar] [CrossRef]
- Bazzano, L.A.; He, J.; Ogden, L.G.; Loria, C.M.; Whelton, P.K. Dietary fiber intake and reduced risk of coronary heart disease in us men and women: The National Health and Nutrition Examination Survey I Epidemiologic Follow-up Study. Arch. Intern. Med. 2003, 163, 1897–1904. [Google Scholar] [CrossRef]
- Skiba, M.B.; Kohler, L.N.; Crane, T.E.; Jacobs, E.T.; Shadyab, A.H.; Kato, I.; Snetselaar, L.; Qi, L.; Thomson, C.A. The Association between Prebiotic Fiber Supplement Use and Colorectal Cancer Risk and Mortality in the Women’s Health Initiative. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1884–1890. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, F.; Pourghazi, F.; Eslami, M.; Gholami, M.; Mohammadian Khonsari, N.; Ejtahed, H.-S.; Larijani, B.; Qorbani, M. Dietary fiber intake and all-cause and cause-specific mortality: An updated systematic review and meta-analysis of prospective cohort studies. Clin. Nutr. 2023, 43, 65–83. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, J.; Zhang, Y.; Qi, H.; Wang, P. Associations between dietary fiber intake and mortality from all causes, cardiovascular disease and cancer: A prospective study. J. Transl. Med. 2022, 20, 344. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.N.; Akerman, A.; Kumar, S.; Diep Pham, H.T.; Coffey, S.; Mann, J. Dietary fibre in hypertension and cardiovascular disease management: Systematic review and meta-analyses. BMC Med. 2022, 20, 139. [Google Scholar] [CrossRef]
- Streppel, M.T.; Arends, L.R.; van’t Veer, P.; Grobbee, D.E.; Geleijnse, J.M. Dietary fiber and blood pressure: A meta-analysis of randomized placebo-controlled trials. Arch. Intern. Med. 2005, 165, 150–156. [Google Scholar] [CrossRef]
- Ganji, V.; Kies, C.V. Psyllium husk fiber supplementation to the diets rich in soybean or coconut oil: Hypocholesterolemic effect in healthy humans. Int. J. Food Sci. Nutr. 1996, 47, 103–110. [Google Scholar] [CrossRef]
- Davidson, M.H.; Maki, K.C.; Kong, J.C.; Dugan, L.D.; Torri, S.A.; Hall, H.A.; Drennan, K.B.; Anderson, S.M.; Fulgoni, V.L.; Saldanha, L.G.; et al. Long-term effects of consuming foods containing psyllium seed husk on serum lipids in subjects with hypercholesterolemia. Am. J. Clin. Nutr. 1998, 67, 367–376. [Google Scholar] [CrossRef]
- Ho, H.V.T.; Sievenpiper, J.L.; Zurbau, A.; Blanco Mejia, S.; Jovanovski, E.; Au-Yeung, F.; Jenkins, A.L.; Vuksan, V. The effect of oat β-glucan on LDL-cholesterol, non-HDL-cholesterol and apoB for CVD risk reduction: A systematic review and meta-analysis of randomised-controlled trials. Br. J. Nutr. 2016, 116, 1369–1382. [Google Scholar] [CrossRef]
- Chambers, E.S.; Byrne, C.S.; Morrison, D.J.; Murphy, K.G.; Preston, T.; Tedford, C.; Garcia-Perez, I.; Fountana, S.; Serrano-Contreras, J.I.; Holmes, E.; et al. Dietary supplementation with inulin-propionate ester or inulin improves insulin sensitivity in adults with overweight and obesity with distinct effects on the gut microbiota, plasma metabolome and systemic inflammatory responses: A randomised cross-over trial. Gut 2019, 68, 1430–1438. [Google Scholar] [CrossRef]
- Reynolds, A.N.; Akerman, A.P.; Mann, J. Dietary fibre and whole grains in diabetes management: Systematic review and meta-analyses. PLoS Med. 2020, 17, e1003053. [Google Scholar] [CrossRef]
- Chandalia, M.; Garg, A.; Lutjohann, D.; von Bergmann, K.; Grundy, S.M.; Brinkley, L.J. Beneficial effects of high dietary fiber intake in patients with type 2 diabetes mellitus. N. Engl. J. Med. 2000, 342, 1392–1398. [Google Scholar] [CrossRef] [PubMed]
- Gibb, R.D.; Sloan, K.J.; McRorie, J.W. Psyllium is a natural nonfermented gel-forming fiber that is effective for weight loss: A comprehensive review and meta-analysis. J. Am. Assoc. Nurse Pract. 2023, 35, 468–476. [Google Scholar] [CrossRef]
- Miketinas, D.C.; Bray, G.A.; Beyl, R.A.; Ryan, D.H.; Sacks, F.M.; Champagne, C.M. Fiber intake predicts weight loss and dietary adherence in adults consuming calorie-restricted diets: The POUNDS lost (preventing overweight using novel dietary strategies) study. J. Nutr. 2019, 149, 1742–1748. [Google Scholar] [CrossRef] [PubMed]
- Ganji, V.; Kies, C.V. Psyllium husk fibre supplementation to soybean and coconut oil diets of humans: Effect on fat digestibility and faecal fatty acid excretion. Eur. J. Clin. Nutr. 1994, 48, 595–597. Available online: https://www.ncbi.nlm.nih.gov/pubmed/7957006 (accessed on 24 December 2023).
