Integration of AI and ML in Tuberculosis (TB) Management: From Diagnosis to Drug Discovery
Abstract
1. Introduction
1.1. Overview of TB
1.2. Fundamental AI Methodologies Highlighted in This Review
1.2.1. Artificial Intelligence
1.2.2. Machine Learning
- Support vector machines (SVMs) are supervised learning algorithms for classification and regression applications [14]. The fundamental objective of an SVM is to determine an optimal hyperplane—a decision boundary—that most effectively separates data points of various classes in a high-dimensional space. Diverse kernel functions enhance the flexibility and efficiency of SVMs in high-dimensional settings [15].
- Random forests (RF) are supervised ensemble learning algorithms that operate by constructing several decision trees during training and generate the mode of the classes for classification or the mean prediction for regression [16]. Bootstrapped aggregation (bagging) is the technique in which each tree in the forest is generated utilizing a randomly selected portion of the training data and a random selection of features [17]. This randomization facilitates generalization and mitigates overfitting. Due to its robustness, ability to handle high-dimensional data, and effectiveness in identifying critical predictive factors such as biomarkers and risk variables in TB diagnosis and treatment outcome prediction, random forests are extensively utilized in biomedical research.
1.2.3. Deep Learning
- Artificial neural networks (ANNs) are computational models inspired by the architecture and function of the human brain [19]. Using weighted connections and activation mechanisms, they consist of interconnected layers of nodes, or “neurons,” that evaluate input data and recognize patterns. Deep learning is fundamentally based on ANNs, which are widely utilized in many biological applications, including pattern identification and disease classification [20].
- Convolutional neural networks (CNNs) are a specialized category of ANNs particularly designed for processing image input [21]. CNNs have demonstrated significant efficacy in autonomously extracting hierarchical features from imaging data, thereby facilitating the interpretation of chest radiographs in TB diagnosis [22]. The design, consisting of convolutional layers, pooling layers, and fully connected layers, facilitates the identification of complex spatial patterns associated with tuberculosis-related abnormalities such as cavities, nodules, and infiltrates. In high-burden, resource-constrained settings, CNN-based models trained on extensive datasets of annotated chest X-rays can achieve diagnostic accuracy that is comparable to or surpasses that of experienced radiologists, thereby facilitating rapid and affordable screening.