- Xie, L.-M.; Ge, Y.-Y.; Huang, X.; Zhang, Y.-Q.; Li, J.-X. Effects of fermentable dietary fiber supplementation on oxidative and inflammatory status in hemodialysis patients. Int. J. Clin. Exp. Med. 2015, 8, 1363–1369. Available online: https://www.ncbi.nlm.nih.gov/pubmed/25785138 (accessed on 24 December 2023).
- Diniz, Y.S.; Cicogna, A.C.; Padovani, C.R.; Silva, M.D.P.; Faine, L.A.; Galhardi, C.M.; Rodrigues, H.G.; Novelli, E.L.B. Dietary restriction and fibre supplementation: Oxidative stress and metabolic shifting for cardiac health. Can. J. Physiol. Pharmacol. 2003, 81, 1042–1048. [Google Scholar] [CrossRef]
- Dong, R.; Peng, K.; Shi, L.; Niu, Q.; Rafique, H.; Liu, Y.; Yuan, L.; Zou, L.; Li, L.; Messia, M.C.; et al. Oat bran prevents high-fat-diet induced muscular dysfunction, systemic inflammation and oxidative stress through reconstructing gut microbiome and circulating metabolome. Food Res. Int. 2023, 172, 113127. [Google Scholar] [CrossRef]
- Wastyk, H.C.; Fragiadakis, G.K.; Perelman, D.; Dahan, D.; Merrill, B.D.; Yu, F.B.; Topf, M.; Gonzalez, C.G.; Van Treuren, W.; Han, S.; et al. Gut-microbiota-targeted diets modulate human immune status. Cell 2021, 184, 4137–4153.e14. [Google Scholar] [CrossRef]
- Park, Y.; Subar, A.F.; Hollenbeck, A.; Schatzkin, A. Dietary fiber intake and mortality in the NIH-AARP diet and health study. Arch. Intern. Med. 2011, 171, 1061–1068. [Google Scholar] [CrossRef]
- Veronese, N.; Solmi, M.; Caruso, M.G.; Giannelli, G.; Osella, A.R.; Evangelou, E.; Maggi, S.; Fontana, L.; Stubbs, B.; Tzoulaki, I. Dietary fiber and health outcomes: An umbrella review of systematic reviews and meta-analyses. Am. J. Clin. Nutr. 2018, 107, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Flint, A.; Pai, J.K.; Forman, J.P.; Hu, F.B.; Willett, W.C.; Rexrode, K.M.; Mukamal, K.J.; Rimm, E.B. Dietary fiber intake and mortality among survivors of myocardial infarction: Prospective cohort study. BMJ 2014, 348, g2659. [Google Scholar] [CrossRef] [PubMed]
- McEligot, A.J.; Largent, J.; Ziogas, A.; Peel, D.; Anton-Culver, H. Dietary fat, fiber, vegetable, and micronutrients are associated with overall survival in postmenopausal women diagnosed with breast cancer. Nutr. Cancer 2006, 55, 132–140. [Google Scholar] [CrossRef]
- Jacobs, D.R.; Pereira, M.A.; Meyer, K.A.; Kushi, L.H. Fiber from whole grains, but not refined grains, is inversely associated with all-cause mortality in older women: The Iowa women’s health study. J. Am. Coll. Nutr. 2000, 19, 326S–330S. [Google Scholar] [CrossRef]
- Zhang, H.R.; Yang, Y.; Tian, W.; Sun, Y.J. Dietary Fiber and All-Cause and Cardiovascular Mortality in Older Adults with Hypertension: A Cohort Study Of NHANES. J. Nutr. Health Aging 2022, 26, 407–414. [Google Scholar] [CrossRef]
- Ricci, C.; Freisling, H.; Leitzmann, M.F.; Taljaard-Krugell, C.; Jacobs, I.; Kruger, H.S.; Smuts, C.M.; Pieters, M. Diet and sedentary behaviour in relation to cancer survival. A report from the national health and nutrition examination survey linked to the U.S. mortality registry. Clin. Nutr. 2020, 39, 3489–3496. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). About the National Health and Nutrition Examination Survey. Available online: https://www.cdc.gov/nchs/nhanes/about/index.html (accessed on 18 June 2025).
- Curtin, L.R.; Mohadjer, L.K.; Dohrmann, S.M.; Montaquila, J.M.; Kruszan-Moran, D.; Mirel, L.B.; Carroll, M.D.; Hirsch, R.; Schober, S.; Johnson, C.L. The National Health and Nutrition Examination Survey: Sample Design, 1999–2006. Vital Health Stat. Ser. 2 2012, 155, 1–39. [Google Scholar]
- Curtin, L.R.; Mohadjer, L.K.; Dohrmann, S.M.; Kruszon-Moran, D.; Mirel, L.B.; Carroll, M.D.; Hirsch, R.; Burt, V.L.; Johnson, C.L. National Health and Nutrition Examination Survey: Sample design, 2007–2010. Vital Health Stat. Ser. 2 2013, 160, 1–23. [Google Scholar]
- Johnson, C.L.; Dohrmann, S.M.; Burt, V.L.; Mohadjer, L.K. National health and nutrition examination survey: Sample design, 2011–2014. Vital Health Stat. Ser. 2 2014, 162, 1–33. [Google Scholar]
- Chen, T.-C.; Clark, J.; Riddles, M.K.; Mohadjer, L.K.; Fakhouri, T.H.I. National Health and Nutrition Examination Survey, 2015–2018: Sample Design and Estimation Procedures. Vital Health Stat. Ser. 2 2020, 184, 1–35. [Google Scholar]
- Rhee, J.J.; Sampson, L.; Cho, E.; Hughes, M.D.; Hu, F.B.; Willett, W.C. Comparison of methods to account for implausible reporting of energy intake in epidemiologic studies. Am. J. Epidemiol. 2015, 181, 225–233. [Google Scholar] [CrossRef]
- Willett, W. Issues in analysis and presentation of dietary data. In Nutritional Epidemiology, 3rd ed.; Oxford University Press: New York, NY, USA, 2012; pp. 305–333. Available online: https://oxford.universitypressscholarship.com/view/10.1093/acprof:oso/9780195122978.001.0001/acprof-9780195122978-chapter-13 (accessed on 18 June 2025).