1.3. Significance of AI in TB Management
| Feature | Traditional Methods | AI-Based Diagnosis | References |
|---|---|---|---|
| Accuracy | 50–70% | 80–95% | [29] |
| Sensitivity | 40–80% | 85–98% | [30] |
| Specificity | 60–85% | 90–99% | [31] |
| Turnaround Time | days to weeks | seconds to minutes | [32] |
| Cost | High | Lower | [33] |
| Human Dependency | High | Low | [34] |
| Scalability | Limited | Highly Scalable | [35] |
| Interpretability | Expert-dependent | Data-driven | [13] |
| Resistance Detection | Requires molecular/genetic testing | Based on imaging and data, AI can detect resistance | [36] |
| Application in Remote Areas | Difficult | Feasible (AI-based mobile applications) | [37] |
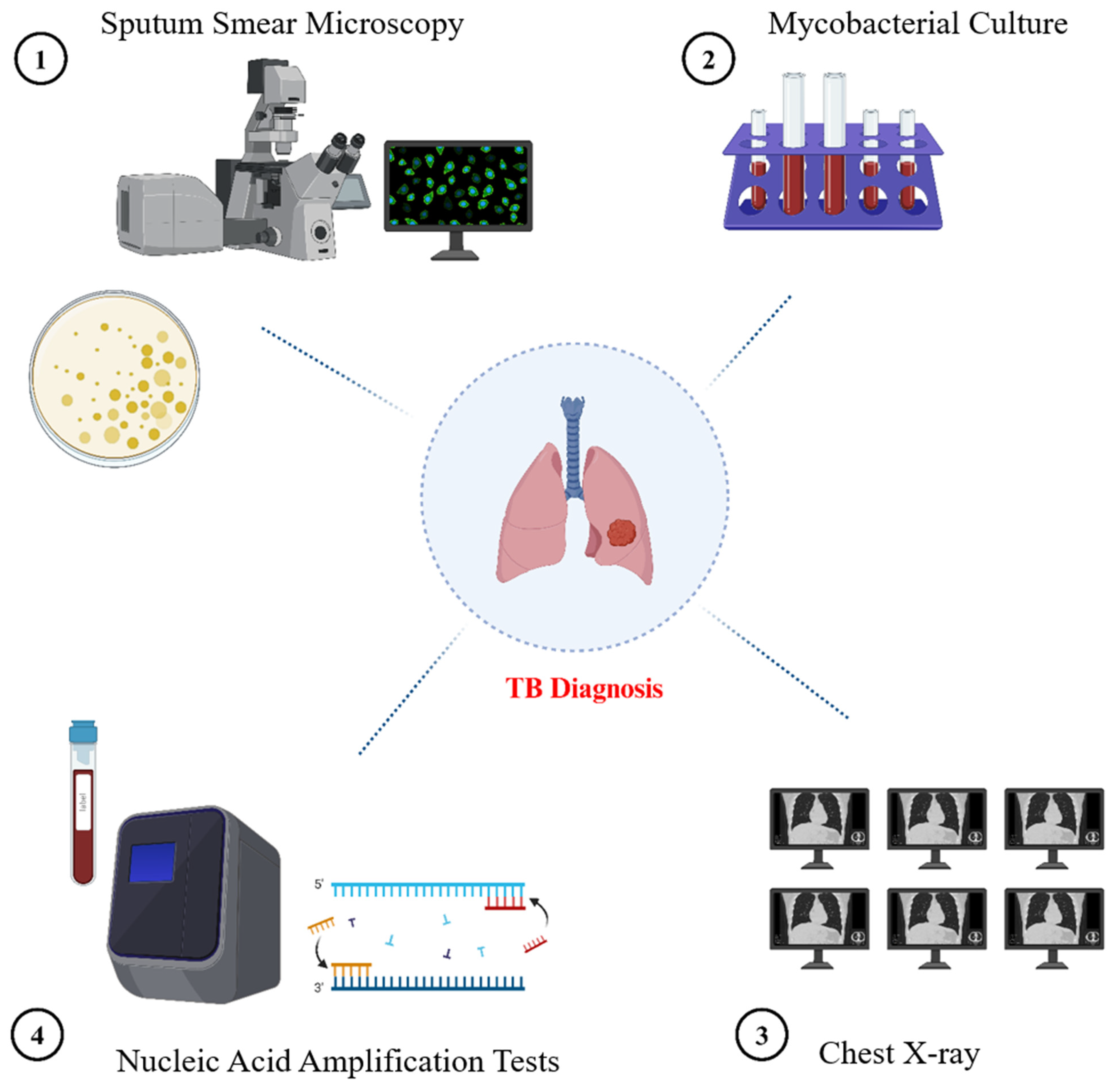
2. Traditional Diagnostic Methods for TB
2.1. Sputum Smear Microscopy
2.2. The Culture of Mycobacteria
2.3. Nucleic Acid Amplification Tests (NAATs)
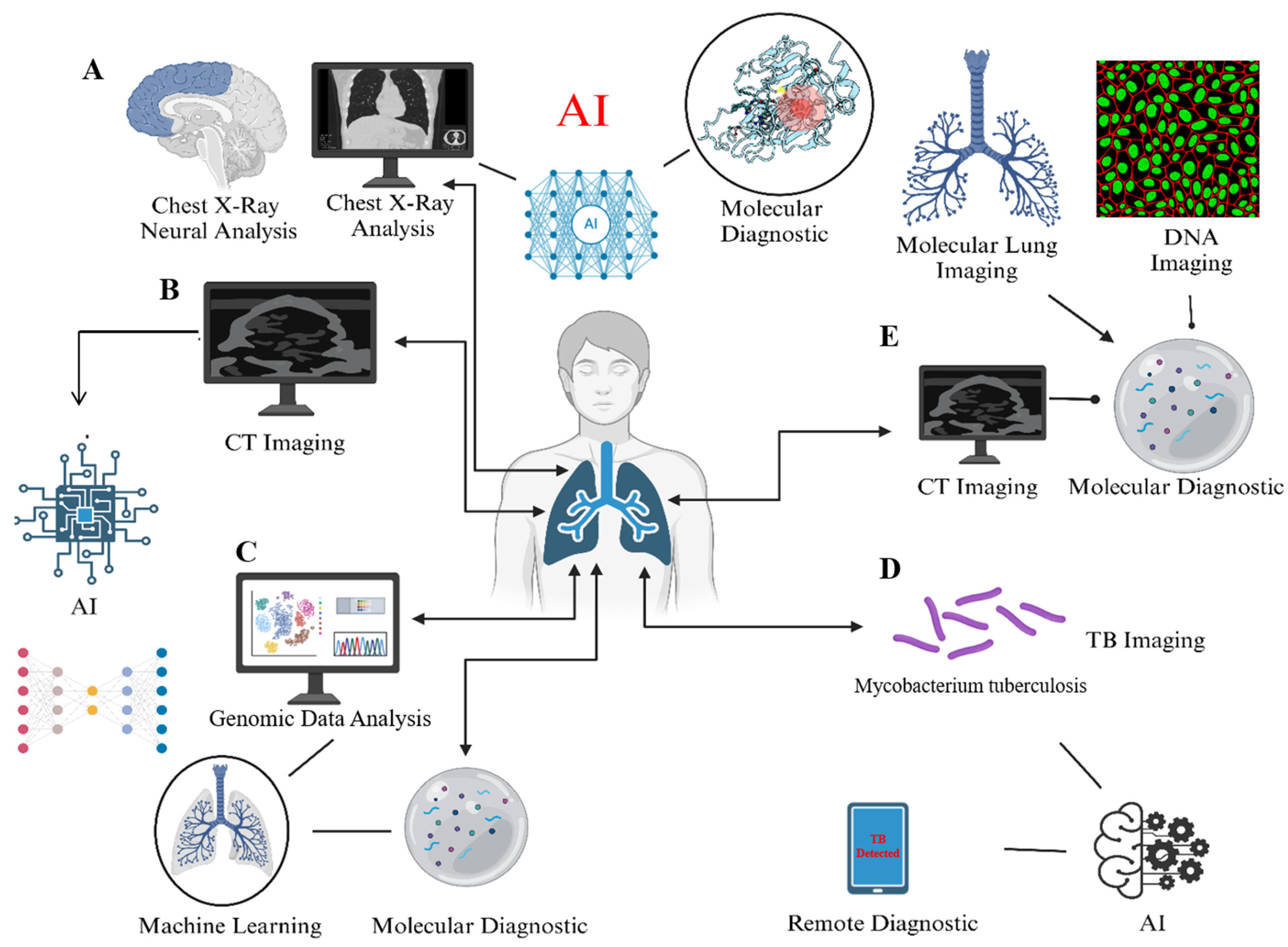
3. AI-Based Methods in TB Management: From Detection to Prognosis
3.1. Chest Radiography (CXR)
3.2. AI-Assisted Diagnosis of CT Imaging
3.3. Molecular Diagnostics
3.3.1. CRISPR–Cas-Based TB Treatment
3.3.2. GeneXpert-Based TB Treatment
3.3.3. TB-LAMP
3.4. Smartphone-Based Imaging Devices (SIDs)
3.5. Nano-Technological Multimodal Diagnosis of TB
4. AI in Treatment Monitoring
4.1. Adherence Tracking
4.2. Monitoring Side-Effects of Treatment
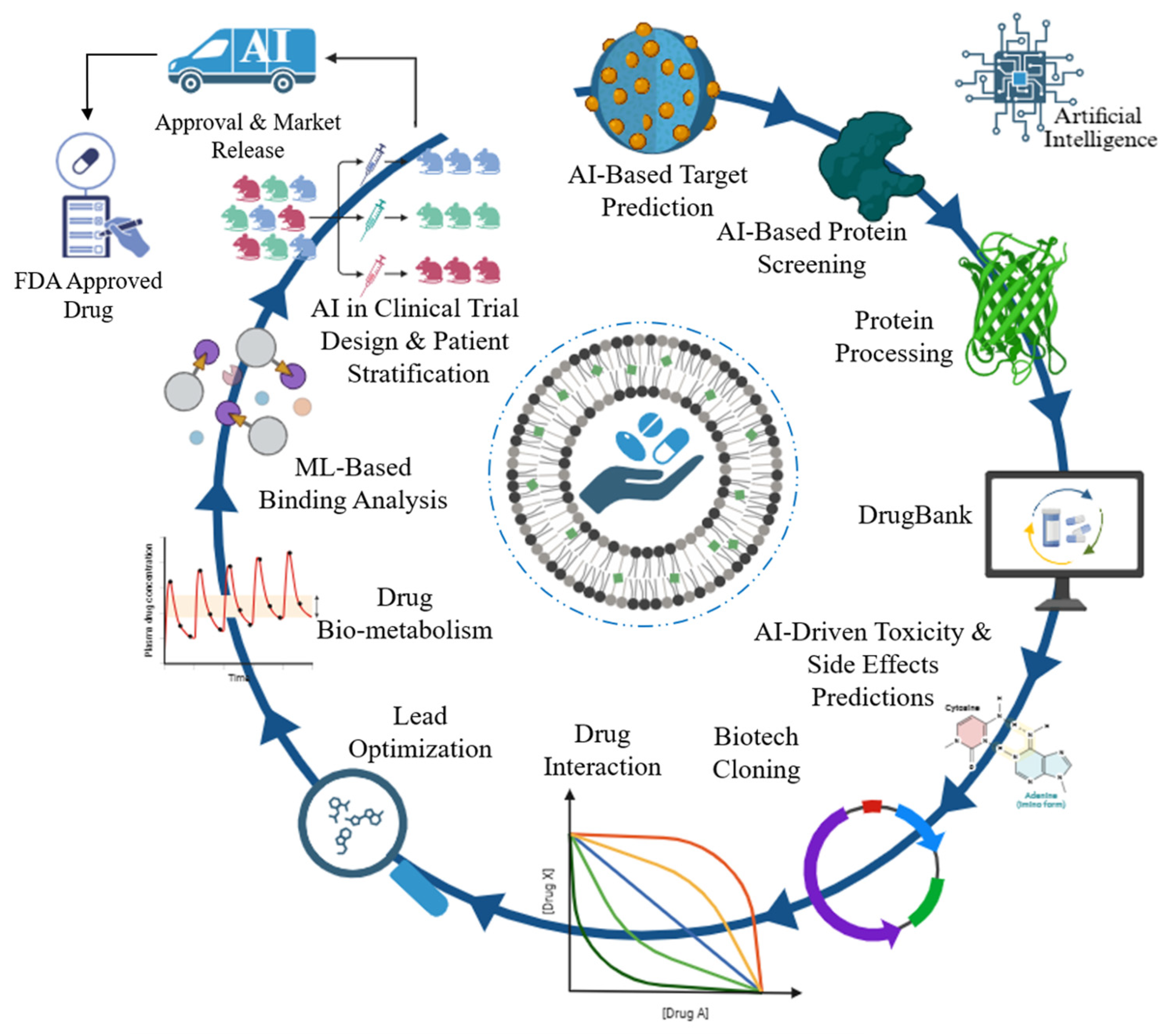
5. AI in TB Drug Discovery
5.1. Identifying Novel Drug Targets
5.2. Virtual Screening of Compounds
5.3. Drug Repurposing
6. Challenges and Limitations
7. Future Directions
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sudre, P.; Ten Dam, G.; Kochi, A. Tuberculosis: A global overview of the situation today. Bull. World Health Organ. 1992, 70, 149. [Google Scholar] [PubMed]
- Flynn, J.L.; Chan, J. Immunology of tuberculosis. Annu. Rev. Immunol. 2001, 19, 93–129. [Google Scholar] [CrossRef] [PubMed]
- Core Curriculum on Tuberculosis; US Department of Health & Human Services, Public Health Service: Washington, DC, USA; Centers for Disease Control and Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, Division of Tuberculosis Elimination: Atlanta, GA, USA, 1991.
- Daniel, T.M. The history of tuberculosis. Respir. Med. 2006, 100, 1862–1870. [Google Scholar] [CrossRef] [PubMed]
- Reported Tuberculosis in the United States; US Department of Health & Human Services, Public Health Service: Washington, DC, USA; Centers for Disease Control and Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, Division of Tuberculosis Elimination: Atlanta, GA, USA, 1975.