- Dwyer, J.; Picciano, M.F.; Raiten, D.J.; Members of the Steering Committee. Collection of Food and Dietary Supplement Intake Data: What We Eat in America-NHANES. J. Nutr. 2003, 133, 590S–600S. [Google Scholar] [CrossRef]
- Ahluwalia, N.; Dwyer, J.; Terry, A.; Moshfegh, A.; Johnson, C. Update on NHANES Dietary Data: Focus on Collection, Release, Analytical Considerations, and Uses to Inform Public Policy. Adv. Nutr. 2016, 7, 121–134. [Google Scholar] [CrossRef]
- Moshfegh, A.J.; Rhodes, D.G.; Baer, D.J.; Murayi, T.; Clemens, J.C.; Rumpler, W.V.; Paul, D.R.; Sebastian, R.S.; Kuczynski, K.J.; Ingwersen, L.A.; et al. The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. Am. J. Clin. Nutr. 2008, 88, 324–332. [Google Scholar] [CrossRef]
- Ahuja, J.K.C.; Moshfegh, A.J.; Holden, J.M.; Harris, E. USDA food and nutrient databases provide the infrastructure for food and nutrition research, policy, and practice. J. Nutr. 2013, 143, 241S–249S. [Google Scholar] [CrossRef]
- Cleland, C.L.; Hunter, R.F.; Kee, F.; Cupples, M.E.; Sallis, J.F.; Tully, M.A. Validity of the global physical activity questionnaire (GPAQ) in assessing levels and change in moderate-vigorous physical activity and sedentary behaviour. BMC Public Health 2014, 14, 1255. [Google Scholar] [CrossRef]
- Yao, F.; Ma, J.; Cui, Y.; Huang, C.; Lu, R.; Hu, F.; Zhu, X.; Qin, P. Dietary intake of total vegetable, fruit, cereal, soluble and insoluble fiber and risk of all-cause, cardiovascular, and cancer mortality: Systematic review and dose-response meta-analysis of prospective cohort studies. Front. Nutr. 2023, 10, 1153165. [Google Scholar] [CrossRef]
- Partula, V.; Deschasaux, M.; Druesne-Pecollo, N.; Latino-Martel, P.; Desmetz, E.; Chazelas, E.; Kesse-Guyot, E.; Julia, C.; Fezeu, L.K.; Galan, P.; et al. Associations between consumption of dietary fibers and the risk of cardiovascular diseases, cancers, type 2 diabetes, and mortality in the prospective NutriNet-Santé cohort. Am. J. Clin. Nutr. 2020, 112, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.J.; Lee, H.S.; Park, G.; Kim, H.M.; Lee, J.W. Association of Dietary Fiber Intake with All-Cause Mortality and Cardiovascular Disease Mortality: A 10-Year Prospective Cohort Study. Nutrients 2022, 14, 3089. [Google Scholar] [CrossRef]
- Reynolds, A.; Mann, J.; Cummings, J.; Winter, N.; Mete, E.; Te Morenga, L. Carbohydrate quality and human health: A series of systematic reviews and meta-analyses. Lancet 2019, 393, 434–445. [Google Scholar] [CrossRef]
- Qi, J.; Gao, J.; Zhang, Y.; Hou, W.; Han, T.; Sun, C. The Association of Dietary Fiber Intake in Three Meals with All-Cause and Disease-Specific Mortality among Adults: The U.S. National Health and Nutrition Examination Survey, 2003–2014. Nutrients 2022, 14, 2521. [Google Scholar] [CrossRef]
- Whitehead, A.; Beck, E.J.; Tosh, S.; Wolever, T.M. Cholesterol-lowering effects of oat β-glucan: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2014, 100, 1413–1421. [Google Scholar] [CrossRef]
- Zhu, B.; Sun, Y.; Qi, L.; Zhong, R.; Miao, X. Dietary legume consumption reduces risk of colorectal cancer: Evidence from a meta-analysis of cohort studies. Sci. Rep. 2015, 5, 8797. [Google Scholar] [CrossRef]