- Burrill, J.; Williams, C.J.; Bain, G.; Conder, G.; Hine, A.L.; Misra, R.R. Tuberculosis: A radiologic review. Radiographics 2007, 27, 1255–1273. [Google Scholar] [CrossRef]
- Hopewell, P.C.; Jasmer, R.M. Overview of clinical tuberculosis. In Tuberculosis and the Tubercle Bacillus; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004; pp. 13–31. [Google Scholar]
- Daniel, T.M.; Bates, J.H.; Downes, K.A. History of tuberculosis. In Tuberculosis: Pathogenesis, Protection, and Control; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1994; pp. 13–24. [Google Scholar]
- Glaziou, P.; Floyd, K.; Raviglione, M.C. Global epidemiology of tuberculosis. In Seminars in Respiratory and Critical Care Medicine; Thieme Medical Publishers: New York, NY, USA, 2018. [Google Scholar]
- Dutour, O. The paleopathology and paleoepidemiology of Upper paleolithic tuberculosis: Review of evidence and hypotheses. Tuberculosis 2023, 143, 102348. [Google Scholar] [CrossRef]
- Meraj, S.S.; Yaakob, R.; Azman, A.; Rum, S.N.M.; Nazri, A.A. Artificial intelligence in diagnosing tuberculosis: A review. Int. J. Adv. Sci. Eng. Inf. Technol. 2019, 9, 81–91. [Google Scholar] [CrossRef]
- Sharma, M.; Singh, P. Use of artificial intelligence in research and clinical decision making for combating mycobacterial diseases. In Artificial Intelligence and Machine Learning in Healthcare; Springer: Berlin/Heidelberg, Germany, 2021; pp. 183–215. [Google Scholar]
- Singh, M.; Pujar, G.V.; Kumar, S.A.; Bhagyalalitha, M.; Akshatha, H.S.; Abuhaija, B.; Alsoud, A.R.; Abualigah, L.; Beeraka, N.M.; Gandomi, A.H. Evolution of machine learning in tuberculosis diagnosis: A review of deep learning-based medical applications. Electronics 2022, 11, 2634. [Google Scholar] [CrossRef]
- Soni, A.; Rai, A.; Ahirwar, S.K. Mycobacterium tuberculosis detection using support vector machine classification approach. In Proceedings of the 2021 10th IEEE International Conference on Communication Systems and Network Technologies (CSNT), Bhopal, India, 18–19 June 2021; IEEE: Piscataway, NJ, USA, 2021. [Google Scholar]
- Kanesamoorthy, K.; Dissanayake, M.B. Prediction of treatment failure of tuberculosis using support vector machine with genetic algorithm. Int. J. Mycobacteriology 2021, 10, 279–284. [Google Scholar] [CrossRef]
- Ma, J.; Yin, H.; Hao, X.; Sha, W.; Cui, H. Development of a random forest model to classify sarcoidosis and tuberculosis. Am. J. Transl. Res. 2021, 13, 6166. [Google Scholar]
- Miller, A.C.; Cavanaugh, J.E.; Arakkal, A.T.; Koeneman, S.H.; Polgreen, P.M. A comprehensive framework to estimate the frequency, duration, and risk factors for diagnostic delays using bootstrapping-based simulation methods. BMC Med. Inform. Decis. Mak. 2023, 23, 68. [Google Scholar] [CrossRef]
- Munadi, K.; Muchtar, K.; Maulina, N.; Pradhan, B. Image enhancement for tuberculosis detection using deep learning. IEEE Access 2020, 8, 217897–217907. [Google Scholar] [CrossRef]
- Khan, M.T.; Kaushik, A.C.; Ji, L.; Malik, S.I.; Ali, S.; Wei, D.-Q. Artificial neural networks for prediction of tuberculosis disease. Front. Microbiol. 2019, 10, 395. [Google Scholar] [CrossRef] [PubMed]
- Dande, P.; Samant, P. Acquaintance to artificial neural networks and use of artificial intelligence as a diagnostic tool for tuberculosis: A review. Tuberculosis 2018, 108, 1–9. [Google Scholar] [CrossRef]
- Oloko-Oba, M.; Viriri, S. Diagnosing tuberculosis using deep convolutional neural network. In Proceedings of the Image and Signal Processing: 9th International Conference, ICISP 2020, Marrakesh, Morocco, 4–6 June 2020; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Meraj, S.S.; Yaakob, R.; Azman, A.; Rum, S.; Shahrel, A.; Nazri, A.; Zakaria, N.F. Detection of pulmonary tuberculosis manifestation in chest X-rays using different convolutional neural network (CNN) models. Int. J. Eng. Adv. Technol. (IJEAT) 2019, 9, 2270–2275. [Google Scholar] [CrossRef]
- Doshi, R.; Falzon, D.; Thomas, B.V.; Temesgen, Z.; Sadasivan, L.; Migliori, G.B.; Raviglione, M. Tuberculosis control, and the where and why of artificial intelligence. ERJ Open Res. 2017, 3, 00056–2017. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, F.; Li, L.; Pang, Y. Clinical utilization of artificial intelligence in predicting therapeutic efficacy in pulmonary tuberculosis. J. Infect. Public Health 2024, 17, 632–641. [Google Scholar] [CrossRef]
- Liao, K.-M.; Liu, C.-F.; Chen, C.-J.; Feng, J.-Y.; Shu, C.-C.; Ma, Y.-S. Using an artificial intelligence approach to predict the adverse effects and prognosis of tuberculosis. Diagnostics 2023, 13, 1075. [Google Scholar] [CrossRef]
- Parreira, P.; Fonseca, A.; Soares, F.; Conte, M.; Rabahi, M. Chest X-ray evaluation using machine learning to support the early diagnosis of pulmonary TB. Int. J. Tuberc. Lung Dis. 2024, 28, 171–175. [Google Scholar] [CrossRef]
- Peng, J.; Song, J.; Wang, F.; Zuo, P.; Lu, Y.; Liu, W.; Tian, L.; Chen, Z.; Zhu, Y.; Wang, X. Harnessing big data to optimize an algorithm for rapid diagnosis of pulmonary tuberculosis in a real-world setting. Front. Cell. Infect. Microbiol. 2021, 11, 650163. [Google Scholar] [CrossRef]
- Lee, J.; Ahn, H.; Choi, D.; Tae, K.S. Evaluation on the usefulness of X-ray computer-aided detection (CAD) system for pulmonary tuberculosis (PTB) using SegNet. J. Biomed. Eng. Res. 2017, 38, 25–31. [Google Scholar]
- Harris, M.; Qi, A.; Jeagal, L.; Torabi, N.; Menzies, D.; Korobitsyn, A.; Pai, M.; Nathavitharana, R.R.; Ahmad Khan, F. A systematic review of the diagnostic accuracy of artificial intelligence-based computer programs to analyze chest X-rays for pulmonary tuberculosis. PLoS ONE 2019, 14, e0221339. [Google Scholar] [CrossRef] [PubMed]
- Ai, J.-W.; Zhou, X.; Xu, T.; Yang, M.; Chen, Y.; He, G.-Q.; Pan, N.; Cai, Y.; Li, Y.; Wang, X. CRISPR-based rapid and ultra-sensitive diagnostic test for Mycobacterium tuberculosis. Emerg. Microbes Infect. 2019, 8, 1361–1369. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Ba, X.; Hou, A.; Zhang, K.; Chen, L.; Li, T. Automatic detection of mycobacterium tuberculosis using artificial intelligence. J. Thorac. Dis. 2018, 10, 1936. [Google Scholar] [CrossRef]
- Moore, D.F.; Guzman, J.A.; Mikhail, L.T. Reduction in turnaround time for laboratory diagnosis of pulmonary tuberculosis by routine use of a nucleic acid amplification test. Diagn. Microbiol. Infect. Dis. 2005, 52, 247–254. [Google Scholar] [CrossRef]