- Jacobs DRJr Andersen, L.F.; Blomhoff, R. Whole-grain consumption is associated with a reduced risk of noncardiovascular, noncancer death attributed to inflammatory diseases in the Iowa Women’s Health Study. Am. J. Clin. Nutr. 2007, 85, 1606–1614. [Google Scholar] [CrossRef]
- Buyken, A.E.; Flood, V.; Empson, M.; Rochtchina, E.; Barclay, A.W.; Brand-Miller, J.; Mitchell, P. Carbohydrate nutrition and inflammatory disease mortality in older adults. Am. J. Clin. Nutr. 2010, 92, 634–643. [Google Scholar] [CrossRef]
- Roy, C.C.; Kien, C.L.; Bouthillier, L.; Levy, E. Short-chain fatty acids: Ready for prime time? Nutr. Clin. Pract. 2006, 21, 351–366. [Google Scholar] [CrossRef]
- Mall, U.P.; Patel, V.H. Evaluation of amla and tomato pomace for polyphenol bioaccessibility and prebiotic effects by in vitro digestion and fermentation. Nutr. Food Sci. 2025, 55, 584–604. [Google Scholar] [CrossRef]
- Lewis, K.; Lutgendorff, F.; Phan, V.; Söderholm, J.D.; Sherman, P.M.; McKay, D.M. Enhanced translocation of bacteria across metabolically stressed epithelia is reduced by butyrate. Inflamm. Bowel Dis. 2010, 16, 1138–1148. [Google Scholar] [CrossRef] [PubMed]
- Mohajeri, M.H.; Brummer, R.J.M.; Rastall, R.A.; Weersma, R.K.; Harmsen, H.J.M.; Faas, M.; Eggersdorfer, M. The role of the microbiome for human health: From basic science to clinical applications. Eur. J. Nutr. 2018, 57, 1–14. [Google Scholar] [CrossRef]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
- Ma, Y.; Griffith, J.A.; Chasan-Taber, L.; Olendzki, B.C.; Jackson, E.; Stanek, E.J.I.I.I.; Li, W.; Pagoto, S.L.; Hafner, A.R.; Ockene, I.S. Association between dietary fiber and serum C-reactive protein. Am. J. Clin. Nutr. 2006, 83, 760–766. [Google Scholar] [CrossRef]
- Ma, Y.; Hébert, J.R.; Li, W.; Bertone-Johnson, E.R.; Olendzki, B.; Pagoto, S.L.; Tinker, L.; Rosal, M.C.; Ockene, I.S.; Ockene, J.K.; et al. Association between dietary fiber and markers of systemic inflammation in the Women’s Health Initiative Observational Study. Nutrition 2008, 24, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, M.; Jensen, M.G. Dietary fibres in the regulation of appetite and food intake. Importance of viscosity. Appetite 2011, 56, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.; Wolever, T.M.; Leeds, A.R.; Gassull, M.A.; Haisman, P.; Dilawari, J.; Goff, D.V.; Metz, G.L.; Alberti, K.G. Dietary fibres, fibre analogues, and glucose tolerance: Importance of viscosity. BMJ 1978, 1, 1392–1394. [Google Scholar] [CrossRef] [PubMed]
- Holt, S.; Carter, D.; Tothill, P.; Heading, R.; Prescott, L. Effect of gel fibre on gastric emptying and absorption of glucose and paracetamol. Lancet 1979, 313, 636–639. [Google Scholar] [CrossRef]
- US Department of Agriculture, Agricultural Research Service. What We Eat in America: Nutrient Intakes from Food by Gender and Age; National Health and Nutrition Examination Survey (NHANES) 2009–2010; USDA: Washington, DC, USA, 2012.
- Quagliani, D.; Felt-Gunderson, P. Closing America’s Fiber Intake Gap: Communication Strategies from a Food and Fiber Summit. Am. J. Lifestyle Med. 2016, 11, 80–85. [Google Scholar] [CrossRef] [PubMed]