- Salcedo, J.; Rosales, M.; Kim, J.S.; Nuno, D.; Suen, S.-c.; Chang, A.H. Cost-effectiveness of artificial intelligence monitoring for active tuberculosis treatment: A modeling study. PLoS ONE 2021, 16, e0254950. [Google Scholar] [CrossRef]
- Das, S.; Biswas, S.; Paul, A.; Dey, A. AI Doctor: An intelligent approach for medical diagnosis. In Industry Interactive Innovations in Science, Engineering and Technology: Proceedings of the International Conference, I3SET 2016; Springer: Singapore, 2017. [Google Scholar]
- Cao, Y.; Liu, C.; Liu, B.; Brunette, M.J.; Zhang, N.; Sun, T.; Zhang, P.; Peinado, J.; Garavito, E.S.; Garcia, L.L. Improving tuberculosis diagnostics using deep learning and mobile health technologies among resource-poor and marginalized communities. In Proceedings of the 2016 IEEE First International Conference on Connected Health: Applications, Systems and Engineering Technologies (CHASE), Washington, DC, USA, 27–29 June 2016; IEEE: Piscataway, NJ, USA, 2016. [Google Scholar]
- Zhang, F.; Han, H.; Li, M.; Tian, T.; Zhang, G.; Yang, Z.; Guo, F.; Li, M.; Wang, Y.; Wang, J. Revolutionizing diagnosis of pulmonary Mycobacterium tuberculosis based on CT: A systematic review of imaging analysis through deep learning. Front. Microbiol. 2025, 15, 1510026. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, Y.; Yang, Z.; Liu, R.; Li, S.; Pang, Y.; Li, L. The impact of maximum cross-sectional area of lesion on predicting the early therapeutic response of multidrug-resistant tuberculosis. J. Infect. Public Health 2025, 18, 102628. [Google Scholar] [CrossRef]
- Swai, H.F.; Mugusi, F.M.; Mbwambo, J.K. Sputum smear negative pulmonary tuberculosis: Sensitivity and specificity of diagnostic algorithm. BMC Res. Notes 2011, 4, 475. [Google Scholar] [CrossRef]
- Steingart, K.R.; Henry, M.; Ng, V.; Hopewell, P.C.; Ramsay, A.; Cunningham, J.; Urbanczik, R.; Perkins, M.; Aziz, M.A.; Pai, M. Fluorescence versus conventional sputum smear microscopy for tuberculosis: A systematic review. Lancet Infect. Dis. 2006, 6, 570–581. [Google Scholar] [CrossRef]
- Steingart, K.R.; Ramsay, A.; Pai, M. Optimizing sputum smear microscopy for the diagnosis of pulmonary tuberculosis. Expert Rev. Anti-Infect. Ther. 2007, 5, 327–331. [Google Scholar] [CrossRef][Green Version]
- WHO. WHO operational handbook on tuberculosis. In Module 3: Diagnosis-Rapid Diagnostics for Tuberculosis Detection; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Steingart, K.R.; Ng, V.; Henry, M.; Hopewell, P.C.; Ramsay, A.; Cunningham, J.; Urbanczik, R.; Perkins, M.D.; Aziz, M.A.; Pai, M. Sputum processing methods to improve the sensitivity of smear microscopy for tuberculosis: A systematic review. Lancet Infect. Dis. 2006, 6, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Van Deun, A.; Salim, A.H.; Cooreman, E.; Hossain, M.A.; Rema, A.; Chambugonj, N.; Hye, M.; Kawria, A.; Declercq, E. Optimal tuberculosis case detection by direct sputum smear microscopy: How much better is more? Int. J. Tuberc. Lung Dis. 2002, 6, 222–230. [Google Scholar] [PubMed]
- Van Deun, A.; Tahseen, S.; Affolabi, D.; Hossain, M.; Joloba, M.; Angra, P.; Ridderhof, J.; de Jong, B.; Rieder, H. Sputum smear microscopy in the Xpert® MTB/RIF era. Int. J. Tuberc. Lung Dis. 2019, 23, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, A.; Nathavitharana, R.R. Addressing TB-related mortality in adults living with HIV: A review of the challenges and potential solutions. Ther. Adv. Infect. Dis. 2022, 9, 20499361221084163. [Google Scholar] [CrossRef]
- Kulka, K.; Hatfull, G.; Ojha, A.K. Growth of Mycobacterium tuberculosis biofilms. J. Vis. Exp. JoVE 2012, 60, 3820. [Google Scholar]
- Ghodbane, R.; Raoult, D.; Drancourt, M. Dramatic reduction of culture time of Mycobacterium tuberculosis. Sci. Rep. 2014, 4, 4236. [Google Scholar] [CrossRef]
- Sorlozano, A.; Soria, I.; Roman, J.; Huertas, P.; Soto, M.J.; Piedrola, G.; Gutierrez, J. Comparative evaluation of three culture methods for the isolation of mycobacteria from clinical samples. J. Microbiol. Biotechnol. 2009, 19, 1259–1264. [Google Scholar] [CrossRef]
- Syhre, M.; Chambers, S.T. The scent of Mycobacterium tuberculosis. Tuberculosis 2008, 88, 317–323. [Google Scholar] [CrossRef]
- Braissant, O.; Theron, G.; Friedrich, S.O.; Diacon, A.H.; Bonkat, G. Comparison of isothermal microcalorimetry and BACTEC MGIT960 for the detection of the metabolic activity of Mycobacterium tuberculosis in sputum samples. J. Appl. Microbiol. 2020, 128, 1497–1502. [Google Scholar] [CrossRef]
- Musisi, E. Evaluation of the Tuberculosis-Molecular Bacterial Load Assay for Tuberculosis Diagnosis and Monitoring Response to Standard Anti-Tuberculosis Therapy. Ph.D. Thesis, University of St Andrews, St Andrews, UK, 2023. [Google Scholar]
- Rakhmawatie, M.D.; Wibawa, T.; Lisdiyanti, P.; Pratiwi, W.R. Evaluation of crystal violet decolorization assay and resazurin microplate assay for antimycobacterial screening. Heliyon 2019, 5, e02263. [Google Scholar] [CrossRef]
- Cornfield, D.B.; Beavis, K.G.; Greene, J.A.; Bojak, M.; Bondi, J. Mycobacterial growth and bacterial contamination in the mycobacteria growth indicator tube and BACTEC 460 culture systems. J. Clin. Microbiol. 1997, 35, 2068–2071. [Google Scholar] [CrossRef] [PubMed]
- Greco, S.; Girardi, E.; Navarra, A.; Saltini, C. Current evidence on diagnostic accuracy of commercially based nucleic acid amplification tests for the diagnosis of pulmonary tuberculosis. Thorax 2006, 61, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Ling, D.I.; Flores, L.L.; Riley, L.W.; Pai, M. Commercial nucleic-acid amplification tests for diagnosis of pulmonary tuberculosis in respiratory specimens: Meta-analysis and meta-regression. PLoS ONE 2008, 3, e1536. [Google Scholar] [CrossRef] [PubMed]
- Daley, P.; Thomas, S.; Pai, M. Nucleic acid amplification tests for the diagnosis of tuberculous lymphadenitis: A systematic review. Int. J. Tuberc. Lung Dis. 2007, 11, 1166–1176. [Google Scholar]
- WHO. Update on the Use of Nucleic acid Amplification Tests to Detect TB and Drug-Resistant TB: Rapid Communication; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Hwang, E.J.; Park, S.; Jin, K.-N.; Kim, J.I.; Choi, S.Y.; Lee, J.H.; Goo, J.M.; Aum, J.; Yim, J.-J.; Park, C.M. Development and validation of a deep learning–based automatic detection algorithm for active pulmonary tuberculosis on chest radiographs. Clin. Infect. Dis. 2019, 69, 739–747. [Google Scholar] [CrossRef]
- Ridho, A.; Alfian, S.D.; van Boven, J.F.; Levita, J.; Yalcin, E.A.; Le, L.; Alffenaar, J.-W.; Hak, E.; Abdulah, R.; Pradipta, I.S. Digital health technologies to improve medication adherence and treatment outcomes in patients with tuberculosis: Systematic review of randomized controlled trials. J. Med. Internet Res. 2022, 24, e33062. [Google Scholar] [CrossRef]
- Subbaraman, R.; de Mondesert, L.; Musiimenta, A.; Pai, M.; Mayer, K.H.; Thomas, B.E.; Haberer, J. Digital adherence technologies for the management of tuberculosis therapy: Mapping the landscape and research priorities. BMJ Glob. Health 2018, 3, e001018. [Google Scholar] [CrossRef]
- Geethalakshmi, S.; Yadav, S. Advancements in artificial intelligence for the diagnosis of multidrug resistance and extensively drug-resistant tuberculosis: A comprehensive review. Cureus 2024, 16, e60280. [Google Scholar]
- Nathavitharana, R.R.; Lederer, P.; Tierney, D.B.; Nardell, E. Treatment as prevention and other interventions to reduce transmission of multidrug-resistant tuberculosis. Int. J. Tuberc. Lung Dis. 2019, 23, 396–404. [Google Scholar] [CrossRef]
- Chinagudaba, S.N.; Gera, D.; Dasu, K.K.V.; Singarajpure, A.; Chadda, V.K. Predictive Analysis of Tuberculosis Treatment Outcomes Using Machine Learning: A Karnataka TB Data Study at a Scale. arXiv 2024, arXiv:2403.08834. [Google Scholar]
- Singh, A.; Lall, B.; Panigrahi, B.K.; Agrawal, A.; Agrawal, A.; Thangakunam, B.; Christopher, D.J. Deep learning for automated screening of tuberculosis from Indian chest x-rays: Analysis and update. arXiv 2020, arXiv:2011.09778. [Google Scholar]
- Seeram, E.; Kanade, V. Artificial Intelligence in Medical Imaging Technology; Springer Nature: Cham, Switzerland, 2024. [Google Scholar]
- Qin, Z.Z.; Ahmed, S.; Sarker, M.S.; Paul, K.; Adel, A.S.S.; Naheyan, T.; Barrett, R.; Banu, S.; Creswell, J. Tuberculosis detection from chest x-rays for triaging in a high tuberculosis-burden setting: An evaluation of five artificial intelligence algorithms. Lancet Digit. Health 2021, 3, e543–e554. [Google Scholar] [CrossRef] [PubMed]
- Geric, C.; Qin, Z.; Denkinger, C.; Kik, S.; Marais, B.; Anjos, A.; David, P.; Ahmad Khan, F.; Trajman, A. The rise of artificial intelligence reading of chest X-rays for enhanced TB diagnosis and elimination. Int. J. Tuberc. Lung Dis. 2023, 27, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Creswell, J.; Vo, L.N.Q.; Qin, Z.Z.; Muyoyeta, M.; Tovar, M.; Wong, E.B.; Ahmed, S.; Vijayan, S.; John, S.; Maniar, R. Early user perspectives on using computer-aided detection software for interpreting chest X-ray images to enhance access and quality of care for persons with tuberculosis. BMC Glob. Public Health 2023, 1, 30. [Google Scholar] [CrossRef]
- Gelaw, S.M.; Kik, S.V.; Ruhwald, M.; Ongarello, S.; Egzertegegne, T.S.; Gorbacheva, O.; Gilpin, C.; Marano, N.; Lee, S.; Phares, C.R. Diagnostic accuracy of three computer-aided detection systems for detecting pulmonary tuberculosis on chest radiography when used for screening: Analysis of an international, multicenter migrants screening study. PLOS Glob. Public Health 2023, 3, e0000402. [Google Scholar] [CrossRef]
- Qin, Z.Z.; Barrett, R.; Ahmed, S.; Sarker, M.S.; Paul, K.; Adel, A.S.S.; Banu, S.; Creswell, J. Comparing different versions of computer-aided detection products when reading chest X-rays for tuberculosis. PLOS Digit. Health 2022, 1, e0000067. [Google Scholar] [CrossRef]
- Du, J.; Su, Y.; Qiao, J.; Gao, S.; Dong, E.; Wang, R.; Nie, Y.; Ji, J.; Wang, Z.; Liang, J. Application of artificial intelligence in diagnosis of pulmonary tuberculosis. Chin. Med. J. 2024, 137, 559–561. [Google Scholar] [CrossRef]
- Acharya, V.; Dhiman, G.; Prakasha, K.; Bahadur, P.; Choraria, A.; Prabhu, S.; Chadaga, K.; Viriyasitavat, W.; Kautish, S. AI-Assisted Tuberculosis Detection and Classification from Chest X-Rays Using a Deep Learning Normalization-Free Network Model. Comput. Intell. Neurosci. 2022, 2022, 2399428. [Google Scholar] [CrossRef]
- Yan, C.; Wang, L.; Lin, J.; Xu, J.; Zhang, T.; Qi, J.; Li, X.; Ni, W.; Wu, G.; Huang, J. A fully automatic artificial intelligence–based CT image analysis system for accurate detection, diagnosis, and quantitative severity evaluation of pulmonary tuberculosis. Eur. Radiol. 2022, 32, 2188–2199. [Google Scholar] [CrossRef]
- Zhan, Y.; Wang, Y.; Zhang, W.; Ying, B.; Wang, C. Diagnostic accuracy of the artificial intelligence methods in medical imaging for pulmonary tuberculosis: A systematic review and meta-analysis. J. Clin. Med. 2022, 12, 303. [Google Scholar] [CrossRef]
- Nijiati, M.; Ma, J.; Hu, C.; Tuersun, A.; Abulizi, A.; Kelimu, A.; Zhang, D.; Li, G.; Zou, X. Artificial intelligence assisting the early detection of active pulmonary tuberculosis from chest X-rays: A population-based study. Front. Mol. Biosci. 2022, 9, 874475. [Google Scholar] [CrossRef] [PubMed]
- Premananthan, G.; Nagaraj, B.; Jaya, J. A new AI assisted medical molecular image diagnostic model. J. Intell. Fuzzy Syst. 2023, 44, 9027–9037. [Google Scholar] [CrossRef]
- Solovchuk, D.R. Advances in AI-assisted biochip technology for biomedicine. Biomed. Pharmacother. 2024, 177, 116997. [Google Scholar]
- de Jesus Ximenes, N.A. Evaluating HAMNASA’s Tuberculosis Screening Services and Patient Management by District Assistants in Dili, Timor-Leste. Master’s Thesis, University of Washington, Seattle, WA, USA, 2024. [Google Scholar]
- Nath, A.; Hashim, Z.; Shukla, S.; Poduvattil, P.A.; Singh, M.; Misra, N.; Shukla, A. Assessing Diagnostic Accuracy and Viability of AI-Assisted Tuberculosis Detection in Northern Indian Healthcare Facilities: A Multicenter Study. Sci. Rep. 2024. [Google Scholar] [CrossRef]
- Shi, L.; Gu, R.; Long, J.; Duan, G.; Yang, H. Application of CRISPR–cas-based technology for the identification of tuberculosis, drug discovery and vaccine development. Mol. Biol. Rep. 2024, 51, 466. [Google Scholar] [CrossRef]
- Zhang, X.; He, X.; Zhang, Y.; Chen, L.; Pan, Z.; Huang, Y.; Li, H. A new method for the detection of Mycobacterium tuberculosis based on the CRISPR/Cas system. BMC Infect. Dis. 2023, 23, 680. [Google Scholar] [CrossRef]
- Abavisani, M.; Karbas Foroushan, S.; Khayami, R.; Sahebkar, A. Mycobacterium tuberculosis Detection Using CRISPR Technology: An Updated Systematic Review and Meta-analysis. Mol. Diagn. Ther. 2024, 28, 777–790. [Google Scholar] [CrossRef]