| Q1 | Q2 | Q3 | Q4 | p-Value | |
|---|---|---|---|---|---|
| n = 6474 | n = 6484 | n = 6476 | n = 6434 | ||
| Fiber intake, g/d | 7.6 ± 2 | 12.6 ± 1.2 | 17.4 ± 1.6 | 27.7 ± 7.3 | <0.001 |
| Fat intake, g/d | 57.2 ± 25.4 | 70.4 ± 29 | 79.5 ± 32.3 | 90 ± 35.9 | <0.001 |
| Energy intake, kcal/d | 1527 ± 551 | 1856 ± 603 | 2109 ± 639 | 2460 ± 695 | <0.001 |
| Age, y | 49.2 ±18.5 | 49.8 ±18.4 | 50.9 ± 18.1 | 50.1 ± 17.2 | <0.001 |
| Sex | <0.001 | ||||
| Men, n (%) | 2458 (38) | 2689 (41.5) | 3162 (48.8) | 3828 (59.5) | |
| Women, n (%) | 4016 (62) | 3795 (58.5) | 3314 (51.2) | 2606 (40.5) | |
| Ethnicity | <0.001 | ||||
| Non-Hispanic White, n (%) | 2993 (46.2) | 3107 (47.9) | 3295 (50.9) | 3065 (47.6) | |
| Non-Hispanic Black, n (%) | 1912 (29.5) | 1501 (23.1) | 1136 (17.5) | 773 (12) | |
| Hispanic/Mexican American, n (%) | 681 (10.5) | 898 (13.8) | 2 (16.2) | 1527 (23.7) | |
| Others, n (% | 888 (13.7) | 978 (15.1) | 993 (15.3) | 1069 (16.6) | |
| Smoking | <0.001 | ||||
| Never, n (%) | 3067 (47.4) | 3536 (54.6) | 3689 (57) | 3767 (58.6) | |
| Former, n (%) | 1400 (21.6) | 1597 (24.6) | 1760 (27.2) | 1879 (29.2) | |
| Current smoker, n (%) | 2004 (31) | 1347 (20.8) | 1026 (15.8) | 786 (12.2) | |
| Alcohol drinking | <0.001 | ||||
| No, n (%) | 1348 (20.8) | 1257 (19.4) | 1199 (18.5) | 1118 (17.4) | |
| Yes, n (%) | 3813 (58.9) | 3993 (61.6) | 4072 (62.9) | 4155 (64.6) | |
| Missing, n (%) | 1313 (20.3) | 1234 (19) | 1205 (18.6) | 1161 (18) | |
| Season | <0.001 | ||||
| Winter, n (%) | 2914 (45) | 2943 (45.4) | 2933 (45.3) | 3162 (49.1) | |
| Summer, n (%) | 3560 (55) | 3541 (54.6) | 3543 (54.7) | 3272 (50.9) | |
| BMI, kg/m2 | 29.5 ± 7.3 | 29.6 ± 7 | 28.8 ± 6.5 | 28.3 ± 6.2 | <0.001 |
| Leisure time physical activity (METs min/wk) | <0.001 | ||||
| <600, n (%) | 3240 (50) | 2998 (46.2) | 2698 (41.7) | 2183 (33.9) | |
| 600–1200, n (%) | 696 (10.8) | 851 (13.1) | 840 (13) | 844 (13.1) | |
| ≥1200, n (%) | 2538 (39.2) | 2635 (40.6) | 2938 (45.4) | 3407 (53) | |
| Ratio of family income to poverty | <0.001 | ||||
| <1.30, n (%) | 2352 (36.3) | 105 (27.8) | 1608 (24.8) | 1519 (23.6) | |
| 1.3–3.5, n (%) | 2310 (35.7) | 2397 (37) | 2247 (34.7) | 2100 (32.6) | |
| >3.5, n (%) | 1381 (21.3) | 1845 (28.5) | 2140 (33) | 2370 (36.8) | |
| Missing, n (%) | 431 (6.7) | 437 (6.7) | 481 (7.4) | 445 (6.9) | |
| Hypertension, n (%) | 2461 (39.5) | 2404 (38.5) | 2351 (37.5) | 2077 (33.2) | <0.001 |
| Q1 | Q2 | Q3 | Q4 | p for Trend | |
|---|---|---|---|---|---|
| All-cause mortality | |||||
| Cases | 768 | 664 | 646 | 442 | |
| Rate (per 1000) | 12.61 | 9.93 | 9.87 | 6.82 | |
| Model A 2 | 1 | 0.71 (0.62–0.82) | 0.66 (0.58–0.75) | 0.47 (0.4–0.55) | <0.001 |
| Model B 3 | 1 | 0.83 (0.72–0.95) | 0.83 (0.72–0.95) | 0.65 (0.55–0.77) | <0.001 |
| Model C 4 | 1 | 0.83 (0.72–0.96) | 0.87 (0.75–1.01) | 0.67 (0.56–0.8) | <0.001 |
| Cancer mortality | |||||
| Cases | 160 | 140 | 152 | 109 | |
| Rate (per 1000) | 2.47 | 2.09 | 2.46 | 1.73 | |
| Model A 2 | 1 | 0.75 (0.56–1) | 0.8 (0.59–1.07) | 0.55 (0.39–0.78) | 0.003 |
| Model B 3 | 1 | 0.89 (0.67–1.18) | 1.05 (0.77–1.42) | 0.81 (0.56–1.17) | 0.51 |
| Model C 4 | 1 | 0.87 (0.65–1.17) | 1.04 (0.76–1.43) | 0.80 (0.55–1.17) | 0.51 |