- Xiao, J.; Li, J.; Quan, S.; Wang, Y.; Jiang, G.; Wang, Y.; Huang, H.; Jiao, W.; Shen, A. Development and preliminary assessment of a CRISPR–Cas12a-based multiplex detection of Mycobacterium tuberculosis complex. Front. Bioeng. Biotechnol. 2023, 11, 1233353. [Google Scholar] [CrossRef]
- Nsengiyumva, N.P.; Hussain, H.; Oxlade, O.; Majidulla, A.; Nazish, A.; Khan, A.J.; Menzies, D.; Ahmad Khan, F.; Schwartzman, K. Triage of persons with tuberculosis symptoms using artificial intelligence–based chest radiograph interpretation: A cost-effectiveness analysis. In Open Forum Infectious Diseases; Oxford University Press: Oxford, UK, 2021. [Google Scholar]
- Naidoo, K.; Dookie, N. Can the GeneXpert MTB/XDR deliver on the promise of expanded, near-patient tuberculosis drug-susceptibility testing? Lancet Infect. Dis. 2022, 22, e121–e127. [Google Scholar] [CrossRef]
- Mvelase, N.R.; Mlisana, K.P. Xpert MTB/XDR for rapid detection of drug-resistant tuberculosis beyond rifampicin. Lancet Infect. Dis. 2022, 22, 156–157. [Google Scholar] [CrossRef]
- Shete, P.B.; Farr, K.; Strnad, L.; Gray, C.M.; Cattamanchi, A. Diagnostic accuracy of TB-LAMP for pulmonary tuberculosis: A systematic review and meta-analysis. BMC Infect. Dis. 2019, 19, 268. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Ma, Y.; Liu, R.; Shang, Y.; Ma, L.; Huo, F.; Li, Y.; Shu, W.; Wang, Y.; Gao, M. Diagnostic yield of oral swab testing by TB-LAMP for diagnosis of pulmonary tuberculosis. Infect. Drug Resist. 2021, 14, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.K.; Albahra, S.; May, L.; Waldman, S.; Crabtree, S.; Bainbridge, S.; Rashidi, H. Evolving applications of artificial intelligence and machine learning in infectious diseases testing. Clin. Chem. 2022, 68, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Raju, G.; Ranjan, A.; Banik, S.; Poddar, A.; Managuli, V.; Mazumder, N. A commentary on the development and use of smartphone imaging devices. Biophys. Rev. 2024, 16, 151–163. [Google Scholar] [CrossRef]
- Kumar, U.; Kesharwani, R.K.; Pal, M.K. The Most Recent Developments and Applications of Smartphone-based Devices in Biomedical and mHealth. In Advanced Research in Electronic Devices for Biomedical and mHealth; Apple Academic Press: Palm Bay, FL, USA, 2024; p. 127. [Google Scholar]
- Kanbes-Dindar, C.; Demirtaş, T.T.; Uslu, B. Nanostructured materials-modified electrochemical biosensing devices for determination of neurochemicals. In Novel Nanostructured Materials for Electrochemical Bio-Sensing Applications; Elsevier: Amsterdam, The Netherlands, 2024; pp. 331–365. [Google Scholar]
- Banik, S.; Melanthota, S.K.; Arbaaz; Vaz, J.M.; Kadambalithaya, V.M.; Hussain, I.; Dutta, S.; Mazumder, N. Recent trends in smartphone-based detection for biomedical applications: A review. Anal. Bioanal. Chem. 2021, 413, 2389–2406. [Google Scholar] [CrossRef]
- Albert, H.; Manabe, Y.; Lukyamuzi, G.; Ademun, P.; Mukkada, S.; Nyesiga, B.; Joloba, M.; Paramasivan, C.; Perkins, M.D. Performance of three LED-based fluorescence microscopy systems for detection of tuberculosis in Uganda. PLoS ONE 2010, 5, e15206. [Google Scholar] [CrossRef]
- Graham, C.F. Fluorescence microscopy of Mycobacterium tuberculosis: A study of basic factors. Am. Rev. Tuberc. 1943, 48, 421–434. [Google Scholar]
- Van Deun, A.; Chonde, T.; Gumusboga, M.; Rienthong, S. Performance and acceptability of the FluoLED Easy™ module for tuberculosis fluorescence microscopy. Int. J. Tuberc. Lung Dis. 2008, 12, 1009–1014. [Google Scholar]
- Bhatia, D.; Acharjee, T.; Shukla, S.; Bhatia, M. Nano-technological advancements in multimodal diagnosis and treatment. AIMS Biophys. 2024, 11, 464–507. [Google Scholar] [CrossRef]
- Capo, A.; Sepe, R.; Pellino, G.; Milone, M.; Malapelle, U.; Pellecchia, S.; Pepe, F.; Cacciola, N.A.; Manigrasso, M.; Bruzzaniti, S. Setting up and exploitation of a nano/technological platform for the evaluation of HMGA1b protein in peripheral blood of cancer patients. Nanomed. Nanotechnol. Biol. Med. 2019, 15, 231–242. [Google Scholar] [CrossRef]
- Tapeinos, C.; Battaglini, M.; Marino, A.; Ciofani, G. Smart diagnostic nano-agents for cerebral ischemia. J. Mater. Chem. B 2020, 8, 6233–6251. [Google Scholar] [CrossRef] [PubMed]
- Sonowal, L.; Gautam, S. Advancements and challenges in carbon nanotube-based drug delivery systems. Nano-Struct. Nano-Objects 2024, 38, 101117. [Google Scholar] [CrossRef]
- Tay, A.; Schweizer, F.E.; Di Carlo, D. Micro-and nano-technologies to probe the mechano-biology of the brain. Lab Chip 2016, 16, 1962–1977. [Google Scholar] [CrossRef] [PubMed]
- Innes, A.L.; Lebrun, V.; Hoang, G.L.; Martinez, A.; Dinh, N.; Nguyen, T.T.H.; Huynh, T.P.; Quach, V.L.; Nguyen, T.B.; Trieu, V.C. An effective health system approach to end TB: Implementing the double X strategy in Vietnam. Glob. Health Sci. Pract. 2024, 12, e2400024. [Google Scholar] [CrossRef]
- Vijayan, S.; Jondhale, V.; Pande, T.; Khan, A.; Brouwer, M.; Hegde, A.; Gandhi, R.; Roddawar, V.; Jichkar, S.; Kadu, A. Implementing a chest X-ray artificial intelligence tool to enhance tuberculosis screening in India: Lessons learned. PLoS Digit. Health 2023, 2, e0000404. [Google Scholar] [CrossRef]
- Aung, S.T.; Nyunt, W.W.; Moe, M.M.; Aung, H.L.; Lwin, T. The fourth national tuberculosis prevalence survey in Myanmar. PLoS Glob. Public Health 2022, 2, e0000588. [Google Scholar] [CrossRef]
- Sekandi, J.N.; Shi, W.; Zhu, R.; Kaggwa, P.; Mwebaze, E.; Li, S. Application of artificial intelligence to the monitoring of medication adherence for tuberculosis treatment in Africa: Algorithm development and validation. JMIR AI 2023, 2, e40167. [Google Scholar] [CrossRef]
- Deutsch-Feldman, M. Tuberculosis—United States, 2020. MMWR. Morb. Mortal. Wkly. Rep. 2021, 70, 409–414. [Google Scholar] [CrossRef]
- Kulkarni, M.; Golechha, S.; Raj, R.; Sreedharan, J.K.; Bhardwaj, A.; Rathod, S.; Vadera, B.; Kurada, J.; Mattoo, S.; Joshi, R. Predicting Treatment Adherence of Tuberculosis Patients at Scale. In Proceedings of the Machine Learning for Health, PMLR, New Orleans, LA, USA, 28 November 2022. [Google Scholar]
- Chen, E.C.; Owaisi, R.; Goldschmidt, L.; Maimets, I.-K.; Daftary, A. Patient perceptions of video directly observed therapy for tuberculosis: A systematic review. J. Clin. Tuberc. Other Mycobact. Dis. 2024, 35, 100406. [Google Scholar] [CrossRef]