| CVD mortality | |||||
| Cases | 162 | 130 | 132 | 87 | |
| Rate (per 1000) | 2.42 | 1.91 | 1.99 | 1.27 | |
| Model A 2 | 1 | 0.77 (0.57–1.02) | 0.78 (0.57–1.06) | 0.56 (0.39–0.79) | 0.002 |
| Model B 3 | 1 | 0.89 (0.66–1.21) | 0.97 (0.68–1.4) | 0.79 (0.53–1.19) | 0.42 |
| Model C 4 | 1 | 0.9 (0.64–1.26) | 1.03 (0.70–1.5) | 0.84 (0.53–1.33) | 0.67 |
| Quartiles of Fiber Intake | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | p Trend | p Interaction | ||||
| Ethnicity | 0.071 | ||||||||
| Non-Hispanic White | 1 | 0.8 | (0.68–0.94) | 0.82 | (0.69–0.98) | 0.64 | (0.51–0.8) | ≤0.001 | |
| Non-Hispanic Black | 1 | 0.99 | (0.76–1.28) | 1.04 | (0.75–1.43) | 0.80 | (0.52–1.22) | 0.58 | |
| Hispanic/Mexican American | 1 | 1.01 | (0.67–1.52) | 0.9 | (0.56–1.47) | 0.79 | (0.52–1.2) | 0.22 | |
| Others | 1 | 0.84 | (0.47–1.48) | 1.2 | (0.74–1.95) | 0.59 | (0.29–0.2) | 0.36 | |
| Sex | 0.71 | ||||||||
| Men | 1 | 0.8 | (0.65–0.98) | 0.8 | (0.65–0.98) | 0.65 | (0.51–0.83) | 0.001 | |
| Women | 1 | 0.87 | (0.71–1.06) | 0.99 | (0.79–1.24) | 0.69 | (0.52–0.92) | 0.1 | |
| Age group | 0.28 | ||||||||
| 20~59 | 1 | 0.73 | (0.53–1.01) | 0.93 | (0.67–1.28) | 0.61 | (0.41–0.93) | 0.08 | |
| ≥60 | 1 | 0.88 | (0.76–1.02) | 0.84 | (0.72–0.99) | 0.70 | (0.57–0.85) | 0.001 | |
| Ratio of family income to poverty | 0.17 | ||||||||
| <1.30 | 1 | 0.97 | (0.74–1.26) | 1.03 | (0.77–1.37) | 0.92 | (0.68–1.24) | 0.77 | |
| 1.3–3.5 | 1 | 0.75 | (0.62–0.91) | 0.72 | (0.57–0.89) | 0.53 | (0.41–0.7) | <0.001 | |
| >3.5 | 1 | 0.87 | (0.63–1.22) | 0.86 | (0.62–1.19) | 0.69 | (0.44–1.11) | 0.14 | |
| Missing | 1 | 0.55 | (0.3–0.99) | 1.37 | (0.71–2.67) | 0.58 | (0.23–1.48) | 0.99 | |
| Education | 0.13 | ||||||||
| <11 grade | 1 | 0.82 | (0.64–1.03) | 1.13 | (0.9–1.43) | 0.84 | (0.62–1.15) | 0.85 | |
| High school | 1 | 0.88 | (0.68–1.15) | 0.72 | (0.52–1) | 0.60 | (0.42–0.86) | 0.005 | |
| Some college | 1 | 0.73 | (0.59–0.90) | 0.68 | (0.52–0.88) | 0.56 | (0.38–0.83) | 0.003 | |
| Higher than college | 1 | 0.83 | (0.5–1.38) | 0.94 | (0.6–1.48) | 0.68 | (0.4–1.18) | 0.24 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akbar, Z.; Fituri, S.; Shi, Z.; Ganji, V. Dietary Fiber Intake Was Inversely Associated with All-Cause Mortality but Not with Cancer and Cardiovascular Disease Mortalities in the US. Diseases 2025, 13, 272. https://doi.org/10.3390/diseases13080272
Akbar Z, Fituri S, Shi Z, Ganji V. Dietary Fiber Intake Was Inversely Associated with All-Cause Mortality but Not with Cancer and Cardiovascular Disease Mortalities in the US. Diseases. 2025; 13(8):272. https://doi.org/10.3390/diseases13080272
Chicago/Turabian StyleAkbar, Zoha, Sundus Fituri, Zumin Shi, and Vijay Ganji. 2025. "Dietary Fiber Intake Was Inversely Associated with All-Cause Mortality but Not with Cancer and Cardiovascular Disease Mortalities in the US" Diseases 13, no. 8: 272. https://doi.org/10.3390/diseases13080272
APA StyleAkbar, Z., Fituri, S., Shi, Z., & Ganji, V. (2025). Dietary Fiber Intake Was Inversely Associated with All-Cause Mortality but Not with Cancer and Cardiovascular Disease Mortalities in the US. Diseases, 13(8), 272. https://doi.org/10.3390/diseases13080272