- Rao, J.S.; Diwan, V.; Kumar, A.A.; Varghese, S.S.; Sharma, U.; Purohit, M.; Das, A.; Rodrigues, R. Acceptability of video observed treatment vs. directly observed treatment for tuberculosis: A comparative analysis between South and central India. Wellcome Open Res. 2022, 7, 152. [Google Scholar] [CrossRef]
- Sekandi, J.N.; McDonald, A.; Nakkonde, D.; Zalwango, S.; Kasiita, V.; Kaggwa, P.; Kakaire, R.; Atuyambe, L.; Buregyeya, E. Acceptability, Usefulness, and Ease of Use of an Enhanced Video Directly Observed Treatment System for Supporting Patients With Tuberculosis in Kampala, Uganda: Explanatory Qualitative Study. JMIR Form. Res. 2023, 7, e46203. [Google Scholar] [CrossRef] [PubMed]
- Molton, J.S.; Pang, Y.; Wang, Z.; Qiu, B.; Wu, P.; Rahman-Shepherd, A.; Ooi, W.T.; Paton, N.I. Prospective single-arm interventional pilot study to assess a smartphone-based system for measuring and supporting adherence to medication. BMJ Open 2016, 6, e014194. [Google Scholar] [CrossRef]
- Rabinovich, L.; Molton, J.S.; Ooi, W.T.; Paton, N.I.; Batra, S.; Yoong, J. Perceptions and acceptability of digital interventions among tuberculosis patients in Cambodia: Qualitative study of video-based directly observed therapy. J. Med. Internet Res. 2020, 22, e16856. [Google Scholar] [CrossRef]
- Zaka-Ur-Rehman, Z.; Jamshaid, M.; Chaudhry, A. Clinical evaluation and monitoring of adverse effects for fixed multidose combination against single drug therapy in pulmonary tuberculosis patients. Pak. J. Pharm. Sci. 2008, 21, 185–194. [Google Scholar]
- Ai, X.; Men, K.; Guo, L.; Zhang, T.; Zhao, Y.; Sun, X.; Zhang, H.; He, G.; van der Werf, M.J.; Van Den Hof, S. Factors associated with low cure rate of tuberculosis in remote poor areas of Shaanxi Province, China: A case control study. BMC Public Health 2010, 10, 112. [Google Scholar] [CrossRef]
- Pramanik, A.; Singh, A.; Rani, G.; Dubey, S.; Aluvala, S. Revolutionizing Tuberculosis Treatment: Smart Technology Innovations for Mycobacterium tuberculosis Management. In Proceedings of the 2023 International Conference on Smart Devices (ICSD), Dehradun, India, 2–3 May 2024; IEEE: Piscataway, NJ, USA, 2024. [Google Scholar]
- Märtson, A.-G.; Burch, G.; Ghimire, S.; Alffenaar, J.-W.C.; Peloquin, C.A. Therapeutic drug monitoring in patients with tuberculosis and concurrent medical problems. Expert Opin. Drug Metab. Toxicol. 2021, 17, 23–39. [Google Scholar] [CrossRef]
- Pandey, S.K.; Singh, K.U.; Dingankar, R.S.; Jadhav, K.; Gupta, K.; Yadav, R.K. Prediction of Tuberculosis Disease Progression with AI Analysis of Clinical Data. In Proceedings of the 2023 International Conference on Artificial Intelligence for Innovations in Healthcare Industries (ICAIIHI), Raipur, India, 29–30 December 2023; IEEE: Piscataway, NJ, USA, 2023. [Google Scholar]
- Blanco-Gonzalez, A.; Cabezon, A.; Seco-Gonzalez, A.; Conde-Torres, D.; Antelo-Riveiro, P.; Pineiro, A.; Garcia-Fandino, R. The role of AI in drug discovery: Challenges, opportunities, and strategies. Pharmaceuticals 2023, 16, 891. [Google Scholar] [CrossRef]
- Ejalonibu, M.A.; Ogundare, S.A.; Elrashedy, A.A.; Ejalonibu, M.A.; Lawal, M.M.; Mhlongo, N.N.; Kumalo, H.M. Drug discovery for Mycobacterium tuberculosis using structure-based computer-aided drug design approach. Int. J. Mol. Sci. 2021, 22, 13259. [Google Scholar] [CrossRef]
- Winkler, D.A. The impact of machine learning on future tuberculosis drug discovery. Expert Opin. Drug Discov. 2022, 17, 925–927. [Google Scholar] [CrossRef]
- Nayyar, A.; Shrivastava, R.; Jain, S. Drug Discovery for Mycobacterium Tuberculosis: An Experimental Analysis. In Proceedings of the 2024 IEEE Region 10 Symposium (TENSYMP), New Delhi, India, 27–29 September 2024; IEEE: Piscataway, NJ, USA, 2024. [Google Scholar]
- Singh, V.; Mizrahi, V. Identification and validation of novel drug targets in Mycobacterium tuberculosis. Drug Discov. Today 2017, 22, 503–509. [Google Scholar] [CrossRef]
- Liang, S.; Ma, J.; Wang, G.; Shao, J.; Li, J.; Deng, H.; Wang, C.; Li, W. The application of artificial intelligence in the diagnosis and drug resistance prediction of pulmonary tuberculosis. Front. Med. 2022, 9, 935080. [Google Scholar] [CrossRef] [PubMed]
- Winkler, D.A. Use of artificial intelligence and machine learning for discovery of drugs for neglected tropical diseases. Front. Chem. 2021, 9, 614073. [Google Scholar] [CrossRef] [PubMed]
- Program, T.D.S. Search for new drugs for treatment of tuberculosis. Antimicrob. Agents Chemother. 2001, 45, 1943–1946. [Google Scholar]
- Lane, T.R.; Urbina, F.; Rank, L.; Gerlach, J.; Riabova, O.; Lepioshkin, A.; Kazakova, E.; Vocat, A.; Tkachenko, V.; Cole, S. Machine learning models for mycobacterium tuberculosis in vitro activity: Prediction and target visualization. Mol. Pharm. 2021, 19, 674–689. [Google Scholar] [CrossRef]
- Anishetty, S.; Pulimi, M.; Pennathur, G. Potential drug targets in Mycobacterium tuberculosis through metabolic pathway analysis. Comput. Biol. Chem. 2005, 29, 368–378. [Google Scholar] [CrossRef]
- Turon, G.; Hlozek, J.; Woodland, J.G.; Kumar, A.; Chibale, K.; Duran-Frigola, M. First fully-automated AI/ML virtual screening cascade implemented at a drug discovery centre in Africa. Nat. Commun. 2023, 14, 5736. [Google Scholar] [CrossRef]
- Jamal, S.; Khubaib, M.; Gangwar, R.; Grover, S.; Grover, A.; Hasnain, S.E. Artificial Intelligence and Machine learning based prediction of resistant and susceptible mutations in Mycobacterium tuberculosis. Sci. Rep. 2020, 10, 5487. [Google Scholar]
- Nagamani, S.; Sastry, G.N. Mycobacterium tuberculosis cell wall permeability model generation using chemoinformatics and machine learning approaches. ACS Omega 2021, 6, 17472–17482. [Google Scholar] [CrossRef]
- Ye, Q.; Chai, X.; Jiang, D.; Yang, L.; Shen, C.; Zhang, X.; Li, D.; Cao, D.; Hou, T. Identification of active molecules against Mycobacterium tuberculosis through machine learning. Brief. Bioinform. 2021, 22, bbab068. [Google Scholar] [CrossRef]
- Chikhale, R.V.; Eldesoky, G.E.; Kolpe, M.S.; Suryawanshi, V.S.; Patil, P.C.; Bhowmick, S. Identification of Mycobacterium tuberculosis transcriptional repressor EthR inhibitors: Shape-based search and machine learning studies. Heliyon 2024, 10, e26802. [Google Scholar] [CrossRef]
- Villemagne, B.; Flipo, M.; Blondiaux, N.; Crauste, C.; Malaquin, S.; Leroux, F.; Piveteau, C.; Villeret, V.; Brodin, P.; Villoutreix, B.O. Ligand efficiency driven design of new inhibitors of Mycobacterium tuberculosis transcriptional repressor EthR using fragment growing, merging, and linking approaches. J. Med. Chem. 2014, 57, 4876–4888. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.K.; Elma, F. In silico identification of novel chemical compounds with anti-TB potential for the inhibition of InhA and EthR from Mycobacterium tuberculosis. J. Clin. Tuberc. Other Mycobact. Dis. 2021, 24, 100246. [Google Scholar] [CrossRef]
- Aloufi, B.H.; Alshammari, A.M. In Silico Approaches. Drugs 2022, 21, 25. [Google Scholar]
- Patel, M.N.; Patel, A.J.; Nandpal, M.N.; Raval, M.A.; Patel, R.J.; Patel, A.A.; Paudel, K.R.; Hansbro, P.M.; Singh, S.K.; Gupta, G. Advancing against drug-resistant tuberculosis: An extensive review, novel strategies and patent landscape. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 398, 2127–2150. [Google Scholar] [CrossRef]
- Guglielmetti, L.; Chiesi, S.; Eimer, J.; Dominguez, J.; Masini, T.; Varaine, F.; Veziris, N.; Ader, F.; Robert, J. Bedaquiline and delamanid for drug-resistant tuberculosis: A clinician’s perspective. Future Microbiol. 2020, 15, 779–799. [Google Scholar] [CrossRef]
- Negi, A.; Perveen, S.; Gupta, R.; Singh, P.P.; Sharma, R. Unraveling Dilemmas and Lacunae in the Escalating Drug Resistance of Mycobacterium tuberculosis to Bedaquiline, Delamanid, and Pretomanid. J. Med. Chem. 2024, 67, 2264–2286. [Google Scholar] [CrossRef]
- McClean, M.; Panciu, T.C.; Lange, C.; Duarte, R.; Theis, F. Artificial intelligence in tuberculosis: A new ally in disease control. Breathe 2024, 20, 240056. [Google Scholar] [CrossRef]
- Chin, K.L.; Anibarro, L.; Sarmiento, M.E.; Acosta, A. Challenges and the Way forward in Diagnosis and Treatment of Tuberculosis Infection. Trop. Med. Infect. Dis. 2023, 8, 89. [Google Scholar] [CrossRef]
- Jonathan, J.; Barakabitze, A.A. ML technologies for diagnosing and treatment of tuberculosis: A survey. Health Technol. 2023, 13, 17–33. [Google Scholar] [CrossRef]
- Deshpande, A.; Likhar, R.; Khan, T.; Omri, A. Decoding drug resistance in mycobacterium tuberculosis complex: Genetic insights and future challenges. Expert Rev. Anti-Infect. Ther. 2024, 22, 511–527. [Google Scholar] [CrossRef]
- MacGregor-Fairlie, M.; Wilkinson, S.; Besra, G.S.; Goldberg Oppenheimer, P. Tuberculosis diagnostics: Overcoming ancient challenges with modern solutions. Emerg. Top. Life Sci. 2020, 4, 435–448. [Google Scholar]
- David, P.-M.; Onno, J.; Pourraz, J.; Ahmad Khan, F. Tweaking algorithms. Technopolitical issues associated with artificial intelligence based tuberculosis detection in global health. Digit. Health 2024, 10, 20552076241239778. [Google Scholar] [CrossRef] [PubMed]
- Bartolomeu-Gonçalves, G.; Souza, J.M.d.; Fernandes, B.T.; Spoladori, L.F.A.; Correia, G.F.; Castro, I.M.d.; Borges, P.H.G.; Silva-Rodrigues, G.; Tavares, E.R.; Yamauchi, L.M. Tuberculosis diagnosis: Current, ongoing, and future approaches. Diseases 2024, 12, 202. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.; Ye, Z.; Li, L.; Yang, L.; Gong, W. Next-generation TB vaccines: Progress, challenges, and prospects. Vaccines 2023, 11, 1304. [Google Scholar] [CrossRef]
- Schwalbe, N.; Wahl, B. Artificial intelligence and the future of global health. Lancet 2020, 395, 1579–1586. [Google Scholar] [CrossRef]
- Palanivel, J.; Manikkam, R.; Sounderrajan, V.; Jayaraj, S.; Rao, S.S.; Thangam, T.; Parthasarathy, K. An Emerging Artificial Intelligence Tool for the Advancement of Modern Health Care in Tuberculosis. In Translational Research in Biomedical Sciences: Recent Progress and Future Prospects; Springer: Berlin/Heidelberg, Germany, 2024; pp. 111–120. [Google Scholar]
- Mahmoudi, S.; García, M.J.; Drain, P.K. Current approaches for diagnosis of subclinical pulmonary tuberculosis, clinical implications and future perspectives: A scoping review. Expert Rev. Clin. Immunol. 2024, 20, 715–726. [Google Scholar] [CrossRef]
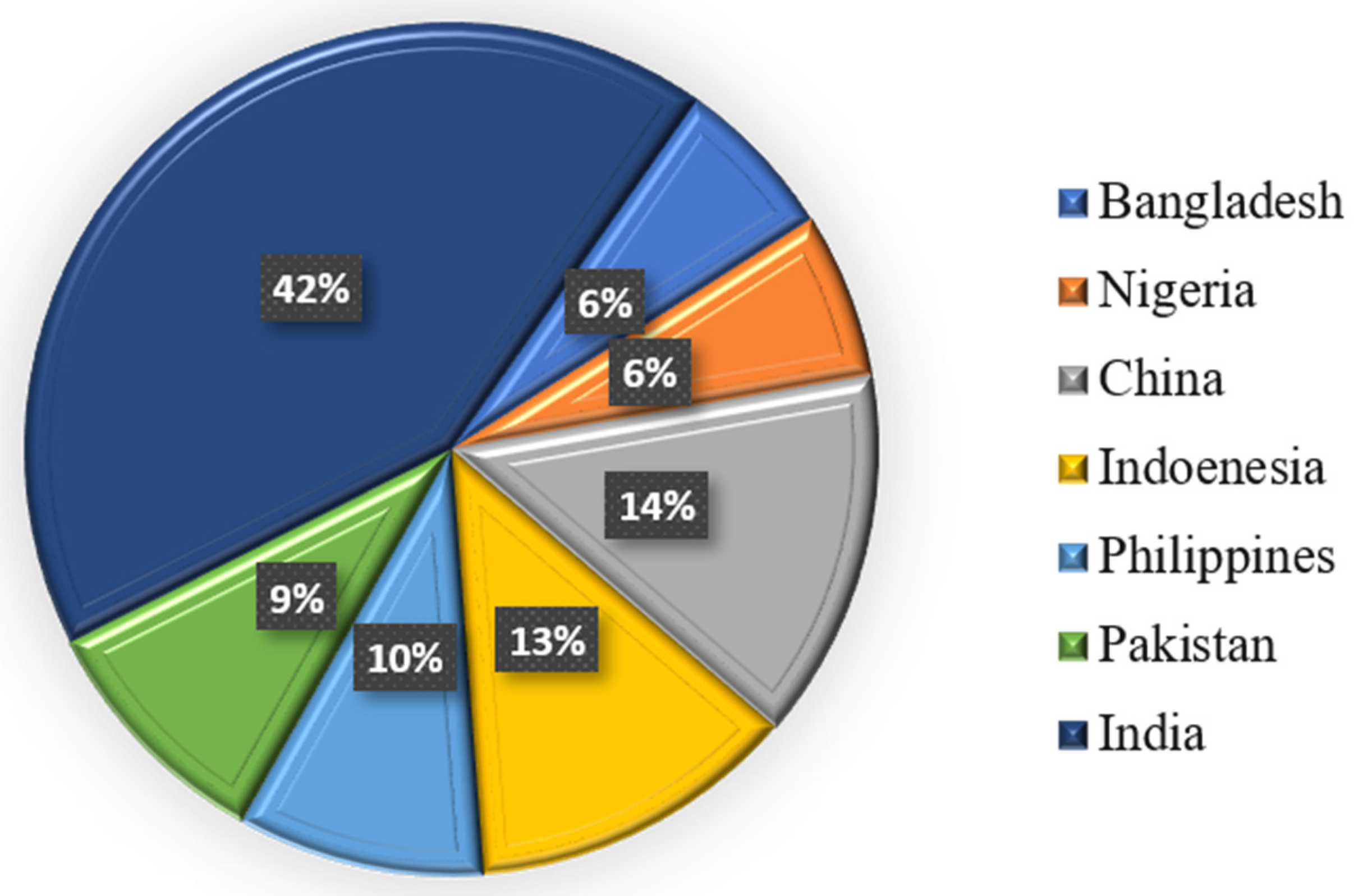
| Studies | AI Models | Important Results | References |
|---|---|---|---|
| An Analysis of Six Countries Using Machine Learning to Forecast Treatment Performance in Tuberculosis | Artificial Neural Networks, Random Forests, and Support Vector Machines |
| [16,63,64] |
| A Multicenter Cohort Study Using Artificial Intelligence-Based Radiographic Extent Evaluation to Forecast Tuberculosis Treatment Results | AI-Powered Radiography Evaluation |
| [24,65] |
| Countries | AI Implementation | Influences | References |
|---|---|---|---|
| Vietnam | AI software combined with X-ray analysis of the chest | Enhanced chest X-ray interpretation quality, which resulted in the discovery of more TB patients during community screening initiatives | [102] |
| India | Wadhwani AI’s AI technologies for TB screening and forecasting outcomes | Improved TB screening procedures and forecasted patient results, which helped develop more potent treatment plans | [103] |
| Myanmar | AI-powered study of chest X-rays | Improved patient care by addressing the lack of radiologists and expediting TB diagnosis | [104] |
| Africa | AI-powered drug adherence monitoring system | Assessed patient compliance with TB therapy, helping to guarantee the efficacy and completion of treatment | [105] |
| United States | AI-powered radiography rating to forecast the effectiveness of treatment | Culture conversion and treatment outcome predictions for pulmonary tuberculosis patients, supporting individualized treatment planning | [106] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Memon, S.; Bibi, S.; He, G. Integration of AI and ML in Tuberculosis (TB) Management: From Diagnosis to Drug Discovery. Diseases 2025, 13, 184. https://doi.org/10.3390/diseases13060184
Memon S, Bibi S, He G. Integration of AI and ML in Tuberculosis (TB) Management: From Diagnosis to Drug Discovery. Diseases. 2025; 13(6):184. https://doi.org/10.3390/diseases13060184
Chicago/Turabian StyleMemon, Sameeullah, Shabana Bibi, and Guozhong He. 2025. "Integration of AI and ML in Tuberculosis (TB) Management: From Diagnosis to Drug Discovery" Diseases 13, no. 6: 184. https://doi.org/10.3390/diseases13060184
APA StyleMemon, S., Bibi, S., & He, G. (2025). Integration of AI and ML in Tuberculosis (TB) Management: From Diagnosis to Drug Discovery. Diseases, 13(6), 184. https://doi.org/10.3390/diseases13060184





